Distribution of Selected Trace Elements in the Bayer Process †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling and Technological Data
2.2. Analytical Methods
2.3. Compiling of the Mass Balance
- C: product-normalised concentration of trace element, mg/kg;
- c: measured concentration of trace element in solid, mg/kg; or liquid, mg/L;
- m1: mass flow of material on dry basis, kg/d; or liquor flow m3/d;
- m2: mass flow of aluminium hydroxide on dry calcined basis, kg/d.
3. Results and Discussion
3.1. Metals (and Metalloids) that Accumulate to Liquor
3.2. Metals that Do Not Accumulate to Liquor
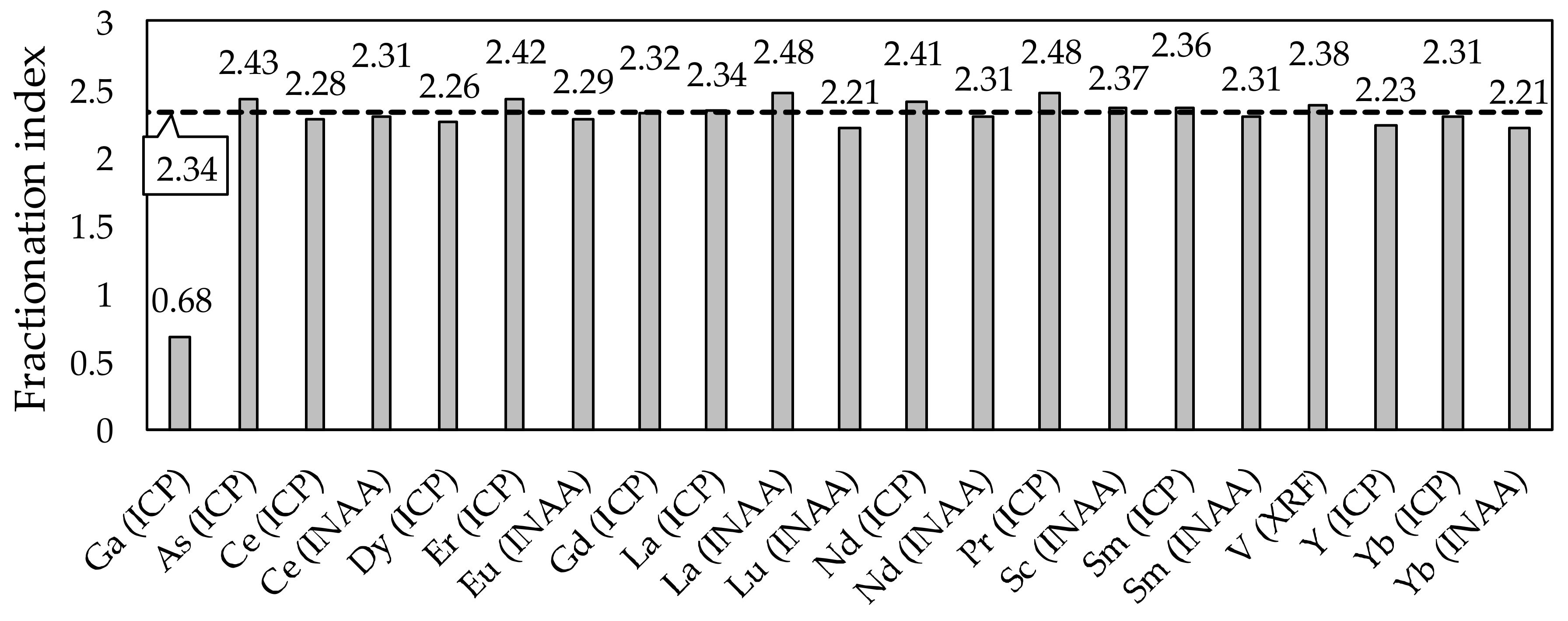
3.3. Fractionation Indexes and Systemic Predictions
- CBR: Predicted concentration of trace element in bauxite residue, mg/kg;
- CFe_BR: Fe2O3 concentration in bauxite residue, %;
- CFe_BX: Fe2O3 concentration in bauxite, %;
- CBX: Average concentration of trace element in bauxite feed, mg/kg.
- CBR: Predicted concentration of trace element in bauxite residue, mg/kg;
- Mtot_BX: Total dry mass of bauxite fed into system, kg;
- Mtot_BR: Total dry mass of bauxite residue leaving the system, kg;
- CBX: Average concentration of trace element in bauxite feed, mg/kg.
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Binnemans, K.; Jones, P.T.; Müller, T.; Yurramendi, L. Rare Earths and the Balance Problem: How to Deal with Changing Markets? J. Sustain. Metall. 2018, 4, 126–146. [Google Scholar] [CrossRef]
- Christmann, P.; Arvanitidis, N.; Martins, L.; Recoché, G.; Solar, S. Towards the Sustainable Use of Mineral Resources: A European Geological Surveys Perspective. Miner. Energy Raw Mater. Rep. 2007, 22, 88–104. [Google Scholar] [CrossRef]
- Christmann, P. Towards a More Equitable Use of Mineral Resources. Nat. Resour. Res. 2018, 27, 159–177. [Google Scholar] [CrossRef]
- European Commission; Deloitte Sustainability; TNO; British Geological Survey; Bureau de Recherches Géologiques et Minières. Study on the Review of the List of Critical Raw Materials; Publications Office of the European Union: Luxembourg, 2017; pp. 1–93.
