The Effect of Cobalt Incorporation on the Microstructure and Properties of Cu(Co) Alloys for Use in Hybrid Bonding
Abstract
1. Introduction
2. Experimental Section
2.1. Film Growth
2.2. Film Characterization and Measurements
3. Results and Discussion
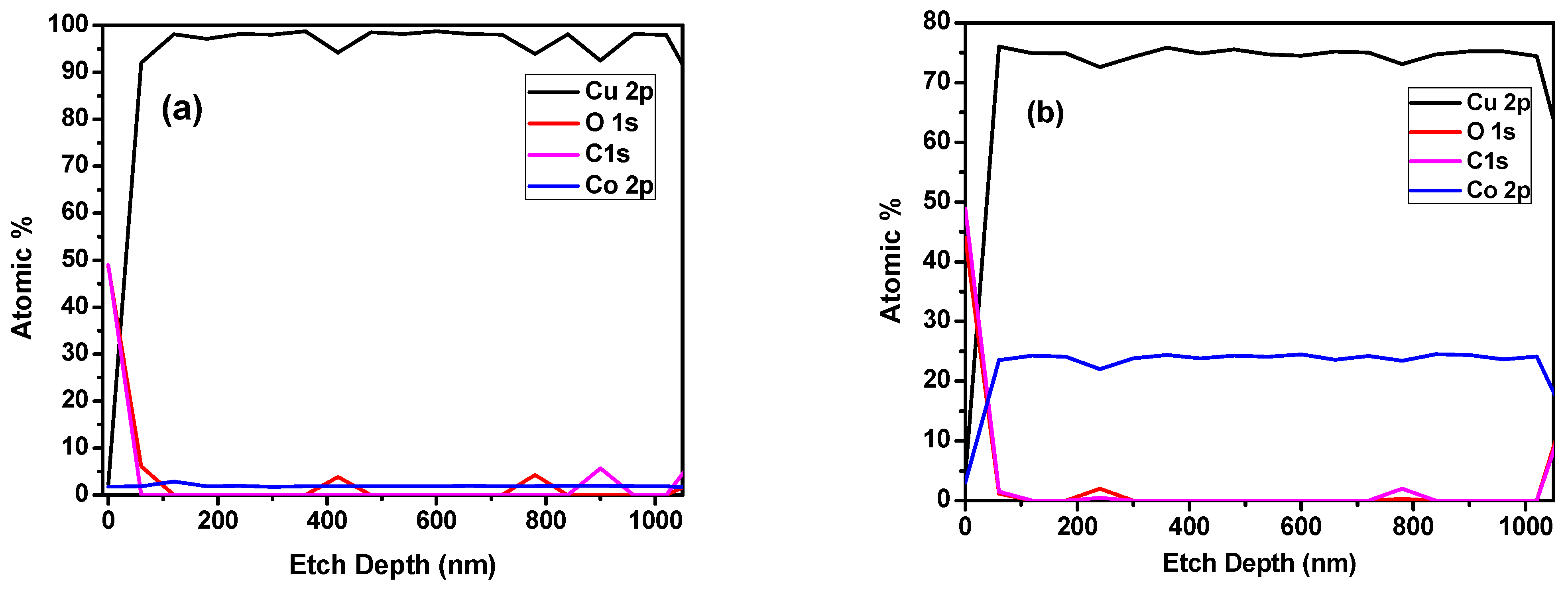
3.1. Effect of Deposition Parameters on Film Characteristics
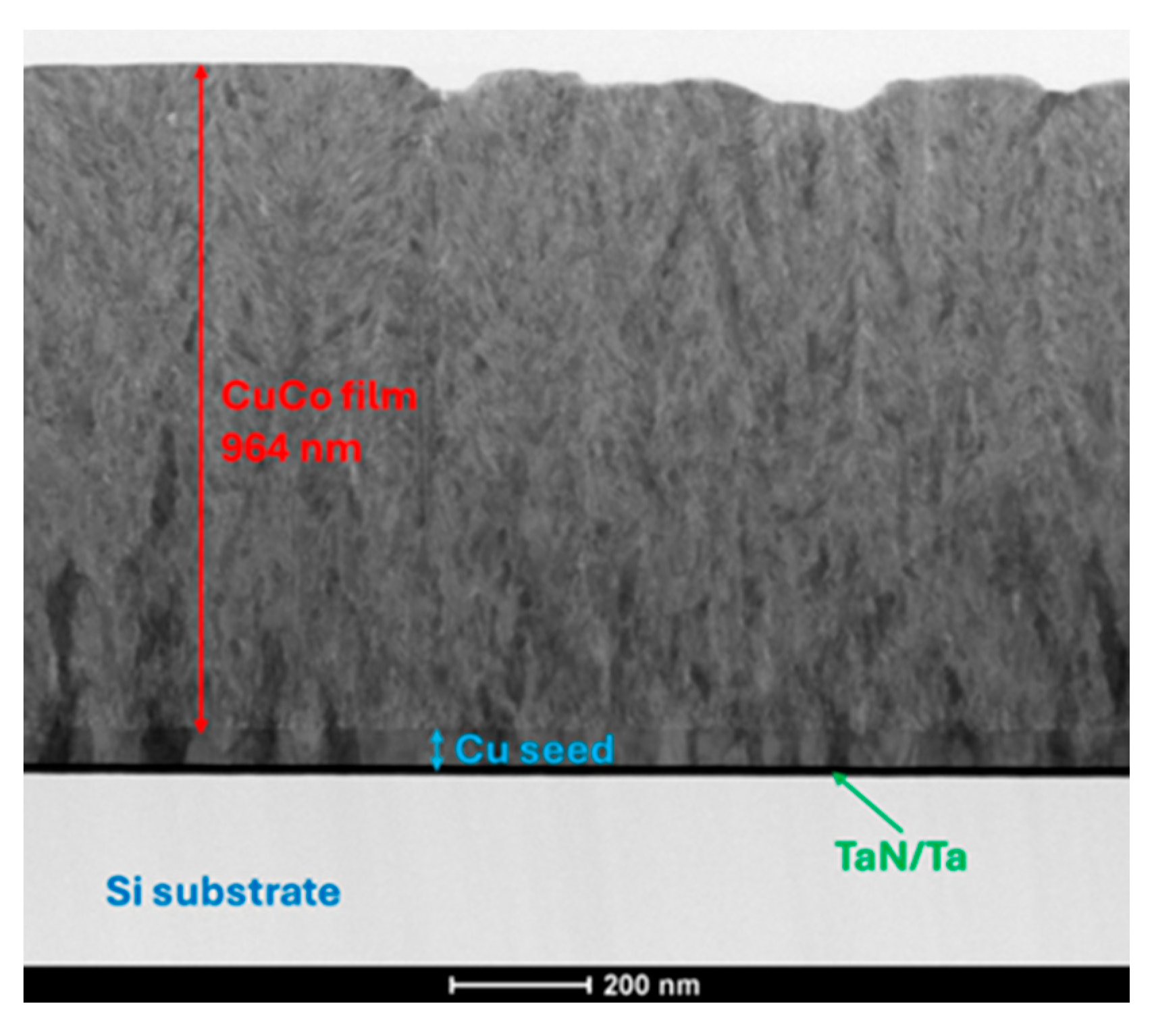
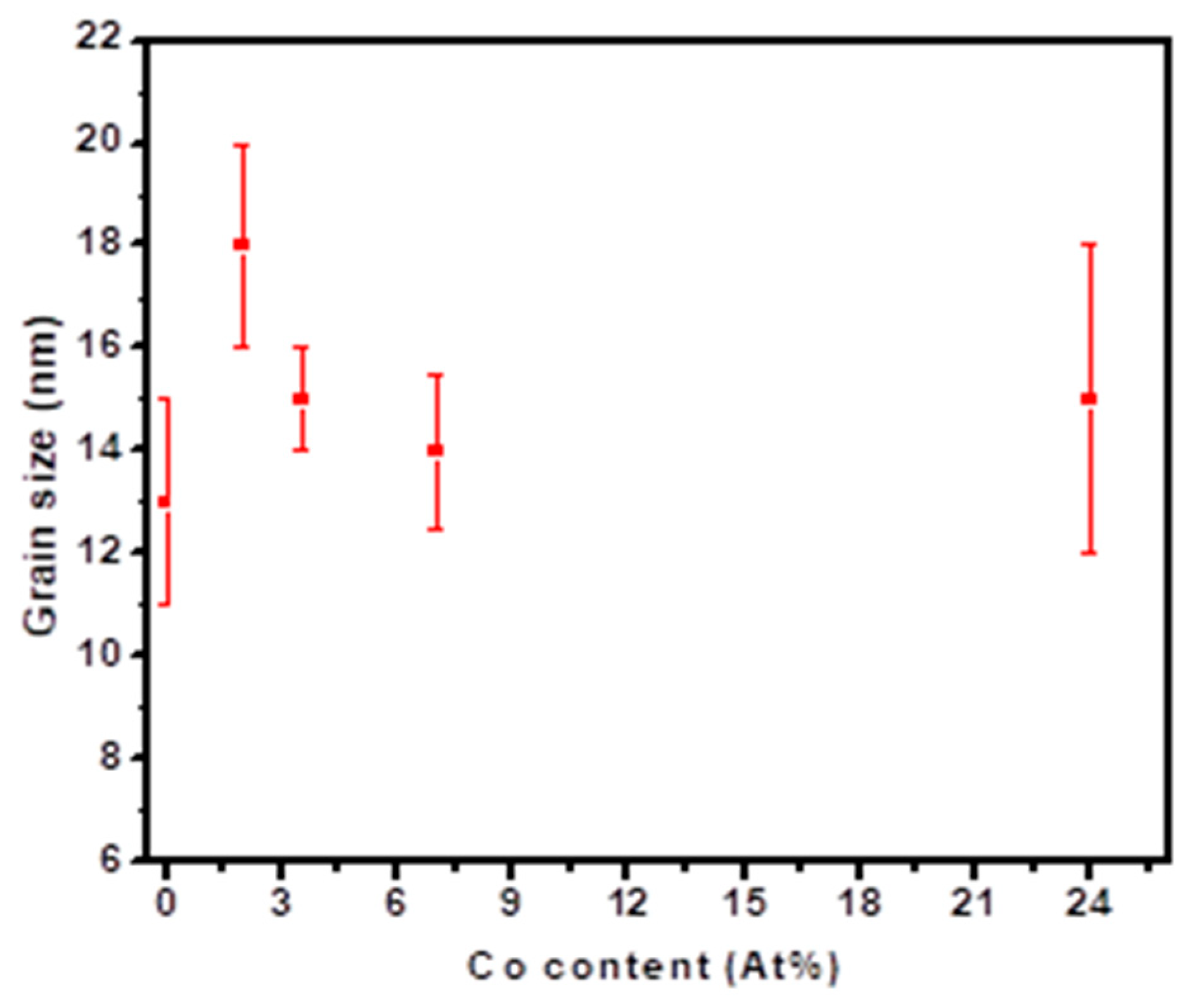
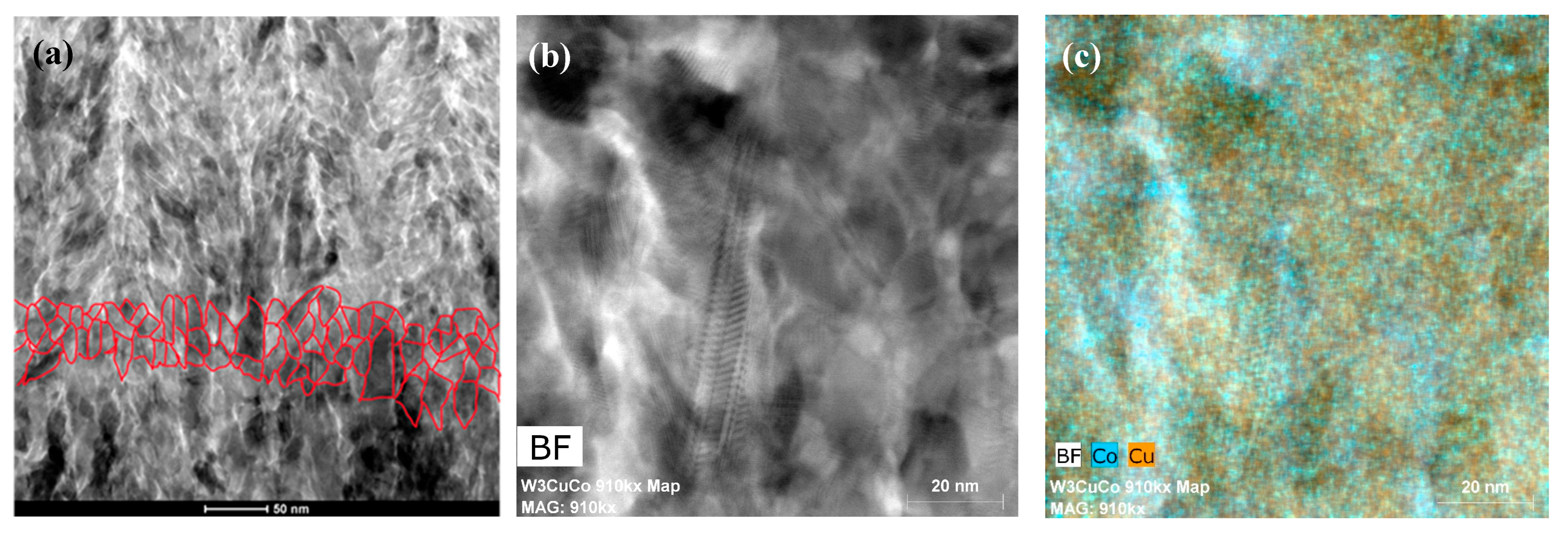
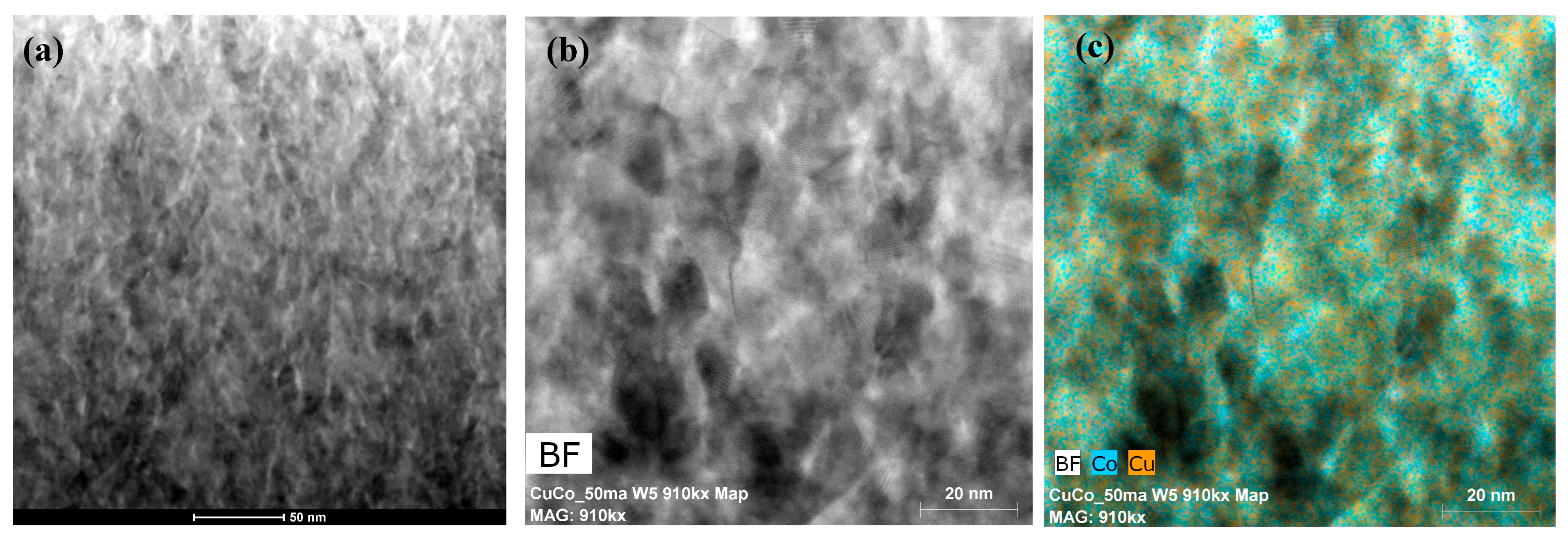
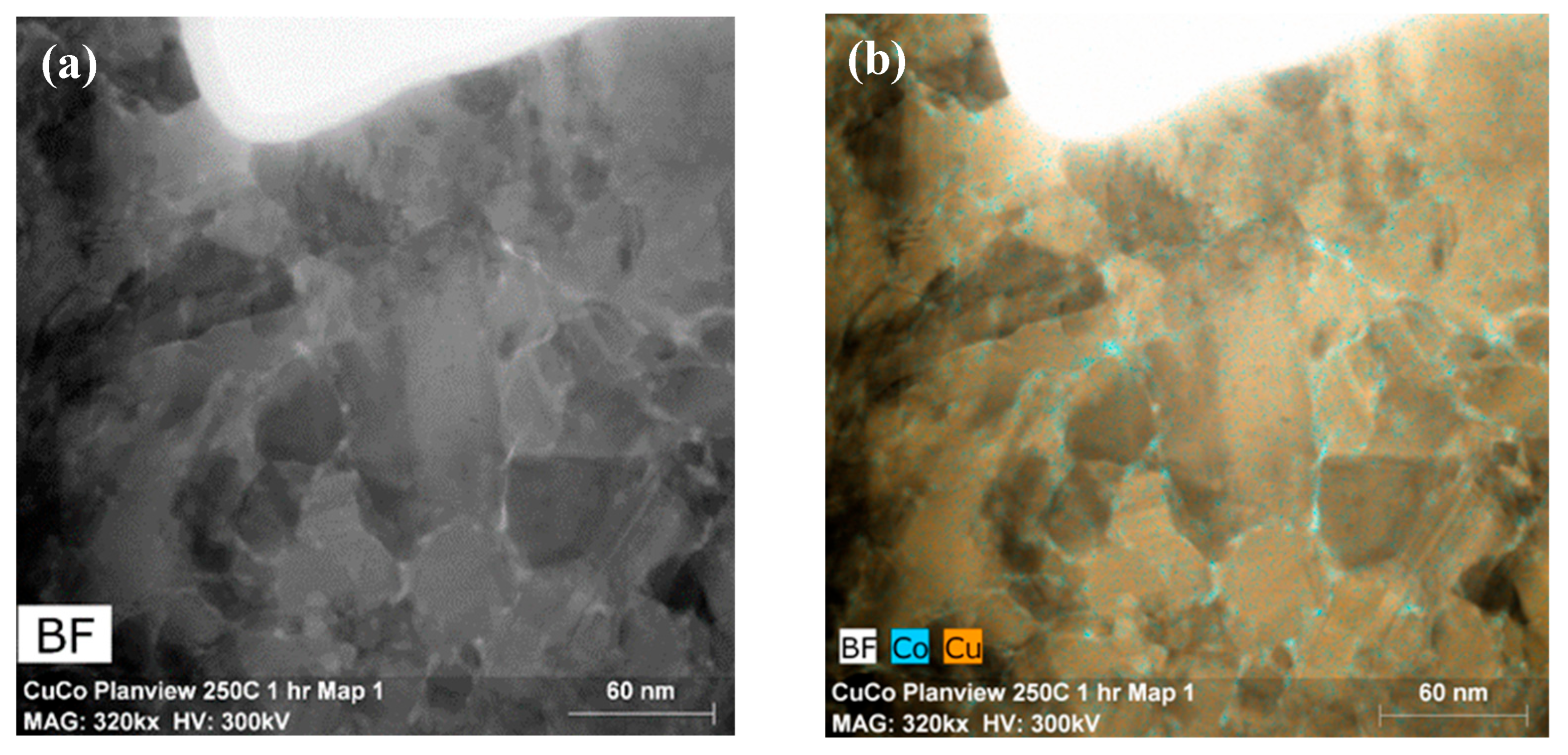
3.2. Microstructure Analysis
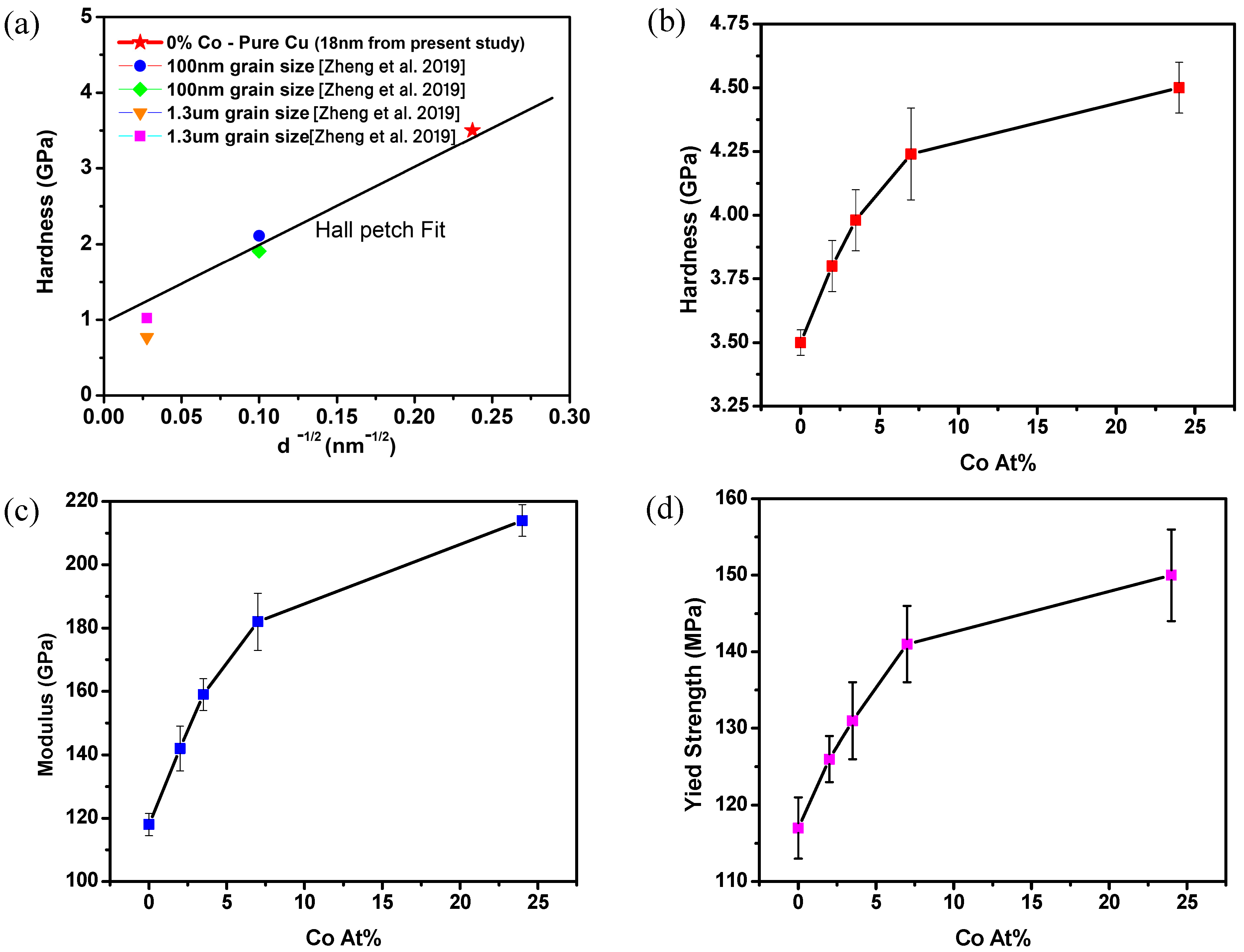
3.3. Mechanical Properties: Hardness and Modulus
3.4. CTE Variation with Co Incorporation
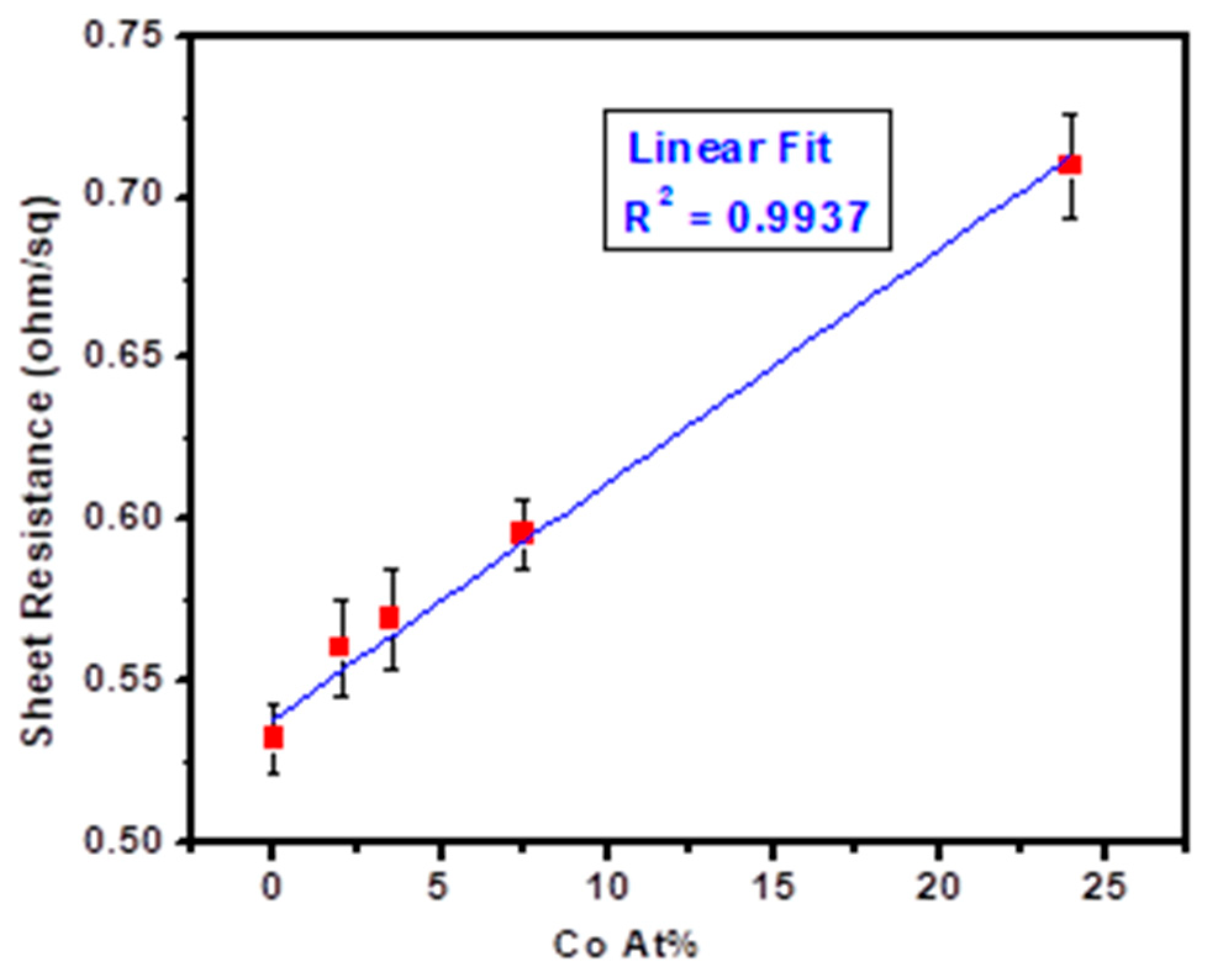
3.5. Electrical Measurements
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hsiung, C.K.; Chen, K.N. A Review on Hybrid Bonding Interconnection and Its Characterization. IEEE Nanotechnol. Mag. 2024, 18, 41–50. [Google Scholar] [CrossRef]
- Morrow, P.R.; Park, C.M.; Ramanathan, S.; Kobrinsky, M.J.; Harmes, M. Three-Dimensional Wafer Stacking via Cu-Cu Bonding Integrated with 65-Nm Strained-Si/Low-k CMOS Technology. IEEE Electron Device Lett. 2006, 27, 335–337. [Google Scholar] [CrossRef]
- Ko, C.T.; Chen, K.N. Low Temperature Bonding Technology for 3D Integration. Microelectron. Reliab. 2012, 52, 302–311. [Google Scholar] [CrossRef]
- Zhang, S.; Li, Z.; Zhou, H.; Li, R.; Wang, S.; Paik, K.W.; He, P. Challenges and Recent Prospectives of 3D Heterogeneous Integration. In E-Prime—Advances in Electrical Engineering, Electronics and Energy; Elsevier Ltd.: Amsterdam, The Netherlands, 2022. [Google Scholar] [CrossRef]
- Le, X.B.; Choa, S.H. Assessment of the Risk of Crack Formation at a Hybrid Bonding Interface Using Numerical Analysis. Micromachines 2024, 15, 1332. [Google Scholar] [CrossRef] [PubMed]
