Abstract
The electric arc furnace (EAF) is a key technology in the steel production industry, particularly for recycling scrap iron. It plays a crucial role in the shift to low-carbon metallurgy, responding to the growing demand for more sustainable production methods. Alongside its environmental and energy benefits, the EAF process generates significant amounts of solid by-products, including dust (EAFD) and slag (EAFS). These wastes are not only rich in base metals but also contain critical elements, which have attracted increasing scientific and industrial interest. Depending on the waste type, key metals such as zinc (from EAFD) and chromium, vanadium, and titanium (from EAFS) are targeted for recovery. This review examines the chemical and phase compositions of these wastes, various leaching techniques (often combined with pretreatment stages), and methods for final metal recovery, either in their pure form or as compounds. Key challenges in hydrometallurgical routes include chloride contamination, the dissolution of refractory zinc ferrite, and impurity management. Despite current limited industrial adoption, hydrometallurgical approaches show significant promise as efficient and environmentally friendly solutions for resource recycling, offering high-purity metal recovery.
1. Introduction
The electric arc furnace (EAF) is widely used for steel production from recycled scrap iron [1]. It is considered a key technology in the transition toward low-carbon metallurgy [2], as it generates only 0.7 t of CO2 per tonne of crude steel, compared to 2.4 t for the conventional blast furnace and basic oxygen furnace processes [3]. Global EAF steel production is expected to reach 1340 million metric tons by 2050, raising its share of global crude steel output from 29% in 2024 to 50% in 2050 [3]. Although the prevalence of EAF-based steel production varies significantly across world regions [3], its growing adoption is driven by several factors, including scrap availability [4], stronger regulatory and economic reasons to reduce greenhouse gas emissions [2], and the expanding integration of renewable energy sources into industrial processes [5,6]. Alongside its environmental and energy advantages, the EAF process generates substantial amounts of solid by-products, like slags (60–270 kg/t liquid steel), dusts (10–30 kg/t liquid steel), and refractory materials (1.6–23 kg/t liquid steel) [7]. These waste streams contain not only considerable amounts of iron but also appreciable concentrations of various non-ferrous metals like zinc, chromium, molybdenum, vanadium, manganese, niobium, and titanium [8,9,10]. In the context of rising global demand and the search for secondary sources of raw materials, EAF wastes have become a subject of scientific and industrial interest.
The EAF dust (EAFD) is classified as hazardous waste and is listed under the European Waste Catalogue number 10 02 07* [11]. Due to the potential leaching of heavy metals and the associated risk of environmental contamination [12] and human health problems [13], its disposal is costly and requires the use of specialized treatment for secure permanent disposal [14]. Nonetheless, the utilization of EAF dust in the production of ceramics, concrete, cement clinker, and industrial glass has recently attracted increasing research interest [15]. At present, the EAF dust containing adequate levels of zinc is primarily processed for the recovery of zinc oxide using pyrometallurgical methods [16], with the Waelz kiln process being the most applied technology [17]. In turn, electric arc furnace slag (EAFS) is classified as non-hazardous waste, despite containing certain toxic elements such as chromium, lead or cadmium [18]. It is predominantly reused as a low-value-added aggregate in construction or as a clinker material in the cement industry, which results in minimal landfilling [9]. Nevertheless, alternative approaches are increasingly being explored, aiming at the recovery of metals [9,10] classified as critical raw materials [19,20,21,22,23].
This review focuses on the recovery of metals from EAF dust and slag using hydrometallurgical methods, which are increasingly viewed as a viable alternative to traditional pyrometallurgical treatment [24]. Pyrometallurgical processing of EAF waste is burdened with important drawbacks: some amounts of zinc and lead may remain in the slag; iron is not sufficiently enriched after vaporization-based zinc separation; a zinc content above 15 wt% is required to ensure process economics; and the method is highly energy-intensive due to the elevated temperatures needed [17]. In contrast, hydrometallurgy offers key advantages, such as lower energy consumption, scalability, improved selectivity, and the capacity to extract metals from low-grade or compositionally complex materials [8]. This article presents chemical and phase compositions of the EAF dust and slag, which significantly influence the efficiency of metal recovery. It also discusses various strategies aimed at enhancing the metal leachability, including various pretreatment methods. Particular attention is given to the recovery of zinc, whose content in the EAF dusts can reach several tens of percent. Although zinc is not considered a strategic or critical metal in many countries, including the European Union [19] and India [20], it remains economically important for Canada [21], the United States [22], and Australia [23]. Its significance stems from a wide range of applications, from conventional uses such as steel galvanizing and alloy production to emerging technologies including precision die-casting, zinc-air batteries, and components for renewable energy systems [25]. As a result, zinc is projected to rank as the fourth most valuable metal on the global market among selected critical materials between 2027 and 2030, following copper, lithium, and nickel [26]. Thus, recent advances in hydrometallurgical recycling of EAF by-products are especially important, since numerous zinc recovery attempts have failed due to the presence of franklinite, a zinc-bearing phase that is difficult to dissolve under conventional leaching conditions [27].
2. Materials and Methods
The review is based on a comprehensive analysis of publications on the hydrometallurgical recovery of metals from EAF by-products, with a particular focus on studies published in the last few years to show significant developments in the field. The literature search employed keywords related to leaching methods, pre-treatment techniques, and strategies for improving recovery efficiency. Source materials included peer-reviewed scientific journal articles and books. The primary database used was Web of Science, complemented by searches on major scientific publishing platforms, e.g., Elsevier, Springer, Taylor & Francis, Wiley. Each publication was evaluated for scientific quality, originality, and relevance to the review’s scope and discussion.
3. EAF Dust
3.1. General Characterization
3.1.1. Particles
Electric arc furnace dust [28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70] is a reddish brown powder (Figure 1a) of a bulk density in a range of 1.1–2.5 g/cm3. It is collected by large bag filters, gravity separators or electrostatic collectors within de-dusting systems of industrial steelmaking plants [1,17]. The primary mechanism of dust emission is the splashing of molten steel and slag caused by the rupture of CO bubbles (coming from decarburization of the liquid steel) at the surface of the molten bath [28,29]. This results in the formation of two types of droplets: ‘thin film droplets’ ranging from 0.3 μm to 500 μm in size and larger ‘jet droplets’, which act as precursors of dust particles [28]. Fine particles can also arise from gas-phase condensation. In consequence, the EAFD consists of particles exhibiting a wide range of shapes and sizes (Figure 1b and Table 1), from below one micrometer to several hundred micrometers [29,30,31,32,33], often displaying a bimodal particle size distribution [32,33,34,40,46,48,49]. The average particle size of EAFD varies depending on the collection method [28,30], with gravity separation yielding the largest particles, bag filters producing intermediate sizes, and electrostatic precipitators capturing the finest fractions.
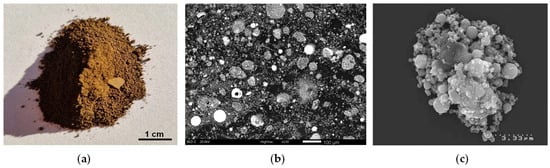
Figure 1.
Electric arc furnace dust: (a) macroscopic view (photo by the author); (b) SEM micrograph showing particles of different shapes and sizes (Adapted from Ref. [35]); (c) SEM micrograph showing a particle aggregate (Reprinted from Ref. [36]).

Table 1.
Particle size distribution of EAF dusts.
The dusts can contain irregular particles originating from the direct fly-off of solids during the introduction of powder materials (e.g., scrap, coal, recycled dusts, additions) into the furnace, sphere-like particles formed from slag droplets, and individual or aggregated (Figure 1c) small spheres (often hollow) composed of either homogeneous or heterogeneous phases such as zinc/iron oxides, slag or/and steel [29,35]. Dendritic structures may be present within the spherical particles, consisting of an iron-chromium spinel embedded in a slag matrix [29].
3.1.2. Chemical Composition
The chemical composition of electric arc furnace dust depends on the type of recycled scrap and includes elements originating from vaporized and subsequently oxidized metals, as well as slag components. The predominant elements in EAFD are zinc, iron, calcium, and silicon, accompanied by varying amounts of heavy metals like lead, chromium, manganese, and cadmium (Table 2). In some dusts, chlorine [35,46,47,51,68,69] and fluorine [12,46,68] were also detected. Since zinc is the primary metal targeted for recovery, EAFD is commonly classified into three categories based on zinc content: low-zinc dust (below 8 wt%), medium-zinc dust (8–20 wt%), and high-zinc dust (above 20 wt%) [16].

Table 2.
Concentration (in wt%) of main elements in EAF dusts from different plants.
Lanzerstorfer [54] demonstrated that zinc tends to accumulate (by a factor of 1.6) in the fine fraction (d50 = 0.85 μm) of the dust collected in bag filters, although its content remains significantly lower (16%) than that of iron (36%). In contrast, Gargul et al. [69] observed a relatively uniform zinc distribution of 21–23% across nine particle size fractions ranging from 0.056 to over 1 mm, with only 15% Zn detected in the finest particles (<0.056 mm). Meanwhile, iron concentrations ranged from 31% to 36% without any clear trend.
3.1.3. Phase Composition
The phase composition of EAFD is quite complex, with several dozen compounds potentially present [12,28,49,55,56]. A major challenge in interpreting X-ray diffraction data is the assignment of overlapping diffraction peaks to different phases; therefore, more advanced analytical methods should be employed to ensure accurate phase identification. Nonetheless, the most commonly identified phases include zincite ZnO, franklinite (zinc ferrite) ZnFe2O4, magnetite Fe3O4, and silica SiO2 [32,33,37,39,40,45,46,47,51,53,57]. Other phases detected involve iron [62], lead [38], manganese [37,59,70] and magnesium [69,70] oxides, calcium oxide [46,53], carbonate [46] or hydroxide [40], sodium and potassium chlorides [46,52], basic chlorides of zinc (simonkolleite) [35] or lead (Pb(OH)Cl) [47,59].
Sofilić et al. [49,55] investigated the mineral composition of different particle fractions of EAFD (ranging from below 50 μm to above 125 μm). In all size ranges, the presence of zinc oxide, iron oxides (Fe3O4, Fe2O3, FeO), silica, and metallic iron was confirmed. Compounds containing manganese (MnO, MnS2, MnO·SiO2) and silicates such as 3Al2O3·2SiO2 were identified almost exclusively in one or two intermediate fractions, specifically those between 50 μm and 90 μm.
Machado et al. [70] applied a range of analytical techniques, including SEM observations, chemical and EDS analysis, X-ray mapping, X-ray diffractometry (XRD), and Mössbauer spectroscopy. XRD analysis revealed the presence of the following phases: ZnFe2O4, Fe3O4, MgFe2O4, FeCr2O4, Ca0.15Fe2.85O4, MgO, Mn3O4, SiO2, and ZnO. In contrast, Mössbauer spectroscopy identified ZnFe2O4, Fe3O4, Ca0.15Fe2.85O4, and FeCr2O4. Notably, magnesium ferrite MgFe2O4, observed in the XRD pattern as overlapping peaks, was not detected by Mössbauer spectroscopy. Simonyan et al. [56] analyzed EAFD using a combination of X-ray diffraction and Mössbauer spectroscopy, supported by thermochemical modeling software to predict the presence of compounds that may not be detectable experimentally. XRD analysis revealed a series of oxides (ZnO, Mn2O3, Mn3O4, Fe3O4, SiO2) and ferrites (ZnFe2O4, MnFe2O4, Fe2CuO4, (Zn,Fe)Fe2O4, (Zn,Mn)Fe2O4). Mössbauer spectroscopy additionally identified phases such as Fe3O4, Fe2O3, FeSiO3, and 3Fe2O3·4H2O. Thermochemical modeling indicated the possible presence of sulfides (ZnS, PbS), oxides (Fe3O4, ZnO, MnO, Cr2O3), and various silicates of potassium, sodium, manganese, calcium, or aluminum.
By combining various methods or relying on quantitative XRD analysis, the percentage composition of individual phases in EAF dust can be determined. Several studies [35,39,62,66,67,70] have reported varying phase compositions (Table 3), with iron-containing phases typically dominating, though the exact proportions depend on both the methodology and the dust source.

Table 3.
Concentration of main phases in EAF dusts.
Bruckard et al. [46] separated EAFD into magnetic (18%) and non-magnetic (64%) fractions. They reported that the magnetic fraction consisted of franklinite (16%), magnetite (69%), hematite (11%), and zincite (3%), while the non-magnetic fraction mainly accumulated franklinite (56%), zincite (28%), and calcite (6%).
3.2. Leaching
Due to the significant zinc content, EAFD is subjected to leaching under various conditions, including both aqueous and non-aqueous solutions. The greatest challenge is posed by zinc ferrite (franklinite), which is highly resistant to chemical attack [58,59], although it is thermodynamically feasible [37,53]:
ZnFe2O4 + 8H+ → Zn2+ + 2Fe3+ + 4H2O ΔG°298K = −47.0 kJ/mol
ZnFe2O4 is a spinel oxide that forms at elevated temperatures from pure iron droplets and zinc vapors in the off-gas, through various possible mechanisms [29,60]. Suetens [60] demonstrated the existence of a zinc concentration gradient within iron oxide particles, which suggests that franklinite may form via a gas (zinc vapor)-solid (iron oxide) reaction. Further oxidation of these particles leads to fully oxidized structures with a decreasing zinc concentration gradient from the edge toward the center. The latter is important from a leaching perspective, as the presence of a zinc concentration gradient can suggest that the inner layer of the franklinite may be less accessible to dissolution under conventional leaching conditions. This can lead to incomplete zinc recovery from pure zinc ferrites, even more aggressive chemical agents and high temperature are applied [58,59].
