Effect of Electrochemical Hydrogen Charging on the Notch Tensile Properties of Natural Gas Transportation Pipeline Steel with Electroless-Plated Coatings and Their Adhesiveness Characterization
Abstract
1. Introduction
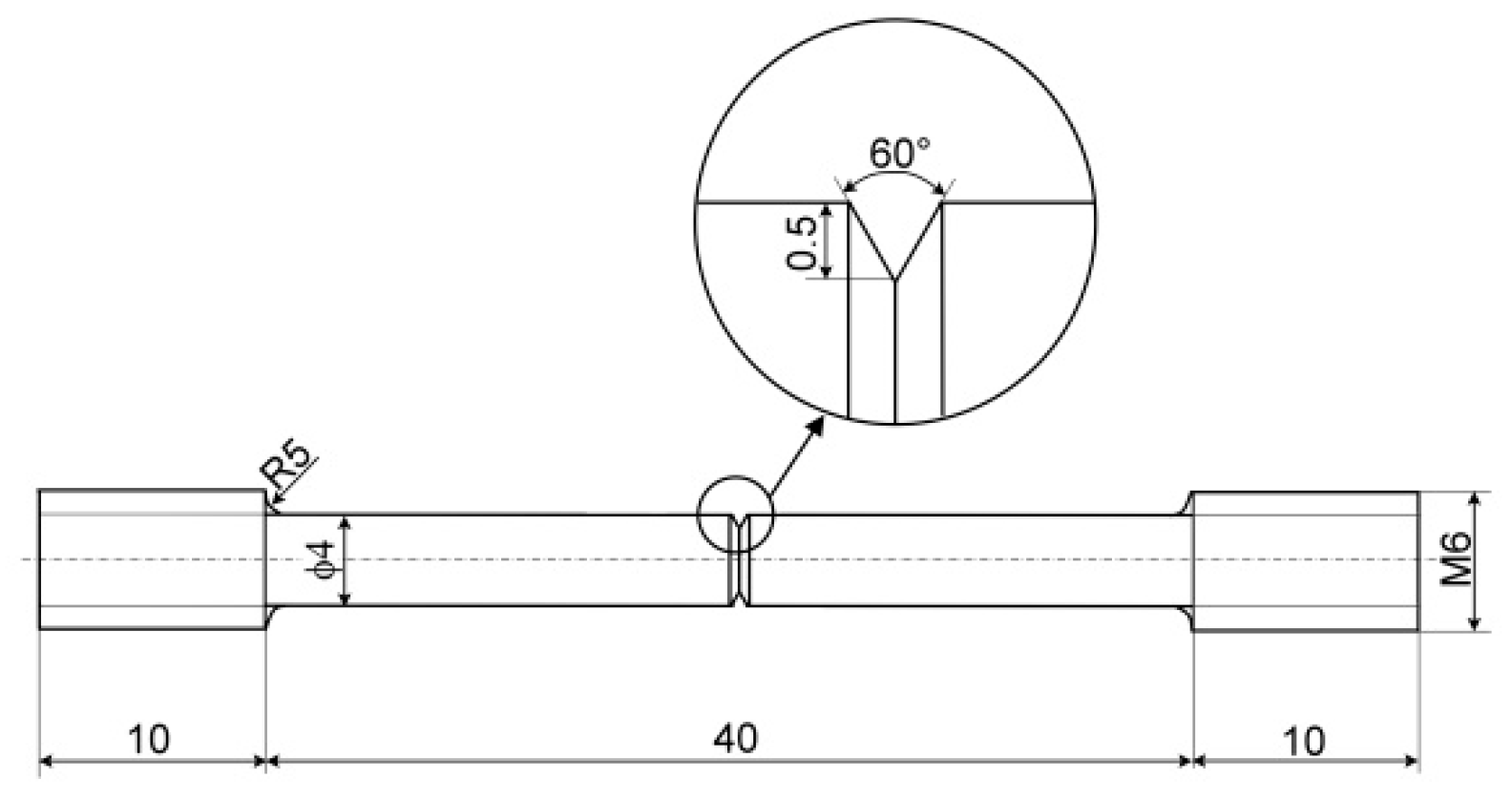
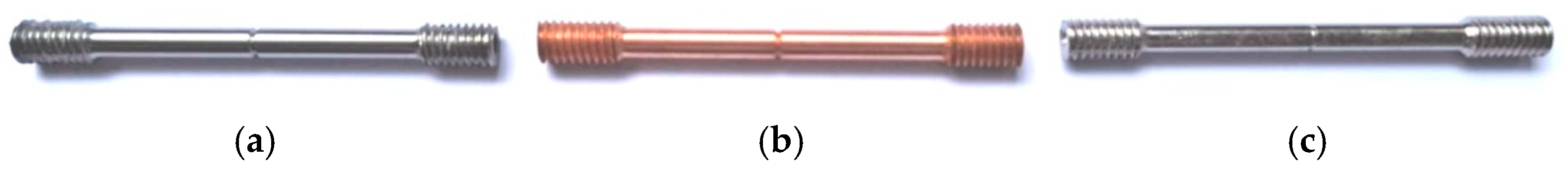
2. Experimental Material and Methods
3. Results and Discussion
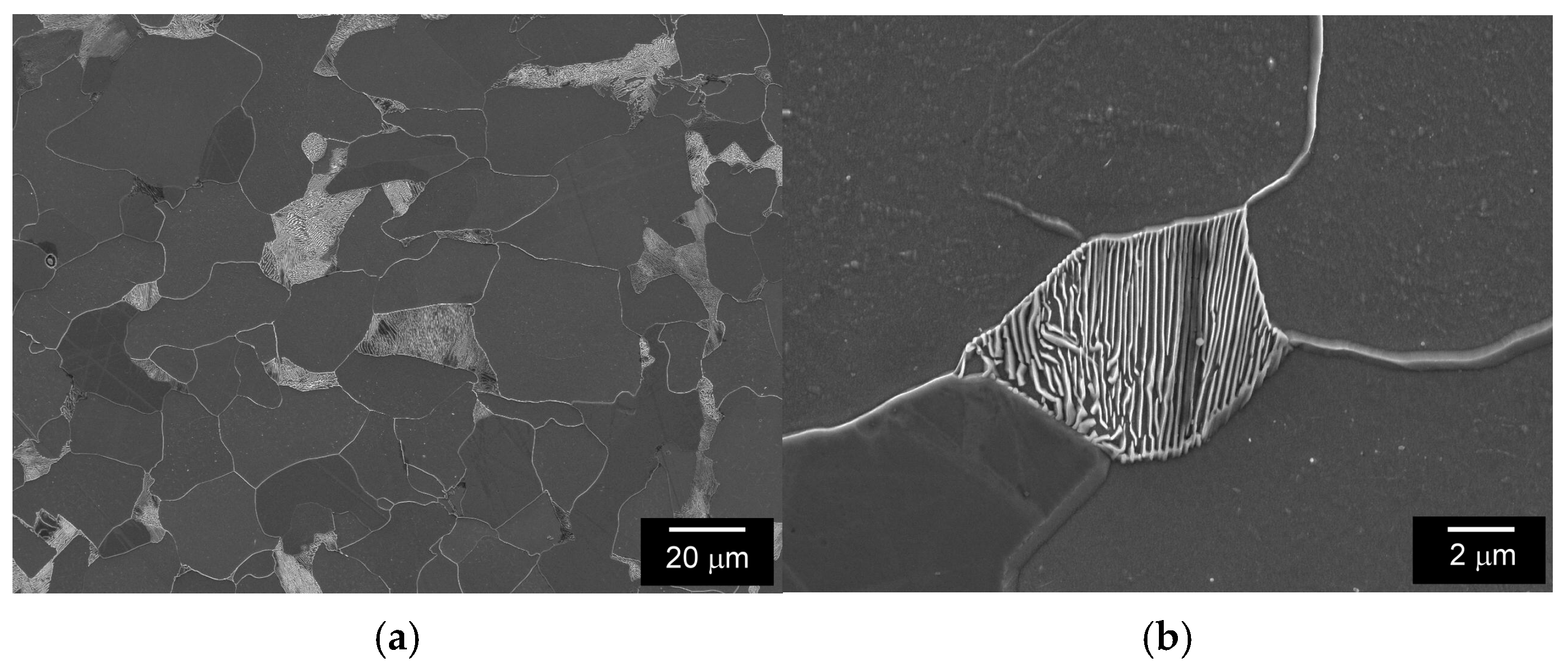
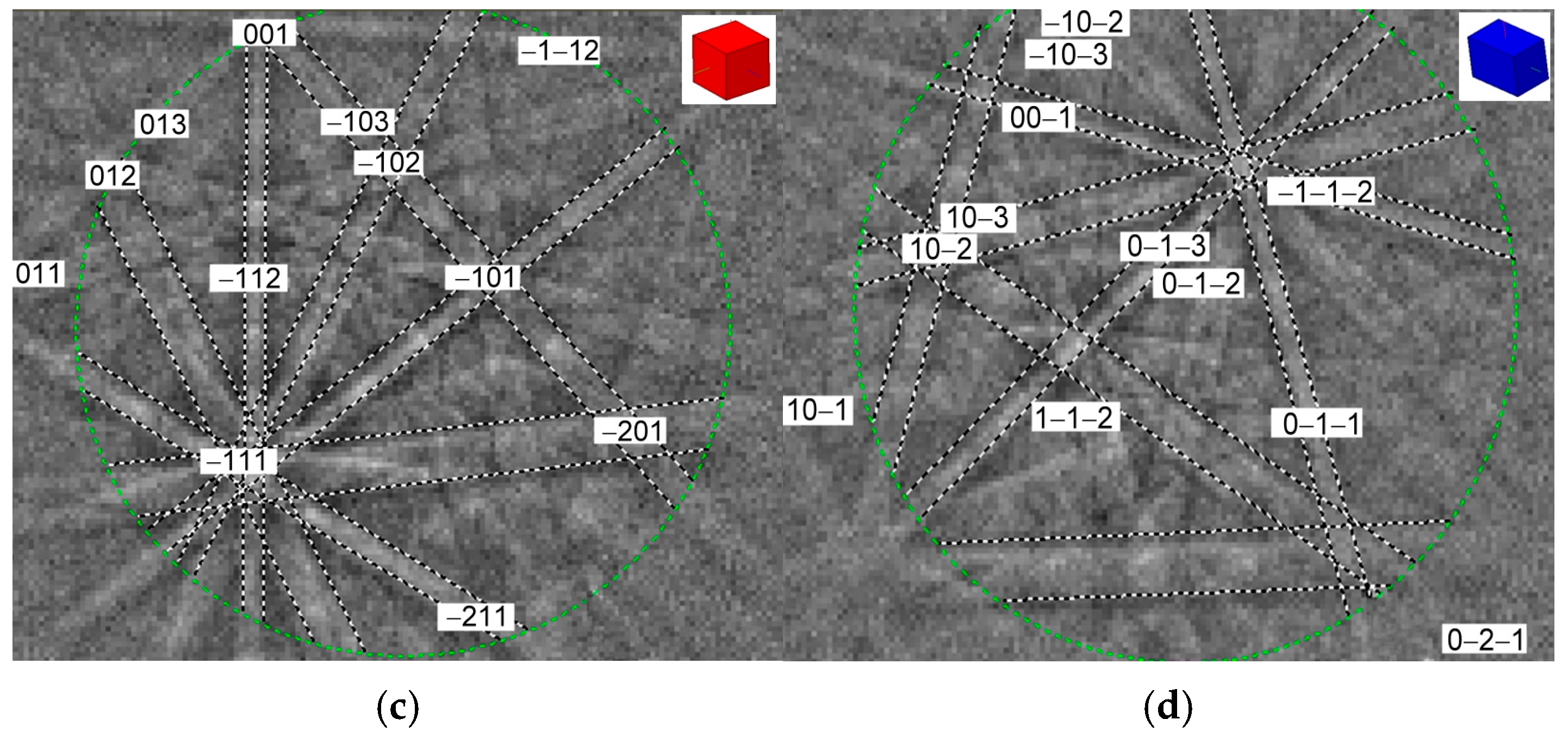