- Balomenos, E.; Davris, P.; Deady, E.; Yang, J.; Panias, D.; Friedrich, B.; Binnemans, K.; Seisenbaeva, G.; Dittrich, C.; Kalvig, P.; et al. The EURARE Project: Development of a Sustainable Exploitation Scheme for Europe’s Rare Earth Ore Deposits. Johns. Matthey Technol. Rev. 2017, 61, 142–153. [Google Scholar] [CrossRef]
- Davris, P.; Balomenos, E.; Taxiarchou, M.; Panias, D.; Paspaliaris, I. Current and Alternative Routes in the Production of Rare Earth Elements. Berg. Huettenmaenn. Monatsh. 2017, 162, 245–251. [Google Scholar] [CrossRef]
- Evans, K. The history, challenges, and new developments in the management and use of bauxite residue. J. Sustain. Metall. 2016, 2, 316–331. [Google Scholar] [CrossRef]
- Frenzel, M.; Ketris, M.P.; Seifert, T.; Gutzmer, J. On the current and future availability of gallium. Resour. Policy 2016, 47, 38–50. [Google Scholar] [CrossRef]
- Authier-Martin, M.; Forte, G.; Ostap, S.; See, J. The mineralogy of bauxite for producing smelter-grade alumina. JOM 2001, 53, 36–40. [Google Scholar] [CrossRef]
- Teas, E.B.; Kotte, J.J. The effect of impurities on process efficiency and methods for impurity control and removal. In Proceedings of the JBI-JGS Symposium Titled “Bauxite/Alumina Industry in the Americas”, Kingston, Jamaica, 1980; Volume 23, pp. 100–129. [Google Scholar]
- Davris, P.; Balomenos, E.; Panias, D.; Paspaliaris, I. Selective leaching of rare earth elements from bauxite residue (red mud), using a functionalized hydrophobic ionic liquid. Hydrometallurgy 2016, 164, 125–135. [Google Scholar] [CrossRef]
- Hudson, L.K. Gallium as a by-product of alumina manufacture. J. Met. 1965, 17, 948–951. [Google Scholar] [CrossRef]
- Suss, A.; Kuznetsova, N.V.; Kozyrev, A.; Panov, A.; Gorbachev, S. Specific features of scandium behavior during sodium bicarbonate digestion of red mud. In Proceedings of the 35th International ICSOBA Conference, Hamburg, Germany, 2–5 October 2017; Volume 42, pp. 491–504. [Google Scholar]
- Suss, A.; Paromova, I.; Panov, A.; Shipova, O.V.; Kutkova, N.N. Behaviour of Berylium in Alumina Production from Bauxites by Bayer Process and Development of Reliable Method of Its Determination. In Proceedings of the Light Metals Conference, New Orleans, LA, USA, 9–12 March 2008; pp. 107–112. [Google Scholar]
- Løvik, A.N.; Restrepo, E.; Müller, D.B. The global anthropogenic gallium system: Determinants of demand, supply and efficiency improvements. Environ. Sci. Technol. 2015, 49, 5704–5712. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pradhan, R.J.; Das, S.N.; Thakur, R.S. Vanadium sludge—An useful byproduct of alumina plant. J. Sci. Ind. Res. 1999, 58, 948–953. [Google Scholar]
- Binnemans, K.; Jones, P.T.; Blanpain, B.; Van Gerven, T.; Pontikes, Y. Towards zero-waste valorisation of rare-earth-containing industrial process residues: A critical review. J. Clean. Prod. 2015, 99, 17–38. [Google Scholar] [CrossRef] [Green Version]
- Borra, C.R.; Blanpain, B.; Pontikes, Y.; Binnemans, K.; Van Gerven, T. Recovery of rare earths and other valuable metals from bauxite residue (red mud): A review. J. Sustain. Metall. 2016, 2, 365–386. [Google Scholar] [CrossRef]
- Atwood, D.A. The Rare Earth Elements: Fundamentals and Applications; John Wiley & Sons: Lexington, KY, USA, 2012; ISBN 978-1-119-95097-4. [Google Scholar]
- Gray, F.; Kramer, D.A.; Bliss, J.D. Gallium and gallium compounds. In Kirk-Othmer Encyclopedia of Chemical Technology; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2013. [Google Scholar] [CrossRef]
- Bauer, G.; Güther, V.; Hess, H.; Otto, A.; Roidl, O.; Roller, H.; Sattelberger, S.; Köther-Becker, S.; Beyer, T.; Bauer, G.; et al. Vanadium and Vanadium Compounds. In Ullmann’s Encyclopedia of Industrial Chemistry; John Wiley & Sons: Hoboken, NJ, USA, 2017; ISBN 978-3-527-30673-2. [Google Scholar]
- Wang, W.; Pranolo, Y.; Cheng, C.Y. Metallurgical processes for scandium recovery from various resources: A review. Hydrometallurgy 2011, 108, 100–108. [Google Scholar] [CrossRef]
- Habashi, F. Gallium update. In Proceedings of the 17th International Symposium ICSOBA, Montréal, QC, Canada, 1–4 October 2006; Volume 33, pp. 141–153. [Google Scholar]
- Deady, É.A.; Mouchos, E.; Goodenough, K.; Williamson, B.J.; Wall, F. A review of the potential for rare-earth element resources from European red muds: Examples from Seydişehir, Turkey and Parnassus-Giona, Greece. Mineral. Mag. 2016, 80, 43–61. [Google Scholar] [CrossRef] [Green Version]
- Derevyankin, V.A.; Porotnikova, T.P.; Kocherova, E.K.; Yumasheva, I.V.; Moiseev, V.E. Behaviour of scandium and lanthanum in the production of alumina from bauxite (in Russian). Izvestiya Vysshikh Uchebnykh Zavedenii Tsvetnaya Metallurgiya 1981, 4, 86–89. [Google Scholar]
- Mohapatra, B.K.; Mishra, B.K.; Mishra, C.R. Studies on metal flow from khondalite to bauxite to alumina and rejects from an alumina refinery, India. In Light Metals; Suarez, C.E., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2012; pp. 87–91. ISBN 978-1-118-35925-9. [Google Scholar]
- Ochsenkühn-Petropulu, M.; Lyberopulu, T.; Parissakis, G. Direct determination of lanthanides, yttrium and scandium in bauxites and red mud from alumina production. Anal. Chim. Acta 1994, 296, 305–313. [Google Scholar] [CrossRef]
- Wagh, A.S.; Pinnock, W.R. Occurrence of scandium and rare earth elements in Jamaican bauxite waste. Econom. Geol. 1987, 82, 757–761. [Google Scholar] [CrossRef]
- Feret, F.R.; See, J. A comparative study of analytical methods of trace elements in bauxite and red mud. In Proceedings of the 18th International Symposium ICSOBA Travaux, Zhengzhou, China, 25–28 November 2010; pp. 68–83. [Google Scholar]
- Logomerac, V.G. Distribution of rare-earth and minor elements in some bauxite and red mud produced. In Proceedings of the Second International Symposium of ICSOBA, Budapest, Hungary, 6–10 October 1969; Volume 3, pp. 383–393. [Google Scholar]
- Papp, E.; Zsindely, S.; Tomcsanyi, L. Molybdenum and zinc traces in the Bayer process. In Proceedings of the 2nd International Symposium ICSOBA, Budapest, Hungary, 6–10 October 1969; Volume 3, pp. 395–402. [Google Scholar]
- Eyer, S.; Nunes, M.; Dobbs, C.; Russo, A.; Burke, K. The analysis of beryllium in Bayer solids and liquids. In Proceedings of the 7th International Alumina Quality Workshop, Perth, Australia, 16–21 October 2005; Volume 1, pp. 254–257. [Google Scholar]
- Sato, C.; Kazama, S.; Sakamoto, A.; Hirayanagi, K. Behavior of radioactive elements (uranium and thorium) in Bayer process. In Reprinted in Essential Readings in Light Metals; Donaldson, D., Raahauge, B.E., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2013; pp. 191–197. ISBN 978-1-118-64786-8. [Google Scholar]
- Bansal, N.; Vaughan, J.; Tam Wai Yin, P.; Leong, T.; Boullemant, A. Chemical thermodynamics of mercury in the Bayer process. In Proceedings of the 7th International Symposium on Hydrometallurgy (Hydro2014), Victoria, BC, Canada, 22–25 June 2014; Volume II, pp. 559–569. [Google Scholar]
- Bansal, N.; Vaughan, J.; Boullemant, A.; Leong, T. Determination of total mercury in bauxite and bauxite residue by flow injection cold vapour atomic absorption spectrometry. Microchem. J. 2014, 113, 36–41. [Google Scholar] [CrossRef]
- Bárdossy, G. Karst Bauxites: Bauxite Deposits on Carbonate Rocks; Developments in Economic Geology 14; Elsevier Scientific Pub. Co.: Amsterdam, The Netherlands, 1982; ISBN 978-0-444-99727-2. [Google Scholar]
- Valeton, I. Bauxites; Developments in Soil Science 1; Elsevier Publishing Company: Amsterdam, The Netherlands, 1972; ISBN 978-0-444-40888-4. [Google Scholar]
- Adamson, A.N.; Bloore, E.J.; Carr, A.R. Basic Principles of Bayer Process Design. In Reprinted in Essential Readings in Light Metals; Donaldson, D., Raahauge, B.E., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2013; pp. 100–117. ISBN 978-1-118-64786-8. [Google Scholar]
- Chin, L.A.D. The state-of-the-art in Bayer process technology. Light Met. 1988, 1988, 49–53. [Google Scholar]
- Power, G.; Gräfe, M.; Klauber, C. Bauxite residue issues: I. Current management, disposal and storage practices. Hydrometallurgy 2011, 108, 33–45. [Google Scholar] [CrossRef]
- S&P Global Platts, a Division of S&P Global Inc. Methodology and Specifications Guide, Nonferrous. Available online: https://www.platts.com/methodology-specifications/metals (accessed on 3 March 2018).