3.2.1. Acid Leaching
The application of strong inorganic acids is a common practice for direct EAFD leaching [27,32,33,35,37,61,62,63,64,65]. Sulfuric acid is most frequently used due to its low cost and widespread availability as a leaching agent (Table 4). Acid leaching typically provides rapid leaching kinetics, allowing for stable zinc concentration levels in the leach liquors to be achieved within 10–20 min [37,45,53]. However, a significant drawback of this method is the simultaneous dissolution of iron compounds, which is problematic during the subsequent electrowinning stage, as iron ion concentrations in acidic solutions above 10 mg/L can severely reduce zinc current efficiency and increase energy consumption [37,71].

Table 4.
Acid leaching of EAF dusts.
Conventional leaching in 1–3 M H2SO4 at ambient or slightly elevated temperatures under atmospheric pressure typically results in up to 80% zinc recovery, and the most efficient processes were observed for EAFD with lower Fe/Zn ratios [33,62], increased temperatures [37,53] and higher liquid-to-solid ratios [37]. As leaching progresses, the solution pH slightly increases due to acid consumption. Lee et al. [33] found that with 0.1–1.4 M H2SO4, pH can rise to 9–4.5 after 30 min, leading to iron removal via secondary precipitation and zinc extraction of 5–80%. With more concentrated acid (1.5–3 M), zinc extraction increased to 99%, with 10% iron dissolution. The high zinc leachability was attributed to its presence mainly as oxide, while zinc ferrite remained in the residue. In turn, Wang et al. [72] demonstrated highly efficient zinc extraction (84%) from reduction-roasted EAFD using 0.8 M H2SO4, which was further enhanced to 94% when water pretreatment was added as a first stage. These improvements were attributed to the decomposition of zinc ferrite into ZnO during heat treatment at 600 °C under a hydrogen atmosphere (to prevent further reduction of ZnO to zinc gas and volatilization of the oxide), followed by the recovery of soluble zinc compounds through water leaching.
Increasing the temperature to about 250 °C and thus raising the pressure in a closed system allows the use of H2SO4 solutions with concentrations below 0.5 M [45,62]. Under these conditions, the dissolution of zinc into the solution is accompanied by a significantly lower transfer of iron, especially at low acid-to-dust ratios, thus greatly enhancing the selectivity of the leaching process [45]. Langová and Matýsek [62] observed that at 250–260 °C, Fe(II) ions remained in the solution, while Fe(III) precipitated hydrolytically as hematite, decreasing iron ions in the electrolyte with simultaneous acidification within the leaching duration. They also found that the concentration of iron ions in the leach liquor further decreased markedly when hydrogen peroxide was added at the start of the experiment, and in this case, zinc extraction reached nearly 99%.
Shawabek [51] compared zinc extraction from EAFD using mineral acids of different concentrations (0.1–5 M). He observed varying trends depending on the acid concentration, although zinc extraction efficiency remained low (20–23%). For diluted acids (below 1 M), the zinc leaching efficiency followed the order: HCl < HNO3 < H2SO4. However, for more concentrated solutions, HCl became increasingly effective, while H2SO4 became less efficient, ultimately establishing the order: H2SO4~HNO3 < HCl. In turn, Halli et al. [65] conducted long-term (168 h) leaching experiments under ambient conditions using a variety of inorganic acids (12 M and 1.2 M HCl, 15 M and 1.5 M HNO3, 18 M and 1.8 M H2SO4, 19.2 M and 1.9 M H3PO4, 100% and 10% aqua regia) and organic acids (0.9 M and 0.09 M citric acid, 17.5 M and 1.75 M acetic acid, 2.7 M and 0.27 M formic acid, 1.6 M and 0.16 M oxalic acid). Total zinc and iron yields were achieved only with concentrated HCl and aqua regia, while concentrated HNO3 resulted in around 90% metals dissolution. Zinc recoveries of 75–80% were observed for H2SO4, more diluted HCl and HNO3, 0.9 M citric acid, and 10% aqua regia, with a 60% zinc recovery for 1.75 M acetic acid. Other acid solutions showed lower zinc and iron leachability. For high zinc (above 75%) and low iron (below 5%) extractions, 1.2 M HCl and 10% aqua regia were identified as the optimal leachants. Nevertheless, if higher iron solubility (15–17%) could be tolerated while maintaining high zinc extraction levels, 0.94 M citric acid and 1.5 M HNO3 could also be considered.
Among organic acids, citric acid solutions appear to be the most promising EAFD leachants [69,72]. Direct leaching with 1 M citric acid enables 60–70% zinc dissolution with iron extraction ranging from 20 to 25% at temperatures between 25 and 75 °C [69]. However, pre-roasting of EAFD with NaOH prior to leaching can transform the refractory zinc ferrite into soluble sodium zincate Na2ZnO2 and sodium ferrate NaFeO2 [73]. Under optimal conditions (0.8 M citric acid, 40 °C, oxygen purging), 100% of zinc and 8% of iron can be extracted into the solution within only 15 min. In comparison, 71% zinc and 12% iron were dissolved from raw EAFD under the same leaching conditions.
Kinetic studies [37,38,53] of EAFD leaching in strong acids reveal that zinc dissolves rapidly, reaching its maximal extraction within the first minutes of the process. Kukurguya and Havlik [53] found that the reaction with H2SO4 proceeds in two steps. The first rate-limiting stage is the diffusion of acid during the reaction with ZnO, while the second step, involving the reaction of acid with ZnFe2O4, is controlled by the chemical reaction. In contrast, iron dissolves into the solution much more slowly than zinc, and its rate-limiting step is the chemical reaction. Zoraga et al. [38] reported that the leaching of zincite from EAFD in HNO3 occurs so rapidly that the kinetics of the reaction could not be determined, whereas the dissolution of zinc ferrite follows a pseudohomogeneous reaction model. Calorimetric measurements showed that the EAFD reaction with acid is weakly exothermic [37].
3.2.2. Alkaline Leaching
Alkaline leaching with strong bases is considered the second main method for zinc recovery from EAFD [34,39,42,52,57,66,67,74,75,76,77,78,79]. This process is more selective towards iron, as it remains in the solid residues and does not transfer practically into the solution. However, zinc leaching is accompanied by the dissolution of lead compounds [34,57,78], although the latter are typically present in small amounts [52]. A key feature of typical alkaline EAFD leaching is the use of concentrated sodium hydroxide solutions, which can pose technical challenges and difficulties during regeneration. In such leachant, zinc dissolves easily from its oxide due to amphoteric nature of the compound:
while franklinite is still difficult to react [34,52,57,78]. To overcome this concern, pretreatment of EAFD with calcium oxide is often employed to convert ZnFe2O4 into more leachable ZnO and stable Ca2Fe2O5 [42]. Alternatively, more aggressive strategies, such as pressure-assisted or microwave-assisted leaching [34,75], or two-step processes [39,66,75,77], have been proposed to enhance leaching efficiency under harsher conditions (Table 5).
ZnO + 2NaOH + H2O → Na2[Zn(OH)4]

Table 5.
Alkaline zinc leaching from EAF dusts.
Kinetic studies of EAFD direct leaching in NaOH solutions have shown that the rate of zinc dissolution increases with base concentration, temperature, and liquid-to-solid ratio [52,57,74]. However, the agitation rate does not significantly affect the process, indicating that the reaction is chemically controlled according to the shrinking core model [67,74]. Siame et al. [67] confirmed that the leaching kinetics of EAFD follow a first-order reaction model, with the reaction being endothermic in nature.
Dutra et al. [75] compared different alkaline leaching strategies: (i) conventional agitation leaching, (ii) pressure leaching, (iii) conventional leaching of microwave-treated EAFD, and (iv) leaching with ultrasonic agitation. After 4 h of leaching with 6 M NaOH at 90 °C, the most significant result was a 74% zinc recovery through conventional leaching, while only 60% of zinc was dissolved from microwave-pretreated EAFD. Pressure leaching (120–200 °C, 4 h) and ultrasonic agitation (55 °C, 1 h) in NaOH solutions of the same concentration resulted in only 50% and 56% zinc recovery, respectively, as most of the franklinite remained unaffected. Application of microwave heating during leaching [34,78] significantly increased the zinc dissolution rate compared to conventional leaching, reaching maximal concentration values within only a few minutes. However, recovery efficiency remained relatively low (80% in 6–8 M NaOH), mainly due to the nonreactivity of the refractory phase.
Zhang et al. [39,77] implemented a two-stage leaching procedure, starting with conventional leaching followed by pressure or hydrothermal leaching in the presence of reductants as a second step. When leaching zinc from EAFD under normal pressure (6 M NaOH, 70 °C), 66% of the zinc was dissolved, with almost all ZnO being extracted from the dust while the ferrite remained unaffected. The addition of iron powder during pressure leaching of first-stage residues resulted in 66% zinc recovery, giving a total of 89% leaching efficiency. Notably, iron powder demonstrated its effectiveness during the leaching of pure zinc ferrite, increasing zinc recovery from 38% to 84% (6 M NaOH, 260 °C) due to the conversion of ferrite into magnetite. Hydrothermal reduction conditions (6 M NaOH, 260 °C) in the presence of starch as the second stage led to 69% zinc recovery. The addition of the reductant converted zinc ferrite into Fe3O4, and starch proved effective, increasing zinc recovery from 27% to 67% during the leaching of pure zinc ferrite.
Roasting of EAFD with CaO [42,68] or NaOH [66,76] as a pretreatment method has recently gained more attention due to the formation of ZnO and Na2ZnO2, respectively. Gamutan et al. [42] and Chairaksa-Fujimoto et al. [68] leached the lime-pretreated product with NaOH, KOH, and LiOH solutions (2 M, 70 °C). Stable zinc concentrations in the leachates were achieved within 2 h, with no iron or calcium transferring to the leachates, as they remained in the solid residues as Ca3Fe2(OH)12. However, it is noteworthy that the leaching efficiency of the solutions increased in the order of LiOH < KOH < NaOH and improved with higher temperatures. Kinetic analysis of the leaching process revealed that the rate-determining step was the chemical reaction in the temperature range of 25–50 °C, while diffusion through the remaining Ca2Fe2O5 layer became the main controlling step at higher temperatures (up to 70 °C). In turn, Bayoumi et al. [79] used cement bypass dust (46% CaO, 9% SiO2, 3% Fe2O3, 1% MgO) as a CaO-rich additive for thermal pretreatment of EAFD, promoting the conversion of zinc ferrite into zinc oxide and Ca2Fe2O5. Subsequent leaching with hot 2 M NaOH achieved zinc extraction efficiencies ranging from 35% to 82%, with higher recovery observed at low solid loadings.
Caustic-roasted EAFD leaching has been shown to be primarily influenced by roasting temperature, followed by the NaOH/EAF ratio and roasting time [66,76], due to the impact these parameters have on the dissociation of zinc ferrite. By adjusting leaching time and NaOH concentration, up to 80% of zinc can be recovered. Youcai and Standford [76] demonstrated that if the dust was hydrolyzed with water before fusion, the zinc leaching efficiency increased to 95%. However, the addition of sodium fluoride, NaF or disodium phosphate, Na2HPO4, as additives to the hydrolyzed dust, followed by fusion and leaching, did not improve the extraction efficiency.
3.2.3. Water Leaching
Water is the cheapest and most readily available leaching agent, although it can only be used for the recovery of metals from easily soluble compounds. Nevertheless, water is used in two main cases of EAFD treatment: (i) for the removal of some impurities from the dust [35,46,47,72,80,81], and (ii) for leaching of specifically pre-treated dusts [41,43,44,82,83,84,85].
- EAFD Purification
EAF dusts often contain soluble contaminants, such as metal salts, with alkali metal chlorides being the most common [35,46,68,81]. Chloride concentrations in EAFD can reach as high as 5–7% [47,68], while fluorine can be present in amounts up to 6% [12]. Wang et al. [72] showed that NaCl can constitute nearly 16% of the dust phases, and soluble zinc sulfate is also present, accounting for 2.5%. In turn, Buckard et al. [46] reported 2.4% NaCl and 0.9% KCl as the only detectable soluble phases.
Lis and Nowacki [80] demonstrated that water extracts from EAFD can contain chloride, fluoride, sulfate, lead, and zinc ions in concentrations well above the highest permissible values (Figure 2). This is important not only from an environmental perspective, as it addresses landfilled waste, but also for the hydrometallurgical treatment of the dust. Chloride and fluoride ions are the most undesirable contaminants, as they can accelerate anode corrosion, promote harmful anode reactions (such as the evolution of gases other than oxygen), and pose health risks to plant workers. Additionally, these ions negatively affect the quality of the zinc cathode deposit during the electrowinning stage [86,87,88]. Alternatively, chloride-based hydrometallurgical processing can be used [89,90].
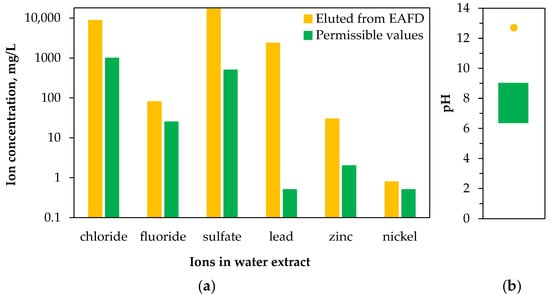
Figure 2.
