Abstract
MnFe2O4/Fe soft magnetic composites (SMCs) were designed by the surface oxidation method, and the MnFe2O4 layer was utilized as the insulation coating. The microstructure of SMCs and the chemical composition of the insulation layer were observed using scanning electron microscopy and energy-dispersive spectroscopy. The surface phase composition of SMCs was characterized using X-ray diffraction, X-ray photoelectron spectrometry, and Raman spectroscopy. The effect of annealing temperature on the insulation layer was investigated, and its relationship with the magnetic properties of the MnFe2O4/Fe SMCs was explored. The best overall performances were obtained at 50 mT and 100 kHz with saturation magnetization Ms = 205 emu/g, amplitude permeability μa = 100, and a core loss of 234.9 W/kg. Therefore, this work can provide a method to develop a novel insulating coating to reduce core loss, which is of great significance to the investigation of other Fe-based soft magnetic composites for applications in high-frequency magnetic fields.
1. Introduction
Soft magnetic composites (SMCs) are a new type of soft magnetic material made by insulating and coating the surface of metal in soft magnetic powder with a high saturation magnetic induction intensity using high-resistivity organic or inorganic compounds, and pressing the coated powder using powder metallurgy technology [1]. SMCs are widely used in transformers, relay electronic devices, and measuring instruments due to their high saturation magnetization, high electrical resistivity, low core loss, and three-dimensional isotropic magnetic properties [2,3,4,5,6,7,8]. Therefore, SMCs are attracting increasing attention from researchers due to these excellent electromagnetic properties [9,10,11,12,13,14].
Eddy current loss is the main component of core loss in SMCs. For SMCs, the most effective and convenient method to reduce eddy current loss is to insulate the surface of the powder particles. Usually, insulation coating is mainly divided into organic, inorganic insulating, and ferromagnetic materials. Organic compounds include phenolic resin [15], epoxy resin [16], and silicone resin [17]. Generally, organic matter is dissolved in an organic solvent, then magnetic powder is added and stirred evenly. Finally, the solvent is evaporated to allow the organic matter to coat the surface of the magnetic powder. Although organic resins have good adhesion with magnetic powders, their poor thermal stability limits their application. Inorganic insulating coating materials, possessing high thermal stability and high resistivity, among other characteristics, have become a focal point in recent research on insulating coatings. Yu et al. [18] prepared an Al2O3 insulation layer coating on the surface of FeSiCr powder using the sol–gel method and investigated the effect of the insulation layer on the magnetic properties. Peng et al. [19] investigated the effect of calcination temperature on the microstructure and magnetic properties of FeNiMo@Al2O3 prepared by the sol–gel method. Lee et al. [20] utilized MgO as an insulating buffer layer to enhance the thermal stability of Fe-Ni soft magnetic composites and investigated the effect of high-temperature heat treatment on those soft magnetic properties. Han et al. [21] utilized SiO2 as an insulating layer to fabricate FeSiCr@SiO2 soft magnetic composites and investigated their magnetic properties. Inorganic oxides, such as oxides (e.g., ZrO2, AlN, Al2O3, Cr2O3, TiO2, SiO2, and MgO) [22,23,24,25,26,27,28,29,30], are often non-ferromagnetic, causing magnetic dilution, which reduces the permeability and saturation magnetic flux density of the core, making it disadvantageous for achieving miniaturization of devices. As a magnetic material (e.g., Fe3O4, NiZnFe2O4, MgFe2O4, CoFe2O4, ZnFe2O4, and MnZnFe2O4) [31,32,33,34,35,36], ferrite has high resistivity and low eddy current loss, making it an ideal insulation coating for SMCs without significantly reducing magnetic performance.
In this work, a uniform MnFe2O4 layer was carefully fabricated on the surface of Fe powder using the surface oxidation method. The coating is produced through a direct chemical reaction with Fe, which prevents the detachment of the insulation layer during the subsequent compression molding process and achieves the goal of reducing core loss. By controlling the concentration of ammonia solution, the MnFe2O4 insulating layer thickness was adjusted. The microstructure and magnetic properties of SMCs with different thicknesses of MnFe2O4 insulation layers were investigated. The results show that the MnFe2O4/ESR organic–inorganic composite insulation coating effectively reduced the core loss of SMCs. This study provides a feasible solution with simple operation and low cost for preparing low-core-loss SMCs.
2. Experiments
The MnFe2O4 insulating layer was coated with Fe powder through the surface oxidation method. The water-atomized iron powder used in this paper comes from Heganas (China) Co., Ltd. (Shanghai, China), with an average particle size of about 57 µm. Its specific composition is 99.946 wt% iron, 0.004 wt% carbon, and 0.050 wt% oxygen. A 100 g amount of Fe powder was mixed with 300 mL of different concentrations of NH3·H2O (1.5, 2.5, and 3.5 wt%) with the addition of MnCl2·4H2O (1.98 g and 0.01 mol). The mixture was placed into a 500 mL autoclave and heated at 180 °C for 1 h to complete the surface oxidation reaction. The obtained powder was washed with deionized water and ethanol three times prior to mixing with an epoxy-modified silicone resin (ESR) (2 wt%) dissolved in dimethyl benzene. The mixture was stirred at 70 °C until complete evaporation of the solution occurred. The toroidal powder cores with an outer diameter of 26.1 mm, an inner diameter of 18.1 mm, and a thickness of 5.0 mm were fabricated by a cold pressure of 800 MPa at room temperature. Then, the compacted cores were annealed at various temperatures (500–600 °C) for 1 h in an Ar atmosphere to reduce the internal stress caused by pressing.
