Abstract
Electric arc furnace slag is a major by-product of steelmaking, yet its industrial utilization remains limited due to its complex chemical and mineralogical composition. This study presents a hydrogen-based approach to recover metallic components from EAF slag for potential reuse in steelmaking. Laboratory experiments were conducted by melting 50 g of industrial slag samples at 1600 °C and injecting hydrogen gas through a ceramic tube into the liquid slag. After cooling, both the slag and the metallic phases were analyzed for their chemical and phase compositions. Additionally, the reduction process was modeled using a combination of approaches, including the thermochemical software FactSage 8.1, models for density, surface tension, and viscosity, as well as a diffusion model. The injection of hydrogen resulted in the reduction of up to 40% of the iron oxide content in the liquid slag. In addition, the fraction of reacted hydrogen gas was calculated.
1. Introduction
The utilization of hydrogen as a reducing agent, capable of replacing carbon in steel production, has been acknowledged as a pivotal transformation in the steelmaking process, undertaken to achieve net-zero emissions by 2050 [1]. In line with this objective, various projects such as ULCOS (Ultra-low CO2 steelmaking), SALCOS (Salzgitter), MaxH2DR, and tkH2Steel (ThyssenKrupp) have been initiated, all with the aim of achieving low-carbon steel production. Additionally, ThyssenKrupp Steel has carried out hydrogen injection in its operational blast furnace (BF), resulting in a reduction of CO2 emissions through the displacement of CO with H2 [2,3,4,5].
Developed by Tata Steel, the HIsarna process is another example of a process with a lower carbon footprint than regular BFs. It enables the reduction of iron ore using thermal coal and charcoal, which leads to avoiding the use of coke [6], thereby resulting in a drastic decrease in CO2 emissions in steel manufacturing. The pilot plant with a 60 ktpa capacity was constructed in the Netherlands in 2010 [7]. Although the plant can utilize low-cost coal and low-quality iron ore, no other plants have been built since that time. The published chemical composition of a HIsarna slag shows a similar chemical composition to a primary BF slag in the cohesive zone [7,8,9]. Nevertheless, a predominant way to cut CO2 emissions by the current dominant, highly CO2-intense BF–BOF route, which emits ~ 70% of CO2, is a scrap and hydrogen-based direct reduced iron (DRI)—EAF route [10,11].
Steelmaking slag, a primary by-product generated during the steelmaking process, holds significant importance for the sustainability of the metallurgical industry. In 2024, Europe produced a total of 129.58 million metric tons of crude steel [12], which corresponds to approximately 19.44 million metric tons of steelmaking slag (calculated from a proportion of 150 kg of slag per 1 ton of crude steel).
In practice, most electric arc furnace (EAF) slag is used as an aggregate in civil engineering projects, substituting natural rock in landfill covers, road sub-bases, and heavy concrete aggregates [13]. Numerous studies have demonstrated that the physical and mechanical properties of EAF slag satisfy high-performance material standards, establishing it as a high-quality alternative to natural aggregates [14,15].
Beyond construction, EAF slag is also applied as a soil amendment, supplying essential nutrients such as iron, phosphorus, magnesium, calcium, and silicate, which contribute to improved crop yields and enhanced plant quality. Notably, calcium and magnesium in slag exhibit higher water solubility than those in natural dolomite or magnesium carbonate, increasing their bioavailability [13]. Similarly, basic oxygen furnace (BOF) slag is also applied as a fertilizer [16].
However, the reuse of EAF slag is hindered by chemical and physical variability. Slag composition is highly dependent on the scrap feed and furnace operating conditions, leading to inconsistent properties across batches [17]. In addition, EAF slag is limited by the presence of heavy metals, which can leach under environmental exposure and contaminate soil and groundwater. Comprehensive leaching studies have shown that, depending on slag composition and weathering conditions, elements such as Cr, V, Pb, Zn, and Cd in eluates can approach or exceed regulatory thresholds, restricting unencapsulated applications [18]. Of particular concern is hexavalent chromium (Cr(VI)), which is more soluble and toxic than Cr(III) and has been repeatedly identified as a hazardous leachable species in certain EAF slags [19]. Consequently, the environmental safety of slag reuse is regulated through standardized leaching tests [20].
The effective utilization of steel slag requires modifications to its chemical composition to enable internal recycling within the steelmaking process and to expand its applications in road construction, the cement industry, and agriculture [21]. In addition, given that EAF slags typically contain 14–29 wt.% total iron, their potential use in agriculture and construction remains limited [22].
Consequently, the extraction of metals from slag offers the dual benefits of expanding slag applications in construction and recovering valuable metals. This approach helps reduce energy consumption and the demand for fossil-based raw materials, thereby enhancing raw material resilience in a market that heavily relies on imports.
Various methods have been employed to recover valuable substances from slag. One study [23] explored the reduction of EAF steelmaking slag using blends of metallurgical coke and waste plastic as a reducing agent. The findings indicated that waste plastic can partially substitute conventional metallurgical coke, proving advantageous as a reductant in EAF steelmaking. Moreover, a blend consisting of approximately 40 wt.% waste plastic and the remainder as metallurgical coke exhibited significantly improved reduction rates compared to pure metallurgical coke. Notably, the off-gas exhibited a marked reduction in CO2 content due to the augmented presence of H2 gas resulting from polymer decomposition.
Min et al. [24] conducted research on the reduction of FeO in slag using solid carbon and examined the impact of FeO contents on the reduction rate of FeO-CaO-SiO2 slag. Meanwhile, Liu et al. [21] delved into the carbothermic reduction of an industrial BOF slag with high basicity, aiming to obtain reduced Fe- and P-containing compounds. The study also explored the influence of SiO2 and Al2O3 additions on microstructural modifications and the reduction process.
Ye et al. [25] investigated the reduction of steelmaking slags to recover valuable metals and oxide materials. A DC furnace with a hollow electrode was utilized as a reactor to treat steel slags using coke, anthracite, and pet-coke as reductants. This approach yielded a metal phase (alloy) containing iron, vanadium, chromium, and nickel. Furthermore, the oxide phase produced following treatment demonstrated applicability as clinker cement material, hydraulic binder, and high-quality metallurgical powders.