Concentration of ions (a) and pH (b) in water extracts of electric arc furnace dust (yellow) and permissible values for discharging sewage into waters or ground (green) (based on [80]).
Bruckard et al. [46] investigated chlorine removal from EAFD (2.5% Cl) using water at a natural pH of 12 (i.e., achieved upon contact with the material). They found that at ambient temperature, 99% of the chlorine was extracted within 60 min, resulting in a residue containing 200 ppm of chlorine. The wash water also contained low concentrations of metal ions (of order mg/L), such as Fe, Zn, Pb, Cd, and Cr. Trifunović et al. [35] grouped water-soluble elements into three categories: (i) Na, K, and Cl, which can be leached with efficiencies of 60–90%; (ii) Cd, Ca, and F, which have leachability of 10–20%; and (iii) Zn, Fe, Pb, Cu, and Ni, which are relatively insoluble. Notably, maximal and stable levels of leached elements were achieved within 30 min, and the liquid-to-solid ratio (5–25) did not significantly affect the results. Interestingly, the effect of temperature showed that leaching of elements from groups (i) and (ii) decreased slightly with increased temperature, although no explanation for this behavior was provided. Notably, the monitored metals could be further leached from the solid residues using sulfuric acid, with fluorine solubility efficiency reaching 22%, although no data were provided for chlorine (raw dust contained 2.5% Cl and 0.023% F).
Chen et al. [47] reported that water washing of natural pH (9–12) reduced the chlorine concentration from 7.02% to 1.75% within 40 min at ambient temperature. Incomplete chlorine removal was attributed to the dissolution of NaCl and KCl, while the remaining chlorine was found as insoluble Pb(OH)Cl. Notably, lead chloride carbonate PbCl2·PbCO3 was detected in the solids when the raw material was weathered after CO2 aging. In turn, Yokoyama et al. [81] conducted experiments with water leaching accompanied by CO2 purging. They found that the maximal zinc and chlorine concentrations increased with higher EAFD addition, but the extraction percentages decreased. At a liquid-to-solid ratio of 1000, 100% of chlorine and 60% of zinc could be extracted, while no iron was leached.
- 2.
- Pretreatment and Leaching
Recently, a new direction in the treatment of EAF dust involves thermal processing to convert insoluble zinc-bearing compounds into chloride, bromide or sulfate forms, which can then be dissolved by water (Table 6).

Table 6.
Zinc leaching by water.
Al-Harahsheh et al. [44,82,83] performed oxidative thermal treatment or pyrolysis using a mixture of EAFD (31% Zn, 20% Fe, 3.5% Pb) and poly(vinyl chloride) PVC. The HCl released during PVC decomposition (200–400 °C, 21 kPa O2, 0.5 h) reacted with metal oxides, converting them into chlorides, primarily ZnCl2 and PbCl2, while iron oxides mainly form stable Fe2O3 [82]. The subsequent water leaching achieved almost total zinc recovery from the product treated at 250 °C and 300 °C. However, as the pretreatment temperature increased, zinc recovery decreased to below 60% due to the formation of volatile chloride (an inverse trend in metal recovery was observed when washing handling equipment). The leaching process was not selective, as lead (70–85%) and iron (10–30%) also transferred into the solution. Notably, the solution’s pH was found to significantly influence the extraction of both lead and iron, especially at pH values above 4.0, where a sharp drop in metal recovery was observed. Further studies [44] showed that thermal treatment in air resulted in only slightly higher zinc recovery compared to nitrogen treatment. In an inert atmosphere [83], solely ZnCl2 was formed, and after pyrolysis, main phases such as zincite, franklinite, magnetite, sylvite KCl, and halite NaCl disappeared in the diffractogram, with the residue being enriched in PbCl2.
Application of tetrabromobisphenol A TBBPA as an additive in EAFD pyrolysis [84,85] leads to the formation of hydrogen bromide HBr, which reacts with metal oxides in the dust, transforming them into bromides. The primary bromide phase identified was PbBr2, while ZnBr2 was not detected directly, likely due to its amorphous nature [84]. Interestingly, microwave-assisted pyrolysis [85] produced various bromides, including NaBr, KBr, K2ZnBr4, K2PbBr4, Na2ZnBr4, and Na2PbB4, whereas iron remained as various oxides. Although zinc bromides are generally water-soluble, leaching even at boiling temperature resulted in only moderate zinc recovery, while lead remained in the solid phase as Pb(OH)Br [84,85]. The addition of H2O2 during water leaching [84] slightly decreased zinc extraction but improved lead recovery and promoted Fe(II) oxidation to Fe(III), facilitating hematite precipitation (pH 2–2.5) and partial bromide oxidation. It was suggested that adjusting the pH to ~5.5–6 after leaching may precipitate lead as Pb(OH)Br, allowing zinc to remain in solution.
Wu et al. [41] employed roasting of EAFD (9% Zn, 40% Fe, 0.1% Pb) with NH4HSO4 (600 °C, 3 h). During the thermal treatment, zinc phases were transformed into ZnSO4, while iron phases were converted into Fe2O3. Subsequent water leaching resulted in high separation of zinc (91%) from iron (below 5%). In turn, Ju et al. [43] used (NH4)2SO4 for sulfating roasting, with similar reaction products, although the optimal roasting temperature was slightly higher at a shorter time (650 °C, 1.5 h). High selective extractions were achieved, with 98% zinc recovery and only 0.8% for iron.
3.2.4. Leaching with Salts
In addition to traditional acid, alkaline, or neutral agents, solutions of salts can be used for EAFD treatment. These include ammonium salts [91,92,93], iron salts [89,94], or their double salts [95] (Table 7).

Table 7.
Zinc leaching by salt solutions.
Application of ammoniacal solutions is beneficial for zincite leaching due to the possible formation of soluble zinc complexes:
ZnO + nNH3 + H2O → [Zn(NH3)n]2+ + 2OH−, n = 1 ÷ 4
This is a selective process, as iron compounds do not react with ammonia and do not form complexes. However, the main problem with such solutions is the temperature limitation due to the potential release of ammonia resulting from ammonium salt decomposition above 60 °C [91]. Nevertheless, ammoniacal solutions are cost-effective and easy to regenerate, especially ammonium carbonate solutions [96].
Havlik et al. [91] demonstrated that zinc dissolution from EAFD in ammonium carbonate solution reached maximal extraction after 0.5 h. Simultaneously, XRD analysis of the solid residues revealed a gradual disappearance of the ZnO peak, while the peaks for franklinite remained unchanged, confirming the selective nature of the reaction. Based on these observations, it was estimated that approximately one-third of the zinc in EAFD can exist in the form of ferrite. Ruiz et al. [92] investigated the effect of pulp density on zinc extraction using the same type of ammonium salt. They observed that at EAFD concentrations of 2% and 5% in the leaching solution, the average efficiency was 45%. However, at higher pulp densities (9%), the efficiency decreased to 30%. At the same time, they observed the leaching of Pb (12–20%), Cd (33–99%), and Cu (48–76%) into the solution, although the concentrations of these metals in the EAFD were low (Pb 4%, Cd 0.07%, Cu 0.3%). For comparison, Halli et al. [65] observed that less than 20% of zinc was dissolved from EAFD in a 25% ammonia solution, even after 168 h of treatment at room temperature. Under such conditions, no iron, lead, or manganese was transferred to the solution, while nearly 40% of chromium was extracted.
In turn, Miki et al. [93] compared the leachability of raw and CaO-roasted EAFD using ammonium chloride solution. Thermal conversion of all franklinite into zincite resulted in an increase in zinc recovery from 50% to nearly complete zinc recovery. The leaching rate of zinc in the pre-treated dust was dependent on the NH4Cl concentration (1–5 M) and temperature (25–70 °C) only in the early stage of leaching (0.5 h), but then the dissolution rate became constant. Less than 20% of calcium was leached within 15 min, with only a slight increase observed with time (2 h), higher temperature, and NH4Cl concentration. No iron was leached under any condition.
Leclerc et al. [94] proposed an unconventional composition of the leaching solution to extract all zinc from EAFD without destroying the iron oxide matrix. Initially, leaching was carried out using the nitrilotriacetate chelating agent (protonated form: N(CH2COOH)(CH2CO2)22−, HNTA2−), which effectively dissolved ZnO and Pb(OH)Cl:
ZnO + HNTA2− → ZnNTA− + H2O + NTA3−
Pb(OH)Cl + HNTA2− → PbNTA− + H2O + Cl−
At this stage, all ZnO was successfully dissolved, resulting in the recovery of 45% of zinc and 48% of lead (precipitated as sulfides for further metallurgical processing), while only 3% of iron was extracted at room temperature. The remaining solid residue was then treated with molten hydrated ferric chloride to break down zinc ferrite:
followed by water leaching to extract almost completely the soluble zinc chloride, while the remaining iron oxides stayed in the solid state.
ZnFe2O4 + 2FeCl3·6H2O → ZnCl2 + 2Fe2O3 + 4HCl + 10H2O
Acidic ferric salts are known to be effective leachants due to the high oxidative properties of the Fe3+/Fe2+ pair, which, combined with the aggressive action of hydrogen ions. FeCl3 solution (5 g/L, 80 °C) was proposed for atmospheric leaching of EAFD to dissolve zinc, lead, and cadmium oxides. This was followed by autoclave treatment of the slurry under conditions of 140 °C for 1 h, which resulted in the formation of a free-settling, filterable residue consisting mainly of hematite and silica, with over 99% zinc extraction efficiency [89].
Wang et al. [95] proposed a novel method for EAFD treatment using a bifunctional reagent, solid ammonium ferric sulfate NH4Fe(SO4)2·12H2O in a hydrothermal process. After chlorine removal by water washing, the dust was treated in an autoclave at elevated temperatures (180–220 °C). During the process, the ammonium ferric salt dissolved in its own crystal water, releasing ionic species:
NH4Fe(SO4)2·12H2O → NH4+ + Fe3+ + 2SO42− + 12H2O
Simultaneously, Fe3⁺ ions underwent hydrolysis:
generating an acidic solution that leached metals from the dust:
leaving zinc in the solution, while iron was effectively removed from the solution by co-precipitation as jarosite:
2Fe3+ + 3H2O → Fe2O3 + 6H+
ZnO + 2H+ → Zn2+ + H2O
ZnFe2O4 + 8H+ → Zn2+ + 2Fe3+ + 4H2O
Fe3O4 + 8H+ → Fe2+ + 2Fe3+ + 4H2O
NH4+ + 3Fe3+ + 2SO42− + 6H2O → NH4Fe3(SO4)2(OH)6 + 6H+
This resulted in high selectivity of the process, with 95% zinc recovery. Less than 2% of iron was extracted, confirming the effective removal of iron from the solution. Phase analysis of the solid residue revealed the presence of only jarosite and hematite.
3.2.5. Bioleaching
Biohydrometallurgical approaches have recently been developed to utilize microbiologically-assisted aqueous extraction of metals from EAF dusts as environmentally friendly technologies. Kinnunen et al. [97] conducted comparative experiments using both raw EAF dust and dust subjected to sulfidation with gaseous hydrogen sulfide (300 °C, 0.5 vol% H2S). The bioleaching culture contained primarily Marinobacter sp., Acidithiobacillus spp. (including At. ferrooxidans, At. thiooxidans, At. albertensis, At. ferrivorans), and Leptospirillum sp. (L. ferrooxidans). After 45 days of bioleaching under acidic conditions (pH ~2, 30 °C), the sulfidated dust showed selective and almost total zinc recovery, while the non-pretreated dust typically leached about 80% of zinc along with iron and lead (each with less than 20% extraction). The significant difference in zinc extraction efficiency was attributed to the transformation of ZnO in the raw dust into ZnS in the sulfidated product. The sulfide formed is more susceptible to bioleaching, as the bacteria oxidize sulfur species (e.g., S2−, S0) to sulfuric acid, which easily reacts with oxide, thus enhancing zinc extraction.
In turn, Mousavi et al. [98] selected Yarrowia lipolytica, a non-pathogenic yeast known for its rapid growth rate, remarkable substrate tolerance, and resistance to heavy metals. This fungus can thrive in diverse environments, including those rich in fats, marine habitats, and substrates like hydrocarbons, fatty acids, alcohols, and acetates. Furthermore, Y. lipolytica produces a variety of organic acids (such as citric, malic, and succinic acids) that act as effective leaching agents. The biolixiviant, used in combination with crude sunflower oil as a hydrophobic carbon source, successfully removed manganese (94%), dysprosium (23%), and erbium (7%) from EAFD at a 50 g/L pulp density. The bioleached residues primarily consisted of zinc ferrite, unaffected by biologically produced weak organic acids.
