Exploring the Difference in Bainite Transformation with Varying the Prior Austenite Grain Size in Low Carbon Steel
Abstract
1. Introduction
2. Experimental Method
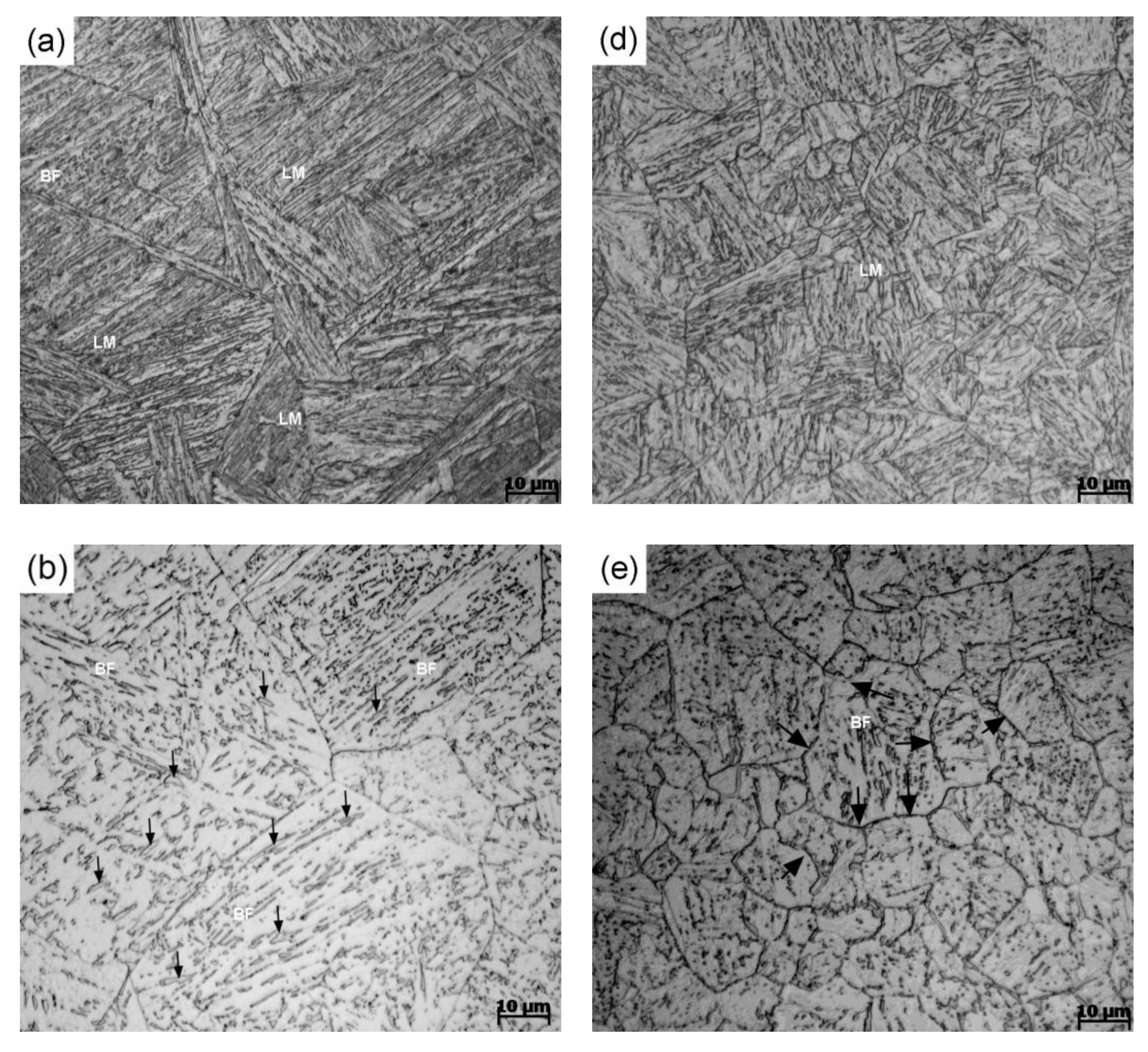
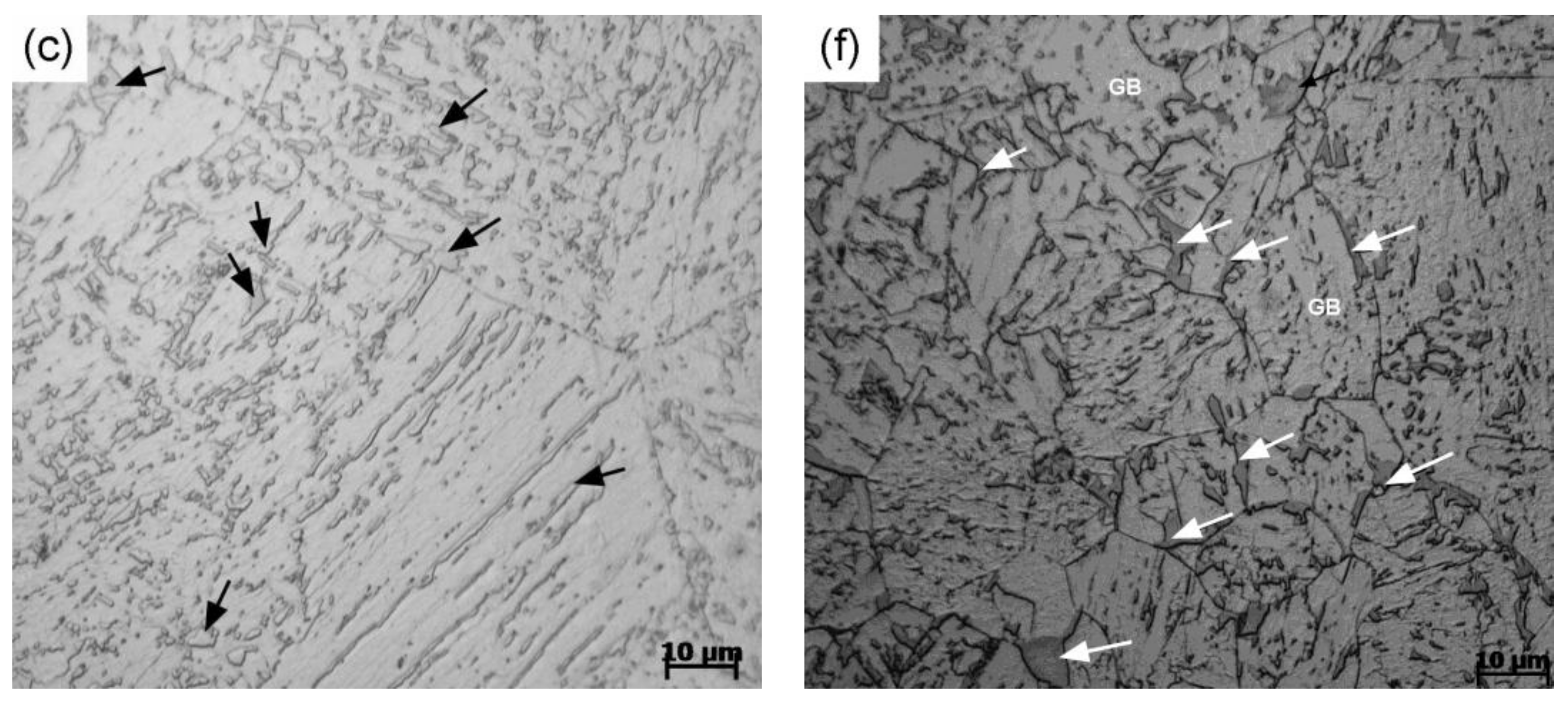
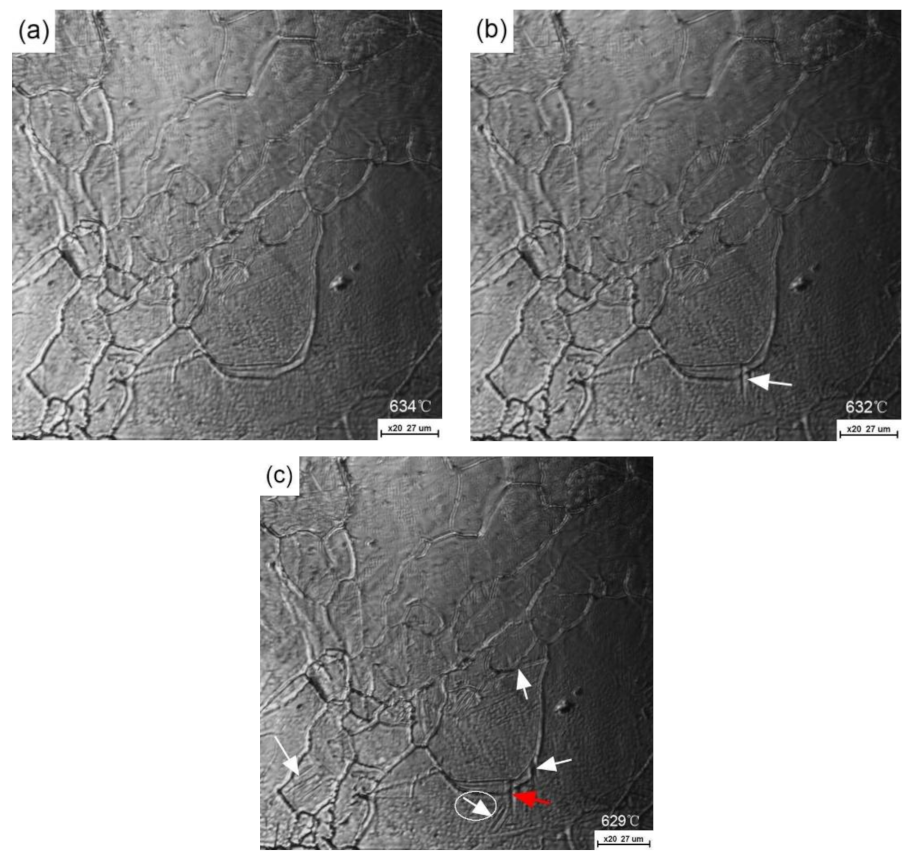
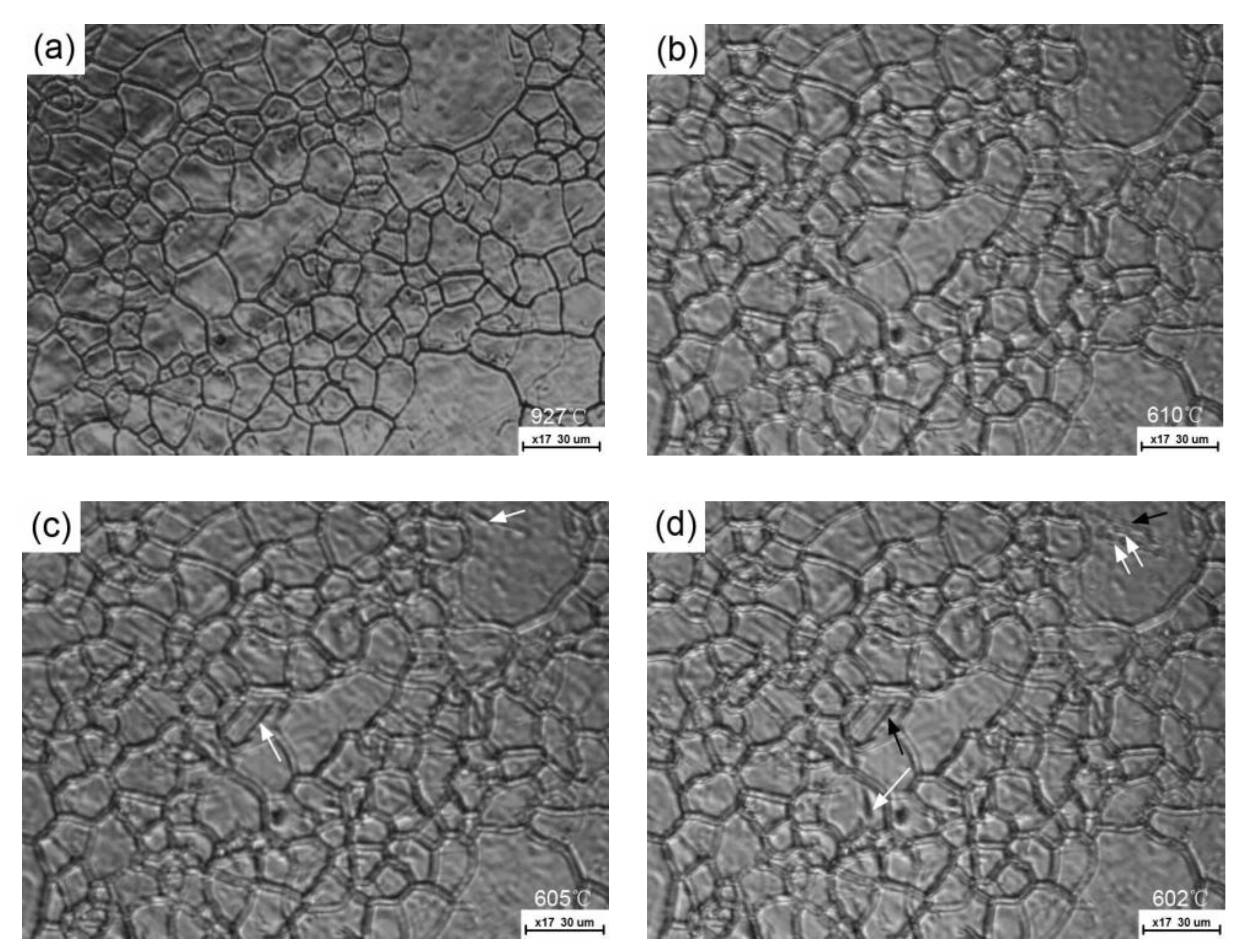
3. Results and Discussion
4. Overall Discussion
5. Conclusions
- (1)
- The predominantly bainite microstructure can be obtained over a very wide range of cooling rates for this studied steel. The hardness decreases rapidly and then gradually levels off with the decrease in cooling rate and FGHAZ always results in a slightly higher hardness than the CGHAZ at any given cooling rate.
- (2)
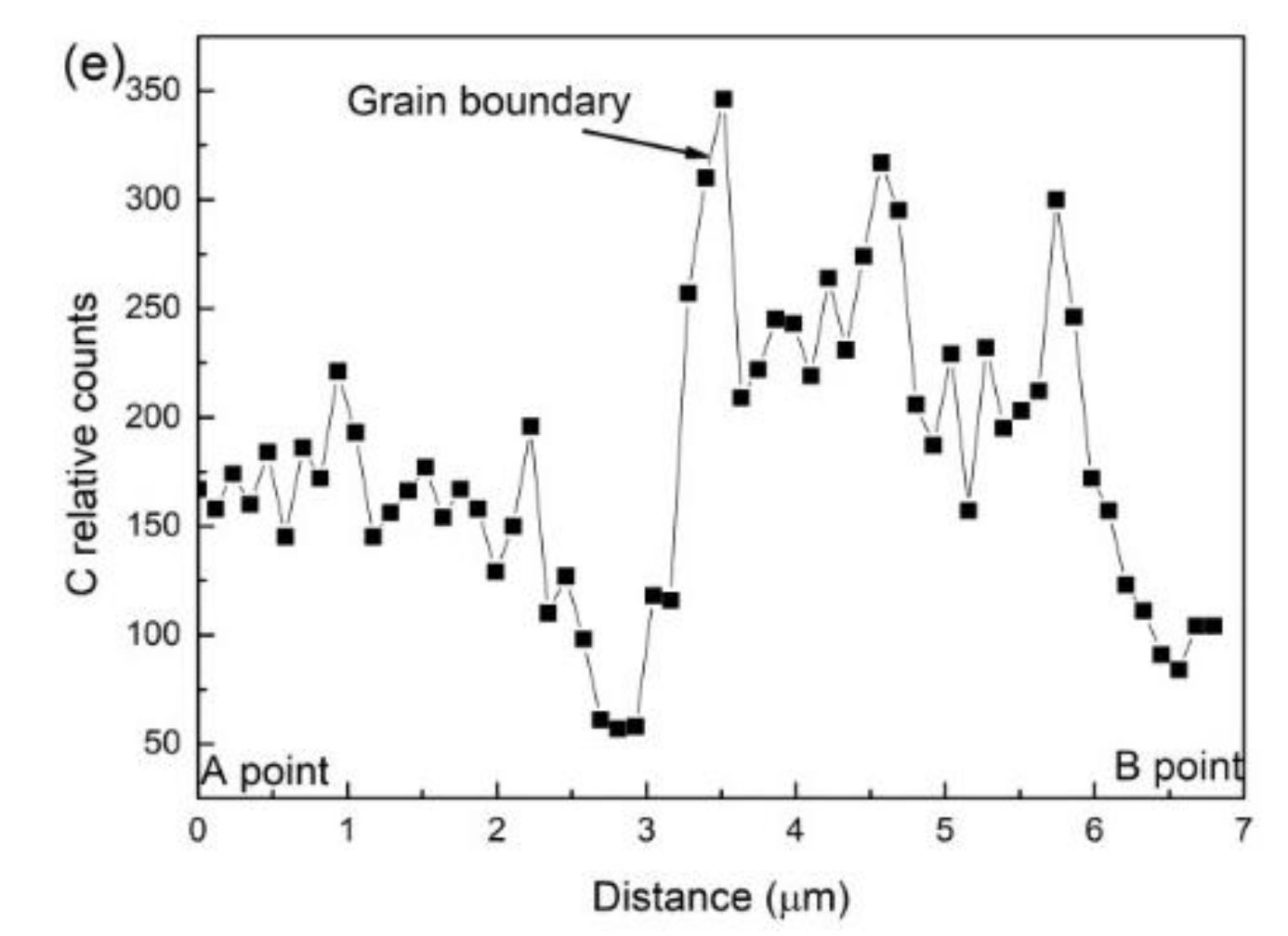
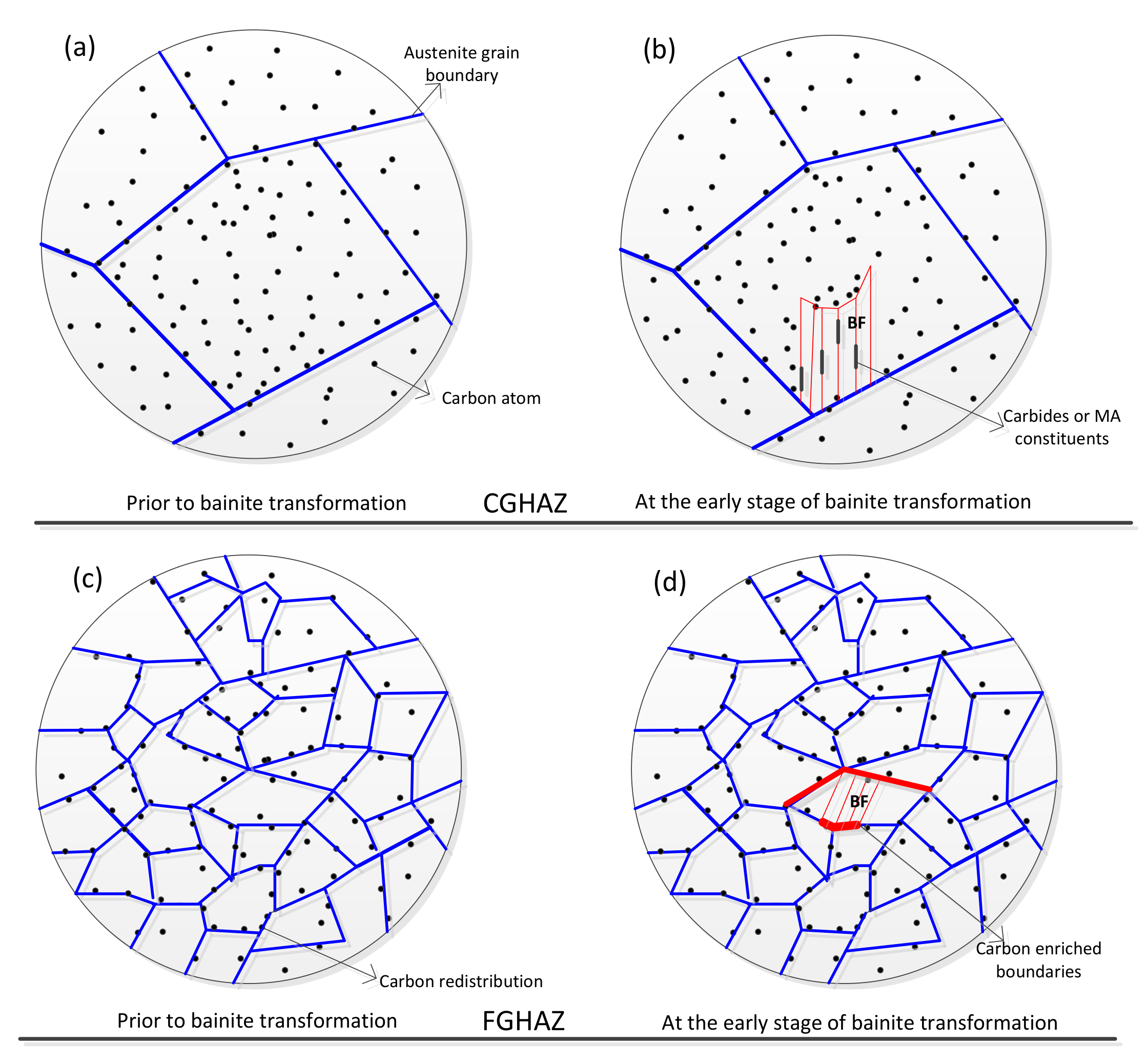
- For a given cooling condition, FGHAZ always has a lower Bs temperature than CGHAZ, which is proven by in situ LSCM observation and partial bainite transformation tests. The prior austenite grain boundaries were always decorated by many long strip MA constituents in FGHAZ, indicating that the carbon segregation occurred at the early stage of bainite transformation. Besides the Hall–Petch strengthening effect in FGHAZ, the increasing interfacial energy for the grain boundary nucleation due to carbon enrichment is probably another factor that lowers the Bs temperature.