3.1. Microstructural and Phase Characterization
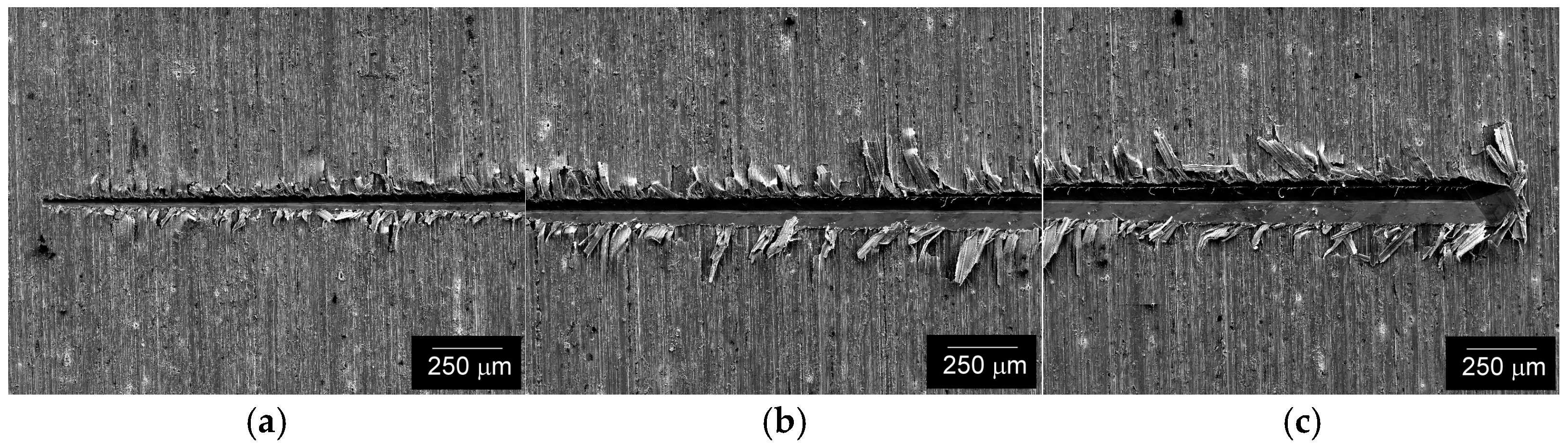
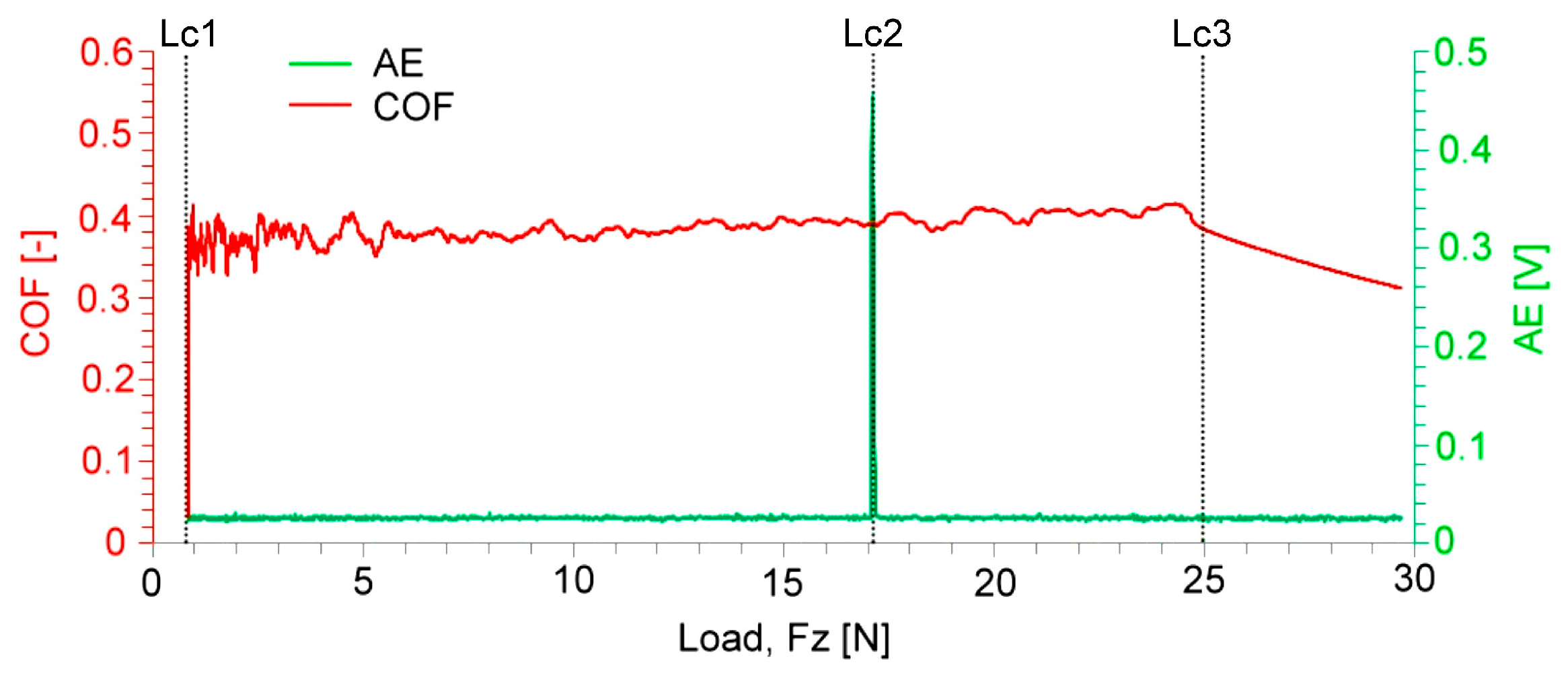
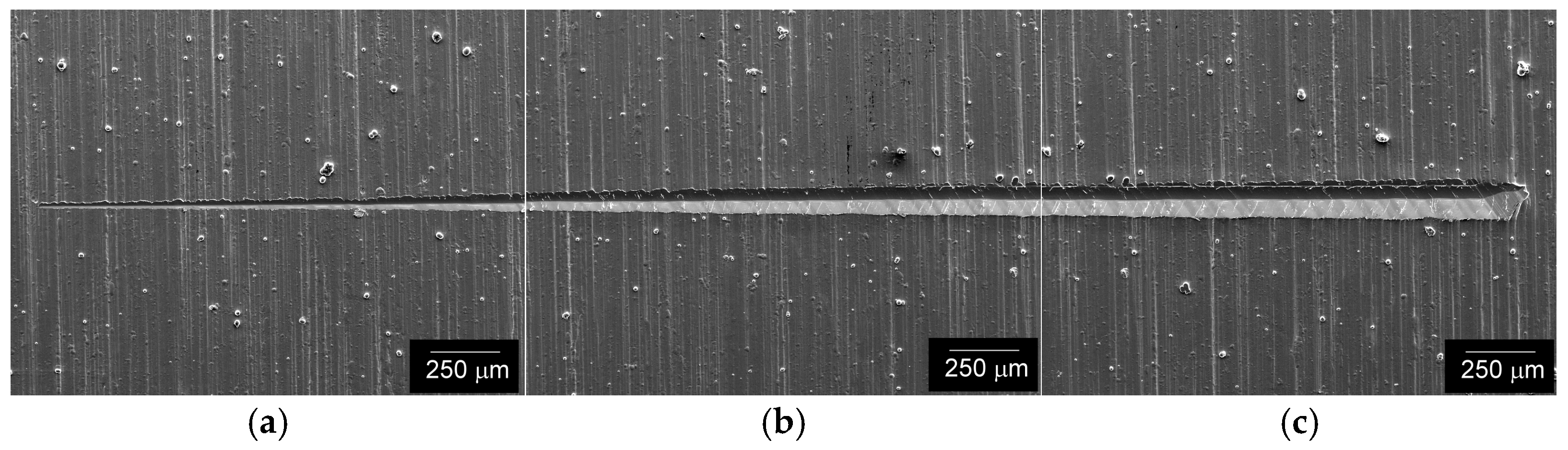
3.2. Tribological Scratching Behavior
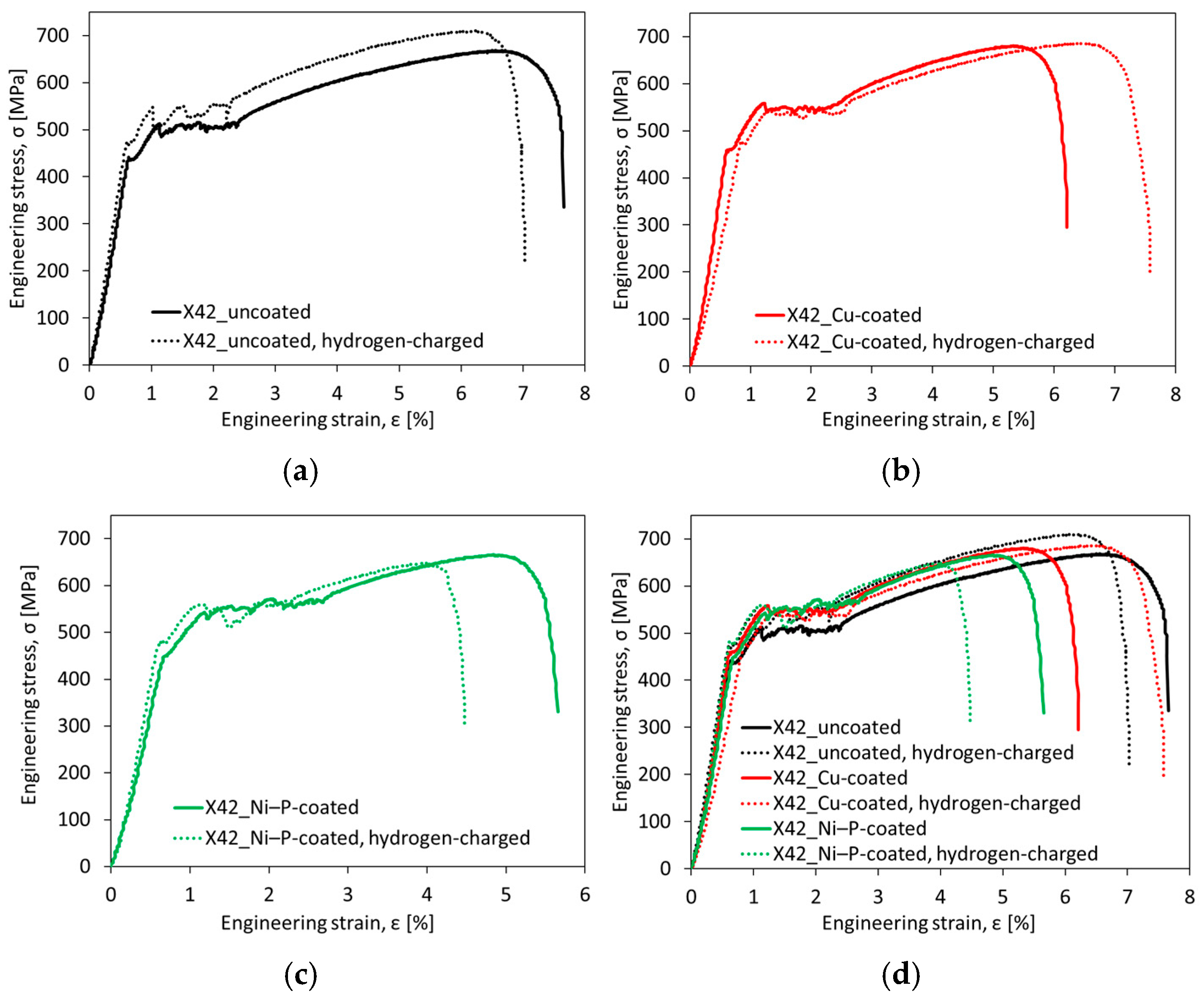
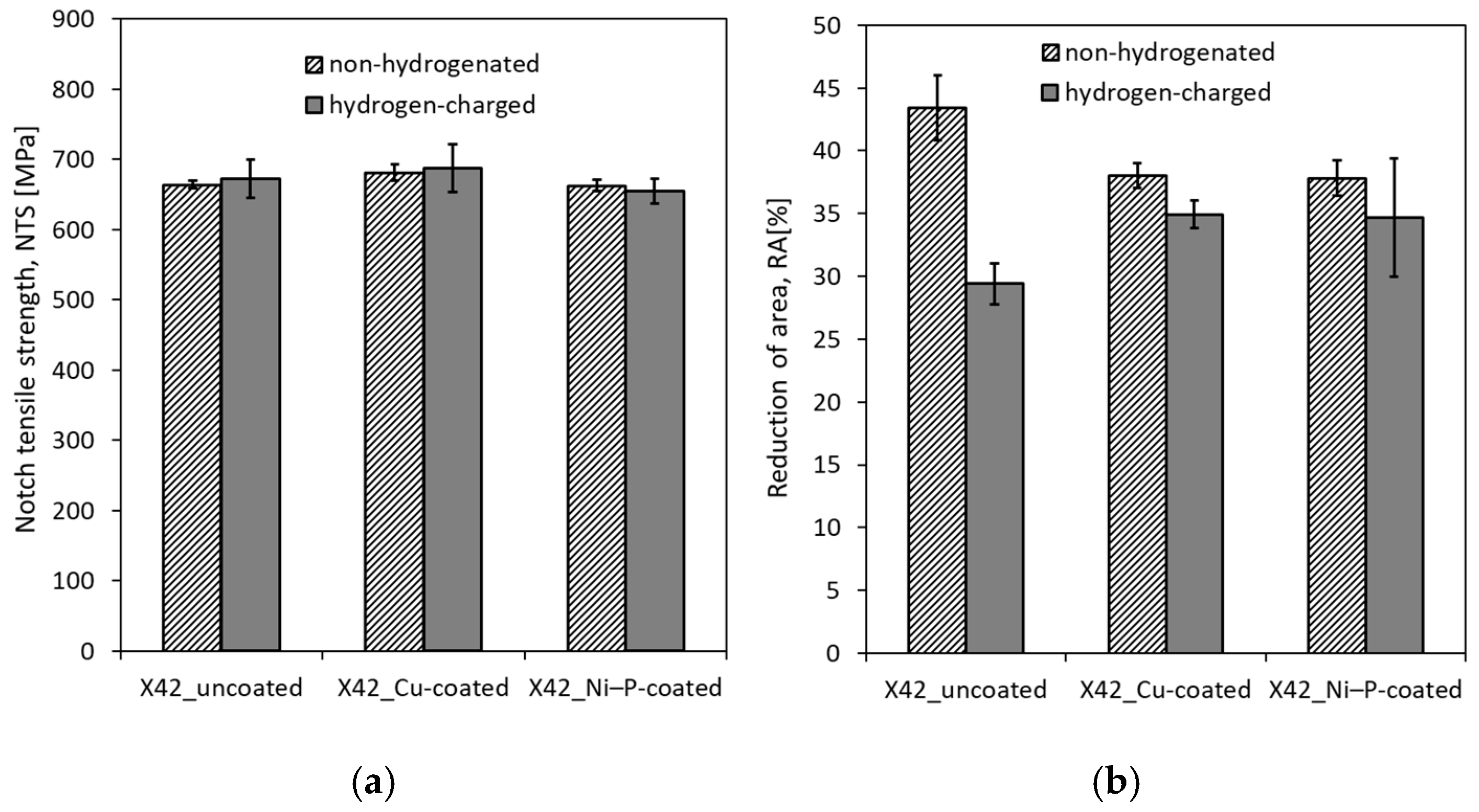
3.3. Notch Tensile Properties and HE Sensitivity
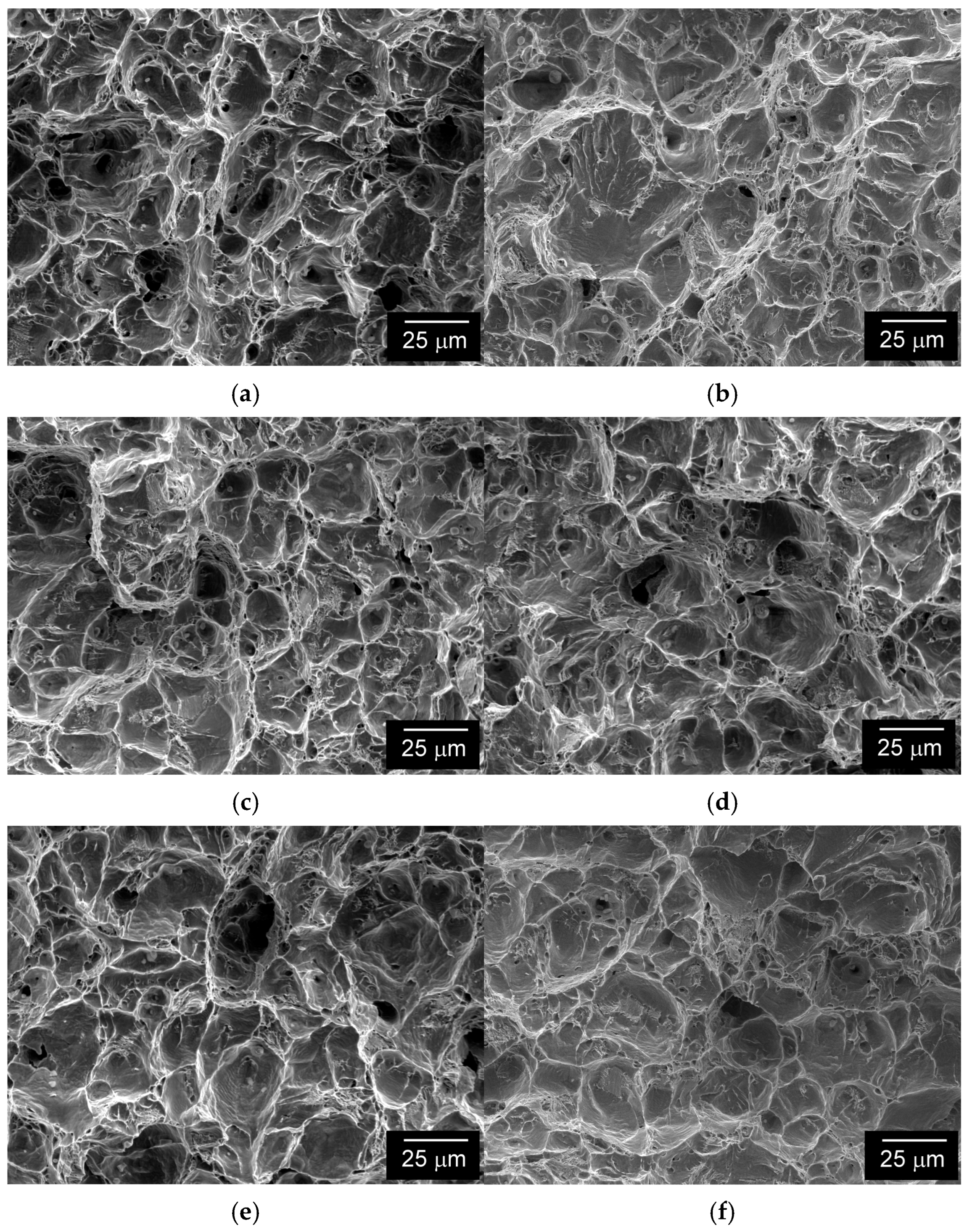
3.4. Fracture Micro-Mechanisms
4. Conclusions