3.2.6. Leaching by Waste-Derived Reagents
The growing interest in sustainable and circular approaches to waste management has led to the exploration of waste-derived reagents for metal recovery. In the context of EAFD leaching, recent studies have investigated the use of industrial by-products as cost-effective leaching agents. Harris et al. [90] reported a method for zinc recovery from EAFDs (14–40% Zn, 18–33% Fe, and up to 3.6% Pb) using spent pickling liquor generated from the hydrochloric acid treatment of galvanized steel. This acidic waste, enriched in zinc (about 11%), generates environmental and recycling problems and is often discarded through methods such as deep well injection. Laboratory and pilot-scale tests demonstrated rapid leaching completed within 0.5–1 h with over 95% extraction of metals (except iron, at ~80%). The process continued with atmospheric-pressure hydrolysis (180 °C), allowing simultaneous precipitation of iron and other impurities as hematite-based residues and recovery of concentrated HCl (30–35%). Quenching the slurry in water yielded a ZnCl2-rich solution containing some Ca(II) and Mg(II) ions. Final zinc recovery was achieved by precipitation as zinc hydroxide chloride Zn5(OH)8Cl2·H2O or zinc carbonate, using recycled magnesia or sodium carbonate, followed by calcination in a steam atmosphere to produce good-quality ZnO. Lately, Yang et al. [99] investigated the use of EAFD-water slurry for leaching metals during wet desulfurization. The process involved purging SO2 gas through the suspension under oxygen-rich conditions, resulting in the production of an acid solution, in fact. This method not only utilized the waste gas but also led to a maximum zinc recovery of 40–50% (4 h) at a range of temperatures (25–55 °C).
3.2.7. Solvoleaching
Although aqueous-based processes have been well established, novel non-aqueous solvents have also been investigated for metal extraction, including solvoleaching techniques using ionic liquids IL and deep eutectic solvents DES [100]. These solvents are low-temperature melting systems (below 100 °C) that offer both chemical and thermal stability. Ionic liquids are salts composed of large, sterically demanding organic cations (e.g., imidazolium, pyridazinium, thiazolium) and smaller organic (e.g., bistrifluoromethanesulfonimide) or inorganic (e.g., BF4−, HSO4−) anions. However, due to the elaborate and costly special synthesis methods required for their production, ionic liquids tend to be relatively expensive. In contrast, deep eutectic solvents are eutectic mixtures of two or more molecular compounds that have a melting point lower than that of their individual components. Most DESs consist of choline chloride combined with a hydrogen-bond donor (e.g., urea, ethylene glycol, malonic acid) or a hydrated metal salt (e.g., chloride). DESs share similar properties with ionic liquids, but their simpler preparation methods make them significantly cheaper and easier to produce. Given that non-aqueous solvents are non-flammable, non-volatile, and biodegradable, they are considered to be safe and environmentally friendly leachants for metal extraction. However, their implementation on an industrial scale has not yet been realized in any metallurgical process [101].
As solvoleaching methods have been developed only within the last two decades, the data on their applicability remain quite limited [40,102]. Teimouri et al. [40] compared the efficiency of two ionic liquids, 1-butyl-3-methylimidazolum hydrogen sulfate [Bmim+HSO4−] and 1-butyl-3-methylimidazolum chloride [Bmim+Cl−], in combination with three oxidants, Fe2(SO4)3, KMnO4, and H2O2 for the extraction of zinc, iron, indium, and gallium from EAF dust (Figure 3).
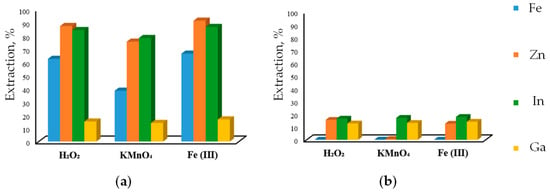
Figure 3.
Effect of oxidants on leaching efficiency of metals in ionic liquids: (a) [Bmim+HSO4−], (b) [Bmim+Cl−] (Adapted from Ref. [40] under License CC BY 4.0).
The results showed that the best performance was achieved with [Bmim+HSO4−] and Fe2(SO4)3, but the ZnFe2O4 phase remained quite refractory in comparison to the dissolution of ZnO and Fe2O3:
ZnO + 2H+ + SO42− → ZnSO4 + H2O
Fe3O4 + 8H+ + 4SO42− → FeSO4 + Fe2(SO4)3 + 4H2O
ZnFe2O4 + 8H+ + 4SO42− → ZnSO4 + Fe2(SO4)3 + 4H2O
The fastest reaction rate was observed during the first hour of leaching, and the process kinetics analysis indicated that it was a diffusion-controlled reaction. The study also showed that, compared to sulfuric acid, the ionic liquid [Bmim+HSO4−] achieved similar or slightly higher zinc extraction (Table 8), with faster dissolution kinetics and lower activation energies.

Table 8.
Solvoleaching of EAF dusts.
In contrast, Bakkar [102] used a deep eutectic solvent (choline chloride-urea) for the leaching of low-iron EAFD. It was observed that after leaching, the concentrations of ZnO and PbO in the solids decreased, indicating the transfer of both metals to the solution. In turn, the concentrations of some oxides, such as Fe2O3, SiO2, and TiO2, increased, suggesting no reaction with the solvent, while CaO showed partial dissolution. Notably, the next stage involved zinc electrowinning in potentiostatic conditions directly from the leaching liquor. The composition of the cathodic deposits was dependent on the applied potential, with lead being deposited at potentials between −0.5 V and −1.0 V, while Pb-Zn alloys formed at more negative potentials up to −2.0 V (versus Al). These two potential ranges also defined the changes in current efficiency, with minimal values around 20% at −1.1 ± 0.1 V, located between two maximal values of nearly 70% at −0.6 V and −1.5 V.
3.3. Leachate Purification and Zinc Recovery
As EAFD leaching is not selective, the resulting pregnant solutions contain a variety of impurities at varying concentrations (Table 9). These concentrations depend not only on the initial concentration of metals in the dust material but also on the type of leachant and the leaching conditions. The primary contaminants are Fe(II,III) and Mn(II) ions in acidic solutions, while Pb(II) ions are present in alkaline leaching liquors. These ionic contaminants must be removed before the final recovery step to obtain high-purity zinc products under energy-efficient conditions.

Table 9.
Concentration of main ionic species in EAFD pregnant leaching solutions.
Several strategies have been proposed for zinc recovery from leachates, involving classical methods such as precipitation of chemical compounds, cementation, solvent extraction, and electrolysis [37,103,104,105,106,107,108]. Typically, zinc oxide, basic carbonate, or metal is obtained as the final product of EAFD treatment (Figure 4). Due to the co-leaching of iron alongside zinc in H2SO4 solutions, a typical first stage in the purification process involves removing Fe(III) ions by precipitating hematite through the addition of alkali or ammonium [37,62,106,107,108], or jarosite formation [99,107]. This stage can be followed by further selective zinc solvent extraction using Cyanex 272 [103]. The final stage of the sulfuric acid-based process involves electrolytic zinc recovery at current densities of 2–5 A/dm2 [37,103]. Another method for zinc recovery involves the precipitation of zinc compounds. For example, Darezereshki et al. [108] precipitated basic zinc sulfate, 3Zn(OH)2·ZnSO4·5H2O, using ammonia from a purified leachate (Fe(III) removal with NaOH followed by D2EHPA solvent extraction). After calcination at 850 °C, they obtained ZnO nanoparticles with a flake-like structure. In turn, Xanthopoulos et al. [104] used sodium carbonate, ammonium carbonate, and sodium bicarbonate for precipitating basic zinc carbonates. The precipitation rate was found to depend on the type of precipitating agent, with the effectiveness decreasing in the order listed above. The final products were mixtures of 3ZnCO3·3Zn(OH)2 and ZnCO3·3Zn(OH)2·H2O, which were precipitated using Na2CO3 solutions [103,106]. Meanwhile, Lutandula and Kashala [50], after filtering PbSO4 from the leachate, precipitated zinc hydroxide with NaOH, which was then converted into oxide (93% ZnO) during calcination (300 °C, 15 min).
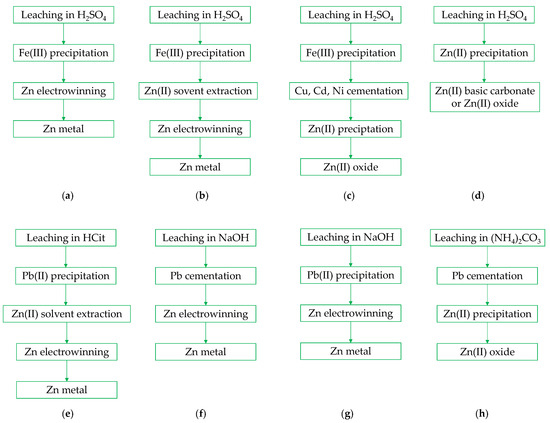
Figure 4.
Strategies for purification of EAFD leach liquors and zinc products recovery, Adapted from Refs.: (a) [37]; (b) [103,108], (c) [106]; (d) [50,104]; (e) [105]; (f) [52,57]; (g) [76]; (h) [92].
A different approach was proposed by Halli et al. [105] for treating citrate leachate. They first removed lead by sulfate precipitation with sulfuric acid, followed by zinc solvent extraction. Two extractants were tested: D2EHPA and Cyanex 572, with the former showing better extraction efficiency and very high selectivity for iron separation at pH 6 (separation factor Zn/Fe = 2629). The optimal extraction conditions (20 vol% D2EHPA, pH 5, O/A = 1, 25 °C, 5 min) and stripping conditions (1 M H2SO4, O/A = 3, 25 °C, 15 min) were developed for enriching the zinc solution (from 20 g/L to 55 g/L Zn(II)), which can then be used in zinc electrowinning.
A different approach is used in the case of NaOH-based leachates, where lead cementation is typically performed using zinc dust [52,57] or lead sulfide precipitation with Na2S [92] as a first step. The resulting solution is then subjected to electrolysis to obtain zinc with 99–99.9% purity. On the other hand, from ammonium carbonate solutions, after removing impurities through cementation, zinc carbonate can easily be precipitated by simple solution evaporation, which, after calcination, yields a final oxide product with over 99% purity [92].
3.4. Commercial Hydrometallurgical Processes
Only a few hydrometallurgical processes for commercial use and pilot-plant operations have been developed, although they are currently not widely applied in industry. The presence of chlorides in EAFD requires their removal, often through water washing, as demonstrated in laboratory-scale studies. To mitigate issues caused by chloride ions in aqueous solutions, two chloride-based technologies have been proposed.
The first one was industrialized by Metals Recycling Technologies (USA, 1995) [109] and involved leaching EAFD in hot ammonium chloride, leaving behind unleached iron oxides and zinc ferrite, which were either recycled in steel mills or stockpiled. The leachate was purified from cadmium and lead to produce saleable metals, and the cleaned solution was then heated to crystallize high-purity zinc oxide (Figure 5a).
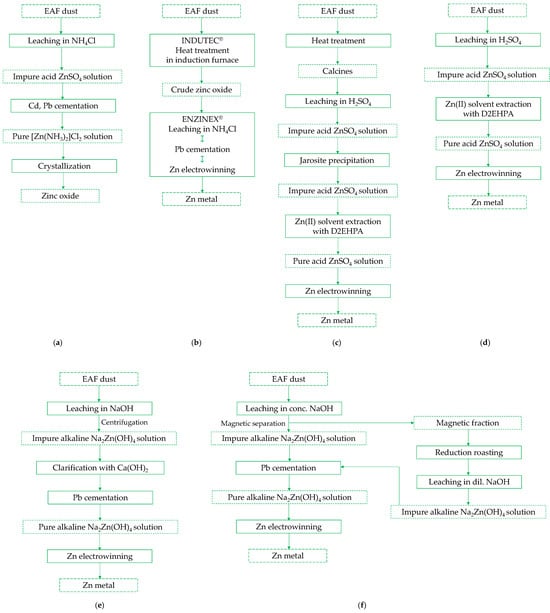
Figure 5.
Schemes of hydrometallurgical processes for zinc recovery from secondary zinc-bearing materials, including EAFD: (a) MRT Process (Adapted from Ref. [109]); (b) INDUTEC®/ENZINEX® Integrate Process (Adapted from Ref. [110]); (c) ZINCEX Process (Adapted from Ref. [111]); (d) Modified ZINCEX Process (Adapted from Ref. [108]); (e) CEBEDEAU Process (Adapted from Ref. [109]); (f) CARDIFF Process (Adapted from Ref. [109]).
Thirty years ago, the ENZINEX® process was also commissioned by Engitec Impianti (Italy) [110]. This process involves chloride leaching in NH4Cl solution at 70–80 °C, combined with cadmium and lead cementation (65–75% Pb purity) using zinc powder and an electrowinning system (5–15 g/L Zn(II), min. 65 °C, 3–4 A/dm2, titanium cathode, graphite anode, 94–98% current efficiency), with the overall reaction:
Zn(NH3)2Cl2 + ⅔NH3 → Zn + ⅓N2 + 2NH4Cl
The ENZINEX® process offers lower cell voltage compared to sulfuric electrowinning, resulting in reduced energy consumption. However, this energy saving is partially offset by ammonia usage, which is eventually scrubbed and recycled within the plant.
The ENZINEX® process is integrated with the INDUTEC® process, a thermal method that converts secondary zinc-bearing materials, including EAFD, into crude zinc oxide C.Z.O. in an induction furnace. The C.Z.O. is then used as feed material in the leaching stage (Figure 5b).
The CASHMAN Process, developed by American Metals Recovery Corp. (USA), was adapted for EAFD treatment [109]. It involved pressure leaching with a CaCl2 solution, followed by the cementation of cadmium and lead using zinc dust. The purified solution was subsequently used to precipitate high-purity zinc oxide.
McElroy and McClaren [89] reported the development of a Canadian process involving pressure leaching of EAFD in ferric chloride at 175 °C, achieving over 99% zinc recovery. This was accompanied by the simultaneous precipitation of FeOOH:
followed by lead recovery as PbCl2 by cooling/crystallization of hot filtrate. Zinc was recovered through solvent extraction using Acorga ZNX-50, followed by stripping with hot acidified water (max. pH 2) and concentration to commercial-grade ZnCl2 solutions (60–70 wt%). These solutions were planned to be sold directly or further concentrated and converted to zinc sulfate using spent electrolyte from a nearby conventional electrolytic zinc plant.