Htet et al. [26] studied hydrogen smelting reduction behavior in synthetic molten HIsarna slag. The slag with a basicity (CaO/SiO2) of 1.25, 13 wt.% Al2O3, 6 wt.% MgO, and 6–12 wt.% FeO was reduced in an alumina crucible through hydrogen injection. As a result, some amount of reduced metallic iron was obtained. The high Al2O3 content in slag allowed Htet et al. [26] to reduce the slag in an alumina crucible. However, the Al2O3 content in the slag increased significantly after the reduction process, reaching up to 40 wt.%. As a result, the thermophysical properties of the slag change substantially due to polymerization [27,28], which significantly affects the reduction kinetics.
Considering the fact that the quality of iron ore is declining (less than 58 wt.% Fe), it can be assumed that the amount of slag produced in operating EAFs will increase since the beneficiation of iron ore entails iron losses due to the tailing grade and the separated gangue [29]. Moreover, the higher gangue content in iron ore pellets decreases the reduction degree [2], resulting in higher residual FeOx content and eventually increased FeOx content in the EAF slag. In addition, the FeOx content during the production of low-carbon steel in an EAF can reach up to 40 wt.% [30]. Therefore, the hydrogen reduction of liquid steelmaking slag can lead to increased efficiency and further CO2 emission reduction.
The use of hydrogen and its mixtures with nitrogen as reductants for copper smelting slags has been extensively studied [31,32,33,34]. However, no literature has been found on the reduction of liquid steelmaking slags from EAF using H2 gas as a reductant. Although EAF steelmaking exhibits a lower CO2 emission rate compared to the traditional BF–BOF route that relies on carbon sources, EAF is considered a major production technology because it utilizes electric energy as the heat source. Furthermore, many companies are expanding their production capacity by implementing EAF technology. Therefore, this work aims to investigate the feasibility of using H2 gas as a reducing agent to recover metallic components from industrial EAF slag through experimental melting and hydrogen injection, in order to enhance slag valorization and promote circularity in steelmaking. Thermodynamic calculations, combined with other models, were employed to support and interpret the experimental results.
2. Materials and Methods
2.1. Material for Reduction Experiments
The material used in this research is industrial EAF slag supplied by a German steelmaking company that produces steel based on scrap melting. The thermophysical properties of this slag were already studied and published in our other study [35]. The as-delivered slag was first ground into a powder and then dried at 120 °C for 12 h to remove any residual moisture. Afterwards, the dried slag was thoroughly mixed to achieve a homogeneous chemical composition throughout the entire volume of slag. The chemical composition is provided in Table 1.

Table 1.
Chemical composition of initial EAF slag in wt.%.
2.2. Reduction Experiments
Slag samples weighing 50 g were placed into high-density MgO crucibles (99.8% MgO, with an outer diameter of 38 mm, an inner diameter of 32 mm, and a height of 60 mm) and installed inside a graphite crucible, which was used as the heating medium in an induction furnace equipped with a medium-frequency (22–40 kHz) generator with a power output of 40 kW. Temperature control was performed using a B-type thermocouple positioned in the graphite crucible at the height of the sample. Since the same experimental setup geometry was used for all experiments, the temperature difference between the B-type thermocouple and the liquid sample was determined by melting metallic nickel in an MgO crucible and subsequently inserting another B-type thermocouple, covered by a silica protective tube, into the liquid melt. The temperature was measured at different heights of the molten metal, and the average temperature difference was estimated.
The slag sample was heated to 1600 °C at a heating rate of 15 K·min−1 under a protective Ar 5.0 atmosphere, with an Ar flow rate of 4 L·min−1 to prevent oxidation in the furnace. After reaching the target temperature, a tube made of pure Al2O3 with an inner diameter of 2 mm and an outer diameter of 4 mm was immersed into the MgO crucible to a pre-established depth, with the tip positioned 3 mm from the bottom of the crucible. After positioning the tube, a supply of H2 gas at a flow rate of 500 cm3·min−1 was started, initiating the reduction reaction. The reduction was carried out for 5, 10, 15, and 30 min in separate experiments.
After stopping the H2 injection, the slag was held at the target temperature for 10 min, while the alumina tube was lifted from the melt. Subsequently, the crucible was cooled in an Ar atmosphere at a cooling rate of 15 K·min−1 to room temperature.
2.3. Sample Characterization
After cooling, the MgO crucible was crushed into smaller pieces, and the slag, metal, and crucible fragments were separated from each other. To study the chemical composition of the resulting slag, a sample was taken, ground, and analyzed using a Wavelength Dispersive X-Ray Fluorescence spectrometer (WDXRF; S8 Tiger; Bruker AXS, Karlsruhe, Germany) on a fused bead. Due to limitations in quantifying elements with atomic numbers lower than 11, all phases were assumed to be in oxide form; however, oxygen could not be measured directly [36].
To investigate the phases present in the slag, X-ray diffraction (XRD) analysis was additionally performed using a Bruker D8 Discover diffractometer (Bruker AXS, Karlsruhe, Germany) with Bragg–Brentano geometry, a goniometer radius of 280 mm, a cobalt anode, and a measuring range of 10–75° (2θ), with a step size of 0.017° and a counting time of 2 s per step. Phase identification was carried out using the ICDD PDF-4+ database, and quantitative phase analysis was performed using the Rietveld refinement.
To analyze the metallic phase, an Inductively Coupled Plasma Optical Emission Spectrometer (ICP-OES 5100 VDV Dual View; Agilent Technologies, Waldbronn, Germany) was utilized.
3. Methodology of Modeling
3.1. Calculation of Bubble Residence Time in Liquid Slag
The diameter of a bubble (Db) formed via an orifice in liquid slag during H2 injection into molten EAF slag is a critical parameter influencing bubble rise behavior and mass transfer. Under low gas flow rates, bubbles may be approximated as spheres. Therefore, Db can be estimated using a theoretical approach based on Tate’s law (Equation (1)) [37]:
where dcapillary is the inner diameter of capillary used for gas injection, is surface tension of molten slag at 1600 °C, g is the gravitational acceleration, and and are the densities of molten slag and hydrogen gas at 1600 °C, respectively.