- Beilliard, Y.; Estevez, R.; Parry, G.; McGarry, P.; Di Cioccio, L.; Coudrain, P. Thermomechanical Finite Element Modeling of Cu–SiO2 Direct Hybrid Bonding with a Dishing Effect on Cu Surfaces. Int. J. Solids Struct. 2017, 117, 208–220. [Google Scholar] [CrossRef]
- Panchenko, I.; Wenzel, L.; Mueller, M.; Rudolph, C.; Hanisch, A.; Wolf, J.M. Microstructure Development of Cu/SiO Hybrid Bond Interconnects After Reliability Tests. IEEE Trans. Compon. Packag. Manuf. Technol. 2022, 12, 410–421. [Google Scholar] [CrossRef]
- Singh, S.; Dunn, K. Composition-Tunable Properties of Cu(Ag) Alloy for Hybrid Bonding Applications. Materials 2023, 16, 7481. [Google Scholar] [CrossRef]
- Dag, S.; Liu, M.; Jiang, L.; Kiaee, A.; See, G.; Lianto, P.; Ayyagari-Sangamalli, B.; Bazizi, E.M. Development of Copper Thermal Coefficient For Low Temperature Hybrid Bonding. In Proceedings of the Electronic Components and Technology Conference, Orlando, FL, USA, 30 May–2 June 2023; Institute of Electrical and Electronics Engineers Inc.: New York, NY, USA, 2023; Volume 2023, pp. 342–349. [Google Scholar] [CrossRef]
- Mirkarimi, L.; Uzoh, C.; Suwito, D.; Lee, B.; Fountain, G.; Workman, T.; Theil, J.; Gao, G.; Buckalew, B.; Oberst, J.; et al. The Influence of Cu Microstructure on Thermal Budget in Hybrid Bonding. In Proceedings of the Electronic Components and Technology Conference, San Diego, CA, USA, 31 May–3 June 2022; Institute of Electrical and Electronics Engineers Inc.: New York, NY, USA, 2022; pp. 162–167. [Google Scholar] [CrossRef]
- Sachs, E.; Cima, M.; Cornie, J.; Brancazio, D.; Bredt, J.; Curodeau, A.; Fan, T.; Khanuja, S.; Lauder, A.; Lee, J.; et al. Three dimensional Printing: The Physics and Implications of Additive Manufacturing. CIRP Ann. 1993, 42, 257–260. [Google Scholar] [CrossRef]