2FeCl3 + 3MO → 2FeOOH + 3MCl2, M = Zn, Cd, Pb
The Modified ZINCEX Process™ (licensed by Técnicas Reunidas, Spain) is a simplified version of the original ZINCEX Process developed in the 1970s [111,112,113,114]. It eliminates the need for roasting, furnaces, and jarosite precipitation (Figure 5c,d). The process consists of three main stages: (i) direct atmospheric leaching with H2SO4 solution, (ii) elective solvent extraction of zinc ions with D2EHPA in kerosene-based diluent (zinc stripping with spent electrolyte from subsequent step; over 99% zinc extraction efficiency), and (iii) conventional electrowinning in sulfate electrolyte (93% efficiency at about 4 A/dm2 using aluminum cathodes). This process can recover 67–90% of zinc from EAFD, producing zinc of at least 99.98% purity [113].
Two examples of technologies utilizing alkaline leaching have also been reported [109,115]. The CEBEDEAU Process, developed in France (1980s), involved leaching EAFD with a hot concentrated NaOH solution (6–12 M, 95 °C), followed by centrifugal filtration using a starch flocculant. Since part of the zinc remained undissolved, the overall recovery depended on the amount of zinc bonded in ferrite. The leachate was clarified with Ca(OH)2 to precipitate calcium silicate and then subjected to cementation with zinc powder to remove lead, cadmium, and copper. Finally, the purified solution, after the addition of a small amount of sodium stannate (~0.2 g/L) to enhance current efficiency, was used in the electrowinning stage (2.5–3.75 A/dm2, 3.2 V) to produce high-quality zinc powder (Figure 5e).
The CARDIFF Process, developed in the United Kingdom in the early 1980s, was similar to the previous technology but included two key modifications: magnetic separation instead of centrifugation for phase separation and the implementation of reduction roasting to decompose zinc ferrite, ultimately increasing zinc recovery to 80% (Figure 5f).
Commercial hydrometallurgical processes for zinc recovery from EAFD offer efficient, scalable solutions that reduce waste and enable the extraction of high-purity zinc while mitigating environmental impacts. The implementation of closed-loop systems was also demonstrated, including solvent extraction circuits in the Modified ZINCEX ProcessTM [111,112] and the recycling of spent electrolyte from electrowinning back into the leaching stage in the CEBEDEAU and CARDIFF processes [109]. However, hydrometallurgical technologies still remain in the minority [27,115], with pyrometallurgical methods dominating the industry.
4. EAF Slag
4.1. General Characterization
4.1.1. Particles
Electric arc furnace slag forms as a single liquid phase on the surface of the molten bath during the high-temperature processing of scrap steel, typically in the presence of limestone [1]. This slag phase accumulates impurities such as silicon, manganese, aluminum, phosphorus, and others. After steel refining is completed, the molten steel is discharged from the EAF through a submerged tap hole. The EAF slag, which floats on top of the molten bath, is removed through the slag door. Upon cooling, the slag solidifies into a rock-like material with a density ranging from 2.8 to 3.9 g/cm3 and a color varying from gray to black, depending on the Fe2O3 content (Figure 6). EAF slag contains particles with a size from a few millimeters up to a few centimeters [116]. The particles are characterized by a rough and porous surface, with pore diameters reaching up to 10 μm. This porosity results from the entrapment of carbon oxides (mainly CO and CO2), which are produced by the reaction of carbon in the steel scrap with oxygen at elevated temperatures.

Figure 6.
Electric arc furnace slag: (a) macroscopic view (Reprinted from Ref. [117]); (b) SEM micrograph showing rough particles of different shapes and sizes (Reprinted from Ref. [118]).
EAF slag is not classified as hazardous waste and is most commonly disposed of; however, there is potential environmental risk due to the leaching of heavy metals (e.g., Fe, Mn, Cr, Cd, Cu, Hg, Mo, Ni, Pb, Sn, V), potentially at concentrations exceeding regulatory thresholds [18,119]. Although EAFS is mostly landfilled, it has significant potential as a multifunctional material [9,116,117,120]. Valuable applications include its use as a supplementary cementitious material blended with clinker to produce Portland slag cement, as aggregate in hot and warm mix asphalt and bituminous emulsion mixtures, and as a cost-effective base or sub-base in road construction. With the growing need to secure secondary sources of metals, hydrometallurgical recycling of industrial waste has attracted increasing attention in recent years [9,10,115,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136].
4.1.2. Composition
EAF slag is a non-metallic material that contains a wide range of elements, with their concentrations varying considerably (Table 10). The contents of major elements are typically expressed as their corresponding oxides, i.e., 3–50% CaO, 1–15% MgO, 1–15% Al2O3, 5–40% SiO2, 15–50% Fe2O3, 0.03–30% Cr2O3, 0.05–2% V2O5, 0.6–1.3% TiO2, and 0.4–5% MnO [120,122,123,124,128,129,130,132,133,134,135]. Other elements like Ti, Zr, Nb, Mo, Ba, Sr, Li, U, Ir, Rb, Cu, Zn, Th, and Y have also been detected in small or trace amounts [10,123,126,127,129].

Table 10.
Concentration (in wt%) of main elements in EAF slags from different plants.
Analysis of four grain size fractions (with d50 in a range of 0.1–70 μm) of EAF slag did not reveal significant differences in the content of major elements [131]. However, the tendency of critical metals to accumulate in specific particle size fractions remains unclear. Similarly, no notable variations in phase composition have been observed.
The EAF slags primarily contain metal oxides and silicates; however, detailed analysis has revealed a highly complex phase composition, with dozens of different compounds identified [10,116,123,132,137]. Typical crystalline phases include larnite Ca2SiO4, wollastonite CaSiO3, wustite FeO, gehlenite Ca2Al(AlSi)O7, bredigite Ca7Mg(SiO4)4, brownmillerite Ca2(Al,Fe)2O5, and (Mg,Al,Fe,Cr) spinels, among others. Due to the complex composition and diverse origins of EAF slag, along with challenges in accurately attributing diffraction peaks to specific phases, quantitative phase analysis yields different results depending on the sample. For example, Zhao et al. [124] analyzed Chinese slag and reported 43.3% wollastonite, 20.4% diopside CaMg(SiO3)2, 13% Cr2O3, 12% augite (Ca,Mg,Fe)Si2O6, 6.6% FeO·Cr2O3 spinel, and 4.3% Fe3O4. In contrast, Teo et al. [122] presented results for Malaysian slag, identifying 45.5% gehlenite, 21% larnite, 20.3% wustite, and 13.4% hematite.
4.2. Recovery of Metals
In contrast to EAF dusts, interest in the hydrometallurgical recovery of metals from EAF slags has emerged only in recent years [10,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136]. Research efforts have primarily focused on the recovery of Cr, V, Mn, Ti, and Nb. Both direct leaching of the material and combined pyrometallurgical–hydrometallurgical treatments have been applied (Table 11). Notably, valuable critical metals are present in small or trace amounts and are often associated with major mineral phases. Therefore, understanding the behavior of these host minerals is essential for predicting the leachability of target metals. For example, Mombelli et al. [123] reported that the mineral composition of slag significantly affects its leaching behavior under standard conditions. They found that larnite is prone to natural leaching of vanadium, while brownmillerite, although less soluble, contributes to the release of chromium. In turn, Kaim and Azimi [10] observed that titanium and niobium are primarily associated with calcium-based minerals, either forming compounds such as CaTiO3 or occurring as substituent elements within the mineral structure.

Table 11.
Leaching of metals from EAF slags.
Manganese is an important critical and strategic component of EAF slags, with concentrations reaching up to approximately 5% (Table 10). Su et al. [125] proposed a hydrometallurgical treatment consisting of several steps: (i) dissolution of EAF slag in a mixed acid solution of 2 M HCl and 2.8 M HNO3 (70 °C); (ii) precipitation of calcium as calcium sulfate using concentrated H2SO4 at pH 0.1; (iii) hydrothermal precipitation of hematite (180 °C in the presence of a methanol/ethanol mixture); and finally (iv) precipitation of hausmannite Mn3O4 using NaOH at pH 8.
Chromium, considered a critical metal, is present in EAF slag at sufficiently high concentrations (0.5–2%) to warrant recovery efforts. Zhao et al. [124] used undersized EAF slag (<75 μm) for high-pressure alkaline leaching under an oxygen atmosphere (20–50 wt% NaOH, 80–180 °C, 0.8–1.4 MPa, L/S 5, 1–6 h). Under strongly alkaline conditions, the main chromium-bearing phases (Cr2O3, FeCr2O4) were converted into soluble sodium chromate Na2CrO4. Metal extraction increased with temperature (up to 160 °C), leaching time (up to 4 h), and NaOH concentration, while oxygen pressure had no significant effect. Most of the silicate phases, such as wollastonite, diopside, and augite, dissolved during the process, exposing encapsulated chromium oxides and iron-chromium spinels to the oxidation reaction. However, with prolonged treatment, a secondary layer composed mainly of iron and oxygen formed on the residue surface, hindering further chromium leaching, thus the maximal process efficiency reached up to 60%. The leaching process was found to be controlled by diffusion through the solid product layer, consistent with the shrinking core model of reaction kinetics.
In turn, Menad et al. [128] investigated various pre-treatment methods (grinding, roasting) prior to water leaching to enhance the recovery of chromium, vanadium, and molybdenum from EAF slag after magnetic separation of iron scrap. Among the grinding additives tested (NaOH, KOH, NaOH + Na2CO3, KOH + K2CO3) combined with roasting temperatures (400–800 °C), different effects on subsequent metal leaching were observed. As a result, no single set of optimal conditions was identified for the simultaneous recovery of all target metals. Further studies [130] focused on the separation of Cr and V using a modified approach, in which mixtures of EAF slag and alkaline reagents were subjected to microwave treatment followed by water leaching. Although different pre-treatment conditions were still required for individual metals, higher leaching efficiencies were achieved with significantly shorter treatment times (Table 11).
Kokko et al. [131] applied a two-stage direct leaching method to selectively recover Ca and V from EAFS produced by pilot-scale hydrogen-reduced iron smelting. In the first stage, a HNO3 + NH4NO3 solution was used, which resulted in over 99% of the dissolved elements being Ca and Mg. For the second stage, Na2CO3 + NaOH and (NH4)2CO3 solutions were tested as alternative media for vanadium leaching. Experimental designs were used to study the effects of medium composition, temperature, and liquid-to-solid ratio on element recoveries and selectivity. Under optimal conditions, 55% of calcium was recovered with 91% selectivity, while 39% vanadium recovery with 90% selectivity in the second step of leaching in the (NH4)2CO3 solution. As an interesting aside, Tong et al. [134] demonstrated that 50–98% Ca can be leached from EAFS in a 2 M NH4Cl solution under microwave irradiation, with the highest efficiency achieved at the highest power setting (900 W, 40 min).
Titanium and niobium are critical metals that exist in EAF slag primarily in trace amounts as refractory phases, making their recovery particularly challenging. Kim and Azimi [10] proposed a two-stage treatment of EAF slag, called “acid-baking water leaching,” to extract trace amounts of titanium and niobium. The process involved thermal treatment of the raw material with concentrated H2SO4, which converts refractory metal phases into soluble sulfates:
CaTiO3 + H2SO4 → CaSO4 + TiO2 + H2O
TiO2 + H2SO4 → TiOSO4 + H2O
4TiOSO4 + 2H2SO4 → 2Ti2(SO4)3 + 2H2O + O2
Nb2O5 + 2H2SO4 → Nb2O3(SO4)2 + 2H2O
By optimizing the baking conditions (200 or 400 °C, acid-to-slag ratio 1–3, duration 0.5–2 h) and subsequent water leaching under ambient conditions (pulp density 50–200 g/L, agitation rate 150–500 rpm), they developed a process capable of simultaneously extracting both metals with efficiencies exceeding 93%.
A further study [136] focused on the recovery of titanium, magnesium, and aluminum and proposed a three-stage process, which included an additional pre-treatment step: carbothermic reduction of EAF slag to remove iron residues [126]. During the pyrometallurgical reduction with lignite coal, metals were redistributed between the metallic and slag (EAFS-slag) phases. This resulted in decreasing contents of certain elements (Fe, Mn, Cr, Nb, Ni, C) in the non-metallic product, accumulation of others (Ca, Mg, Al, Sr), and comparable distribution between both phases for some elements (Ti, Cu). Under acid-baking conditions, metals in the EAFS slag were converted into sulfates, enabling selective leaching of Ti, Mg, and Al with water, while CaSO4·H2O remained as a component of the solid residue [126]. The resulting leach liquor contained the target metals at relatively low concentrations: 0.1 g/L Ti(III), 5.2 g/L Mg(II), and 2.2 g/L Al(III) [136]. The concentrations of impurities (Ca, Fe, Cr, Sr) ranged from 0.005 g/L to 0.4 g/L. By exploiting the differences in hydroxide precipitation pH (2.5–3.0 for Ti, 3.5–4.9 for Al, and 6.7–7.3 for Mg), selective metal separation using NaOH (1 M, 80 °C, 20 min) was achieved. The final results showed a titanium recovery efficiency of 85% as TiO2 with a purity of 83%, and 100% recovery of magnesium and aluminum as hydroxides with purities exceeding 90%.
