1. Introduction
Nowadays, some medical treatments require implants that meet specific characteristics, such as high biocompatibility, fatigue resistance, a bone-like modulus of elasticity, functionality, high corrosion resistance, and zero systemic histopathological response [
1]. For decades, metal alloys such as stainless steel and titanium have been used due to their mechanical properties and corrosion resistance [
2,
3]. However, titanium and its alloys have become substitutes for stainless steel due to their ability to adjust their microstructure and adapt to desired mechanical properties. In particular, the Ti6Al4V alloy offers excellent biocompatibility and immediate availability [
4,
5,
6].
Research in the field of PVD hard metal coatings has generated materials that are used as implants. Despite the advantages of titanium, its elastic modulus is much higher than that of human cortical bone, which can lead to premature implant failure and the release of toxic elements such as Al and V into the human body [
7,
8,
9]. To mitigate this, studies have focused on reducing the Young’s modulus of titanium alloys by minimizing the effects of mechanical stress. However, this reduction may compromise fatigue resistance. Furthermore, the lifespan of implants made of stainless steel and cobalt–titanium alloys is approximately 20 to 25 years, with a high failure rate after 15 years of service [
9,
10,
11].
Alternative materials have been explored, such as rhenium, a refractory metal with a high melting temperature and excellent corrosion resistance [
12,
13,
14,
15,
16].
Recent research has investigated the biocompatibility of rhenium, showing good osteoconductivity and osteogenesis properties compared to other materials used in orthopedic implants reported on recent advances in the synthesis and application of rhenium-based nanomaterials, highlighting their potential in electrochemical sensors, catalysis, surface-enhanced Raman spectroscopy (SERS), and biomedical applications [
12,
17]. Despite the growing interest in these materials, the literature remains limited regarding synthesis procedures and the characterization of their physical and chemical properties. In this context, various studies have explored rhenium-based polypyridine complexes due to their luminescent properties and applicability in biomedical imaging. Moherane et al. [
18] reported the use of polypyridyl-coordinated Re(I) tricarbonyl complexes as model probes for cancer diagnosis and treatment, while Sharma et al. [
19] found that mononuclear and binuclear Re(I) tricarbonyl complexes exhibit promising characteristics as targeted anticancer agents. These studies highlight that the combination of a prolonged luminescent lifespan, a large Stokes shift, a high quantum yield, and strong absorption in the infrared transparent window makes these materials attractive for bioanalytical and therapeutic applications. However, to date, no studies on thin films of rhenium for biomedical applications have been reported, underscoring the novelty of our work and the need for further research to establish its potential in this field.
In addition to its biocompatibility, rhenium exhibits high valence electron density and omnidirectional metallic bonding, making it highly incompressible and resistant to plastic deformation. The addition of interstitial atoms can enhance the hardness of rhenium, making it an attractive material for application as an orthopedic implant [
16].
Despite the abovementioned advantages, some studies have pointed out the limitations of using pure rhenium in hard coatings. However, research on rhenium compounds, such as rhenium carbide, has shown super hardness and superconductivity properties, suggesting a vast potential for applications in biomaterials [
13,
17].
Although rhenium is an expensive and relatively scarce material, more efficient production and recovery methods have been developed. These features will enable future viability for biomedical applications [
19]. In summary, rhenium emerges as a promising material for use in biomaterials due to its good biocompatibility, high mechanical strength, and unique properties [
20]. Additionally, its potential as a coating material has been explored, despite challenges related to cost and availability.
This study synthesized ReN (rhenium nitride) films on silicon and Ti6Al4V substrates by reactive magnetron sputtering at different nitrogen pressures (120, 140, 160, and 180 mTorr). The electrochemical, mechanical, and biocompatibility properties were characterized. The novelty of this study lies in the comprehensive characterization of ReN coatings synthesized by reactive magnetron sputtering, focusing on their electrochemical, mechanical, and biocompatibility properties. This research demonstrates that optimizing nitrogen pressure at 180 mTorr leads to the complete formation of cubic ReN, which significantly enhances corrosion resistance and provides an optimal combination of hardness and elastic modulus.