- Ganesh, K.J.; Darbal, A.D.; Rajasekhara, S.; Rohrer, G.S.; Barmak, K.; Ferreira, P.J. Effect of Downscaling Nano-Copper Interconnects on the Microstructure Revealed by High Resolution TEM-Orientation-Mapping. Nanotechnology 2012, 23, 135702. [Google Scholar] [CrossRef]
- Pu, W.; He, X.; Ren, J.; Wan, C.; Jiang, C. Electrodeposition of Sn-Cu Alloy Anodes for Lithium Batteries. Electrochim. Acta 2005, 50, 4140–4145. [Google Scholar] [CrossRef]
- Ivanova, A.R.; Nuesca, G.; Chen, X.; Goldberg, C.; Kaloyeros, A.E.; Arkles, B.; Sullivan, J.J. The Effects of Processing Parameters in the Chemical Vapor Deposition of Cobalt from Cobalt Tricarbonyl Nitrosyl. J. Electrochem. Soc. 1999, 146, 2139. [Google Scholar] [CrossRef]
- Kaloyeros, A.E. Method of Interlayer Mediated Epitaxy of Cobalt Silicide from Low Temperature Chemical Vapor Deposition of Cobalt. U.S. Patent US6346477B1, 12 February 2002. [Google Scholar]
- Lane, M.W.; Liniger, E.G.; Lloyd, J.R. Relationship between Interfacial Adhesion and Electromigration in Cu Metallization. J. Appl. Phys. 2003, 93, 1417–1421. [Google Scholar] [CrossRef]
- Zhang, L.; Zhou, J.P.; Im, J.; Ho, P.S.; Aubel, O.; Hennesthal, C.; Zschech, E. Effects of Cap Layer and Grain Structure on Electromigration Reliability of Cu/Low-k Interconnects for 45 Nm Technology Node. In Proceedings of the 2010 IEEE International Reliability Physics Symposium, Anaheim, CA, USA, 2–6 May 2010; IEEE: New York, NY, USA, 2010; pp. 581–585. [Google Scholar] [CrossRef]
- Zhang, L.; Kraatz, M.; Aubel, O.; Hennesthal, C.; Zschech, E.; Ho, P.S. Grain Size and Cap Layer Effects on Electromigration Reliability of Cu Interconnects: Experiments and Simulation. In Proceedings of the AIP Conference Proceedings, Annapolis, MD, USA, 1–4 November 2010; Volume 1300, pp. 3–11. [Google Scholar] [CrossRef]
