The Impact of Hydrogen Charging Time on Microstructural Alterations in Pipeline Low-Carbon Ferrite–Pearlite Steel
Abstract
1. Introduction
- Hydrogen-Induced Crackingor Hydrogen Environment-Assisted Cracking occurs due to the recombination of atomic hydrogen into a molecule inside the material [12];
- Hydrogen blistering arises when hydrogen enters the metal from hydrogen-containing environments and accumulates around inclusions or other defects. With increasing hydrogen pressure, the integrity of the metal is eventually compromised. The susceptibility to this type of damage depends on the structure of the metallic material. Ferritic, martensitic, and duplex steels are generally less resistant to this form of damage compared to austenitic steels [13];
- Stress-Oriented Hydrogen-Induced Cracking [12] leads to premature failure of ductile steels and alloys under sustained loading at stress levels below the yield strength. The resulting cracks are oriented perpendicular to the direction of hydrogen diffusion;
- Sulfide Stress Cracking [12] manifests as longitudinal cracking due to stress from externally applied loads. This phenomenon is particularly critical in industries that process crude oil and natural gas containing hydrogen sulfide. Austenitic and duplex steels (austenitic–ferritic) are particularly susceptible to this form of corrosion, especially at elevated temperatures [13]. This type of degradation also affects steels under load, resulting in crack propagation parallel to the direction of hydrogen diffusion;