- (3)
- A large bainitic ferrite packet can be transformed only in fine austenite grains because the ferritic laths grow in a “side-by-side” mode, although each of the laths may consist of several sub-units. In contrast, the interlocked distribution of bainitic ferrite packets can frequently be found in coarse austenite grains, which is attributed to the mechanism of sympathetic nucleation.
Author Contributions
Funding
Conflicts of Interest
References
- Garcia-Junceda, A.; Capdevila, C.; Caballero, F.G.; Garcia de Andres, C. Dependence of martensite start temperature on fine austenite grain size. Scr. Mater. 2008, 58, 134–137. [Google Scholar] [CrossRef]
- Morito, S.; Saito, H.; Ogawa, T.; Furuhara, T.; Maki, T. Effect of austenite grain size on the morphology and crystallography of lath martensite in low carbon steels. ISIJ Int. 2005, 45, 91–94. [Google Scholar] [CrossRef]
- Furuhara, T.; Kikumoto, K.; Saito, H.; Sekine, T.; Ogawa, T.; Morito, S.; Maki, T. Phase transformation from fine-grained austenite. ISIJ Int. 2008, 48, 1038–1045. [Google Scholar] [CrossRef]
- Hanamura, T.; Torizuka, S.; Tamura, S.; Enokida, S.; Takechi, H. Effect of austenite grain size on transformation behavior, microstructure and mechanical properties of 0.1C-5Mn martensitic steel. ISIJ Int. 2013, 53, 2218–2225. [Google Scholar] [CrossRef]
- Hidalgo, J.; Santofimia, M.J. Effect of prior austenite grain size refinement by thermal cycling on the microstructural features of as-quenched lath martensite. Metall. Mater. Trans. A 2016, 47, 5288–5301. [Google Scholar] [CrossRef]
- Matsuzaki, A.; Bhadeshia, H.K.D.H. Effect of austenite grain size and bainite morphology on overall kinetics of bainite transformation in steels. Mater. Sci. Technol. 1999, 15, 518–522. [Google Scholar] [CrossRef]
- Rees, G.I.; Bhadeshia, H.K.D.H. Bainite transformation kinetics Part 1 Modified model. Mater. Sci. Technol. 1992, 8, 985–993. [Google Scholar] [CrossRef]
- Lan, L.Y.; Qiu, C.L.; Zhao, D.W.; Gao, X.H.; Du, L.X. Effect of austenite grain size on isothermal bainite transformation in low carbon microalloyed steel. Mater. Sci. Technol. 2011, 27, 1657–1663. [Google Scholar] [CrossRef]
- Shome, M.; Gupta, O.P.; Mohanty, O.N. Effect of simulated thermal cycles on the microstructure of the heat-affected zone in HSLA-80 and HSLA-100 steel plates. Metall. Mater. Trans. A 2004, 35, 985–996. [Google Scholar] [CrossRef]
