Controlled Formation of Nanoislands During Microwave Annealing of Au Thin Films
Abstract
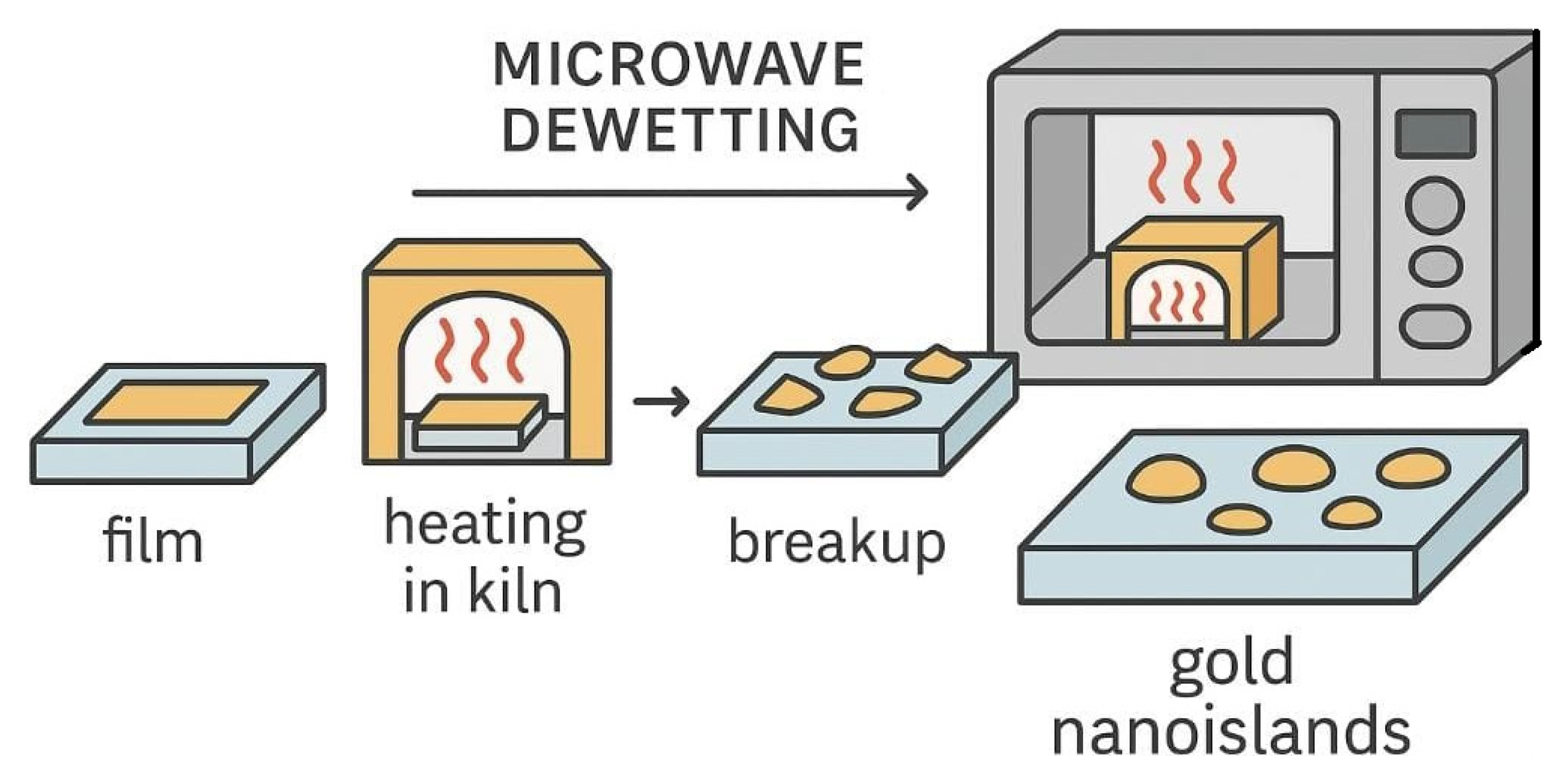
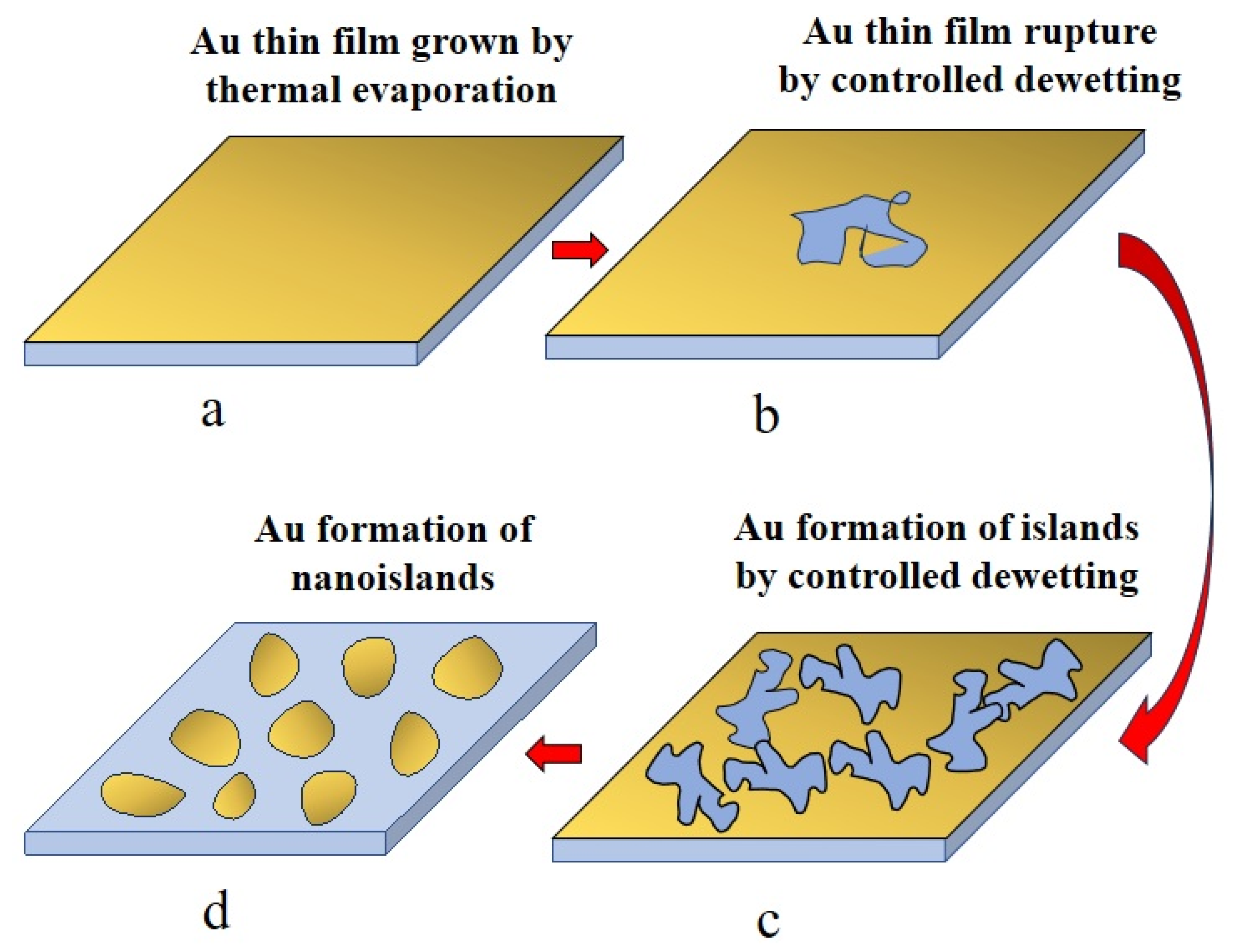
1. Introduction
2. Materials and Methods
3. Results and Discussion
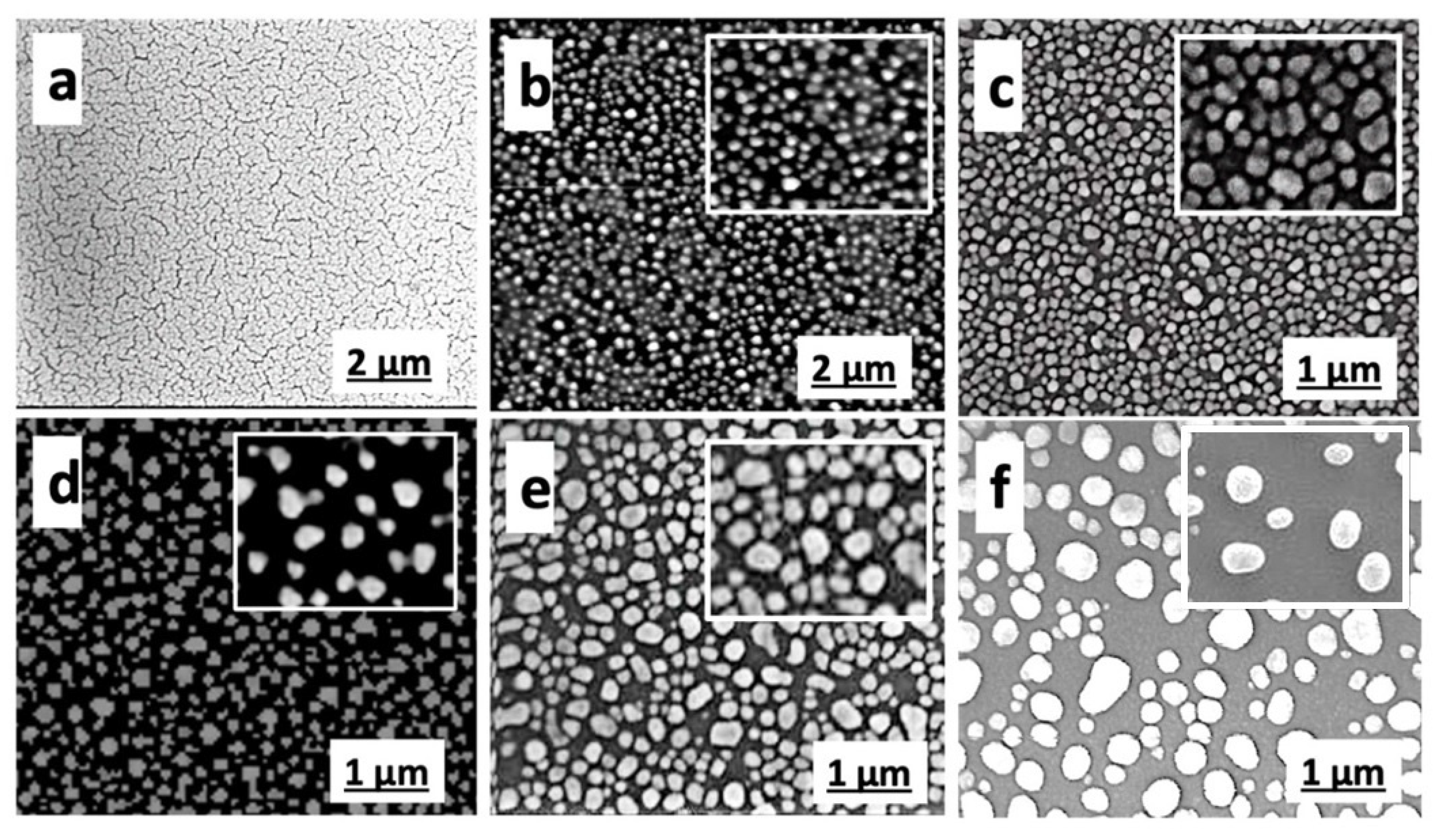
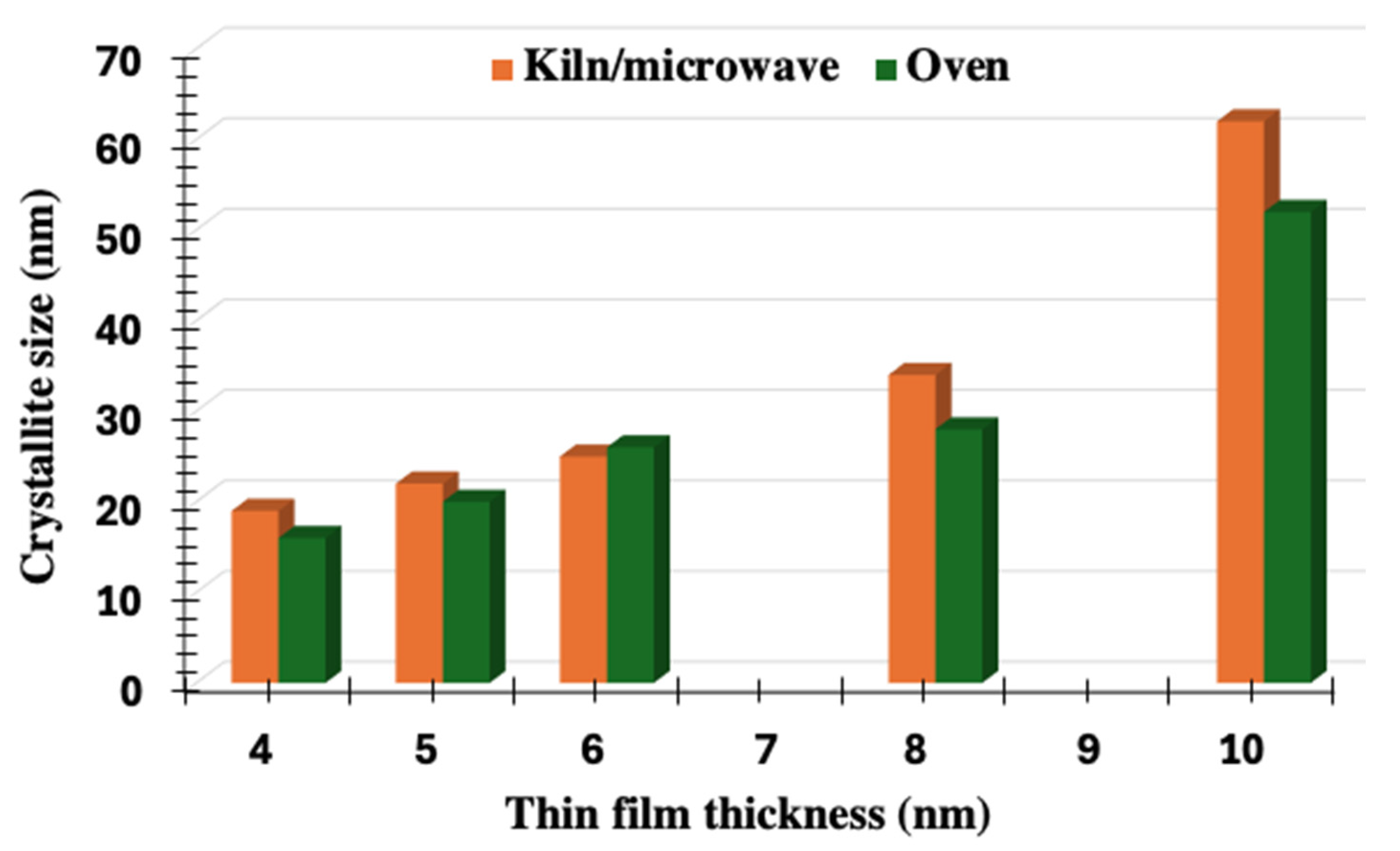
3.1. Morphological and Structural Analysis: SEM, AFM, and XRD
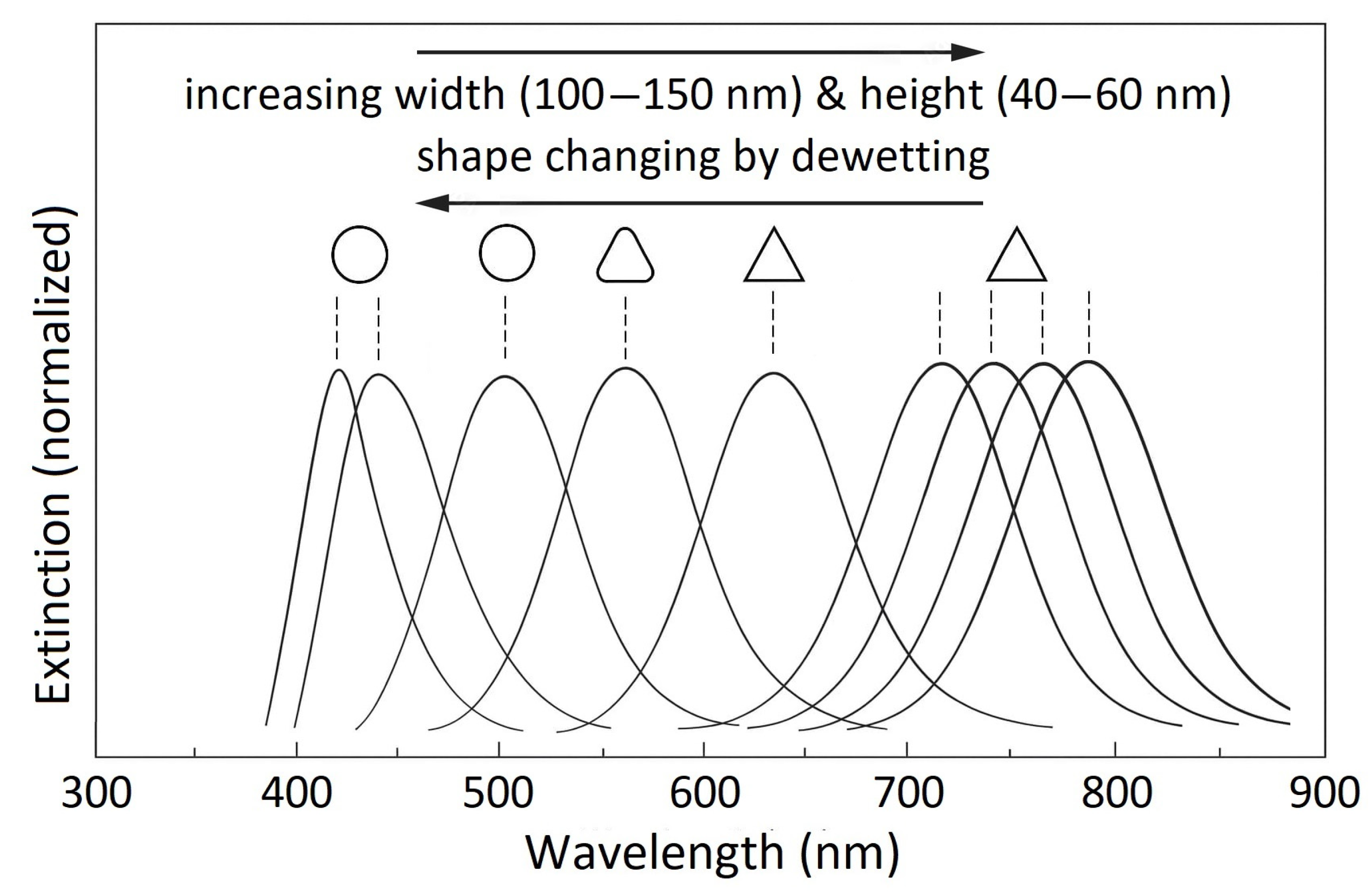
3.2. Optical Properties of the Nanoislands
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schmidbaur, H.; Cihonski, J.L. Noble Metals (Chemistry). In Encyclopedia of Physical Science and Technology, 3rd ed.; Academic Press: Cambridge, MA, USA, 2003; pp. 463–492. [Google Scholar] [CrossRef]
- Bernardo-Gavito, R.; Serrano, A.; García, M.A.; Miranda, R.; Granados, D. Local characterization of the optical properties of annealed Au films on glass substrates. J. Appl. Phys. 2013, 114, 164312. [Google Scholar] [CrossRef]
- Piwnuan, C.; Muangphat, C.; Wootthikanokkhan, J. Enhancing NIR Shielding Properties of Au/CsWO3 Composite via Physical Mixing and Solvothermal Processes. Materials 2024, 17, 2746. [Google Scholar] [CrossRef] [PubMed]
- Freestone, I.; Meeks, N.; Sax, M.; Higgitt, C. The Lycurgus Cup—A Roman nanotechnology. Gold Bull. 2007, 40, 270–277. [Google Scholar] [CrossRef]
- Hammami, I.; Alabdallah, N.M.; Al-Jomaa, A.; Kamoun, M. Gold nanoparticles: Synthesis properties and applications. J. King Saud Univ.-Sci. 2021, 33, 101560. [Google Scholar] [CrossRef]
