Anodizing 3D-Printed AlSi10Mg Alloy and Its Fatigue Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Material
2.2. Test Specimen
2.3. Anodizing
2.4. Fatigue Test
2.5. Corrosion Test
3. Results and Discussion
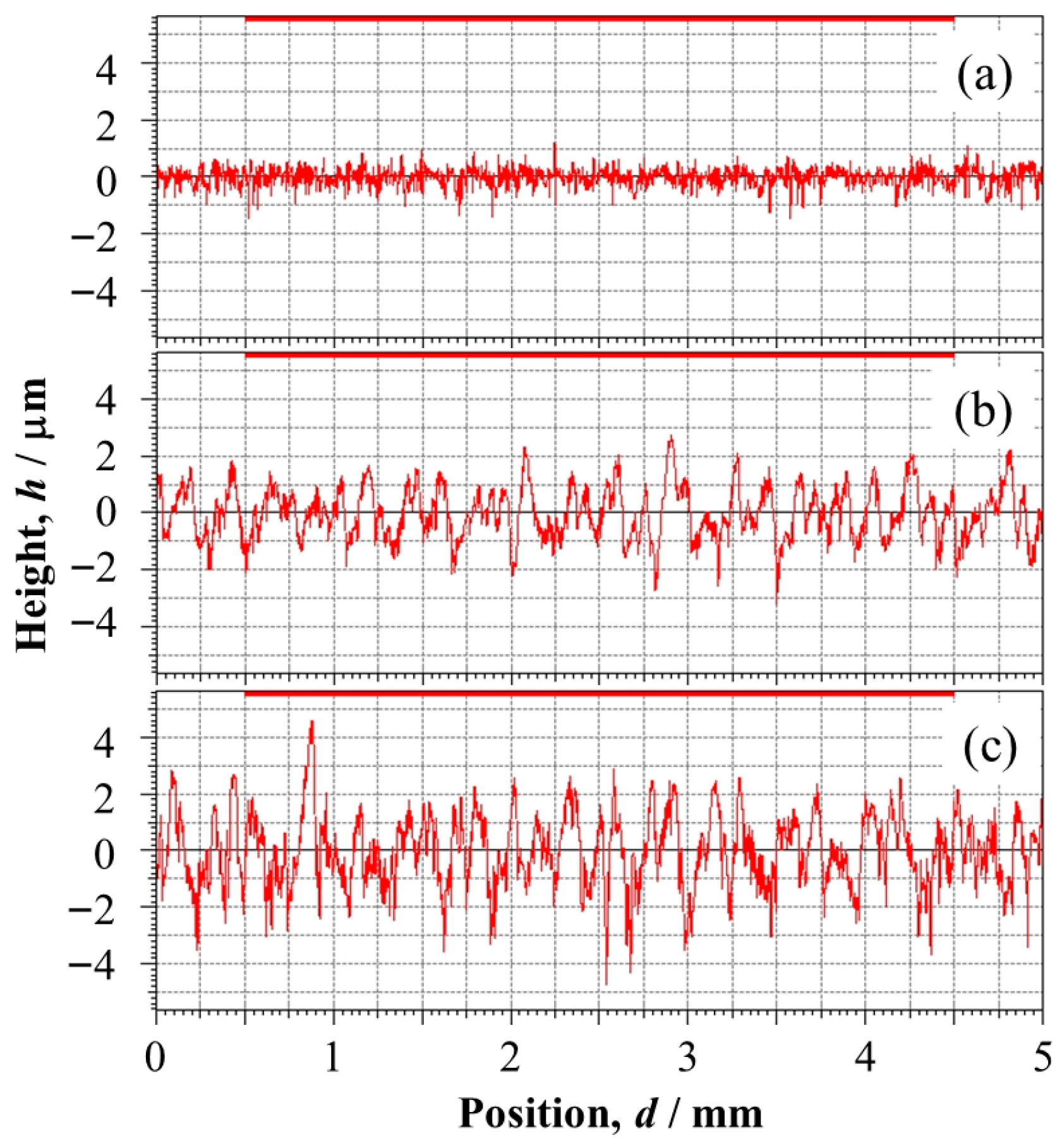
3.1. Relative Density and Residual Stress
3.2. Anodized Layer
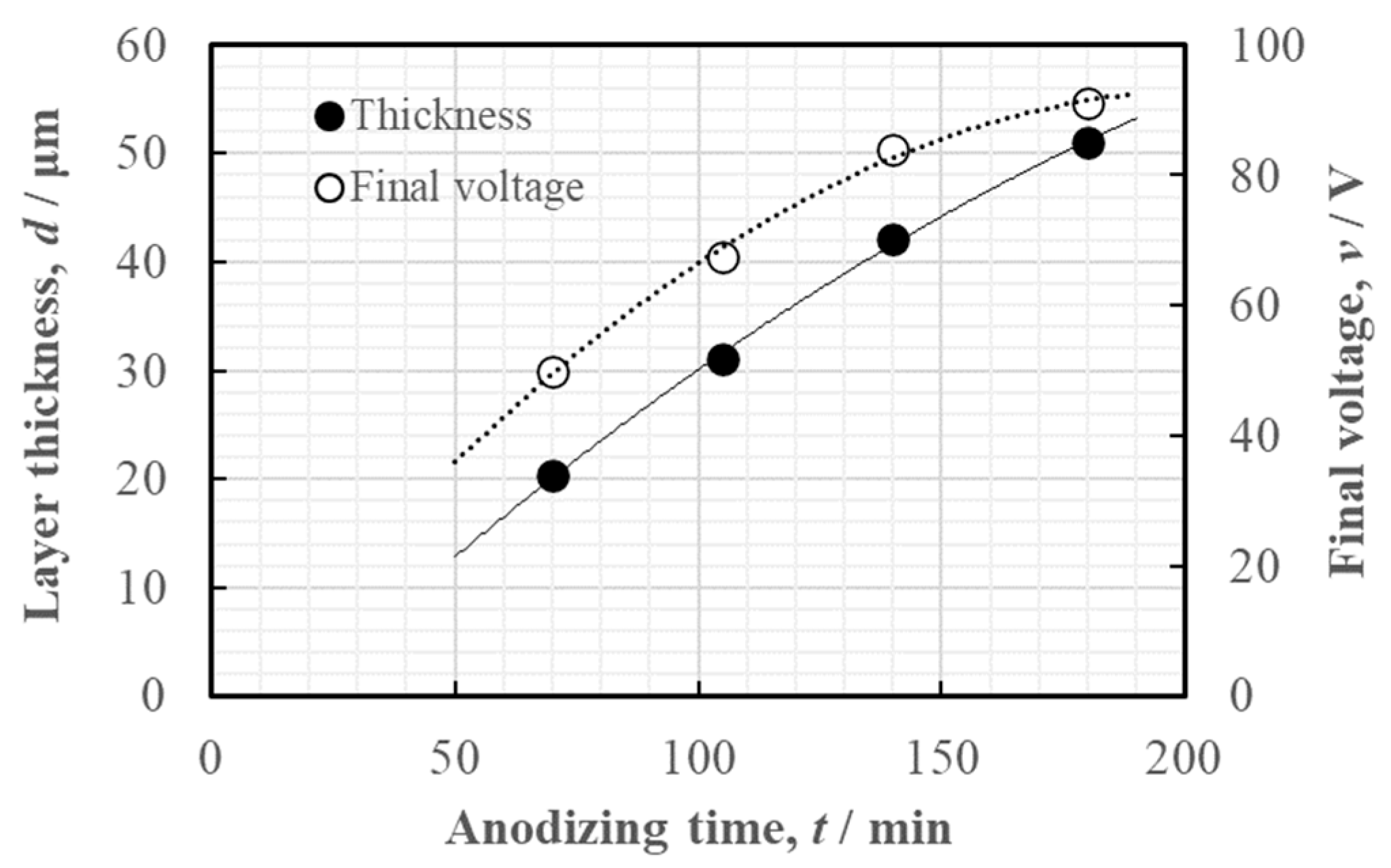
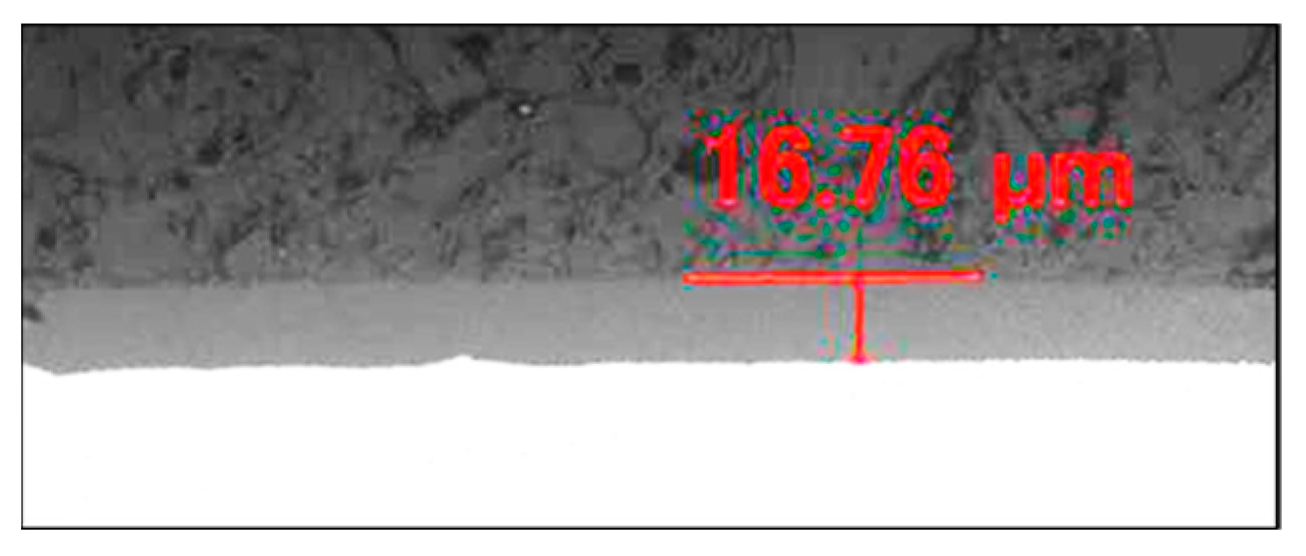
3.2.1. Anodized Layer Thickness and Growth Rate
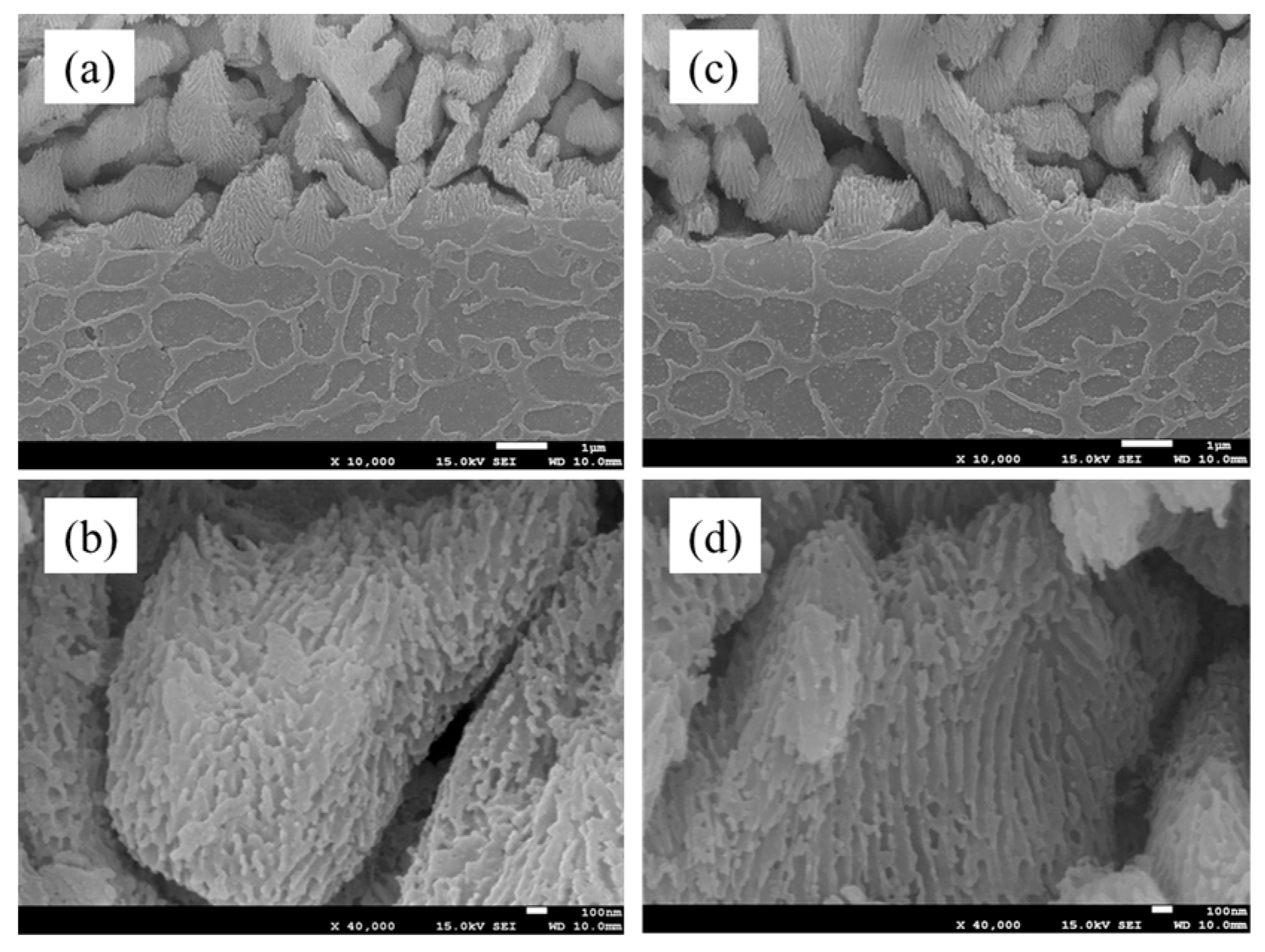
3.2.2. Cross-Section of Anodized Layer
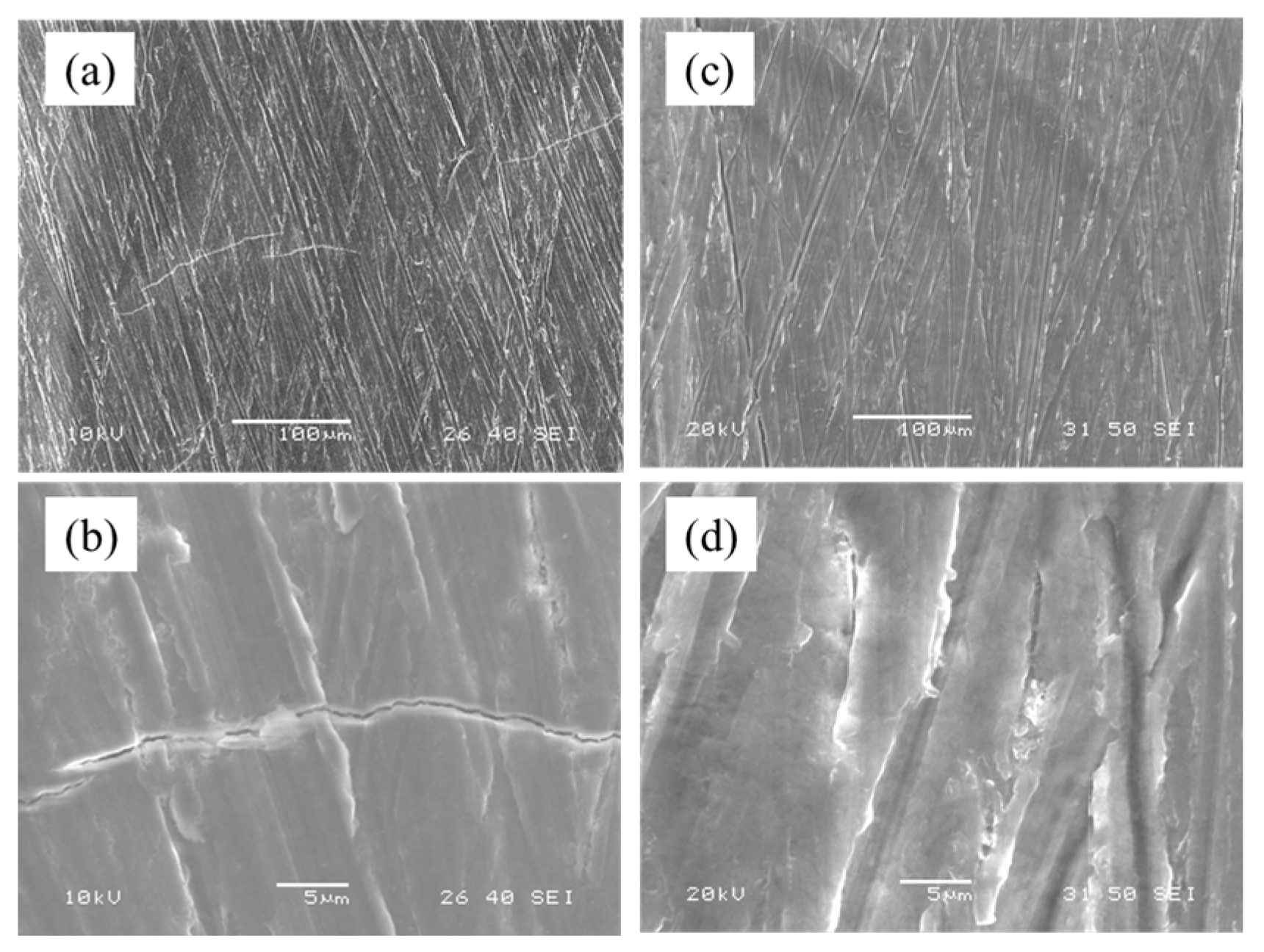
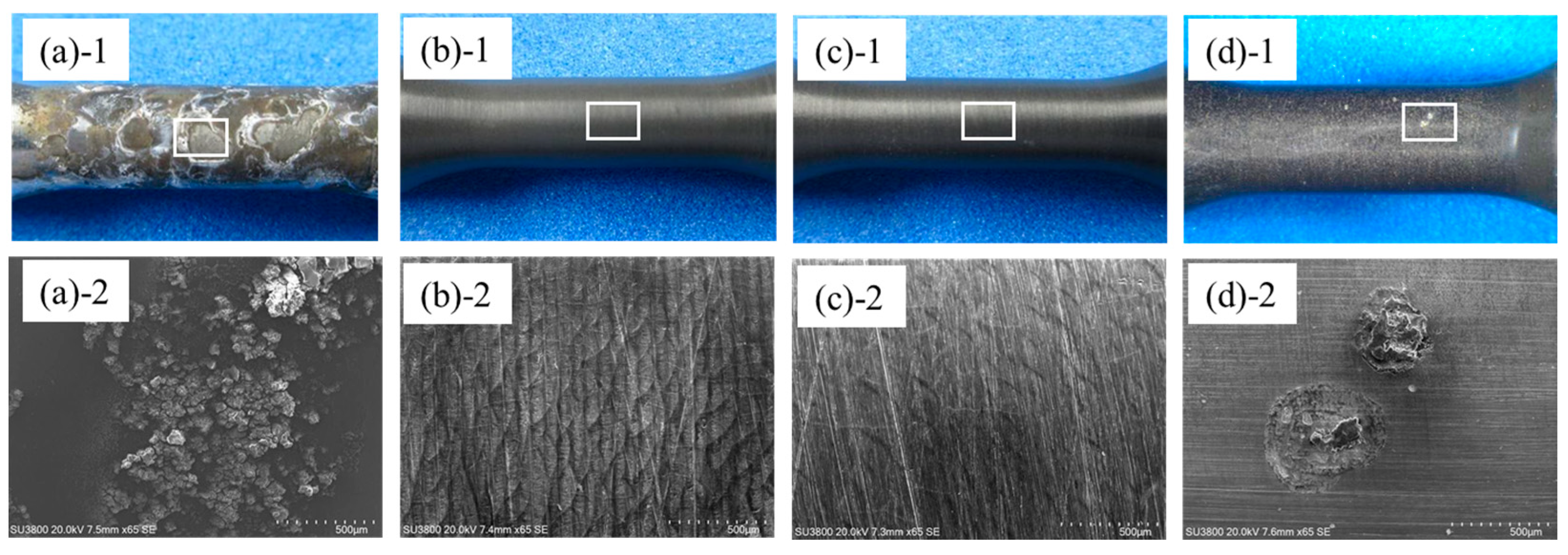
3.2.3. Surface of Anodized Layer
3.2.4. Hardness of Anodized Layer
3.3. Fatigue Property
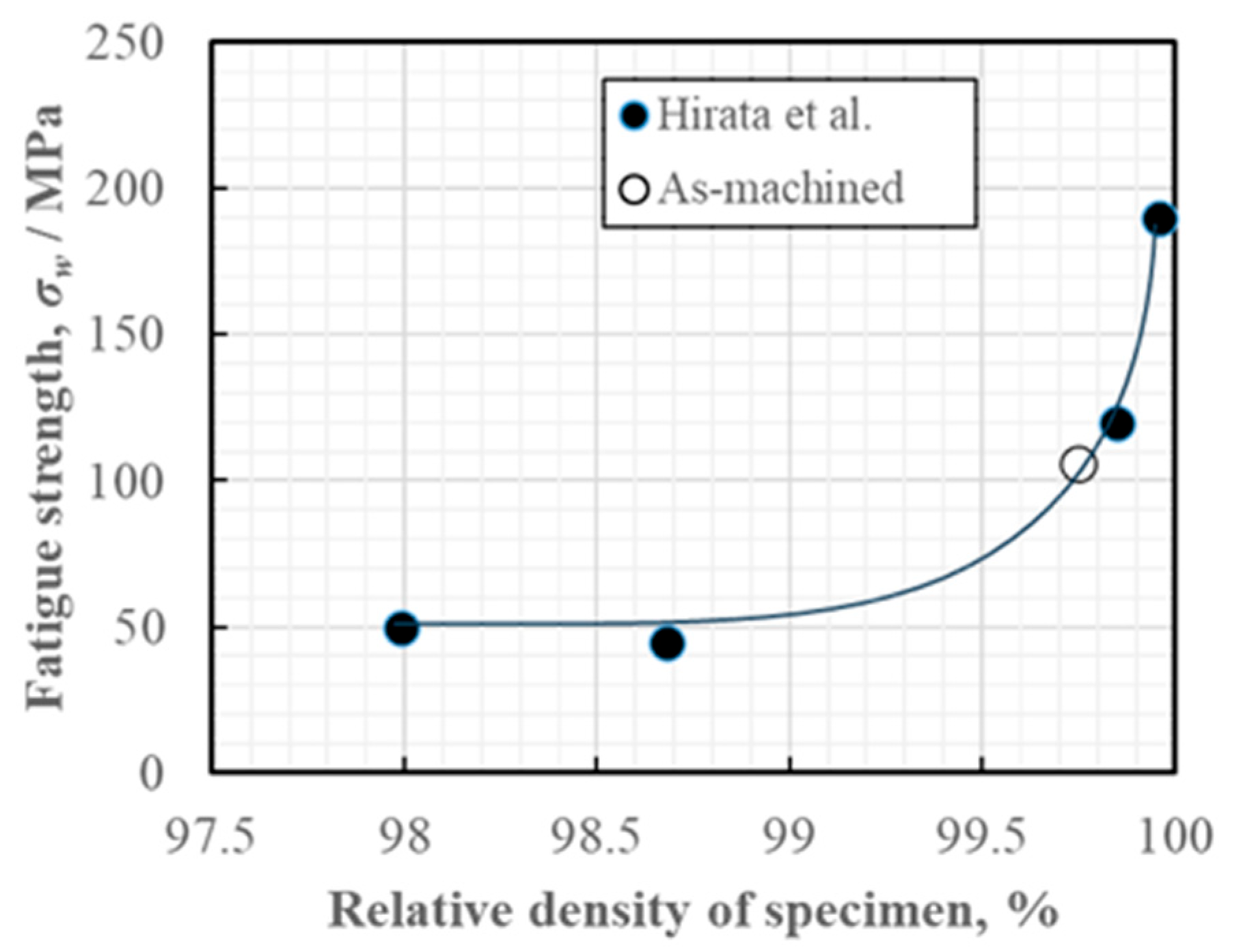
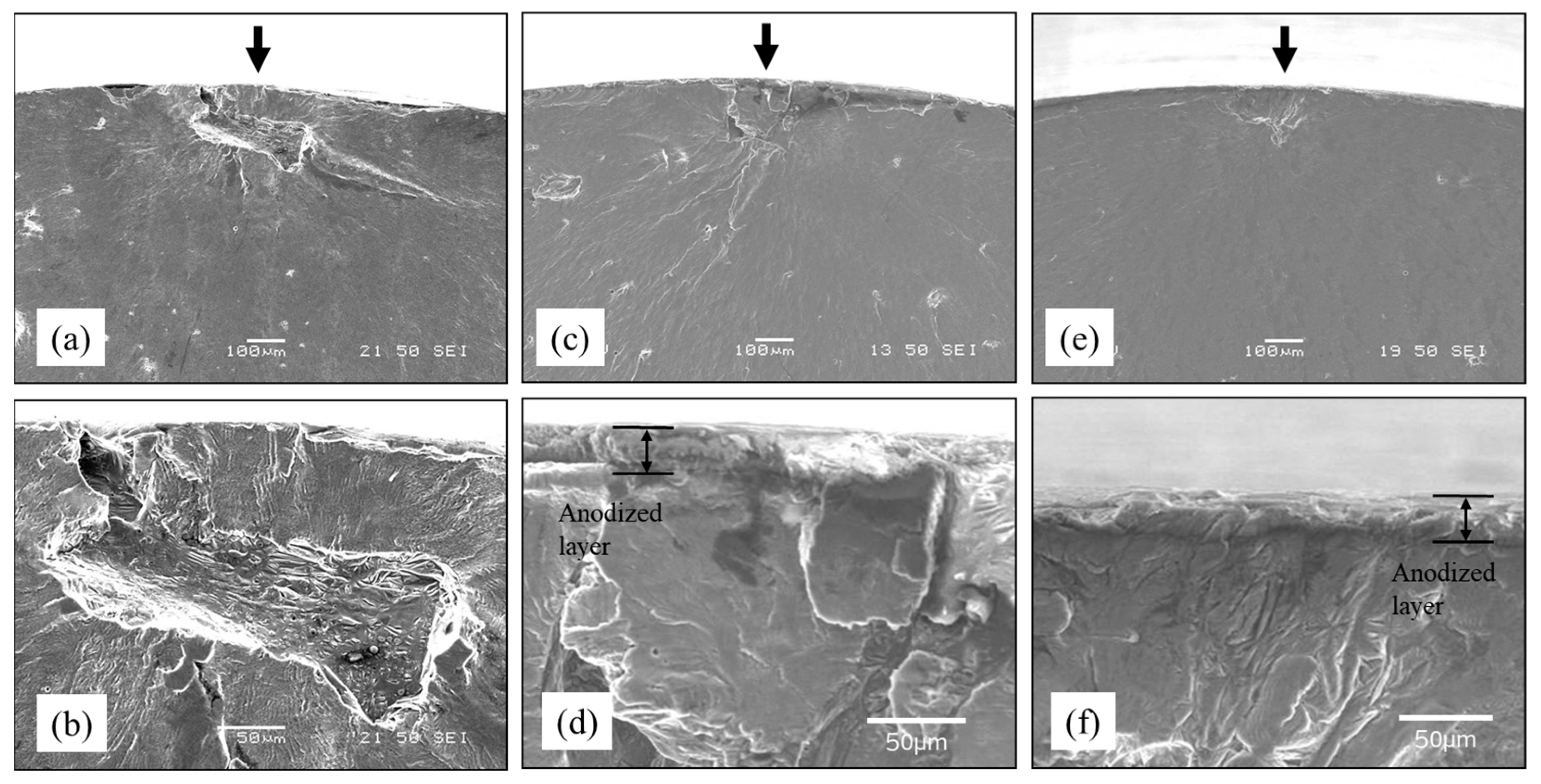
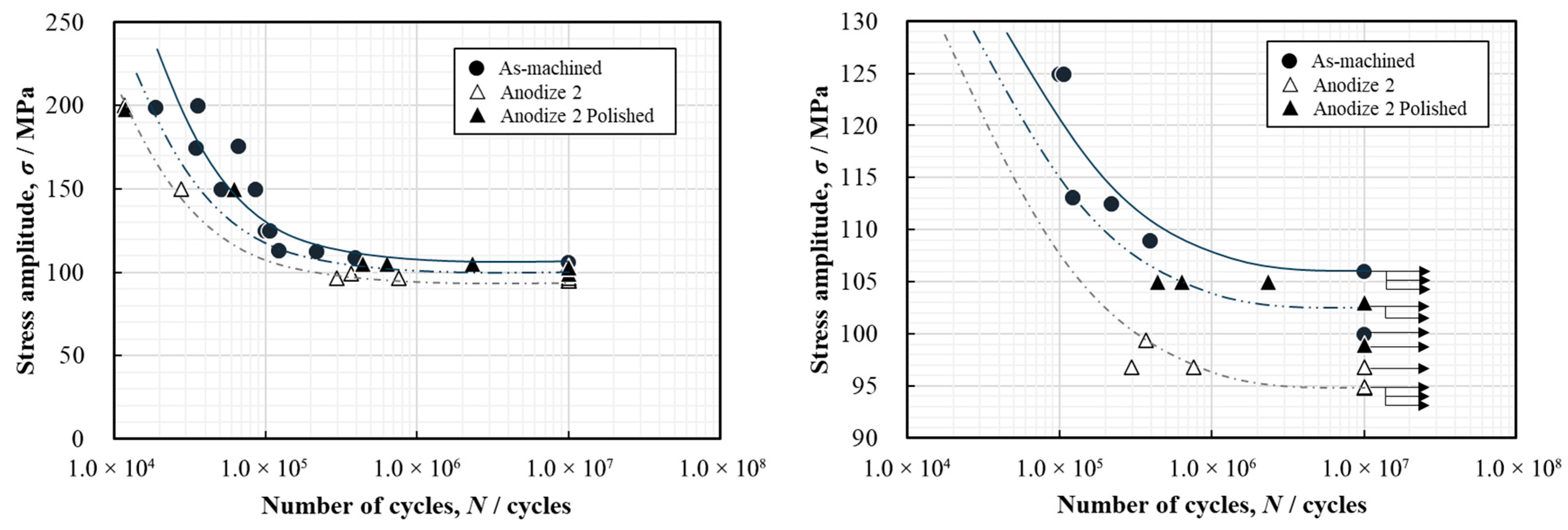
3.3.1. Rotating Bending Fatigue Test Result
3.3.2. Improvement in Fatigue Properties by Smoothing the Surface
3.4. Corrosion Property
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- McMahon, M. Aluminium Additive Manufacturing: How a new generation of alloys will fuel industry growth. Metal AM 2024, 10, 107–123. [Google Scholar]
