Abstract
This study investigates the synergistic effects of single- and binary-additive systems on the morphology and nucleation mechanism of Sn-Pb alloy electrodeposited coatings. Scanning electron microscopy (SEM), energy-dispersive spectroscopy (EDS), linear sweep voltammetry (LSV), electrochemical impedance spectroscopy (EIS), and chronoamperometry were applied in order to obtain more information on the action mechanisms of single-additive systems (cinnamaldehyde, PEG-2000, gelatin, vanillin) and binary ones (0.1 g/L cinnamaldehyde + 0.2 g/L PEG-2000) in Sn-Pb electroplating. Results showed that the use of binary-additive systems based on cinnamaldehyde and PEG-2000 significantly improved coating quality, leading to a smooth and uniform surface, dense grains, and a near-eutectic composition (Sn 63.10 wt.%, Pb 36.90 wt.%). This was because the composite additive, through synergistic effects, exhibited the highest cathodic polarization and the largest charge transfer resistance (189.20 Ω cm2), thus inhibiting the electrodeposition process of Sn2+ and Pb2+. Chronoamperometry revealed that, unlike single additives (PEG-2000 or cinnamaldehyde), the binary-additive system promoted a transition of nucleation mode to instantaneous nucleation, accompanied by a decrease in the peak current and an extension of the corresponding time. This study provides a theoretical basis and experimental support for understanding the nucleation mode of Sn-Pb electroplating, as well as optimizing the synergistic mechanism of additives.
1. Introduction
As the integrated circuit industry continues to develop, Moore’s Law faces challenges in the traditional semiconductor industry. To maintain the sustainable development of the semiconductor industry, the International Technology Roadmap for Semiconductors (ITRS) proposed the concept of “More-than-Moore” in its 2005 technology roadmap [1,2]. In the More-than-Moore era, advanced packaging technologies have emerged as a key research focus for enhancing chip performance [3]. While various advanced packaging forms further increase the packaging density and reduce the package volume, the diameter of bumps in packaging continues to decrease. Metal micro-bumps, which play key roles in electrical interconnection, heat transfer, and mechanical support, are critical for packaging. Common bump preparation methods include electroplating, solder jetting, screen printing, ball planting, the C4NP method, etc. [4,5,6]. Electroplating is particularly advantageous for preparing fine-pitch (<25 μm) and high-density (>104 bumps/cm2) packaging [7,8,9].
The solders for bump preparation by electroplating include binary or multicomponent alloys such as Sn-Pb, Sn-Zn, Sn-Bi, Sn-Ag, and Sn-Ag-Cu [10,11,12]. In Sn-Pb solders, the addition of Pb reduces the surface tension of pure tin, improves the wettability of the solder alloy, and inhibits the transformation of β-tin (white tin) to α-tin (gray tin), maintaining structural integrity. Due to its high electrical conductivity, low melting point, and excellent mechanical properties, Sn-Pb solder remains irreplaceable in specific fields [13,14]. For example, deep-space detectors experience extreme environments involving extremely low temperatures, high temperatures, and large temperature differences during their service life. The temperature on the lunar surface has been observed to reach up to 150 °C under solar irradiation and quickly drops to −180 °C at night. Moreover, with changes in the relative position and orientation to the sun, deep-space detectors are subjected to multiple alternating extreme temperature changes [15,16].
The deposition potentials of Sn and Pb are very close, with Sn2+ at −0.137 V and Pb2+ at −0.126 V, making them prone to codeposition [17]. However, in the absence of additives, uneven deposition and compositional segregation easily occur. This phenomenon can be attributed to the minimal overvoltage exhibited by Sn2+ and Pb2+. In the process of electroplating, the coating mainly deposits on dominant crystal planes with low overvoltage, making it difficult to transform to other crystal planes and prone to forming acicular or dendritic coatings with unidirectional growth. Therefore, to obtain a dense coating, it is necessary to increase the resistance of the electrode reduction reaction and enhance the cathodic polarization overpotential by adding additives to inhibit the reduction reaction rate of metal ions [18,19]. Kohl [20] added polymeric sulfide to Sn-Pb electroplating baths to achieve dendrite inhibition and obtained uniform current distribution by adding lactone. Fujimura et al. [21] added explosively synthesized nanodiamond to the electrolyte for Sn-Pb electrodeposition, achieving granular dense coatings with a 30% improvement in both microhardness and plasticity. Danilov et al. [22] developed a methanesulfonate-based electrolyte system. This system incorporates organic additives, which effectively suppress the crystallization delay effect of Sn2+ ions and reduce the discharge overpotential. This approach facilitates the fabrication of compositionally stable alloy coatings, even from solutions exhibiting low Sn2+ ion concentrations. Abdel-Aziz et al. [23] employed a methanesulfonic acid (MSA) system for electrodepositing Sn-Pb alloy coatings, utilizing EDTA as a complexing agent and gelatin as an additive, which improved the coating surface morphology and compactness. Electrochemical impedance spectroscopy (EIS) analysis demonstrated that the coating quality achieved from the MSA system was comparable to that obtained from fluoroborate baths.
