Ag/ZrO2 Hybrid Coating for Tribological and Corrosion Protection of Ti45Nb Alloy in Biomedical Environments
Abstract
1. Introduction
2. Materials and Methods
2.1. Material and Hybrid Solution Preparation
2.2. Spin Coating Process
2.3. Coating Characterization
3. Results and Discussion
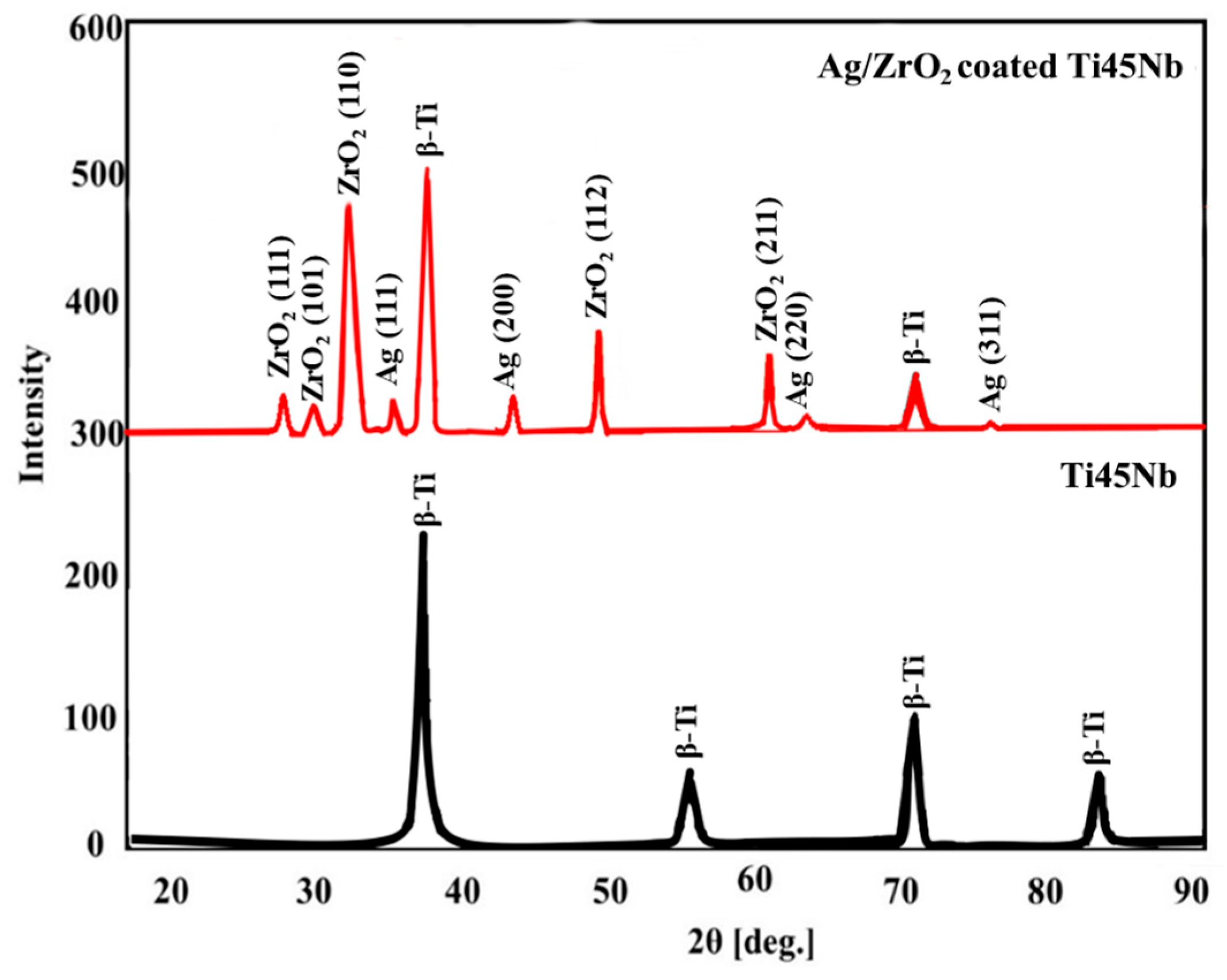
3.1. Surface Analysis
3.2. Electrochemical Corrosion Analyses
3.3. Tribological Properties
4. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hossain, N.; Islam, M.A.; Ahmed, M.M.S.; Chowdhury, M.A.; Mobarak, M.H.; Rahman, M.M.; Hossain, M.H. Advances and significances of titanium in dental implant applications. Results Chem. 2024, 7, 101394. [Google Scholar] [CrossRef]
- Siddiqi, A.; Payne, A.G.T.; De Silva, R.K.; Duncan, W.J. Titanium implants: An overview of clinical applications and complications. J. Biomed. Mater. Res. Part B Appl. Biomater. 2011, 97, 396–408. [Google Scholar]
- Sun, Y.; Liu, Q.; Yu, Z.; Ren, L.; Zhao, X.; Wang, J. Study on Osseointegration Capability of β-Type Ti–Nb–Zr–Ta–Si Alloy for Orthopedic Implants. Materials 2024, 17, 472. [Google Scholar] [CrossRef] [PubMed]
- Frutos, E.; Karlík, M.; Jiménez, J.A.; Langhansová, H.; Lieskovská, J.; Polcar, T. Development of new β/α″-Ti-Nb-Zr biocompatible coating with low Young’s modulus and high toughness for medical applications. Mater. Des. 2018, 142, 44–55. [Google Scholar] [CrossRef]
- Jawed, S.F.; Rabadia, C.D.; Liu, Y.J.; Wang, L.Q.; Li, Y.H.; Zhang, X.H.; Zhang, L.C. Beta-type Ti-Nb-Zr-Cr alloys with large plasticity and significant strain hardening. Mater. Des. 2019, 181, 108064. [Google Scholar] [CrossRef]
- Mohammed, M.T.; Khan, Z.A.; Siddiquee, A.N. Beta titanium alloys: The lowest elastic modulus for biomedical applications—A review. Int. J. Chem. Mol. Nucl. Mater. Metall. Eng. 2014, 8, 726–731. [Google Scholar]
- Yilmazer, H.; Niinomi, M.; Nakai, M.; Cho, K.; Hieda, J.; Todaka, Y.; Miyazaki, T. Mechanical properties of a medical β-type titanium alloy with specific microstructural evolution through high-pressure torsion. Mater. Sci. Eng. C 2013, 33, 2499–2507. [Google Scholar] [CrossRef] [PubMed]
