Forming and Oxidation Behavior During Forging with Consideration of Carbon Content of Steel
Abstract
1. Introduction
2. Finite Element Model
2.1. Material Data
2.2. Forming Behavior
2.3. Recrystallization Kinetics
- DRX:
- MDRX:
- SRX:
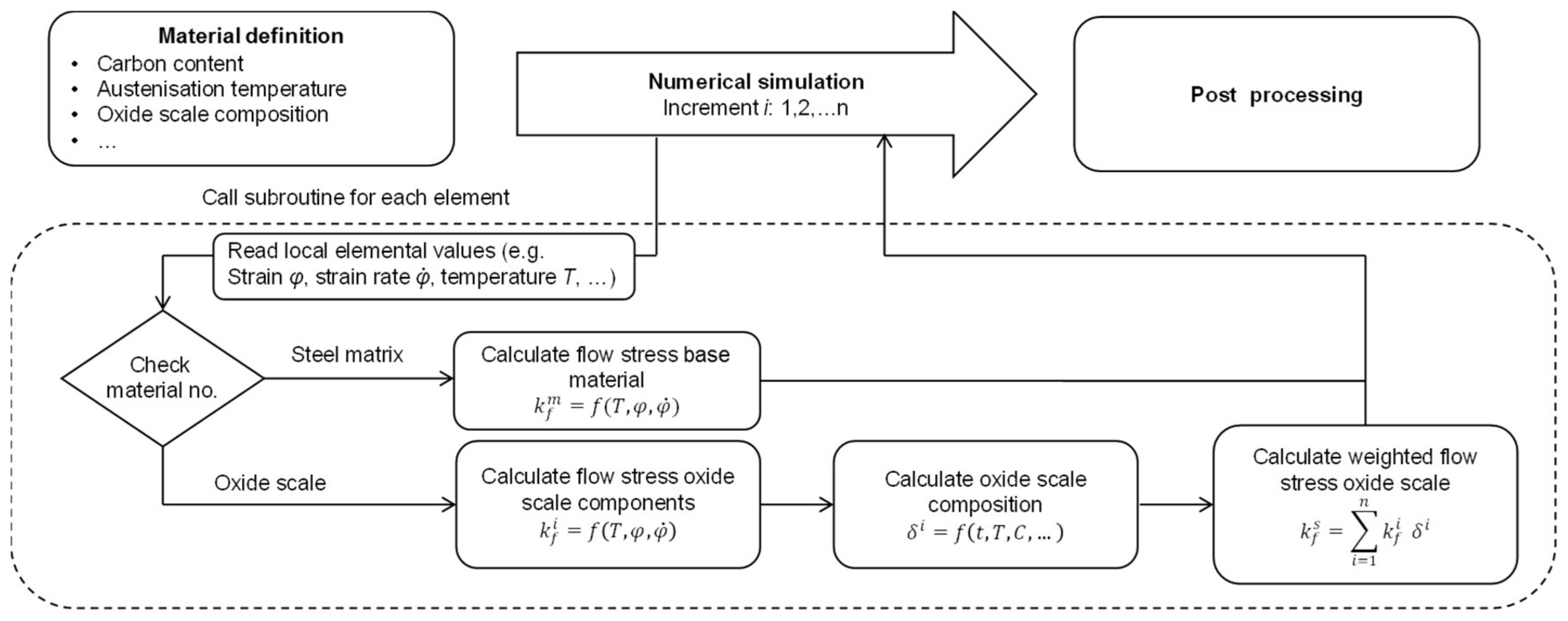
2.4. Oxidation Behavior
3. Numerical and Experimental Simulation of Die Forging Processes
3.1. FE Model
3.2. Simulation of Ring Compression Tests
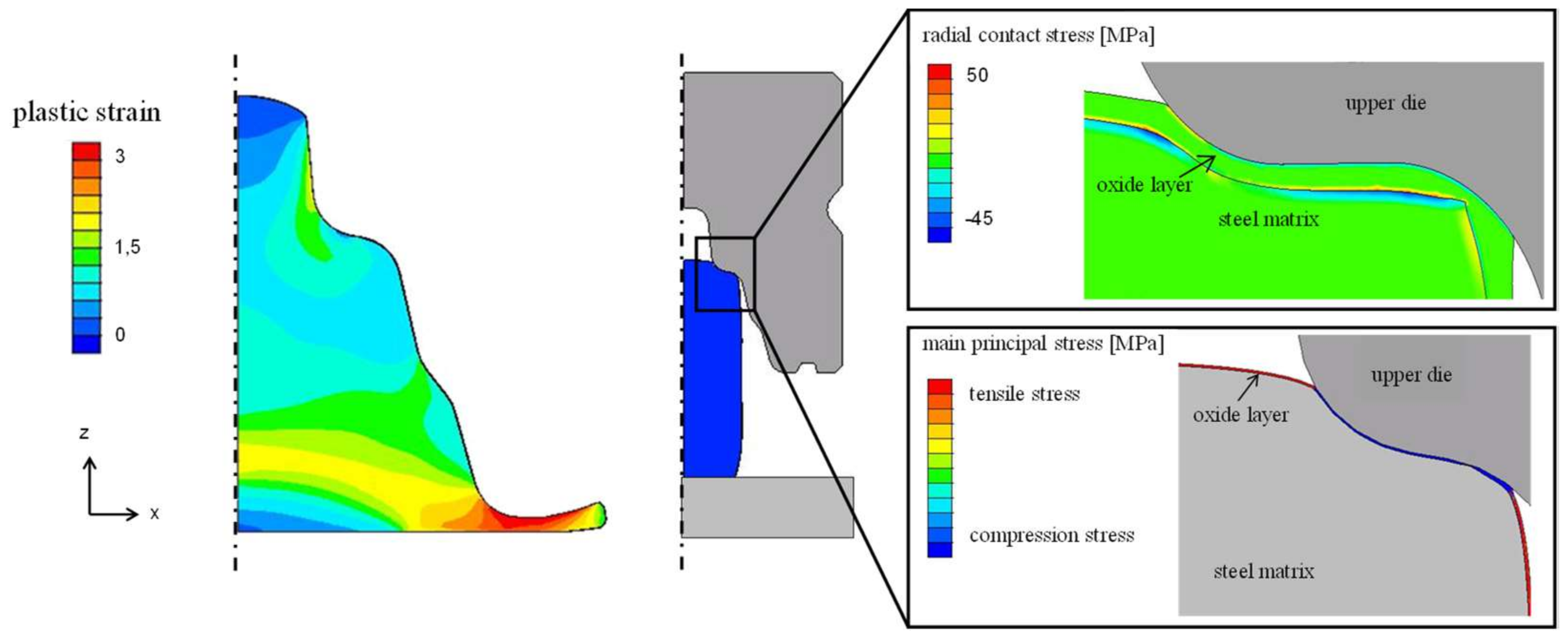
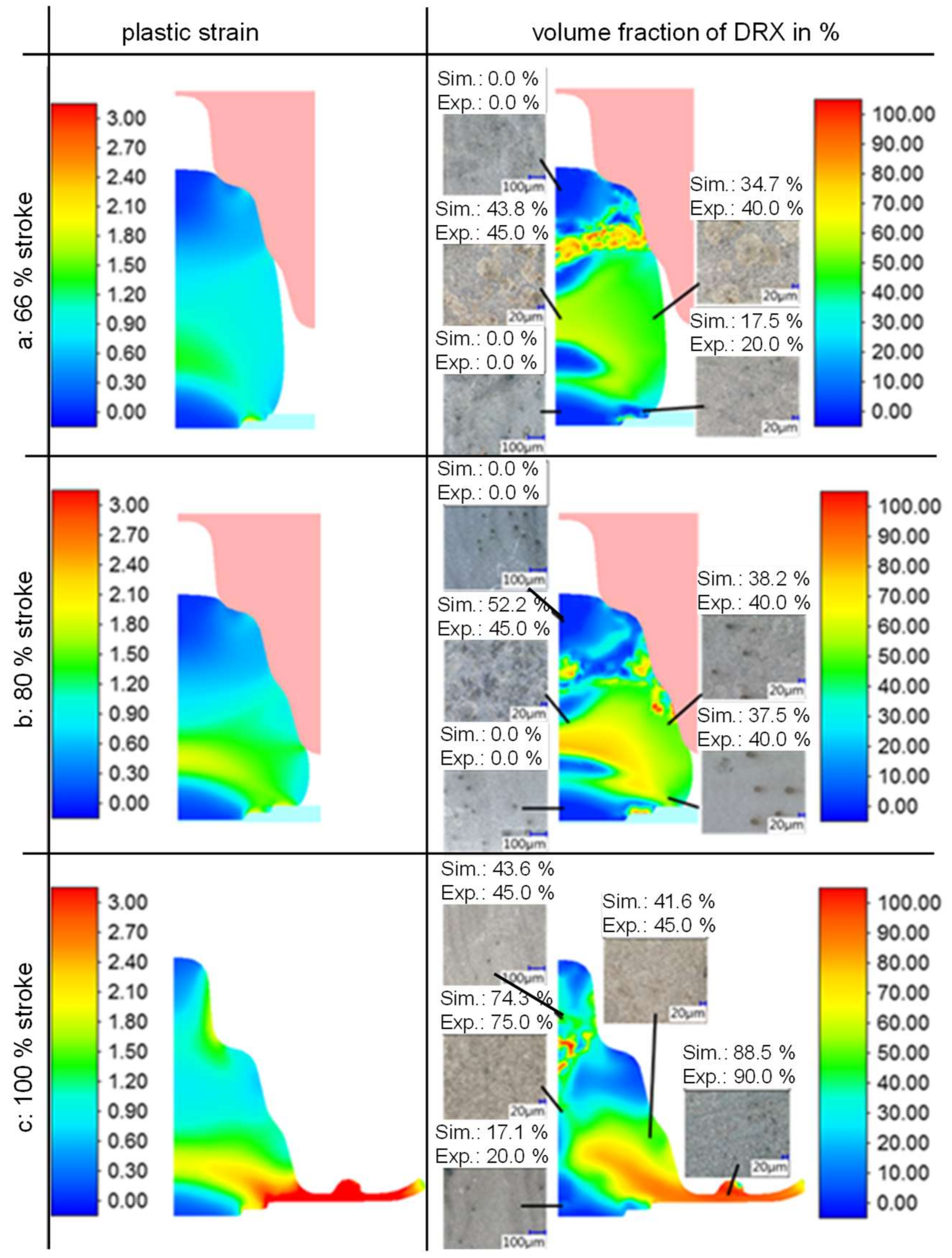
3.3. Simulation of Laboratory Die Forging
4. Conclusions
Author Contributions
Funding
Acknowledgements
Conflicts of Interest
References
- Kawalla, R.; Steinert, F. Untersuchung des Einflusses von Prozessparametern in der Fertigstrasse auf die Tertiärzunderausbildung. Materialwiss. Werkstofftech. 2007, 38, 36–42. (In German) [Google Scholar] [CrossRef]
- Suarez, L.; Schneider, J.; Houbaert, Y. High-Temperature Oxidation of Fe-Si Alloys in the Temperature Range 900–1250 °C. Defect Diffus. Forum 2008, 273–276, 661–666. [Google Scholar]
- Yuan, Q.; Xu, G.; Zhou, M.; He, B. The Effect of the Si Content on the Morphology and Amount of Fe2SiO4 in Low Carbon Steels. Metals 2016, 6, 94. [Google Scholar] [CrossRef]
- Uran, S.; Veal, B.; Grimsditch, M.; Pearson, J.; Berger, A. Effect of Surface Roughness on Oxidation: Changes in Scale Thickness, Composition, and Residual Stress. Oxid. Met. 2000, 54, 73–85. [Google Scholar] [CrossRef]
- Sun, W.; Tieu, A.K.; Jiang, Z.; Lu, C. High temperature oxide scale characteristics of low carbon steel in hot rolling. J. Mater. Process. Technol. 2004, 155–156, 105–113. [Google Scholar] [CrossRef]