- Lee, S.J.; Park, J.S.; Lee, Y.K. Effect of austenite grain size on the transformation kinetics of upper and lower bainite in a low-alloy steel. Scr. Mater. 2008, 59, 87–90. [Google Scholar] [CrossRef]
- Lan, L.Y.; Qiu, C.L.; Zhao, D.W.; Gao, X.H.; Du, L.X. Effect of reheat temperature on continuous cooling bainite transformation behavior in low carbon microalloyed steel. J. Mater. Sci. 2013, 48, 4356–4364. [Google Scholar] [CrossRef]
- abbasi, M.; Nelson, T.W.; Sorensen, C.D. Analysis of variant selection in friction-stir-processed high-strength low-alloy steels. J. Appl. Crystallogr. 2013, 46, 716–725. [Google Scholar] [CrossRef]
- Lan, L.Y.; Kong, X.W.; Qiu, C.L. Characterization of coarse bainite transformation in low carbon steel during simulated welding thermal cycles. Mater. Charact. 2015, 105, 95–103. [Google Scholar] [CrossRef]
- Zhao, H.; Wynne, B.P.; Palmiere, E.J. Effect of austenite grain size on the bainitic ferrite morphology and grain refinement of a pipeline steel after continuous cooling. Mater. Charact. 2017, 123, 128–136. [Google Scholar] [CrossRef]
- Garcia de Andres, C.; Caballero, F.G.; Capdevila, C.; Alvarez, L.F. Application of dilatometric analysis to the study of solid-solid phase transformations in steels. Mater. Charact. 2002, 48, 101–111. [Google Scholar] [CrossRef]
