Mechanochemical Activation as a Key Step for Enhanced Ammonia Leaching of Spent LiCoO2 Cathodes
Abstract
1. Introduction
2. Materials and Methods
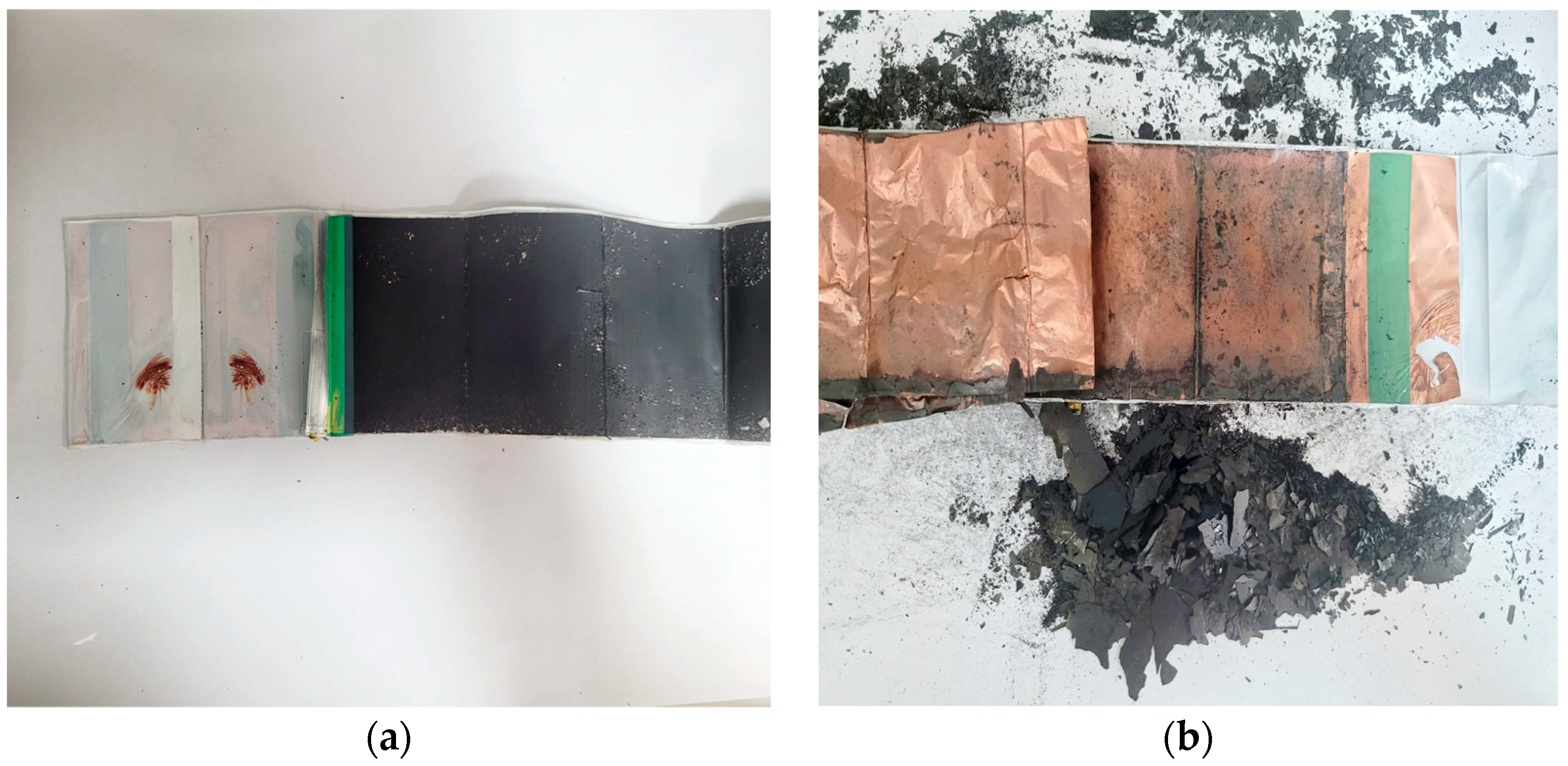
2.1. Materials
2.2. Mechanical Activation
2.3. Analytical Techniques
2.4. Leaching Experiments
3. Results and Discussion
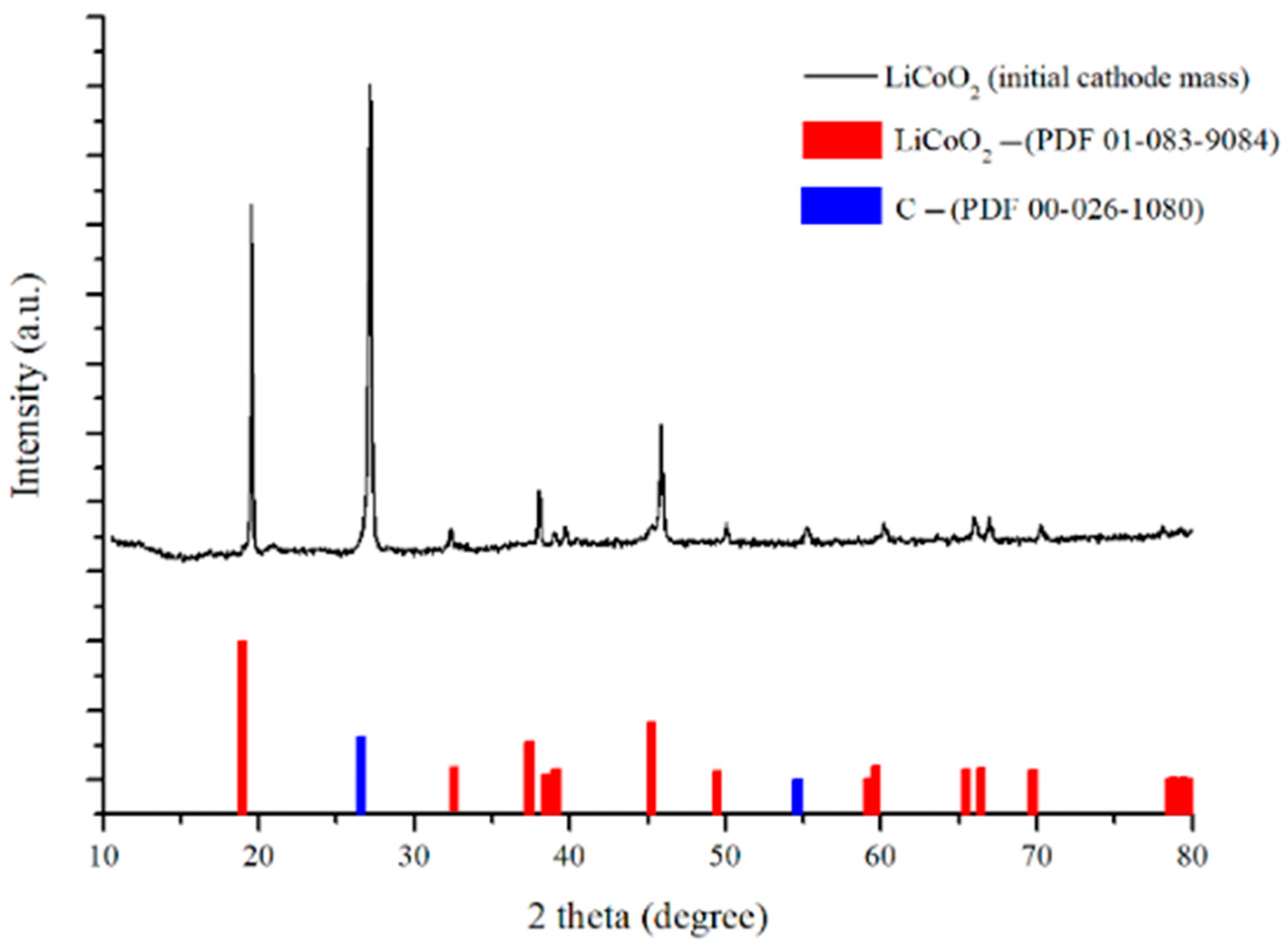
3.1. LCO Cathode Material Characterization
3.2. Characterization of the LCO Cathode Material After Mechanical Activation
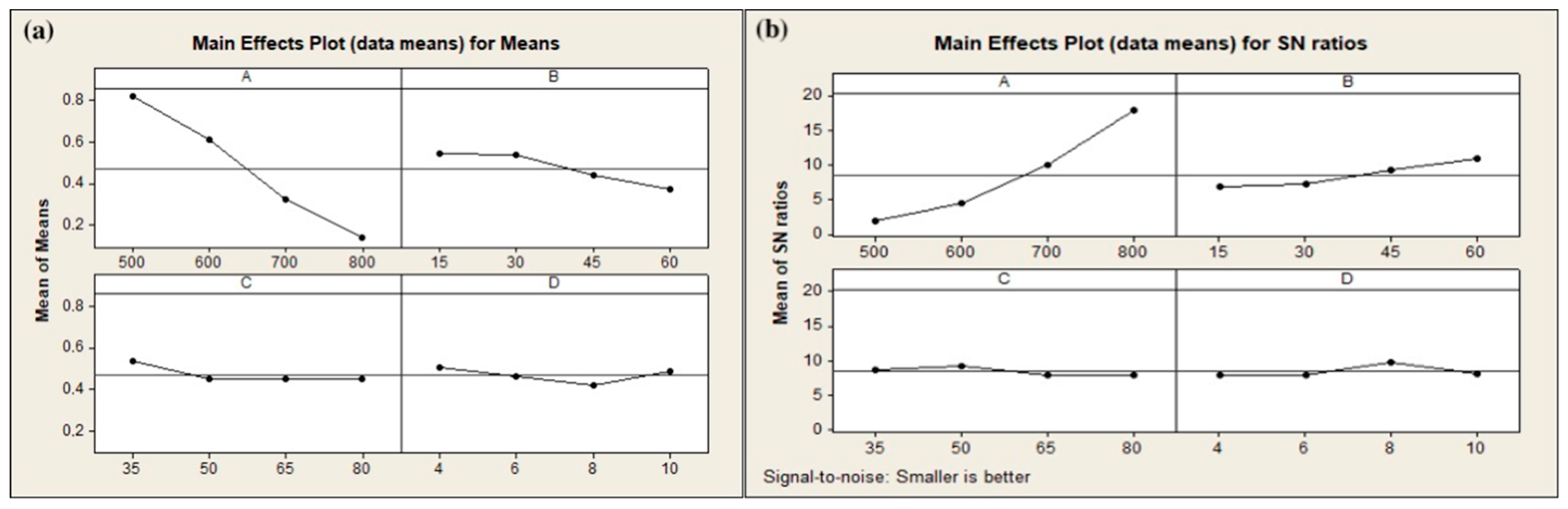
3.2.1. Optimization of Mechanical Activation Conditions
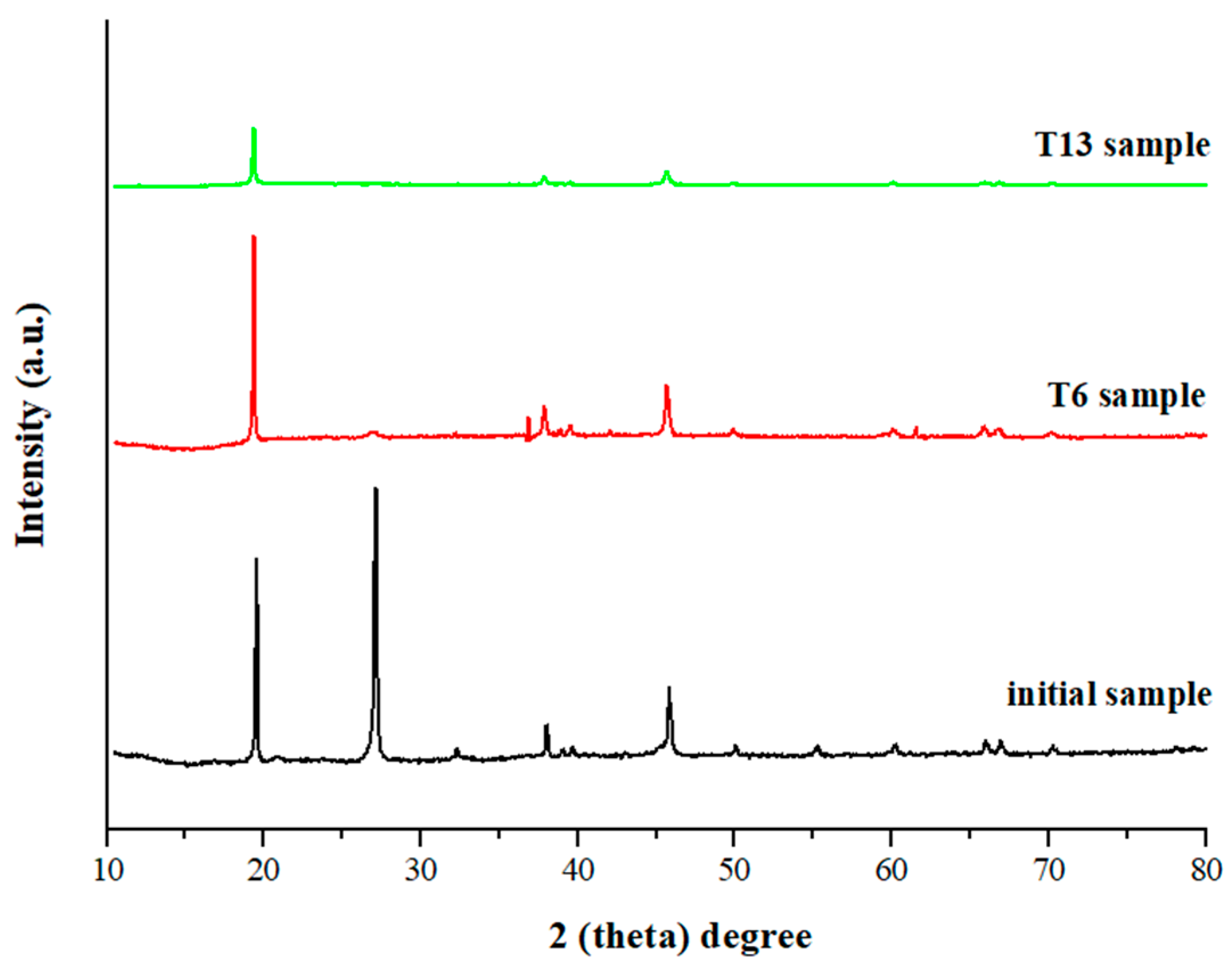
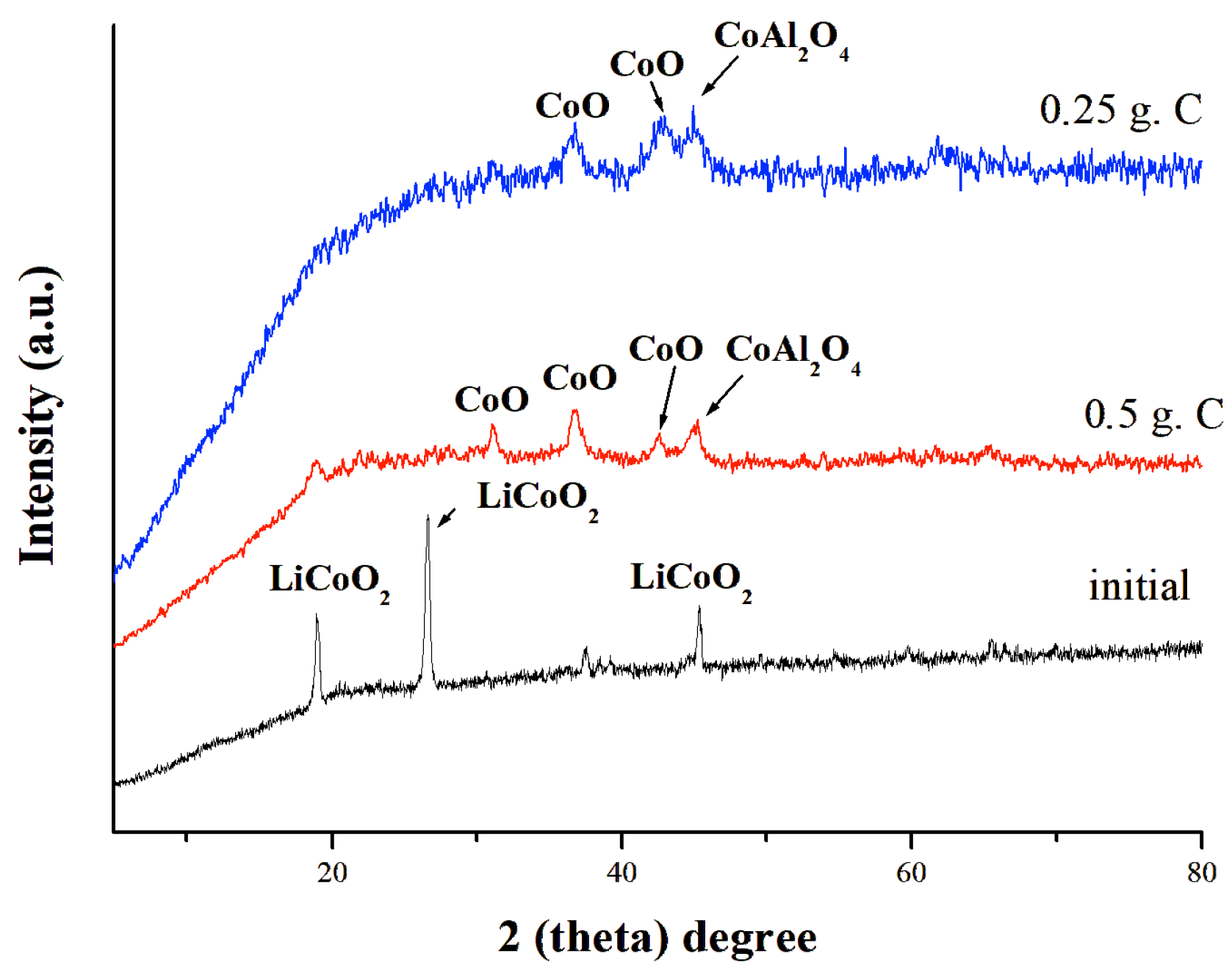
3.2.2. Structural Changes After Mechanical Activation
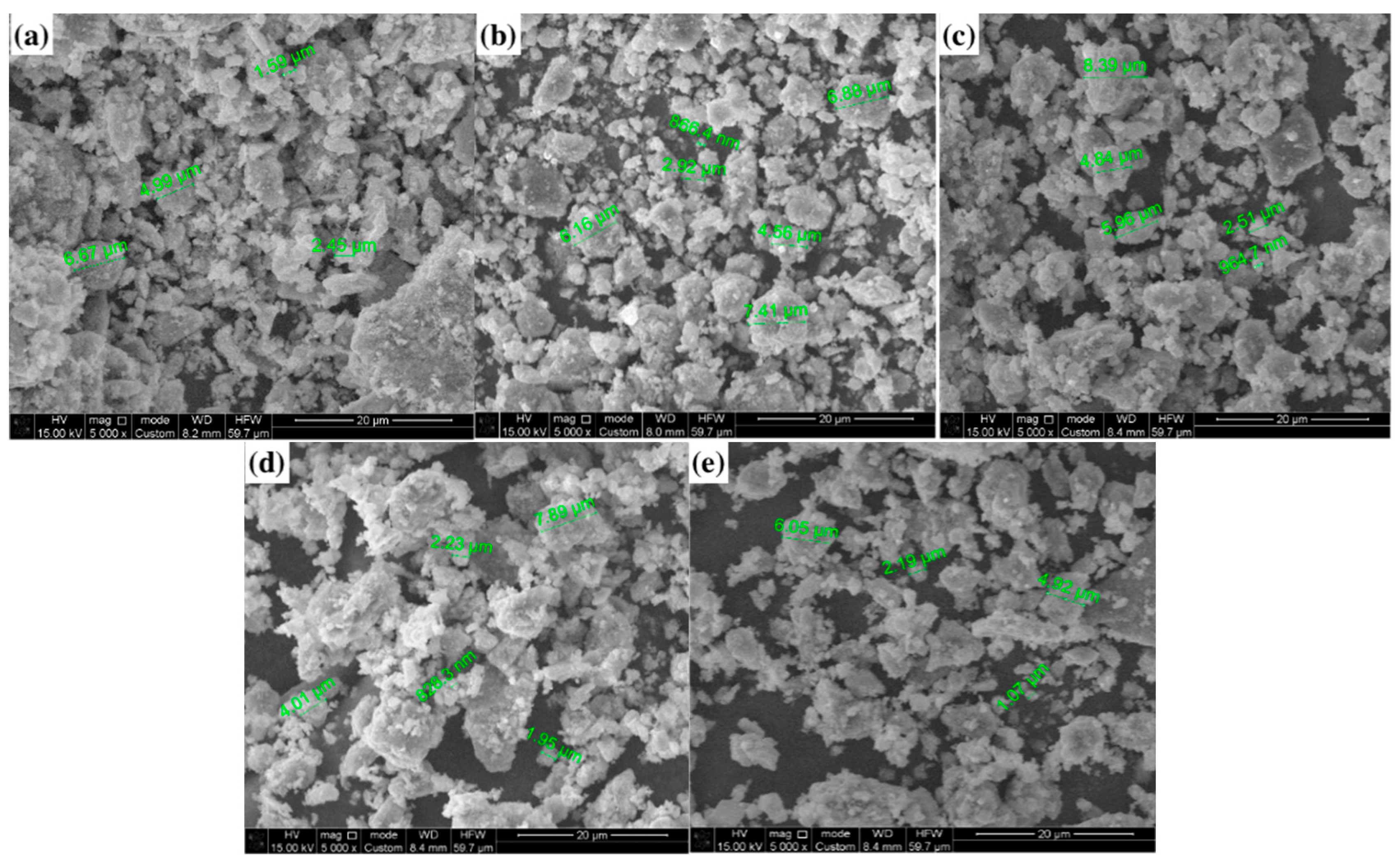
3.2.3. Changes in Morphology After Mechanical Activation
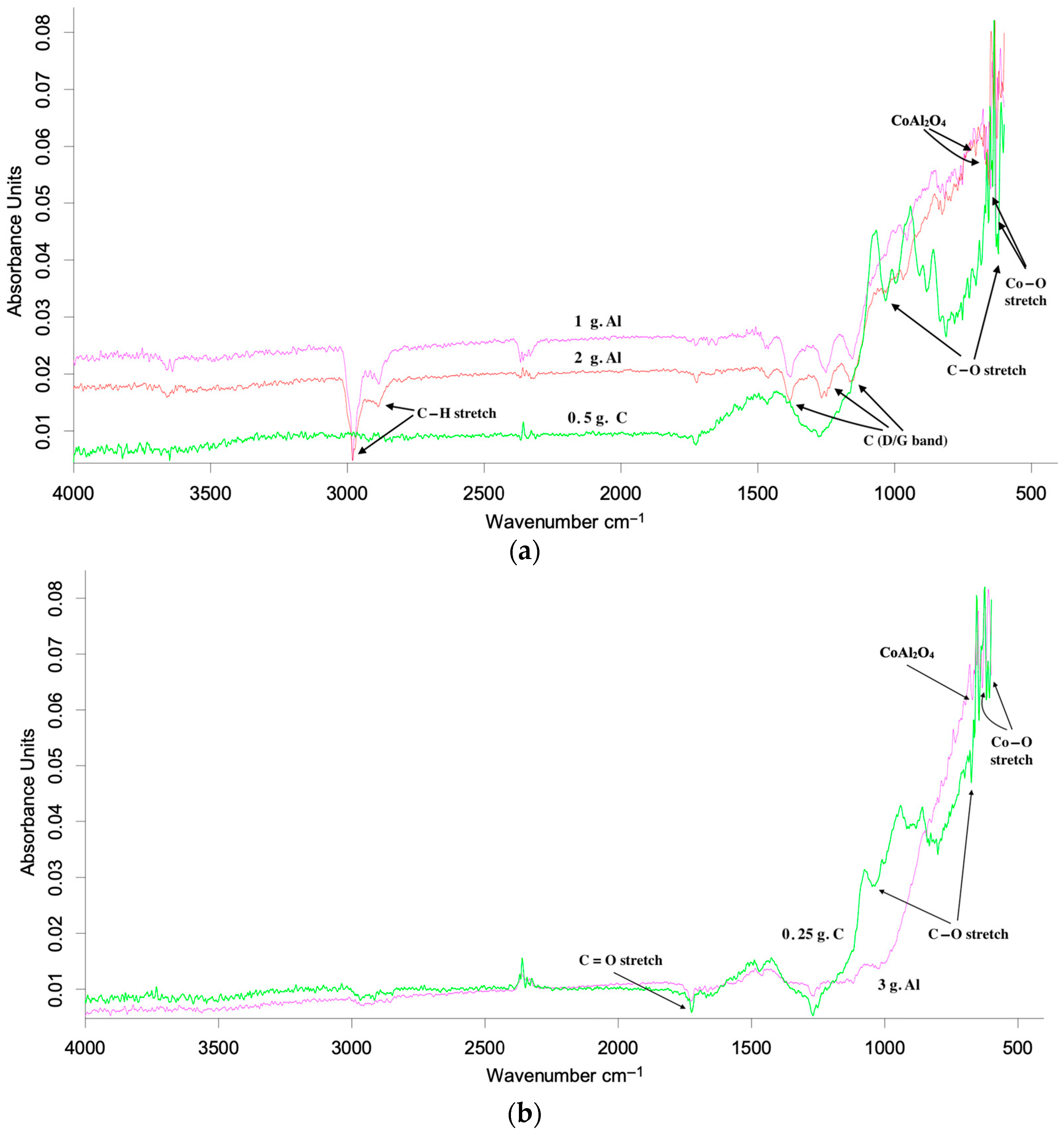
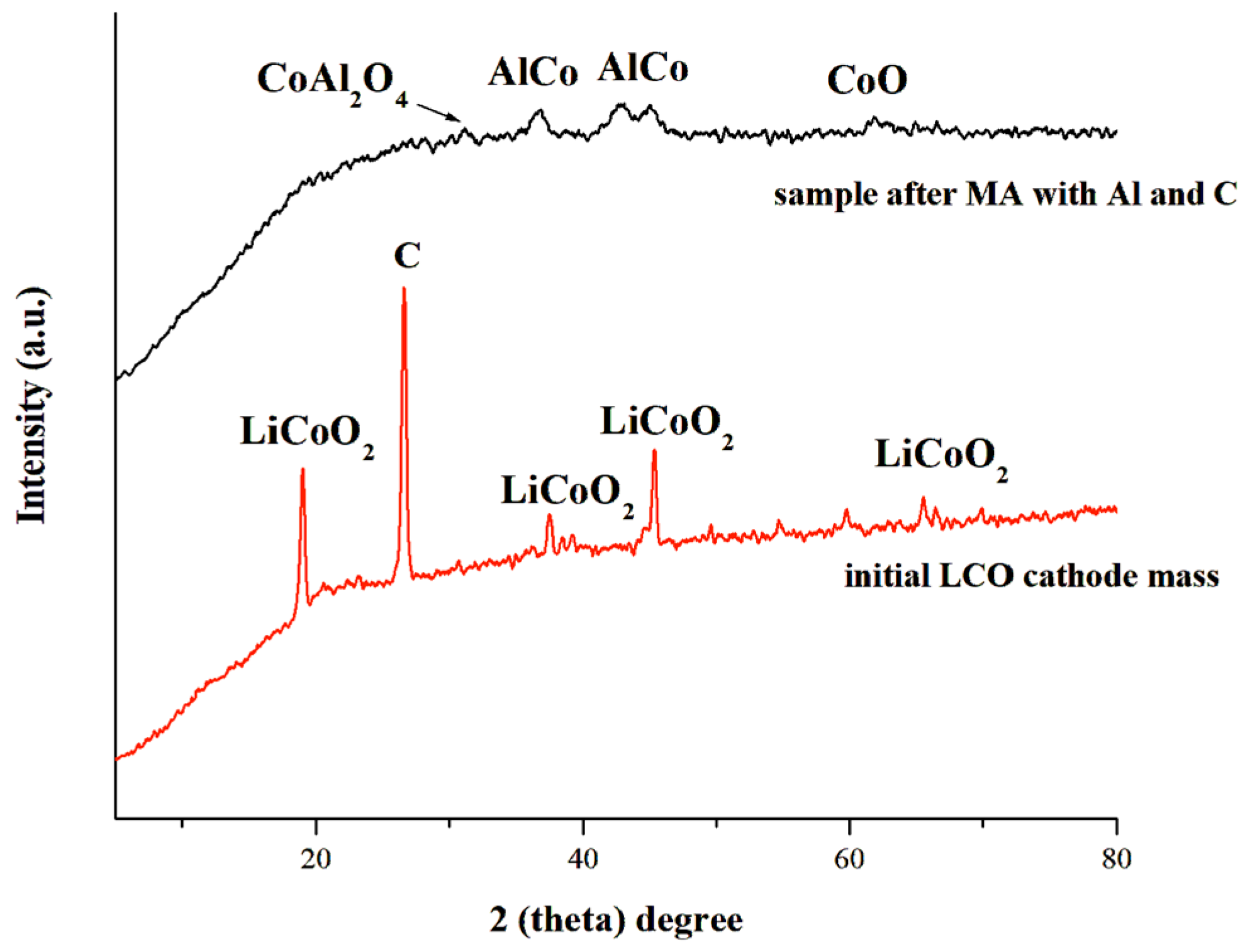
3.2.4. Influence of Aluminum and Carbon Additives on the Phase Composition of the Initial Cathode Material
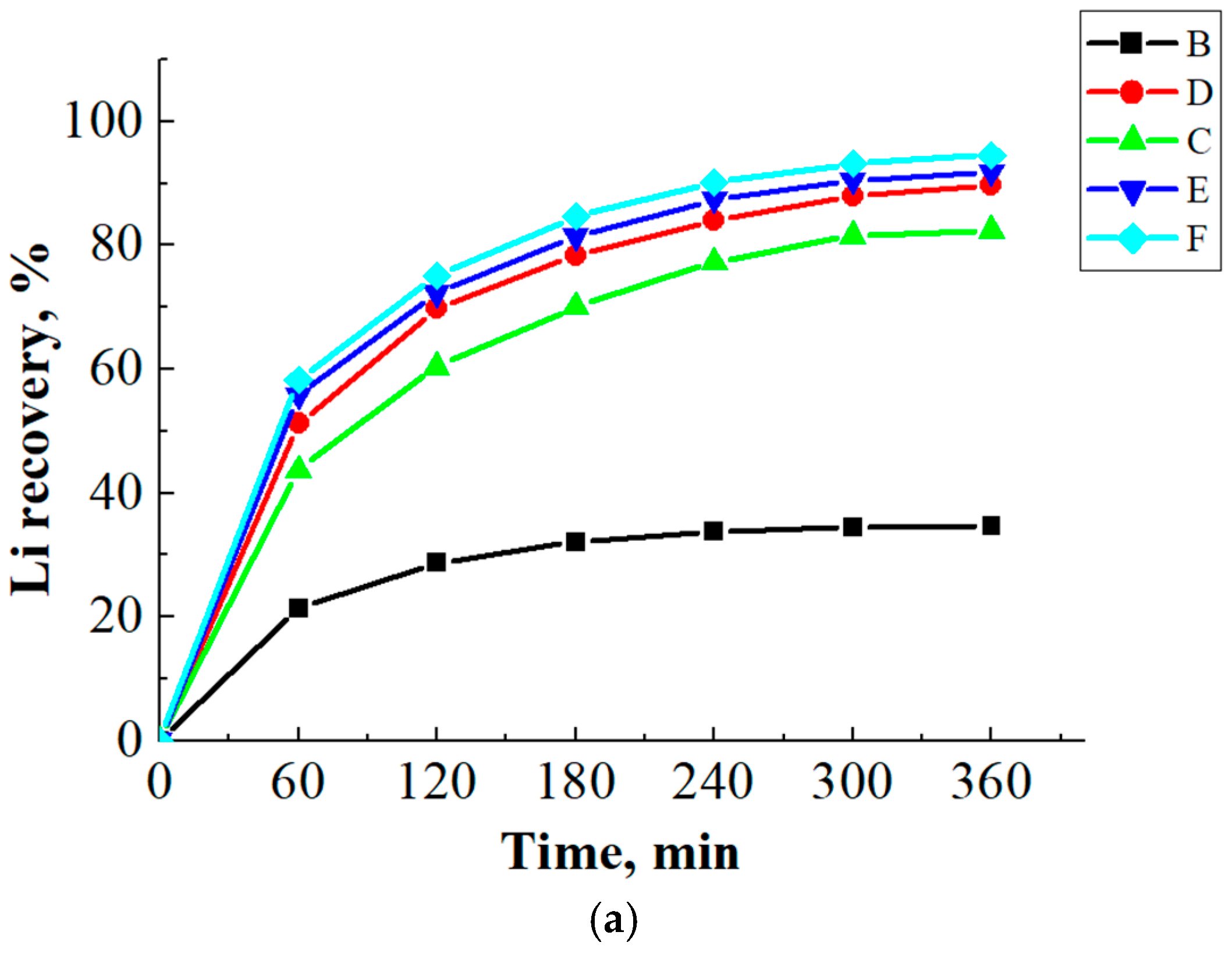
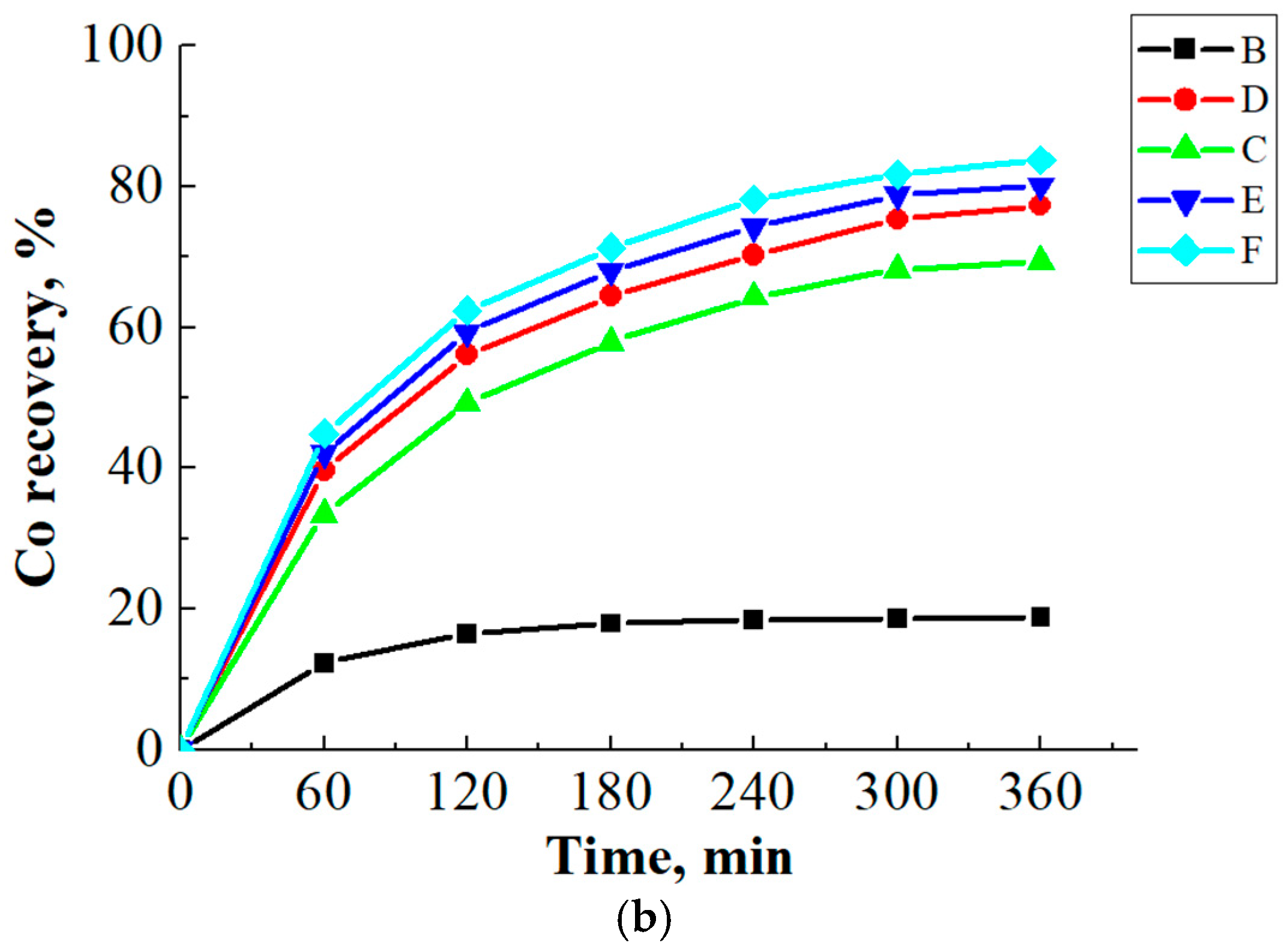
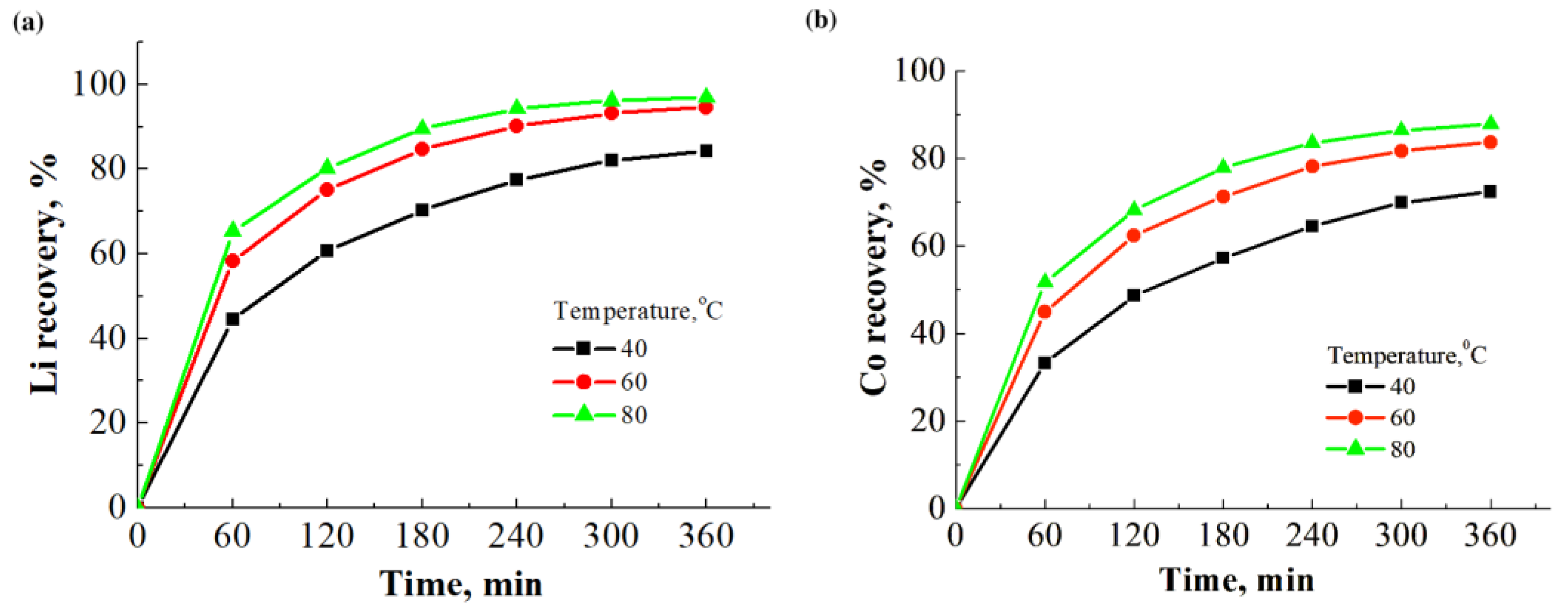
3.3. Ammonia Leaching of Spent LCO Cathode Material
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Biswal, B.K.; Zhang, B.; Tran, P.T.M.; Zhang, J.; Balasubramanian, R. Recycling of spent lithium-ion batteries for a sustainable future: Recent advancements. Chem. Soc. Rev. 2024, 53, 5552–5592. [Google Scholar] [CrossRef] [PubMed]
- Tian, G.; Yuan, G.; Aleksandrov, A.; Zhang, T.; Li, Z.; Fathollahi-Fard, A.M.; Ivanov, M. Recycling of spent Lithium-ion Batteries: A comprehensive review for identification of main challenges and future research trends. Sustain. Energy Technol. Assess. 2022, 53, 102447. [Google Scholar]