- Gräfe, M.; Power, G.; Klauber, C. Bauxite residue issues: III. Alkalinity and associated chemistry. Hydrometallurgy 2011, 108, 60–79. [Google Scholar] [CrossRef]
- Hudson, L.K.; Misra, C.; Perrotta, A.J.; Wefers, K.; Williams, F.S. Aluminum Oxide. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co.: Wienheim, Germany, 2000; Volume 2, pp. 607–645. ISBN 978-3-527-30673-2. [Google Scholar]
- Lavalou, E.; Bosca, B.; Keramidas, O. Alumina production from diasporic bauxites. Light Met. 1999, CD-ROM Collection, 55–62. [Google Scholar]
- Papanastassiou, D.; Contaroudas, D.; Solymár, K. Processing and marketing of Greek diasporic bauxite for metallurgical and non-metallurgical applications. In Proceedings of the 17th International Symposium of ICSOBA, “Aluminium: From Raw Materials to Applications”, Montréal, QC, Canada, 1–4 October 2006. [Google Scholar]
- Whittington, B.I. The chemistry of CaO and Ca(OH)2 relating to the Bayer process. Hydrometallurgy 1996, 43, 13–35. [Google Scholar] [CrossRef]
- Bánvölgyi, G. Scale formation in alumina refineries. In Proceedings of the 34th International Conference and Exhibition ICSOBA, Quebec, QC, Canada, 3–6 October 2016; pp. 1–14. [Google Scholar]
- Laskou, M.; Economou-Eliopoulos, M. The role of microorganisms on the mineralogical and geochemical characteristics of the Parnassos-Ghiona bauxite deposits, Greece. J. Geochem. Explor. 2007, 93, 67–77. [Google Scholar] [CrossRef]
- Boulangé, B.; Carvalho, A. The bauxite of Porto Trombetas. In Brazilian Bauxites; Carvalho, A., Boulangé, B., Melfi, A.J., Lucas, Y., Eds.; NUPEGEL, Departamento de Geologia Geral Universidade de Sao Paulo, Brasil: São Paulo, Brazil, 1997. [Google Scholar]
- Adam, C.; Krüger, O. Challenges in analysing rare earth elements in different waste matrices to determine recovery potentials. In Book of Abstracts, 2nd Conference on European Rare Earth Resources; Heliotopos Conferences Ltd.: Santorini, Greece, 2017; pp. 237–239. [Google Scholar]
- Ochsenkühn-Petropoulou, M.; Ochsenkühn, K.; Luck, J. Comparison of inductively coupled plasma mass spectrometry with inductively coupled plasma atomic emission spectrometry and instrumental neutron activation analysis for the determination of rare earth elements in Greek bauxites. Spectrochim. Acta Part B At. Spectrosc. 1991, 46, 51–65. [Google Scholar] [CrossRef]
- Hoffman, E.L. Instrumental neutron activation in geoanalysis. J. Geochem. Explor. 1992, 44, 297–319. [Google Scholar] [CrossRef]
- Govindaraju, K. Report (1967–1981) on four ANRT rock reference samples: Diorite DR-N, serpentine UB-N, bauxite BX-N and disthene DT-N. Geostand. Newsl. 1982, 6, 91–159. [Google Scholar] [CrossRef]
- Govindaraju, K.; Roelandts, I. 1988 compilation report on trace elements in six ANRT rock reference samples: Diorite DR-N, serpentine UB-N, bauxite BX-N, disthene DT-N, granite GS-N and potash feldspar FK-N. Geostand. Geoanal. Res. 1989, 13, 5–67. [Google Scholar] [CrossRef]
- Singh, U.; Mishra, R.S. Simultanious multielemental analysis of alumina process samples using inductively coupled plasma spectrometry (ICP-AES). Anal. Chem. Indian J. 2012, 11, 1–5. [Google Scholar]
- Yamada, Y. X-ray fluorescence analysis by fusion bead method for ores and rocks. Rigaku J. 2010, 26, 15–23. [Google Scholar]
- Metrohm, Determination of Total Caustic, Total Soda and Alumina in Bayer Process Liquors with 859 Titrotherm. Available online: http://partners.metrohm.com/GetDocument?action=get_dms_document&docid=693348 (accessed on 23 March 2018).