- Chen, X.W.; Qiao, G.Y.; Han, X.L.; Wang, X.; Xiao, F.R.; Liao, B. Effects of Mo, Cr and Nb on microstructure and mechanical properties of heat affected one for Nb-bearing X80 pipeline steels. Mater. Des. 2014, 53, 888–901. [Google Scholar] [CrossRef]
- Lan, L.Y.; Yu, M.; Qiu, C.L. On the local mechanical properties of isothermally transformed bainite in low carbon steel. Mater. Sci. Eng. A 2019, 742, 442–450. [Google Scholar] [CrossRef]
- Li, X.D.; Shang, C.J.; Ma, X.P.; Gault, B.; Subramanian, S.V.; Sun, J.B.; Misra, R.D.K. Elemental distribution in the martensite-austenite constituent in intercritically reheated coarse-grained heat-affected zone of a high-strength pipeline steel. Scr. Mater. 2017, 139, 67–70. [Google Scholar] [CrossRef]
- Biss, V.; Cryderman, R.L. Martensite and retained austenite in hot-rolled low-carbon bainitic steel. Metall. Mater. Trans. 1971, 2, 2267–2276. [Google Scholar] [CrossRef]
- Zhang, M.X.; Kelly, P.M. Determination of carbon content in bainitic ferrite and carbon distribution in austenite by using CBKLDP. Mater. Charact. 1998, 40, 159–168. [Google Scholar] [CrossRef]