- Oddy, W.A. Gilding: An outline of the technological history of the plating of gold on to silver or copper in the Old World. Endeavour 1991, 15, 29–33. [Google Scholar] [CrossRef]
- Hanawa, T. Overview of metals and applications. In Metals for Biomedical Devices; Niinomi, M., Ed.; Woodhead Publishing Limited: Cambridge, UK, 2010; ISBN 978-1-84569-434-0. [Google Scholar] [CrossRef]
- Wang, H.H.; Su, C.H.; Wu, Y.J.; Lin, C.A.J.; Lee, C.H.; Shen, J.L.; Chan, W.H.; Chang, W.H.; Yeh, H.I. Application of Gold in Biomedicine: Past, Present and Future. Int. J. Gerontol. 2012, 6, 1–4. [Google Scholar] [CrossRef]
- Balfourier, A.; Kolosnjaj-Tabi, J.; Luciani, N.; Gazeau, F. Gold-based therapy: From past to present. Proc. Natl. Acad. Sci. USA 2020, 117, 22639–22648. [Google Scholar] [CrossRef]
- Schatz, G.C.; Van Duyne, R.P. Electromagnetic Mechanism of Surface-Enhanced Spectroscopy; John Wiley & Sons, Ltd.: New York, NY, USA, 2002. [Google Scholar] [CrossRef]
- Badilescu, S.; Raju, D.; Bathini, S.; Packirisamy, M. Gold Nano-Island Platforms for Localized Surface Plasmon Resonance Sensing: A Short Review. Molecules 2020, 25, 4661. [Google Scholar] [CrossRef]
- Madou, M. Fundamentals of Microfabrication: The Science of Miniaturization, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2002; Available online: https://books.google.co.in/books?id=9bk3gJeQKBYC (accessed on 24 May 2025).
