CeO2-Cobalt Ferrite Composite as a Dual-Function Catalyst for Hydrogen Peroxide Decomposition and Organic Pollutants Degradation
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Synthesis
2.3. Characterization Techniques
2.4. Hydrogen Peroxide Decomposition Tests
2.5. Fenton-like Oxidation of Congo Red and Oxytetracycline
3. Results
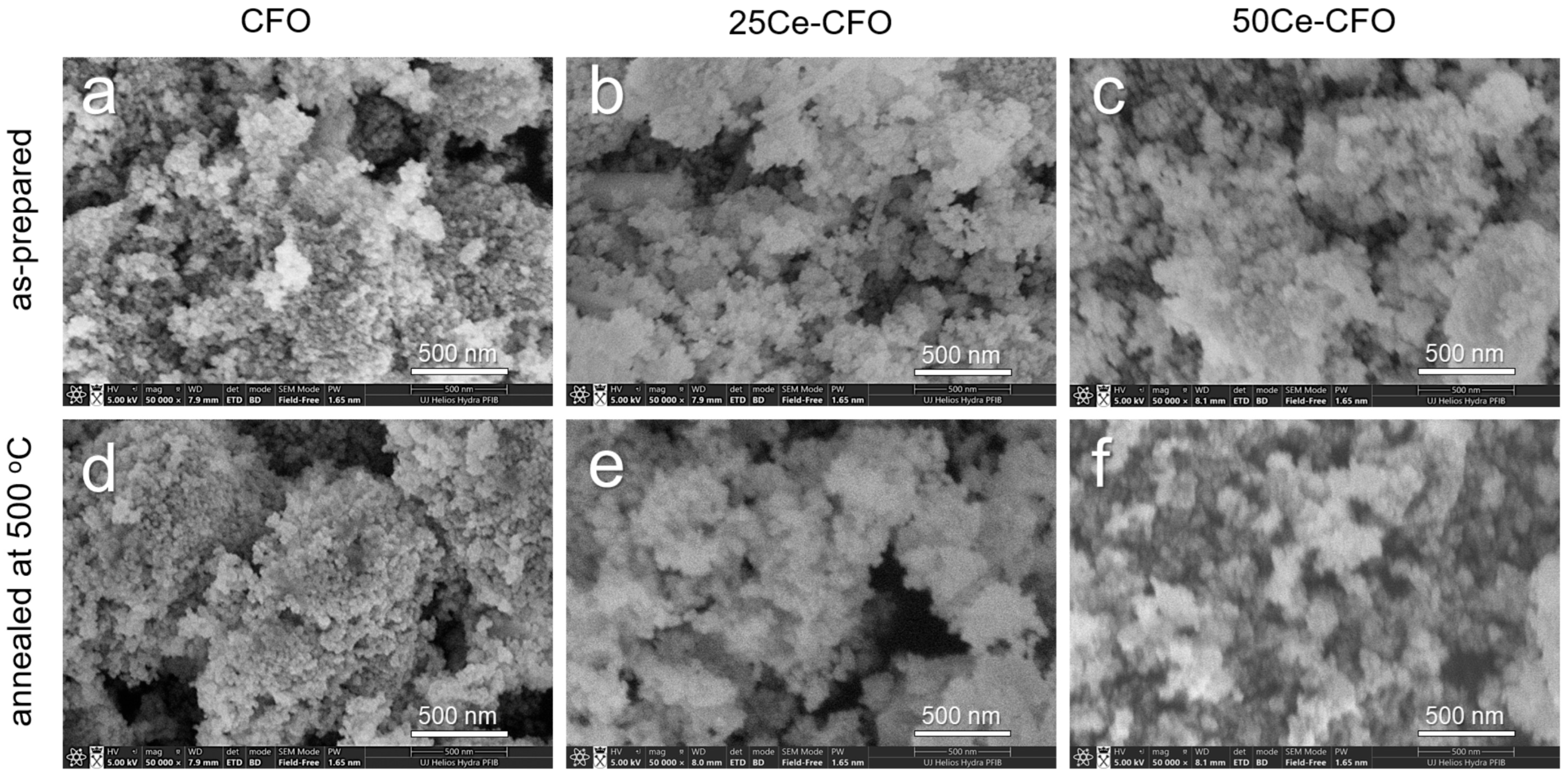
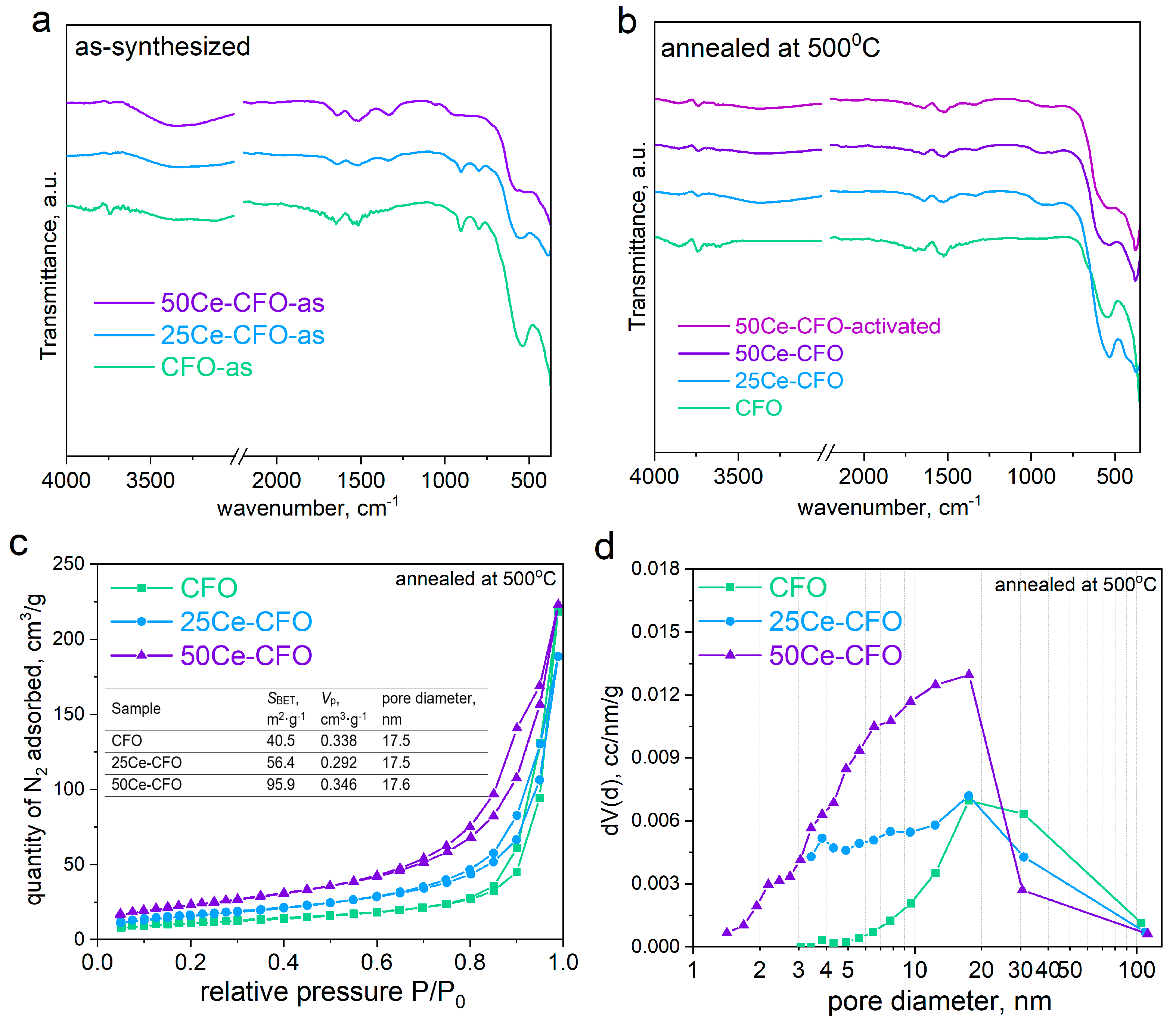
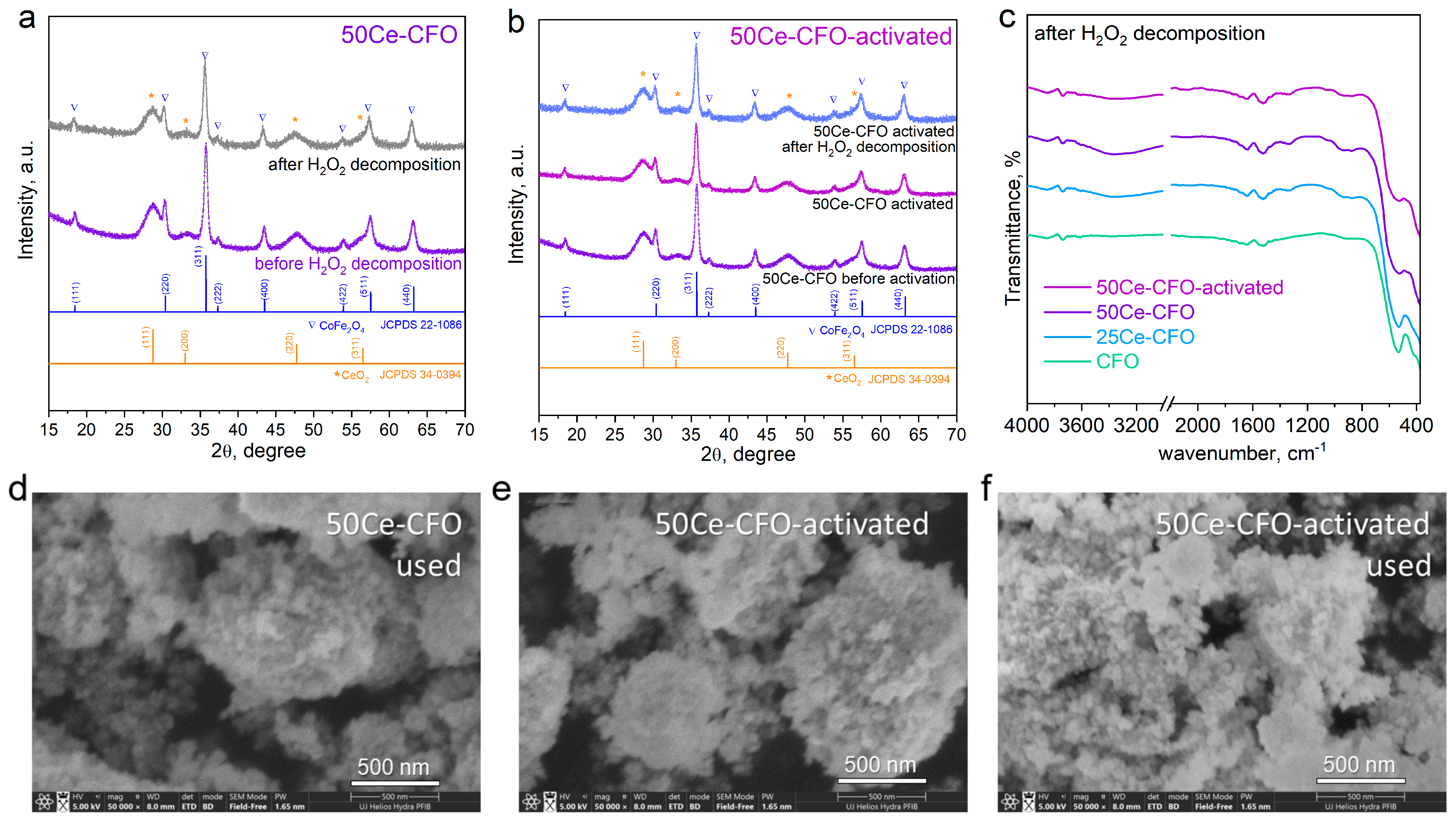
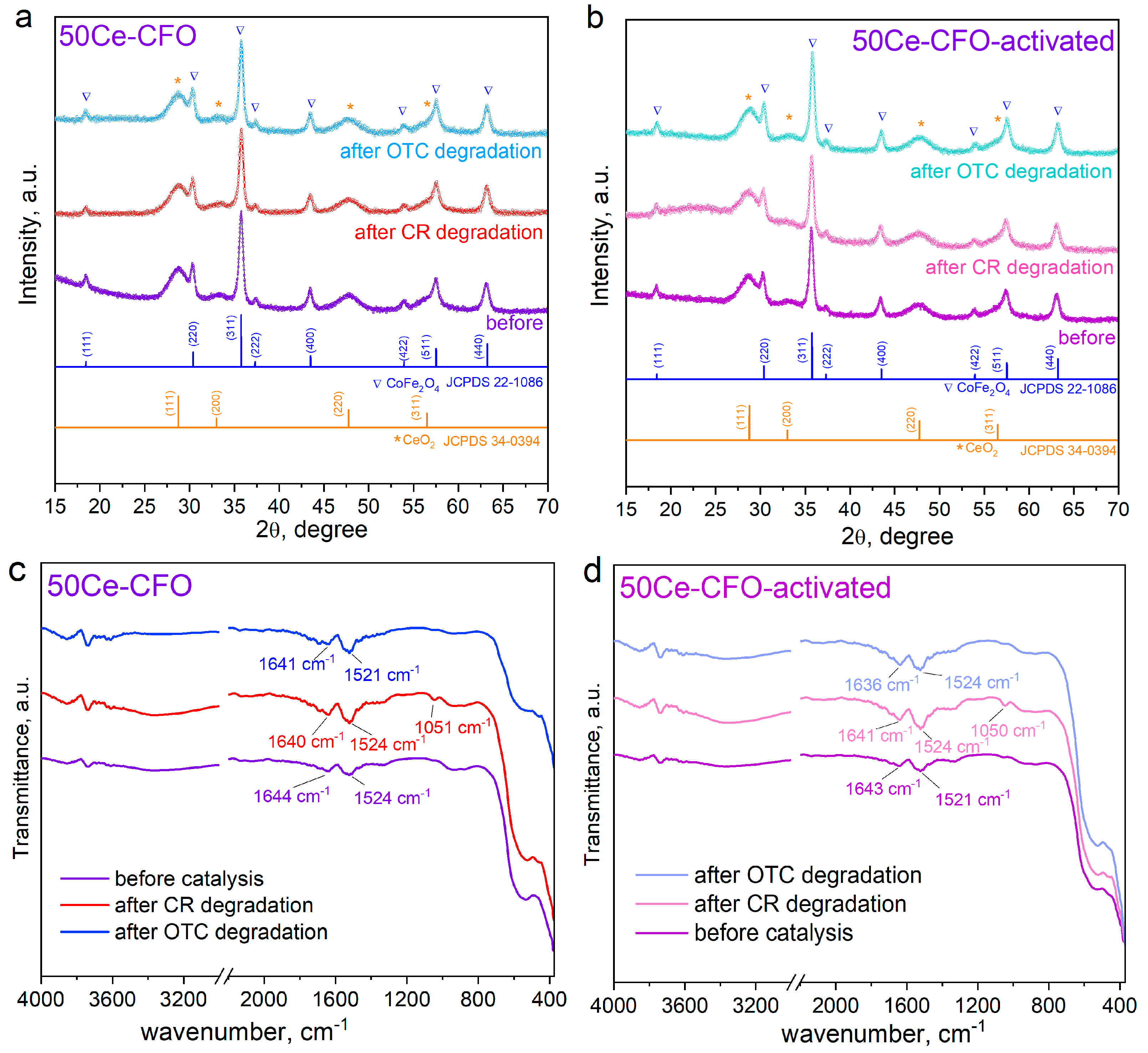
3.1. Structure and Morphology Studies
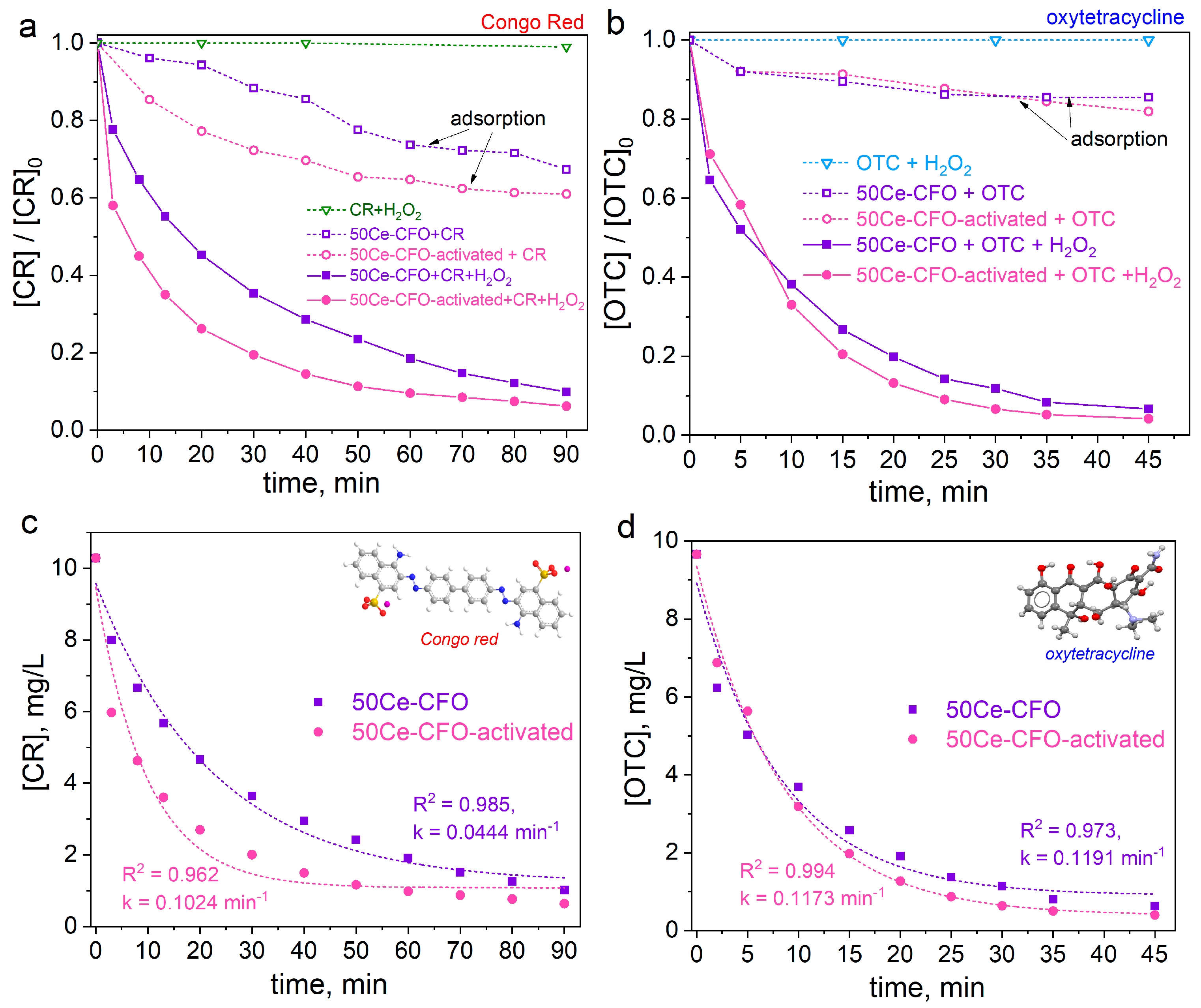
3.2. Catalytic Activity in H2O2 Decomposition
3.3. Catalytic Degradation of Congo Red Dye and Antibiotic Oxytetracycline
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Andersen, H.L.; Granados-Miralles, C.; Jensen, K.M.Ø.; Saura-Múzquiz, M.; Christensen, M. The Chemistry of Spinel Ferrite Nanoparticle Nucleation, Crystallization, and Growth. ACS Nano 2024, 18, 9852–9870. [Google Scholar] [CrossRef]
- Tatarchuk, T.; Bilovol, V.; Shyichuk, A.; Danyliuk, I.; Sokołowski, K.; Gajewska, M. Mesoporous Co-Mn Ferrites as Highly Radical-Forming Catalysts for Wet Peroxide Oxidation of 4-Nitrophenol. Appl. Surf. Sci. 2025, 690, 162610. [Google Scholar] [CrossRef]
- Ma, D.; Lai, C.; Yi, H.; Huo, X.; Li, L.; Zhang, M.; Xu, F.; Yan, H.; Hu, S.; Luo, Y. Confinement Strategies for the Design of Efficient Heterogeneous Fenton-like Catalysts: From Nano-Space to Atomic Scale. Coord. Chem. Rev. 2025, 522, 216241. [Google Scholar] [CrossRef]
- Soufi, A.; Hajjaoui, H.; Boumya, W.; Elmouwahidi, A.; Baillón-García, E.; Abdennouri, M.; Barka, N. Recent Trends in Magnetic Spinel Ferrites and Their Composites as Heterogeneous Fenton-like Catalysts: A Review. J. Environ. Manage. 2024, 367, 121971. [Google Scholar] [CrossRef]