- Giggins, C.S.; Pettit, F.S. Corrosion of metals and alloys in mixed gas environments at elevated temperatures. Oxid. Met. 1980, 14, 363–413. [Google Scholar] [CrossRef]
- Eubanks, A.G.; Moore, D.G.; Pennington, W.A. Effect of Surface Roughness on the Oxidation Rate of Iron. J. Electrochem. Soc. 1962, 109, 382–389. [Google Scholar] [CrossRef]
- Barnes, D.J.; Wilson, J.E.; Stott, F.H.; Wood, G.C. The influence of oxide films on the friction and wear of Fe-5% Cr alloy in controlled environments. Wear 1977, 45, 161–176. [Google Scholar] [CrossRef]
- Fernández, L.A.; Houbart, Y. Hot rolling of high-silicon steel: Scale growth and its plastic deformation. In Proceedings of the Conference WMM, Cardiff, UK, 17–19 May 2014; pp. 55–74. [Google Scholar]
- Hinslex, C.F.; Male, A.T.; Rowe, G.W. Frictional properties of metal oxides at high temperatures. Wear 1968, 11, 233–238. [Google Scholar] [CrossRef]
- Kong, H.; Yoon, E.-S.; Kwon, K. Self-formation of protective oxide films at dry sliding mild steel surfaces under a medium vacuum. Wear 1995, 181–183, 325–333. [Google Scholar] [CrossRef]
- Krzyzanowski, M.; Beynon, J.H.; Farrugia, D.C.J. Oxide Scale Behavior in High Temperature Metal Processing; Wiley-VCH Verlag: Weinheim, Germany, 2010. [Google Scholar]
- Vergne, C.; Boher, C.; Gras, R.; Levaillant, C. Influence of oxides on friction in hot rolling: Experimental investigations and tribological modelling. Wear 2006, 260, 957–975. [Google Scholar] [CrossRef]
- Hensger, K.-E.; Klimanek, P.; Böhme, D. Beitrag zur metallkundlichen Interpretation von Warmfließkurven. Neue Hütte 1983, 28, 15–20. (In German) [Google Scholar]
- Graf, M.; Kawalla, R. Approaches for a Fast Analysis System for Hot Rolling Processes. Steel Res. Int. 2014, 85, 1364–1368. [Google Scholar] [CrossRef]