- Kim, T.J.; Jung, Y.H.; Zhang, H.; Kim, K.; Lee, J.; Ma, Z. Photolithography-Based Nanopatterning Using Re-entrant Photoresist Profile. ACS Appl. Mater. Interfaces 2018, 10, 8117–8123. [Google Scholar] [CrossRef]
- Qin, D.; Xia, Y.; Whitesides, G.M. Soft lithography for micro- and nanoscale patterning. Nat. Protoc. 2010, 5, 491–502. [Google Scholar] [CrossRef]
- Alasadi, A.; Claeyssens, F.; Allwood, D.A. Rapid Fabrication of 2-D Magnetic Microstructures by Laser Direct Writing (LDW). IEEE Trans. Magn. 2021, 57, 1–6. [Google Scholar] [CrossRef]
- Haes, A.J.; Haynes, C.L.; Van Duyne, R.P. Nanosphere Lithography: Self-Assembled Photonic and Magnetic Materials. MRS Online Proc. Libr. 2000, 636, 481. [Google Scholar] [CrossRef]
- Bercea, A.I. Periodic Multi-Scale Structuring of Surfaces by Self-Assembly of Colloidal Masks: Shape-Size Coupling Effects. Ph.D. Thesis, Université de Limoges, Limoges, France, 2021. Available online: https://theses.fr/2021LIMO0103 (accessed on 24 May 2025).
- Jambhulkar, S.; Ravichandran, D.; Zhu, Y.; Thippanna, V.; Ramanathan, A.; Patil, D.; Fonseca, N.; Thummalapalli, S.V.; Sundaravadivelan, B.; Sun, A.; et al. Nanoparticle Assembly: From Self-Organization to Controlled Micropatterning for Enhanced Functionalities. Small 2024, 20, e2306394. [Google Scholar] [CrossRef]