- Etude, M.C.; Ikeuba, A.I.; Njoku, C.N.; Yakubu, E.; Uzoma, H.C.; Mgbemere, C.E.; Udunwa, D.I. Recycling lithium-ion batteries: A review of current status and future directions. Sustain. Chem. One World 2024, 4, 100027. [Google Scholar] [CrossRef]
- Fan, E.; Li, L.; Wang, Z.; Lin, J.; Huang, Y.; Yao, Y.; Chen, R.; Wu, F. Sustainable recycling technology for Li-ion batteries and beyond: Challenges and future prospects. Chem. Rev. 2020, 120, 7020–7063. [Google Scholar] [CrossRef]
- Li, P.; Luo, S.; Lin, Y.; Xiao, J.; Xia, X.; Liu, X.; Wang, L.; He, X. Fundamentals of the recycling of spent lithium-ion batteries. Chem. Soc. Rev. 2024, 53, 11967–12013. [Google Scholar] [CrossRef]
- Lv, W.; Wang, Z.; Cao, H.; Sun, Y.; Zhang, Y.; Sun, Z. A critical review and analysis on the recycling of spent lithium-ion batteries. ACS Sustain. Chem. Eng. 2018, 6, 1504–1521. [Google Scholar] [CrossRef]
- Zeng, X.; Li, J.; Liu, L. Solving spent lithium-ion battery problems in China: Opportunities and challenges. Renew. Sustain. Energy Rev. 2015, 52, 1759–1767. [Google Scholar] [CrossRef]
- Ordoñez, J.; Gago, E.J.; Girard, A. Processes and technologies for the recycling and recovery of spent lithium-ion batteries. Renew. Sustain. Energy Rev. 2016, 60, 195–205. [Google Scholar] [CrossRef]
- Liu, C.; Lin, J.; Cao, H.; Zhang, Y.; Sun, Z. Recycling of spent lithium-ion batteries in view of lithium recovery: A critical review. J. Clean. Prod. 2019, 228, 801–813. [Google Scholar] [CrossRef]
- Huang, B.; Pan, Z.; Su, X.; An, L. Recycling of lithium-ion batteries: Recent advances and perspectives. J. Power Sources 2018, 399, 274–286. [Google Scholar] [CrossRef]