Currently, there is limited literature on Sn-Pb micro-bump electroplating, and existing studies mainly focus on centimeter-scale substrates. The direct application of centimeter-scale electrodeposition processes to micro-bumps results in poor coating quality. The predominant challenge of Sn-Pb electroplating at the microscale is the “edge effect” of electric field lines, as shown in Figure 1. This refers to the phenomenon where the current density is higher in edge regions than in other areas, causing faster metal deposition and thicker coatings at the edges. Particularly under high current densities, this leads to local over-plating or under-plating. This, in turn, has the potential to have a detrimental effect on the conductivity and reliability of the bumps [24,25].
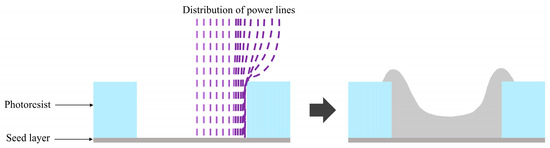
Figure 1.
Current density distribution and plating results within through-holes during additive-free electroplating.
Based on the existing electroplating process for Sn-Pb coatings on large-size substrates, this study conducted optimization experiments on a 500 μm diameter masked substrate. By screening different additives and combining polarization curves with EIS, the effects on coating morphology and performance were discussed. Additionally, the influence of single (cinnamaldehyde, PEG-2000) and binary (cinnamaldehyde + PEG-2000) additive systems on the Sn-Pb electrocrystallization process was examined by chronoamperometry (CA), with particular focus on nucleation mode transition (instantaneous/progressive) and kinetic parameters (peak current, time to reach the peak current).
2. Experimental
2.1. Electrodeposition of Coatings
The chemicals required for the experiments, including Sn(CH3SO3)2 (50 wt.% in H2O, S193794), Pb(CH3SO3)2 (50 wt.% in H2O, L305195), and CH3SO3H, (70 wt.% in H2O, M108502) were all provided by Shanghai Aladdin Biochemical Technology Co., Ltd. (Shanghai, China). The cathode material was a copper foil mask customized by Mengtian Electronics Co., Ltd. (Shenzhen, China). The copper foil was sealed with flexible polyimide, and the blind holes of the mask had a diameter of 500 μm, with the center distance twice the diameter. The anode material was a pure tin sheet (99.99 wt.%) cut into dimensions of 2 cm × 1 cm × 0.1 mm. Electroplating and electrochemical tests were completed by the CS350M electrochemical workstation of Wuhan CorrTest Instrument Co., Ltd. (Wuhan, China). All experiments were independently repeated at least three times (n = 3).
The plating bath was prepared as follows: first, certain amounts of Sn(CH3SO3)2 and Pb(CH3SO3)2 solutions were measured separately. Subsequently, CH3SO3H was weighed according to the respective molar ratios and added to the two salt solutions, followed by uniform stirring. Then, the mixtures were stirred under ultrasonic vibration to obtain a homogeneous and clear solution. Finally, additives (one or two of vanillin, gelatin, cinnamaldehyde, or PEG-2000) were added, which are common inhibitors and leveling agents [26,27,28]. The specific composition of the electroplating solution is shown in Table 1. The solution primarily consists of Sn(CH3SO3)2, Pb(CH3SO3)2, CH3SO3H and additives.

Table 1.
Composition of Sn-Pb electroplating solution.
Pre-treatment of the cathode for electroplating involved deionized water washing, pickling, pure water washing, and blow-drying for use. The pickling was performed with a 10 wt.% methanesulfonic acid solution for 20 s. The electroplating was carried out in a galvanostatic mode using an electrochemical workstation with an electroplating time of 30 min, an electroplating temperature of 25 °C, and a current density of 1 A/dm2. The configuration of the electroplating apparatus is depicted in Figure 2.

Figure 2.
Electroplating device.
2.2. Electrochemical Testing and Coating Characterization
All electrochemical tests were conducted using a CS350 electrochemical workstation in a three-electrode system. The temperature of the test environment was maintained at 25 °C. The working electrode was a glassy carbon electrode (the inner core is a circle with a diameter of 3 mm), the counter electrode was a platinum mesh, and the reference electrode was a saturated calomel electrode (SCE). Before each electrochemical measurement, the glassy carbon electrode and platinum mesh electrode were ultrasonically cleaned in ethanol and deionized water, alternating twice for 20 s each time. For linear sweep voltammetry (LSV), the scanning rate was 2 mV/s and the voltage range was −1.0 to 0 V. The EIS spectra were recorded at a potential of −0.45 V vs. SCE, with a 10 mV amplitude voltage signal and a frequency domain range of 106–0.01 Hz. The original data were subjected to equivalent circuit fitting using ZView2 software. During chronoamperometry (CA) investigations, the step potentials were set to −0.70 V, −0.75 V, and −0.80 V (vs. SCE), which were determined based on the deposition potential of the Sn-Pb alloy obtained from LSV. The step potentials that are more negative than the cathode potential can provide sufficient overpotential (η ≥ 200 mV) to ensure the complete reduction of Sn2+/Pb2+ ions, avoid the uncertainty in the mixed control region, and facilitate nuclear kinetics analysis [29,30].
The microstructure and chemical composition of the Sn-Pb coating were analyzed by field emission scanning electron microscopy (FEG-SEM, model SUPRA 55, Zeiss, Oberkochen, Germany) coupled with an X-ray energy-dispersive spectrometer (EDS) to screen out the best additive for optimizing coating quality.
