Study on the Optimization of the Morphology and Nucleation Mechanism of Electroplated Sn-Pb Coatings by the Synergistic Effect of Composite Additives
Abstract
1. Introduction
2. Experimental
2.1. Electrodeposition of Coatings
2.2. Electrochemical Testing and Coating Characterization
3. Results and Discussion
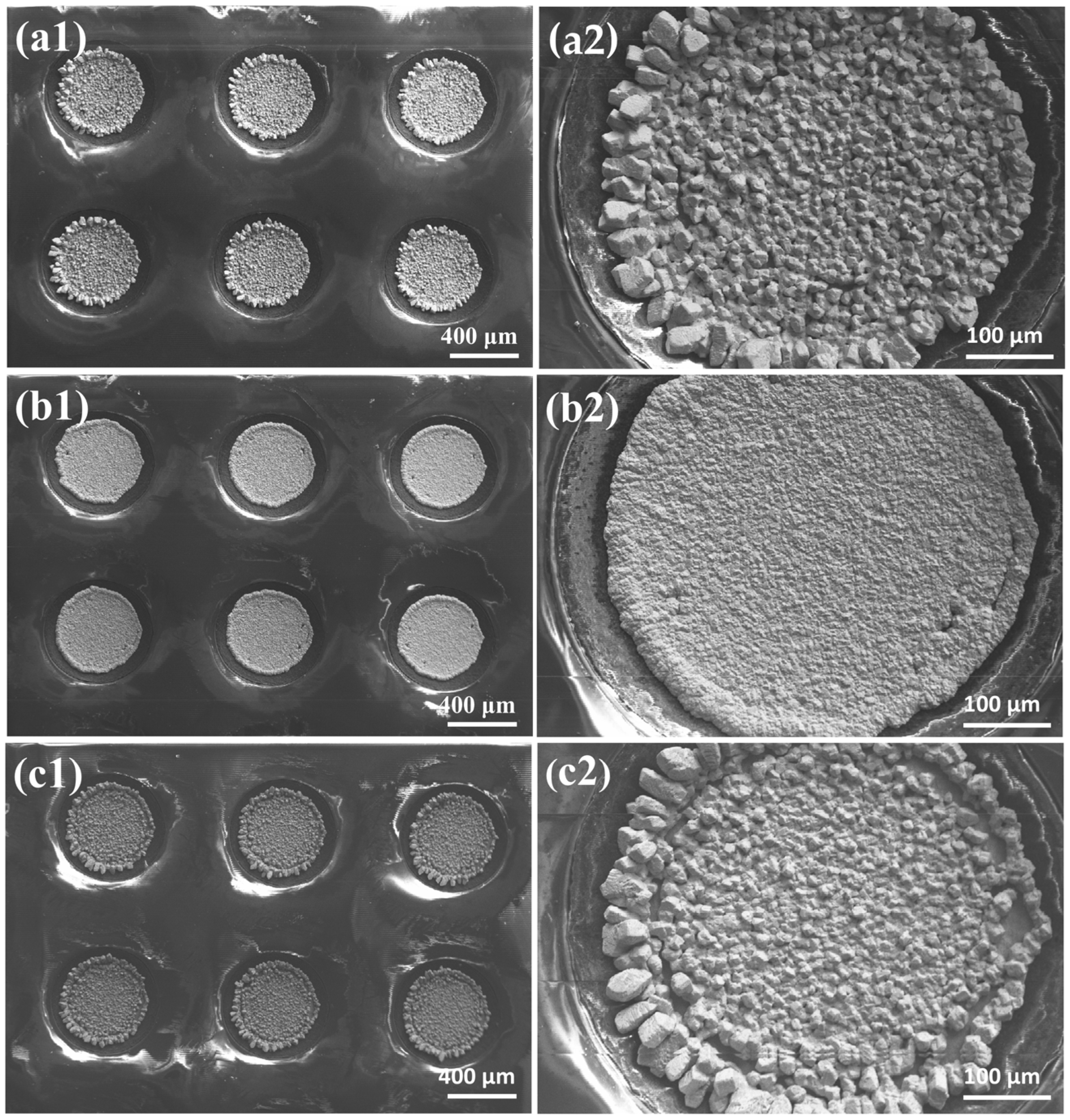
3.1. Effects of Additives on Morphology and Composition of Sn-Pb Micro-Bumps
3.2. Effects of Additives on Electrochemical Behavior
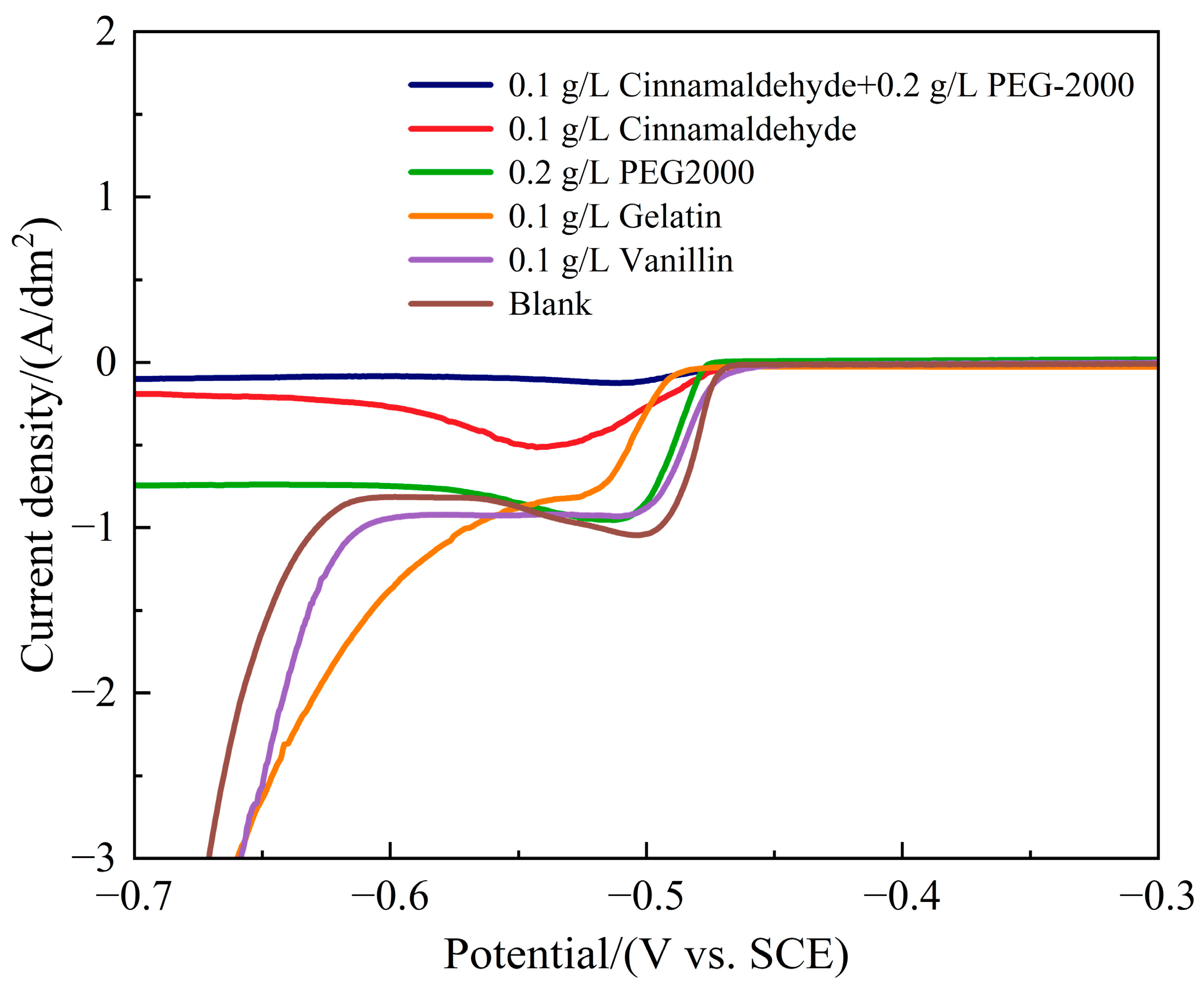