- Schaaf, P.; Constantinescu, C.; Matei, A. Preface on laser material interactions: From basic science to industrial applications (LaserMaterInter2020). Appl. Surf. Sci. Adv. 2021, 6, 100133. [Google Scholar] [CrossRef]
- Constantinescu, C.; Kallepalli, L.N.D.; Delaporte, P.; Utéza, O.; Grojo, D. Laser processing of metal thin films using transparent microsphere arrays. Appl. Surf. Sci. 2015, 336, 112–117. [Google Scholar] [CrossRef]
- Schaaf, P.; Constantinescu, C.; Matei, A. Laser material processing: From fundamental interactions to innovative applications (E-MRS). Appl. Surf. Sci. Adv. 2024, 21, 100592. [Google Scholar] [CrossRef]
- Nedyalkov, N.; Nikov, R.; Koleva, M.; Atanasov, P.A.; Constantinescu, C.; Delaporte, P.; Grojo, D. Nanoparticle-decorated ceramic as substrate in surface enhanced Raman spectroscopy. Appl. Surf. Sci. 2015, 336, 16–20. [Google Scholar] [CrossRef]
- Constantinescu, C.; Kallepalli, L.N.D.; Delaporte, P.; Utéza, O.; Grojo, D. Arrays of metallic micro-/nano-structures by means of colloidal lithography and laser dewetting. Appl. Surf. Sci. 2016, 374, 124–131. [Google Scholar] [CrossRef]
- Yang, L.; Wei, J.; Ma, Z.; Song, P.; Ma, J.; Zhao, Y.; Huang, Z.; Zhang, M.; Yang, F.; Wang, X. The Fabrication of Micro/Nano Structures by Laser Machining. Nanomaterials 2019, 9, 1789. [Google Scholar] [CrossRef]
- Itina, T.E. Understanding mono- and bi-metallic Au and Ni nanoparticle responses to fast heating. Nanoscale Adv. 2024, 6, 5451–5463. [Google Scholar] [CrossRef] [PubMed]