- Schacht, K.; Prahl, U.; Bleck, W. Material Models and their Capability for Process and Material Properties Design in Different Forming Processes. Mater. Sci. Forum 2016, 854, 174–182. [Google Scholar] [CrossRef]
- Papaefthymiou, S.; Goulas, C.; Castro Cerda, F.M.; Geerlofs, N.; Petrov, R. The Effect of Heating Rate on the Microstructure of a Soft-Annealed Medium Carbon Steel. Steel Res. Int. 2017, 88, 1700158. [Google Scholar] [CrossRef]
- Pohlandt, K. Werkstoffe und Werkstoffprüfung für die Kaltmassivumformung; Expert-Verlag: Renningen-Malmsheim, Germany, 2001. (In German) [Google Scholar]
- Ullmann, M.; Kawalla, R.; Schmidtchen, M. Dynamic Recrystallization Behaviour of Twin Roll Cast AZ31 Strips during Hot Deformation. Key Eng. Mater. 2014, 622–623, 569–574. [Google Scholar] [CrossRef]
- Korpala, G. Einfluss der chemischen Zusammensetzung auf die mechanischen Eigenschaften von unlegiertem bainitischen Stahl mit Restaustenit. Ph.D. Thesis, TU Bergakademie Freiberg, Freiberg, Germany, June 2017. [Google Scholar]
- Korpala, G.; Ullmann, M.; Graf, M.; Wester, H.; Bouguecha, A.; Awiszus, B.; Behrens, B.-A.; Kawalla, R. Modelling the Influence of Carbon Content on Material Behavior during Forging. AIP Conf. Proc. 2017, 1896. [Google Scholar] [CrossRef]
- Behrens, B.-A.; Chugreev, A.; Awiszus, B.; Graf, M.; Kawalla, R.; Ullmann, M.; Korpala, G.; Wester, H. Sensitivity Analysis of Oxide Scale Influence on General Carbon Steels during Hot Forging. Metals 2018, 8, 140. [Google Scholar] [CrossRef]
- Tominaga, J.; Wakimoto, K.; Mori, T.; Murakami, M.; Yoshimura, T. Manufacture of wire rods with good descaling property. Trans. Iron Steel Inst. Jpn. 1982, 22, 646–656. [Google Scholar] [CrossRef]