2. Materials and Methods
The ReN films were synthesized using reactive magnetron sputtering. The coatings were deposited on silicon (100) and Ti6Al4V alloy substrates, purchased from Rickard Specialty Metals, in an AJA-ATC 1800 (AJA Int., Hingham, MA, USA) system with a base pressure of 10−8 Torr. For the deposition of the thin films, individual 2-inch diameter targets with 99.999% purity for the rhenium target were used. The samples were cut from a standard titanium alloy bar with a circular section of 25 mm diameter and 6 mm thickness.
Prior to deposition, the substrates were ground with silicon carbide (SiC) paper with grit sizes ranging from 400 to 2000 and subsequently polished to a mirror finish using alumina paste with particle sizes between 1 and 0.5 μm. The samples were then ultrasonically cleaned in isopropyl alcohol for approximately 10 min.
The deposition process was carried out at a substrate temperature of 350 °C, with a distance of 100 mm between the target and the substrate. A negative bias voltage of −50 V was applied to the substrate to improve adhesion and control the film microstructure. The reactive sputtering atmosphere was regulated by varying the nitrogen flow between 22 and 35 mL/min, corresponding to a pressure range of 120 to 180 mTorr, using a mass flow controller. The coatings were deposited at a constant power of 150 W for 70 min.
Ti6Al4V X-ray diffraction analyses were performed in a Brentano-Bragg configuration using Empyrean® XRD. (Panalytical®, Almelo, The Netherlands) equipment. An X-ray source with characteristic lines Kα1 (λ = 1.540598 Å) and Kα2 (λ = 1.544426 Å) was used. An incident beam radius of 240 mm, a potential difference of 45 kV and an intensity of 40 mA, a scanning range of 20° to 90°, a step size set at 0.0400°, and a counting time of 2 s per point were used. In addition, a nickel filter was incorporated into the incident beam, and divergence, anti-dispersion, and soler gratings were used in both beams, with specific angles to optimize signal quality. The results were interpreted using HighScore version 5.3, the Panalytical® ICSD databases, and the accessible version of the COD (Malvern Panalytical, Malvern, UK).
For the mechanical analysis of the ReN coatings, an AIF’s Hysitron Ubi-1® nanoindenter (Östliche Rheinbrückenstr, Karlsruhe, Germany) configured with a Berkovich-type diamond tip, and a maximum applied load of 10 mN was used to evaluate the hardness and elastic modulus.
Applying the potentiodynamic polarization curves technique allowed for the evaluation of the electrochemical activity, corrosion potentials, and corrosion resistance of the ReN films and the Ti6Al4V substrate. In the electrochemical characterization, a Gamry® brand potentiostat model interface 1010 (Gamry Instruments, Warminster, PA, USA) was used, together with an electrochemical cell composed of a working electrode for the sample to be analyzed, a platinum counter electrode, and a Ag/AgCl/KCl (saturated) reference electrode. All tests were performed in Hanks’ lactate solution at 37 °C. The electrolytic composition per 1000 mL was as follows: sodium (131 mmol/L), potassium (5.4 mmol/L), calcium (1.8 mmol/L), chlorides (112 mmol/L), and lactate (28 mmol/L). The initial stabilization period was 180 min to establish the open circuit potential (OPC) or the stable potential state. During the test, a sweep speed of 125 μV/s was applied in a potential range of −0.35 V to 0.40 V concerning the OPC. The electrochemical response was recorded while measurements were obtained in a wide range of potentials, which allowed the polarization curves to be generated, showing the variation of the reaction current as a function of the applied potentials.
To characterize the adhesion and proliferation of HeLa cells on the uncoated and ReN-coated substrates, the sterilization process was initiated using ethanol and ultraviolet (UV) light to eliminate possible contaminants. The sterilized samples were placed in cell culture plates and prepared for cell seeding. HeLa cells, suspended in DMEM (Dulbecco Modified Eagles Minimal Essential Medium) culture medium, were seeded on the surface of the coated and uncoated substrates at a density of 10,000 cells per well. The cell-seeded plates were incubated in a controlled environment at 37 °C with 5% CO2 for 24 h, allowing cell attachment and growth. To assess the viability of HeLa cells on the substrate surfaces, a live/dead staining assay was performed using a staining solution containing calcein-AM and propidium iodide, which was added to the samples. After incubating for 30 min at 37 °C, the cells’ morphologies and the samples’ surfaces were observed using the scanning electron microscope (SEM) JEOL Neoscope® JCM 5000 system (Tokyo, Japan) in low vacuum mode. Before observation, the samples were cleaned and prepared by rinsing with distilled water followed by drying. This analysis allowed the exposed samples to be characterized, analyzing the protein–coating interaction and the surface cell morphology.