3.2.1. Linear Sweep Voltammetry (LSV) Analysis
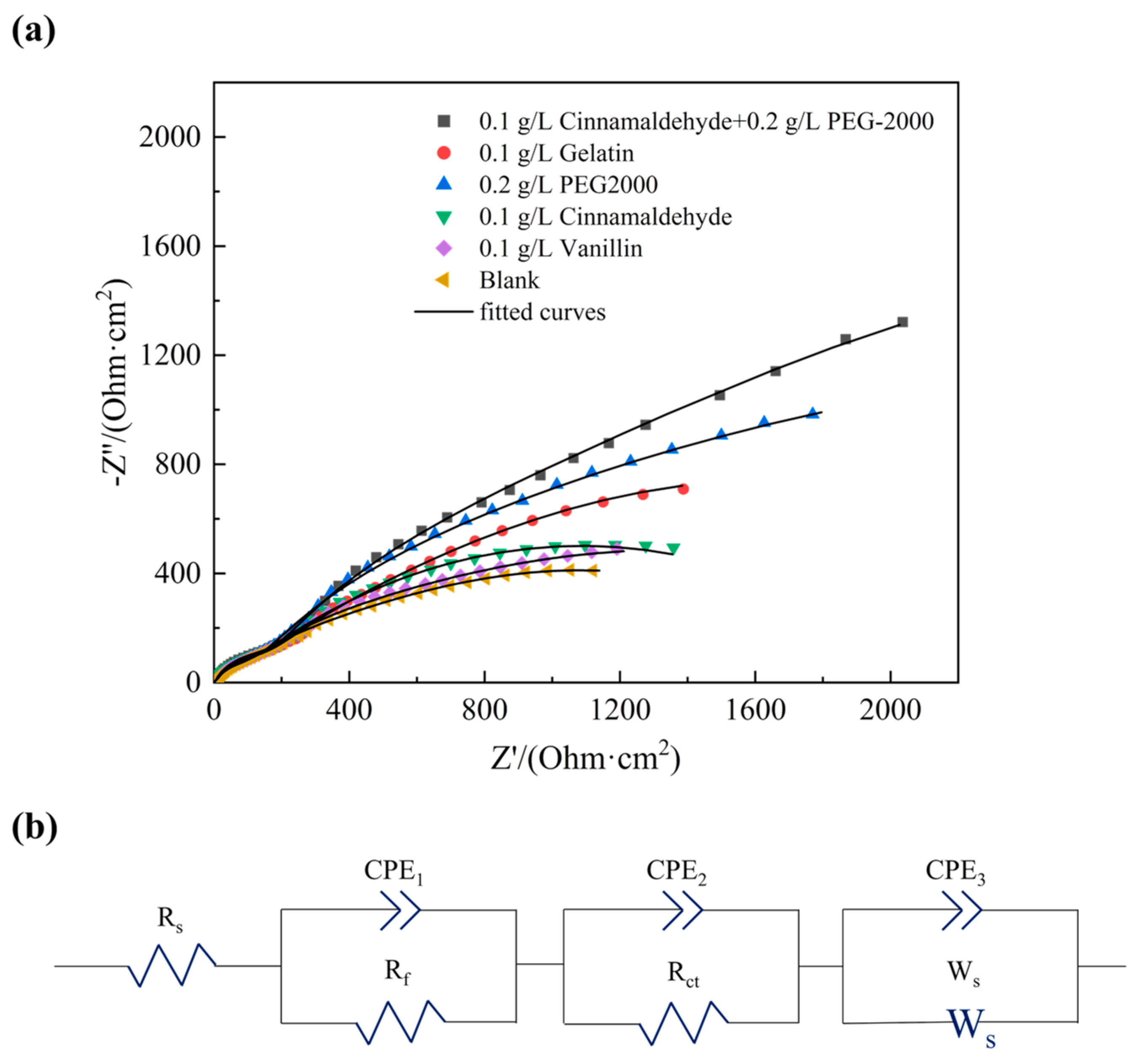
3.2.2. Electrochemical Impedance Spectroscopy (EIS) Analysis
3.3. Effects of Additives on Electrocrystallization Nucleation Mechanism
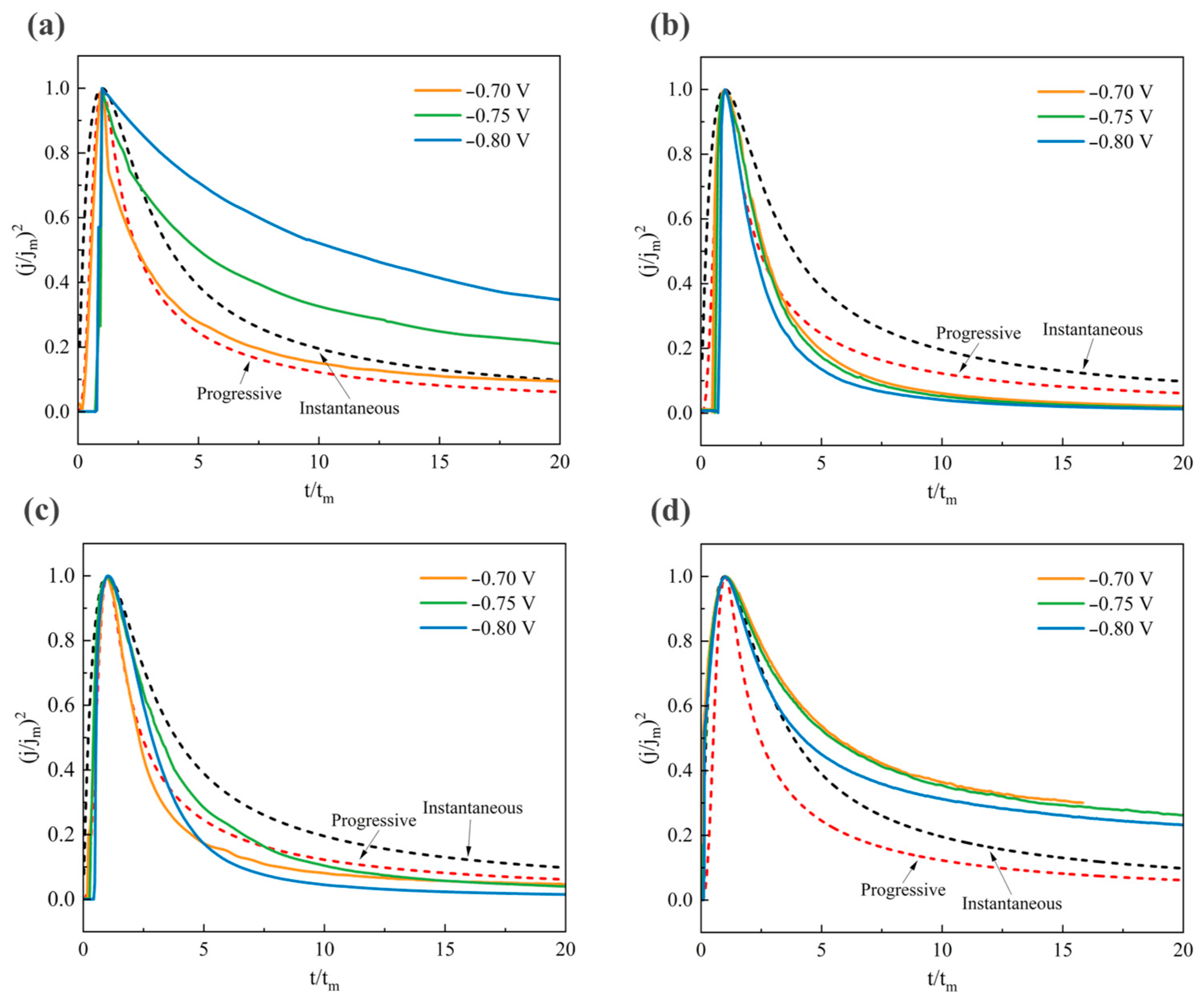
3.3.1. Chronoamperometry Transient Behavior Analysis
3.3.2. Electrodeposition Nucleation Mode
4. Conclusions
- Various additives influence both the morphology and composition of Sn-Pb coatings. When using 0.1 g/L cinnamaldehyde and 0.2 g/L PEG-2000, the composite additives optimize the electric field distribution through double-layer reconstruction. This facilitates the uniform distribution of current density on the electrode surface, thereby suppressing edge effects. The combination of steric hindrance from PEG-2000 and the conjugated adsorption of cinnamaldehyde ensures that the nucleation rate exceeds the crystal growth rate. The resulting coating features a smooth surface, dense grains, no edge effect, and a composition matching the eutectic ratio.