- Tomozawa, M.; Miyahara, Y.; Kako, K. Solute segregation onΣ3 and random grain boundaries in type 316L stainless steel. Mater. Sci. Eng. A 2013, 578, 167–173. [Google Scholar] [CrossRef]
- Thibaux, P.; Metenier, A.; Xhoffer, C. Carbon diffusion measurement in austenite in the temperature range 500 to 900 °C. Metall. Mater. Trans. A 2007, 38, 1169–1176. [Google Scholar] [CrossRef]
- Escobar, J.D.; Faria, G.A.; Wu, L.; Oliveira, J.P.; Mei, P.R.; Ramirez, A.J. Austenite reversion kinetics and stability during tempering of a Ti-stabilized supermartensitic stainless steel: Correlative in situ synchrotron X-ray diffraction and dilatometry. Acta Mater. 2017, 138, 92–99. [Google Scholar] [CrossRef]
- Wan, X.L.; Wu, K.M.; Nune, K.C.; Li, Y.; Cheng, L. In situ observation of acicular ferrite formation and grain refinement in simulated heat affected zone of high strength low alloy steel. Sci. Technol. Weld. Join. 2015, 20, 254–263. [Google Scholar] [CrossRef]
- Sainis, S.; Farahani, H.; Gamsjager, E.; van der Zwaag, S. An In-situ LSCM study on bainite formation in a Fe-0.2C-1.5Mn- 2.0Cr alloy. Metals 2018, 8, 498–512. [Google Scholar] [CrossRef]
- Takayama, N.; Miyamoto, G.; Furuhara, T. Effects of transformation temperature on variant pairing of bainitic ferrite in low carbon steel. Acta Mater. 2012, 60, 2387–2396. [Google Scholar] [CrossRef]