3. Results
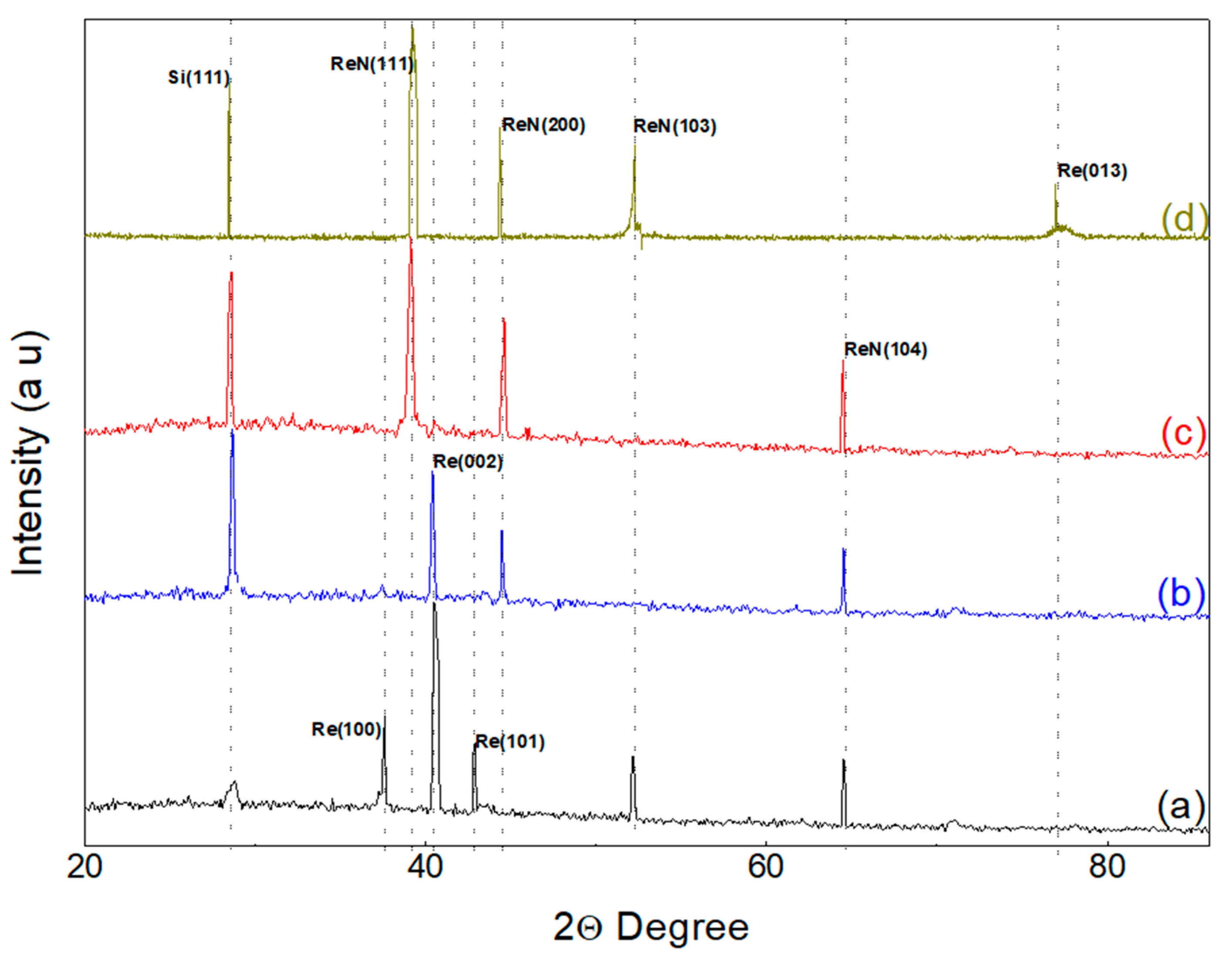
Figure 1 presents the XRD diffractograms obtained by parallel beam geometry and low incidence angle for the analyzed samples. A contribution from the substrate is observed in all diffraction patterns, evidenced by the presence of peaks around 28.40° corresponding to the (111) plane of silicon. The sample deposited at 120 mTorr (
Figure 1a) exhibits three prominent diffraction peaks at 37.51°, 40.4°, and 42.8°, corresponding to the (100), (002), and (101) diffraction planes of the hexagonal close-packed rhenium structure, as per Powder Diffraction File (PDF) #89-2935. Additionally, a low intensity peak at 77.08° is observed, corresponding to the (013) crystallographic plane in the sample deposited at 180 mTorr (
Figure 1d) [
21,
22]. These results suggest a preferential (002) orientation in the pure Re film. Furthermore, peaks at 52.16° and 64.53° are observed, compatible with the cubic phase of ReN where nitrogen occupies interstitial positions [
23]. The obtained results are consistent with the characteristics of cubic rhenium nitride, with nitrogen atoms located in the octahedral interstices corresponding to the
space group. The X-ray diffraction pattern indicates the presence of Re in significant amounts in the treated samples. The presence of hexagonal Re could be attributed to the partial decomposition of ReN
3 due to the nitrogen content or an intermediate product in the ion exchange reaction [
24].
Initially, the central peak is presented at 40.43°. However, as the pressure increases, new peaks emerge at 44.44° and 64.59°, corresponding to the (200) and (104) planes of ReN in its cubic phase. These shifts in the peak positions indicate a modification in the crystalline structure, evidencing the formation of cubic ReN as the nitrogen content increases. The diffraction pattern is consistent with the cubic structure of rhenium nitride, where the nitrogen atoms occupy octahedral interstitial positions in the
space group [
25].
At a pressure of 160 mTorr (