- Bhaduri, B.; Dikshit, A.K.; Kim, T.; Tripathi, K.M. Research Progress and Prospects of Spinel Ferrite Nanostructures for the Removal of Nitroaromatics from Wastewater. ACS Appl. Nano Mater. 2022, 5, 16000–16026. [Google Scholar] [CrossRef]
- Qin, H.; He, Y.; Xu, P.; Huang, D.; Wang, Z.; Wang, H.; Wang, Z.; Zhao, Y.; Tian, Q.; Wang, C. Spinel Ferrites (MFe2O4): Synthesis, Improvement and Catalytic Application in Environment and Energy Field. Adv. Colloid Interface Sci. 2021, 294, 102486. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Yan, Z.; Chen, C.; Chen, J. Spinels: Controlled Preparation, Oxygen Reduction/Evolution Reaction Application, and Beyond. Chem. Rev. 2017, 117, 10121–10211. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Liu, Q.; Mai, Z.; Wang, M.; Zhao, H.; Xu, K. Strategies for Improving Performance of Iron-Based Catalysts in Activating Heterogeneous Fenton-like Oxidation in Pollutants Degradation: From the Perspective of Materials Structure Design. Process Saf. Environ. Prot. 2024, 190, 794–820. [Google Scholar] [CrossRef]
- Tatarchuk, T. Studying the Defects in Spinel Compounds: Discovery, Formation Mechanisms, Classification, and Influence on Catalytic Properties. Nanomaterials 2024, 14, 1640. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, P.; Zhao, K.; Wen, G.; Zhang, X.; Wang, Y. Review of CeO2 Nanostructure/Carbon Composite Catalysts for Wastewater Treatment. ACS Appl. Nano Mater. 2025, 8, 10210–10241. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, T.; Liu, J. Synergistically Boosted Degradation of Organic Dyes by CeO2 Nanoparticles with Fluoride at Low PH. ACS Appl. Nano Mater. 2020, 3, 842–849. [Google Scholar] [CrossRef]