- Borgenstam, A.; Hillert, M.; Agren, J. Metallographic evidence of carbon diffusion in the growth of bainite. Acta Mater. 2009, 57, 3242–3252. [Google Scholar] [CrossRef]
- Slane, J.A.; Wolverton, C.; Gibala, R. Experimental and theoretical evidence for carbon-vacancy binding in austenite. Metall. Mater. Trans. A 2004, 35, 2239–2245. [Google Scholar] [CrossRef]
- Abe, T.; Tsukada, K.; Tagawa, H.; Kozasu, I. Grain boundary segregation behavior of phosphorus and carbon under equilibrium and non-equilibrium conditions in austenitic region of steels. ISIJ Int. 1990, 30, 444–450. [Google Scholar] [CrossRef]
- Timokhina, I.B.; Liss, K.D.; Raabe, D.; Rakha, K.; Beladi, H.; Xiong, X.Y.; Hodgson, P.D. Growth of bainitic ferrite and carbon partitioning during the early stages of bainite transformation in a 2 mass% silicon steel studied by in situ neutron diffraction, TEM and APT. J. Appl. Crystallogr. 2016, 49, 399–414. [Google Scholar] [CrossRef]
- Kempen, A.T.W.; Sommer, F.; Mittemeijer, E.J. The kinetics of the austenite-ferrite phase transformation of Fe-Mn: Differential thermal analysis during cooling. Acta Mater. 2002, 50, 3545–3555. [Google Scholar] [CrossRef]