- Brookins, D.G. Eh-pH Diagrams for Geochemistry; Springer Science & Business Media: Berlin/Heidelberg, Germany, 1988; ISBN 978-3-642-73093-1. [Google Scholar]
- Brookins, D.G. Eh-pH diagrams for the rare earth elements at 25 C and one bar pressure. Geochem. J. 1983, 17, 223–229. [Google Scholar] [CrossRef]
- Vind, J.; Malfliet, A.; Blanpain, B.; Tsakiridis, P.E.; Tkaczyk, A.H.; Vassiliadou, V.; Panias, D. Rare Earth Element Phases in Bauxite Residue. Minerals 2018, 8, 77. [Google Scholar] [CrossRef]
- Gamaletsos, P.N. Mineralogy and Geochemistry of Bauxites from Parnassos-Ghiona Mines and the Impact on the Origin of the Deposits. Ph.D. Thesis, National and Kapodistrian University of Athens, Athens, Greece, 2014. [Google Scholar]
- Wellington, M.; Valcin, F. Impact of Bayer Process Liquor Impurities on Causticization. Ind. Eng. Chem. Res. 2007, 46, 5094–5099. [Google Scholar] [CrossRef]
- Schulte, R.F.; Foley, N.K. Compilation of Gallium Resource Data for Bauxite Deposits; U.S. Geological Survey: Reston, VA, USA, 2014. [CrossRef]
- Shaw, D.M. The geochemistry of gallium, indium, thallium—A review. Phys. Chem. Earth 1957, 2, 164–211. [Google Scholar] [CrossRef]
- Figueiredo, A.M.G.; Avristcher, W.; Masini, E.A.; Diniz, S.C.; Abrão, A. Determination of lanthanides (La, Ce, Nd, Sm) and other elements in metallic gallium by instrumental neutron activation analysis. J. Alloys Compd. 2002, 344, 36–39. [Google Scholar] [CrossRef]
- Riveros, P.A. Recovery of gallium from Bayer liquors with an amidoxime resin. Hydrometallurgy 1990, 25, 1–18. [Google Scholar] [CrossRef]
- Lamerant, J.-M. Process for Extracting and Purifying Gallium from Bayer Liquors. Patent No US5102512 A, 10 April 1991. [Google Scholar]
- Lamerant, J.-M. Process for Extracting Gallium from Bayer Liquors Using an Impregnated Absorbent Resin. Patent No. US5424050 A, 13 June 1995. [Google Scholar]
- Selvi, P.; Ramasami, M.; Samuel, M.H.P.; Sripriya, R.; Senthilkumar, K.; Adaikkalam, P.; Srinivasan, G.N. Gallium recovery from Bayer’s liquor using hydroxamic acid resin. J. Appl. Polym. Sci. 2004, 92, 847–855. [Google Scholar] [CrossRef]
- Ilić, Z.; Mitrović, A. Determination of gallium in Bayer process sodium aluminate solution by inductively coupled plasma atomic emission spectrometry. Anal. Chim. Acta 1989, 221, 91–97. [Google Scholar] [CrossRef]