To estimate Db at the experimental conditions carried out in this work, the following values are used: dcapillary is 0.002 m, is 0.322 N·m−1 (from our previous work [35]), g is 9.81 m·s−2, is 3451 kg·m−3 (from our previous work [35]), and is 0.01311 kg·m−3. is calculated from Charles and Gay–Lussac’s Law based on the initial density of hydrogen gas of 0.08185 kg·m−3 at 26.85 °C or 300 K [38].
Assuming the formed bubble is spherical, its volume (Vb) can be calculated using Equation (2):
Knowing the flow rate of hydrogen gas at 25 °C (), the flow rate of hydrogen gas at 1600 °C () is be calculated via Charles and Gay–Lussac’s Law. Therefore, the time required to form 1 bubble in liquid EAF slag under experimental conditions is given by Equation (3):
To determine the bubble rise velocity (), the equilibrium between the Archimedean and drag forces is used, as described in Equations (4) and (5):
or
where Rb is the bubble radius, Cd is the drag coefficient, A is the reference area, and υ is the flow velocity relative to the object.
Thus, the bubble velocity is given by Equation (6):
The unknown Cd is calculated using the Schiller and Naumann equation [39] (Equation (7)):
where Retp is the Reynolds number, which is based on the thermophysical properties of the slag and is calculated using Equation (8) [40]:
where is viscosity of liquid EAF slag at 1600 °C and it is 0.02 Pa·s [35].
Knowing υ of bubble in slag, the residence time (τ) of bubble in liquid slag is determined by Equation (9):
where 0.003 is the height from the crucible bottom where the bubble forms in meters, and H is the height of liquid slag in the crucible determined by Equation (10):
Here, the volume of liquid slag (Vslag) in the crucible is determined using Equation (11):
where m is the mass of charged slag.
The area (S) of the cylindrical MgO crucible used in the experiments is determined by Equation (12):
where is the inner diameter of MgO crucible.
3.2. Calculation of Reacted H2 with Slag
To estimate the amount of H2 reacting with oxygen () in the slag during bubble rise, an approximate model of a diffusion-controlled reaction on the bubble surface is applied. This model is based on the diffusion of oxygen atoms from the liquid slag to the bubble surface where they react with H2 to form H2O. Considering that the H2/H2O mixture is homogeneous due to mixing in the bubble, the reaction rate is limited only by the mass transfer of oxygen from the liquid slag to the bubble surface [41]. The amount of H2 in bubble reacting with oxygen in the slag during its rising out of the slag can be described by the following equation:
where kl is the mass transfer coefficient, Co is the volume concentration of oxygen in slag, and A is the sphere surface area.
In this equation, kl·Co·A defines the reaction rate driven by mass transfer of oxygen from the bulk to the bubble surface. The reduction reaction considered is follows:
(FeO) +{H2}→[Fe] +{H2O}
This assumes that wüstite (FeO) is the dominant reactive species in slag. Due to the oxygen affinities of slag components (see the Ellingham diagram [42]) and the relative dominance of FeO in terms of its quantity in the slag, H2 primarily reacts with iron-bound oxygen.
To define kl, the Sherwood number (Sh) is used, as proposed by Levich [43] in Equation (15):
Thus,
where DO is the diffusion coefficient of oxygen in liquid slag.
The Reynolds number (Re) is determined from Equation (17) [44]:
The Schmidt number (Sc) is defined using Equation (18) [45]:
DO is obtained from the Stokes–Einstein–Debye relation according to Equation (19) [46]:
Once all parameters are defined, the value of is calculated. This value is then used to determine the fraction of reacted H2 ().
First, the total amount of hydrogen in the bubble () is determined using the following Equation (20):
Thus, the fraction of reacted H2 () is given by Equation (21):
3.3. Modeling-Based Calculations
In this work, the thermochemical software FactSage 8.1 [47] was used to perform equilibrium calculations. The “Equilib” module was employed to simulate the reduction of liquid slag by H2, using the FactPS, FToxid, and FTmisc databases. The resulting liquid and solid phase compositions were then used to calculate the liquid slag viscosity using the Einstein–Roscoe model [48]. This model combines viscosity data obtained from the FactSage “Viscosity” module with the influence of the solid fraction determined by the “Equilib” module.
where η is the viscosity of the liquid melt containing solid particles; η0 is the viscosity of the liquid slag without solid particles; c is the fraction of solids in the slag; and n is a parameter related to the geometrical shape of the solid particles, with the constant commonly assumed to be 2.5 [49].
Furthermore, the liquid slag composition derived from the “Equilib” module was used to model the slag density via molar volumes [50] and the surface tension using a partial molar approach [51].
Finally, the foaming index (Σ) of the liquid slag was calculated using the following empirical equation (Equation (23)) proposed by Kim et al. [52]:
All calculated data presented in Section 3.3 were subsequently used for the calculations performed in Section 3.1 and Section 3.2. A visual representation of the conducted modelling is provided in Figure 1.
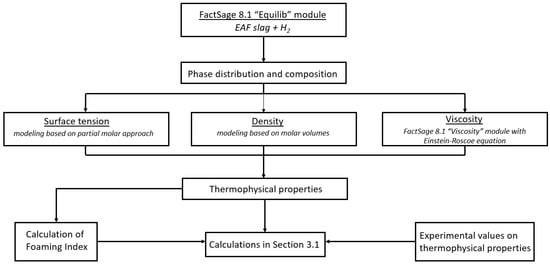
Figure 1.
Block scheme of modelling performed in this work based on thermophysical properties. The experimental values on thermophysical properties are taken from our previous work [35].
4. Results and Discussion
4.1. Reduction Experiments
After the experiments, all crucibles were cooled and crushed to analyze the results of the reduction process. Figure 2a presents the outcome of H2 injection into the liquid slag for 30 min. Clear evidence of a successful reduction process is indicated by the presence of various metallic products in the solidified slag. The largest metallic droplet found was approximately 10 mm in diameter and 5 mm in height, and it weighed about 3.2 g.
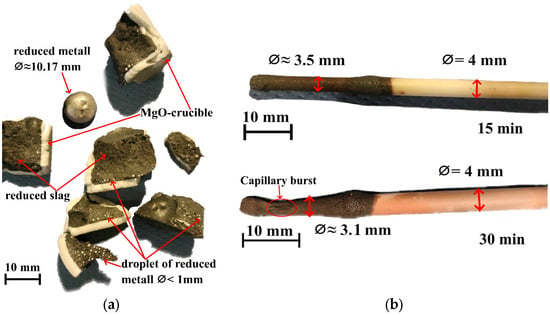
Figure 2.