- McMahon, M.; Williams, N. BMW Group: Laying the foundations for the application of metal Additive Manufacturing in the automotive industry. Metal AM 2024, 10, 131–147. [Google Scholar]
- Mower, T.M.; Long, M.J. Mechanical behavior of additive manufactured, powder-bed laser-fused materials. Mater. Sci. Eng. A 2016, 651, 198–213. [Google Scholar] [CrossRef]
- Martin, J.H.; Yahata, B.D.; Hundley, J.M.; Mayer, J.A.; Schaedler, T.A.; Pollock, T.M. 3D printing of high-strength aluminum alloys. Nature 2017, 549, 365–369. [Google Scholar] [CrossRef]
- Liu, S.; Zhu, H.; Peng, G.; Yin, J.; Zeng, X. Microstructure prediction of selective laser melting AlSi10Mg using finite element analysis. Mater. Des. 2018, 142, 319–328. [Google Scholar] [CrossRef]
- Minkowitz, L.; Arneitz, S.; Effertz, P.S.; Armancio-Filho, S.T. Laser-powder bed fusion process optimization of AlSi10Mg using extra trees regression. Mater. Des. 2023, 227, 111718. [Google Scholar] [CrossRef]
- Gao, C.; Tang, H.; Zhang, S.; Mao, Z.; Bi, Y.; Rao, J.-H. Process optimization for Up-Facing Surface Finish of AlSi10Mg Alloy Produced by Laser Powder Bed Fusion. Metals 2022, 12, 2053. [Google Scholar] [CrossRef]