- Zhao, Z.; Long, H.; Li, X.; Fan, Y.; Han, Z. Precipitation of vanadium from Bayer liquor with lime. Hydrometallurgy 2012, 115–116, 52–56. [Google Scholar] [CrossRef]
- Fenerty, M.J. Production of Fused Alumina. Patent No. US2961296 A, 22 November 1960. [Google Scholar]
- Smith, P. Reactions of lime under high temperature Bayer digestion conditions. Hydrometallurgy 2017, 170, 16–23. [Google Scholar] [CrossRef]
- Okudan, M.D.; Akcil, A.; Tuncuk, A.; Deveci, H. Effect of parameters on vanadium recovery from by-products of the Bayer process. Hydrometallurgy 2015, 152, 76–83. [Google Scholar] [CrossRef]
- Burke, I.T.; Mayes, W.M.; Peacock, C.L.; Brown, A.P.; Jarvis, A.P.; Gruiz, K. Speciation of Arsenic, Chromium, and Vanadium in Red Mud Samples from the Ajka Spill Site, Hungary. Environ. Sci. Technol. 2012, 46, 3085–3092. [Google Scholar] [CrossRef] [PubMed]
- Zhong-Lin, Y.; Song-Qing, G. Development of research on the scaling problem in Bayer process. Light Met. 1995, 155–159. [Google Scholar]
- Vind, J.; Malfliet, A.; Bonomi, C.; Paiste, P.; Sajó, I.E.; Blanpain, B.; Tkaczyk, A.H.; Vassiliadou, V.; Panias, D. Modes of occurrences of scandium in Greek bauxite and bauxite residue. Miner. Eng. 2018, 123, 35–48. [Google Scholar] [CrossRef]
- Gladyshev, S.V.; Akcil, A.; Abdulvaliyev, R.A.; Tastanov, E.A.; Beisembekova, K.O.; Temirova, S.S.; Deveci, H. Recovery of vanadium and gallium from solid waste by-products of Bayer process. Miner. Eng. 2015, 74, 91–98. [Google Scholar] [CrossRef]
- Schlegl, R.; Weber, M.; Wruss, J.; Low, D.; Queen, K.; Stilwell, S.; Lindblad, E.B.; Möhlen, M. Influence of elemental impurities in aluminum hydroxide adjuvant on the stability of inactivated Japanese Encephalitis vaccine, IXIARO®. Vaccine 2015, 33, 5989–5996. [Google Scholar] [CrossRef] [PubMed]
- Santana, F.; Tartarotti, F. Alumina recovery estimation through material balance in Alumar refinery. In Proceedings of the 19th International Symposium ICSOBA, Belém, Brazil, 29 October–2 November 2012; pp. 1–6. [Google Scholar]

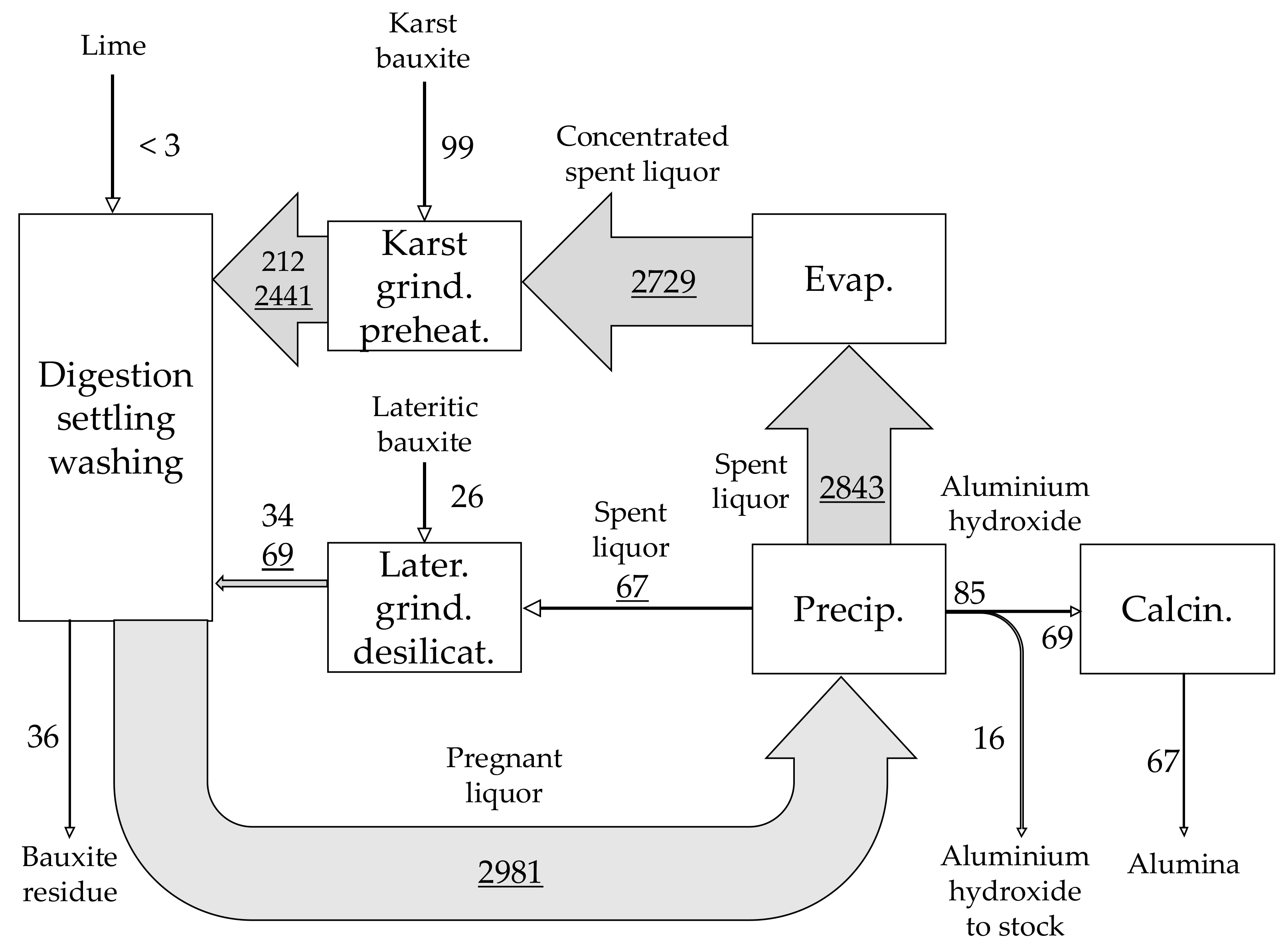

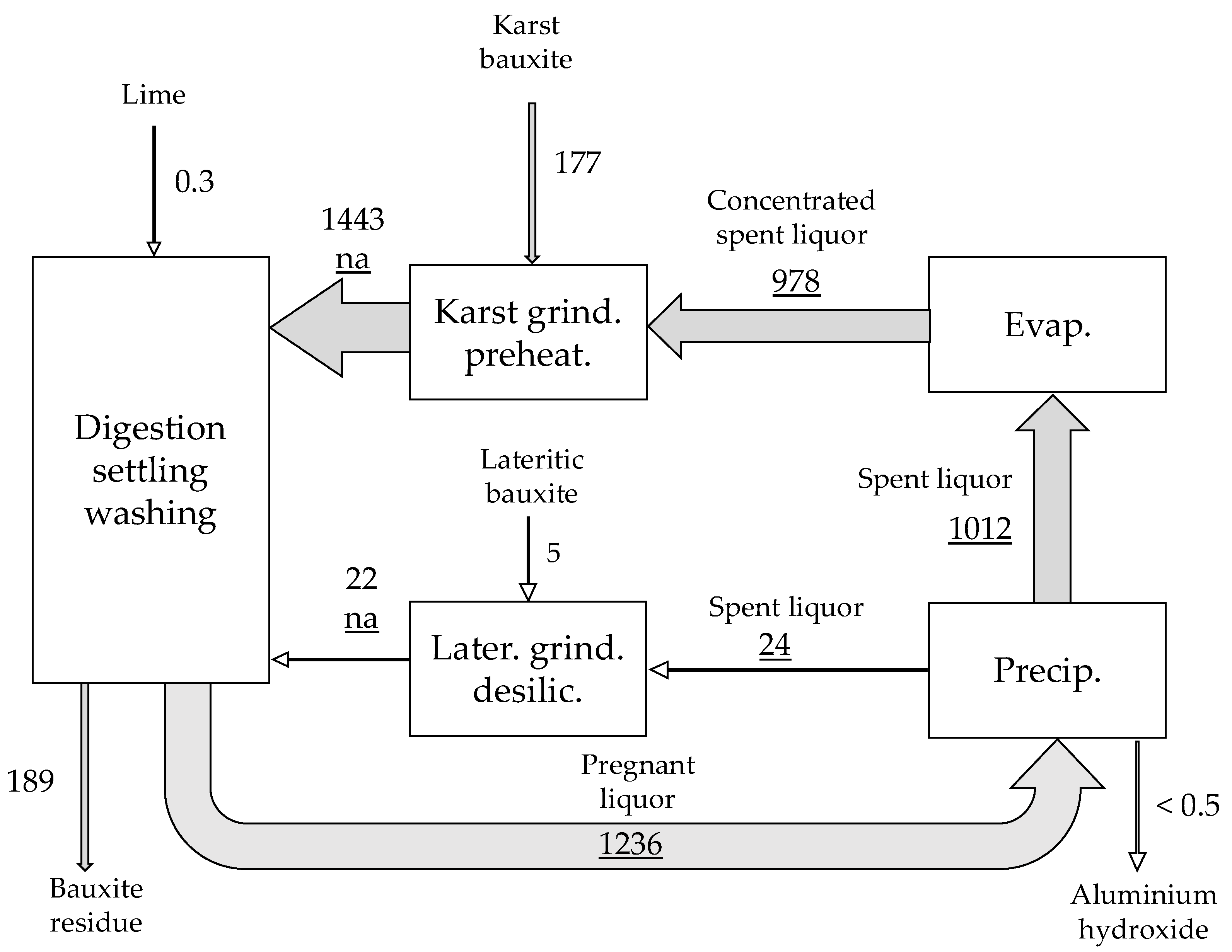
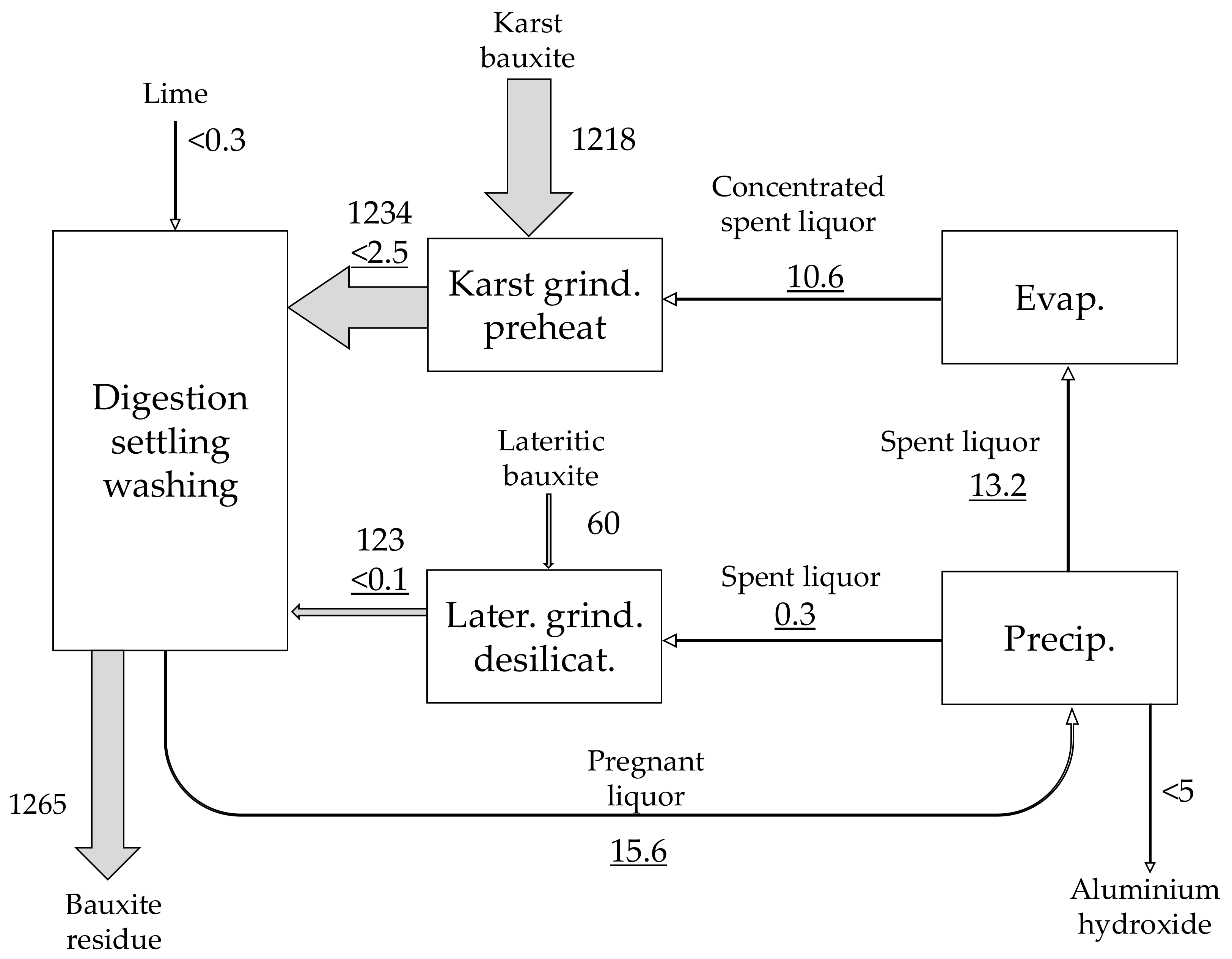
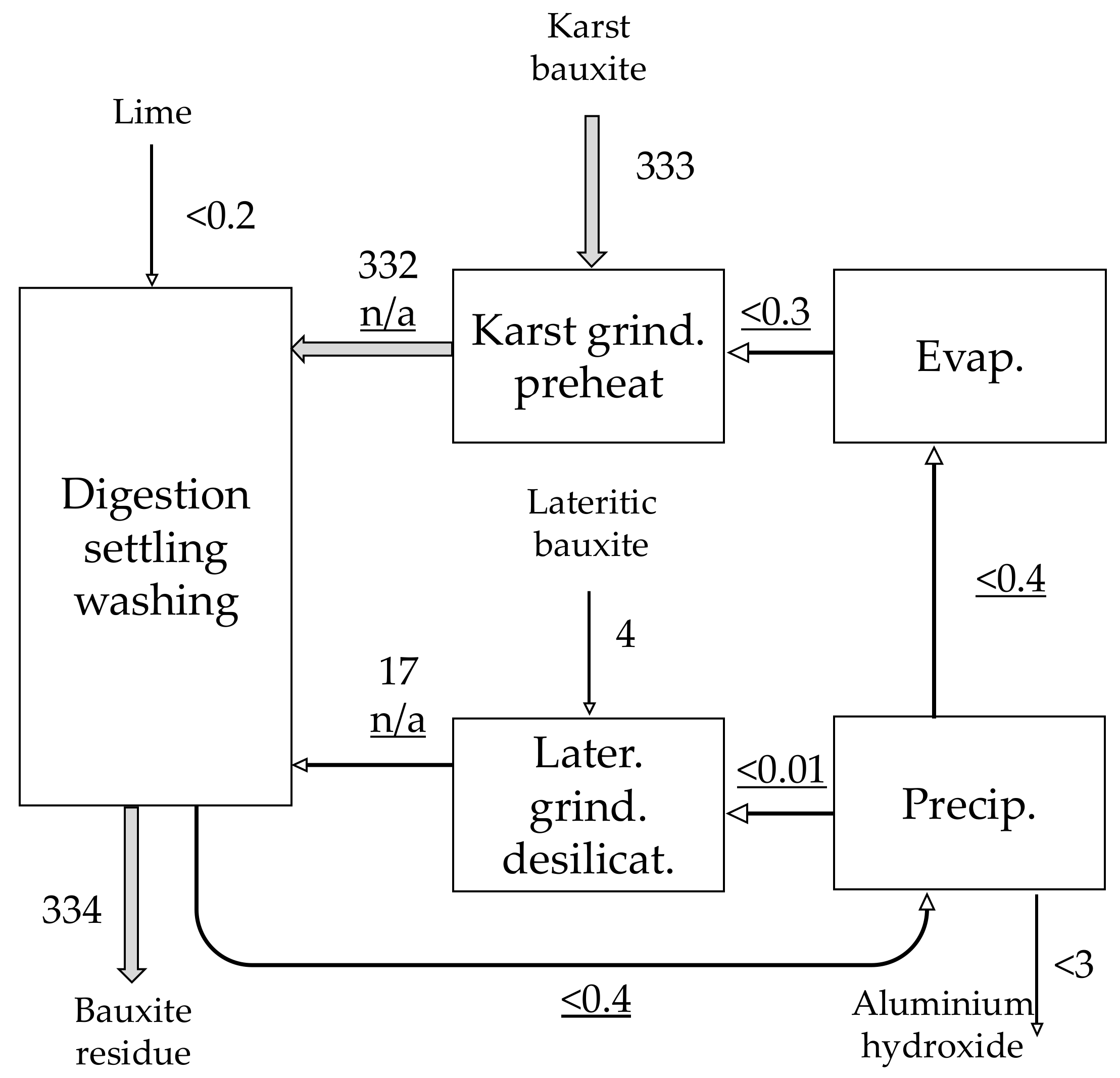
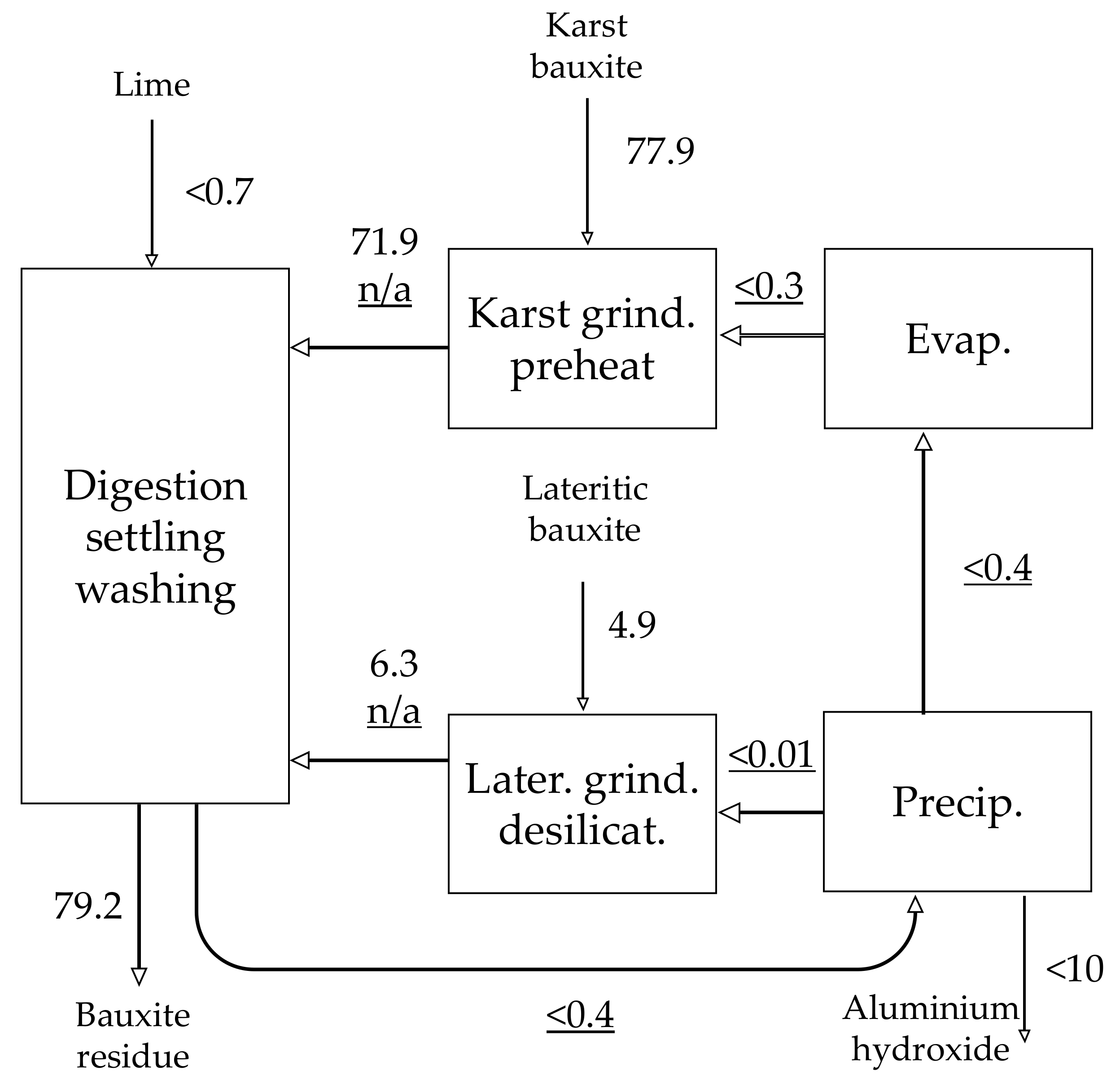
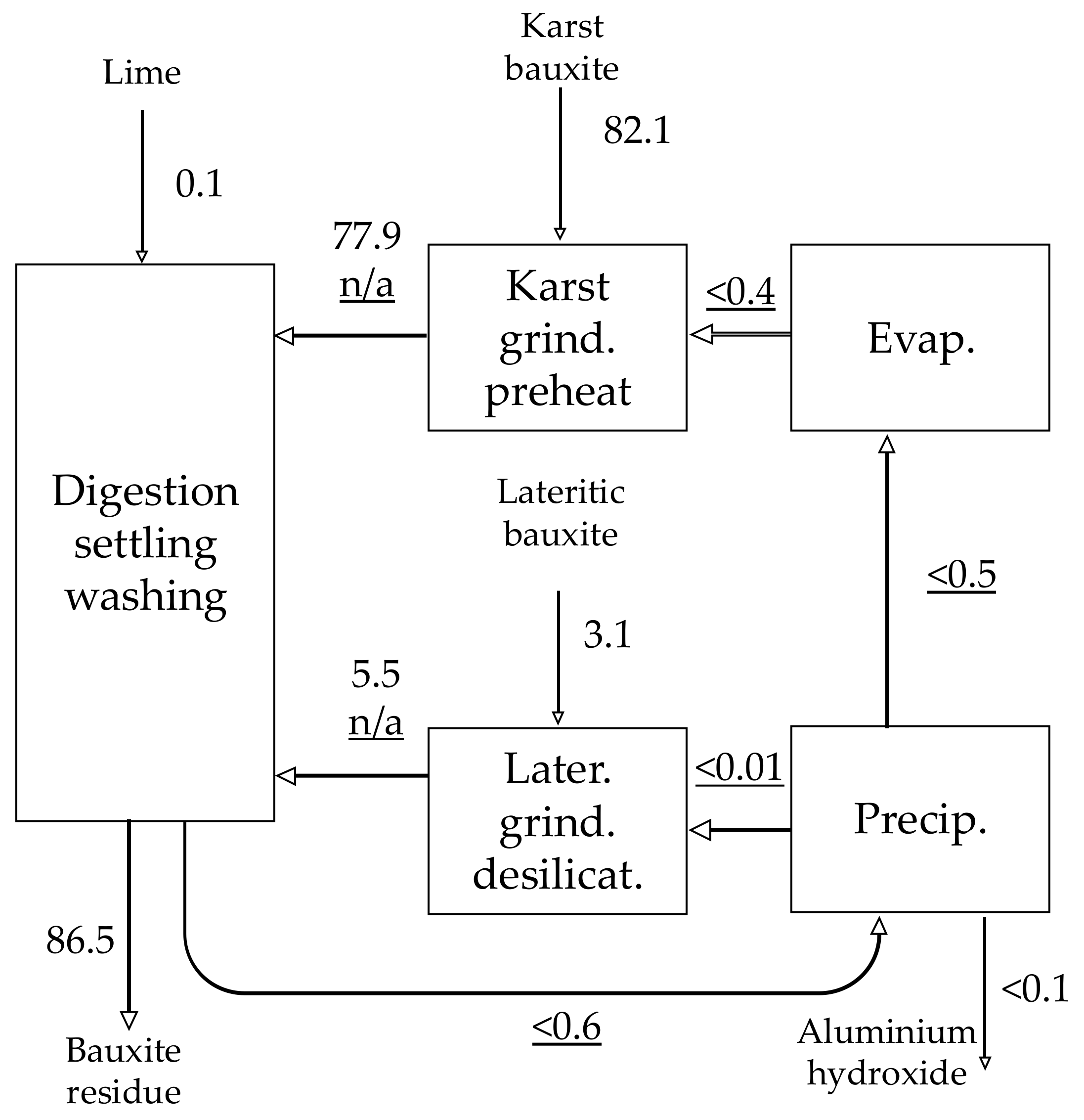