(a) Crushed MgO crucible after 30 min reduction time and (b) pictures of capillaries after experiments.
Nevertheless, it is clearly observed that the slag also contains a large number of smaller metal droplets with diameters less than 1 mm, which did not have sufficient time to agglomerate into larger droplets, possibly due to the lack of liquid mixing at the bottom of the crucible. In addition, metallic shine is observed within the slag, indicating the presence of even smaller metallic particles with diameters in the range of tens to hundreds of micrometers.
Visual observation of the Al2O3 capillaries after the experiments showed thinning of the capillary outer diameter in the zone of contact with the liquid slag, indicating dissolution of the capillary into the slag. Figure 2b shows Al2O3 capillaries after 15 and 30 min of reduction time, clearly demonstrating this tendency. Notably, after 30 min of reduction time, a burst was observed in the thickest part of the capillary, indicating that this side had dissolved to the extent of reaching the inner diameter.
The chemical composition of the reduced slag after the reduction experiments, obtained using WDXRF analysis, is presented in Table 2. The results show a gradual decrease in FeOx content, with 30 min of H2 injection resulting in a reduction of approximately 40% of the FeOx originally present in the slag. The reduced metallic phase mainly consisted of iron, with a minor content of other elements (Table 3). It was possible to measure the chemical composition of the metallic phase via ICP-OES only for the samples from 15 to 30 min, due to the minimum sample quantity required for reliable analysis.

Table 2.
Chemical composition of reduced EAF slag in wt.%.

Table 3.
Chemical composition of reduced metal in wt.% (Fe is the rest).
The reduction mainly took place for FeOx, as confirmed by the chemical composition of the metallic droplets, where the Fe content exceeded 99.96 wt.% after 30 min of reduction. In addition, a slight increase in Mn content was observed, reaching up to 0.17 wt.% after 30 min. However, most of the Mn remained in the slag in oxide form. The content of tramp elements such as P remained unchanged, which is beneficial for the quality of the reduced metal, since it can be reused in steelmaking without requiring additional dephosphorization treatment.
To study the phases present in the slag before and after the reduction, XRD analysis was conducted, and the results are presented in Figure 3.
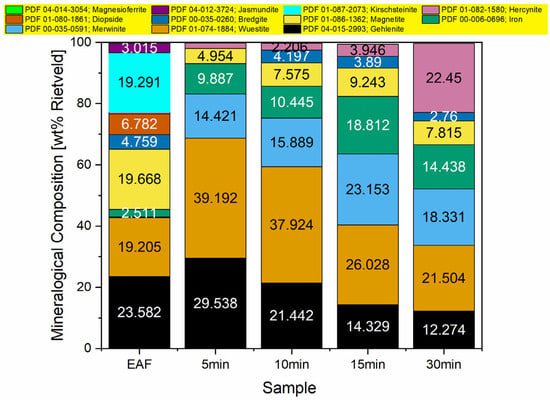
Figure 3.
Mineralogical composition of studied samples obtained from the Rietveld refinement method using XRD.
The initial EAF slag is mainly composed of gehlenite (Ca2Al(Al,SiO7)), wüstite (FeO), magnetite (Fe3O4), and kirschsteinite (CaFeSiO4). As observed after the reduction experiments, the main iron oxide phase was wüstite. However, due to the presence of very small metal particles in the slag—which did not have sufficient time to coagulate into larger particles that would be easier to separate—different amounts of metallic iron were detected in all samples.
Furthermore, with increasing reduction time, the amount of reduced iron in the slag increases, while the amount of wüstite decreases. After hydrogen injection, the slag is mainly composed of wüstite, gehlenite, hercynite (FeAl2O4), and merwinite (Ca3Mg(SiO4)2).
4.2. Results of Modeling
The calculated values show that under the experimental conditions of this work, when a bubble detaches from the capillary, a bubble with a diameter of 4.85 × 10−3 m is formed, corresponding to a volume of 5.97 × 10−8 m3. Given that the hydrogen flow rate at 1600 °C is 5.23 × 10−5 m3·s−1, the time required to form one bubble is approximately 1.1 × 10−3 s.
Substituting these values into Equations (6)–(8) yields a gas bubble velocity in the liquid slag of 0.27 m·s−1. Knowing the mass of the charged slag (0.05 kg) and the density of liquid slag at 1600 °C (3451.84 kg·m−3) [35], the liquid slag occupies a volume of 1.45 × 10−5 m3. Consequently, the height of the liquid slag in a cylindrical crucible with a diameter of 0.033 m is 16.9 × 10−3 m. Therefore, with the capillary submerged at a height of 3 mm from the crucible bottom, a bubble requires approximately 0.05 s to rise to the surface of the liquid slag.
While rising to the surface, the reduction reaction of oxides in the slag by hydrogen can affect the bubble composition and thus its residence time. One such effect is the formation of water vapor inside the gas bubble due to the reduction reaction. Assuming that only FeO in the slag reacts with hydrogen and no other oxides participate, the conversion of H2 gas into H2O is described by Equation (14). Treating the gas inside the bubble as an ideal gas and assuming that 1 mol of H2 produces 1 mol of H2O (per Equation (14)), the volume of the bubble remains unchanged. Since the density of water vapor at 1600 °C is 0.011567 kg·m−3 [53] and ρH2O ≈ ρH2 ˂ ρl, changes in gas composition can be neglected when calculating the bubble diameter and its rising velocity.
The formed H2 gas bubble interacts with the surrounding slag according to Equation (14) at the gas–slag boundary. The reaction rate is driven by the mass transfer of oxygen from the slag bulk to the bubble surface via diffusion. Oxygen arriving at the bubble–slag interface immediately reacts with H2 (assuming zero oxygen concentration in the gas phase inside the bubble, as any O2 reacts instantly to form H2O, and the H2/H2O concentration is uniform throughout the bubble with no gradients). The volume of oxygen available for reduction in the slag boundary layer is determined by the FeO content, which acts as the main oxygen source. However, only a fraction of this oxygen reaches the interface, determined by the molar oxygen transfer coefficient through the stationary liquid film surrounding the bubble surface.