- Mølhave, K.; Madsen, D.N.; Rasmussen, A.M.; Carlsson, A.; Appel, C.C.; Brorson, M.; Bøggild, P. Solid gold nanostructures fabricated by electron beam deposition. Nano Lett. 2003, 3, 1499–1503. [Google Scholar] [CrossRef]
- Theerthagiri, J.; Lee, S.J.; Karuppasamy, K.; Park, J.; Yu, Y.; Kumari, M.A.; Choi, M.Y. Fabrication strategies and surface tuning of hierarchical gold nanostructures for electrochemical detection and removal of toxic pollutants. J. Hazard. Mater. 2021, 420, 126648. [Google Scholar] [CrossRef]
- Losic, D.; Mitchell, J.G.; Voelcker, N.H. Fabrication of gold nanostructures by templating from porous diatom frustules. New J. Chem. 2006, 30, 908–914. [Google Scholar] [CrossRef]
- Hsu, M.-S.; Chen, Y.-L.; Lee, C.-Y.; Chiu, H.-T. Gold nanostructures on flexible substrates as electrochemical dopamine sensors. ACS Appl. Mater. Interfaces 2012, 4, 5570–5575. [Google Scholar] [CrossRef]
- Kim, J.-H.; Kang, T.; Yoo, S.M.; Lee, S.Y.; Kim, B.; Choi, Y.-K. A well-ordered flower-like gold nanostructure for integrated sensors via surface-enhanced Raman scattering. Nanotechnology 2009, 20, 235302. [Google Scholar] [CrossRef]
- Xue, M.; Ma, X.; Xie, Z.; Duan, L.; Jiang, Y.; Zhang, M.; Cao, T. Fabrication of Gold-Directed Conducting Polymer Nanoarrays for High-Performance Gas Sensor. Chem.-Asian J. 2010, 5, 2266–2270. [Google Scholar] [CrossRef]