- Bordbar-Khiabani, A.; Gasik, M. Electrochemical and Biological Characterization of Ti–Nb–Zr–Si Alloy for Orthopedic Applications. Sci. Rep. 2023, 13, 2312. [Google Scholar] [CrossRef] [PubMed]
- Szczęsny, G.; Kopec, M.; Kowalewski, Z.L. Toxicity, irritation, and allergy of metal implants: Historical perspective and modern solutions. Coatings 2025, 15, 361. [Google Scholar] [CrossRef]
- Li, Y.; Tian, P.; Cao, H.; Wang, Y.; Zhao, X.; Han, S.; Wang, C. Remarkable enhancement of corrosion resistance and tribological properties of chitosan-MXene based hydrogel coating on the surface of Ti6Al4V alloy. Tribol. Int. 2024, 192, 109229. [Google Scholar] [CrossRef]
- Srinivasan, G.; Manickam, A.; Sivakumar, S.; Murugan, J.; Elangomannan, S.; Mohan, S. A comprehensive review: Surface modification strategies to enhance corrosion resistance of zirconia-based biomaterials in implant applications. J. Mater. Sci. Mater. Eng. 2025, 20, 76. [Google Scholar] [CrossRef]
- Lee, M.; Han, S.I.; Kim, C.; Velumani, S.; Han, A.; Kassiba, A.H.; Castaneda, H. ZrO2/ZnO/TiO2 nanocomposite coatings on stainless steel for improved corrosion resistance, biocompatibility, and antimicrobial activity. ACS Appl. Mater. Interfaces 2022, 14, 13801–13811. [Google Scholar] [CrossRef] [PubMed]
- Ismail, R.; Arfaoui, J.; Ksibi, Z.; Ghorbel, A.; Delahay, G. Effect of the amount of Ag on the performance of Ag/Ce-ZrO2 catalyst for the total oxidation of toluene. J. Chem. Lett. 2024, 5, 186–191. [Google Scholar]
- Sola, T.; Maurel, P.; Weiss, L.; Fleury, E.; Grosdidier, T. A Comprehensive Investigation of the Tribological Behaviour of α, α+β, and β Titanium Alloys against a Steel Counterpart. Wear 2025, 560, 205595. [Google Scholar] [CrossRef]
- Li, P.; Ma, X.; Wang, D.; Zhang, H. Microstructural and Mechanical Properties of β-Type Ti–Nb–Sn Biomedical Alloys with Low Elastic Modulus. Metals 2019, 9, 712. [Google Scholar] [CrossRef]
- Yilmazer, H.; Niinomi, M.; Cho, K.; Nakai, M.; Hieda, J.; Sato, S.; Todaka, Y. Microstructural Evolution of Precipitation-Hardened β-Type Titani.um Alloy through High-Pressure Torsion. Acta Mater. 2014, 80, 172–182. [Google Scholar] [CrossRef]