- Arnaud, L.; Lamontagne, P.; Bana, F.; Le Friec, Y.; Waltz, P. Study of Electromigration Void Nucleation Time in Cu Interconnects with Doping Elements. Microelectron. Eng. 2013, 107, 145–150. [Google Scholar] [CrossRef]
- Rosenberg, R.; Batson, P.E.; Bruley, J.; Splinter, S.J.; Muller, D.A. Observations on the Influence of Solute on Grain Boundary Diffusion and Electromigration; AIP Publishing: Melville, NY, USA, 2009; pp. 127–134. [Google Scholar] [CrossRef]
- Barmak, K.; Gungor, A.; Rollett, A.D.; Cabral, C.; Harper, J.M.E. Texture of Cu and Dilute Binary Cu-Alloy Films: Impact of Annealing and Solute Content. Mater. Sci. Semicond. Process. 2003, 6, 175–184. [Google Scholar] [CrossRef]
- Barmak, K.; Gungor, A.; Cabral, C.; Harper, J.M.E. Annealing Behavior of Cu and Dilute Cu-Alloy Films: Precipitation, Grain Growth, and Resistivity. J. Appl. Phys. 2003, 94, 1605–1616. [Google Scholar] [CrossRef]
- Harper, J.M.E.; Gupta, J.; Smith, D.A.; Chang, J.W.; Holloway, K.L.; Cabral, C.; Tracy, D.P.; Knorr, D.B. Crystallographic Texture Change during Abnormal Grain Growth in Cu-Co Thin Films. Appl. Phys. Lett. 1994, 65, 177–179. [Google Scholar] [CrossRef]
- Gupta, D. Diffusion Processes in Advanced Technological Materials; Gupta, D., Ed.; Springer: Berlin/Heidelberg, Germany, 2005. [Google Scholar] [CrossRef]
- Cantwell, P.R.; Tang, M.; Dillon, S.J.; Luo, J.; Rohrer, G.S.; Harmer, M.P. Grain Boundary Complexions. Acta Mater. 2014, 62, 1–48. [Google Scholar] [CrossRef]
- Gungor, A.; Barmak, K.; Rollett, A.D.; Cabral, C.; Harper, J.M.E. Texture and Resistivity of Dilute Binary Cu(Al), Cu(In), Cu(Ti), Cu(Nb), Cu(Ir), and Cu(W) Allow Thin Films. J. Vac. Sci. Technol. B Microelectron. Nanometer Struct. 2002, 20, 2314–2319. [Google Scholar] [CrossRef]