- Al-Rubaye, A.G.G. Development of Optical Biosensors Based on Metal Nanostructures for Pollution (Mycotoxins) Detection. Ph.D. Thesis, Sheffield Hallam University, Sheffield, UK, 2019. [Google Scholar] [CrossRef]
- Jin, L.; Liu, B.; Louis, M.E.; Li, G.; He, J. Highly crystalline mesoporous titania loaded with monodispersed gold nanoparticles: Controllable metal–support interaction in porous materials. ACS Appl. Mater. Interfaces 2020, 12, 9617–9627. [Google Scholar] [CrossRef] [PubMed]
- Karakouz, T.; Tesler, A.B.; Bendikov, T.A.; Vaskevich, A.; Rubinstein, I. Highly stable localized plasmon transducers obtained by thermal embedding of gold island films on glass. Adv. Mater. 2008, 20, 3893–3899. [Google Scholar] [CrossRef]
- Vigil, E.; Saadoun, L.; Ayllón, J.A.; Domènech, X.; Zumeta, I.; Rodríguez-Clemente, R. TiO2 thin film deposition from solution using microwave heating. Thin Solid Film. 2000, 365, 12–18. [Google Scholar] [CrossRef]
- Jun, T.; Song, K.; Jeong, Y.; Woo, K.; Kim, D.; Bae, C.; Moon, J. High-performance low-temperature solution-processable ZnO thin film transistors by microwave-assisted annealing. J. Mater. Chem. 2010, 21, 1102–1108. [Google Scholar] [CrossRef]
- Al-Gaashani, R.; Radiman, S.; Tabet, N.; Daud, A.R. Effect of microwave power on the morphology and optical property of zinc oxide nano-structures prepared via a microwave-assisted aqueous solution method. Mater. Chem. Phys. 2011, 125, 846–852. [Google Scholar] [CrossRef]