- Han, Q.; Gu, H.; Soe, S.; Setchi, R.; Lacan, F.; Hill, J. Manufacturability of AlSi10Mg overhang structures fabricated by laser powder bed fusion. Mater. Des. 2018, 160, 1080–1095. [Google Scholar] [CrossRef]
- Nagamoto, H.; Kobayashi, K.; Fujita, H. Stiffness optimization process using topology optimization techniques and lattice structures. In Proceedings of the 26th Small Powertrains and Energy Systems Technology Conference, Hyogo, Japan, 31 October–3 November 2022; p. 2022-32-0078. [Google Scholar]
- Dallari, E.; Bononi, M.; Pola, A.; Tocci, M.; Veronesi, P.; Giovanardi, R. Pulsed Current Effect on the Hard Anodizing of an AlSi10Mg Aluminum Alloy Obtained via Additive Manufacturing. Surfaces 2023, 6, 97–113. [Google Scholar] [CrossRef]
- Rubben, T.; Revilla, R.T.; De Graeve, I. Effect of heat treatments on the anodizing behavior of additive manufactured AlSi10Mg. J. Electrochem. Soc. 2019, 166, C42–C48. [Google Scholar] [CrossRef]
- Hirata, T.; Kimura, T.; Nakamoto, T. Effect of Internal Pores on Fatigue Properties in Selective Laser Melted AlSi10Mg Alloy. Mater. Trans. 2022, 63, 1013–1020. [Google Scholar] [CrossRef]