- Makuza, B.; Tian, Q.; Guo, X.; Chattopadhyay, K.; Yu, D. Pyrometallurgical options for recycling spent lithium-ion batteries: A comprehensive review. J. Power Sources 2021, 491, 229622. [Google Scholar] [CrossRef]
- Assefi, M.; Maroufi, S.; Yamauchi, Y.; Sahajwalla, V. Pyrometallurgical recycling of Li-ion, Ni–Cd and Ni–MH batteries: A minireview. Curr. Opin. Green Sustain. Chem. 2020, 24, 26–31. [Google Scholar] [CrossRef]
- Sethurajan, M.; van Hullebusch, E.D.; Fontana, D.; Akcil, A.; Deveci, H.; Batinic, B.; Leal, J.P.; Gasche, T.A.; Kucuker, M.A.; Kuchta, K.; et al. Recent advances on hydrometallurgical recovery of critical and precious elements from end of life electronic wastes-a review. Crit. Rev. Environ. Sci. Technol. 2019, 49, 212–275. [Google Scholar]
- Jung, J.C.Y.; Sui, P.C.; Zhang, J. A review of recycling spent lithium-ion battery cathode materials using hydrometallurgical treatments. J. Energy Storage 2021, 35, 102217. [Google Scholar] [CrossRef]
- Yao, Y.; Zhu, M.; Zhao, Z.; Tong, B.; Fan, Y.; Hua, Z. Hydrometallurgical processes for recycling spent lithium-ion batteries: A critical review. ACS Sustain. Chem. Eng. 2018, 6, 13611–13627. [Google Scholar] [CrossRef]
- Sattar, R.; Ilyas, S.; Bhatti, H.N.; Ghaffar, A. Resource recovery of critically-rare metals by hydrometallurgical recycling of spent lithium ion batteries. Sep. Purif. Technol. 2019, 209, 725–733. [Google Scholar] [CrossRef]
- Zhang, C.; Zhuang, L.; Wang, J.; Bai, J.; Yuan, W. Extraction of zinc from zinc ferrites by alkaline leaching: Enhancing recovery by mechanochemical reduction with metallic iron. J. S. Afr. Inst. Min. Metall. 2016, 116, 1111–1114. [Google Scholar]