- Internal Hydrogen-Assisted Cracking [14] refers to internal cracking caused by hydrogen that accumulates during manufacturing or assembly processes.
2. Materials and Methods
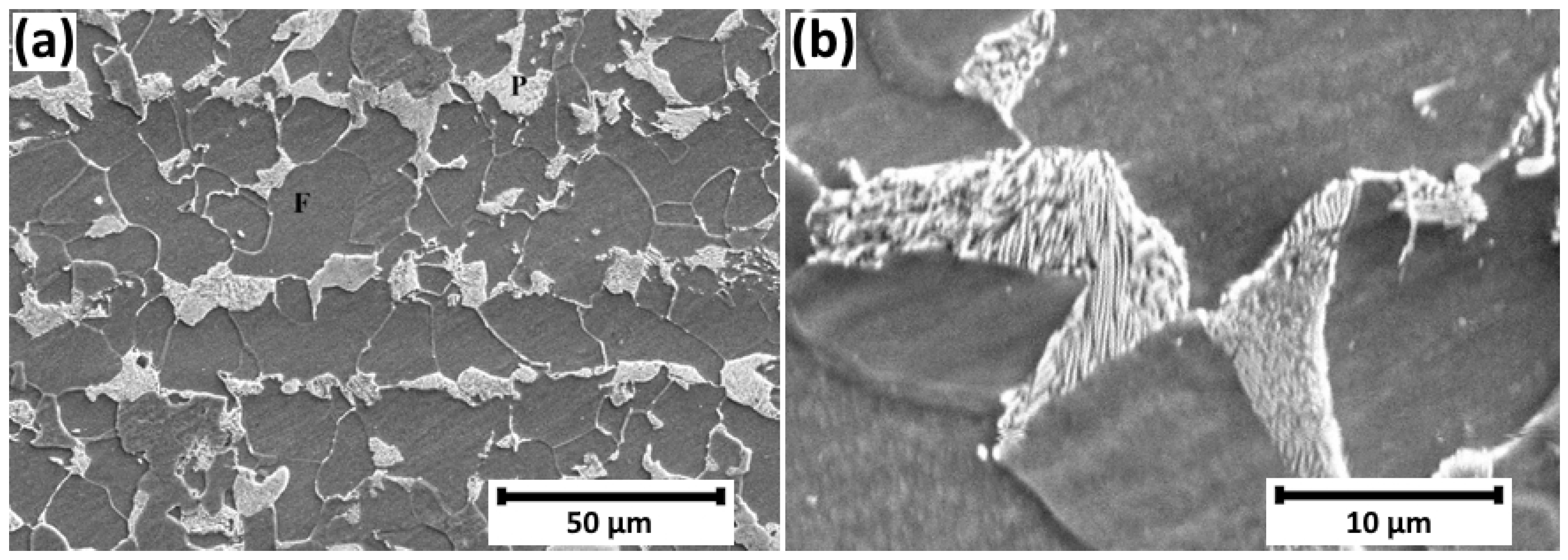
2.1. Material
2.2. Test Methods
2.2.1. Determination of Hydrogen Concentration in Samples of In-Service X52 Pipeline Steel, and of the Effective Diffusion Coefficient of Atomic Hydrogen
2.2.2. In-Situ Hydrogen Charging and Tensile Test
2.3. Characterization Methods
3. Results and Discussion
3.1. Hydrogen Concentration and Effective Diffusion Coefficient of Atomic Hydrogen in In-Service X52 Pipeline Steel
3.2. Mechanical Testing
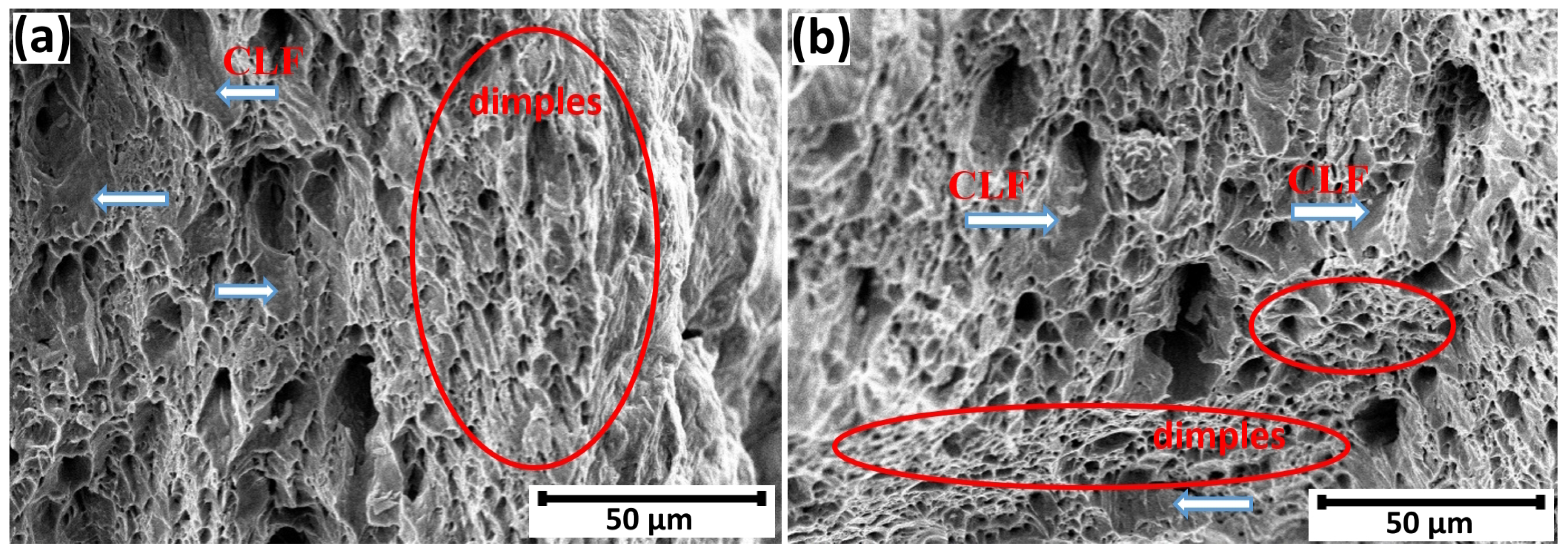
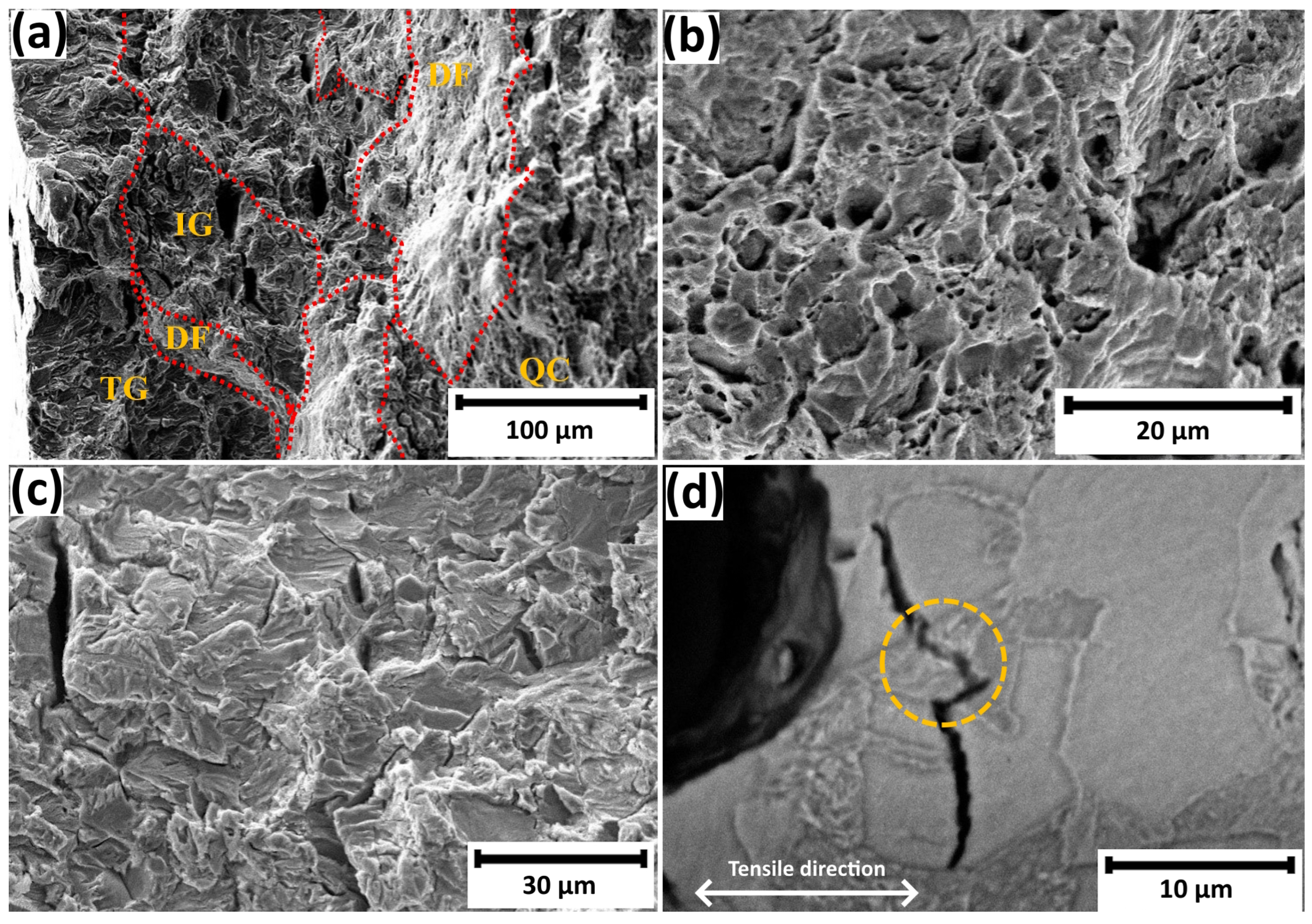
3.3. Failure Analysis
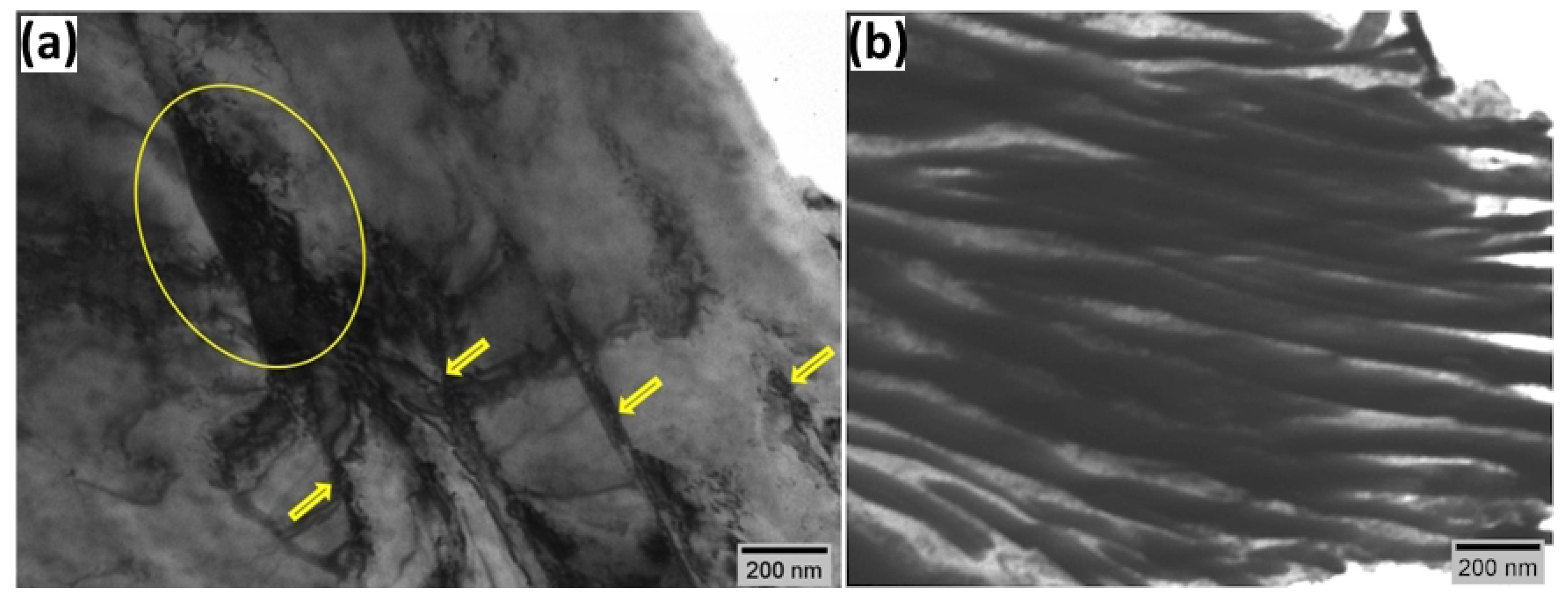