As EAF slag contains high concentrations of iron, Sanda et al. [127] proposed a simple method involving acid leaching (1–4 M HCl, 30–90 °C, 2 h) followed by solvent extraction using trioctylphosphine oxide TOPO in methyl isobutyl ketone MIK or kerosene. Maximum iron dissolution reached approximately 45% within 1 h at 90 °C. The subsequent solvent extraction showed improved performance with increased TOPO concentrations (50–200 g/L), the use of MIBK as the diluent, and elevated chloride ion concentrations in the aqueous phase (added as CaCl2). However, no stripping stage was performed, and thus, overall recovery efficiencies were not reported. In turn, Zulkifli et al. [133] found that 5 M H2SO4 was a more effective leaching agent than 5 M HCl. However, at elevated temperatures, the transfer of iron ions to the solution decreased, with this effect becoming noticeable above 60 °C, likely due to HCl evaporation. On the other hand, increasing the acid concentration (0.5–8 M) enhanced iron solubility. For HCl, solubility stabilized in the 3–8 M range, while for H2SO4, it suddenly dropped at 8 M. The addition of oxidizing agents (H2O2 or KMnO4) to the acid solutions further improved iron leachability, with hydrogen peroxide showing significantly better performance. Unfortunately, the authors did not report leaching efficiencies, but only the final concentrations of iron ions in solution (2–11 g/L).
Finally, it is worth mentioning attempts at bioleaching metals from EAF slag. Hocheng et al. [135] compared the effectiveness of three microbial cultures (bacteria and fungi), showing a decreasing order of efficiency: Acidithiobacillus thiooxidans > Acidithiobacillus ferrooxidans > Aspergillus niger. The differences in effectiveness were attributed to the types of acids produced, i.e., sulfuric acid by At. thiooxidans bacteria and citric acid by A. niger fungi. Notably, bioleaching required water prewashing to reduce the alkalinity of EAFS (from pH 11 to 8) in order to somewhat enhance the action of acids. Optimal metal extraction was achieved within six days using the At. thiooxidans culture supernatant, and the extraction efficiency improved further with repeated bioleaching cycles with fresh bacteria culture. The highest extraction efficiencies were for Mg (75%) and Zn (60%), while for the other tested metals (Al, Ni, Cu, Cr, Mo, Pb, Mn), efficiencies did not exceed 30%. These tests demonstrated the potential of bioleaching as a complementary method for metal recovery from EAF slag, although further optimization and the adaptation of microorganisms to high metal ion concentrations are necessary to enhance efficiency for a wider range of heavy metals.
5. Conclusions
The hydrometallurgical recovery of metals from electric arc furnace dust and slags shows considerable promise for reclaiming valuable metals, such as zinc, chromium, vanadium, titanium, etc. While EAFD is traditionally processed via pyrometallurgical routes, hydrometallurgical methods offer several key advantages, including lower energy consumption, milder operating conditions, and better environmental compatibility. Various leaching systems have been developed under laboratory conditions, although only a few commercial technologies have demonstrated their effectiveness in recycling EAFD. Similarly, in EAFS, the complexity of material chemistry and the low content of critical metals complicates process optimization. However, challenges remain in the widespread industrial adoption of hydrometallurgical methods, particularly in EAFD treatment, where issues such as the low leachability of zinc ferrites and the presence of chloride ions hinder efficient recovery. These problems can be overcome by combining pyrometallurgical and hydrometallurgical methods, utilizing additives that help reduce iron in ferrites, thereby destroying their structures or developing solvometallurgical treatments. Despite these hurdles, hydrometallurgical approaches present a significant opportunity to reduce industrial waste disposal. With continued research and development, hydrometallurgical recovery could become a more viable and economically sustainable alternative offering a more environmentally friendly and resource-efficient solution for metal recovery (Figure 7).
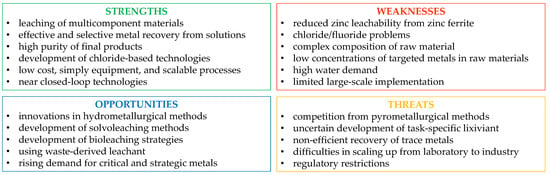
Figure 7.
SWOT analysis of hydrometallurgical treatment of EAF by-products.
Funding
This research received no external funding.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The author declares no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript, in order of appearance:
| EAF | Electric arc furnace |
| EAFD | Electric arc furnace dust |
| EAFS | Electric arc furnace slag |
| C.Z.O. | Crude zinc oxide |
References
- Karbowniczek, M. Electric Arc Furnace Steelmaking; CRC Press: Boca Raton, FL, USA, 2021. [Google Scholar]
- Hasanbeigi, A. Steel Climate Impact—An International Benchmarking of Energy and CO2 Intensities; Global Efficiency Intelligence: St. Petersburg, FL, USA, 2022. [Google Scholar]
- Word Steel in Figures 2025; World Steel Association: Brussels, Belgium, 2025.
- Corneille, A.; Agarwal, A.; Seguineaud, C.; Ventricelli, V.; Conesa, R. Unlocking Potential in the Global Scrap Steel Market: Opportunities and Challenges. OECD Science, Technology and Industry Policy Papers; No. 170; OECD Publishing: Paris, France, 2024. [Google Scholar] [CrossRef]
- Lopez, G.; Farfan, J.; Breyer, C. Trends in the global steel industry: Evolutionary projections and defossilisation pathways through power-to-steel. J. Clean. Prod. 2022, 375, 134182. [Google Scholar] [CrossRef]
- Hu, H.; Yang, L.; Yang, S.; Zou, Y.; Wang, S.; Chen, F.; Guo, Y. Development and assessment of an integrated wind energy system for green steelmaking based on electric arc furnace route. Energy 2024, 302, 131783. [Google Scholar] [CrossRef]
- Remus, R.; Aguado-Monsobet, M.A.; Roudier, S.; Sancho, L.D. Best Available Techniques (BAT) Reference Document for Iron and Steel Production. Industrial Emissions Directive 2010/75/EU; EC Joint Research Centre: Seville, Spain, 2013. [Google Scholar]
- Xue, Y.; Liu, X.; Xu, C.; Han, Y. Hydrometallurgical detoxification and recycling of electric arc furnace dust. Int. J. Miner. Metall. Mater. 2023, 30, 2076–2094. [Google Scholar] [CrossRef]
- Kurecki, M.; Meena, N.; Shyrokykh, T.; Korobeinikov, Y.; Örell, T.J.; Voss, Z.; Pretorius, E.; Jones, J.; Sridhar, S. Recycling perspectives of electric arc furnace slag in the United States: A review. Steel Res. Int. 2025, 2300854. [Google Scholar] [CrossRef]
- Kim, J.; Azimi, G. Technospheric mining of niobium and titanium from electric arc furnace slag. Hydrometallurgy 2020, 191, 105203. [Google Scholar] [CrossRef]
- 2014/955/EU: Commission Decision of 18 December 2014 Amending Decision 2000/532/EC on the List of Waste Pursuant to Directive 2008/98/EC of the European Parliament and of the Council. 2014. Available online: https://eur-lex.europa.eu/eli/dec/2014/955/oj/eng (accessed on 4 June 2025).
- Stagemann, J.A.; Roy, A.; Caldwell, R.J.; Schilling, P.J.; Tittsworth, R. Understanding environmental leachability of electric arc furnace dust. J. Environ. Eng. 2000, 126, 112–120. [Google Scholar] [CrossRef]
- Chung, C.J.; Wu, C.D.; Hwang, B.F.; Wu, C.C.; Huang, P.H.; Ho, C.T.; Hsu, H.T. Effects of ambient PM2.5 and particle-bound metals on the healthy residents living near an electric arc furnace: A community-based study. Sci. Total. Environ. 2020, 728, 138799. [Google Scholar] [CrossRef]
- Salihoglu, G.; Pinarli, V. Steel foundry electric arc furnace dust management: Stabilization by using lime and Portland cement. J. Hazard. Mater. 2008, 153, 1110–1116. [Google Scholar] [CrossRef]
- Nair, A.T.; Mathew, A.; Archana, A.R.; Akbar, M.A. Use of hazardous electric arc furnace dust in the construction industry: A cleaner production approach. J. Clean. Prod. 2022, 377, 134282. [Google Scholar] [CrossRef]
- Lin, X.; Peng, Z.; Yan, J.; Li, Z.; Hwang, J.Y.; Zhang, Y.; Li, G.; Jiang, T. Pyrometallurgical recycling of electric arc furnace dust. J. Clean. Prod. 2017, 149, 1079–1100. [Google Scholar] [CrossRef]
- Antrekowitsch, J.; Steinlechner, S.; Hanke, G.; Brandner, U. Zinc. In Handbook of Recycling, 2nd ed.; Meskers, C., Worell, E., Reuter, M.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2024; pp. 371–384. [Google Scholar]
- Yu, S.; Garrabrants, A.C.; DeLapp, R.C.; Hubner, T.; Thorneloe, S.A.; Kosson, D.S. Evaluation of testing approaches for constituent leaching from electric arc furnace (EAF) slags. J. Environ. Manag. 2025, 373, 123892. [Google Scholar] [CrossRef]
- Grohol, M.; Veeh, C. Study on the Critical Raw Materials for the EU 2023; Publication Office of the European Union: Luxemburg, 2023. [Google Scholar]
- Critical Minerals for India. Report of the Committee on Identification of Critical Minerals, Ministry of Mines of India. 2023. Available online: https://mines.gov.in/admin/download/649d4212cceb01688027666.pdf (accessed on 24 October 2024).
- The Canadian Critical Minerals Strategy. Available online: https://www.canada.ca/en/campaign/critical-minerals-in-canada/canadian-critical-minerals-strategy.html (accessed on 24 June 2025).
- U.S. Geological Survey Releases 2022 List of Critical Minerals. Available online: https://www.usgs.gov/news/national-news-release/us-geological-survey-releases-2022-list-critical-minerals (accessed on 24 October 2024).
- Updates to Australia’s Critical Mineral List. Available online: https://www.industry.gov.au/news/updates-australias-critical-minerals-list (accessed on 24 June 2025).
- Keglevich de Buzin, P.J.W.; Heck, N.C.; Vilela, A.C. EAF dust: An overview on the influences of physical, chemical and mineral features in its recycling and waste incorporation routes. J. Mater. Res. Technol. 2017, 6, 194–202. [Google Scholar] [CrossRef]
- The World Zinc Factbook 2024; International Lead and Zinc Study Group: Lisbon, Portugal, 2024; Available online: https://www.ilzsg.org/ (accessed on 24 June 2025).
- Forecast Market Value of Selected Critical Materials Worldwide from 2027 to 2030. Statista. Available online: https://www.statista.com/statistics/1361230/forecast-global-market-value-of-critical-materials/ (accessed on 24 June 2025).