- Bertrand, E.; Blake, T.; De Coninck, J. Dynamics of dewetting. Colloids Surf. A Physicochem. Eng. Asp. 2010, 369, 141–147. [Google Scholar] [CrossRef]
- Hsu, C.-Y.; Huang, J.-W.; Gwo, S.; Lin, K.-J. The facile fabrication of tunable plasmonic gold nanostructure arrays using microwave plasma. Nanotechnology 2009, 21, 35302. [Google Scholar] [CrossRef]
- Tesler, A.B.; Chuntonov, L.; Karakouz, T.; Bendikov, T.A.; Haran, G.; Vaskevich, A.; Rubinstein, I. Tunable localized plasmon transducers prepared by thermal dewetting of percolated evaporated gold films. J. Phys. Chem. C 2011, 115, 24642–24652. [Google Scholar] [CrossRef]
- Al-Rubaye, A.G.; Nabok, A.; Tsargorodska, A. LSPR Biosensor Based on Nanostructured Gold Films: Detection of Mycotoxins. Procedia Technol. 2017, 27, 131–132. [Google Scholar] [CrossRef]
- Al-Rubaye, A.G.; Nabok, A.; Tsargorodska, A. Spectroscopic ellipsometry study of gold nanostructures for LSPR bio-sensing applications. Sens. Bio-Sens. Res. 2017, 12, 30–35. [Google Scholar] [CrossRef]
- Tsuji, M.; Hashimoto, M.; Nishizawa, Y.; Kubokawa, M.; Tsuji, T. Microwave-assisted synthesis of metallic nanostructures in solution. Chem.-A Eur. J. 2005, 11, 440–452. [Google Scholar] [CrossRef] [PubMed]
- Tesler, A.B.; Maoz, B.M.; Feldman, Y.; Vaskevich, A.; Rubinstein, I. Solid-state thermal dewetting of just-percolated gold films evaporated on glass: Development of the morphology and optical properties. J. Phys. Chem. C 2013, 117, 11337–11346. [Google Scholar] [CrossRef]