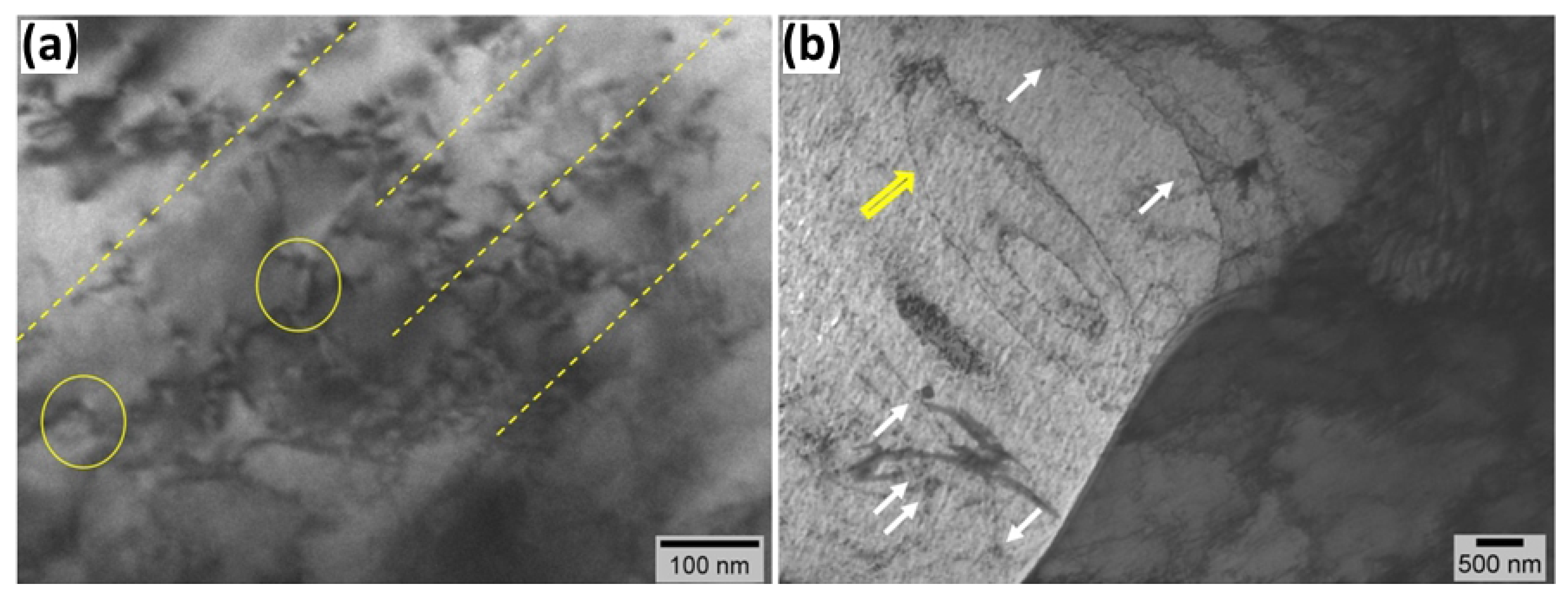
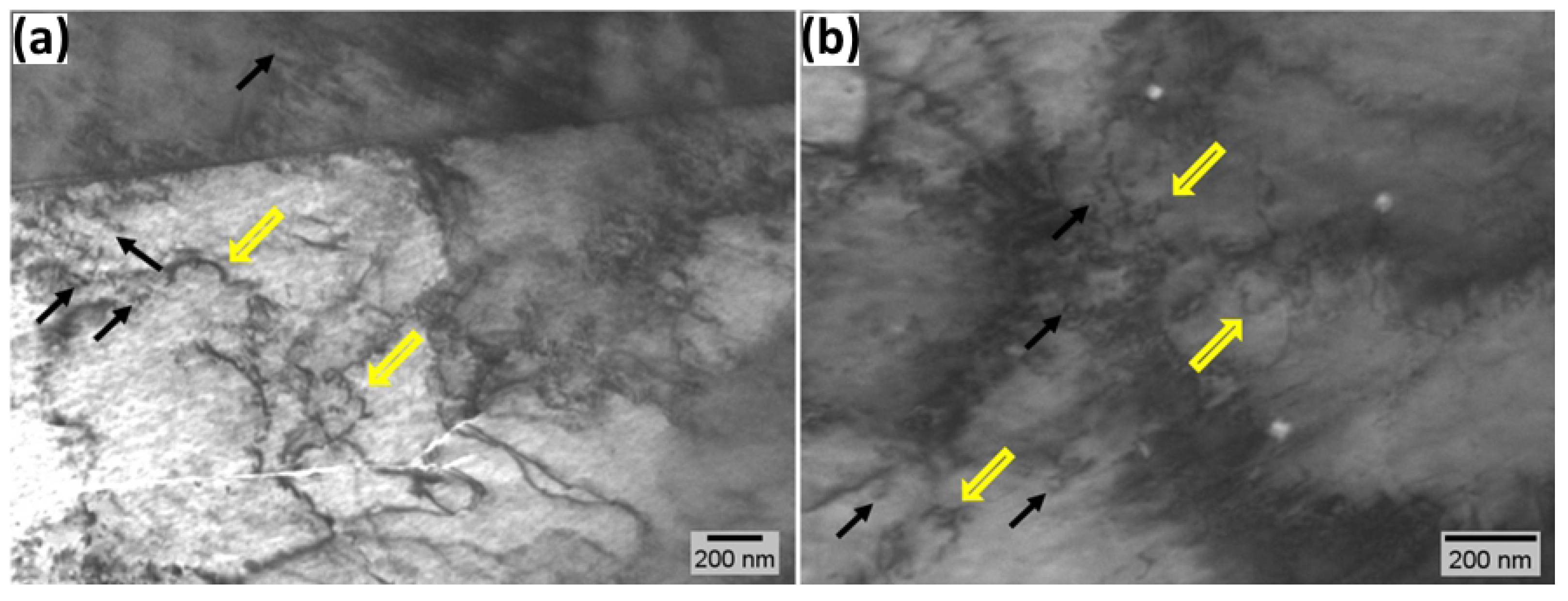
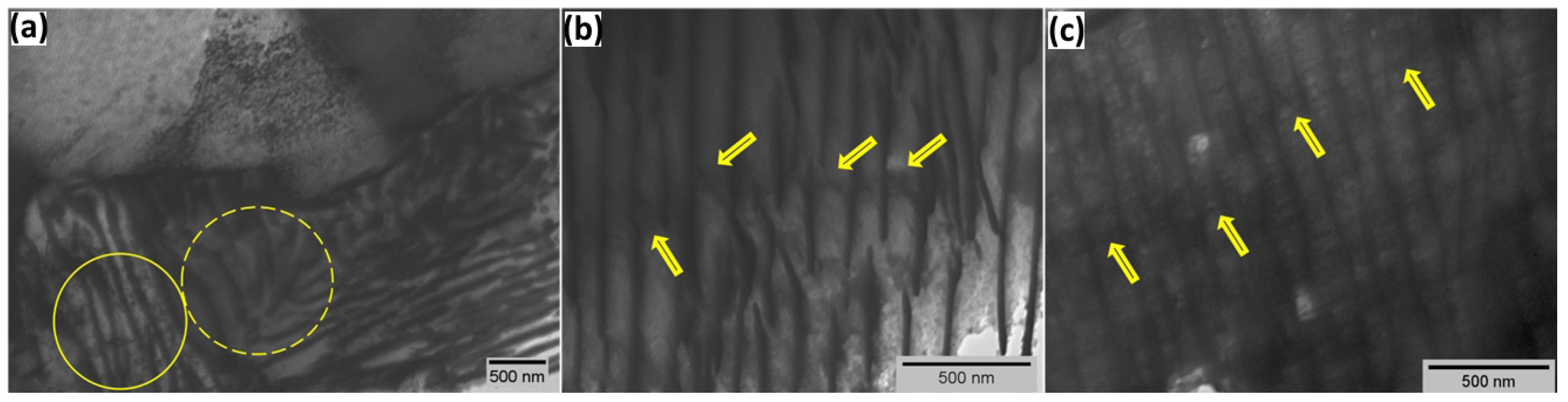
3.4. TEM Analysis
4. Conclusions
- Although charging time had little effect on the overall hydrogen concentration and strength characteristics in the metal, pronounced time-dependent changes in plasticity, fracture morphology and microstructure were observed after tensile testing of specimens charged for 1, 24, and 72 h.
- Fractographic analysis revealed a transition from ductile fracture in uncharged specimens to a predominance of brittle fracture modes (quasi-cleavage, intergranular, and transgranular) in hydrogen-charged samples. The relative area fraction of brittle fracture zones increased from 46% to 65% with an increase in loading time from 1 to 72 h.
- The present microstructural investigations indicated an initial plastic failure in the ferrite grains and then, with an increase in hydrogen charging time, failure at the ferrite–cementite interface.
- TEM analysis of hydrogen-charged ferrite phases further indicates that prolonged charging leads to localized plasticity, dislocation pinning, and the formation of prismatic loop debris, which can be explained by enhanced hydrogen trapping at ferrite-cementite interfaces, grain boundaries, and the formation of hydrogen atmospheres within the dislocation cores. The number of pinned dislocations and prismatic loops increases with increasing hydrogen charging time. Hydrogen facilitates the activation of Frank-Read sources and the generation of dislocations.