- LSV and EIS results show that 0.1 g/L cinnamaldehyde and 0.2 g/L PEG-2000 increase cathodic polarization and charge transfer resistance (Rct = 189.20 Ω·cm2), requiring higher overpotential for the reduction of Sn2+ and Pb2+. This suppresses dendritic growth and increases the resistance for charge transfer across the electrode/solution interface, delaying the electrochemical reaction rate. This process ultimately results in a Sn-Pb coating characterized by fine grains and a smooth surface.
- The composite additives (0.1 g/L cinnamaldehyde + 0.2 g/L PEG-2000) transform the nucleation mechanism of Sn-Pb deposition into instantaneous nucleation. Chronoamperometry results demonstrate a decreased peak current and prolonged time to reach the peak (at −0.70 V, jm = −0.64 A/dm2, tm = 1.89 s). This is because the long chains of PEG-2000 and conjugated molecules of cinnamaldehyde form a dynamic adsorption layer. Its instantaneous desorption promotes the simultaneous formation of numerous critical nuclei by Sn2+ and Pb2+, delaying nucleus growth and refining grains, thus providing kinetic guarantees for obtaining dense and smooth coatings.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Meindl, J.D. Beyond Moore’s Law: The interconnect era. Comput. Sci. Eng. 2003, 5, 20–24. [Google Scholar] [CrossRef]
- Schaller, R.R. Technological innovation in the semiconductor industry: A case study of the International Technology Roadmap for Semiconductors (ITRS). In Proceedings of the Portland International Conference on Management of Engineering and Technology (PICMET), Portland, OR, USA, 29 July–2 August 2001; p. 195. [Google Scholar]
- Lau, J.H. Recent Advances and Trends in Advanced Packaging. IEEE Trans. Compon. Packag. Manuf. Technol. 2022, 12, 228–252. [Google Scholar] [CrossRef]
- Goh, Y.; Haseeb, A.S.M.A.; Faizul Mohd Sabri, M. Electrodeposition of lead-free solder alloys. Solder. Surf. Mt. Technol. 2013, 25, 76–90. [Google Scholar] [CrossRef]
- Thanedburapasup, S.; Wetchirarat, N.; Muengjai, A.; Tengprasert, W.; Wiman, P.; Thublaor, T.; Chandra-ambhorn, S. Fabrication of Mn–Co Alloys Electrodeposited on AISI 430 Ferritic Stainless Steel for SOFC Interconnect Applications. Metals 2023, 13, 612. [Google Scholar] [CrossRef]
- Nagy, P.; Péter, L.; Kolonits, T.; Nagy, A.; Gubicza, J. Combinatorial Design of an Electroplated Multi-Principal Element Alloy: A Case Study in the Co-Fe-Ni-Zn Alloy System. Metals 2024, 14, 700. [Google Scholar] [CrossRef]
- Yang, H.; Wang, Y.; Chen, M.; Liu, J.; Liu, Y.; Chen, T.; Li, J. Research on Electroplating Bonding of Flip-Chip Under the Action of Additives. IEEE Trans. Compon. Packag. Manuf. Technol. 2013, 13, 1324–1331. [Google Scholar] [CrossRef]
- Wu, X.; Zhu, X.; Wang, S.; Tang, X.; Lang, T.; Belyaev, V.; Abduev, A.; Kazak, A.; Lin, C.; Yan, Q.; et al. Bump-Fabrication Technologies for Micro-LED Display: A Review. Materials 2025, 18, 1783. [Google Scholar] [CrossRef]