- Zhang, C.; Zhuang, L.; Yuan, W.; Wang, J.; Bai, J. Extraction of lead from spent leaded glass in alkaline solution by mechanochemical reduction. Hydrometallurgy 2016, 165, 312–317. [Google Scholar] [CrossRef]
- Chen, H.; Zhou, S.; Han, Z.; Jiang, Y.; Yu, H.; Zhou, X.; Li, X. A copper-based sorbent with oxygen-vacancy defects from mechanochemical reduction for carbon disulfide absorption. J. Mater. Chem. A 2016, 4, 17207–17214. [Google Scholar]
- Sohn, M.; Park, E.; Yoo, B.M.; Han, T.H.; Park, H.B.; Kim, H. Metal-assisted mechanochemical reduction of graphene oxide. Carbon 2016, 110, 79–86. [Google Scholar] [CrossRef]
- Van Loy, S.; Önal, M.A.R.; Binnemans, K.; Van Gerven, T. Recovery of valuable metals from NdFeB magnets by mechanochemically assisted ferric sulfate leaching. Hydrometallurgy 2020, 191, 105154. [Google Scholar] [CrossRef]
- Akhgar, B.N.; Pourghahramani, P. Impact of mechanical activation and mechanochemical activation on natural pyrite dissolution. Hydrometallurgy 2015, 153, 83–87. [Google Scholar] [CrossRef]
- Gao, Y.; Li, Y.; Li, J.; Xie, H.; Chen, Y. Direct recovery of LiCoO2 from the recycled lithium-ion batteries via structure restoration. J. Alloys Compd. 2020, 845, 156234. [Google Scholar] [CrossRef]
- Wang, M.; Liu, K.; Yu, J.; Zhang, C.C.; Zhang, Z.; Tan, Q. Recycling spent lithium-ion batteries using a mechanochemical approach. Circ. Econ. 2022, 1, 100012. [Google Scholar] [CrossRef]
- Wang, S.; Yan, S.; Chen, Z.; Ou, Y.; Mahara, B.; Chen, X.; Yang, Y.; Zhou, T. Boosting the sustainable recycling of spent lithium-ion batteries through mechanochemistry. Green Chem. 2025, 27, 9917–9926. [Google Scholar] [CrossRef]