- In the non-charged sample, TEM images show activation of slip bands. Hydrogen exposure increases the mobility of dominant screw dislocations outside the primary slip plane, leading to tangling within slip bands. To minimize elastic strain energy, dislocations rearrange into walls, forming a cellular network. The slip bands and dislocation cells represent sequential stages in hydrogen-induced rearrangement from localized slip to an energy-minimized dislocation configuration.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

References
- Djukic, M.B.; Bakic, G.M.; Zeravcic, V.S.; Sedmak, A.; Rajicic, B. Hydrogen embrittlement of industrial components: Prediction, prevention, and models. Corrosion 2016, 72, 943–961. [Google Scholar] [CrossRef]
- Barrera, O.; Bombac, D.; Chen, Y.; Daff, T.; Galindo-Nava, E.; Gong, P.; Haley, D.; Horton, R.; Katzarov, I.; Kermode, J.R.; et al. Understanding and mitigating hydrogen embrittlement of steels: A review of experimental, modelling and design progress from atomistic to continuum. J. Mater. Sci. 2018, 53, 6251–6290. [Google Scholar] [CrossRef]
- Ali, M.; Ul-Hamid, A.; Alhems, L.M.; Saeed, A. Review of common failures in heat exchangers—Part I: Mechanical and elevated temperature failures. Eng. Fail. Anal. 2020, 109, 104396. [Google Scholar] [CrossRef]
- Adasooriya, N.D.; Tucho, W.M.; Holm, E.; Årthun, T.; Hansen, V.; Solheim, K.G.; Hemmingsen, T. Effect of hydrogen on mechanical properties and fracture of martensitic carbon steel under quenched and tempered conditions. Mater. Sci. Eng. A 2021, 803, 140495. [Google Scholar] [CrossRef]
- Wasim, M.; Djukic, M.B. Hydrogen embrittlement of low carbon structural steel at macro-, micro-and nano-levels. Int. J. Hydrogen Energy 2020, 45, 2145–2156. [Google Scholar] [CrossRef]
- Norouzi, E.; Miresmaeili, R.; Shahverdi, H.R.; Askari-Paykani, M.; Vergani, L.M. Effect of hydrogen on the microstructure and mechanical properties of FeCCrNiBxSi advanced high strength steels. Corros. Sci. 2024, 230, 111897. [Google Scholar] [CrossRef]
- Drímalová, P.; Nový, F.; Medvecká, D.; Váňová, P. The Influence of Hydrogen Embrittlement on Mechanical Properties of Advanced High-Strength Structural Steel S960MC. In Quality Production Improvement and System Safety: QPI 16-CZOTO 10; Materials Research Forum LLC: Lancaster County, PA, USA, 2023; Volume 34, p. 62. [Google Scholar]
- Djukic, M.B.; Zeravcic, V.S.; Bakic, G.M.; Sedmak, A.; Rajicic, B. Hydrogen damage of steels: A case study and hydrogen embrittlement model. Eng. Fail. Anal. 2015, 58, 485–498. [Google Scholar] [CrossRef]
- Tau, L.; Chan, S. Effects of ferrite/pearlite alignment on the hydrogen permeation in a AISI 4130 steel. Mater. Lett. 1996, 29, 143–147. [Google Scholar] [CrossRef]
- Laureys, A.; Pinson, M.; Claeys, L.; Deseranno, T.; Depover, T.; Verbeken, K. Initiation of hydrogen induced cracks at secondary phase particles. Fract. Struct. Integr. 2020, 14, 113–127. [Google Scholar] [CrossRef]
- Laureys, A.; Depraetere, R.; Cauwels, M.; Depover, T.; Hertelé, S.; Verbeken, K. Use of existing steel pipeline infrastructure for gaseous hydrogen storage and transport: A review of factors affecting hydrogen induced degradation. J. Nat. Gas Sci. Eng. 2022, 101, 104534. [Google Scholar] [CrossRef]
- Elboujdaini, M. Hydrogen-induced cracking and sulfide stress cracking. In Uhlig’s Corrosion Handbook; Wiley: Hoboken, NJ, USA, 2011; Volume 3, pp. 183–194. [Google Scholar]
- Outokumpu Oyj. Handbook of Stainless Steel; Outokumpu Oyj: Helsinki, Finland, 2013. [Google Scholar]
- Gangloff, R.P.; Somerday, B.P. Gaseous Hydrogen Embrittlement of Materials in Energy Technologies: The Problem, Its Characterisation and Effects on Particular Alloy Classes; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Troiano, A.R. The Role of Hydrogen and Other Interstitials in the Mechanical Behavior of Metals: (1959 Edward de Mille Campbell Memorial Lecture); Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Boot, T.; Riemslag, T.; Reinton, E.; Liu, P.; Walters, C.L.; Popovich, V. In-situ hollow sample setup design for mechanical characterisation of gaseous hydrogen embrittlement of pipeline steels and welds. Metals 2021, 11, 1242. [Google Scholar] [CrossRef]
- Wan, H.J.; Wu, X.Q.; Ming, H.L.; Wang, J.Q.; Han, E.H. Effects of hydrogen charging time and pressure on the hydrogen embrittlement susceptibility of X52 pipeline steel material. Acta Metall. Sin. (Engl. Lett.) 2024, 37, 293–307. [Google Scholar] [CrossRef]
- Laureys, A.; Van den Eeckhout, E.; Petrov, R.; Verbeken, K. Effect of deformation and charging conditions on crack and blister formation during electrochemical hydrogen charging. Acta Mater. 2017, 127, 192–202. [Google Scholar] [CrossRef]
- Djukic, M.B.; Bakic, G.M.; Zeravcic, V.S.; Sedmak, A.; Rajicic, B. The synergistic action and interplay of hydrogen embrittlement mechanisms in steels and iron: Localized plasticity and decohesion. Eng. Fract. Mech. 2019, 216, 106528. [Google Scholar] [CrossRef]
- Sobola, D.; Dallaev, R. Exploring hydrogen embrittlement: Mechanisms, consequences, and advances in metal science. Energies 2024, 17, 2972. [Google Scholar] [CrossRef]
- ISO 17081:2014(E); Method of Measurement of Hydrogen Permeation and Determination of Hydrogen Uptake and Transport in Metals by an Electrochemical Technique. International Organization for Standardization: Geneva, Switzerland, 2014.