- Kaya, M.; Hussaini, S.; Kursunoglu, S. Critical review on secondary zinc residues and their recycling technologies. Hydrometallurgy 2020, 195, 105362. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Y.; Cui, K.; Fu, T.; Gao, J.; Hussain, S.; AlGarni, T.S. Pyrometallurgical recovery of zinc and valuable metals from electric arc furnace dust—A review. J. Clean. Prod. 2021, 298, 126788. [Google Scholar] [CrossRef]
- Guézennec, A.G.; Huber, J.C.; Patisson, F.; Sessiecq, P.; Birat, J.P.; Ablitzer, D. Dust formation in electric arc furnace: Birth of the particles. Powd. Technol. 2005, 157, 2–11. [Google Scholar] [CrossRef]
- Trifunović, V.; Milić, S.; Avramović, L.; Jonović, R.; Gardić, V.; Dordievski, S.; Dimitrijević, S. Investigation of hazardous waste: A case study of electric arc furnace dust characterization. Hem. Ind. 2022, 76, 237–249. [Google Scholar] [CrossRef]
- Lanzerstrofer, C.; Brunner, C. Electric arc furnace dust: Characterization of flowability. In Proceedings of the 32nd International Conference on Metallurgy and Materials METAL 2023, Brno, Czech Republic, 17–19 May 2023. [Google Scholar]
- Oustadakis, P.; Tsakiridis, P.E.; Katsiapi, A.; Agatzini-Leonardou, S. Hydrometallurgical process for zinc recovery from electric arc furnace dust (EAFD). Part I: Characterization and leaching by diluted sulphuric acid. J. Hazard. Mater. 2010, 179, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Park, D.S.M.; Hwang, Y.; Ha, J.G.; Shin, H.S. Toward high recovery and selective leaching of zinc from electric arc furnace dust with different physicochemical properties. Environ. Eng. Res. 2020, 25, 335–344. [Google Scholar] [CrossRef]
- Laubertova, M.; Havlik, T.; Parilak, L.; Derin, B.; Trpcevska, J. The effects of microvave-assisted leaching on the treatment of electric arc furnace dusts (EAFD). Arch. Metall. Mater. 2020, 65, 321–328. [Google Scholar] [CrossRef]
- Trifunović, V.; Milić, S.; Avramović, L.; Bugarin, M.; Dordievski, S.; Antonijević, M.M.; Radovanović, M.B. Application of a simple pretreatment in the process of acid leaching of electric arc furnace dust. Metals 2024, 14, 426. [Google Scholar] [CrossRef]
- Pickles, C.A.; Marzoughi, O. Thermodynamic investigation of the sulphation rosasting of electric arc furnace dust. Minerals 2019, 9, 18. [Google Scholar]
- Rudnik, E. Recovery of zinc from steelmaking flue dust by hydrometallurgical route. Arch. Metall. Mater. 2020, 65, 601–608. [Google Scholar] [CrossRef]
- Zoraga, M.; Ilhan, S.; Kalpakli, A.O. Leaching kinetics of electric arc furnace dust in nitric acid solutions. Int. J. Chem. Kinet. 2020, 52, 933–942. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, R.; Wang, H.; Liu, W.; Yang, T.; Chen, L. Recovery of zinc from electric arc furnace dust by alkaline pressure leaching using iron as a reductant. J. Cent. South Univ. 2021, 28, 2701–2710. [Google Scholar] [CrossRef]
- Teimouri, S.; Potgieter, J.H.; Lundström, M.; Billing, C.; Wilson, B.P. A new hydrometallurgical process for metal extraction from electric arc furnace dust using ionic liquids. Materials 2022, 15, 8648. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Li, J.; Teng, W.; Chen, Y.; Liu, W.; Ren, S.; Yang, J.; Liu, Q. One-step extraction of zinc and separation of iron from hazardous electric arc furnace dust via sulphating roasting-water leaching. J. Environ. Chem. Eng. 2023, 11, 11155. [Google Scholar] [CrossRef]
- Gamutan, J.; Koide, S.; Sasaki, Y.; Nagasaka, T. Selective dissolution of leaching zinc from lime treated electric arc furnace dust by alkaline media. J. Environ. Chem. Eng. 2024, 12, 111789. [Google Scholar] [CrossRef]
- Ju, J.; Yang, W.; Long, T.; Deng, S.; Yang, C. High-efficiency separation and recovery of Zn and Fe from EAF dust via ammonium salt roasting-water leaching: Process optimization and mechanistic insights. Process. Saf. Environ. Prot. 2025, 199, 107276. [Google Scholar] [CrossRef]
- Al-Harahsheh, M.; Altarawneh, S.; Al-Omari, M. Selective dissolution of zinc and lead from electric arc furnace dust via oxidative thermolysis with polyvinyl chloride and water-leaching process. Hydrometallurgy 2022, 212, 105898. [Google Scholar] [CrossRef]
- Havlik, T.; Friedrich, B.; Stopić, S. Pressure leaching of EAF dust with sulphuric acid. World Metall. 2004, 57, 113–120. [Google Scholar]
- Bruckard, W.J.; Davey, K.J.; Rodopoulos, T.; Woodcock, J.T.; Italiano, J. Water leaching and magnetic separation for decreasing the chloride level and upgrading the zinc content of EAF steelmaking baghouse dusts. Int. J. Miner. Proc. 2005, 75, 1–20. [Google Scholar] [CrossRef]
- Chen, W.S.; Shen, Y.H.; Tsai, M.S.; Chang, F.C. Removal of chloride from electric arc furnace dust. J. Hazard. Mater. 2011, 190, 639–644. [Google Scholar] [CrossRef]
- Santos, E.B.C.; Muniz, D.D.; Barbosa, N.P.; Correira, E.A.S.; Alves, L.D.M.; de Melo, M.B.F.V. Brazilian steel waste: Electrical arc furnace dust chemical, thermal and morphological characterization. J. Mater. Res. Technol. 2021, 14, 2775–2781. [Google Scholar] [CrossRef]
- Sofilić, T.; Rastovčan-Mioč, A.; Cerjan-Stefanović, S.; Novosel-Radović, V.; Jenko, M. Characterization of steel mill electric-arc furnace dust. J. Hazard. Mater. 2004, 109, 59–70. [Google Scholar] [CrossRef]
- Lutandula, M.S.; Kashala, G.N. Zinc oxide production through reprocessing of the electric arc furnace flue dusts. J. Environ. Chem. Eng. 2013, 1, 600–603. [Google Scholar] [CrossRef]
- Shawabkeh, R.A. Hydrometallurgical extraction of zinc from Jordanian electric arc furnace dust. Hydrometallurgy 2010, 104, 61–65. [Google Scholar] [CrossRef]
- Palimąka, P.; Pietrzyk, S.; Stępień, M.; Ciećko, K.; Nejman, I. Zinc recovery from steelmaking dust by hydrometallurgical methods. Metals 2018, 8, 547. [Google Scholar] [CrossRef]
- Kukurugya, F.; Vindt, T.; Havlik, T. Behavior of zinc, iron and calcium from electric arc furnace (EAF) dust in hydrometallurgical processing in sulfuric acid solutions: Thermodynamic and kinetic aspects. Hydrometallurgy 2015, 154, 30–32. [Google Scholar] [CrossRef]
- Lanzerstrofer, C. Electric arc furnace (EAF) dust: Application of air classification for improved zinc enrichment in in-plant recycling. J. Clean. Prod. 2018, 174, 1–6. [Google Scholar] [CrossRef]
- Sofilić, T.; Novosel-Radović, V.; Cerjan-Stefanović, S.; Rastovčan-Mioč, A. The mineralogical composition of dust from an electric arc furnace. Mater. Tehnol. 2005, 39, 149–154. [Google Scholar]
- Simonyan, L.M.; Alpatova, A.A.; Demidova, N.V. The EAF dust chemical and phase composition research techniques. J. Mater. Res. Technol. 2019, 8, 1601–1607. [Google Scholar] [CrossRef]
- Orhan, G. Leaching and cementation of heavy metals from electric arc furnace dust in alkaline medium. Hydrometallurgy 2005, 78, 236–245. [Google Scholar] [CrossRef]
- Langová, Š.; Leško, J.; Matýsek, D. Selective leaching of zinc from zinc ferrite with hydrochloric acid. Hydrometallurgy 2009, 95, 179–182. [Google Scholar] [CrossRef]
- Leclerc, N.; Meux, E.; Lecuire, J.M. Hydrometallurgical extraction from zinc ferrites. Hydrometallurgy 2003, 70, 175–183. [Google Scholar] [CrossRef]
- Suetens, T.; Guo, M.; Van Acker, K.; Blanpain, B. Formation of the ZnFe2O4 phase in an electric arc furnace off-gas treatment system. J. Hazard. Mater. 2015, 287, 180–187. [Google Scholar] [CrossRef]
- Kul, M.; Oskay, K.O.; Simsir, M.; Sübütay, H.; Kirgezen, H. Optimization of selective leaching of Zn from electric arc furnace steelmaking dust using response surface methodology. Trans. Nonferrous. Met. Soc. China 2015, 25, 2753–2762. [Google Scholar] [CrossRef]
- Langová, Š.; Riplová, J.; Vallová, S. Atmospheric leaching of steel-making wastes and the precipitation of goethite from the ferric sulphate solution. Hydrometallurgy 2007, 87, 157–162. [Google Scholar] [CrossRef]
- Langová, Š.; Matýsek, D. Zinc recovery from steel-making wastes by acid pressure leaching and hematite precipitation. Hydrometallurgy 2010, 10, 171–173. [Google Scholar] [CrossRef]
- Teo, Y.Y.; Lee, H.S.; Low, Y.S.; Choong, S.W.; Low, K.O. Hydrometallurgical extraction of zinc and iron from electric arc furnace dust (EAFD) using hydrochloric acid. J. Phys. Sci. 2018, 29, 49–54. [Google Scholar] [CrossRef]
- Halli, P.; Hamuyuni, J.; Revitzer, H.; Lindström, M. Selection of leaching media for metal dissolution from electric arc furnace dust. J. Clean. Prod. 2017, 164, 265–276. [Google Scholar] [CrossRef]
- Sajal, W.R.; Ahmad, S.; Gulshan, F. Optimisation of hybrid process to recover zinc from electric furnace dust using design of experiment approach. Can. Metall. Quart. 2025, 64, 664–679. [Google Scholar] [CrossRef]
- Siame, M.C.; Kaoma, J.; Hlabangana, N.; Danha, G. An attainable region approach for the recovery of iron and zinc from electric arc furnace dust. S. Afr. J. Chem. Eng. 2019, 27, 35–42. [Google Scholar] [CrossRef]
- Chairaksa-Fujimoto, R.; Maruyama, K.; Miki, T.; Nagasaka, T. The selective alkaline leaching of zinc oxide from electric arc furnace dust pre-treated with calcium oxide. Hydrometallurgy 2016, 159, 120–125. [Google Scholar] [CrossRef]
- Gargul, K.; Handzlik, P.; Palimąka, P.; Pawlik, A. The application of citric acid solutions for selective removal of zinc from steelmaking dust. J. Min. Metall. Sec. B-Metall. 2021, 57, 163–173. [Google Scholar] [CrossRef]
- Machado, J.G.M.S.; Brehm, F.A.; Moraes, C.A.M.; dos Santos, C.A.; Vilela, A.C.F.; da Cunha, J.B.M. Chemical, physical, structural and morphological characterization of the electric arc furnace dust. J. Hazard. Mater. 2006, 136, 953–960. [Google Scholar] [CrossRef] [PubMed]
- Saba, A.E.; Elsherief, A.E. Continous electrowinning of zinc. Hydrometallurgy 2000, 54, 91–106. [Google Scholar] [CrossRef]
- Wang, X.; Zhong, Y.; Kang, Y.; Gao, J.; Guo, Z. Promoted acid leaching of Zn from hazardous zinc-containing metallurgical dusts: Focus on transformation of Zn phases in selective reduction roasting. Process. Saf. Environ. Prot. 2022, 163, 353–361. [Google Scholar] [CrossRef]
- Halli, P.; Hamuyuni, J.; Leikola, M.; Lindström, M. Developing a sustainable solution for recycling electric arc furnace dust via organic acid leaching. Min. Eng. 2018, 124, 1–9. [Google Scholar] [CrossRef]
- Jarupisitthorn, C.; Pimtong, T.; Lothongkum, G. Investigation kinetics of zinc leaching from electric arc furnace dust by sodium hydroxide. Mater. Chem. Phys. 2002, 77, 531–535. [Google Scholar] [CrossRef]
- Dutra, A.J.B.; Paiva, P.R.P.; Tavares, L.M. Alkaline leaching of zinc from electric arc furnace steel dust. Min. Eng. 2006, 19, 478–485. [Google Scholar] [CrossRef]
- Youcai, Z.; Stanforth, R. Integrated hydrometallurgical process for production of zinc from electric furnace dust in alkaline medium. J. Hazard. Mater. 2000, 80, 223–240. [Google Scholar] [CrossRef]
- Zhang, D.; Ling, H.; Yang, T.; Liu, W.; Chen, L. Selective leaching of zinc from electric arc furnace dust by a hydrothermal reduction method in a sodium hydroxide system. J. Clean. Prod. 2019, 224, 536–544. [Google Scholar] [CrossRef]
- Xia, D.K.; Pickles, C.A. Microwave caustic leaching of electric arc furnace dust. Min. Eng. 2000, 13, 79–94. [Google Scholar] [CrossRef]
- Bayoumi, R.A.; Abdelmonem, N.M.; Soliman, M.A.; Ismail, I.M.; Refaat, A.A. Hybrid hydro-pyrometallurgical process for Zn recovery from electric arc furnace dust. J. Phys. Conf. Ser. 2022, 2305, 012009. [Google Scholar] [CrossRef]
- Lis, T.; Nowacki, K. Determination of physical and chemical properties of electric arc furnace dusts for the purposed of their utilization. Steel Res. 2012, 83, 842–851. [Google Scholar] [CrossRef]
- Yokoyama, S.; Sasaki, T.; Sasano, J.; Izaki, M. Elution of zinc in dust discharged from electric arc furnace in carbonic acid solution. J. Phys. Conf. Ser. 2012, 352, 012050. [Google Scholar] [CrossRef]
- Al-Harahsheh, M.; Altawneh, S.; Al-Omari, M.; Altarawneh, M.; Kingman, S.; Dodds, C. Leeching behavior of zinc and lead from electric arc furnace dust—Poly(vinyl) chloride residues after oxidative thermal treatment. J. Clean. Prod. 2021, 328, 129622. [Google Scholar] [CrossRef]
- Al-Harahsheh, M.; Orabi, Y.; Al-Asheh, S. Comparative study on the pyrolysis and leachability of washed/unwashed electric furnace dust-PVC mixtures and their residues. J. Environ. Chem. Eng. 2021, 9, 105410. [Google Scholar] [CrossRef]