The surface morphology and composition of the oxidized Fe powders and annealed SMC samples were detected by scanning electron microscopy (SEM) coupled with energy-dispersive X-ray spectroscopy (EDS; Hitachi SU8010, Tokyo, Japan). The identification of the phases in the Fe powders and the oxidized powders was performed by X-ray diffraction (XRD; Bruck Advance D8, Berlin, Gremany) using Cu Kα radiation. The oxidation state of the surface insulation layer of the SMC powders was analyzed by X-ray photoelectron spectrometry (XPS; ESCALAB250Xi, Thermo Fisher Scientific, Waltham, MA, USA) and further analyzed using Fourier-transform infrared (FT-IR) spectroscopy (Bruker VERTEX 70, Billerica, MA, USA) over a frequency range of 400 to 4000 cm−1. Raman measurement was performed in a LABRAM Aramis Raman microscopy system from Jobin Yvon France (Palaiseau, France) in the range from 100 to 1000 cm−1 with a 532 nm laser. The thermal analysis was performed by thermogravimetry and differential scanning calorimetry (TG-DSC, NETZSCH STA 449C, Selb, Germany), in which the samples were heated from room temperature to 700 °C under an Ar atmosphere in alumina crucibles at a rate of 10 °C/min. Magnetic hysteresis was achieved in a vibrating sample magnetometer (VSM; Lake-shore 7400-s, Westerville, OH, USA) in powder form at room temperature within the field range of ±20,000 Oe. The amplitude permeability and core loss of the SMCs were measured with a maximum applied induction of 50 mT over the frequency range from 10 to 170 kHz by an auto testing system for magnetic materials (Iwatsu SY8258B-H/m, Tokyo, Japan).
3. Results and Discussion
Figure 1 shows the SEM morphologies and EDS elemental analysis of the oxidized Fe powders. The surface morphology of oxidized iron powder particles under SEM is rough, and the EDS selective element analysis was performed on the surface oxidation layer, as shown in Figure 1a. The results indicate that the coating layer consists of manganese, iron, and oxygen elements; therefore, it can be inferred that the chemical composition of the insulation layer is manganese ferrite (MnFe2O4). Figure 1b,c show the cross-sectional morphology of the oxidized iron powder after embedding and polishing, indicating that the particle surface was coated with a thin insulating layer. The EDS elemental analysis was conducted on both the interior and surface of the particles, and the comparative test results showed that only iron was present inside the particles, while manganese, oxygen, and iron were present on the surface of the particles. This further indicates that the surface of the oxidized iron powder was insulated with MnFe2O4.
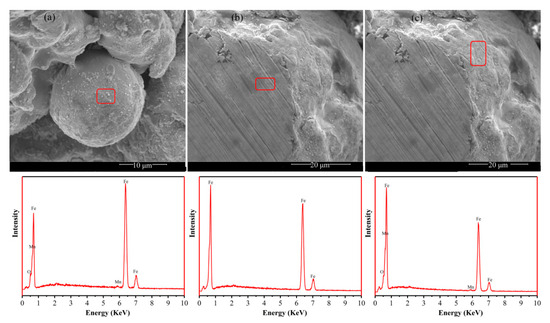
Figure 1.
(a) SEM image of oxidized iron powder particles; (b,c) show SEM images of cross-sections of oxidized iron powder particles. The figures below are EDS element analysis maps of the corresponding red areas.
To further determine the uniformity and integrity of the insulation coating, EDS surface scanning elemental analysis was performed on the surface of the oxidized iron powder particles, and the results are shown in Figure 2. The iron, manganese, and oxygen elements in the coating layer are uniformly distributed on the surface of the particles, which further confirms that the chemical composition of the insulating coating layer is MnFe2O4. The uniform and complete coating layer guarantees the amplitude permeability of SMCs, which have frequency stability and low eddy current losses at high frequencies.
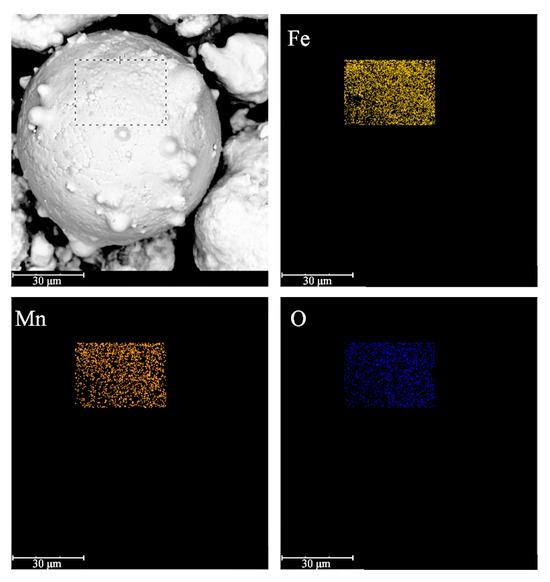
Figure 2.
The SEM and EDS elemental distribution maps of the dotted box for the oxidized Fe powders.
XPS analysis was conducted on iron powder oxidized by 2.5 wt% ammonia solution to further confirm the chemical composition of the insulation layer. The Mn 2p3/2 peak is located at 641.5 eV, as shown in Figure 3, indicating the presence of divalent Mn2+ ions on the surface of the oxidized iron powder [37]. The two peaks corresponding to Fe 2p1/2 and Fe 2p3/2 are shown in the XPS Fe 2p spectrum, located at around 724.6 eV and 711.0 eV, respectively. It is worth noting a weak satellite peak at approximately 719.1 eV, which was assigned to Fe in the 3+ oxidation state [38]. The survey spectrum of oxidized powder is shown in Figure 3c, which indicates the insulating layer comprising MnFe2O4.