- Minakshi, M.; Nallathamby, K.; Mitchell, D.R.G. Electrochemical Characterization of an Aqueous Lithium Rechargeable Battery: The Effect of CeO2 Additions to the MnO2 Cathode. J. Alloys Compd. 2009, 479, 87–90. [Google Scholar] [CrossRef]
- Dong, Y.; Shah, M.A.K.Y.; Khalid, M.; Lu, Y.; Deng, C. Optimizing Low-Temperature Ceramic Fuel Cells with CuFe2O4–CeO2 Heterostructures. ACS Appl. Energy Mater. 2023, 6, 12494–12502. [Google Scholar] [CrossRef]
- Ma, H.; Liu, Z.; Koshy, P.; Sorrell, C.C.; Hart, J.N. Density Functional Theory Investigation of the Biocatalytic Mechanisms of PH-Driven Biomimetic Behavior in CeO2. ACS Appl. Mater. Interfaces 2022, 14, 11937–11949. [Google Scholar] [CrossRef] [PubMed]
- Ravi, G.; Thyagarajan, K. Effect of Calcination Temperature on the Structural, Morphological, and Magnetic Properties of CuFe2O4/CoFe2O4/CeO2 Nanocomposites. Ceram. Int. 2025, 51, 31083–31096. [Google Scholar] [CrossRef]
- Wetchakun, N.; Chaiwichain, S.; Wetchakun, K.; Kangwansupamonkon, W.; Inceesungvorn, B.; Phanichphant, S. Synthesis and Characterization of Novel Magnetically Separable CoFe2O4/CeO2 Nanocomposite Photocatalysts. Mater. Lett. 2013, 113, 76–79. [Google Scholar] [CrossRef]
- Sayed, M.A.; Abdelhameed, R.M.; Badr, I.H.A.; Abdel-Aziz, A.M. Efficient Adsorptive Removal of Hazardous Congo Red Dye Using Ce-BTC@microcrystalline Cellulose Composite. Sci. Rep. 2025, 15, 19734. [Google Scholar] [CrossRef]
- Cao, Y.; Peng, X.; Tan, Z.; Liu, Y.; Wang, X.; Zhao, W.; Jiang, L. Structural Evolution of Active Entities on Co3O4/CeO2 Catalyst during Water Gas Shift Reaction. Ind. Eng. Chem. Res. 2019, 58, 17692–17698. [Google Scholar] [CrossRef]