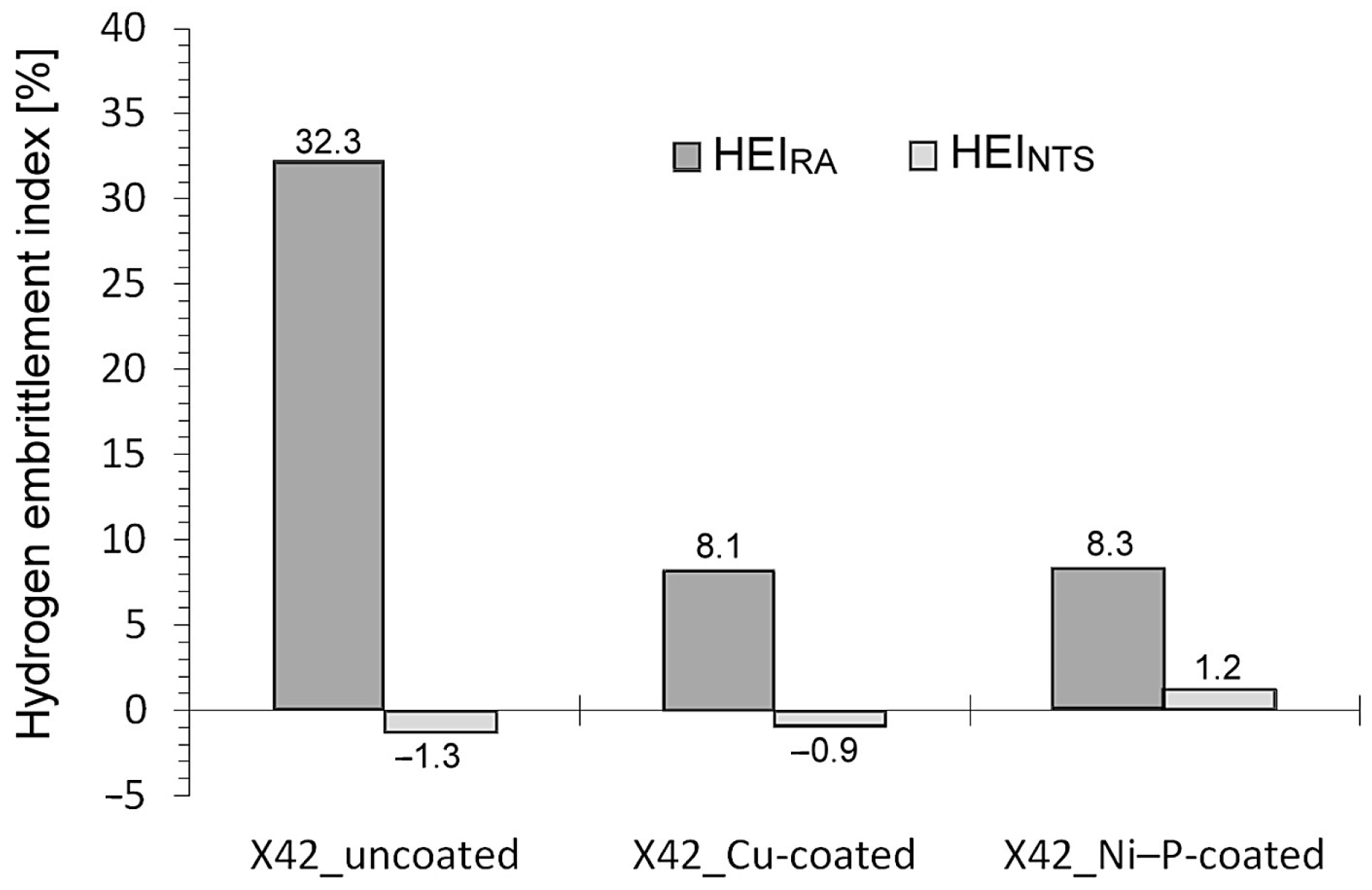
- Neither the application of coatings nor electrochemical hydrogenation resulted in any significant changes in notch tensile strength. However, both aforementioned effects induced noticeable changes in the reduction of area. The sole application of individual coatings without hydrogen charging resulted in a clear decrease of reduction of area compared to the uncoated material, which might be related to hydrogen embrittlement due to the presence of hydrogen ions in the coating solutions as well as the observed superficial imperfections in produced coatings.
- Both coated material configurations after electrochemical hydrogenation exhibited higher reduction of area in comparison with the hydrogenated material without coating, which indicated certain protective barrier effects of both investigated coatings against hydrogen permeation. These findings have been supported by the performed fractographic observations.
- The HEI values calculated according to the changes in notch tensile strength (HEINTS) do not show any significant variations among individual material configurations. On the other hand, the HEI values of the coated material configurations calculated according to the changes in reduction of area (HEIRA) were remarkably (almost four times) reduced in comparison with the uncoated material.