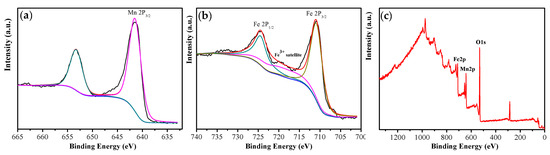
Figure 3.
XPS spectra taken from the Fe powders oxidized by 2.5 wt% NH3·H2O: (a) Mn 2p, (b) Fe 2p, and (c) survey spectrum.
To analyze the effect of ammonia concentration on the insulation coating, the XPS measurements were conducted on iron powder oxidized with different ammonia concentrations, and the results are shown in Figure 4. Comparing the intensity of the satellite peak of 719.1 eV under different concentrations of ammonia, it was found that the intensity of satellite peaks slightly increased with an increase in ammonia water concentration, indicating that the content of Fe3+ in the surface insulation layer increased with an increase in ammonia concentration, and therefore, the thickness of the insulation coating layer gradually increased. No Fe 2p3/2 peak corresponding to metallic Fe0 was observed in the 706.6 eV region of all samples, indicating that the insulation layer was completely coated on the surface of the iron powder.
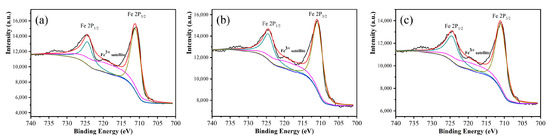
Figure 4.
XPS spectra of the Fe 2p peaks taken from the Fe powders under different reaction concentrations: (a) 1.5 wt%, (b) 2.5 wt%, and (c) 3.5 wt%.
XRD measurements were carried out on raw iron powder and iron powder oxidized with different concentrations of ammonia to analyze the chemical composition of the insulation coating layer, and the results are shown in Figure 5. According to JCPDS Card No. 87-0721, the diffraction peaks at 2θ values of ca. 44.60°, 64.98°, and 82.26° were from the (110), (200), and (211) planes of the α-Fe phase. It is worth noting that no peak corresponding to the oxide was observed; only the peak originating from the raw material powder was detected. This result can be explained by the amount of oxide phase being too low to be detected by XRD.
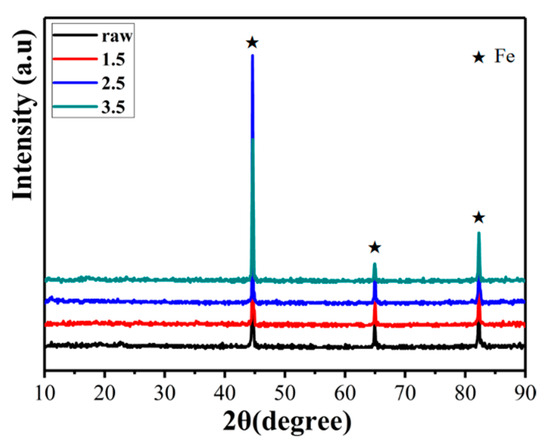
Figure 5.
XRD data for the raw powders and those with different reaction concentrations.
FT-IR spectroscopy of oxidized iron powder was performed in the 4000–400 cm−1 range, as shown in Figure 6. The peaks at 3436, 1633, and 1380 cm−1 correspond to the -OH stretching vibration peaks, which are caused by the adsorption of water from the air on the sample surface. The peaks displayed at 1061 and 572 cm−1 correspond to the Fe-O vibrational stretching peak [39], while the peak at 478 cm−1 corresponds to the Mn-O bond. The test results indicate the presence of Mn-O and Fe-O bonds on the surface of the sample after insulation coating, which further demonstrates that the chemical composition of the insulation layer is MnFe2O4.
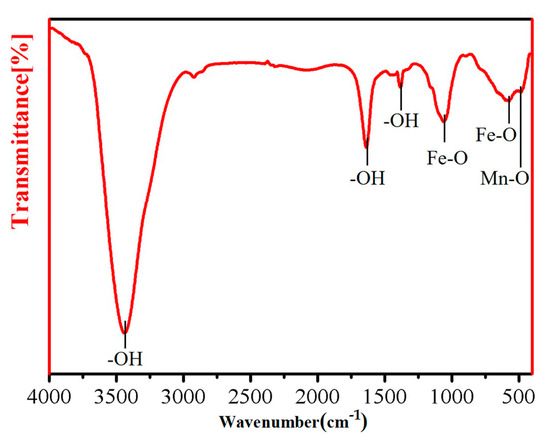
Figure 6.
FT-IR spectrum of the oxidized Fe powders.
The Raman spectra of oxidized iron powder are shown in Figure 7. The sharp characteristic peaks of MnFe2O4 appear at 217 and 279 cm−1, while broad peaks appear simultaneously at 393, 490, and 630 cm−1. This experimental result confirms that MnFe2O4 ferrite has a cubic spinel structure [40]. The Raman test results further indicate that the chemical composition of the surface insulation coating of oxidized iron powder is MnFe2O4.