3. Results and Discussion
3.1. Effects of Additives on Morphology and Composition of Sn-Pb Micro-Bumps
In the micro-bump electroplating process, additives play a crucial role in regulating the coating morphology. The morphology of Sn-Pb coatings is mainly evaluated using two aspects, namely flatness and particle uniformity. Flatness reflects the overall smoothness of the coating surface—an ideal coating should have low surface roughness without obvious undulations or depressions. The term “particle uniformity” is employed to denote the size distribution and agglomeration of metal particles within the coating. A uniform distribution of particles enhances the density and stability of the coating. Figure 3 illustrates a comparative analysis of the Sn-Pb alloy coating surface morphology in the absence and in the presence of the investigated additives by scanning electron microscopy. In terms of flatness and particle uniformity, the morphology from best to worst is as follows: cinnamaldehyde + PEG-2000 > gelatin > cinnamaldehyde ≈ PEG-2000 ≈ blank > vanillin. Results show that both the blank and single-additive systems hardly achieve complete flatness of the coating. Specifically, the edge effect is obvious in Figure 3a,c–e, where edge grains are abnormally coarse and more prominent than the central area, with uneven particle distribution, obvious size differences, and local agglomeration. The grains in Figure 3b are uniform in size, and the edge effect is weakened, but the edge still presents a protruding ring shape. This is because gelatin, as a high-molecular-weight polymer connected by peptide bonds of various amino acids, has the characteristics of high molecular weight and multi-functional groups. Its molecular chain is rich in hydrophilic groups (-COOH, -NH2, -OH), and the chain-like molecular structure can form a dense adsorption layer at the interface. This is effective in inhibiting the disordered deposition of metal ions and promoting uniform nucleation.


Figure 3.
SEM micrographs (secondary electron images) of Sn-Pb alloy coatings obtained in the absence (a) and in the presence of gelatin (b), vanillin (c), cinnamaldehyde (d), PEG-2000 (e), and cinnamaldehyde + PEG-2000 (f). Left column: (1) low-magnification view (100×); right column: (2) high-magnification view (500×) of selected areas from the corresponding left images.
When the additives are 0.1 g/L cinnamaldehyde and 0.2 g/L PEG-2000, as shown in Figure 3f, the coating surface is smooth, morphologically uniform, with dense grains and no edge effect. This is because the composite additives optimize the electric field distribution through double-layer reconstruction. Their structural formulas are shown in Figure 4: the long-chain adsorption of PEG-2000 can “level out” the potential difference on the electrode surface, while the polar groups of cinnamaldehyde regulate the interfacial charge density, weakening the high electric field intensity in the edge area to homogenize the current density distribution on the electrode surface and inhibit edge thickening defects caused by current concentration. The combination of steric hindrance from PEG-2000 and the conjugated adsorption of cinnamaldehyde reduces the nucleation overpotential and decreases the mass transfer rate. Under synergistic action, the nucleation rate exceeds the grain growth rate, forming a morphology with multiple nuclei and fine grains.

Figure 4.
Structural formulas of additives: (a) PEG-2000; (b) cinnamaldehyde; (c) vanillin.
Morphological analysis indicates that the types and combination modes of additives have an impact on the flatness, grain uniformity, and edge effect of Sn-Pb coatings. Among them, the composite system of cinnamaldehyde and PEG-2000 exhibits the optimal synergistic effect. In fact, the differences in coating morphology essentially stem from the selective regulation of the reduction kinetics of Sn2+ and Pb2+ by additives. As shown in Table 2, the Pb content in the Sn-Pb coating without additives is 20.71 wt.%, far lower than the requirement of the eutectic composition. Following the addition of additives, with the exception of vanillin (structural formula shown in Figure 4c), there was a concomitant increase in the Pb content. This is because the methoxy group (-OMe) and aldehyde group (-CHO) of vanillin have a stronger complexing ability with Pb2+ than with Sn2+, reducing the effective concentration of Pb2+ and thereby inhibiting its deposition [31,32,33]. When 0.1 g/L cinnamaldehyde and 0.2 g/L PEG-2000 were added, the aldehyde group of cinnamaldehyde formed a weak coordination bond with Sn2+, modifying the Sn/Pb co-deposition ratio to approach eutectic composition [34,35].

Table 2.
The composition of Sn-Pb coating obtained by an energy-dispersive spectrometer (mean ± SD, n = 5).
3.2. Effects of Additives on Electrochemical Behavior
3.2.1. Linear Sweep Voltammetry (LSV) Analysis
Based on the above characterization results of coating morphology and elemental composition, to further explore the influence mechanism of different additives on the electrochemical behavior of Sn-Pb codeposition, LSV was used to systematically study the cathodic polarization characteristics. Figure 5 shows the cathodic polarization curves of plating baths with different additives. It can be seen from the figure that without additives, the deposition potential of reactants is −0.47 V, and the limiting current density is 1.05 A/dm2. After adding different types of additives, the deposition potential shifts negatively, with gelatin exhibiting the most pronounced effect; the cathodic polarization increases, with cinnamaldehyde + PEG-2000 showing the most significant effect; the limiting current density decreases, indicating that additives hinder the Sn-Pb electroplating process, reduce the rate of electrode reaction, and lead to a decrease in reduction current. When different additives are used, the order of cathodic polarization intensity is as follows: cinnamaldehyde + PEG-2000 > cinnamaldehyde > PEG-2000 > gelatin > vanillin ≈ additive-free, indicating that adding 0.1 g/L cinnamaldehyde and 0.2 g/L PEG-2000 has the best leveling effect, which is consistent with the scanning results. The reason is that cinnamaldehyde, as an aromatic aldehyde, forms a strong adsorption layer on the electrode surface through its benzene ring and aldehyde group, while PEG-2000 is a long-chain polymer that can enhance the compactness of the adsorption layer through entanglement. The synergistic effect of these elements results in a dense composite adsorption layer that complicates the ion diffusion path, reduces the limiting current density to a minimum and maximizes the cathodic polarization.