- Volpert, M.; Henry, D.; Taneja, D.; Chaira, T.; Gueugnot, A.; Hodaj, F. Cu pillar as interconnect for 10 μm pitch and below: Fabrication issues and assembly results. In Proceedings of the 2018 IEEE 7th Electronic System-Integration Technology Conference (ESTC), Dresden, Germany, 18–21 September 2018; pp. 1–7. [Google Scholar]
- Jo, Y.; Kim, S.M.; Jeong, E.S.; Lee, K.T.; Jin, S.; Lee, W.Y.; Lee, M.H. On the role of complexing agents in co-electrodeposition of SnAg alloy with uniform composition. Mater. Sci. Semicond. Process. 2023, 166, 107751. [Google Scholar] [CrossRef]
- Shang, M.; Yao, J.; Zhang, D.; Su, X.; Ma, H.; Wang, Y.; Ma, H. Preparation and soldering performance of SAC305@Sn-bi Core-shell solder balls based on eutectic co-deposition. Mater. Charact. 2024, 211, 113815. [Google Scholar] [CrossRef]
- Hwang, Y.; Lee, J.; Kim, M.J. Pulse electrodeposition of Sn-Bi low-temperature solder with phloroglucinol. Electrochim. Acta 2025, 535, 146661. [Google Scholar] [CrossRef]
- Tu, K.N.; Zeng, K. Tin–lead (SnPb) solder reaction in flip chip technology. Mater. Sci. Eng. R Rep. 2001, 34, 1–58. [Google Scholar] [CrossRef]
- Siviour, C.R.; Walley, S.M.; Proud, W.G.; Field, J.E. Mechanical properties of SnPb and lead-free solders at high rates of strain. J. Phys. D Appl. Phys. 2005, 38, 4131. [Google Scholar] [CrossRef]
- Balint, T.S.; Kolawa, E.A.; Cutts, J.A.; Peterson, C.E. Extreme environment technologies for NASA’s robotic planetary exploration. Acta Astronaut. 2008, 63, 285–298. [Google Scholar] [CrossRef]
- Balint, T.S.; Cutts, J.A.; Kolawa, E.A.; Peterson, C.E. Extreme environment technologies for space and terrestrial applications. In Space Exploration Technologies; SPIE: Orlando, FL, USA, 2008; pp. 36–47. [Google Scholar]
- Petersson, I.; Ahlberg, E. Kinetics of the electrodeposition of Pb-Sn alloys: Part I. At glassy carbon electrodes. J. Electroanal. Chem. 2000, 485, 166–177. [Google Scholar] [CrossRef]
- Arafat, Y.; Sultana, S.T.; Dutta, I.; Panat, R. Effect of Additives on the Microstructure of Electroplated Tin Films. J. Electrochem. Soc. 2018, 165, D816–D824. [Google Scholar] [CrossRef]
- Bučko, M.; Stupar, S.; Bajat, J.B. The Influence of Sm Content on the Surface Morphology and Corrosion Behavior of Zn-Co-Sm Composite Coatings. Metals 2023, 13, 481. [Google Scholar] [CrossRef]
- Kohl, P.A. The High Speed Electrodeposition of Sn/Pb Alloys. J. Electrochem. Soc. 1982, 129, 1196. [Google Scholar] [CrossRef]
- Fujimura, T.; Dolmatov, V.Y.; Burkat, G.K.; Orlova, E.A.; Veretennikova, M.V. Electrochemical codeposition of Sn–Pb–metal alloy along with detonation synthesis nanodiamonds. Diam. Relat. Mater. 2004, 13, 2226–2229. [Google Scholar] [CrossRef]