Figure 1c), the X-ray diffraction (XRD) pattern reveals an increased formation of rhenium nitrides. Three diffraction peaks are observed at 39.21°, 44.51°, and 64.49°, corresponding to the (111), (200), and (104) diffraction planes of the hexagonal ReN structure. This increase in nitride formation suggests that the introduction of nitrogen facilitates the incorporation of nitrogen atoms into the rhenium crystal lattice. The deviations in the positions of the experimental peaks could be attributed to residual stresses in the ceramic film, which is adhered to a metallic substrate with lower hardness and a higher coefficient of thermal expansion. In addition, a low-intensity peak associated with metallic rhenium was detected and assigned to the (002) plane [
26].
The complete formation of rhenium nitride is observed by increasing the pressure to 180 mTorr (
Figure 1d). This change suggests that, at this pressure, the available nitrogen is sufficient to saturate the rhenium crystal structure, resulting in the predominant formation of the cubic phase of rhenium nitride (ReN). Diffraction peaks at 39.21°, 44.30°, and 52.35° are observed, corresponding to the (111), (200), and (103) diffraction planes of the cubic ReN structure. The complete formation of rhenium nitride at this pressure is explained by the saturation and stabilization of the face-centered cubic (FCC) structure, minimizing the presence of metallic rhenium. This phenomenon may also be associated with optimizing deposition conditions that favor the maximum incorporation of nitrogen into the rhenium crystal lattice. The significant presence of rhenium nitrides at this pressure indicates the stability of the FCC phase, where nitrogen atoms are located in interstitial positions of the rhenium matrix. The transition from 160 mTorr to 180 mTorr shows the formation of rhenium nitrides and the definition of the cubic phase of ReN. These characteristics indicate that controlling the nitrogen pressure during the deposition process is essential to determine the phase and purity of the resulting material. From this study, it is possible to conclude that for nitrogen pressures of 180 mTorr, the stable formation of rhenium nitride is obtained [
27].