- The results of tribological scratch tests indicated stable adhesive bonding with the steel substrate in the initial material condition, i.e., the state without application of electrochemical hydrogen charging. The frictional and acoustic emission behaviors of the individual coatings studied corresponded fairly with their microstructural characteristics.
- After the electrolytic hydrogen charging, the adhesiveness of the coatings was lowered. Bubbles and flocks occurred within hydrogenated coatings. Moreover, a local spallation of the Cu coating also occurred after the electrochemical hydrogenation. Thus, further research efforts should be focused on improvements in the coatings’ surface quality and long-term durability, including the study of their cyclic mechanical behavior in pressurized hydrogen gas environments.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tashie-Lewis, B.C.; Nnabuife, S.G. Hydrogen Production, Distribution, Storage and Power Conversion in a Hydrogen Economy—A Technology Review. Chem. Eng. J. Adv. 2021, 8, 100172. [Google Scholar] [CrossRef]
- Akhtar, M.S.; Khan, H.; Liu, J.J.; Na, J. Green hydrogen and sustainable development—A social LCA perspective highlighting social hotspots and geopolitical implications of the future hydrogen economy. J. Clean. Prod. 2023, 395, 136438. [Google Scholar] [CrossRef]
- Nigutová, K.; Oroszová, L.; Molčanová, Z.; Csík, D.; Gáborová, K.; Möllmer, J.; Lange, M.; Saksl, K. Experimental Validation of Hydrogen Affinity as a Design Criterion for Alloys. Materials 2024, 17, 6106. [Google Scholar] [CrossRef] [PubMed]
- Johnson, W.H. On some remarkable changes produced in iron and steel by the action of hydrogen and acids. Proc. R. Soc. Lond. 1875, 23, 168–179. [Google Scholar] [CrossRef]
- Hirth, J.P. Effects of hydrogen on the properties of iron and steel. Met. Trans. A 1980, 11, 861–890. [Google Scholar] [CrossRef]
- Sofronis, P.; Robertson, I.M. Transmission electron microscopy observations and micromechanical/continuum models for the effect of hydrogen on the mechanical behaviour of metals. Philos. Mag. A 2002, 82, 3405–3413. [Google Scholar] [CrossRef]
- Nagumo, M. Effects of Hydrogen on Mechanical Behavior of Steels. Tetsu-to-Hagane 2004, 90, 766–775. [Google Scholar] [CrossRef] [PubMed]
- Song, R.G.; Dietzel, W.; Zhang, B.J.; Liu, W.J.; Tseng, M.K.; Atrens, A. Stress corrosion cracking and hydrogen embrittlement of an Al–Zn–Mg–Cu alloy. Acta Mater. 2004, 52, 4727–4743. [Google Scholar] [CrossRef]
- Liu, Q.; Atrens, A. Reversible hydrogen trapping in a 3.5NiCrMoV medium strength steel. Corros. Sci. 2015, 96, 112–120. [Google Scholar] [CrossRef]
- Lynch, S.P. Interpreting hydrogen-induced fracture surfaces in terms of deformation processes: A new approach. Scr. Mater. 2011, 65, 851–854. [Google Scholar] [CrossRef]
- Zhao, Q.; Xing, Y.; Feng, M.; Chen, Y.; Wang, X.; Yang, Z.; Wang, Z.; Du, Y.; Zhang, L. Study on hydrogen embrittlement behaviour of X52 welded joints in hydrogen transport environments. Eng. Fail. Anal. 2025, 179, 109774. [Google Scholar] [CrossRef]
- Safyari, M.; Horváth, D.; Moshtaghi, M. Enhancing hydrogen embrittlement resistance in steel pipelines through hybrid welding. Weld World 2025, in press. [Google Scholar] [CrossRef]
- Jayasekaran, R.; Mahmoudi, A.; Narasimhan, J.S.M.L.; Sadeghi, F.; England, R.D.; Ren, N. Hydrogen Diffusion, Effusion, and Tensile Fatigue of AISI 52100 Steel. Eng. Fail. Anal. 2025, 179, 109796. [Google Scholar] [CrossRef]
- Lee, J.Y.; Lee, S.M. Hydrogen trapping phenomena in metals with bcc and fcc crystal structures by the desorption thermal-analysis technique. Surf. Coat. Technol. 1986, 28, 301–314. [Google Scholar] [CrossRef]
- Hirata, K.; Iikubo, S.; Koyama, M.; Tsuzaki, K.; Ohtani, H. First principles study on hydrogen diffusivity in BCC, FCC, and HCP iron. Metall. Mater. Trans. A 2018, 49, 5015–5022. [Google Scholar] [CrossRef]
- Wang, H.; Cao, S.; Ming, H.; Wan, H.; Hu, Q.; Wang, J.; Han, E.-H. Insight into the hydrogen permeation and hydrogen embrittlement mechanisms of X52 pipeline steel exposed to gaseous hydrogen. Int. J. Hydrogen Energy 2025, 133, 502–519. [Google Scholar] [CrossRef]
- Ebling, F.; Bratsch, P.; Wagner, S.; Pundt, A.; Preußner, J.; Oesterlin, H.; Wackermann, K.; Michler, T. Assessing hydrogen embrittlement of alloy 718: Hollow and conventional tensile tests. Eng. Frac. Mech. 2025, 319, 111028. [Google Scholar] [CrossRef]
- Fukunaga, A. Hydrogen embrittlement behavior of iron-based superalloy A286. Procedia Struct. Integr. 2024, 54, 115–122. [Google Scholar] [CrossRef]
- Ševc, P.; Falat, L.; Čiripová, L.; Džupon, M.; Vojtko, M. The Effects of Electrochemical Hydrogen Charging on Room-Temperature Tensile Properties of T92/TP316H Dissimilar Weldments in Quenched-and-Tempered and Thermally-Aged Conditions. Metals 2019, 9, 864. [Google Scholar] [CrossRef]
- Dmytrakh, I.; Syrotyuk, A.; Leshchak, R. Specific mechanism of hydrogen influence on deformability and fracture of low-alloyed pipeline steel. Procedia Struct. Integr. 2022, 36, 298–305. [Google Scholar] [CrossRef]
- Eichinger, M.; Zwittnig, D.; Mori, G. Influence of the microstructure on the hydrogen uptake and embrittlement of 42CrMo4 steel under gaseous hydrogen charging. Int. J. Hydrogen Energy 2025, 133, 402–415. [Google Scholar] [CrossRef]
- Dwivedi, S.K.; Vishwakarma, M. Hydrogen embrittlement in different materials: A review. Int. J. Hydrogen Energy 2018, 43, 21603–21616. [Google Scholar] [CrossRef]
- Sobola, D.; Dallaev, R. Exploring Hydrogen Embrittlement: Mechanisms, Consequences, and Advances in Metal Science. Energies 2024, 17, 2972. [Google Scholar] [CrossRef]
- Li, Q.; Deng, C.; Wu, S.; Zhao, H.; Xu, X.; Liu, Y.; Gong, B. The combined mechanism of hydrogen embrittlement and anodic dissolution on the fracture toughness degradation of X65 weld joint in H2S. Mater. Today Commun. 2025, 46, 112635. [Google Scholar] [CrossRef]