- Danilov, F.I.; Vasil’eva, E.A.; Butyrina, T.E.; Protsenko, V.S. Electrodeposition of lead–tin alloy from methanesulphonate bath containing organic surfactants. Prot. Met. Phys. Chem. Surf. 2010, 46, 697–703. [Google Scholar] [CrossRef]
- Abdel-Aziz, A.B.; El-Zomrawy, A.A.; El-Sabbah, M.M.B.; Ghayad, I.M. Electrodeposition of lead and lead-tin alloy on copper using an eco-friendly methanesulfonate plating bath. J. Mater. Res. Technol. 2022, 18, 2166–2174. [Google Scholar] [CrossRef]
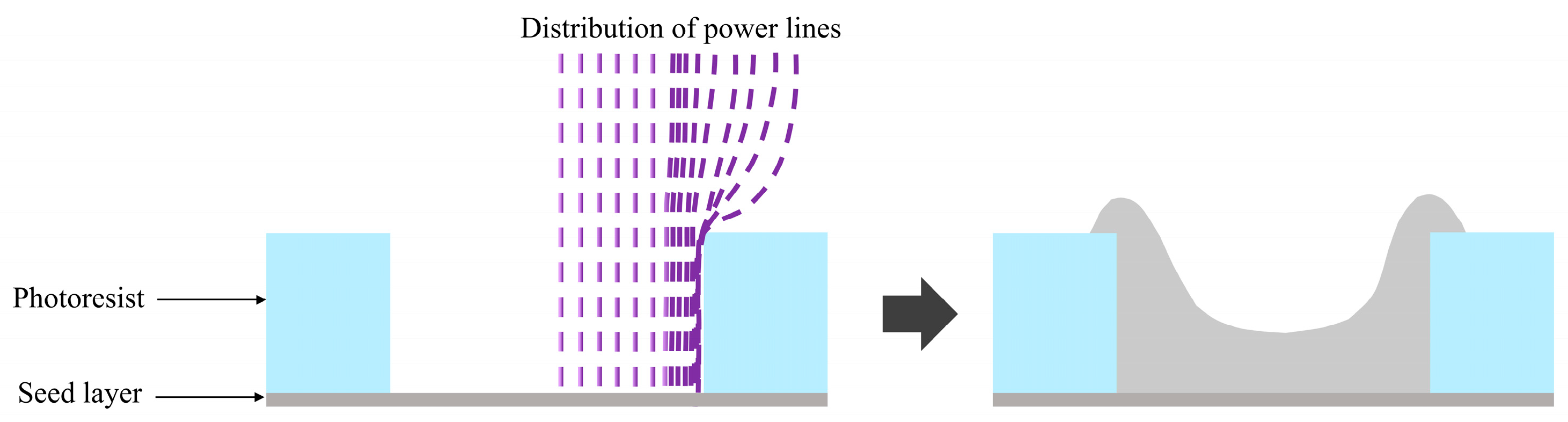
- Luo, X.; Zhang, S.; Liu, Z. Investigating the edge effects of Cu electroplating on the SAMs-coated Si substrate. J. Mater. Sci. Mater. Electron. 2023, 34, 1047. [Google Scholar] [CrossRef]
- Oh, Y.J.; Chung, S.H.; Lee, M.S. Optimization of Thickness Uniformity in Electrodeposition onto a Patterned Substrate. Mater. Trans. 2004, 45, 3005–3010. [Google Scholar] [CrossRef]
- Chen, H.Y.; Chen, C.; Wu, P.W.; Shieh, J.M.; Cheng, S.S.; Hensen, K. Effect of Polyethylene Glycol Additives on Pulse Electroplating of SnAg Solder. J. Electron. Mater. 2008, 37, 224–230. [Google Scholar] [CrossRef]
- Barry, F.J.; Cunnane, V.J. Synergistic effects of organic additives on the discharge, nucleation and growth mechanisms of tin at polycrystalline copper electrodes. J. Electroanal. Chem. 2002, 537, 151–163. [Google Scholar] [CrossRef]
- Kim, S.J.; Kim, H.T.; Park, S.M. Effects of o-Vanillin as a Brightener on Zinc Electrodeposition at Iron Electrodes. J. Electrochem. Soc. 2004, 151, C850. [Google Scholar] [CrossRef]
- Esmailzadeh, S.; Shahrabi, T.; Yaghoubinezhad, Y.; Darband, G.B. An analytical study on nucleation and growth mechanism of nanostructured Ni-Se coating by the chronoamperometry and pulse potential techniques. J. Electrochem. Chem. 2021, 881, 114949. [Google Scholar] [CrossRef]
- Tan, W.; He, H.; Gao, Y.; Peng, Y.; Dai, X. Nucleation and growth mechanisms of an electrodeposited Ni–Se–Cu coating on nickel foam. J. Colloid Interface Sci. 2021, 600, 492–502. [Google Scholar] [CrossRef]
- Huang, Y.; Yang, C.; Tan, X.; Zhang, Z.; Wang, S.; Hu, J.; Chen, Y. Benzaldehyde derivatives on tin electroplating as corrosion resistance for fabricating copper circuit. Nanotechnol. Rev. 2022, 11, 3125–3137. [Google Scholar] [CrossRef]
- Zhou, X.; Li, W.; Hu, J.; Zuo, Z. Electrochemical-voltammetry behavior of several aromatic aldehydes in acid solution. Wuhan Univ. J. Nat. Sci. 2000, 5, 485–490. [Google Scholar] [CrossRef]
- Culita, D.C.; Simonescu, C.M.; Patescu, R.E.; Dragne, M.; Stanica, N.; Oprea, O. o-Vanillin functionalized mesoporous silica–coated magnetite nanoparticles for efficient removal of Pb (II) from water. J. Solid State Chem. 2016, 238, 311–320. [Google Scholar] [CrossRef]
- Meng, Z.; Liu, Z.Q. Preparation of Sn nanosheets by electrodeposition in polycarbonate template. In Proceedings of the 2019 20th International Conference on Electronic Packaging Technology (ICEPT), Hong Kong, China, 12–15 August 2019; pp. 1–4. [Google Scholar]