- Yanachkov, B.; Mourdjeva, Y.; Valuiska, K.; Dyakova, V.; Kolev, K.; Kaleicheva, J.; Lazarova, R.; Katzarov, I. Effect of Hydrogen Content on the Microstructure, Mechanical Properties, and Fracture Mechanism of Low-Carbon Lath Martensite Steel. Metals 2024, 14, 1340. [Google Scholar] [CrossRef]
- Yanachkov, B.; Lyutov, L.; Katzarov, I.; Drenchev, L.; Kolev, K. Effect of Microstructure on the Mechanical Response of Hydrogen-Charged Pure Iron. Metals 2022, 12, 2160. [Google Scholar] [CrossRef]
- Oh, D.K.; Jeong, M.S.; Shin, S.H.; Hwang, B. Effect of Hydrogen on Tensile Properties and Fracture Behavior of Two High-Strength American Petroleum Institute Linepipe Steels. Metals 2024, 14, 1397. [Google Scholar] [CrossRef]
- Pinson, M.; Springer, H.; Depover, T.; Verbeken, K. The effect of quench cracks and retained austenite on the hydrogen trapping capacity of high carbon martensitic steels. Int. J. Hydrogen Energy 2021, 46, 16141–16152. [Google Scholar] [CrossRef]
- Nykyforchyn, H.; Zvirko, O.; Dzioba, I.; Krechkovska, H.; Hredil, M.; Tsyrulnyk, O.; Student, O.; Lipiec, S.; Pala, R. Assessment of operational degradation of pipeline steels. Materials 2021, 14, 3247. [Google Scholar] [CrossRef] [PubMed]
- Katzarov, I.H.; Paxton, A.T. Hydrogen embrittlement II. Analysis of hydrogen-enhanced decohesion across (111) planes in α-Fe. Phys. Rev. Mater. 2017, 1, 033603. [Google Scholar] [CrossRef]
- Zhou, X.; Foster, M.E.; Ronevich, J.A.; San Marchi, C.W. Review and construction of interatomic potentials for molecular dynamics studies of hydrogen embrittlement in Fe-C based steels. J. Comput. Chem. 2020, 41, 1299–1309. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.P.; Aktulga, H.M.; Berger, R.; Bolintineanu, D.S.; Brown, W.M.; Crozier, P.S.; In’t Veld, P.J.; Kohlmeyer, A.; Moore, S.G.; Nguyen, T.D.; et al. LAMMPS-a flexible simulation tool for particle-based materials modeling at the atomic, meso, and continuum scales. Comput. Phys. Commun. 2022, 271, 108171. [Google Scholar] [CrossRef]
- Nagano, M.; Hayashi, Y.; Ohtani, N.; Isshiki, M.; Igaki, K. Hydrogen diffusivity in high purity alpha iron. Scr. Metall. 1982, 16, 973–976. [Google Scholar] [CrossRef]
- Niu, R.; Li, H.; Liu, P.Y.; Burr, P.; Feng, Y.; Yen, H.W.; Huang, C.; Sun, Y.H.; Ma, M.; Guo, A.; et al. Hydrogen-enhanced deformation in pearlite. Acta Mater. 2024, 281, 120327. [Google Scholar] [CrossRef]
- Song, Y.; Han, Z.; Chai, M.; Yang, B.; Liu, Y.; Cheng, G.; Li, Y.; Ai, S. Effect of cementite on the hydrogen diffusion/trap characteristics of 2.25 Cr-1Mo-0.25 V steel with and without annealing. Materials 2018, 11, 788. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, Y.; Hino, M.; Nakamura, M.; Matsunaga, H. Pearlite-driven surface-cracking and associated loss of tensile ductility in plain-carbon steels under exposure to high-pressure gaseous hydrogen. Int. J. Hydrogen Energy 2021, 46, 6945–6959. [Google Scholar] [CrossRef]
- Jia, Y.; Ren, C.; Wu, S.; Mu, Y.; Xu, L.; Jia, Y.; Yan, W.; Yi, J.; Wang, G. Multistage strain-hardening behavior of ultrastrong and ductile lightweight refractory complex-concentrated alloys. J. Mater. Sci. Technol. 2023, 149, 73–87. [Google Scholar] [CrossRef]