- Al-Harahsheh, M.; Aljarrah, M.; Rummanah, F.; Abdel-Latif, K.; Kingman, S. Leaching of valuable metals from electric arc furnace dust—Tetrabromobisphenol A pyrolysis residues. J. Anal. Appl. Pyrol. 2017, 125, 50–60. [Google Scholar] [CrossRef]
- Al-Harahsheh, M.; Kingman, S.; Hamilton, I. Microwave treatment of electric arc furnace dust with tetrabromobisphenol A: Dielectric characterization and pyrolysis-leaching. J. Anal. Appl. Pyrol. 2017, 128, 168–175. [Google Scholar] [CrossRef]
- Nicol, M.; Akilan, C.; Tjandrawan, V.; Gonzalez, J.A. The effects of halides in the electrowinning of zinc. I. Oxidation of chloride on lead-silver anodes. Hydrometallurgy 2017, 173, 125–133. [Google Scholar] [CrossRef]
- Zhao, M.; Wang, S.; Zhang, L. Removal of chlorine from zinc sulfate solution: A review. Environ. Sci. Poll. Res. Int. 2022, 42, 62839–62850. [Google Scholar] [CrossRef]
- Parsonage, D.; Singh, P.; Nikoloski, A.N. Adverse effect of fluoride on hydrometallurgical operations. Min. Process. Extract. Metall. Rev. 2014, 35, 44–65. [Google Scholar] [CrossRef]
- McElroy, R.O.; McClaren, M. Processing of electric furnace dust via chloride hydrometallurgy. In Hydrometallurgy’94; Springer: Dordrecht, The Netherlands, 1994; pp. 993–1010. [Google Scholar]
- Harris, G.B.; White, C.W.; Hofbauer, T.; Dry, M.J. Treatment of electric arc furnace dust via a chloride-processing route. In Proceedings of the 60th Conference of Metallurgists COM 2021, MetSoc, Virtual Online Conference, 17–19 August 2021. [Google Scholar]
- Havlik, T.; Maruskinova, G.; Miskufova, A. Determination of ZnO amount in electric arc furnace dust and temperature dependence of leaching in ammonium carbonate by using of X-ray diffraction. Arch. Metall. Mater. 2018, 63, 653–658. [Google Scholar] [CrossRef]
- Ruiz, O.; Clemente, C.; Alonso, M.; Alugacil, F.J. Recycling of an electric arc furnace flue dust to obtain high grade ZnO. J. Hazard. Mater. 2007, 141, 33–36. [Google Scholar] [CrossRef] [PubMed]
- Miki, T.; Chairaksa-Fujimoto, R.; Maruyama, K.; Nagasaka, T. Hydrometallurgical extraction of zinc from CaO treated EAF dust in ammonium chloride solution. J. Hazard. Mater. 2016, 146, 90–96. [Google Scholar] [CrossRef]
- Leclerc, N.; Meux, E.; Lecuire, J.M. Hydrometallurgical recovery of zinc and lead from electric arc furnace dust using mononitriloacetate anion and hexahydrated ferric chloride. J. Hazard. Mater. 2002, 91, 257–270. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Jia, N.; Liu, W.; Zhang, M.; Guo, M. Efficient and selective hydrothermal extraction of zinc from zinc-containing electric arc furnace dust using a novel bifunctional agent. Hydrometallurgy 2016, 166, 107–112. [Google Scholar] [CrossRef]
- Harvey, T.G. The hydrometallurgical extraction of zinc by ammonium carbonate: A review of the Schnabel process. Min. Process. Extract. Metall. Rev. 2006, 27, 231–279. [Google Scholar] [CrossRef]
- Kinnunen, P.; Miettinen, H.; Frilund, C.; Simell, P. Integration of H2S gas cleaning and bioleaching for zinc recovery from electric arc furnace dust. Hydrometallurgy 2025, 236, 106505. [Google Scholar] [CrossRef]
- Mousavi, S.N.; Mousavi, S.M.; Beolchini, F. A practical green methodology for metal extraction from electric arc furnace dust through Yarrowia Lipolytica biolixviant. Min. Eng. 2024, 217, 108948. [Google Scholar] [CrossRef]
- Yang, H.; Hu, K.; Feng, J.; Ning, P.; Wang, F.; Cui, S. Resource utilization of electric arc furnace dust: Efficient wet desulfurization and valuable metal leaching kinetics investigation. J. Environ. Manag. 2025, 375, 124419. [Google Scholar] [CrossRef]
- Binnemans, K.; Jones, P.T. Solvometallurgy: An emerging branch of extractive metallurgy. J. Sustain. Metall. 2017, 3, 570–600. [Google Scholar] [CrossRef]
- Binnemans, K.; Jones, P.T. Ionic liquids and deep–eutectic solvents in extractive metallurgy: Mismatch between academic research and industrial applicability. J. Sustain. Metall. 2023, 9, 423–438. [Google Scholar] [CrossRef]
- Bakkar, A. Recycling of electric arc furnace dust through dissolution in deep eutectic ionic liquids and electrowinning. J. Hazard. Mater. 2014, 280, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Tsakiridis, P.E.; Oustadakis, P.; Katsiapi, A.; Agatzini-Leonardu, S. Hydrometallurgical process for zinc recovery from electric arc furnace dust (EAFD). Part II: Downstream processing and zinc recovery by electrowinning. J. Hazard. Mater. 2010, 179, 8–14. [Google Scholar] [CrossRef]
- Xanthopoulos, P.; Agatzini-Leonardu, S.; Oustadakis, P.; Tsakiridis, P.E. Zinc recovery from purified electric arc furnace dust leach liquors by chemical precipitation. J. Environ. Chem. Eng. 2017, 5, 3550–3559. [Google Scholar] [CrossRef]
- Halli, P.; Agarwal, V.; Partinen, J.; Lundström, M. Recovery Pb and Zn from a citrate leach liquor of a roasted EAF dust using precipitation and solvent extraction. Sep. Pur. Technol. 2020, 236, 116264. [Google Scholar] [CrossRef]
- Carranza, F.; Romero, R.; Mazuelos, A.; Iglesias, N. Recovery of Zn from acid mine water and electric arc furnace dust in an integrated process. J. Environ. Manag. 2016, 165, 175–183. [Google Scholar] [CrossRef]
- Hazaveh, P.K.; Karimi, S.; Raschi, F.; Sheibani, S. Purification of the leach solution of recycling from the hazardous electric arc furnace dust through an as-bearing jarosite. Ecotox. Environ. Saf. 2020, 202, 110893. [Google Scholar]
- Darezereshki, E.; Vakylabad, A.B.; Koohestani, B. A hydrometallurgical approach to produce nano-ZnO from electrical arc furnace dusts. Min. Metall. Explor. 2021, 38, 1525–1535. [Google Scholar] [CrossRef]
- Xia, D. Recovery of Zinc from Zinc Ferrite Ane Electric Arc Furnace Dust. Ph.D. Thesis, Queens University of Kingston, Kingston, ON, Canada, 1997. [Google Scholar]
- Maccagoni, M.G. INDUTEC®/ENZINEX® integrate process on secondary zinc-bearing materials. J. Sustain. Metall. 2016, 2, 133–140. [Google Scholar] [CrossRef]
- Frias, C.; Diaz, G.; Martin, D.; Sanchez, F.; Onoberhie, C. Highly Efficient and Low Cost Zinc Electrolyte Purification Using ZINCEXTM Solvent Extraction. Available online: https://ddtp.tecnicasreunidas.es/downloads/ (accessed on 18 July 2025).
- Modified Zincex ProcessTM by Técnicas Reunidas. Available online: https://ddtp.tecnicasreunidas.es/wp-content/uploads/2016/11/P-ZINCEX-technology-information-II.pdf (accessed on 18 July 2025).
- Diaz, G.; Martin, D. Modified Zincex Process: The clean, safe and profitable solution to the zinc secondaries treatment. Res. Conserv. Rec. 1994, 10, 43–57. [Google Scholar] [CrossRef]
- Cole, P.M.; Sole, K.C. Zinc solvent extraction in the process industries. Min. Process. Extract. Metall. Rev. 2003, 24, 91–137. [Google Scholar] [CrossRef]
- Binnemans, K.; Jones, P.T.; Fernandez, A.M.; Torres, V.M. Hydrometallurgical processes for the recovery of metals from steel industry by-products: A critical review. J. Sustain. Metall. 2020, 6, 505–540. [Google Scholar] [CrossRef]
- Teo, P.T.; Zakaria, S.K.; Salleh, S.Z.; Taib, M.A.A.; Sharif, N.M.; Seman, A.A.; Mohamed, J.J.; Yusoff, M.; Yusoff, A.H.; Mohamad, M.; et al. Assessment of electric arc furnace (EAF) steel slag waste’s recycling options into value added green products: A review. Metals 2020, 10, 1347. [Google Scholar] [CrossRef]
- Terrones-Saeta, J.M.; Suárez-Macías, J.; Moreno-López, E.R.; Corpas-Iglesias, F.A. Determination of the chemical, physical and mechanical characteristics of electric arc furnace slags and environmental evaluation of the process for their utilization as an aggregate in bituminous mixtures. Materials 2021, 14, 782. [Google Scholar] [CrossRef]
- Kassim, D.; Lamaa, G.; Silva, R.V.; de Brito, J. Performance enhancement of alkali-activated electric arc furnace slag mortars through an accelerated CO2 curing process. Appl. Sci. 2022, 12, 1662. [Google Scholar] [CrossRef]
- Neuhold, S.; van Zomeren, A.; Dijkstra, J.J.; van der Sloot, H.A.; Drissen, P.; Algermissen, D.; Mudersbach, D.; Schüler, S.; Griessacher, T.; Raith, J.G.; et al. Investigation of possible leaching control meachanisms for chromium and vanadium in electric furnace (EAF) slags using combined experimental and modeling approaches. Minerals 2019, 9, 525. [Google Scholar] [CrossRef]
- Kishore, K.; Sheikh, M.N.; Hadi, M.N.S. A critical analysis of electric arc furnace (EAF) slag for sustainable geopolymer concrete production. Mater. Today Sustain. 2025, 29, 101064. [Google Scholar] [CrossRef]
- Samanta, N.S.; Das, P.P.; Dhara, S.; Purkait, M.K. An overview of precious metal recovery from steel industry slag: Recovery strategy and utilization. Ind. Eng. Chem. Res. 2023, 62, 9006–9031. [Google Scholar] [CrossRef]
- Teo, P.T.; Abu Seman, A.; Basu, A.; Sharif, N.M. Chemical, thermal and phase analysis of Malaysia’s electric arc furnace (EAF) slag waste. Mater. Sci. Forum 2016, 840, 399–403. [Google Scholar] [CrossRef]
- Mombelli, D.; Mapelli, C.; Barella, S.; Di Cecca, C.; Le Saout, G.; Garcia-Diaz, E. The effect of microstructure on the leaching behavior of electric arc furnace (EAF) carbon steel slag. Proc. Saf. Environ. Protect. 2016, 102, 810–821. [Google Scholar] [CrossRef]
- Zhao, Q.Z.; Zeng, Y.N.; Li, J.G.; Wang, Y.J. Selective extraction of chromium from EAF stainless steel slag by pressurized oxidation in a NaOH solution. Mater. Trans. 2020, 61, 2030–2039. [Google Scholar] [CrossRef]
- Su, T.; Bian, R.; Chen, Y.; Zhu, S.; Huo, Y.; Liu, J. Improvement of a hydrometallurgical process for recovering Mn from electric-arc furnace slag. Min. Eng. 2020, 159, 103344. [Google Scholar] [CrossRef]
- Kim, J.; Azimi, G. Valorization of electric arc furnace slag via carbothermic reduction followed by acid baking—Water leaching. Res. Conserv. Rec. 2021, 173, 105710. [Google Scholar] [CrossRef]
- Sanda, O.; Tinubu, A.C.; Taiwo, E.A. Recovery of iron from EAF smelter slags via hydrochloric acid leaching and solvent extraction using trioctyl phosphine oxide. Sep. Sci. Technol. 2021, 56, 1026–1034. [Google Scholar] [CrossRef]
- Menad, N.; Kana, N.; Kanari, N.; Pereira, F.; Seron, A. Process for enhancing the valuable metal recovery from „electric arc furnace” (EAF) slags. Waste Biom. Valor. 2021, 12, 5187–5200. [Google Scholar] [CrossRef]
- Akbari, M.; Daneshmand, S.; Vini, M.H.; Azimy, H. Effect of roasting process on the V (anti-tumor agent) recovery from the slag of the electric arc furnace (EAF). Heliyon 2024, 10, e31906. [Google Scholar] [CrossRef]
- Menad, N.E.; Seron, A.; Bensamdi, S. Innovative process for strategic metal recovery from electric arc furnace slag by alkaline leaching. Metals 2024, 14, 1364. [Google Scholar] [CrossRef]
- Kokko, M.; Manninen, M.; Hu, T.; Lassi, U.; Vielma, T.; Pesonen, J. Hydrometallurgical recovery of vanadium and calcium from electric arc furnace slag in hydrogen based steelmaking. Min. Eng. 2024, 217, 108966. [Google Scholar] [CrossRef]
- Kurtulus, R.; Khoei, M.A.; Cantaluppi, M.; Ylieniemi, J. Effects of combined alkalinity and ultrasonication on element release and structural alteration in EAF slag. Min. Eng. 2025, 222, 109154. [Google Scholar] [CrossRef]
- Zulkifili, F.S.; Maarof, H.I.; Nasuha, N.; Puasa, S.W. Leaching of electric arc furnace slag for selective recovery of iron: Effect of temperature, H2SO4/HCl acid, and oxidant concentration. Pertanika J. Sci. Technol. 2022, 30, 2023–2032. [Google Scholar] [CrossRef]
- Tong, Z.; Ma, G.; Zhang, X.; Cai, Y. Microwave-supported leaching of electric arc furnace (EAF) slag by ammonium salts. Minerals 2017, 7, 119. [Google Scholar] [CrossRef]
- Hocheng, H.; Su, C.; Jadhav, U.U. Bioleaching of metals from steel slag by Acidithiobactillus thioxidans culture supernatant. Chemosphere 2014, 117, 652–657. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Azimi, G. Selective precipitation of titanium, magnesium, and aluminum from the steelmaking slag leach liquor. Res. Conserv. Rec. 2022, 180, 106177. [Google Scholar] [CrossRef]
- Yildirim, I.Z.; Prezzi, M. Chemical, mineralogical, and morphological properties of steel slag. Adv. Civ. Eng. 2011, 2011, 463638. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).