- Song, T.; Cooman, B.C.D. Effect of boron on the isothermal bainite transformation. Metall. Mater. Trans. A 2013, 44, 1686–1705. [Google Scholar] [CrossRef]
- Madariaga, I.; Gutierrez, I. Role of the particle-matrix interface on the nucleation of acicular ferrite in a medium carbon microalloyed steel. Acta Mater. 1999, 47, 951–960. [Google Scholar] [CrossRef]





© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lan, L.; Chang, Z.; Fan, P. Exploring the Difference in Bainite Transformation with Varying the Prior Austenite Grain Size in Low Carbon Steel. Metals 2018, 8, 988. https://doi.org/10.3390/met8120988
Lan L, Chang Z, Fan P. Exploring the Difference in Bainite Transformation with Varying the Prior Austenite Grain Size in Low Carbon Steel. Metals. 2018; 8(12):988. https://doi.org/10.3390/met8120988
Chicago/Turabian StyleLan, Liangyun, Zhiyuan Chang, and Penghui Fan. 2018. "Exploring the Difference in Bainite Transformation with Varying the Prior Austenite Grain Size in Low Carbon Steel" Metals 8, no. 12: 988. https://doi.org/10.3390/met8120988
APA StyleLan, L., Chang, Z., & Fan, P. (2018). Exploring the Difference in Bainite Transformation with Varying the Prior Austenite Grain Size in Low Carbon Steel. Metals, 8(12), 988. https://doi.org/10.3390/met8120988