- Zhang, S.-L.; Harper, J.M.E.; Cabral, C.; D’heurle, F.M. In Situ Resistivity Study of Copper-Cobalt Films: Precipitation, Dissolution and Phase Transformation. Thin Solid Film. 2001, 401, 298–305. [Google Scholar] [CrossRef]
- De Messemaeker, J.; Witters, L.; Zhang, B.; Tsau, Y.W.; Fodor, F.; De Vos, J.; Beyer, G.; Croes, K.; Beyne, E. New Cu “Bulge-Out” Mechanism Supporting SubMicron Scaling of Hybrid Wafer-to-Wafer Bonding. In Proceedings of the Electronic Components and Technology Conference, Orlando, FL, USA, 30 May–2 June 2023; Institute of Electrical and Electronics Engineers Inc.: New York, NY, USA, 2023; Volume 2023, pp. 109–113. [Google Scholar] [CrossRef]
- Joi, A.; Akolkar, R.; Landau, U. Pulse Electrodeposition of Copper-Manganese Alloy for Application in Interconnect Metallization. J. Electrochem. Soc. 2013, 160, D3145–D3148. [Google Scholar] [CrossRef]
- Paunovic, M.; Schlesinger, M.; Synder, D. Modern Electroplating; Schlesinger, M., Paunovic, M., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2010. [Google Scholar] [CrossRef]
- O’Brien, B. The Impact of Seed Layer Structure on the Recrystallization of ECD Cu and Its Alloys. Ph.D. Thesis, University At Albany, State University of New York, Albany, NY, USA, 2015. [Google Scholar]
- Singh, S.; Dunn, K. Tuning of Copper Grain Sizes for Integration in Hybrid Bonding Applications. In Proceedings of the 2024 IEEE 10th Electronics System-Integration Technology Conference, ESTC 2024—Proceedings, Berlin, Germany, 11–13 September 2024; Institute of Electrical and Electronics Engineers Inc.: New York, NY, USA, 2024. [Google Scholar] [CrossRef]