- Sun, W. A Study on the Characteristics of Oxide Scale in Hot Rolling of Steel. Ph.D. Thesis, University of Wollongong, Wollongong, Australia, 2005. [Google Scholar]
- Krzyzanowski, M.; Beyon, J.H. Oxide Behaviour in hot rolling. In Metal Forming Science and Practice; Lenard, J., Ed.; Elsevier: Amsterdam, The Netherlands, 2002; pp. 259–295. [Google Scholar]
- Frolish, M.F.; Krzyzanowski, M.; Beyon, J.H. Oxide scale behaviour on aluminium and steel under hot working conditions. J. Mater. Process. Technol. 2006, 177, 36–40. [Google Scholar] [CrossRef]

| Steel | C | Si | Mn | P | S | Cr | Mo | Ni | Cu | Al |
|---|---|---|---|---|---|---|---|---|---|---|
| C15 | 0.160 | 0.210 | 0.420 | 0.001 | <0.001 | 0.100 | 0.045 | 0.087 | 0.108 | 0.017 |
| C45 | 0.490 | 0.200 | 0.420 | 0.004 | 0.001 | 0.100 | 0.034 | 0.081 | 0.096 | 0.021 |
| C60 | 0.630 | 0.200 | 0.420 | <0.001 | <0.001 | 0.100 | 0.035 | 0.076 | 0.100 | 0.017 |
| 3275 | 0.03662 | 0.03642 | 0.00270 | 0.41618 | −0.04210 | −0.08004 | 0.07959 |
| 894 | −0.01367 | 0.00276 | 0.00236 | 0.17777 |
| 1.83501 | 0.00015 | 0.13325 | 0.17776 | 0.15951 |
| Steel | ||||||||
|---|---|---|---|---|---|---|---|---|
| C15 | ||||||||
| C45 | ||||||||
| C60 |
| Steel | nm | m1 | m2 | QMDRX |
|---|---|---|---|---|
| C15 | 1.15 | 5.55 × 10−7 | −0.84 | 147700 |
| C45 | 1.29 | 5.76 × 10−7 | −0.81 | 141400 |
| C60 | 1.34 | 5.87 × 10−7 | −0.78 | 135900 |
| Steel | |||||
|---|---|---|---|---|---|
| C15 | 3.16 × 10−14 | ||||
| C45 | 7.15 × 10−15 | ||||
| C60 | 1.76 × 10−15 |
| Steel | kp0 [µm²/s] | Qp [J/mol] | kl0 [µm/s] | Ql [J/mol] | r² |
|---|---|---|---|---|---|
| C15 | 8794612 | 132539 | 9.855 × 1014 | 344434 | 0.966 |
| C45 | 5218083 | 129089 | 9.852 × 1014 | 350988 | 0.918 |
| C60 | 677343 | 107488 | 5.600 × 1014 | 322481 | 0.792 |