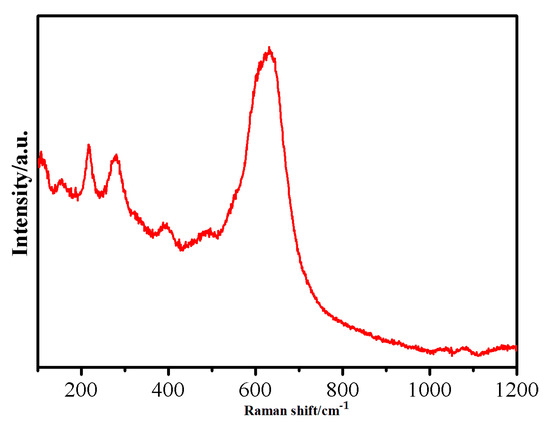
Figure 7.
Raman spectroscopy result for the oxidized Fe powder.
Based on the above analysis results, the reaction mechanism of surface oxidation can be inferred. The possible reaction for forming MnFe2O4 in this process can be expressed as the following equation. According to the Pourbaix diagram [41], the Fe reacted with H2O to form Fe(OH)2 and was further oxidized to [Fe(OH)n]−(n−3) (n = 2, 3) by a small amount of dissolved O2 in the solution. Moreover, the Mn2+ ions formed Mn(NH3)62+ due to the strong coordinating ability of Mn2+ with NH3. Finally, Mn(NH3)62+ reacted with hydroxy complexes [Fe(OH)n]−(n−3) to form ferromagnetic MnFe2O4 [42]:
According to the above surface oxidation reaction mechanism, as the concentration of ammonia increases, OH− and NH4+ in the solution increase, causing the insulation coating to become thicker. Therefore, the reaction concentration will affect the thickness of the insulating coating and then affect the magnetic properties of the magnetic powder after insulating coating. Figure 8 shows the hysteresis loops of raw iron powder and those oxidized with different concentrations of ammonia, with a test magnetic field range of −20,000 Oe to 20,000 Oe. The saturated magnetization (Ms) value of the raw iron powder was 210 emu/g, while the Ms of the iron powder after insulation coating decreased from 205 emu/g to 198 emu/g when the ammonia water concentration increased from 1.5 wt% to 3.5 wt%, as shown in the left inset. On the one hand, the slight change in the Ms of iron powder after insulation coating is due to the thin thickness of the insulation layer. On the other hand, the Ms of the insulating layer MnFe2O4 is lower than that of the iron powder, resulting in a gradual decrease in the saturation magnetic induction intensity of the coated magnetic powder with increasing ammonia concentration. As shown in the right inset, the coercive field of the oxidized Fe powders increased with increasing ammonia concentration, mainly owing to the increasing content of MnFe2O4 in the insulating layer.
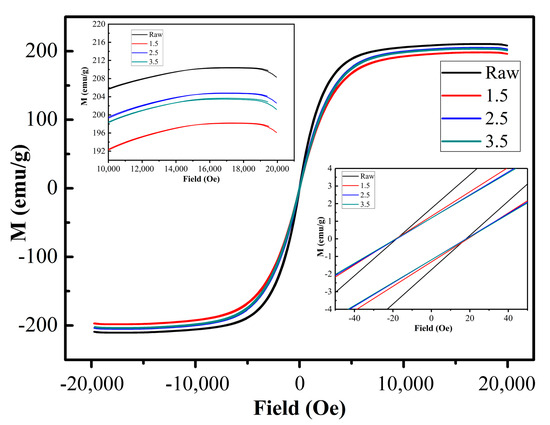
Figure 8.
Hysteresis loops for the Fe powders and those in different reaction concentrations.
In order to analyze the thermal stability of the coating, TG-DSC measurements were conducted on the iron powder coated with MnFe2O4 and the iron powder coated with MnFe2O4/ESR, as shown in Figure 9. According to Figure 9a, it can be seen that the mass of iron powder after MnFe2O4 insulation coating remains unchanged during the process of increasing the temperature from room temperature to 700 °C. The results indicate that MnFe2O4 does not decompose below 700 °C. As shown in Figure 9b, an apparent decrease in the mass was observed at approximately 200 °C due to thermal degradation of the ESR layer, which decomposed slowly with increasing temperature. When the temperature rises to 700 °C, the decrease in mass is 0.97%, which is less than the mass fraction of 2 wt% ESR, which is because the ESR decomposes and generates silicon dioxide.
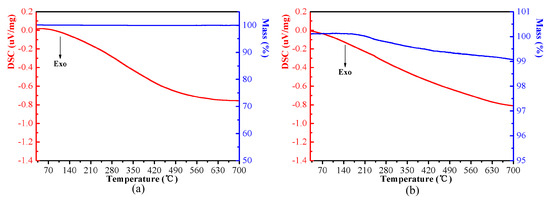
Figure 9.
DSC-TG curves of the Fe powders coated with MnFe2O4 (a) and MnFe2O4/ESR (b).
To further analyze the impact of annealing temperature on the insulating coating, SEM morphology observation and corresponding EDS elemental analysis were conducted on the cross-section of the SMCs at different annealing temperatures; the results are shown in Figure 10. The presence of gold in the EDS test results of all samples is due to the gold spraying treatment on the samples. The EDS test results of the magnetic core cross-section at different annealing temperatures show that the interior (1) of the particles only contains iron, while the surface (2) of the particles contains manganese, oxygen, iron, and silicon. This is due to the presence of an insulating layer of MnFe2O4 on the particle surface and the decomposition of silicone resin into silicon dioxide after annealing. In addition, the peak intensity of silicon increases with an increase in annealing temperature because the gradual decomposition of silicone resin increases the content of silicon dioxide. The peak intensity of manganese also increases with the increase in annealing temperature, which is due to the gradual decomposition of silicone resin, exposing more MnFe2O4 on the surface of the particles.