- Batkal, A.; Kamunur, K.; Mussapyrova, L.; Mukanov, Y.; Nadirov, R. Efficient Extraction of Lithium, Cobalt, and Nickel from Nickel-Manganese-Cobalt Oxide Cathodes with Cholin Chloride/Pyrogallol-Based Deep Eutectic Solvent. Recycling 2025, 10, 88. [Google Scholar] [CrossRef]
- Ketegenov, T.; Kamunur, K.; Mussapyrova, L.; Batkal, A.; Nadirov, R. Enhanced recovery of lithium and cobalt from spent lithium-ion batteries using ultrasound-assisted deep eutectic solvent leaching. Metals 2024, 14, 1052. [Google Scholar] [CrossRef]
- Xiao, W.; Yang, S.; Zhang, P.; Li, P.; Wu, P.; Li, M.; Chen, N.; Jie, K.; Huang, C.; Zhang, N.; et al. Facile synthesis of highly porous metal oxides by mechanochemical nanocasting. Chem. Mater. 2018, 30, 2924–2929. [Google Scholar] [CrossRef]
- Dolotko, O.; Hlova, I.Z.; Mudryk, Y.; Gupta, S.; Balema, V.P. Mechanochemical recovery of Co and Li from LCO cathode of lithium-ion battery. J. Alloys Compd. 2020, 824, 153876. [Google Scholar] [CrossRef]
- Deng, Y.; Kang, T.; Ma, Z.; Tan, X.; Song, X.; Wang, Z.; Pang, P.; Shu, D.; Zuo, X.; Nan, J. Safety influences of the Al and Ti elements modified LiCoO2 materials on LiCoO2/graphite batteries under the abusive conditions. Electrochim. Acta 2019, 295, 703–709. [Google Scholar] [CrossRef]
- Dang, S.; Zhou, P.; Shi, P.; Min, Y.; Xu, Q. In situ aluminothermic reduction induced by mechanochemical activation enhances the ability of the spent LiCoO2 cathode to activate peroxymonosulfate. ACS Sustain. Chem. Eng. 2021, 9, 15375–15385. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, Y.; Liu, X.; Xu, S. A simplified process for recovery of Li and Co from spent LiCoO2 cathode using Al foil as the in situ reductant. ACS Sustain. Chem. Eng. 2019, 7, 12222–12230. [Google Scholar] [CrossRef]