- Wu, Z.; Wu, S.; Bao, J.; Qian, W.; Karabal, S.; Sun, W.; Withers, P. The effect of defect population on the anisotropic fatigue resistance of AlSi10Mg alloy fabricated by laser powder bed fusion. Int. J. Fatigue 2021, 151, 106317. [Google Scholar] [CrossRef]
- Lehner, P.; Blinn, B.; Beck, T. Improving the Defect Tolerance and Fatigue Strength of AM AlSi10Mg. Adv. Eng. Mater. 2023, 25, 2201855. [Google Scholar] [CrossRef]
- Roveda, I.; Serrano-Munoz, I.; Haubrich, J.; Requena, G.; Nadia, M. Investigation on the fatigue strength of AlSi10Mg fabricated by PBF-LB/M and subjected to low temperature heat treatments. Mater. Des. 2024, 244, 113170. [Google Scholar] [CrossRef]
- Sausto, F.; Tezzele, C.; Beretta, S. Analysis of Fatigue Strength of L-PBF AlSi10Mg with Different Surface Post-Processes: Effect of Residual Stresses. Metals 2022, 12, 898. [Google Scholar] [CrossRef]
- Brandão, A.D.; Gumpinger, J.; Gschweitl, M.; Seyfert, C.; Hofbauer, P.; Ghidini, T. Fatigue Properties Of Additively Manufactured AlSi10Mg—Surface Treatment Effect. Procedia Struct. Integr. 2017, 7, 58–66. [Google Scholar] [CrossRef]