- Çomaklı, O.; Yazıcı, M.; Demir, M.; Yetim, A.F.; Çelik, A. Effect of Bilayer Numbers on Structural, Mechanical, Tribological and Corrosion Properties of TiO2–SiO2 Multilayer Film-Coated β-Type Ti45Nb Alloys. Ceram. Int. 2023, 49, 3007–3015. [Google Scholar] [CrossRef]
- Peta, K.; Bartkowiak, T.; Rybicki, M.; Galek, P.; Mendak, M.; Wieczorowski, M.; Brown, C.A. Scale-Dependent Wetting Behavior of Bioinspired Lubricants on Electrical Discharge Machined Ti6Al4V Surfaces. Tribol. Int. 2024, 194, 109562. [Google Scholar] [CrossRef]
- Alagarsamy, K.; Vishwakarma, V.; Kaliaraj, G.S.; Vasantha, N.C.; Samuel, S.J.R. Biological adhesion and electrochemical behavior of Ag-ZrO2 bioceramic coatings for biomedical applications. J. Adhes. Sci. Technol. 2020, 34, 349–368. [Google Scholar] [CrossRef]
- Kaliyaperumal, R.; Nagaraj, K.; Poovan, V.K.; Sakthikumar, K.; Govindasamy, C.; Sivakumar, A.S. Hydrothermal implementation with zirconia: Synthesis, characterization and investigation of biocidal activity of Ag/ZrO2 nanocomposites. Z. Phys. Chem. 2024, 238, 209–221. [Google Scholar] [CrossRef]
- Sredojević, D.; Lazić, V.; Pirković, A.; Periša, J.; Murafa, N.; Spremo-Potparević, B.; Živković, L.; Topalović, D.; Zarubica, A.; Krivokuća, M.J.; et al. Toxicity of silver nanoparticles supported by surface-modified zirconium dioxide with dihydroquercetin. Nanomaterials 2022, 12, 3195. [Google Scholar] [CrossRef] [PubMed]
- Aslan Çakır, M. Investigations of the wettability and electrochemical corrosion behavior of Nb2O5 thin films on a Ti45Nb alloy. J. Mater. Eng. Perform. 2023, 32, 9198–9205. [Google Scholar] [CrossRef]
- Venkatesham, M.; Ayodhya, D.; Madhusudhan, A.; Veerabhadram, G. Synthesis of stable silver nanoparticles using gum acacia as reducing and stabilizing agent and study of its microbial properties: A novel green approach. Int. J. Green Nanotechnol. 2012, 4, 199–206. [Google Scholar] [CrossRef]
- ASTM G133-05; Standard Test Method for Linearly Reciprocating Ball-on-Flat Sliding Wear. ASTM International: West Conshohocken, PA, USA, 2005.