- Thompson, C.V. Solid-State Dewetting of Thin Films. Annu. Rev. Mater. Res. 2012, 42, 399–434. [Google Scholar] [CrossRef]
- Sundaresan, S.; Liu, X.; Campbell, R.; Caride, B.; Look, D.C.; Myers-Ward, R.L.; Gaskill, D.K.; Hite, J.; Eddy, C.; Caldwell, J.D.; et al. Comparison of Solid-State Microwave Annealing with Conventional Furnace Annealing of Ion-Implanted SiC. J. Appl. Phys. 2021, 129, 135701. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Rubaye, A.G.G.; Alasadi, A.; Muhammed, K.R.; Constantinescu, C.-D. Controlled Formation of Nanoislands During Microwave Annealing of Au Thin Films. Metals 2025, 15, 1030. https://doi.org/10.3390/met15091030
Al-Rubaye AGG, Alasadi A, Muhammed KR, Constantinescu C-D. Controlled Formation of Nanoislands During Microwave Annealing of Au Thin Films. Metals. 2025; 15(9):1030. https://doi.org/10.3390/met15091030
Chicago/Turabian StyleAl-Rubaye, Ali Ghanim Gatea, Alaa Alasadi, Khalid Rmaydh Muhammed, and Catalin-Daniel Constantinescu. 2025. "Controlled Formation of Nanoislands During Microwave Annealing of Au Thin Films" Metals 15, no. 9: 1030. https://doi.org/10.3390/met15091030
APA StyleAl-Rubaye, A. G. G., Alasadi, A., Muhammed, K. R., & Constantinescu, C.-D. (2025). Controlled Formation of Nanoislands During Microwave Annealing of Au Thin Films. Metals, 15(9), 1030. https://doi.org/10.3390/met15091030