| Initial State for FEM | Steel Grad | Experiment | Simulation | Deviation | |||
|---|---|---|---|---|---|---|---|
| Inner ø in mm | Height in mm | Inner ø in mm | Height in mm | Inner ø in % | Height in % | ||
| Oxidation temperature: 1000 °C Oxide thickness: 50 µm | C15 | 3.70 | 1.61 | 3.91 | 1.63 | 5.1 | 1.2 |
| C45 | 3.75 | 1.67 | 3.86 | 1.70 | 2.9 | 1.8 | |
| C60 | 3.80 | 1.68 | 3.77 | 1.66 | −0.8 | −1.2 | |
| Oxidation temperature: 900 °C Oxide thickness: 30 µm | C15 | 3.65 | 1.59 | 3.74 | 1.59 | 2.5 | 1.9 |
| C45 | 3.71 | 1.82 | 4.00 | 1.82 | 7.8 | −1.1 | |
| C60 | 3.80 | 1.67 | 3.81 | 1.68 | 0.3 | 0.6 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Graf, M.; Ullmann, M.; Korpala, G.; Wester, H.; Awiszus, B.; Kawalla, R.; Behrens, B.-A. Forming and Oxidation Behavior During Forging with Consideration of Carbon Content of Steel. Metals 2018, 8, 996. https://doi.org/10.3390/met8120996
Graf M, Ullmann M, Korpala G, Wester H, Awiszus B, Kawalla R, Behrens B-A. Forming and Oxidation Behavior During Forging with Consideration of Carbon Content of Steel. Metals. 2018; 8(12):996. https://doi.org/10.3390/met8120996
Chicago/Turabian StyleGraf, Marcel, Madlen Ullmann, Grzegorz Korpala, Hendrik Wester, Birgit Awiszus, Rudolf Kawalla, and Bernd-Arno Behrens. 2018. "Forming and Oxidation Behavior During Forging with Consideration of Carbon Content of Steel" Metals 8, no. 12: 996. https://doi.org/10.3390/met8120996
APA StyleGraf, M., Ullmann, M., Korpala, G., Wester, H., Awiszus, B., Kawalla, R., & Behrens, B.-A. (2018). Forming and Oxidation Behavior During Forging with Consideration of Carbon Content of Steel. Metals, 8(12), 996. https://doi.org/10.3390/met8120996