- Fini, S.; Croccolo, D.; De Agostinis, M.; Olmi, G.; Paiardini, L.; Scapecchi, C.; Mele, M. Fatigue response of AlSi10Mg by laser powder bed fusion: Influence of build orientation, heat, and surface treatments. Prog. Addit. Manuf. 2024, 10, 1385–1403. [Google Scholar] [CrossRef]
- Linder, C.; Vucko, F.; Ma, T.; Proper, S.; Dartfelt, E. Corrosion-Fatigue Performance of 3D-Printed (L-PBF) AlSi10Mg. Materials 2023, 16, 5694. [Google Scholar] [CrossRef]
- Zhang, L.; Jiang, B.; Zhang, P.; Yan, H.; Xu, X.; Liu, R.; Tang, J.; Ren, C. Methods for fatigue-life estimation: A review of the current status and future trends. Nanotechnol. Precis. Eng. 2023, 6, 025001. [Google Scholar] [CrossRef]
- Lee, J.; Park, S.-Y.; Choi, B.-H. Evaluation of Fatigue Characteristics of Aluminum Alloys and Mechanical Components Using Extreme Value Statistics and C-Specimens. Metals 2021, 11, 1915. [Google Scholar] [CrossRef]
- Srinivasan, D.V.; Moradi, M.; Komninos, P.; Zarouchas, D.; Vassilopoulos, A.P. A generalized machine learning framework to estimate fatigue life across materials with minimal data. Mater. Des. 2024, 246, 113355. [Google Scholar] [CrossRef]
- JIS B0601:1994; Geometrical Product Specifications (GPS)-Surface Roughness-Definitions and Parameters. Japanese Standards Association: Minato-ku, Tokyo, Japan, 1994.