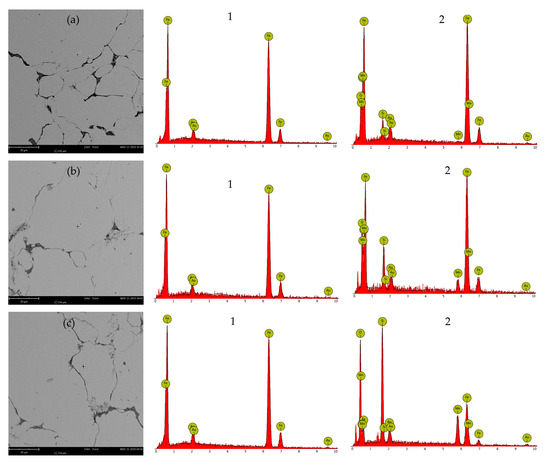
Figure 10.
SEM micrographs and EDS analysis of samples annealed at different temperatures: (a) 500 °C, (b) 550 °C, and (c) 600 °C.
Figure 11a shows the variation curves of the amplitude permeability of the Fe SMCs with frequency under different annealing temperatures, where the Fe SMCs were prepared by insulation oxidation with an ammonia concentration of 2.5 wt%. The external magnetic field applied during testing was 50 mT. All samples exhibited high amplitude permeability with frequency stability, indicating that the insulation layer was completely coated on the surface of the iron powder particles. It can also be observed that as the annealing temperature increased from 500 °C to 550 °C, the amplitude permeability of the core significantly increased. However, when the annealing temperature was further raised to 600 °C, the amplitude permeability of the core slightly decreased. On the one hand, the release of internal stress in the core became more thorough with increasing temperature, which was beneficial for improving the amplitude permeability. On the other hand, the increase in annealing temperature led to an increase in the porosity of the core, which reduced the amplitude permeability. Therefore, when the annealing temperature increased from 500 °C to 550 °C, the elimination of internal stress played a dominant role in enhancing the amplitude permeability. When the annealing temperature increased from 550 °C to 600 °C, the increase in porosity had a more pronounced effect on reducing the amplitude permeability, indicating that the internal stress in the core was fully eliminated at an annealing temperature of 550 °C.
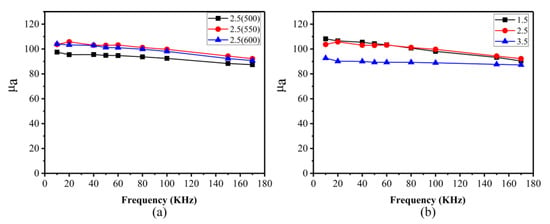
Figure 11.
The amplitude permeability for the Fe SMCs at different frequencies. (a) Annealed at different temperatures; (b) oxidized by different ammonia concentrations with annealing at 550 °C.
The variation curves of amplitude permeability with frequency for the Fe SMCs prepared at different ammonia concentrations under an annealing temperature of 550 °C, as shown in Figure 11b. As the ammonia concentration increased from 1.5 wt% to 2.5 wt%, the amplitude permeability of the Fe SMCs slightly decreased, which was attributed to the increase in the thickness of the insulation layer. Additionally, when the ammonia concentration was 1.5 wt%, the amplitude permeability of the Fe SMCs gradually decreased with increasing frequency because of the thin insulation coating layer, which resulted in weaker frequency stability of the amplitude permeability. While the ammonia concentration reached 3.5 wt%, the amplitude permeability of the core exhibited excellent frequency stability, indicating that the insulation layer was complete and had a certain thickness. Furthermore, the amplitude permeability of the Fe SMCs significantly decreased due to the increased content of MnFe2O4 in the magnetic powder.
Figure 12 shows the core loss (Ps) versus the frequency of the Fe SMCs annealed at different temperatures, oxidized by different ammonia concentrations, and annealed at 550 °C. The Ps of the SMCs mainly consists of the hysteresis loss Ph and eddy current loss Pe [43]. Figure 12a shows the Ps of the SMC samples after oxidation with an ammonia concentration of 2.5 wt% under different annealing temperatures, with the applied external magnetic field being 50 mT. At the frequency of 100 kHz, the core loss decreased from 267.6 W/kg to 234.9 W/kg as the annealing temperature increased from 500 °C to 550 °C. This mainly resulted from the reduced residual stresses and decreased coercivity caused by the annealing treatment, decreasing the hysteresis loss of Ps. When the annealing temperature rose to 600 °C, the core loss increased to 268.0 W/kg. This was mainly caused by the further decomposition of the coating layer, leading to an increase in the eddy current loss. Therefore, 550 °C is the optimal annealing temperature for obtaining the lowest core loss of the SMCs.
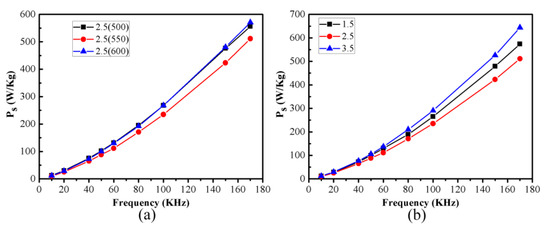
Figure 12.
Core loss of the Fe SMCs at different frequencies. (a) Annealed at different temperatures; (b) oxidized by different ammonia concentrations with annealing at 550 °C.