- Haessner, F. (Ed.) Recrystallization of Metallic Materials, 2nd ed.; Riederer-Verlag: Stuttgart, Germany, 1978. [Google Scholar]
- Massalski, T.B.; Murray, J.L.; Baker, H.; Bennett, L.H. (Eds.) Binary Alloy Phase Diagrams, 2nd ed.; ASM International: Materials Park, OH, USA, 1990. [Google Scholar]
- Ashby, M.F.; Harper, J.; Lewis, J. The Interaction of Crystal Boundaries with Second-Phase Particles. Transactions of the American Institute of Mining, Metallurgical and Petroleum Engineers. Metall. Soc. 1969, 245, 413–420. [Google Scholar]
- Zheng, Z.; Chiang, P.C.; Huang, Y.T.; Wang, W.T.; Li, P.C.; Tsai, Y.H.; Chen, C.M.; Feng, S.P. Study of Grain Size Effect of Cu Metallization on Interfacial Microstructures of Solder Joints. Microelectron. Reliab. 2019, 99, 44–51. [Google Scholar] [CrossRef]
- Hakamada, M.; Nakamoto, Y.; Matsumoto, H.; Iwasaki, H.; Chen, Y.; Kusuda, H.; Mabuchi, M. Relationship between Hardness and Grain Size in Electrodeposited Copper Films. Mater. Sci. Eng. A 2007, 457, 120–126. [Google Scholar] [CrossRef]
- Sanders, P.G.; Eastman, J.A.; Weertman, J.R. Tensile Behavior of Nanocrystalline Copper. 1995. Available online: https://digital.library.unt.edu/ark:/67531/metadc668453/ (accessed on 5 June 2025).[Green Version]
- Sun, X.; Reglero, R.; Sun, X.; Yacaman, M.J. Microhardness of Bulk and Higher Density Nanocrystalline Copper Obtained by Hot Compaction. Mater. Chem. Phys. 2000, 63, 82–87. [Google Scholar] [CrossRef]
- Sui, F.; Sandström, R. Creep Strength Contribution Due to Precipitation Hardening in Copper–Cobalt Alloys. J. Mater. Sci. 2019, 54, 1819–1830. [Google Scholar] [CrossRef]
- Bachmaier, A.; Rathmayr, G.B.; Schmauch, J.; Schell, N.; Stark, A.; De Jonge, N.; Pippan, R. High Strength Nanocrystalline Cu-Co Alloys with High Tensile Ductility. J. Mater. Res. 2019, 34, 58–68. [Google Scholar] [CrossRef]