- Murali, R.; Bonar, S.F.; Kirsh, G.; Walter, W.K.; Walter, W.L. Osteolysis in Third-Generation Alumina Ceramic-on-Ceramic Hip Bearings with Severe Impingement and Titanium Metallosis. J. Arthroplast. 2008, 23, 1240.e13–1240.e19. [Google Scholar] [CrossRef] [PubMed]
- Tonna, C.; Wang, C.; Mei, D.; Lamaka, S.V.; Zheludkevich, M.L.; Buhagiar, J. Biodegradation behaviour of Fe-based alloys in Hanks’ balanced salt solutions: Part I. material characterisation and corrosion testing. Bioact. Mater. 2022, 7, 426–440. [Google Scholar] [CrossRef] [PubMed]
- Basahel, S.N.; Ali, T.T.; Mokhtar, M.; Narasimharao, K. Influence of crystal structure of nanosized ZrO2 on photocatalytic degradation of methyl orange. Nanoscale Res. Lett. 2015, 10, 73. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Mei, D.; Li, Y.; Chen, L.; Wang, H.; Huang, W.; Wang, L.; Zhu, S.; Guan, S. Protective nature of cerium-based oxides coating against Mg corrosion in Hanks’ balanced salt solution. Corros. Sci. 2023, 219, 111255. [Google Scholar] [CrossRef]
- Hussain, C.M.; Verma, C.; Aslam, J.; Aslam, R.; Zehra, S. Corrosion protective coatings. In Corrosion Protection at the Nanoscale; Elsevier BV: Amsterdam, The Netherlands, 2023; pp. 283–321. [Google Scholar] [CrossRef]
- Liu, X.; Chu, P.K.; Ding, C. Surface modification of titanium, titanium alloys, and related materials for biomedical applications. Mater. Sci. Eng. R Rep. 2004, 47, 49–121. [Google Scholar] [CrossRef]
- Mischler, S. Triboelectrochemical techniques and interpretation methods in tribocorrosion: A comparative evaluation. Tribol. Int. 2008, 41, 573–583. [Google Scholar] [CrossRef]
- Bai, H.; Zhong, L.; Kang, L.; Liu, J.; Zhuang, W.; Lv, Z.; Xu, Y. A review on wear-resistant coating with high hardness and high toughness on the surface of titanium alloy. J. Alloys Compd. 2021, 882, 160645. [Google Scholar] [CrossRef]
- Landolt, D.; Mischler, S. Tribocorrosion of Passive Metals and Coatings; Elsevier: Amsterdam, The Netherlands, 2011. [Google Scholar]





| Component | Concentration (mM) |
|---|---|
| NaCl | 137.93 |
| KCl | 5.36 |
| CaCl2·2H2O | 1.26 |
| MgSO4·7H2O | 0.81 |
| Na2HPO4 | 0.34 |
| KH2PO4 | 0.44 |
| NaHCO3 | 4.17 |
| Glucose | 5.55 |
| Samples | Coating Thickness (µm) | Hardness Value (HV0.1) | Roughness Value (Ra-μm) |
|---|---|---|---|
| Uncoated Ti45Nb | - | 200 ± 0.2 | 0.09 ± 0.01 |
| Ag/ZrO2 coated Ti45Nb | 1.8 ± 0.09 | 390 ± 0.4 | 0.18 ± 0.02 |
| Material | Ecorr (V) | Icorr (mA/cm2) | βa (mV/dec) | βa (mV/dec) | Corrosion Rate (mpy) |
|---|---|---|---|---|---|
| Uncoated Ti45Nb | −0.35 | 1.53 × 10−5 | 145 | 240 | 5.56 |
| Ag/ZrO2 hybrid coated Ti45Nb | −0.16 | 4.94 × 10−6 | 128 | 360 | 1.48 |
| Zr | O | Ag | Ca | P | Ti | Nb | |
|---|---|---|---|---|---|---|---|
| Uncoated Ti45Nb (at.%) | - | 49.56 | - | 2.25 | 1.65 | 39.51 | 7.03 |
| Ag/ZrO2 coated Ti45Nb (at.%) | 31.75 | 53.40 | 10.45 | 2.54 | 1.96 | - | - |
| Parameters | Average Coefficient of Friction (μ) | |||
|---|---|---|---|---|
| DRY | HBSS | DRY | HBSS | |
| Uncoated Ti45Nb | 0.32 ± 0.04 | 0.22 ± 0.04 | 1.12 ± 0.04 | 0.85 ± 0.04 |
| Ag/ZrO2 coated Ti45Nb | 0.34 ± 0.05 | 0.27 ± 0.03 | 0.91 ± 0.03 | 0.78 ± 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aslan Çakir, M. Ag/ZrO2 Hybrid Coating for Tribological and Corrosion Protection of Ti45Nb Alloy in Biomedical Environments. Metals 2025, 15, 831. https://doi.org/10.3390/met15080831
Aslan Çakir M. Ag/ZrO2 Hybrid Coating for Tribological and Corrosion Protection of Ti45Nb Alloy in Biomedical Environments. Metals. 2025; 15(8):831. https://doi.org/10.3390/met15080831
Chicago/Turabian StyleAslan Çakir, Mevra. 2025. "Ag/ZrO2 Hybrid Coating for Tribological and Corrosion Protection of Ti45Nb Alloy in Biomedical Environments" Metals 15, no. 8: 831. https://doi.org/10.3390/met15080831
APA StyleAslan Çakir, M. (2025). Ag/ZrO2 Hybrid Coating for Tribological and Corrosion Protection of Ti45Nb Alloy in Biomedical Environments. Metals, 15(8), 831. https://doi.org/10.3390/met15080831