Figure 12b shows the Ps with frequency for the SMCs prepared under different ammonia concentrations at an annealing temperature of 550 °C, with the applied external magnetic field being 50 mT. At the frequency of 100 kHz, the core loss decreases and then increases with an increase in ammonia concentration, reaching a minimum of 234.9 W/kg at an ammonia concentration of 2.5 wt%. The thickness of the insulating coating increases with the ammonia solution concentration. On the one hand, an increase in the thickness of the MnFe2O4 insulating layer leads to reduced eddy current loss, thereby decreasing the core loss. On the other hand, an increase in the thickness of the insulating layer leads to an increase in hysteresis loss, thereby increasing the core loss. The analysis of the amplitude permeability of the SMCs with changes in ammonia concentration indicates that the insulating layer thickness of the core prepared at an ammonia concentration of 1.5 wt% is very thin. When the ammonia concentration increases from 1.5 wt% to 2.5 wt%, the former effect is more significant, thereby reducing the core loss. When the ammonia concentration increases to 3.5 wt%, the insulating layer thickness further increases, and the latter effect dominates, causing the core loss to rise. Combined with the annealing temperature and ammonia concentration, 550 °C and 2.5 wt% were the optimal conditions for obtaining good soft magnetic properties with a high amplitude permeability (approximately 100) and low core loss (234.9 W/kg, 50 mT 100 kHz) in the SMCs.
4. Conclusions
In this work, Fe soft magnetic composites based on powder coated with MnFe2O4/ESR insulating layers were prepared by surface oxidation and mixing with the ESR. The chemical composition of the insulation layer was analyzed by characterization methods such as SEM, EDS, and XPS, and the results showed that the insulation layer was made of manganese ferrite (MnFe2O4). The influence of ammonia concentration and annealing temperature on the magnetic properties of Fe SMCs was investigated. The SMCs prepared with an ammonia concentration of 2.5 wt% had the best comprehensive magnetic properties after annealing at 550 °C. The SMCs exhibited an excellent amplitude permeability of 99.7 and significantly low core loss of 234.9 W/kg (at 100 kHz, 50 mT). This study provides a new method for developing SMCs that can operate at high frequencies with high magnetic permeability and low loss, which may be beneficial for the miniaturization, integration, and energy saving of electronic components.
Author Contributions
Conceptualization, S.L. and R.L.; methodology, R.L.; software, S.L.; validation, S.L., R.L. and X.X.; formal analysis, X.X.; data curation, S.L.; writing—original draft preparation, S.L.; writing—review and editing, X.X.; visualization, S.L., R.L., and X.X.; supervision, R.L. and X.X.; project administration, X.X.; funding acquisition, R.L. and X.X. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Key Research and Development Program (2016YFB0700302).
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding authors.
Conflicts of Interest
Author Shigeng Li was employed by the company Jiangxi Huanyu Ceramic Technology Research Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Zhang, Z.; Xu, W.; Guo, T.; Jiang, Y.; Yan, M. Effect of processing parameters on the magnetic properties and microstructures of molybdenum permalloy compacts made by powder metallurgy. J. Alloys Compd. 2014, 594, 153–157. [Google Scholar] [CrossRef]
- Feng, H.; Jin, X.; Li, T.; Cui, H.; Meng, C.; Liu, Z.; Xue, D. MHz mechanism of permeability in Fe-Si-Al soft magnetic composites. J. Alloys Compd. 2025, 1014, 178742. [Google Scholar] [CrossRef]
- Yang, Z.; Luo, Z.; Jin, Q.; Wang, J.; Wu, Z.; Fan, X. Microstructure and magnetic properties of Fe/phosphate soft magnetic composites with layered structure. J. Alloys Compd. 2025, 1014, 178676. [Google Scholar] [CrossRef]
- Wang, R.; Jin, Z.; Guo, W.; Wu, Y.; Jia, J.; Shao, Y.; Chen, N.; Yao, K. Effects of magnetic volume fraction induced by compaction pressure on magnetic properties of FeBCPSiMoCr-based soft magnetic composites. J. Magn. Magn. Mater. 2025, 616, 172832. [Google Scholar] [CrossRef]
- Zhu, Z.; Wang, P.; Zhang, J. Preparation of FeSiBC soft magnetic composites with excellent magnetic properties and environmental reliability based on mechanofusion method. J. Mater. Res. Technol. 2025, 36, 4112–4124. [Google Scholar] [CrossRef]