- Bachmaier, A.; Pfaff, M.; Stolpe, M.; Aboulfadl, H.; Motz, C. Phase Separation of a Supersaturated Nanocrystalline Cu-Co Alloy and Its Influence on Thermal Stability. Acta Mater. 2015, 96, 269–283. [Google Scholar] [CrossRef]
- Shen, T.D.; Koch, C.C. Formation, Solid Solution Hardening and Softening of Nanocrystalline Solid Solutions Prepared by Mechanical Attrition. Acta Mater. 1996, 44, 753–761. [Google Scholar] [CrossRef]
- Dieter, G.E. Mechanical Metallurgy, 3rd ed.; Mc Graw-Hill Book Co.: New York, NY, USA, 1988. [Google Scholar][Green Version]
- Katot, M.; Moris, T.; Schwartz, L.H. Hardening by Spinodal Modulated Structure. Acta Metall. 1980, 28, 285–290. [Google Scholar] [CrossRef]
- Zhou, X.; Darvishi Kamachali, R.; Boyce, B.L.; Clark, B.G.; Raabe, D.; Thompson, G.B. Spinodal Decomposition in Nanocrystalline Alloys. Acta Mater. 2021, 215, 117054. [Google Scholar] [CrossRef]
- Tabor, D. The physical meaning of indentation and scratch hardness. Br. J. Appl. Phys. 1956, 7, 159–166. [Google Scholar] [CrossRef]
- Lu, L.; Wang, L.B.; Ding, B.Z.; Lu, K. High-Tensile Ductility in Nanocrystalline Copper. J. Mater. Res. 2000, 15, 270–273. [Google Scholar] [CrossRef]
- Butt, M.Z.; Feltham, P. Solid-Solution Hardening. J. Mater. Sci. 1993, 28, 2557–2576. [Google Scholar] [CrossRef]
- Lin, H.E.; Tran, D.P.; Chiu, W.L.; Chang, H.H.; Chen, C. Enhanced Thermal Expansion with Nanocrystalline Cu in SiO2 Vias for Hybrid Bonding. Appl. Surf. Sci. 2024, 672, 160784. [Google Scholar] [CrossRef]
- Beyne, E.; Kim, S.-W.; Peng, L.; Heylen, N.; De Messemaeker, J.; Okudur, O.O.; Phommahaxay, A.; Kim, T.-G.; Stucchi, M.; Velenis, D.; et al. Scalable, Sub 2 μm Pitch, Cu/SiCN to Cu/SiCN Hybrid Wafer-to-Wafer Bonding Technology. In Proceedings of the 2017 IEEE International Electron Devices Meeting (IEDM), San Francisco, CA, USA, 2–6 December 2017; IEEE: New York, NY, USA, 2017; pp. 32.4.1–32.4.4. [Google Scholar] [CrossRef]