By calculating these parameters (listed in Table 4), the fraction of H2 that reacts with oxygen in the slag during its rise to the surface is estimated to be 16.64%. Therefore, nearly 84% of the H2 exits the slag system without participating in reduction reactions.

Table 4.
Defined values for parameters obtained from the calculations in Section 3.1 and Section 3.2.
However, reduction reactions induced by H2 injection alter the chemical composition and, consequently, the thermophysical properties of the slag. To study these changes, thermochemical software FactSage 8.1 with the “Equilib” module was utilized. Figure 4a,b show the changes in the liquid phase composition and phase fractions as a result of the reduction reaction, with points 1, 2, 3, and 4 corresponding to 5, 10, 15, and 30 min of injection time, respectively. After 30 min of reduction, FactSage predicts that 29.04% of FeO remains in the liquid slag, while the metallic phase weighs 10.35 g. Concurrently, the fraction of solid spinel phase gradually increases, which may enhance foam stability [54]. Figure 4a further illustrates that the primary changes in the liquid phase composition are due to the reduction of iron oxide to metallic iron, while the content of other components shifts only due to the redistribution caused by FeO reduction.
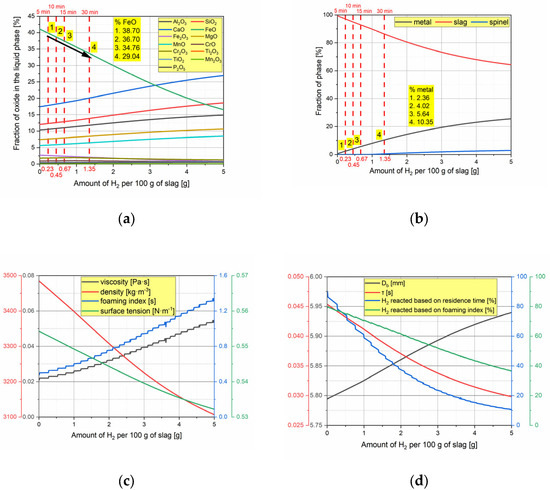
Figure 4.
Results of modeling. (a) Change in composition of liquid slag; (b) change in the phase fraction; (c) change in the thermophysical properties of slag and the foaming index; (d) change in the characteristics of a H2 gas bubble.
The changes in the slag system caused by the reduction reaction were used to model variations in the viscosity, density, and surface tension of the liquid slag, with the results presented in Figure 4c. It is evident that the ongoing reduction reaction decreases the slag’s density and surface tension while increasing its viscosity. The modeled values closely align with those measured in our previous work [35]; however, the partial molar approach used to estimate surface tension yielded higher values—about 200 mN·m−1 greater than those measured using the maximum bubble pressure method.
Consequently, when using the modeled values to calculate the fraction of reacted H2, the result is only 7.5%, which is significantly lower than the previously estimated values (Figure 4d). This discrepancy is mainly due to the increased surface tension, which reduces the diameter of the bubbles formed and thus increases their velocity. As a result, the residence time decreases significantly, causing the fraction of reacted H2 to drop to low values. With further progress of the reduction reaction, the residence time of the bubbles in the slag decreases simultaneously with a decrease in the oxygen concentration in the slag. According to the calculations, this results in only 0.81% of H2 reacted in a bubble after the addition of 5 g of H2 to the slag.
However, the main factor contributing to the decrease in the fraction of reacted H2 is the reduction in the height of the liquid slag. This change is primarily caused by the utilization of oxygen from the slag into the atmosphere and the simultaneous formation of liquid Fe, which tends to settle at the bottom of the crucible. As a result, the height of the liquid slag in the crucible can decrease from 16.6 cm to 12 cm.
It is well-known that gas injection into liquid slag leads to slag foaming. The foaming index and average foam life concepts were introduced by Ito and Fruehan in 1989 [54,55]. Essentially, this concept represents the residence time of gas bubbles within the liquid slag, which depends on the slag’s thermophysical properties. The foaming index was calculated based on the modeled values, with results shown in Figure 4a. It is clear that H2 injection increases the foaming index due to lowered surface tension and increased viscosity, conditions that promote more stable foam. Consequently, the residence time of H2-filled bubbles available for reduction reactions will increase.
Therefore, we also performed calculations, in which the residence time was replaced with the foaming index. The results, presented in Figure 4a, show that an increase in the foaming index is beneficial for the reduction process. At the beginning of the reaction, the fraction of H2 gas reacting with the oxygen in the slag reached 79.23%, while after introducing 5 g of H2 into the slag, this value dropped to 36.62%. However, these values remain significantly higher compared to the values calculated using the bubble residence time.
However, this model has limitations. Although the reduction process model incorporates components that have been validated in previous studies, the integrated framework itself has not been directly validated here. This can constrain conclusions about its robustness and generalizability. Nevertheless, the objective was to present the framework and demonstrate its potential applications. Rigorous validation using additional datasets will be crucial in future studies.
5. Conclusions
In this work, reduction experiments were conducted on EAF steelmaking slag. Modeling-based calculations were performed to study the reduction process, complemented by thermochemical software to further investigate slag reduction behavior. As a result, injecting 500 cm3·min−1 of H2 into the liquid slag achieved a 40% reduction in FeOx content compared to the initial slag. Other oxides were not reduced, and thus, a metal with Fe content exceeding 99.96 wt.% and very low levels of tramp elements was obtained.
The calculations indicated that although the reduction of FeOx increases the slag’s viscosity and decreases its surface tension—factors that would typically improve gas residence time in bubbles—the simultaneous decrease in oxygen concentration in the slag and the decrease in the height of the liquid slag led to a higher fraction of unreacted H2 in the bubbles. Consequently, the efficiency of H2 as a reductant decreased, and the fraction of reacted H2 dropped from 7.5% to 0.8% as the bubble residence time decreased from 0.045 s to 0.029 s after introducing 5 g of H2 into 100 g of liquid slag.
However, substituting the bubble residence time in liquid slag with the slag foaming index in the calculations yielded significantly higher values that were more consistent with the experimental results. In this case, the fraction of reacted H2 was 79.13% and 36.62% after introducing 5 g of H2 into 100 g of liquid slag.