- Kudryavtsev, V.N.; Tyutina, K.M.; Popov, A.N.; Maksimenko, S.; Zonin, V.A. Electrodeposition of tin-based alloys as functional coatings. Plat. Surf. Finish. 1992, 79, 57–61. [Google Scholar]
- Zhao, Z.; Zou, Y.; Liu, P.; Lai, Z.; Wen, L.; Jin, Y. EIS equivalent circuit model prediction using interpretable machine learning and parameter identification using global optimization algorithms. Electrochim. Acta 2022, 418, 140350. [Google Scholar] [CrossRef]
- Lukács, Z.; Kristóf, T. A generalized model of the equivalent circuits in the electrochemical impedance spectroscopy. Electrochim. Acta 2020, 363, 137199. [Google Scholar] [CrossRef]
- Gao, Y.; Patel, R.L.; Shen, K.Y.; Wang, X.; Axelbaum, R.L.; Liang, X. Boosting the electrochemical performance of Li1.2Mn0.54Ni0.13Co0.13O2 by atomic layer-deposited CeO2 coating. ACS Omega 2018, 3, 906–916. [Google Scholar] [CrossRef]
- Song, C.; Wang, W.; Peng, H.; Wang, Y.; Zhao, C.; Zhang, H.; Dou, Y. Improving the electrochemical performance of LiNi0.80Co0.15Al0.05O2 in lithium ion batteries by LiAlO2 surface modification. Appl. Sci. 2018, 8, 378. [Google Scholar] [CrossRef]
- Sun, Y.; Zangari, G. Commentary and Notes on the Original Derivations of the Scharifker-Hills Model. J. Electrochem. Soc. 2023, 170, 032503. [Google Scholar] [CrossRef]
- Khelladi, M.R.; Mentar, L.; Azizi, A.; Sahari, A.; Kahoul, A. Electrochemical nucleation and growth of copper deposition onto FTO and n-Si (1 0 0) electrodes. Mater. Chem. Phys. 2009, 115, 385–390. [Google Scholar] [CrossRef]
- Grishenkova, O.V.; Kosov, A.V.; Zaikov, Y.P.; Isaev, V.A. Model for the Nucleation and Diffusion-Controlled Growth of Binary Alloy Nuclei under Potentiostatic Conditions. Russ. Metall. (Metally) 2020, 2020, 914–917. [Google Scholar] [CrossRef]
- Löw, M.; Maroni, F.; Zaubitzer, S.; Dongmo, S.; Marinaro, M. Nucleation Mechanisms of Electrodeposited Magnesium on Metal Substrates. Batter. Supercaps 2024, 7, e202400250. [Google Scholar] [CrossRef]
- Scharifker, B.; Hills, G. Theoretical and experimental studies of multiple nucleation. Electrochim. Acta 1983, 28, 879–889. [Google Scholar] [CrossRef]
- Petit, C.; Solh, O.; Tsobmene, B.; Diliberto, S.; Rezai, C.; Thomas, M.; Boulanger, C. The effect of surface preparation on the nucleation of zinc-nickel alloy electroplated in acidic electrolyte on steel substrate. J. Appl. Electrochem. 2024, 54, 2389–2400. [Google Scholar] [CrossRef]



| Component | Concentration (g/L) | Function |
|---|---|---|
| Basic electroplating solution | ||
| Sn(CH3SO3)2 | 24.00 | Sn2+ source |
| Pb(CH3SO3)2 | 9.00 | Pb2+ source |
| CH3SO3H | 115.15 | Conductor |
| Additive system | ||
| Blank | —— | Blank control |
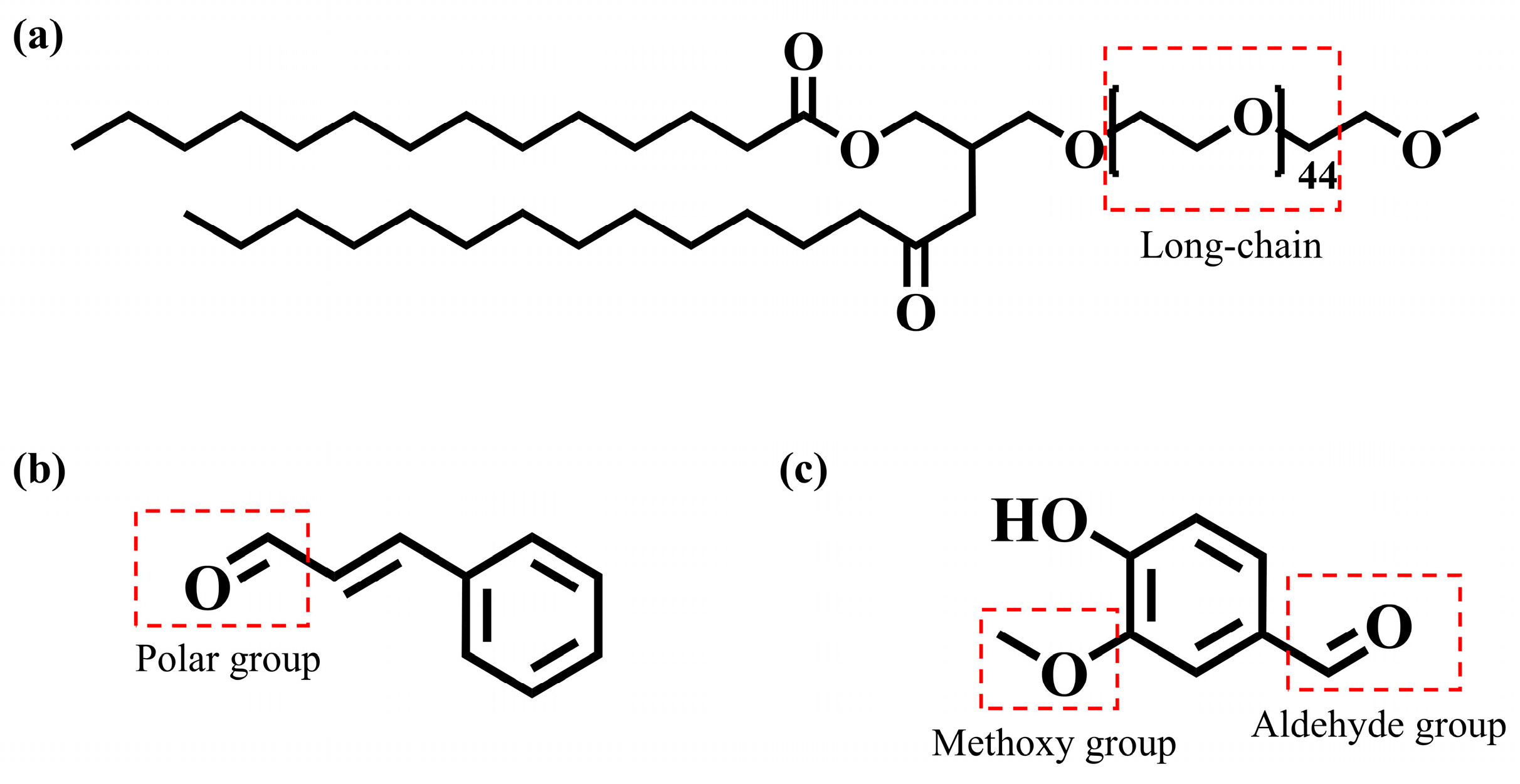
| Gelatin | 0.1 | Inhibitor |
| Vanillin | 0.1 | Leveler |