- Sun, Q.; Liu, Y.; Li, X.; Guo, X.; Huang, W.-H.; Zhu, Y.; Wang, Z.; Chueh, C.-C.; Chen, C.-L.; Peng, Y.-K.; et al. Highly Disordered Fe-Doped CeO2 with Oxygen Vacancies Facilitates Electrocatalytic Water Oxidation. Energy Fuels 2023, 37, 9434–9443. [Google Scholar] [CrossRef]
- Urooj, I.; Sohail, M.; Shah, W.A.; Ali, H.; An, X.; Liu, S.; Wahab, M.A. Nanorectangular NiFe2O4 Decorated with CeO2 Nanoparticles and Modified with Phosphate Ions as an Electrocatalyst for Water Electrolysis. ACS Appl. Nano Mater. 2025, 8, 3769–3786. [Google Scholar] [CrossRef]
- Luo, Y.; Alves, D.; Barwa, T.N.; Dempsey, E.; Breslin, C.B. A CeFe2O4-CeO2 Composite for the Electrochemical Detection and Advanced Oxidation of the Antibiotic Sparfloxacin. J. Environ. Manage. 2025, 380, 125167. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Ji, Q.; Lei, Y.; Ma, J.; Xiao, Q.; Yang, Y.; Komarneni, S. Efficient Degradation of Orange II by Core Shell CoFe2O4–CeO2 Nanocomposite with the Synergistic Effect from Sodium Persulfate. Chemosphere 2022, 291, 132765. [Google Scholar] [CrossRef]
- Liu, B.; Li, J.; Wang, C.; Li, Y.; Gao, L.; Yang, Q. Efficient Activation of Peroxymonosulfate for Trichloroethylene Degradation by Cobalt Ferrites Anchored on CeO2 Surfaces: Radical to Non-Radical Pathway Shift. J. Environ. Chem. Eng. 2024, 12, 112280. [Google Scholar] [CrossRef]
- Yuan, B.; Tan, Z.; Guo, Q.; Shen, X.; Zhao, C.; Chen, J.L.; Peng, Y.-K. Regulating the H2O2 Activation Pathway on a Well-Defined CeO2 Nanozyme Allows the Entire Steering of Its Specificity between Associated Enzymatic Reactions. ACS Nano 2023, 17, 17383–17393. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Liu, J.; Chen, Z.; Lin, P.; Ou, W.; Wang, Z.; Xiao, W.; Chen, Y.; Cao, D. Mechanism and Application of Surface-Charged Ferrite Nanozyme-Based Biosensor toward Colorimetric Detection of l-Cysteine. Langmuir 2022, 38, 8266–8279. [Google Scholar] [CrossRef] [PubMed]