- JIS Z2371:2015; Corrosion Tests in Artificial Atmospheres-Salt Spray Test. Japanese Standards Association: Minato-ku, Tokyo, Japan, 2015.
- Chernyakova, K.; Tzaneva, B.; Vrublevsky, I.; Videkov, V. Effect of Aluminum Anode Temperature on Growth Rate and Structure of Nanoporous Anodic Alumina. J. Electrochem. Soc. 2020, 167, 103506. [Google Scholar] [CrossRef]
- Robbins, H.; Schwartz, B. Chemical Etching of Silicon I. The System HF, HNO3 and H2O. J. Electrochem. Soc. 1959, 106, 505–508. [Google Scholar] [CrossRef]
- Keller, F.; Hunter, M.S.; Robinson, D.L. Structural Features of Oxide Coatings on Aluminum. J. Electrochem. Soc. 1953, 100, 411–419. [Google Scholar] [CrossRef]
- Iwai, M.; Kikuchi, T. Fabrication of unique porous alumina films with extremely high porosity and an ultra-flat barrier layer by anodizing aluminum in sodium metaborate. Electrochim. Acta 2021, 399, 139440. [Google Scholar] [CrossRef]
- Schneider, M.; Fürbeth, W. Anodizing—The pore makes the difference. Mater. Corros. 2022, 73, 1752. [Google Scholar] [CrossRef]
- Maejima, M. Causes of Cracks in Aluminum Anodic Oxide Coating. J. Alum. Finish. Soc. Kinki 2018, 310, 7–12. (In Japanese) [Google Scholar]
- Mora-Sanchez, H.; del Olmo, R.; Rams, J.; Torres, B.; Mohedano, M.; Matykina, E.; Arrabal, R. Hard Anodizing and Plasma Electrolytic Oxidation of an Additively Manufactured Al-Si alloy. Surf. Coat. Technol. 2021, 420, 127339. [Google Scholar] [CrossRef]
- Zhang, P.; Zuo, Y. Effects of pore parameters on performance of anodic film on 2024 aluminum alloy. Mater. Chem. Phys. 2019, 231, 9. [Google Scholar] [CrossRef]
- Winter, L.; Lampke, T. Fatigue Resistance of an Anodized and Hardanodized 6082 Aluminum Alloy Depending on the Coating Thickness in the High Cycle Regime. Adv. Eng. Mater. 2023, 25, 2300394. [Google Scholar] [CrossRef]
- Malek, B.; Chaussumier, M.; Mabru, C. Effect of anodization and loading on fatigue life of 2618-T851 aluminum alloy. Int. J. Fract. 2022, 240, 209–220. [Google Scholar] [CrossRef]
- Shahzad, M.; Chaussumier, M.; Chieragatti, R.; Mabru, C.; Rezai-Aria, F. Influence of Anodizing Process on Fatigue Life of Machined Aluminium Alloy. Procedia Eng. 2010, 2, 1015–1024. [Google Scholar] [CrossRef]
- Sadeler, R. Effect of a commercial hard anodizing on the fatigue property of a 2014-T6 aluminium alloy. J. Mater. Sci. 2006, 41, 5803–5809. [Google Scholar] [CrossRef]
- Nakamura, Y.; Sakai, T.; Hirano, H.; Chandran, K.S.R. Effect of alumite surface treatments on long-life fatigue behavior of a cast aluminum in rotating bending. Int. J. Fatigue 2010, 32, 621–626. [Google Scholar] [CrossRef]