- Huang, Q. Effects of Impurity Elements on Isothermal Grain Growth of Electroplated Copper. J. Electrochem. Soc. 2018, 165, D251–D257. [Google Scholar] [CrossRef]
- Gall, D. Electron Mean Free Path in Elemental Metals. J. Appl. Phys. 2016, 119, 085101. [Google Scholar] [CrossRef]
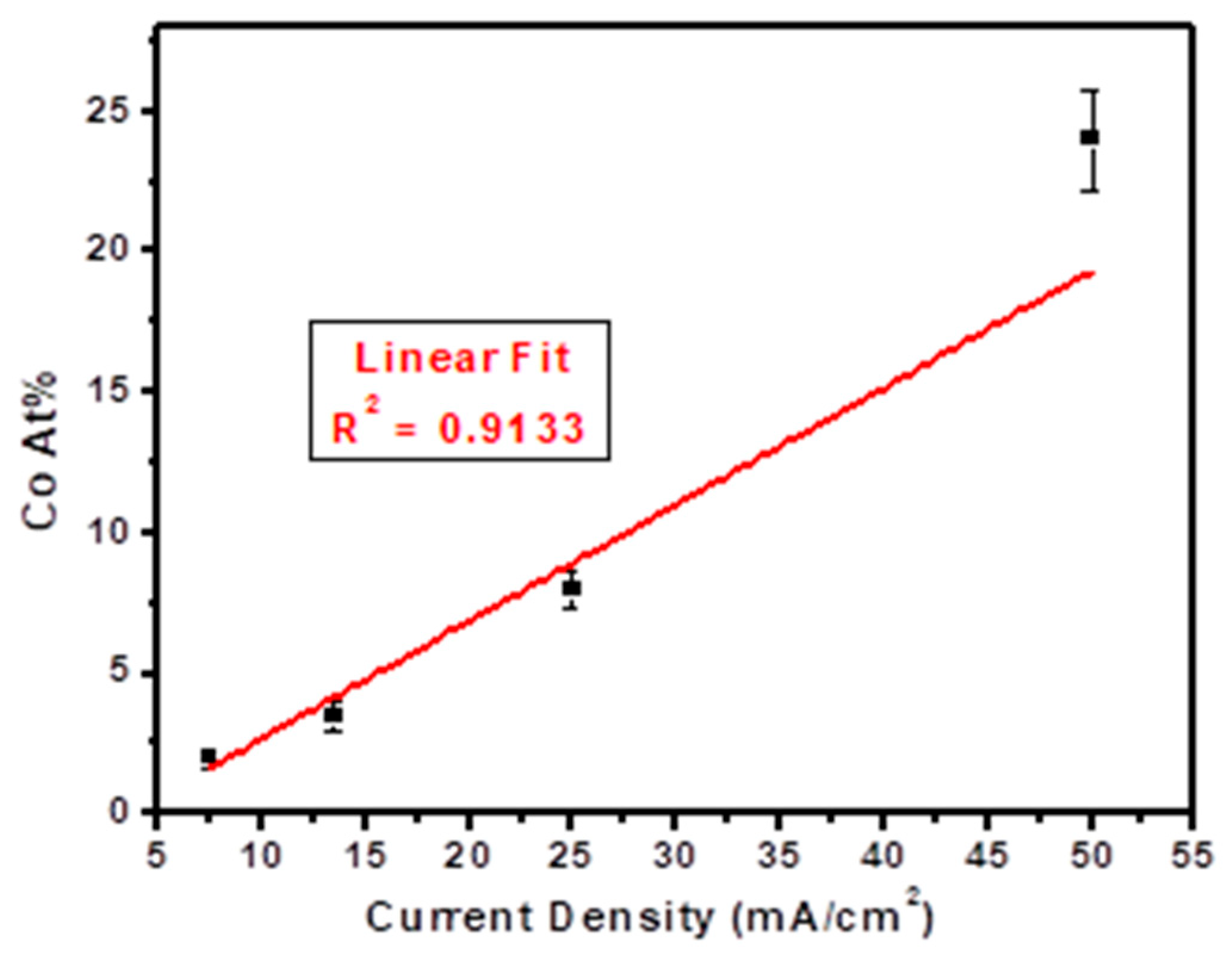

| Film Number | Current Density (mA/cm2) | Co at.% |
|---|---|---|
| 1 | 7.5 | 0 |
| 2 | 7.5 | 2 |
| 3 | 13 | 3.5 |
| 4 | 25 | 7 |
| 5 | 50 | 24 |
| S. No. | Co Content (%) | Hardness (H) [GPa] | Modulus [GPa] | Calculated Yield Strength (τyield) [MPa] |
|---|---|---|---|---|
| 1 | 0 | 3.5 | 118 | 117 |
| 2 | 2 | 3.8 | 142 | 126 |
| 3 | 3.5 | 3.95 | 159 | 131 |
| 4 | 7 | 4.24 | 183 | 141 |
| 5 | 24 | 4.5 | 214 | 150 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, S.; Dunn, K. The Effect of Cobalt Incorporation on the Microstructure and Properties of Cu(Co) Alloys for Use in Hybrid Bonding. Metals 2025, 15, 1023. https://doi.org/10.3390/met15091023
Singh S, Dunn K. The Effect of Cobalt Incorporation on the Microstructure and Properties of Cu(Co) Alloys for Use in Hybrid Bonding. Metals. 2025; 15(9):1023. https://doi.org/10.3390/met15091023
Chicago/Turabian StyleSingh, Sarabjot, and Kathleen Dunn. 2025. "The Effect of Cobalt Incorporation on the Microstructure and Properties of Cu(Co) Alloys for Use in Hybrid Bonding" Metals 15, no. 9: 1023. https://doi.org/10.3390/met15091023
APA StyleSingh, S., & Dunn, K. (2025). The Effect of Cobalt Incorporation on the Microstructure and Properties of Cu(Co) Alloys for Use in Hybrid Bonding. Metals, 15(9), 1023. https://doi.org/10.3390/met15091023