- Du, X.; Zhou, L.; Li, J.; Xie, Z.; Li, S.; Xiao, H.; Li, M.; Zhao, Y. Achieving high strength and low yield ratio by constructing the network martensite-ferrite heterogeneous in low carbon steels. Mater. Sci. Eng. A 2025, 920, 147526. [Google Scholar] [CrossRef]
- Gong, P.; Katzarov, I.H.; Nutter, J.; Paxton, A.T.; Rainforth, W.M. The influence of hydrogen on plasticity in pure iron-theory and experiment. Sci. Rep. 2020, 10, 10209. [Google Scholar] [CrossRef]
- Fitzgerald, S.; Aubry, S.; Dudarev, S.; Cai, W. Dislocation dynamics simulation of Frank-Read sources in anisotropic α-Fe. Model. Simul. Mater. Sci. Eng. 2012, 20, 045022. [Google Scholar] [CrossRef]
- Zheng, Z.; Liang, S.; Huang, M.; Zhao, L.; Zhu, Y.; Li, Z. Studying the effects of hydrogen on dislocation mobility and multiplication in nickel by phase-field method. Mech. Mater. 2022, 173, 104443. [Google Scholar] [CrossRef]
- Caillard, D.; Legros, M.; Couret, A. Extrinsic obstacles and loop formation in deformed metals and alloys. Philos. Mag. 2013, 93, 203–221. [Google Scholar] [CrossRef]



| Element | C | Si | Mn | S | P | Cr | Cu | Ni |
|---|---|---|---|---|---|---|---|---|
| Wt. % | 0.154 | 0.48 | 1.30 | 0.016 | 0.014 | 0.02 | 0.07 | 0.03 |
| Specimen No. | Hydrogen Charging Conditions (Electrolyte, Current Density, Charging Time) | Hydrogen Concentration, [wppm] |
|---|---|---|
| H*-0 | without hydrogen charging | 1.13 ± 0.20 |
| H*-1 | 0.5 M H2SO4, 5 mA/, 1 h | 4.04 ± 0.13 |
| H*-24 | 0.5 M H2SO4, 5 mA/, 24 h | 4.01 ± 0.67 |
| H*-72 | 0.5 M H2SO4, 5 mA/, 72 h | 3.85 ± 0.61 |
| Specimen No. | , [MPa] | , [%] | , [%] |
|---|---|---|---|
| H-0 | 540 | 12.9 | 18.9 |
| H-1 | 554 | 8.1 | 9.7 |
| H-24 | 552 | 8.2 | 9.6 |
| H-72 | 560 | 8.7 | 10.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dyakova, V.; Yanachkov, B.; Valuiska, K.; Mourdjeva, Y.; Krastev, R.; Simeonova, T.; Kolev, K.; Lazarova, R.; Katzarov, I. The Impact of Hydrogen Charging Time on Microstructural Alterations in Pipeline Low-Carbon Ferrite–Pearlite Steel. Metals 2025, 15, 1079. https://doi.org/10.3390/met15101079
Dyakova V, Yanachkov B, Valuiska K, Mourdjeva Y, Krastev R, Simeonova T, Kolev K, Lazarova R, Katzarov I. The Impact of Hydrogen Charging Time on Microstructural Alterations in Pipeline Low-Carbon Ferrite–Pearlite Steel. Metals. 2025; 15(10):1079. https://doi.org/10.3390/met15101079
Chicago/Turabian StyleDyakova, Vanya, Boris Yanachkov, Kateryna Valuiska, Yana Mourdjeva, Rumen Krastev, Tatiana Simeonova, Krasimir Kolev, Rumyana Lazarova, and Ivaylo Katzarov. 2025. "The Impact of Hydrogen Charging Time on Microstructural Alterations in Pipeline Low-Carbon Ferrite–Pearlite Steel" Metals 15, no. 10: 1079. https://doi.org/10.3390/met15101079
APA StyleDyakova, V., Yanachkov, B., Valuiska, K., Mourdjeva, Y., Krastev, R., Simeonova, T., Kolev, K., Lazarova, R., & Katzarov, I. (2025). The Impact of Hydrogen Charging Time on Microstructural Alterations in Pipeline Low-Carbon Ferrite–Pearlite Steel. Metals, 15(10), 1079. https://doi.org/10.3390/met15101079