Future studies should validate these findings through a broader set of reduction experiments, while also examining the influence of varying slag compositions and operating parameters to optimize hydrogen utilization and improve model robustness.
Author Contributions
Conceptualization, M.L.; Methodology, M.L. and O.V.; Software, M.L. and M.S.; Validation, M.L., H.P.M., M.S. and O.V.; Formal analysis, M.L. and M.S.; Investigation, M.L., M.S. and M.G.; Resources, H.P.M., M.G. and O.V.; Data curation, M.L.; Writing—original draft, M.L.; Writing—review & editing, M.L., H.P.M., M.S., M.G. and O.V.; Visualization, M.L. and M.S.; Supervision, H.P.M. and O.V.; Project administration, O.V.; Funding acquisition, H.P.M. and O.V. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by ESF Elbe-Stahlwerke Feralpi GmbH, project number 051201112A and the German Research Foundation (DFG), project number 461482547.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
Author Hans Peter Markus was employed by the company ESF Elbe-Stahlwerke Feralpi GmbH. The authors declare that this study received funding from ESF Elbe-Stahlwerke Feralpi GmbH. The funder had the following involvement with the study: providing research materials. The funder was not involved in the design of the study, the collection or analysis of the data, the decision to publish, or the preparation of the manuscript.
Correction Statement
This article has been republished with a minor correction to the Data Availability Statement. This change does not affect the scientific content of the article.
Nomenclature
| Db | Bubble diameter, m |
| dcapillary | Capillary diameter, m |
| Surface tension of molten slag at 1600 °C, N·m−1 | |
| g | Gravitational acceleration, m·s−2 |
| Density of liquid slag at 1600 °C, kg·m−3 | |
| Density of hydrogen gas at 1600 °C, kg·m−3 | |
| Vb | Bubble volume, m3 |
| Hydrogen gas flow rate at 25 °C, m3·s | |
| Hydrogen gas flow rate at 1600 °C, m3·s | |
| t | Time for formation of 1 gas bubble, s |
| Bubble rise velocity, m·s−1 | |
| Rb | Bubble radius, m |
| Cd | Drag coefficient, (-) |
| A | Reference area, m2 |
| Retp | Reynolds number based on thermophysical properties, (-) |
| Viscosity of liquid slag at 1600 °C, Pa·s | |
| τ | Residence time of a gas bubble in liquid slag, s |
| H | Height of liquid slag in a crucible, m |
| Vslag | Volume of liquid slag, m3 |
| S | Area of a crucible, m2 |
| m | Mass of charged slag, kg |
| Inner diameter of a crucible | |
| Amount of reacted H2, mol | |
| kl | Mass transfer coefficient, m·s−1 |
| Co | Volume concentration of oxygen in slag, mol·m−3 |
| Sh | Sherwood number, (-) |
| Do | Diffusion coefficient of oxygen in liquid slag, m2·s−1 |
| Sc | Schmidt number, (-) |
| kb | Boltzman constant, J·K−1 |
| Total amount of H2 gas in a bubble, mol | |
| T | Absolute temperature, K |
| P | Pressure, Pa |
| R | Gas constant, J·K−1·mol−1 |
| Fraction of reacted H2 gas, % | |
| Σ | Foaming index, s |
| η | Viscosity of liquid slag containing solid particles, Pa·s |
| η0 | Viscosity of liquid slag without solid particles, Pa·s |
| c | Fraction of solid phase, (-) |
| n | Parameter related to the geometrical shape of the solid particles, (-) |
References
- International Energy Agency. Global Hydrogen Review 2021; OECD: Paris, France, 2021; ISBN 978-92-64-51931-2. [Google Scholar]
- Kovtun, O.; Levchenko, M.; Oldinski, E.; Gräbner, M.; Volkova, O. Swelling Behavior of Iron Ore Pellets during Reduction in H2 and N2/H2 Atmospheres at Different Temperatures. Steel Res. Int. 2023, 94, 2300140. [Google Scholar] [CrossRef]
- Injection of Hydrogen into Blast Furnace: Thyssenkrupp Steel Concludes First Test Phase Successfully. Available online: https://www.thyssenkrupp-steel.com/en/newsroom/press-releases/thyssenkrupp-steel-concludes-first-test-phase-successfully.html (accessed on 14 July 2025).
- Reallabor der Energiewende H2Stahl Bringt Wasserstoff in die Stahlindustrie. Available online: https://www.energieforschung.de/de/aktuelles/projekteinblicke/2021/reallabor-der-energiewende-h2-stahl-bringt-wasserstoff-in-die-stahl-industrie (accessed on 14 July 2025).
- MAXH2DR. Available online: https://www.estep.eu/clean-steel-partnership/maxh2dr (accessed on 25 August 2025).
- Meijer, K.; Guenther, C.; Dry, R.J. HIsarna Pilot Plant Project. In Proceedings of the 1st International Conference on Energy and CO2 Reduction in the Steel Industry, Düsseldorf, Germany, 27 June–1 July 2011. [Google Scholar]
- Khasraw, D.; Yan, Z.; Hage, J.L.T.; Meijer, K.; Li, Z. Reduction of FeO in Molten Slag by Solid Carbonaceous Materials for HIsarna Alternative Ironmaking Process. Metall. Mater. Trans. B 2022, 53, 3246–3261. [Google Scholar] [CrossRef]
- Fan, X.; Jiao, K.; Zhang, J.; Wang, K.; Chang, Z. Phase Transformation of Cohesive Zone in a Water-Quenched Blast Furnace. ISIJ Int. 2018, 58, 1775–1780. [Google Scholar] [CrossRef]
- Htet, T.T.; Yan, Z.; Khasraw, D.; Hage, J.; Meijer, K.; Li, Z. Kinetic Study on Reduction of FeO in a Molten HIsarna Slag by Various Solid Carbon Sources. Metall. Mater. Trans. B 2022, 54, 163–177. [Google Scholar] [CrossRef]
- Nurdiawati, A.; Zaini, I.N.; Wei, W.; Gyllenram, R.; Yang, W.; Samuelsson, P. Towards Fossil-Free Steel: Life Cycle Assessment of Biosyngas-Based Direct Reduced Iron (DRI) Production Process. J. Clean. Prod. 2023, 393, 136262. [Google Scholar] [CrossRef]
- Fan, Z.; Friedmann, S.J. Low-Carbon Production of Iron and Steel: Technology Options, Economic Assessment, and Policy. Joule 2021, 5, 829–862. [Google Scholar] [CrossRef]
- European Steel in Figures 2025. Available online: https://www.eurofer.eu/publications/brochures-booklets-and-factsheets/european-steel-in-figures-2025 (accessed on 1 July 2025).