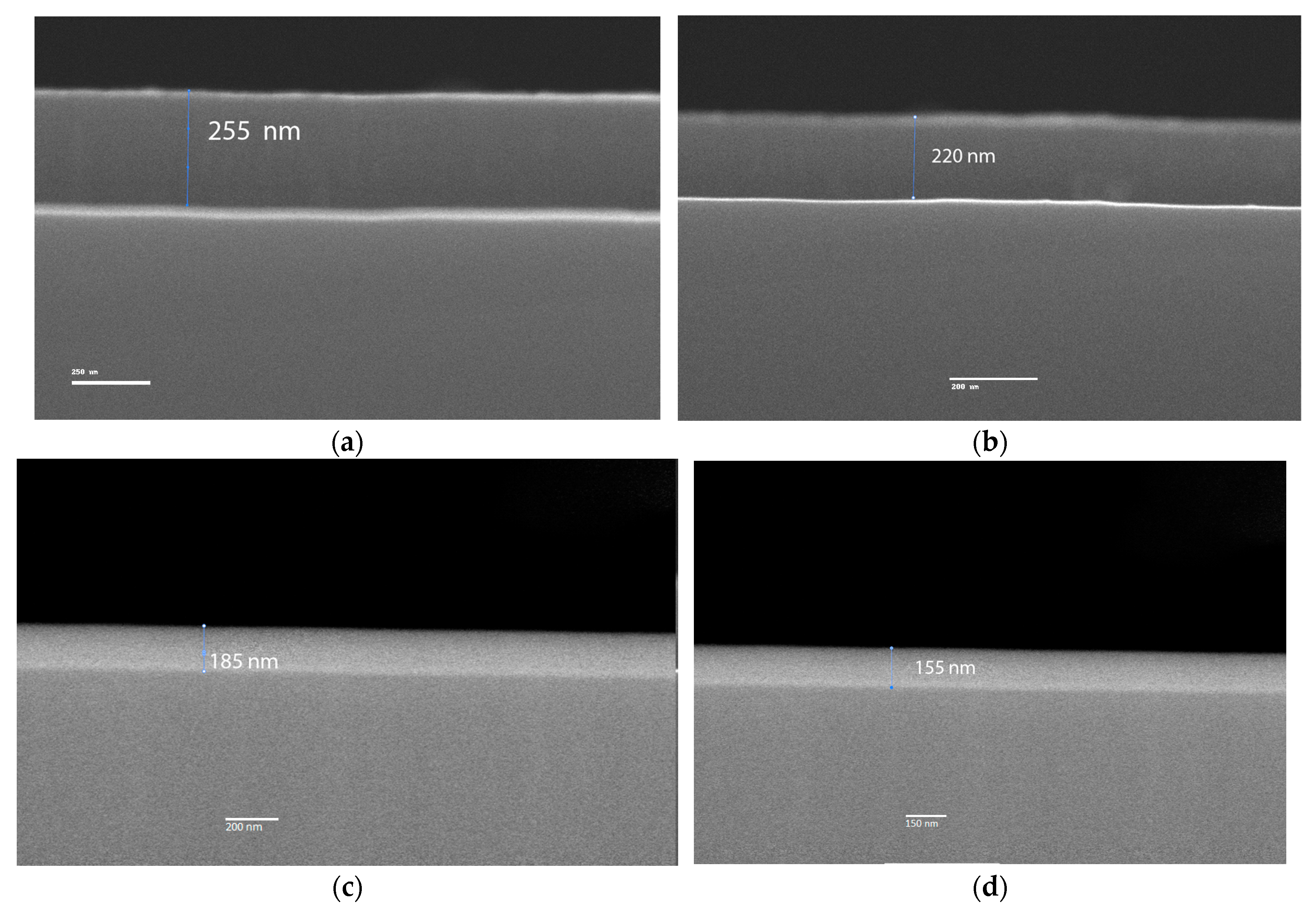
In the
Figure 2 SEM images, the coating thickness are evaluated. The cross-sectional images reveal a well-defined coating-substrate interface, with a continuous layer along the cross-section, free of cracks or severe deformations. The nitrogen flow variation during the sputtering process primarily influences the deposition rate, which correlates with the measured thicknesses in the samples. Specifically, the sample deposited at 180 mTorr exhibits a thickness of 255 ± 4 nm, making it thicker than those deposited at 160 mTorr, 140 mTorr, and 120 mTorr, which have thicknesses of approximately 220 ± 3 nm, 185 ± 5 nm, and 155 ± 4 nm, respectively [
28]. This trend is attributed to the fact that as the nitrogen flow increases, the deposition rate decreases due to the reduction in the argon percentage in the gas mixture, which is the primary gas responsible for sputtering the rhenium target.