| Material | No. | Code | Description | |
|---|---|---|---|---|
| Input bauxite | Karst/diasporic | 1 | DD-BX | Parnassos-Ghiona bauxite from B3 horizon. Extracted by company Delphi-Distomon S.A., a subsidiary of Mytilineos S.A. In the plant jargon it is termed as “Delphi-Distomo” bauxite. This material is subjected to limestone removal before the Bayer process. |
| 2 | ST-BX | Parnassos-Ghiona bauxite from B3 horizon. Extracted by S&B Industrial Minerals S.A. In the plant jargon it is termed as “standard” bauxite. This material is subjected to limestone removal before the Bayer process. | ||
| 3 | HS-BX | Parnassos-Ghiona bauxite from horizon B2. It represents a minor input (1%) to the process. At present day, it is no longer exploited. | ||
| 4 | TU-BX | Bauxite from Turkey, western Taurides range and Milas area. | ||
| Lateritic/gibbsitic | 5 | TR-BX | Bauxite from Brazil, Porto Trombetas deposit. It represents the main input of lateritic bauxite to the process (21% of total bauxite input). Input of lateritic bauxite alters between this (Porto Trombetas) and Ghanaian (Awaso) bauxite. | |
| 6 | GH-BX | Bauxite from Ghana, Awaso deposit. At the time of sampling, this material was not processed. | ||
| HMS unit | 7 | DC-BX | Mixed karst/diasporic bauxite, from which limestone is separated (“decalcitated”). Inputs to this unit are DD-BX and ST-BX. It represents the main bauxite input (73% of total bauxite input) to the Bayer process as a mixture of karst bauxite from two different mining locations that exploit B3 horizon Parnassos-Ghiona bauxite. | |
| 8 | DC-RE | HMS residue. Limestone residue that is separated from Parnassos-Ghiona B3 horizon bauxite. Besides limestone, contains also a significant proportion of rejected bauxite material. | ||
| Intermediate materials/additives | 9 | BF-DG | Solid fraction of karst bauxite slurry, from grinding and preheating units of karst bauxite. | |
| 10 | SW-DS | Solid fraction of lateritic/gibbsitic bauxite slurry from pre-desilication unit. | ||
| 11 | CA-OX | Lime, CaO. | ||
| Products/by-products | 12 | HY-AL | Aluminium hydroxide, Al(OH)3, output from precipitation unit. | |
| 13 | CA-AL | Calcined alumina, Al2O3, output from calcination unit. | ||
| 14 | RM-FP | Bauxite residue after the filterpressing of residue slurry. | ||