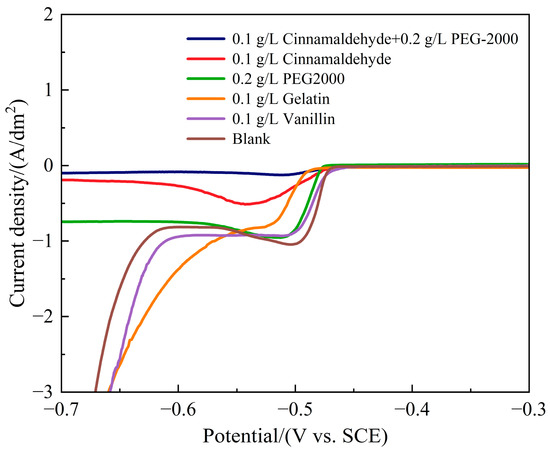
Figure 5.
Cathodic polarization curves with different additives.
3.2.2. Electrochemical Impedance Spectroscopy (EIS) Analysis
The EIS spectra in Figure 6 exhibit three characteristic regions: A semicircle in the high-frequency region (>103 Hz) corresponds to the rapid adsorption/desorption process of additive molecules on the electrode surface. Its diameter varies with additive concentration, consistent with the Langmuir adsorption model; a smaller Rf value indicates weaker hindrance of the adsorption layer to ion transport (Rf//CPE1). A flattened semicircle in the intermediate-frequency region (100–103 Hz) reflects the charge transfer process of Sn2+/Pb2+ (Rct//CPE2). A diffusion tail with a slope <45° in the low-frequency region (<100 Hz) indicates that mass transfer is hindered by the adsorption layer (Ws//CPE3). Based on the above analysis, the equivalent circuit shown in Figure 6b was established, where Rs represents the solution resistance between the working electrode and counter electrode, CPE1 denotes the adsorption layer capacitance, Rf is the resistance of the adsorption layer formed on the cathode surface, CPE2 represents the double-layer capacitance between the working electrode and solution, Rct is the charge transfer resistance of Sn2+ and Pb2+, CPE3 is the non-ideal capacitance related to diffusion, and Ws represents non-ideal Warburg impedance. The Nyquist diagram in the low-frequency region (k < 45°) and the phase angle (30°~45°) both indicate that the diffusion process is hindered. This is different from the traditional Warburg impedance because the adsorption layer hinders the diffusion. Ws (generalized finite-length Warburg impedance) combined with CPE3 can better fit the non-ideal diffusion behavior [36,37,38,39]. As shown in Figure 6a, the Nyquist plot exhibits excellent agreement between the experimental data points (hollow symbols) and fitting curves (black solid lines), verifying the applicability of the equivalent circuit model. The relative errors of all fitting parameters are <15%, among which the relative errors of key parameters such as charge transfer resistance are <5%. The consistency between experimental data and the theoretical model is further confirmed by the chi-squared value (χ2 < 3.2 × 10−3). Detailed fitting parameters are listed in Table 3.

Figure 6.
The EIS spectra recorded in the absence and presence of organic additives are presented as Nyquist diagrams: (a) Nyquist plots; (b) equivalent circuit models.

Table 3.
Values of equivalent circuit parameters by fitting impedance results.
As shown in Figure 6, the high-frequency capacitive loop is typically associated with the charge transfer resistance (Rct), which represents the reduction in ions to be deposited and acts as the rate-determining step in electroplating. The radius of the capacitive arc varies with different additives—the larger the radius, the stronger the inhibitory effect of the corresponding additive on Sn-Pb electrodeposition and the better the leveling effect. Notably, when 0.1 g/L cinnamaldehyde and 0.2 g/L PEG-2000 are used as additives, the capacitive arc radius is the largest, with a charge transfer resistance of 189.20 Ω⋅cm2, indicating the strongest deposition inhibition and optimal leveling effect. This result is consistent with the findings from SEM images and cathodic polarization curves.
3.3. Effects of Additives on Electrocrystallization Nucleation Mechanism
3.3.1. Chronoamperometry Transient Behavior Analysis
To investigate the effects of different additives on the Sn-Pb electrodeposition process, chronoamperometric experiments under different stepped potentials are conducted, as shown in Figure 7. Figure 7 presents the chronoamperometric curves without additives, with PEG-2000, cinnamaldehyde, and PEG-2000 + cinnamaldehyde, respectively. When the stepped potential is applied, the current exhibits an instantaneous peak within an extremely short time, followed by a rapid decay. As time progresses, all curves show typical nucleation characteristics. The current density first increases to a peak value (jmax, denoted as jm, and the time when jm appears is denoted as tm), then gradually decreases until it stabilizes. This is because during nucleation and crystal growth, the current density gradually increases with the continuous increase in the number of nuclei. After a period of time tm, the diffusion regions of nuclei start to overlap, causing the current density to reach the peak. When the diffusion regions completely overlap, no new nucleation sites exist, and only crystal growth occurs, leading to a gradual decrease in current density until stabilization.