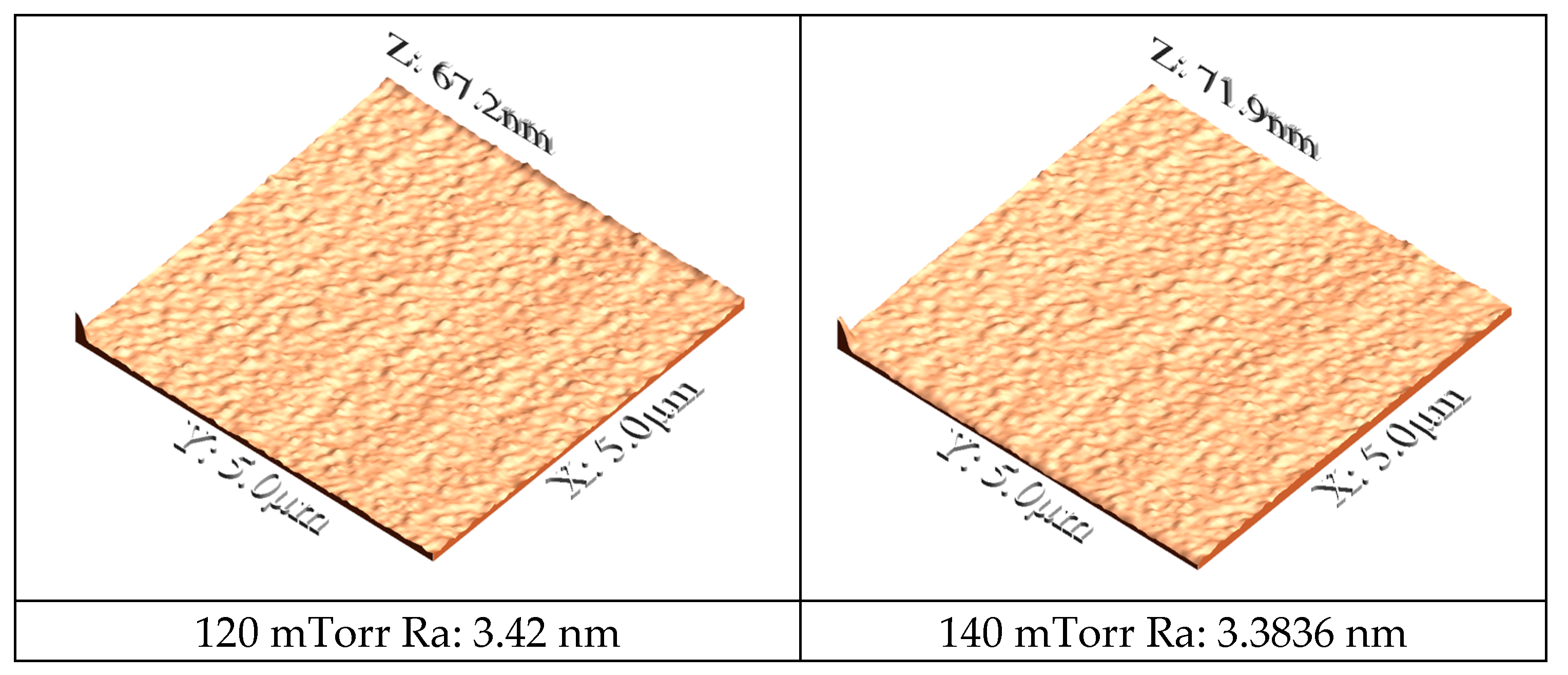
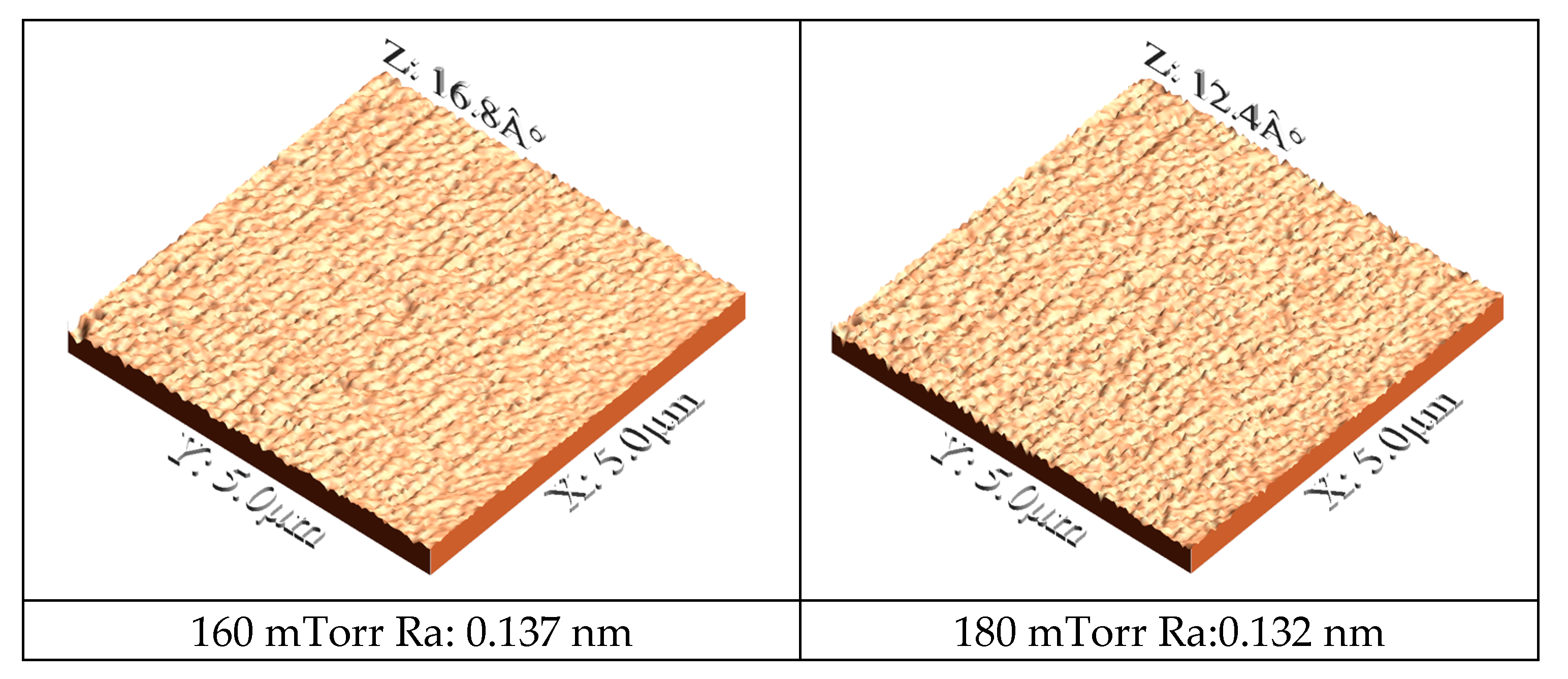
Figure 3 presents AFM images utilized to quantitatively evaluate the surface morphology of the coatings deposited at various nitrogen pressures. A clear correlation is observed between the deposition conditions, the film morphology, and its crystallographic structure. Furthermore, the AFM data (
Figure 3) reveal that the surface roughness decreases markedly with increasing nitrogen pressure, with Ra values of 0.132 nm and 0.137 nm recorded for the films deposited at 180 mTorr and 160 mTorr, respectively, in contrast, to significantly higher values of 3.42 nm and 3.3836 nm for those deposited at 120 mTorr and 140 mTorr. These morphological trends are consistent with the XRD diffractograms (
Figure 1), which indicate that at low nitrogen pressures (120 mTorr) the diffraction patterns are dominated by peaks corresponding to the hexagonal close-packed structure of rhenium, whereas at 180 mTorr the complete formation of the cubic phase of rhenium nitride is observed [
29]. The transition to the cubic phase at higher pressures is likely to promote a denser and more homogeneous film growth, accounting for both the increased film thickness and the reduced surface roughness [
30,
31].