- Shlapa, Y.; Siposova, K.; Veltruska, K.; Maraloiu, V.-A.; Garcarova, I.; Rajnak, M.; Musatov, A.; Belous, A. Design of Magnetic Fe3O4/CeO2 “Core/Shell”-Like Nanocomposites with Pronounced Antiamyloidogenic and Antioxidant Bioactivity. ACS Appl. Mater. Interfaces 2023, 15, 49346–49361. [Google Scholar] [CrossRef]
- Xu, L.; Wang, J. Magnetic Nanoscaled Fe3O4/CeO2 Composite as an Efficient Fenton-Like Heterogeneous Catalyst for Degradation of 4-Chlorophenol. Environ. Sci. Technol. 2012, 46, 10145–10153. [Google Scholar] [CrossRef]
- Li, J.; Gou, G.; Zhao, H.; Liu, C.; Li, N.; Li, L.; Tan, B.; Lai, B. Efficient Peroxymonosulfate Activation by CoFe2O4-CeO2 Composite: Performance and Catalytic Mechanism. Chem. Eng. J. 2022, 435, 134840. [Google Scholar] [CrossRef]
- Gao, P.; Chen, X.; Hao, M.; Xiao, F.; Yang, S. Oxygen Vacancy Enhancing the Fe2O3-CeO2 Catalysts in Fenton-like Reaction for the Sulfamerazine Degradation under O2 Atmosphere. Chemosphere 2019, 228, 521–527. [Google Scholar] [CrossRef]
- Chen, J.; Wan, J.; Li, C.; Wei, Y.; Shi, H. Synthesis of Novel Fe0-Fe3O4/CeO2/C Composite Cathode for Efficient Heterogeneous Electro-Fenton Degradation of Ceftriaxone Sodium. J. Hazard. Mater. 2022, 437, 129393. [Google Scholar] [CrossRef]
- Li, D.; Zhao, Z.; Guo, J.; Shen, R.; Xu, F. Magnetic Nanoscaled Fe3O4-CeO2-TiO2 Composite: UV-Fenton Reaction to Degrade AO-7 Dye. Inorg. Chem. Commun. 2023, 149, 110389. [Google Scholar] [CrossRef]
- Shen, C.; Zhu, Q.; Chen, H.; Zhang, Y.; Du, M.; Li, F.; Ma, J. Insights into the Synergistic Effect of Fe and Ce in Fenton-like Reactions Catalyzed by Chitosan/FeOOH/CeO2 Microspheres. J. Clean. Prod. 2024, 451, 142058. [Google Scholar] [CrossRef]