- Nasab, M.H.; Giussani, A.; Gastaldi, D.; Tirelli, V.; Vedani, M. Effect of Surface and Subsurface Defects on Fatigue Behavior of AlSi10Mg Alloy Processed by Laser Powder Bed Fusion (L-PBF). Metals 2019, 9, 1063. [Google Scholar] [CrossRef]
- Strauß, L.; Pang, G.A.; Löwisch, G. Fatigue life prediction of additively manufactured AlSi10Mg based on surface roughness and residual stress. Fatigue Fract. Eng. Mater. Struct. 2024, 47, 4465–4477. [Google Scholar] [CrossRef]
- Afroz, L.; Qian, M.; Forsmark, J.; Li, Y.; Easton, M.; Das, R. Fatigue life of laser powder bed fusion (L-PBF) AlSi10Mg alloy: Effects of surface roughness and porosity. Prog. Addit. Manuf. 2025, 10, 2423–2441. [Google Scholar] [CrossRef]
- Du Plessis, A.; Beretta, S. Killer notches: The effect of as-built surface roughness on fatigue failure in AlSi10Mg produced by laser powder bed fusion. Addit. Manuf. 2020, 35, 101424. [Google Scholar] [CrossRef]
- Alwitt, R.S.; Xu, J.; McClung, R.C. Stresses in Sulfuric Acid Anodized Coatings on Aluminum. J. Electrochem. Soc. 1993, 140, 1241–1246. [Google Scholar] [CrossRef]
- Laieghi, H.; Kvvssn, V.; Butt, M.M.; Ansari, P.; Salamci, M.U.; Patterson, A.E.; Salamci, E. Corrosion in laser powder bed fusion AlSi10Mg alloy. Eng. Rep. 2024, 6, e12984. [Google Scholar] [CrossRef]
- Martinez-Viademonte, M.P.; Abrahami, S.T.; Hack, T.; Burchardt, M.; Terryn, H. A Review on Anodizing of Aerospace Aluminum Alloys for Corrosion Protection. Coatings 2020, 10, 1106. [Google Scholar] [CrossRef]
- Ofoegbu, S.U.; Fernandes, F.A.O.; Pereira, A.B. The Sealing Step in Aluminum Anodizing: A Focus on Sustainable Strategies for Enhancing Both Energy Efficiency and Corrosion Resistance. Coatings 2020, 10, 226. [Google Scholar] [CrossRef]
- Jo, H.; Lee, S.; Kim, D.; Lee, J. Low Temperature Sealing of Anodized Aluminum Alloy for Enhancing Corrosion Resistance. Materials 2020, 13, 4904. [Google Scholar] [CrossRef]
- De Sousa Araujo, J.V.; Milagre, M.X.; Zhou, X.; Costa, I. An assessment of pitting corrosion in anodized aluminum alloys: It might not be what it seems. Mater. Corros. 2023, 75, 599–613. [Google Scholar] [CrossRef]
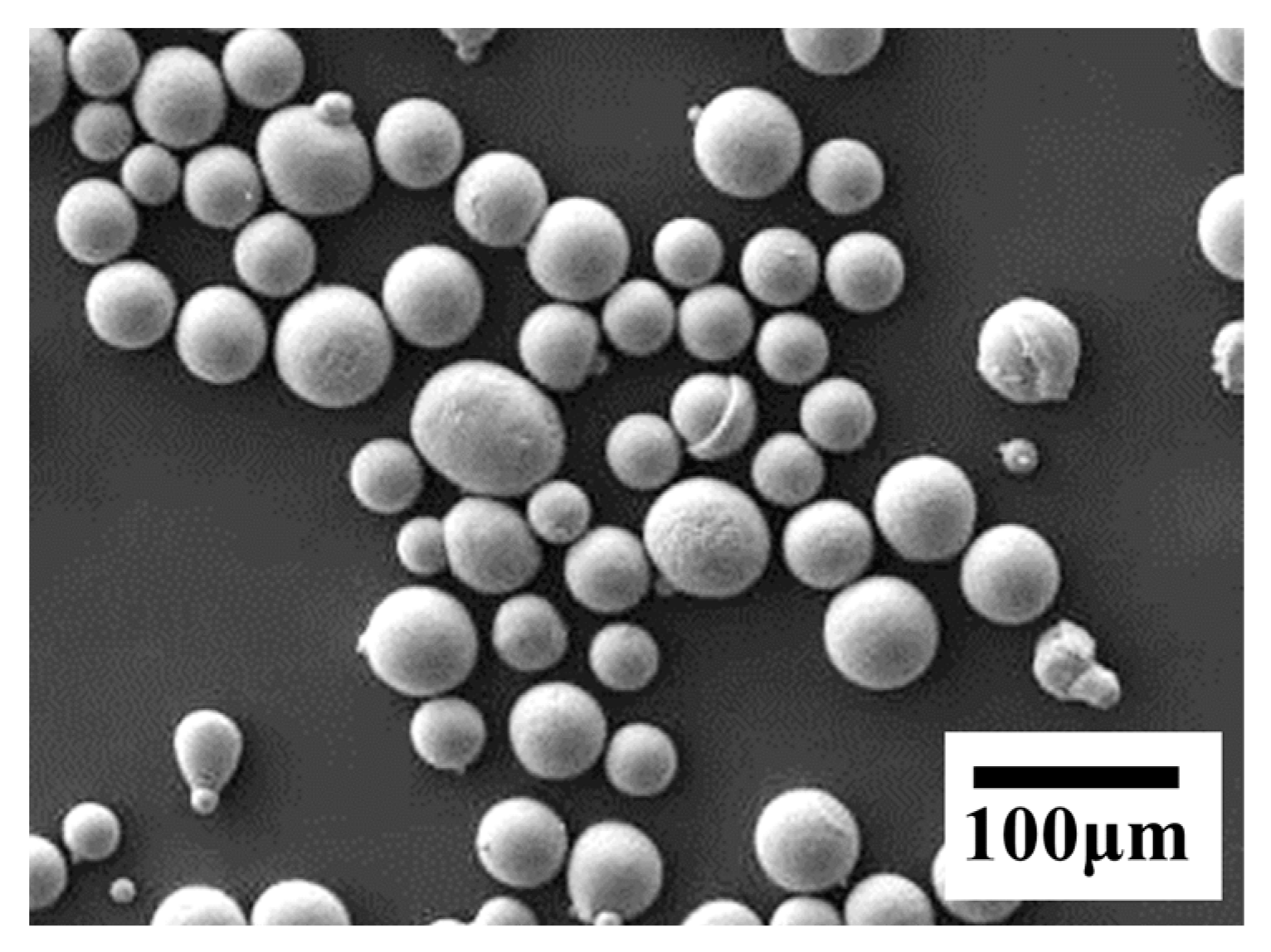

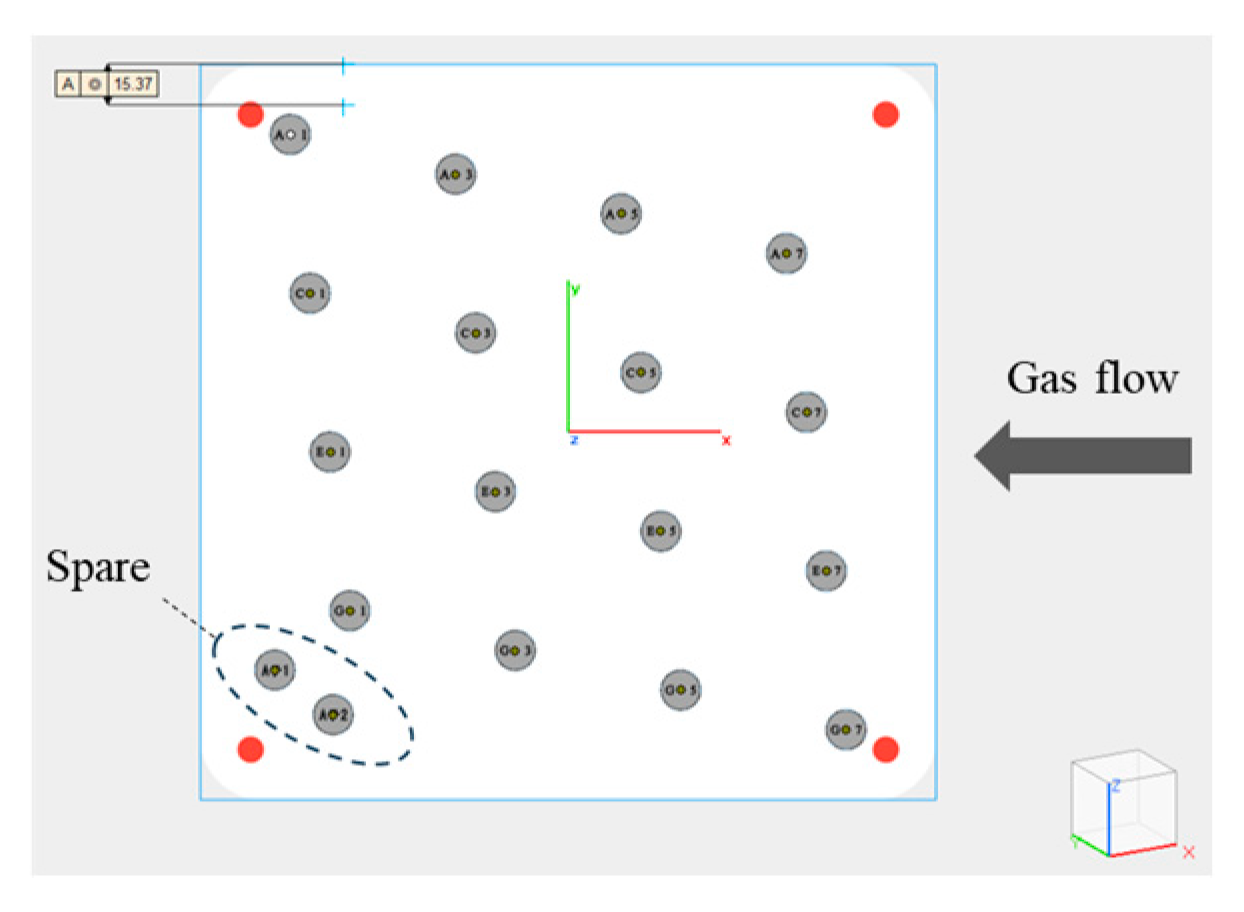











| Si | Mg | Fe | Ti, Mn, Ni, Cu, Zn | Al |
|---|---|---|---|---|
| 10.00 | 0.36 | 0.09 | 0.01 | Bal. |
| D10 | D50 | D90 |
|---|---|---|
| 27.68 | 44.87 | 64.83 |
| Apparent Density (g/cm3) | Packing Density (%) |
|---|---|
| 1.44 | 53.9 |
| 3D Printer | SLM280 (Nikon SLM Solutions) |
|---|---|
| Laser power | 650 W |
| Scan speed | 1850 mm/s |