Figure 7.
Chronoamperometric curves at different voltages: (a) blank; (b) PEG-2000; (c) cinnamaldehyde; (d) cinnamaldehyde + PEG-2000.
Table 4 shows the peak current (jm) and corresponding time (tm) at different stepped potentials. As shown in Table 4, in contrast to plating baths that contain additives, the absence of additives (Figure 7a) leads to a decline in peak current with increasing voltage. This is because the concentration polarization on the electrode surface intensifies. Following the application of voltage, Sn2+ and Pb2+ undergo rapid reduction at the electrode surface, resulting in a significant decrease in reactant concentration near the electrode. As the diffusion mass transfer rate fails to keep up, ion mass transfer is hindered, leading to concentration polarization and a decrease in current with increasing voltage.

Table 4.
Peak current (jm) and corresponding time (tm) under different stepped potentials.
In the plating bath with both PEG-2000 and cinnamaldehyde (Figure 7d), jm is the smallest and tm is the longest (at an applied voltage of −0.70 V, jm = −0.64 A/dm2, tm = 1.89 s). At this point, the mixed additives form a composite adsorption layer on the electrode surface. The long-chain molecules of PEG-2000 slow down the diffusion rate of Sn2+ and Pb2+ through steric hindrance, while the conjugated groups of cinnamaldehyde form an ordered adsorption film at active sites via π-π stacking interactions. The synergistic effect of both increases the path resistance for ion migration, thereby reducing the instantaneous current density and prolonging the time to reach the peak current, ultimately exhibiting the dual effects of peak current decay and time delay.
From a process requirement perspective, a smaller peak current indicates that the electrodeposition process occurs under low overpotential conditions. This has the effect of effectively suppressing hydrogen evolution reactions and dendritic growth. A longer peak time suggests a slow nucleation process in the initial deposition stage, which is conducive to the formation of a dense and uniform grain structure. The synergistic effect of PEG-2000 and cinnamaldehyde achieves smoothing of the deposition current and controllability of the nucleation process, facilitating the formation of a uniform, flat, and fine-grained Sn-Pb coating.
3.3.2. Electrodeposition Nucleation Mode
To further clarify the nucleation mode of Sn2+ and Pb2+ in this system, the Scharifker–Hills (S-H) mathematical model was employed to analyze the current transient curves [40,41,42,43]. Nucleation mechanisms generally conform to two classic models, which are progressive nucleation and instantaneous nucleation [44,45], with their theoretical equations as follows:
Progressive nucleation:
Instantaneous nucleation:
In the equations, j represents the current density and t denotes the corresponding time.
All current transient curves were subjected to dimensionless processing using the (j/jm)2 − (t/tm) method and compared with the standard curves derived from the aforementioned equations, allowing for the nucleation mechanisms under different conditions to be determined: when a curve is consistent with the standard curve of the continuous nucleation model (Equation (1)), it indicates that the system follows the continuous nucleation mechanism; when it matches the standard curve of the instantaneous nucleation model (Equation (2)), the dominant mechanism is judged to be the instantaneous nucleation. The specific analysis is as follows: in the absence of additives (Figure 8a), the nucleation mode approaches progressive nucleation at −0.7 V and transitions to instantaneous nucleation with increasing voltage. When PEG-2000 is added alone (Figure 8b), progressive nucleation is observed consistently, as the adsorption of PEG-2000 on the electrode surface continuously occupies new active sites during the reaction, and the long-chain molecules inhibit burst nucleation via steric hindrance. For a single addition of cinnamaldehyde (Figure 8c), progressive nucleation is observed at −0.7 V; with increasing voltage, the charge transfer rate increases, and the weak adsorption of conjugated groups has a limited effect on overpotential, leading to transitional nucleation characteristics between progressive and instantaneous nucleation. When both PEG-2000 and cinnamaldehyde are added (Figure 8d), instantaneous nucleation occurs within the voltage range of −0.70 to −0.80 V, thereby effecting a complete change in the nucleation mode of Sn2+ and Pb2+. This is because the long-chain structure of PEG-2000 provides sites for the conjugated molecules of cinnamaldehyde, forming a dense composite adsorption layer that rapidly desorbs and adsorbs on the electrode surface. When the adsorption layer disintegrates instantaneously, all blocked sites are released explosively, enabling Sn2+ and Pb2+ to complete critical nucleus formation within an extremely short time.

Figure 8.
Fitting results of (j2/jm2) − (t/tm) dimensionless nucleation curve with Scharifker–Hills nucleation model: (a) blank; (b) PEG-2000; (c) cinnamaldehyde; (d) cinnamaldehyde + PEG-2000.
In summary, as the applied voltage increases, the nucleation mode exhibits a trend of transitioning from progressive nucleation to instantaneous nucleation. Furthermore, the individual addition of PEG-2000 or cinnamaldehyde does not alter the nucleation mode of Sn-Pb. In contrast, the simultaneous addition of PEG-2000 and cinnamaldehyde not only changes the nucleation mode but also affects the peak current of nucleation and the time to reach the peak.