- Hosseini, S.; Soltani, S.M.; Fennell, P.S.; Choong, T.S.Y.; Aroua, M.K. Production and Applications of Electric-Arc-Furnace Slag as Solid Waste in Environmental Technologies: A Review. Environ. Technol. Rev. 2016, 5, 1–11. [Google Scholar] [CrossRef]
- Loureiro, C.D.A.; Moura, C.F.N.; Rodrigues, M.; Martinho, F.C.G.; Silva, H.M.R.D.; Oliveira, J.R.M. Steel Slag and Recycled Concrete Aggregates: Replacing Quarries to Supply Sustainable Materials for the Asphalt Paving Industry. Sustainability 2022, 14, 5022. [Google Scholar] [CrossRef]
- Maharaj, C.; White, D.; Maharaj, R.; Morin, C. Re-Use of Steel Slag as an Aggregate to Asphaltic Road Pavement Surface. Cogent Eng. 2017, 4, 1416889. [Google Scholar] [CrossRef]
- Matino, I.; Branca, T.A.; Fornai, B.; Colla, V.; Romaniello, L. Scenario Analyses for By-Products Reuse in Integrated Steelmaking Plants by Combining Process Modeling, Simulation, and Optimization Techniques. Steel Res. Int. 2019, 90, 1900150. [Google Scholar] [CrossRef]
- Teo, P.T.; Zakaria, S.K.; Salleh, S.Z.; Taib, M.A.A.; Mohd Sharif, N.; Abu Seman, A.; Mohamed, J.J.; Yusoff, M.; Yusoff, A.H.; Mohamad, M.; et al. Assessment of Electric Arc Furnace (EAF) Steel Slag Waste’s Recycling Options into Value Added Green Products: A Review. Metals 2020, 10, 1347. [Google Scholar] [CrossRef]
- Yu, S.; Garrabrants, A.C.; DeLapp, R.C.; Hubner, T.; Thorneloe, S.A.; Kosson, D.S. From Leaching Data to Release Estimates: Screening and Scenario Assessments of Electric Arc Furnace (EAF) Slag under Unencapsulated Use. J. Hazard. Mater. 2024, 479, 135522. [Google Scholar] [CrossRef] [PubMed]
- Lateef, K.B.; Sheikh, A.R.; Nurulakmal, M.S.; Baharun, N. Evaluation of the Leaching Behavior of Hexavalent Chromium from Malaysian Electric Arc Furnace Steel Slag. Adv. Mater. Res. 2013, 652–654, 1628–1632. [Google Scholar] [CrossRef]
- EN 12457-2: 2002; Characterization of Waste–Leaching–Compliance Test for Leaching of Granular Waste Materials and Sludges–Part 2: One Stage Batch Test at a Liquid to Solid Ratio of 10 L/Kg for Materials with Particle Size below 4 mm (Without or with Size Reduction). European Committee for Standardization (CEN): Brussels, Belgium, 2002.
- Liu, C.; Huang, S.; Wollants, P.; Blanpain, B.; Guo, M. Valorization of BOF Steel Slag by Reduction and Phase Modification: Metal Recovery and Slag Valorization. Metall. Mater. Trans. B 2017, 48, 1602–1612. [Google Scholar] [CrossRef]
- Shen, H.; Forssberg, E. An Overview of Recovery of Metals from Slags. Waste Manag. 2003, 23, 933–949. [Google Scholar] [CrossRef]
- Dankwah, J.R.; Koshy, P.; Saha-Chaudhury, N.M.; O’Kane, P.; Skidmore, C.; Knights, D.; Sahajwalla, V. Reduction of FeO in EAF Steelmaking Slag by Metallurgical Coke and Waste Plastics Blends. ISIJ Int. 2011, 51, 498–507. [Google Scholar] [CrossRef]
- Min, D.J.; Han, J.W.; Chung, W.S. A Study of the Reduction Rate of FeO in Slag by Solid Carbon. Metall. Mater. Trans. B 1999, 30, 215–221. [Google Scholar] [CrossRef]
- Ye, G.; Burstrom, E.; Kuhn, M.; Piret, J. Reduction of Steel-Making Slags for Recovery of Valuable Metals and Oxide Materials. Scand. J. Metall. 2003, 32, 7–14. [Google Scholar] [CrossRef]
- Htet, T.T.; Yan, Z.; Sampath Kumar, B.; Hage, J.; Meijer, K.; Li, Z. Study on Hydrogen Smelting Reduction Behaviour in Synthetic Molten HIsarna Slag. Ironmak. Steelmak. 2023, 50, 1590–1600. [Google Scholar] [CrossRef]
- Bartzsch, G.; Christian, R.; Mohanty, S.R.; Shukla, A.K.; Volkova, O. Effect of Al2O3 and TiO2 on Viscosity, Surface Tension, and Density of Blast Furnace Slag with CaO/SiO2 = 1.13. Steel Res. Int. 2023, 94, 2200798. [Google Scholar] [CrossRef]
- Kovtun, O.; Korobeinikov, I.; C, S.; Shukla, A.K.; Volkova, O. Viscosity of BOF Slag. Metals 2020, 10, 982. [Google Scholar] [CrossRef]
- Xiong, D.; Lu, L.; Holmes, R.J. Developments in the Physical Separation of Iron Ore. In Iron Ore; Elsevier: Amsterdam, The Netherlands, 2015; pp. 283–307. ISBN 978-1-78242-156-6. [Google Scholar]
- Yildirim, I.Z.; Prezzi, M. Chemical, Mineralogical, and Morphological Properties of Steel Slag. Adv. Civ. Eng. 2011, 2011, 463638. [Google Scholar] [CrossRef]
- Qu, G.; Wei, Y.; Li, B.; Wang, H.; Yang, Y.; McLean, A. Distribution of Copper and Iron Components with Hydrogen Reduction of Copper Slag. J. Alloys Compd. 2020, 824, 153910. [Google Scholar] [CrossRef]
- Attah-Kyei, D.; Klemettinen, L.; Michallik, R.; Salminen, J.; Taskinen, P.; Lindberg, D. Hydrogen as Carbon-Free Reducing Agent in Non-Ferrous Slag Fuming. Metall. Mater. Trans. B 2022, 53, 3775–3792. [Google Scholar] [CrossRef]