| Layer thickness | 60 μm |
| Hatch distance | 0.17 mm |
| Focus | −4 mm |
| Plate temperature | 150 °C |
| Atmosphere | N2 |
| Anodize 1 | |||||
| Process | Chemicals | Concentration | Temperature | Treatment time | Current Density (Voltage) |
| Degreasing | TOP ALCLEAN 101 (EDCM) Weak Alkaline type | 30 g/L | 60 °C | 2 min | N/A |
| Anodizing | H2SO4 | 180 g/L | 20 ± 1 °C | 85 min | 1.0 A/dm2 (15–35 V) |
| Sealing | TOP SEAL H-298 Ni Acetate type | 40 mL/L | 92 °C | 30 min | N/A |
| Anodize 2 | |||||
| Process | Chemicals | Concentration | Temperature | Treatment time | Current Density (Voltage) |
| Degreasing | TOP ALCLEAN 101 (EDCM) Weak Alkaline type | 30 g/L | 60 °C | 2 min | N/A |
| Anodizing | H2SO4 | 180 g/L | 5 ± 1 °C | 70 min | 1.0 A/dm2 (35–50 V) |
| Sealing | TOP SEAL H-298 Ni Acetate type | 40 mL/L | 92 °C | 30 min | N/A |
| Average | 99.75 |
|---|---|
| Maximum | 99.78 |
| Minimum | 99.69 |
| σ | 0.02 |
| Anodize 1 | Anodize 2 | |
|---|---|---|
| Average | 19.75 | 20.30 |
| Maximum | 20.64 | 21.13 |
| Minimum | 19.04 | 19.11 |
| σ | 0.59 | 0.76 |
| Parameter | As-Machined | Anodize 1 | Anodize 2 |
|---|---|---|---|
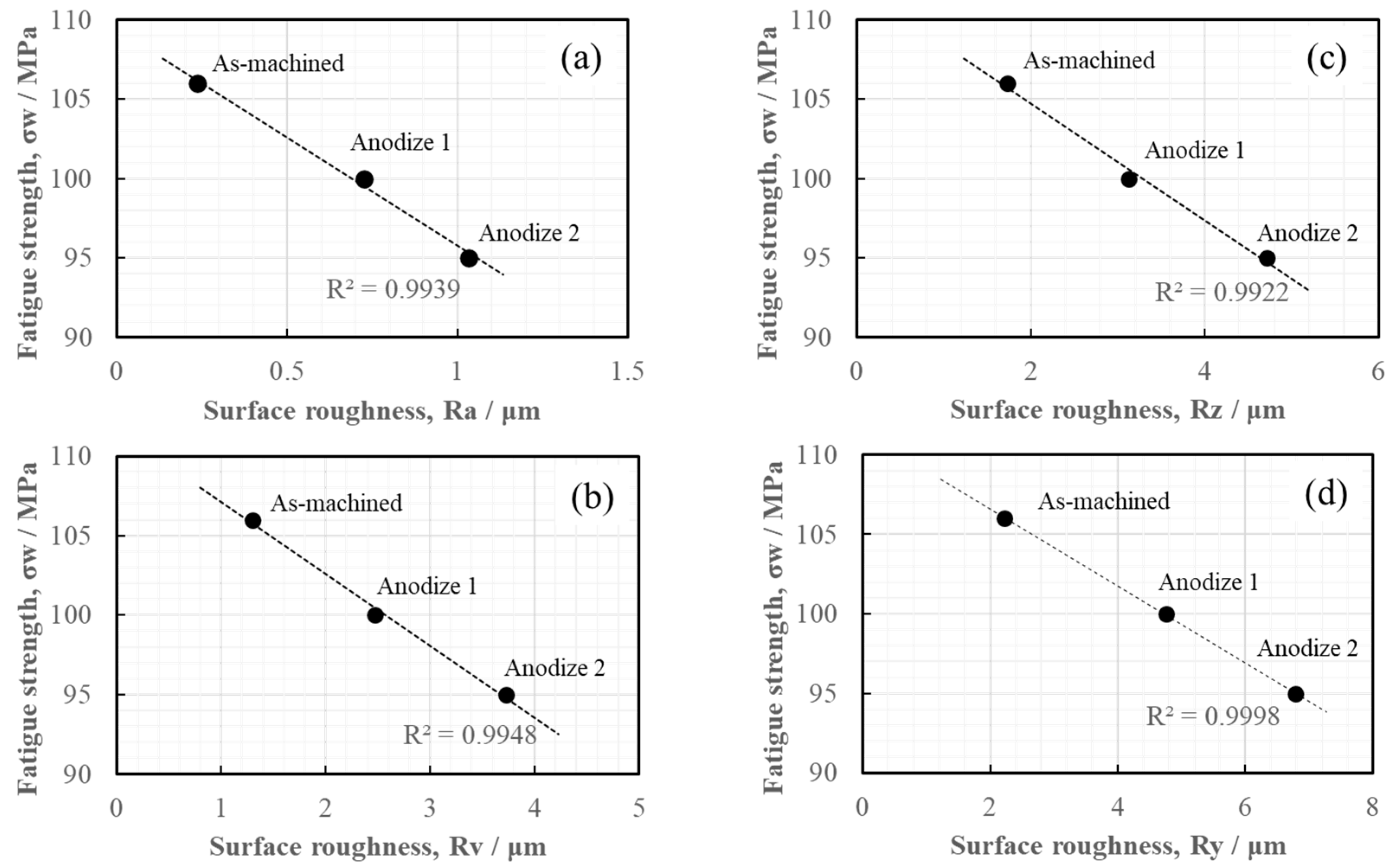
| Ra | 0.235 | 0.725 | 1.033 |
| Rz | 1.728 | 3.127 | 4.711 |
| Rv | 1.300 | 2.471 | 3.726 |
| Ry | 2.228 | 4.763 | 6.799 |
| S | 15.329 | 30.353 | 32.874 |
| Anodize 1 | Anodize 2 | |
|---|---|---|
| Average | 285 | 343 |
| Maximum | 297 | 367 |
| Minimum | 265 | 324 |
| Parameter | Polished Anodize 2 |
|---|---|
| Ra | 0.097 |
| Rz | 0.454 |
| Rv | 0.397 |
| Ry | 0.705 |
| S | 22.110 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kurita, H.; Tako, S.; Tanaka, C.; Hara, K.; Matsushima, K.; Satsukawa, K.; Watanabe, K.; Kyogoku, H. Anodizing 3D-Printed AlSi10Mg Alloy and Its Fatigue Properties. Metals 2025, 15, 1022. https://doi.org/10.3390/met15091022
Kurita H, Tako S, Tanaka C, Hara K, Matsushima K, Satsukawa K, Watanabe K, Kyogoku H. Anodizing 3D-Printed AlSi10Mg Alloy and Its Fatigue Properties. Metals. 2025; 15(9):1022. https://doi.org/10.3390/met15091022
Chicago/Turabian StyleKurita, Hirotaka, Shinya Tako, Chika Tanaka, Kenji Hara, Kazunori Matsushima, Koji Satsukawa, Keita Watanabe, and Hideki Kyogoku. 2025. "Anodizing 3D-Printed AlSi10Mg Alloy and Its Fatigue Properties" Metals 15, no. 9: 1022. https://doi.org/10.3390/met15091022
APA StyleKurita, H., Tako, S., Tanaka, C., Hara, K., Matsushima, K., Satsukawa, K., Watanabe, K., & Kyogoku, H. (2025). Anodizing 3D-Printed AlSi10Mg Alloy and Its Fatigue Properties. Metals, 15(9), 1022. https://doi.org/10.3390/met15091022