- Li, Q.; Zheng, S.; Mao, Y.; Xu, D.; Peng, Y.; Zhao, Y. Microstructure and properties of amorphous FeSiCrBC soft magnetic composites prepared by using HNO3 solution. J. Mater. Sci. Mater. Electron. 2023, 34, 862. [Google Scholar] [CrossRef]
- Huang, H.; Wang, J.; Cui, Z.; Gao, Z.; Huang, Z.; Wu, Z. Selective oxidation of rare metal oxide insulation layers on particle substrates for optimizing the performance of FeSiCr-based soft magnetic composites. Mater. Des. 2023, 230, 111984. [Google Scholar] [CrossRef]
- Muñoz, Á.; Burgos, N.; Zhukova, V.; Talaat, A.; Martín, J.; González, J.; Osinalde, M. Magnetic properties and power losses of inorganic, organic and hybrid-based soft magnetic composites. Ceram. Int. 2024, 50, 55099–55112. [Google Scholar] [CrossRef]
- Oyama, H.; Kawada, N.; Sato, T. Magnetic Properties of Soft Magnetic Powder/Epoxy Composite Sheet. IEEE Trans. Magn. 2023, 59, 2001205. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, Y.; Feng, S.; Kan, X.; Zhu, Y.; Li, Y.; Sun, W.; Shen, J.; Liu, X. Improvement of electromagnetic properties of FeSiAl soft magnetic composites. J. Mater. Sci. 2023, 58, 9698–9707. [Google Scholar] [CrossRef]
- Yu, H.; Zhou, S.; Zhang, G.; Dong, B.; Meng, L.; Li, Z.; Dong, Y.; Cao, X. The phosphating effect on the properties of FeSiCr alloy powder. J. Magn. Magn. Mater. 2022, 552, 168741. [Google Scholar] [CrossRef]
- Hsiang, H.-I.; Chuang, K.-H.; Lee, W.-H. FeSiCr Alloy Powder to Carbonyl Iron Powder Mixing Ratio Effects on the Magnetic Properties of the Iron-Based Alloy Powder Cores Prepared Using Screen Printing. Materials 2021, 14, 1034. [Google Scholar] [CrossRef] [PubMed]
- Bai, R.; Shao, L.; Ding, H.; Li, X.; Zhou, J.; Xue, Z.; Ke, H.; Wang, W. Optimizing the high-frequency magnetic properties of soft magnetic composites via surface nanoengineering. J. Mater. Sci. Technol. 2025, 211, 82–88. [Google Scholar] [CrossRef]
- Zhu, Q.; Zhang, C.; Peng, X.; Li, J.; Wu, Q.; Xu, J.; Hong, B.; Wang, X.; Ge, H. Preparation, structure and magnetic properties of spherical FeSiB/Al2O3 soft magnetic composites prepared in a magnetic field. J. Mater. Sci. 2024, 59, 6354–6364. [Google Scholar] [CrossRef]
- Birčáková, Z.; Füzer, J.; Kollár, P.; Szabó, J.; Jakubčin, M.; Streckova, M.; Bureš, R.; Fáberová, M. Preparation and characterization of iron-based soft magnetic composites with resin bonded nano-ferrite insulation. J. Alloys Compd. 2020, 828, 154416. [Google Scholar] [CrossRef]
- Zhang, J.; Qiao, L.; Zheng, J.; Ying, Y.; Cai, W.; Yu, J.; Li, W.; Che, S. The Magnetic Properties of FeSiCr/Epoxy Resin Soft Magnetic Composites for Injection Molding in a Broad Frequency Up To 2000 kHz. J. Electron. Mater. 2022, 52, 1024–1035. [Google Scholar] [CrossRef]
- Choi, K.; Lee, S.; Hwang, J.; Yang, S.; Huh, J.; Yi, K.; Byun, J. Magnetic properties of Fe soft magnetic composites with a double-insulating layer comprising Fe3O4 and silicone resin. J. Alloys Compd. 2023, 936, 168255. [Google Scholar] [CrossRef]
- Xie, Q.; Yu, H.; Yuan, H.; Han, G.; Chen, X.; Liu, Z. Enhanced Magnetic Properties and Thermal Conductivity of FeSiCr Soft Magnetic Composite with Al2O3 Insulation Layer Prepared by Sol-Gel Process. Metals 2023, 13, 813. [Google Scholar] [CrossRef]
- Yao, Z.; Peng, Y.; Xia, C.; Yi, X.; Mao, S.; Zhang, M. The effect of calcination temperature on microstructure and properties of FeNiMo@Al2O3 soft magnetic composites prepared by sol-gel method. J. Alloys Compd. 2020, 827, 154345. [Google Scholar] [CrossRef]
- Park, J.; Kim, H.; Lee, J.; Jeong, J. Improved Soft Magnetic Properties in FeNi@MgO Composites by Sol-Gel-Based Surface Coating and High-Temperature Heat Treatment. Metals 2023, 13, 1383. [Google Scholar] [CrossRef]
- Li, C.; Yu, H.; Han, G.; Liu, Z. FeSiCr-Based Soft Magnetic Composites with SiO2 Insulation Coating Prepared Using the Elemental Silicon Powder Hydrolysis Method. Metals 2023, 13, 1444. [Google Scholar] [CrossRef]
- Geng, K.; Xie, Y.; Xu, L.; Yan, B. Structure and magnetic properties of ZrO2-coated Fe powders and Fe/ZrO2 soft magnetic composites. Adv. Powder Technol. 2017, 28, 2015–2022. [Google Scholar] [CrossRef]
- Wu, S.; Dong, Z.; Li, J.; Fan, J.; Liang, Y.; Liu, J. Preparation and Magnetic Properties of AlN/Phenolic Resin-Coated Iron-Based Soft Magnetic Composites. J. Electron. Mater. 2023, 52, 8086–8094. [Google Scholar] [CrossRef]
- Li, H.; Bai, G.; Zhao, M.; Yu, S.; Lu, Z.; Yang, H.; Cheng, M.; Zhang, Z.; Liu, X.; Chen, W.; et al. FeSiAl soft magnetic composite with double Al2O3 insulation layers for simultaneous high mechanical and magnetic properties. J. Mater. Sci. Technol. 2025, 206, 307–316. [Google Scholar] [CrossRef]