- Perrin, C.L. Linear or Nonlinear Least-Squares Analysis of Kinetic Data? J. Chem. Educ. 2017, 94, 669–672. [Google Scholar] [CrossRef]
- Kale, G.H.; Humbe, A.V.; Birajdar, S.D.; Shinde, A.B.; Jadhav, K.M. L-Ascorbic Acid Assisted Synthesis and Characterization of CoFe2O4 Nanoparticles at Different Annealing Temperatures. J. Mater. Sci. Mater. Electron. 2016, 27, 2151–2158. [Google Scholar] [CrossRef]
- Seeharaj, P.; Pasupong, P.; Choojun, K. Magnetically Separable CeO2/CoFe2O4 Heterojunction Photocatalysts for Dye Degradation: Characterization and Mechanism. J. Asian Ceram. Soc. 2024, 12, 44–58. [Google Scholar] [CrossRef]
- Li, Y.; Wang, L.; Chen, D.; Liu, J.; Fan, W.; Sun, J. Experimental and Theoretical Study on Performance of Cu-Fe Spinel Supported with Certain Inerts during Chemical Looping Combustion. Chem. Eng. J. 2024, 495, 153591. [Google Scholar] [CrossRef]
- Tatarchuk, T.; Starko, I. Mesoporous La-Substituted Nickel-Cobalt Ferrites Synthesized via Reduction Method Resulting in Significantly Enhanced Adsorption Properties. J. Environ. Chem. Eng. 2025, 13, 115657. [Google Scholar] [CrossRef]
- Dharmalingam, S.T.; Dar, M.A.; Gul, R.; Minakshi Sundaram, M.; Alnaser, I.A.; Sivasubramanian, R. Nano-Octahedron Cobalt Oxide Decorated Graphene Nanocomposites for the Selective/Simultaneous Detection of Dopamine. Adv. Mater. Interfaces 2025, 12, 2400981. [Google Scholar] [CrossRef]
- Ebenezer, J.; Velayudham, P.; Schechter, A. Electrochemical Nitrogen Fixation Using CeFeO3 and CeO2 for Ammonia Synthesis and Nitrate Remediation. ACS Appl. Mater. Interfaces 2025, 17, 36796–36809. [Google Scholar] [CrossRef]