| Liquid Samples | ||||
| Liquors | 15 | CL | Concentrated spent liquor, from the output of evaporation unit, routed to karst bauxite grinding. | |
| 16 | PL | Pregnant liquor, from the outlet of settling and security filtration. | ||
| 17 | SL | Spent liquor, from the outlet of precipitation. Largest proportion is routed to evaporation and a small proportion to lateritic bauxite grinding. | ||
| Slurries | 18 | BF | Liquid phase from grinding and preheating units of karst bauxite, corresponds to sampling point 9 and solid sample “BF-DG”. | |
| 19 | SW | Liquid phase from grinding and desilication unit of lateritic bauxite, corresponds to sampling point of 10 “SW-DS”. | ||
| Abbreviation | Method | Preparation and Specifications | |
|---|---|---|---|
| Solid samples | XRF-st | X-ray fluorescence, standardised (Perform’X, Thermo Fisher Scientific™, Waltham, MA, USA) | Fusion of solids with Li2B4O7/LiBO2 (66:33) flux, sample to flux ratio 1:11 [56]. Standardised with appropriate standard materials. |
| ICP-MS | Inductively coupled plasma mass spectrometry (Xseries 2, Thermo Fisher Scientific™, Waltham, MA, USA) | Fusion of solids with Li2B4O7/LiBO2 (66:33) flux, sample to flux ratio 1:20, glass bead dissolved in 10% v/v nitric acid. | |
| INAA | Instrumental neutron activation analysis (Activation Laboratories Ltd., Ancaster, ON, Canada) | About 2 g of sample is inserted in a polyethylene vial [52]. | |
| titr. | Thermometric acid-base titration (855 Robotic Titrosampler, Metrohm, Herisau, Switzerland) | Details given in method description [57]. | |
| Liquor samples | AAS | Atomic absorption spectrophotometer (2100, PerkinElmer, Waltham, MA, USA) | Appropriate dilution with deionised water. |
| ICP-MS | Inductively coupled plasma mass spectrometer (specified above) | Sample is diluted with deionised water and then acidified with concentrated nitric acid while gently heating the sample in proportions 1:10:1, additional dilutions are made [55]. | |
| ICP-OES | Inductively coupled plasma optical emission spectrometer (Optima 8000, PerkinElmer, Waltham, MA, USA) | Sample is diluted with deionised water and then acidified with concentrated nitric acid while gently heating the sample in proportions 1:10:1, additional dilutions are made [55]. | |