- Liu, D.; Wu, C.; Yan, M. Investigation on sol-gel Al2O3 and hybrid phosphate-alumina insulation coatings for FeSiAl soft magnetic composites. J. Mater. Sci. 2015, 50, 6559–6566. [Google Scholar] [CrossRef]
- Chaudhari, A.; Singh, V. Improvement in different properties of the permalloy by nano-Cr2O3 incorporation. J. Appl. Electrochem. 2017, 47, 1009–1021. [Google Scholar] [CrossRef]
- Sun, H.; Zhou, G.; Guo, Z.; Wang, C.; Wang, J.; Zong, C. Efficient synthesis of TiO2-coated layer for Fe-based soft magnetic composites and their regulation mechanism analysis on magnetic properties. J. Mater. Sci-Mater. Electron. 2022, 33, 13956–13967. [Google Scholar] [CrossRef]
- Yu, H.; Lai, X.; Zhong, X.; Liu, Z.; Guo, B.; Chen, X.; Han, G. Optimization of sol-gel prepared SiO2 coating and FeSiCr@SiO2 soft magnetic composites based on critical ammonia concentration. Mater. Chem. Phys. 2023, 303, 127765. [Google Scholar] [CrossRef]
- Bures, R.; Neslusan, M.; Faberova, M.; Čilliková, M.; Birčáková, Z.; Kollar, P.; Fuzer, J.; Milyutin, V. Formation of Effective Non-ferromagnetic Barrier in Fe/MgO Soft Magnetic Composite. ACS Appl. Electron. Mater. 2024, 6, 1928–1939. [Google Scholar] [CrossRef]
- Bureš, R.; Fáberová, M.; Kollár, P.; Füzer, J.; Dobák, S.; Onderko, F.; Kurek, P. Microwave Sintered Fe/MgO Soft Magnetic Composite. Acta Phys. Pol. A 2017, 131, 780–782. [Google Scholar] [CrossRef]
- Qian, L.; Peng, J.; Xiang, Z.; Pan, Y.; Lu, W. Effect of annealing on magnetic properties of Fe/Fe3O4 soft magnetic composites prepared by in-situ oxidation and hydrogen reduction methods. J. Alloys Compd. 2019, 778, 712–720. [Google Scholar] [CrossRef]
- Busharat, M.; Shukrullah, S.; Naz, M.; Khan, Y.; Ibrahim, A.; Al-Arainy, A.; Shoaib, M. Study of Cation Distribution and Photocatalytic Activity of Nonthermal Plasma-Modified NiZnFe2O4 Magnetic Nanocomposites. ACS Omega 2024, 9, 14791–14804. [Google Scholar] [CrossRef]
- Li, S.; Liu, R.; Xiong, X. Preparation and characterization of carbonyl iron soft magnetic composites with magnesioferrite insulating coating layer. Trans. Nonferrous Met. Soc. China 2020, 30, 3067–3077. [Google Scholar] [CrossRef]
- Kummari, S.; Srikanth, V. CoFe content-controlled magnetic exchange coupling in CoFe2O4-CoFe composites prepared by a simple and single-step sonochemical reduction method. Mater. Lett. 2023, 347, 134626. [Google Scholar] [CrossRef]
- Li, S.; Liu, R.; Xiong, X. Fe-based soft magnetic composites with high permeability and low core loss by in situ coating ZnFe2O4 layer. J. Mater. Sci. 2020, 55, 274–282. [Google Scholar] [CrossRef]
- Lauda, M.; Füzer, J.; Kollár, P.; Strečková, M.; Bureš, R.; Kováč, J.; Baťková, M.; Baťko, I. Magnetic properties and loss separation in FeSi/MnZnFe2O4 soft magnetic composites. J. Magn. Magn. Mater. 2016, 411, 12–17. [Google Scholar] [CrossRef]
- Bennet, J.; Tholkappiyan, R.; Vishista, K.; Jaya, N.; Hamed, F. Attestation in self-propagating combustion approach of spinel AFe2O4 (A = Co, Mg and Mn) complexes bearing mixed oxidation states: Magnetostructural properties. Appl. Surf. Sci. 2016, 383, 113–125. [Google Scholar] [CrossRef]
- Zhou, M.; Han, Y.; Guan, W.; Han, S.; Meng, Q.; Xu, T.; Su, H.; Guo, X.; Zou, Z.; Yang, F.; et al. Magnetic properties and loss mechanism of Fe-6.5wt%Si powder core insulated with magnetic Mn-Zn ferrite nanoparticles. J. Magn. Magn. Mater. 2019, 482, 148–154. [Google Scholar] [CrossRef]
- Zhou, G.; Wang, Y.; Zhou, R.; Wang, C.; Jin, Y.; Qiu, J.; Hua, C.; Cao, Y. Synthesis of amino-functionalized bentonite/CoFe2O4@MnO2 magnetic recoverable nanoparticles for aqueous Cd2+ removal. Sci. Total Environ. 2019, 682, 505–513. [Google Scholar] [CrossRef]
- Wang, W.; Ding, Z.; Zhao, X.; Wu, S.; Li, F.; Yue, M.; Liu, J. Microstructure and magnetic properties of MFe2O4 (M = Co, Ni, and Mn) ferrite nanocrystals prepared using colloid mill and hydrothermal method. J. Appl. Phys. 2015, 117, 17A328. [Google Scholar] [CrossRef]
- Pourbaix, M. Atlas of Electrochemical Equilibria in Aqueous Solution, 2nd ed.; National Association of Corrosion Engineers: Houston, TX, USA, 1974; Volume 141, pp. 313–409. [Google Scholar]
- Yu, S.; Yoshimura, M. Direct fabrication of ferrite MFe2O4 (M = Zn, Mg)/Fe composite thin films by soft solution processing. Chem. Mater. 2000, 12, 3805–3810. [Google Scholar] [CrossRef]
- Xiao, L.; Sun, Y.; Ding, C.; Yang, L.; Yu, L. Annealing effects on magnetic properties and strength of organic-silicon epoxy resin-coated soft magnetic composites. J. Mech. Eng. Sci. 2014, 228, 2049–2058. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).