4. Conclusions
This study demonstrates that the composite additives (0.1 g/L cinnamaldehyde + 0.2 g/L PEG-2000) enhance the electroplating performance of Sn-Pb via synergistic effects. The system exhibits the highest cathodic polarization and maximum charge transfer resistance (189.20 Ω·cm2), thereby effectively regulating the co-deposition process of Sn2+ and Pb2+ to obtain high-quality coatings with a smooth surface, dense grains, and a composition close to the eutectic ratio (Sn 63.10 wt.%, Pb 36.90 wt.%). The synergistic regulation mechanism of composite additives is interpreted from the perspectives of electrochemical behavior and the crystalline nucleation mechanism. This carries important guiding significance for the optimization of coating processes in precision electronic packaging and the design of new additives.
- Various additives influence both the morphology and composition of Sn-Pb coatings. When using 0.1 g/L cinnamaldehyde and 0.2 g/L PEG-2000, the composite additives optimize the electric field distribution through double-layer reconstruction. This facilitates the uniform distribution of current density on the electrode surface, thereby suppressing edge effects. The combination of steric hindrance from PEG-2000 and the conjugated adsorption of cinnamaldehyde ensures that the nucleation rate exceeds the crystal growth rate. The resulting coating features a smooth surface, dense grains, no edge effect, and a composition matching the eutectic ratio.
- LSV and EIS results show that 0.1 g/L cinnamaldehyde and 0.2 g/L PEG-2000 increase cathodic polarization and charge transfer resistance (Rct = 189.20 Ω·cm2), requiring higher overpotential for the reduction of Sn2+ and Pb2+. This suppresses dendritic growth and increases the resistance for charge transfer across the electrode/solution interface, delaying the electrochemical reaction rate. This process ultimately results in a Sn-Pb coating characterized by fine grains and a smooth surface.
- The composite additives (0.1 g/L cinnamaldehyde + 0.2 g/L PEG-2000) transform the nucleation mechanism of Sn-Pb deposition into instantaneous nucleation. Chronoamperometry results demonstrate a decreased peak current and prolonged time to reach the peak (at −0.70 V, jm = −0.64 A/dm2, tm = 1.89 s). This is because the long chains of PEG-2000 and conjugated molecules of cinnamaldehyde form a dynamic adsorption layer. Its instantaneous desorption promotes the simultaneous formation of numerous critical nuclei by Sn2+ and Pb2+, delaying nucleus growth and refining grains, thus providing kinetic guarantees for obtaining dense and smooth coatings.
Author Contributions
Conceptualization, X.L. and H.M.; methodology, X.L. and C.L.; software, J.Y. (Jie Yu) and J.Y. (Jinye Yao); validation, R.L., M.S., and X.S.; formal analysis, X.L., M.S., and C.L.; investigation, J.Y. (Jie Yu) and R.L.; resources, X.L. and X.S.; data curation, J.Y. (Jinye Yao); writing—original draft preparation, X.L.; writing—review and editing, all authors; visualization, C.L.; supervision, H.M.; project administration, H.M.; funding acquisition, H.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to privacy.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Meindl, J.D. Beyond Moore’s Law: The interconnect era. Comput. Sci. Eng. 2003, 5, 20–24. [Google Scholar] [CrossRef]
- Schaller, R.R. Technological innovation in the semiconductor industry: A case study of the International Technology Roadmap for Semiconductors (ITRS). In Proceedings of the Portland International Conference on Management of Engineering and Technology (PICMET), Portland, OR, USA, 29 July–2 August 2001; p. 195. [Google Scholar]
- Lau, J.H. Recent Advances and Trends in Advanced Packaging. IEEE Trans. Compon. Packag. Manuf. Technol. 2022, 12, 228–252. [Google Scholar] [CrossRef]
- Goh, Y.; Haseeb, A.S.M.A.; Faizul Mohd Sabri, M. Electrodeposition of lead-free solder alloys. Solder. Surf. Mt. Technol. 2013, 25, 76–90. [Google Scholar] [CrossRef]
- Thanedburapasup, S.; Wetchirarat, N.; Muengjai, A.; Tengprasert, W.; Wiman, P.; Thublaor, T.; Chandra-ambhorn, S. Fabrication of Mn–Co Alloys Electrodeposited on AISI 430 Ferritic Stainless Steel for SOFC Interconnect Applications. Metals 2023, 13, 612. [Google Scholar] [CrossRef]