| INAA | Instrumental neutron activation analysis (specified above) | Dewatering of liquor until the creation of dry pulps (Büchi Syncore, vacuum pump Büchi V-700, controller Büchi V-850; Flawil, Switzerland). Then, about 2 g of sample is inserted in a polyethylene vial. | |
| XRF | X-ray fluorescence, no standardisation, semi-quantitative (Xepos, Spectro, Kleve, Germany) | Dewatering of liquor until the creation of dry pulps (specified above). | |
| UV | UV Photometer (Cary 60 UV–Vis, Agilent, Santa Clara, CA, United States) | Acidification with conc. HCl and dilution with deionised water in proportions 1:3:20; only for analysing Fe. |
| Sample | Al2O3 * | Na2O ** | As | Br | Ca | Ce | Cr | Fe | Ga | Gd | K |
|---|---|---|---|---|---|---|---|---|---|---|---|
| g/L | g/L | mg/L | mg/L | mg/L | mg/L | mg/L | mg/L | mg/L | mg/L | g/L | |
| titr. | titr. | INAA | INAA | AAS | ICP-MS | ICP-MS | UV | ICP-OES | ICP-MS | AAS | |
| PL | 192.2 | 159.4 | 110.8 | 33.6 | 14.9 | <0.04 | 1.4 | 9.6 | 267.2 | <0.04 | 13.7 |
| SL | 108.6 | 171.7 | 99.6 | 31.4 | 16.5 | <0.04 | 1.3 | 3.4 | 279.7 | <0.04 | 13.8 |
| La | Mg | Mo | Ni | Sc | Si | Th | U | V | W | Y | |
| mg/L | mg/L | mg/L | mg/L | mg/L | mg/L | mg/L | mg/L | mg/L | mg/L | mg/L | |
| ICP-MS | AAS | INAA | AAS | INAA | AAS | INAA | ICP-MS | ICP-OES | INAA | ICP-MS | |
| PL | <0.04 | <0.1 | 318 | 4.8 | <0.05 | 544 | <0.1 | 1.28 | 295.2 | 27 | <0.04 |
| SL | <0.04 | <0.1 | 273 | <4 | <0.05 | 520 | <0.1 | 1.27 | 314.7 | 21 | <0.04 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vind, J.; Alexandri, A.; Vassiliadou, V.; Panias, D. Distribution of Selected Trace Elements in the Bayer Process. Metals 2018, 8, 327. https://doi.org/10.3390/met8050327
Vind J, Alexandri A, Vassiliadou V, Panias D. Distribution of Selected Trace Elements in the Bayer Process. Metals. 2018; 8(5):327. https://doi.org/10.3390/met8050327
Chicago/Turabian StyleVind, Johannes, Alexandra Alexandri, Vicky Vassiliadou, and Dimitrios Panias. 2018. "Distribution of Selected Trace Elements in the Bayer Process" Metals 8, no. 5: 327. https://doi.org/10.3390/met8050327
APA StyleVind, J., Alexandri, A., Vassiliadou, V., & Panias, D. (2018). Distribution of Selected Trace Elements in the Bayer Process. Metals, 8(5), 327. https://doi.org/10.3390/met8050327