- Krishnaiah, K.; Shahabudeen, P. Applied Design of Experiments and Taguchi Methods; PHI Learning Pvt. Ltd.: Delhi, India, 2012. [Google Scholar]
- Nowak, S.; Winter, M. Elemental analysis of lithium ion batteries. J. Anal. At. Spectrom. 2017, 32, 1833–1847. [Google Scholar] [CrossRef]
- Lakshmanan, R.; Gangulibabu; Bhuvaneswari, D.; Kalaiselvi, N. Temperature dependent surface morphology and lithium diffusion kinetics of LiCoO2 cathode. Met. Mater. Int. 2012, 18, 249–255. [Google Scholar] [CrossRef]
- Tuinstra, F.; Koenig, J.L. Raman spectrum of graphite. J. Chem. Phys. 1970, 53, 1126–1130. [Google Scholar] [CrossRef]
- Setoudeh, N.; Nosrati, A.; Welham, N.J. Lithium recovery from mechanically activated mixtures of lepidolite and sodium sulfate. Miner. Process. Extr. Metall. 2021, 130, 354–361. [Google Scholar] [CrossRef]
- Alyosif, B.; Uysal, T.; Aydemir, M.K.; Erdemoğlu, M. Contribution of mechanical activation for obtaining potassium chloride from microcline. Min. Metall. Explor. 2023, 40, 1311–1319. [Google Scholar] [CrossRef]
- Vieceli, N.; Nogueira, C.A.; Pereira, M.F.; Dias, A.P.S.; Durao, F.O.; Guimarães, C.; Margarido, F. Effects of mechanical activation on lithium extraction from a lepidolite ore concentrate. Miner. Eng. 2017, 102, 1–14. [Google Scholar] [CrossRef]
- Liu, X.; Huang, K.; Xiong, H.; Dong, H. Ammoniacal leaching process for the selective recovery of value metals from waste lithium-ion batteries. Environ. Technol. 2023, 44, 211–225. [Google Scholar] [CrossRef]
- Wang, M.M.; Zhang, C.C.; Zhang, F.S. An environmental benign process for cobalt and lithium recovery from spent lithium-ion batteries by mechanochemical approach. Waste Manag. 2016, 51, 239–244. [Google Scholar] [CrossRef]