- Chowdhury, M.F.W.; Tapia-Bastidas, C.V.; Hoschke, J.; Venezuela, J.; Atrens, A. A review of influence of hydrogen on fracture toughness and mechanical properties of gas transmission pipeline steels. Int. J. Hydrogen Energy 2025, 102, 181–221. [Google Scholar] [CrossRef]
- Nykyforchyn, H.; Tsyrulnyk, O.; Venhryniuk, O.; Zvirko, O. Techniques for investigation of hydrogen influence on fracture toughness and embrittlement of pipeline steels. Procedia Struct. Integr. 2024, 59, 125–130. [Google Scholar] [CrossRef]
- Laureys, A.; Depraetere, R.; Cauwels, M.; Depover, T.; Hertelé, S.; Verbeken, K. Use of existing steel pipeline infrastructure for gaseous hydrogen storage and transport: A review of factors affecting hydrogen induced degradation. J. Nat. Gas Sci. Eng. 2022, 101, 104534. [Google Scholar] [CrossRef]
- Cauwels, M.; Depraetere, R.; De Waele, W.; Hertelé, S.; Verbeken, K.; Depover, T. Effect of stress triaxiality on the hydrogen embrittlement micromechanisms in a pipeline steel evaluated by fractographic analysis. Mater. Sci. Eng. A 2023, 886, 145689. [Google Scholar] [CrossRef]
- Liu, L.; Zhai, C.; Lu, C.; Ding, W.; Hirose, A.; Kobayashi, K.F. Study of the effect of δ phase on hydrogen embrittlement of Inconel 718 by notch tensile tests. Corros. Sci. 2005, 47, 355–367. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, H.; Liu, C.; Wang, C.; Fan, X.; Cheng, Y.F.; Li, Y. Effects of defect on the hydrogen embrittlement behavior of X80 pipeline steel in hydrogen-blended natural gas environments. Int. J. Hydrogen Energy 2024, 58, 158–173. [Google Scholar] [CrossRef]
- Tarzimoghadam, Z.; Rohwerder, M.; Merzlikin, S.V.; Bashir, A.; Yedra, L.; Eswara, S.; Ponge, D.; Raabe, D. Multi-scale and spatially resolved hydrogen mapping in a Ni–Nb model alloy reveals the role of the δ phase in hydrogen embrittlement of alloy 718. Acta Mater. 2016, 109, 69–81. [Google Scholar] [CrossRef]
- Krieger, W.; Merzlikin, S.V.; Bashir, A.; Szczepaniak, A.; Springer, H.; Rohwerder, M. Spatially resolved localization and characterization of trapped hydrogen in zero to three dimensional defects inside ferritic steel. Acta Mater. 2018, 144, 235–244. [Google Scholar] [CrossRef]
- Deng, Y.; Barnoush, A. Hydrogen embrittlement revealed via novel in situ fracture experiments using notched micro-cantilever specimens. Acta Mater. 2018, 142, 236–247. [Google Scholar] [CrossRef]
- Jothi, S.; Croft, T.N.; Brown, S.G.R. Multiscale multiphysics model for hydrogen embrittlement in polycrystalline nickel. J. Alloys Compd. 2014, 645, S500–S504. [Google Scholar] [CrossRef]
- Matsumoto, R.; Seki, S.; Taketomi, S.; Miyazaki, N. Hydrogen-related phenomena due to decreases in lattice defect energies-Molecular dynamics simulations using the embedded atom method potential with pseudo-hydrogen effects. Comp. Mater. Sci. 2014, 92, 362–371. [Google Scholar] [CrossRef]
- Solanki, K.N.; Ward, D.K.; Bammann, D.J. A nanoscale study of dislocation nucleation at the crack tip in the nickel-hydrogen system. Met. Mater. Trans. A 2011, 42, 340–347. [Google Scholar] [CrossRef]
- Djukic, M.B.; Bakic, G.M.; Zeravcic, V.S.; Sedmak, A.; Rajicic, B. The synergistic action and interplay of hydrogen embrittlement mechanisms in steels and iron: Localized plasticity and decohesion. Eng. Fract. Mech. 2019, 216, 106528. [Google Scholar] [CrossRef]
- Ghadiani, H.; Farhat, Z.; Alam, T.; Islam, M.A. Fracture Toughness Assessment of Pipeline Steels Under Hydrogen Exposure for Blended Gas Applications. Metals 2025, 15, 29. [Google Scholar] [CrossRef]
- Islam, A.; Alam, T.; Sheibley, N.; Edmonson, K.; Burns, D.; Hernandez, M. Hydrogen blending in natural gas pipelines: A comprehensive review of material compatibility and safety considerations. Int. J. Hydrogen Energy 2024, 93, 1429–1461. [Google Scholar] [CrossRef]
- Wu, X.; Zhang, H.; Yang, M.; Jia, W.; Qiu, Y.; Lan, L. From the perspective of new technology of blending hydrogen into natural gas pipelines transmission: Mechanism, experimental study, and suggestions for further work of hydrogen embrittlement in high-strength pipeline steels. Int. J. Hydrogen Energy 2022, 47, 8071–8090. [Google Scholar] [CrossRef]
- Mahajan, D.; Tan, K.; Venkatesh, T.; Kileti, P.; Clayton, C.R. Hydrogen Blending in Gas Pipeline Networks—A Review. Energies 2022, 15, 3582. [Google Scholar] [CrossRef]
- Cui, D.; Bai, Y.; Xiong, L.; Yu, B.; Wei, B.; Sun, C. Effects of hydrogen blending ratios and CO2 on hydrogen embrittlement of X65 steel in high-pressure offshore hydrogen-blended natural gas pipelines. J. Mater. Res. Technol. 2024, 33, 4763–4771. [Google Scholar] [CrossRef]
- Kim, W.J.; Park, Y.; Park, D.J. Quantitative risk assessments of hydrogen blending into transmission pipeline of natural gas. J. Loss Prev. Process Ind. 2024, 91, 105412. [Google Scholar] [CrossRef]
- Rosa, N.; Fereidani, N.A.; Cardoso, B.J.; Martinho, N.; Gaspar, A.; Silva, M.G. Advances in hydrogen blending and injection in natural gas networks: A review. Int. J. Hydrogen Energy 2025, 105, 367–381. [Google Scholar] [CrossRef]
- Ghadiani, H.; Farhat, Z.; Alam, T.; Islam, M.A. Assessing Hydrogen Embrittlement in Pipeline Steels for Natural Gas-Hydrogen Blends: Implications for Existing Infrastructure. Solids 2024, 5, 375–393. [Google Scholar] [CrossRef]
- Nnoka, M.; Jack, T.A.; Szpunar, J. Effects of different microstructural parameters on the corrosion and cracking resistance of pipeline steels: A review. Eng. Fail. Anal. 2024, 159, 108065. [Google Scholar] [CrossRef]
- Lima dos Santos, M.; Filgueira de Almeida, A.; de Sousa Figueiredo, G.G.; da Silva, M.M.; Maciel, T.M.; Santos, T.F.A.; de Santana, R.A.C. Influence of Centerline Segregation Region on the Hydrogen Embrittlement Susceptibility of API 5L X80 Pipeline Steels. Metals 2024, 14, 1154. [Google Scholar] [CrossRef]