- Chen, F.; Shen, X.; Wang, Y.; Zhang, J. CeO2/H2O2 System Catalytic Oxidation Mechanism Study via a Kinetics Investigation to the Degradation of Acid Orange 7. Appl. Catal. B Environ. 2012, 121–122, 223–229. [Google Scholar] [CrossRef]
- Lousada, C.M.; Yang, M.; Nilsson, K.; Jonsson, M. Catalytic Decomposition of Hydrogen Peroxide on Transition Metal and Lanthanide Oxides. J. Mol. Catal. A Chem. 2013, 379, 178–184. [Google Scholar] [CrossRef]
- Tatarchuk, T.; Shyichuk, A.; Danyliuk, N.; Naushad, M.; Kotsyubynsky, V.; Boychuk, V. Cobalt Ferrite as an Electromagnetically Boosted Metal Oxide Hetero-Fenton Catalyst for Water Treatment. Chemosphere 2023, 326, 138364. [Google Scholar] [CrossRef] [PubMed]
- Rajeendre Katugampalage, T.; Waribam, P.; Opaprakasit, P.; Kaewsaneha, C.; Hsu, S.-H.; Chooaksorn, W.; Jong, C.-A.; Sooksaen, P.; Ratanatawanate, C.; Sreearunothai, P. Bimetallic Fe:Co Metal–Organic Framework (MOF) with Unsaturated Metal Sites for Efficient Fenton-like Catalytic Degradation of Oxytetracycline (OTC) Antibiotics. Chem. Eng. J. 2024, 479, 147592. [Google Scholar] [CrossRef]
- Bracco, E.B.; Marco-Brown, J.L.; Butler, M.; Candal, R.J. Degradation of Oxytetracycline and Characterization of Byproducts Generated by Fenton or Photo-Fenton like Processes after Adsorption on Natural and Iron(III)-Modified Montmorillonite Clays. Environ. Nanotechnol. Monit. Manag. 2023, 19, 100778. [Google Scholar] [CrossRef]
- Solano, A.M.S.; Garcia-Segura, S.; Martínez-Huitle, C.A.; Brillas, E. Degradation of Acidic Aqueous Solutions of the Diazo Dye Congo Red by Photo-Assisted Electrochemical Processes Based on Fenton’s Reaction Chemistry. Appl. Catal. B Environ. 2015, 168–169, 559–571. [Google Scholar] [CrossRef]
- Moores, L.C.; Kaur, D.; Smith, M.D.; Poole, J.S. Regioselectivity of Hydroxyl Radical Reactions with Arenes in Nonaqueous Solutions. J. Org. Chem. 2019, 84, 3260–3269. [Google Scholar] [CrossRef]