| Cinnamaldehyde | 0.1 | Brightener, grain refiner |
| PEG-2000 | 0.2 | Inhibitor, wetting agent |
| Cinnamaldehyde + PEG-2000 | 0.1 + 0.2 | Synergistic brightener |
| Samples | Sn (wt.%) | Pb (wt.%) | Total Quantity (wt.%) |
|---|---|---|---|
| Blank | 79.29 ± 0.36 | 20.71 ± 0.36 | 100 |
| Gelatin | 73.83 ± 0.23 | 26.17 ± 0.23 | 100 |
| Vanillin | 85.40 ± 0.30 | 14.60 ± 0.30 | 100 |
| Cinnamaldehyde | 72.00 ± 0.18 | 28.00 ± 0.18 | 100 |
| PEG-2000 | 77.58 ± 0.24 | 22.42 ± 0.24 | 100 |
| Cinnamaldehyde + PEG-2000 | 63.10 ± 0.11 | 36.90 ± 0.11 | 100 |
| Samples | Rs (Ω⋅cm2) | C1 (10−5 F/cm2) | n1 | Rf (Ω⋅cm2) | C2 (10−5 F/cm2) | n2 | Rct (Ω⋅cm2) | C3 (10−5 F/cm2) | n3 |
|---|---|---|---|---|---|---|---|---|---|
| Blank | 0.57 | 11.23 | 0.90 | 6.83 | 31.80 | 0.84 | 70.86 | 53.60 | 0.84 |
| Vanillin | 0.57 | 10.00 | 0.91 | 6.05 | 30.10 | 0.82 | 85.78 | 70.35 | 0.83 |
| Cinnamaldehyde | 0.52 | 6.93 | 0.95 | 3.94 | 35.82 | 0.81 | 113.67 | 84.50 | 0.83 |
| PEG-2000 | 0.55 | 7.15 | 0.96 | 4.08 | 27.95 | 0.80 | 121.80 | 92.76 | 0.82 |
| Gelatin | 0.65 | 12.26 | 0.89 | 7.48 | 38.36 | 0.80 | 141.30 | 108.33 | 0.84 |
| Cinnamaldehyde + PEG-2000 | 0.51 | 2.18 | 1.00 | 2.15 | 26.13 | 0.80 | 189.20 | 80.93 | 0.95 |
| Electrolyte | E (V) | jm (A/dm2) | tm (s) |
|---|---|---|---|
| Blank | −0.70 | −9.30 | 0.30 |
| −0.75 | −6.86 | 0.14 | |
| −0.80 | −4.67 | 0.14 | |
| PEG-2000 | −0.70 | −14.33 | 0.21 |
| −0.75 | −19.37 | 0.17 | |
| −0.80 | −26.84 | 0.14 | |
| Cinnamaldehyde | −0.70 | −2.59 | 0.59 |
| −0.75 | −4.90 | 0.38 | |
| −0.80 | −13.50 | 0.23 | |
| Cinnamaldehyde + PEG-2000 | −0.70 | −0.64 | 1.89 |
| −0.75 | −0.85 | 1.27 | |
| −0.80 | −1.35 | 1.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Li, C.; Yu, J.; Liu, R.; Shang, M.; Su, X.; Yao, J.; Ma, H. Study on the Optimization of the Morphology and Nucleation Mechanism of Electroplated Sn-Pb Coatings by the Synergistic Effect of Composite Additives. Metals 2025, 15, 936. https://doi.org/10.3390/met15090936
Liu X, Li C, Yu J, Liu R, Shang M, Su X, Yao J, Ma H. Study on the Optimization of the Morphology and Nucleation Mechanism of Electroplated Sn-Pb Coatings by the Synergistic Effect of Composite Additives. Metals. 2025; 15(9):936. https://doi.org/10.3390/met15090936
Chicago/Turabian StyleLiu, Xiangqing, Chenyu Li, Jie Yu, Ruiqi Liu, Min Shang, Xiaolin Su, Jinye Yao, and Haitao Ma. 2025. "Study on the Optimization of the Morphology and Nucleation Mechanism of Electroplated Sn-Pb Coatings by the Synergistic Effect of Composite Additives" Metals 15, no. 9: 936. https://doi.org/10.3390/met15090936
APA StyleLiu, X., Li, C., Yu, J., Liu, R., Shang, M., Su, X., Yao, J., & Ma, H. (2025). Study on the Optimization of the Morphology and Nucleation Mechanism of Electroplated Sn-Pb Coatings by the Synergistic Effect of Composite Additives. Metals, 15(9), 936. https://doi.org/10.3390/met15090936