- Rahimi, S.; Verbeken, K.; Depover, T.; Proverbio, E. Hydrogen embrittlement of pipeline steels under gaseous and electrochemical charging: A comparative review on tensile properties. Eng. Fail. Anal. 2025, 167, 108956. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Park, J.S.; Nahm, S.H.; Baek, U.B. Evaluation of hydrogen related degradation of API X42 pipeline under hydrogen/natural gas mixture conditions using small punch test. Theor. Appl. Fract. Mech. 2021, 113, 102961. [Google Scholar] [CrossRef]
- Walallawita, R.; Hinchliff, M.C.; Sediako, D.; Quinn, J.; Chou, V.; Walker, K.; Hill, M. Evaluating the Effect of Blended and Pure Hydrogen in X60 Pipeline Steel for Low-Pressure Transmission Using Hollow-Specimen Slow-Strain-Rate Tensile Testing. Metals 2024, 14, 1132. [Google Scholar] [CrossRef]
- Chen, J.-M.; Wu, J.-K. Hydrogen diffusion through copper-plated AISI 4140 steels. Corros. Sci. 1992, 33, 657–666. [Google Scholar] [CrossRef]
- Khan, H.H.; Wang, T.; Su, L.; Li, H.; Zhu, Q.; Yang, A.; Li, Z.; Wang, W.; Zhu, H. In Situ Thermal Interactions of Cu-Based Anti-Corrosion Coatings on Steel Implemented by Surface Alloying. Coatings 2024, 14, 722. [Google Scholar] [CrossRef]
- Keech, P.G.; Vo, P.; Ramamurthy, S.; Chen, J.; Jacklin, R.; Shoesmith, D.W. Design and development of copper coatings for long term storage of used nuclear fuel. Corros. Eng. Sci. Technol. 2014, 49, 425–430. [Google Scholar] [CrossRef]
- Hall, D.S.; Keech, P.G. An overview of the Canadian corrosion program for the long-term management of nuclear waste. Corros. Eng. Sci. Technol. 2017, 52, 2–5. [Google Scholar] [CrossRef]
- Standish, T.E.; Zagidulin, D.; Ramamurthy, S.; Keech, P.G.; Noël, J.J.; Shoesmith, D.W. Galvanic corrosion of copper-coated carbon steel for used nuclear fuel containers. Corros. Eng. Sci. Technol. 2017, 52, 65–69. [Google Scholar] [CrossRef]
- Samanta, S.; Singh, C.; Banerjee, A.; Mondal, K.; Dutta, M.; Singh, S.B. Development of amorphous Ni-P coating over API X70 steel for hydrogen barrier application. Surf. Coat. Technol. 2020, 403, 126356. [Google Scholar] [CrossRef]
- Biggio, D.; Elsener, B.; Rossi, A. Ni-P Coatings as Hydrogen Permeation Barriers—A Review. Coatings 2025, 15, 365. [Google Scholar] [CrossRef]
- Michler, T.; Naumann, J. Coatings to reduce hydrogen environment embrittlement of 304 austenitic stainless steel. Surf. Coat. Technol. 2009, 203, 1819–1828. [Google Scholar] [CrossRef]
- Falat, L.; Čiripová, L.; Kromka, F.; Homolová, V.; Džunda, R.; Motýľová, M. Hydrogen Embrittlement Resistance of Ferritic–Pearlitic Pipeline Steel with Non-Electrochemically Deposited Copper- or Nickel–Phosphorus-Based Coating. Coatings 2025, 15, 585. [Google Scholar] [CrossRef]
- ASTM C1624-22; Standard Test Method for Adhesion Strength and Mechanical Failure Modes of Ceramic Coatings by Quantitative Single Point Scratch Testing. ASTM International: West Conshohocken, PA, USA, 2022. [CrossRef]
- ISO 6892-1:2019; Metallic Materials—Tensile Testing Part 1: Method of Test at Room Temperature. ISO: London, UK, 2019. Available online: https://www.iso.org/standard/78322.html (accessed on 4 August 2025).
- Zafra, A.; Álvarez, G.; Benoit, G.; Henaff, G.; Martinez-Pañeda, E.; Rodríguez, C.; Belzunce, J. Hydrogen-assisted fatigue crack growth: Pre-charging vs in-situ testing in gaseous environments. Mater. Sci. Eng. A 2023, A871, 144885. [Google Scholar] [CrossRef]
- Arniella, V.; Zafra, A.; Álvarez, G.; Belzunce, J.; Rodríguez, C. Comparative study of embrittlement of quenched and tempered steels in hydrogen environments. Int. J. Hydrogen Energy 2022, 47, 17056–17068. [Google Scholar] [CrossRef]
- Burnett, P.J.; Rickerby, D.S. The relationship between hardness and scratch adhesion. Thin Solid Film. 1987, 154, 403–416. [Google Scholar] [CrossRef]
- Cottrell, A.H.; Bilby, B.A. Dislocation theory of yielding and strain ageing of iron. Proc. Phys. Soc. 1949, 62, 49–62. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhang, B.; Wang, D.; Zhou, Y.; Wang, J.; Han, E.-H.; Ke, W. Hydrogen-assisted fracture features of a high strength ferrite-pearlite steel. J. Mater. Sci. Technol. 2019, 35, 1081–1087. [Google Scholar] [CrossRef]
- Yu, L.; Feng, H.; Li, S.; Guo, Z.; Chi, Q. Study on Hydrogen Embrittlement Behavior of X65 Pipeline Steel in Gaseous Hydrogen Environment. Metals 2025, 15, 596. [Google Scholar] [CrossRef]
- Štamborská, M.; Lapin, J.; Bajana, O.; Losertová, M. Tensile deformation behaviour of ferritic-pearlitic steel studied by digital image correlation method. Kov. Mater. 2015, 53, 399–407. [Google Scholar] [CrossRef]


| C | N | Mn | Si | P | S | Cu | Cr | Mo | V | Ni | Al | Sn | Fe |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.16 | 0.009 | 0.51 | 0.24 | 0.014 | 0.010 | 0.19 | 0.09 | 0.02 | 0.007 | 0.08 | 0.027 | 0.012 | rest |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Falat, L.; Čiripová, L.; Puchý, V.; Petrišinec, I.; Džunda, R. Effect of Electrochemical Hydrogen Charging on the Notch Tensile Properties of Natural Gas Transportation Pipeline Steel with Electroless-Plated Coatings and Their Adhesiveness Characterization. Metals 2025, 15, 1032. https://doi.org/10.3390/met15091032
Falat L, Čiripová L, Puchý V, Petrišinec I, Džunda R. Effect of Electrochemical Hydrogen Charging on the Notch Tensile Properties of Natural Gas Transportation Pipeline Steel with Electroless-Plated Coatings and Their Adhesiveness Characterization. Metals. 2025; 15(9):1032. https://doi.org/10.3390/met15091032
Chicago/Turabian StyleFalat, Ladislav, Lucia Čiripová, Viktor Puchý, Ivan Petrišinec, and Róbert Džunda. 2025. "Effect of Electrochemical Hydrogen Charging on the Notch Tensile Properties of Natural Gas Transportation Pipeline Steel with Electroless-Plated Coatings and Their Adhesiveness Characterization" Metals 15, no. 9: 1032. https://doi.org/10.3390/met15091032
APA StyleFalat, L., Čiripová, L., Puchý, V., Petrišinec, I., & Džunda, R. (2025). Effect of Electrochemical Hydrogen Charging on the Notch Tensile Properties of Natural Gas Transportation Pipeline Steel with Electroless-Plated Coatings and Their Adhesiveness Characterization. Metals, 15(9), 1032. https://doi.org/10.3390/met15091032