- Guan, J.; Li, Y.; Guo, Y.; Su, R.; Gao, G.; Song, H.; Yuan, H.; Liang, B.; Guo, Z. Mechanochemical process enhanced cobalt and lithium recycling from wasted lithium-ion batteries. ACS Sustain. Chem. Eng. 2017, 5, 1026–1032. [Google Scholar]
- Batkal, A.; Kamunur, K.; Mussapyrova, L.; Milikhat, B.; Nadirov, R. Optimized Ammonia Leaching and Energy-Efficient Stripping for Lithium and Cobalt Recovery from Spent LiCoO2 Cathodes. Metals 2025, 15, 690. [Google Scholar] [CrossRef]




| Component | (wt.%) | Component | (wt.%) |
|---|---|---|---|
| O | 26.278 | P | 0.38 |
| Na | 5.595 | Cl | 5.222 |
| Mg | 0.303 | Ti | 0.164 |
| Al | 10.198 | Cu | 3.155 |
| Si | 0.077 | Zn | 0.015 |
| Fe | 0.038 | ||
| Co | 44.891 | ||
| Li | 3.684 | ||
| Total | 100 |
| Factor | Level 1 | Level 2 | Level 3 | Level 4 |
|---|---|---|---|---|
| Rotation speed (rpm) | 500 | 600 | 700 | 800 |
| Milling time (min) | 15 | 30 | 45 | 60 |
| Ball-to-powder ratio | 35 | 50 | 65 | 80 |
| Ball diameter (mm) | 4 | 6 | 8 | 10 |
| № | rpm (A) | Time (B) | BPR (C) | Ball Diameter (mm) (D) | Relative Intensity of LiCoO2 (003) Diffraction |
|---|---|---|---|---|---|
| T1 | 500 | 15 | 35 | 4 | 1.00 |
| T2 | 500 | 30 | 50 | 6 | 0.88 |
| T3 | 500 | 45 | 65 | 8 | 0.73 |
| T4 | 500 | 60 | 80 | 10 | 0.66 |
| T5 | 600 | 15 | 50 | 8 | 0.55 |
| T6 | 600 | 30 | 35 | 10 | 0.77 |
| T7 | 600 | 45 | 80 | 4 | 0.61 |
| T8 | 600 | 60 | 65 | 6 | 0.50 |
| T9 | 700 | 15 | 65 | 10 | 0.42 |
| T10 | 700 | 30 | 80 | 8 | 0.33 |
| T11 | 700 | 45 | 35 | 6 | 0.29 |
| T12 | 700 | 60 | 50 | 4 | 0.25 |
| T13 | 800 | 15 | 80 | 6 | 0.19 |
| T14 | 800 | 30 | 65 | 4 | 0.16 |
| T15 | 800 | 45 | 50 | 10 | 0.11 |
| T16 | 800 | 60 | 35 | 8 | 0.08 |