- Masi, A.; Capobianco, A.; Bobrowski, K.; Peluso, A.; Chatgilialoglu, C. Hydroxyl Radical vs. One-Electron Oxidation Reactivities in an Alternating GC Double-Stranded Oligonucleotide: A New Type Electron Hole Stabilization. Biomolecules 2023, 13, 1493. [Google Scholar] [CrossRef]
- Elumalai, S.; Muthuraman, G. Recovery of Methyl Orange and Congo Red from Aqueous Solutions Using Tri-Octyl Amine (TOA) in Benzene as Carrier. Process Saf. Environ. Prot. 2015, 96, 177–183. [Google Scholar] [CrossRef]
- Olusegun, S.J.; Mohallem, N.D.S. Comparative Adsorption Mechanism of Doxycycline and Congo Red Using Synthesized Kaolinite Supported CoFe2O4 Nanoparticles. Environ. Pollut. 2020, 260, 114019. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tatarchuk, T.; Kotsyubynsky, V. CeO2-Cobalt Ferrite Composite as a Dual-Function Catalyst for Hydrogen Peroxide Decomposition and Organic Pollutants Degradation. Metals 2025, 15, 985. https://doi.org/10.3390/met15090985
Tatarchuk T, Kotsyubynsky V. CeO2-Cobalt Ferrite Composite as a Dual-Function Catalyst for Hydrogen Peroxide Decomposition and Organic Pollutants Degradation. Metals. 2025; 15(9):985. https://doi.org/10.3390/met15090985
Chicago/Turabian StyleTatarchuk, Tetiana, and Volodymyr Kotsyubynsky. 2025. "CeO2-Cobalt Ferrite Composite as a Dual-Function Catalyst for Hydrogen Peroxide Decomposition and Organic Pollutants Degradation" Metals 15, no. 9: 985. https://doi.org/10.3390/met15090985
APA StyleTatarchuk, T., & Kotsyubynsky, V. (2025). CeO2-Cobalt Ferrite Composite as a Dual-Function Catalyst for Hydrogen Peroxide Decomposition and Organic Pollutants Degradation. Metals, 15(9), 985. https://doi.org/10.3390/met15090985