- Lee, T.-H.; Joo, S.-H.; Nersisyan, H.H.; Kong, M.-S.; Lee, J.-W.; Park, K.-W.; Lee, J.-H. Reduction Kinetics of Zinc Powder from Brass Converter Slag by Pyrometallurgical Method Using Hydrogen Gas. KONA Powder Part. J. 2016, 33, 278–286. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, T.; Zheng, C. Reduction Kinetics of Copper Slag by H2. Minerals 2022, 12, 548. [Google Scholar] [CrossRef]
- Levchenko, M.; Kovtun, O.; Angelini, A.; Markus, H.P.; Sosin, D.; Endo, R.; Volkova, O. Effect of SiO2 and Al2O3 on the Thermophysical Properties and the Foaming Index of Electric Arc Furnace Slag from the Production of Construction Steel. Steel Res. Int. 2025, 96, 2400476. [Google Scholar] [CrossRef]
- Otto, M. Analytische Chemie; 5. Auflage; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2019; ISBN 978-3-527-34465-9. [Google Scholar]
- Hernandez-Aguilar, J.R.; Cunningham, R.; Finch, J.A. A Test of the Tate Equation to Predict Bubble Size at an Orifice in the Presence of Frother. Int. J. Miner. Process. 2006, 79, 89–97. [Google Scholar] [CrossRef]
- Holman, J.P. Heat Transfer, 9th ed.; McGraw-Hill Series in Mechanical Engineering; McGraw-Hill: New York, NY, USA, 2002; ISBN 0-07-240655-0. [Google Scholar]
- Schiller, L. A Drag Coefficient Correlation. Zeit Ver Dtsch. Ing 1933, 77, 318–320. [Google Scholar]
- Islam, M.T.; Nguyen, A.V. Bubble-Assisted Matte Transportation into Slag Phase: A Numerical Investigation. Miner. Eng. 2023, 202, 108286. [Google Scholar] [CrossRef]
- Roussel, M.R. Reaction-Diffusion Equations. In Nonlinear Dynamics; IOP Publishing: Bristol, UK, 2005; pp. 2053–2571. [Google Scholar]
- Hasegawa, M. Chapter 3.3—Ellingham Diagram. In Treatise on Process Metallurgy; Seetharaman, S., Ed.; Elsevier: Boston, MA, USA, 2014; pp. 507–516. ISBN 978-0-08-096986-2. [Google Scholar]
- Levich, V.G.; Tobias, C.W. Physicochemical Hydrodynamics. J. Electrochem. Soc. 1963, 110, 251C. [Google Scholar] [CrossRef]
- Sommerfeld, A. Ein Beitrag zur Hydrodynamischen Erklaerung der Turbulenten Fluessigkeitsbewegungen; Accademia dei Lince: Rome, Italy, 1909. [Google Scholar]
- Incropera, F.P.; DeWitt, D.P. Fundamentals of Heat and Mass Transfer, International ed.; Wiley: New York, NY, USA, 1990; ISBN 978-0-471-51729-0. [Google Scholar]
- Köddermann, T.; Ludwig, R.; Paschek, D. On the Validity of Stokes–Einstein and Stokes–Einstein–Debye Relations in Ionic Liquids and Ionic-Liquid Mixtures. ChemPhysChem 2008, 9, 1851–1858. [Google Scholar] [CrossRef]
- Bale, C.W.; Bélisle, E.; Chartrand, P.; Decterov, S.A.; Eriksson, G.; Gheribi, A.E.; Hack, K.; Jung, I.-H.; Kang, Y.-B.; Melançon, J.; et al. FactSage Thermochemical Software and Databases, 2010–2016. Calphad 2016, 54, 35–53. [Google Scholar] [CrossRef]
- Roscoe, R. The Viscosity of Suspensions of Rigid Spheres. Br. J. Appl. Phys. 1952, 3, 267–269. [Google Scholar] [CrossRef]
- Reddy, R.G.; Weizenbach, R.N.; Landolt, C.A.; Queneau, P.E. Metallurgical Society of CIM Extractive Metallurgy of Copper, Nickel and Cobalt. In Proceedings of Paul E. Queneau International Symposium; Minerals, Metals & Materials Society: Warrendale, PA, USA, 1993; ISBN 0-87339-218-3. [Google Scholar]
- Persson, M.; Matsushita, T.; Zhang, J.; Seetharaman, S. Estimation of Molar Volumes of Some Binary Slags from Enthalpies of Mixing. Steel Res. Int. 2007, 78, 102–108. [Google Scholar] [CrossRef]
- Mills, K.C. Estimation of Physicochemical Properties of Coal Slags and Ashes. In Mineral Matter and Ash in Coal; ACS Publications: Washington, DC, USA, 1986; ISBN 1947-5918. [Google Scholar]
- Kim, H.S.; Min, D.J.; Park, J.H. Foaming Behavior of CaO-SiO2-FeO-MgOsatd-X (X = Al2O3, MnO, P2O5, and CaF2) Slags at High Temperatures. ISIJ Int. 2001, 41, 317–324. [Google Scholar] [CrossRef]
- Wagner, W.; Pruß, A. The IAPWS Formulation 1995 for the Thermodynamic Properties of Ordinary Water Substance for General and Scientific Use. J. Phys. Chem. Ref. Data 2002, 31, 387–535. [Google Scholar] [CrossRef]
- Ito, K.; Fruehan, R.J. Study on the Foaming of CaO-SiO2-FeO Slags: Part I. Foaming Parameters and Experimental Results. Metall. Trans. B 1989, 20, 509–514. [Google Scholar] [CrossRef]
- Ito, K.; Fruehan, R.J. Study on the Foaming of CaO-SiO2-FeO Slags: Part II. Dimensional Analysis and Foaming in Iron and Steelmaking Processes. Metall. Trans. B 1989, 20, 515–521. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).