| Source | DF | SS | MS | F | p |
|---|---|---|---|---|---|
| rpm | 3 | 1.0946 | 0.3649 | 32.64 | 0.000 |
| Error | 12 | 0.1341 | 0.0112 | ||
| Total | 15 | 1.2287 | |||
| S = 0.1057; R − Sq = 89.08% | |||||
| time, min | 3 | 0.0794 | 0.0265 | 0.28 | 0.841 |
| Error | 12 | 1.1493 | 0.0958 | ||
| Total | 15 | 1.2287 | |||
| S = 0.3095; R − Sq = 6.46% | |||||
| BPR | 3 | 0.022 | 0.007 | 0.07 | 0.973 |
| Error | 12 | 1.207 | 0.101 | ||
| Total | 15 | 1.229 | |||
| S = 0.3171; R − Sq = 1.80% | |||||
| ball size, mm | 3 | 0.016 | 0.005 | 0.05 | 0.984 |
| Error | 12 | 1.213 | 0.101 | ||
| Total | 15 | 1.229 | |||
| S = 0.3179; R − Sq = 1.27% | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mussapyrova, L.; Milikhat, B.; Baláž, M.; Batkal, A.; Kamunur, K.; Nadirov, R. Mechanochemical Activation as a Key Step for Enhanced Ammonia Leaching of Spent LiCoO2 Cathodes. Metals 2025, 15, 1021. https://doi.org/10.3390/met15091021
Mussapyrova L, Milikhat B, Baláž M, Batkal A, Kamunur K, Nadirov R. Mechanochemical Activation as a Key Step for Enhanced Ammonia Leaching of Spent LiCoO2 Cathodes. Metals. 2025; 15(9):1021. https://doi.org/10.3390/met15091021
Chicago/Turabian StyleMussapyrova, Lyazzat, Bagdatgul Milikhat, Matej Baláž, Aisulu Batkal, Kaster Kamunur, and Rashid Nadirov. 2025. "Mechanochemical Activation as a Key Step for Enhanced Ammonia Leaching of Spent LiCoO2 Cathodes" Metals 15, no. 9: 1021. https://doi.org/10.3390/met15091021
APA StyleMussapyrova, L., Milikhat, B., Baláž, M., Batkal, A., Kamunur, K., & Nadirov, R. (2025). Mechanochemical Activation as a Key Step for Enhanced Ammonia Leaching of Spent LiCoO2 Cathodes. Metals, 15(9), 1021. https://doi.org/10.3390/met15091021