- Nagy, P.; Péter, L.; Kolonits, T.; Nagy, A.; Gubicza, J. Combinatorial Design of an Electroplated Multi-Principal Element Alloy: A Case Study in the Co-Fe-Ni-Zn Alloy System. Metals 2024, 14, 700. [Google Scholar] [CrossRef]
- Yang, H.; Wang, Y.; Chen, M.; Liu, J.; Liu, Y.; Chen, T.; Li, J. Research on Electroplating Bonding of Flip-Chip Under the Action of Additives. IEEE Trans. Compon. Packag. Manuf. Technol. 2013, 13, 1324–1331. [Google Scholar] [CrossRef]
- Wu, X.; Zhu, X.; Wang, S.; Tang, X.; Lang, T.; Belyaev, V.; Abduev, A.; Kazak, A.; Lin, C.; Yan, Q.; et al. Bump-Fabrication Technologies for Micro-LED Display: A Review. Materials 2025, 18, 1783. [Google Scholar] [CrossRef]
- Volpert, M.; Henry, D.; Taneja, D.; Chaira, T.; Gueugnot, A.; Hodaj, F. Cu pillar as interconnect for 10 μm pitch and below: Fabrication issues and assembly results. In Proceedings of the 2018 IEEE 7th Electronic System-Integration Technology Conference (ESTC), Dresden, Germany, 18–21 September 2018; pp. 1–7. [Google Scholar]
- Jo, Y.; Kim, S.M.; Jeong, E.S.; Lee, K.T.; Jin, S.; Lee, W.Y.; Lee, M.H. On the role of complexing agents in co-electrodeposition of SnAg alloy with uniform composition. Mater. Sci. Semicond. Process. 2023, 166, 107751. [Google Scholar] [CrossRef]
- Shang, M.; Yao, J.; Zhang, D.; Su, X.; Ma, H.; Wang, Y.; Ma, H. Preparation and soldering performance of SAC305@Sn-bi Core-shell solder balls based on eutectic co-deposition. Mater. Charact. 2024, 211, 113815. [Google Scholar] [CrossRef]
- Hwang, Y.; Lee, J.; Kim, M.J. Pulse electrodeposition of Sn-Bi low-temperature solder with phloroglucinol. Electrochim. Acta 2025, 535, 146661. [Google Scholar] [CrossRef]
- Tu, K.N.; Zeng, K. Tin–lead (SnPb) solder reaction in flip chip technology. Mater. Sci. Eng. R Rep. 2001, 34, 1–58. [Google Scholar] [CrossRef]
- Siviour, C.R.; Walley, S.M.; Proud, W.G.; Field, J.E. Mechanical properties of SnPb and lead-free solders at high rates of strain. J. Phys. D Appl. Phys. 2005, 38, 4131. [Google Scholar] [CrossRef]
- Balint, T.S.; Kolawa, E.A.; Cutts, J.A.; Peterson, C.E. Extreme environment technologies for NASA’s robotic planetary exploration. Acta Astronaut. 2008, 63, 285–298. [Google Scholar] [CrossRef]
- Balint, T.S.; Cutts, J.A.; Kolawa, E.A.; Peterson, C.E. Extreme environment technologies for space and terrestrial applications. In Space Exploration Technologies; SPIE: Orlando, FL, USA, 2008; pp. 36–47. [Google Scholar]
- Petersson, I.; Ahlberg, E. Kinetics of the electrodeposition of Pb-Sn alloys: Part I. At glassy carbon electrodes. J. Electroanal. Chem. 2000, 485, 166–177. [Google Scholar] [CrossRef]
- Arafat, Y.; Sultana, S.T.; Dutta, I.; Panat, R. Effect of Additives on the Microstructure of Electroplated Tin Films. J. Electrochem. Soc. 2018, 165, D816–D824. [Google Scholar] [CrossRef]
- Bučko, M.; Stupar, S.; Bajat, J.B. The Influence of Sm Content on the Surface Morphology and Corrosion Behavior of Zn-Co-Sm Composite Coatings. Metals 2023, 13, 481. [Google Scholar] [CrossRef]
- Kohl, P.A. The High Speed Electrodeposition of Sn/Pb Alloys. J. Electrochem. Soc. 1982, 129, 1196. [Google Scholar] [CrossRef]
- Fujimura, T.; Dolmatov, V.Y.; Burkat, G.K.; Orlova, E.A.; Veretennikova, M.V. Electrochemical codeposition of Sn–Pb–metal alloy along with detonation synthesis nanodiamonds. Diam. Relat. Mater. 2004, 13, 2226–2229. [Google Scholar] [CrossRef]
- Danilov, F.I.; Vasil’eva, E.A.; Butyrina, T.E.; Protsenko, V.S. Electrodeposition of lead–tin alloy from methanesulphonate bath containing organic surfactants. Prot. Met. Phys. Chem. Surf. 2010, 46, 697–703. [Google Scholar] [CrossRef]
- Abdel-Aziz, A.B.; El-Zomrawy, A.A.; El-Sabbah, M.M.B.; Ghayad, I.M. Electrodeposition of lead and lead-tin alloy on copper using an eco-friendly methanesulfonate plating bath. J. Mater. Res. Technol. 2022, 18, 2166–2174. [Google Scholar] [CrossRef]
- Luo, X.; Zhang, S.; Liu, Z. Investigating the edge effects of Cu electroplating on the SAMs-coated Si substrate. J. Mater. Sci. Mater. Electron. 2023, 34, 1047. [Google Scholar] [CrossRef]
- Oh, Y.J.; Chung, S.H.; Lee, M.S. Optimization of Thickness Uniformity in Electrodeposition onto a Patterned Substrate. Mater. Trans. 2004, 45, 3005–3010. [Google Scholar] [CrossRef]
- Chen, H.Y.; Chen, C.; Wu, P.W.; Shieh, J.M.; Cheng, S.S.; Hensen, K. Effect of Polyethylene Glycol Additives on Pulse Electroplating of SnAg Solder. J. Electron. Mater. 2008, 37, 224–230. [Google Scholar] [CrossRef]
- Barry, F.J.; Cunnane, V.J. Synergistic effects of organic additives on the discharge, nucleation and growth mechanisms of tin at polycrystalline copper electrodes. J. Electroanal. Chem. 2002, 537, 151–163. [Google Scholar] [CrossRef]
- Kim, S.J.; Kim, H.T.; Park, S.M. Effects of o-Vanillin as a Brightener on Zinc Electrodeposition at Iron Electrodes. J. Electrochem. Soc. 2004, 151, C850. [Google Scholar] [CrossRef]
- Esmailzadeh, S.; Shahrabi, T.; Yaghoubinezhad, Y.; Darband, G.B. An analytical study on nucleation and growth mechanism of nanostructured Ni-Se coating by the chronoamperometry and pulse potential techniques. J. Electrochem. Chem. 2021, 881, 114949. [Google Scholar] [CrossRef]
- Tan, W.; He, H.; Gao, Y.; Peng, Y.; Dai, X. Nucleation and growth mechanisms of an electrodeposited Ni–Se–Cu coating on nickel foam. J. Colloid Interface Sci. 2021, 600, 492–502. [Google Scholar] [CrossRef]
- Huang, Y.; Yang, C.; Tan, X.; Zhang, Z.; Wang, S.; Hu, J.; Chen, Y. Benzaldehyde derivatives on tin electroplating as corrosion resistance for fabricating copper circuit. Nanotechnol. Rev. 2022, 11, 3125–3137. [Google Scholar] [CrossRef]
- Zhou, X.; Li, W.; Hu, J.; Zuo, Z. Electrochemical-voltammetry behavior of several aromatic aldehydes in acid solution. Wuhan Univ. J. Nat. Sci. 2000, 5, 485–490. [Google Scholar] [CrossRef]
- Culita, D.C.; Simonescu, C.M.; Patescu, R.E.; Dragne, M.; Stanica, N.; Oprea, O. o-Vanillin functionalized mesoporous silica–coated magnetite nanoparticles for efficient removal of Pb (II) from water. J. Solid State Chem. 2016, 238, 311–320. [Google Scholar] [CrossRef]
- Meng, Z.; Liu, Z.Q. Preparation of Sn nanosheets by electrodeposition in polycarbonate template. In Proceedings of the 2019 20th International Conference on Electronic Packaging Technology (ICEPT), Hong Kong, China, 12–15 August 2019; pp. 1–4. [Google Scholar]
- Kudryavtsev, V.N.; Tyutina, K.M.; Popov, A.N.; Maksimenko, S.; Zonin, V.A. Electrodeposition of tin-based alloys as functional coatings. Plat. Surf. Finish. 1992, 79, 57–61. [Google Scholar]
- Zhao, Z.; Zou, Y.; Liu, P.; Lai, Z.; Wen, L.; Jin, Y. EIS equivalent circuit model prediction using interpretable machine learning and parameter identification using global optimization algorithms. Electrochim. Acta 2022, 418, 140350. [Google Scholar] [CrossRef]
- Lukács, Z.; Kristóf, T. A generalized model of the equivalent circuits in the electrochemical impedance spectroscopy. Electrochim. Acta 2020, 363, 137199. [Google Scholar] [CrossRef]
- Gao, Y.; Patel, R.L.; Shen, K.Y.; Wang, X.; Axelbaum, R.L.; Liang, X. Boosting the electrochemical performance of Li1.2Mn0.54Ni0.13Co0.13O2 by atomic layer-deposited CeO2 coating. ACS Omega 2018, 3, 906–916. [Google Scholar] [CrossRef]
- Song, C.; Wang, W.; Peng, H.; Wang, Y.; Zhao, C.; Zhang, H.; Dou, Y. Improving the electrochemical performance of LiNi0.80Co0.15Al0.05O2 in lithium ion batteries by LiAlO2 surface modification. Appl. Sci. 2018, 8, 378. [Google Scholar] [CrossRef]
- Sun, Y.; Zangari, G. Commentary and Notes on the Original Derivations of the Scharifker-Hills Model. J. Electrochem. Soc. 2023, 170, 032503. [Google Scholar] [CrossRef]
- Khelladi, M.R.; Mentar, L.; Azizi, A.; Sahari, A.; Kahoul, A. Electrochemical nucleation and growth of copper deposition onto FTO and n-Si (1 0 0) electrodes. Mater. Chem. Phys. 2009, 115, 385–390. [Google Scholar] [CrossRef]
- Grishenkova, O.V.; Kosov, A.V.; Zaikov, Y.P.; Isaev, V.A. Model for the Nucleation and Diffusion-Controlled Growth of Binary Alloy Nuclei under Potentiostatic Conditions. Russ. Metall. (Metally) 2020, 2020, 914–917. [Google Scholar] [CrossRef]
- Löw, M.; Maroni, F.; Zaubitzer, S.; Dongmo, S.; Marinaro, M. Nucleation Mechanisms of Electrodeposited Magnesium on Metal Substrates. Batter. Supercaps 2024, 7, e202400250. [Google Scholar] [CrossRef]
- Scharifker, B.; Hills, G. Theoretical and experimental studies of multiple nucleation. Electrochim. Acta 1983, 28, 879–889. [Google Scholar] [CrossRef]
- Petit, C.; Solh, O.; Tsobmene, B.; Diliberto, S.; Rezai, C.; Thomas, M.; Boulanger, C. The effect of surface preparation on the nucleation of zinc-nickel alloy electroplated in acidic electrolyte on steel substrate. J. Appl. Electrochem. 2024, 54, 2389–2400. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).