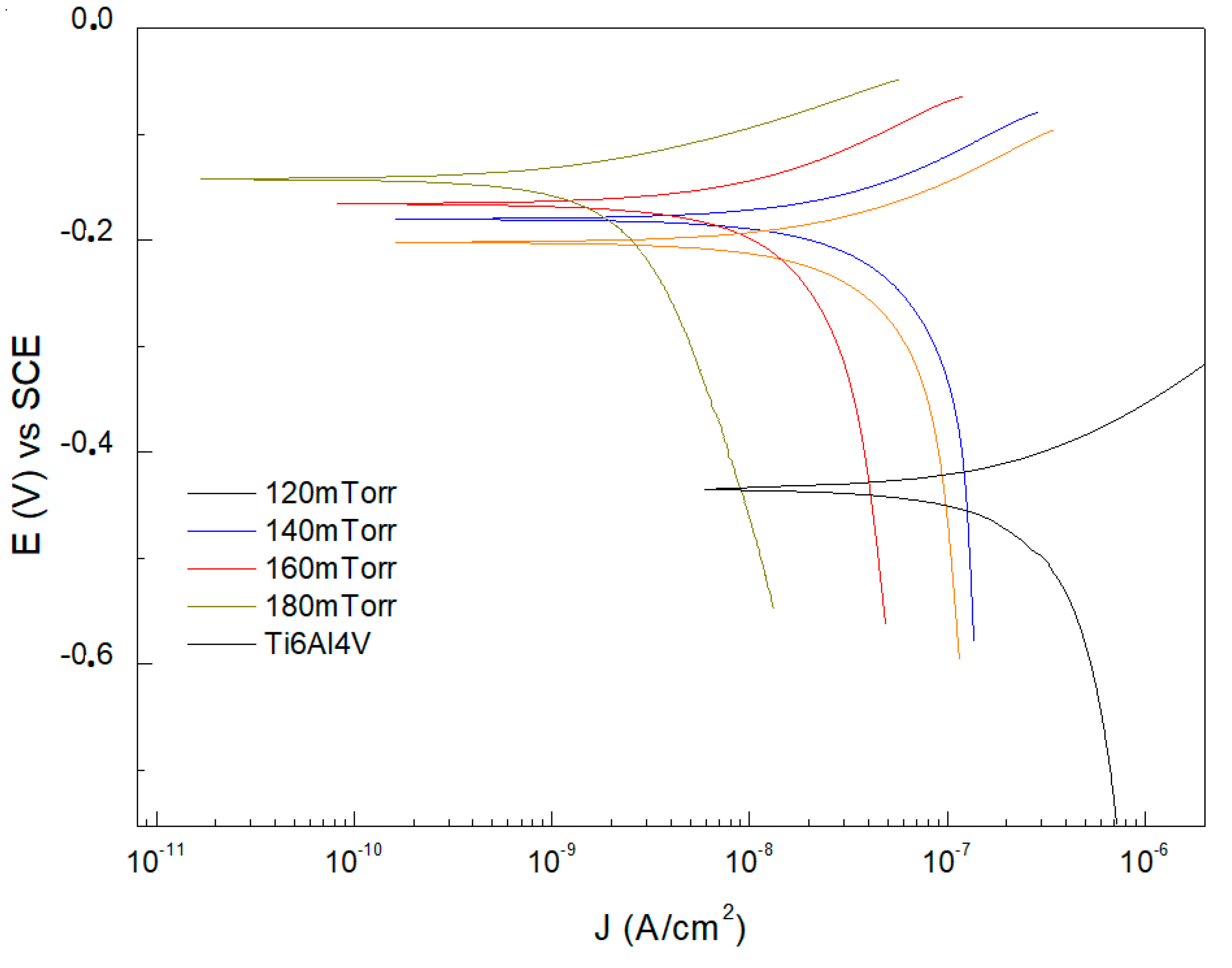
Figure 4 shows the results of the corrosion tests performed on Ti6Al4V substrates coated with ReN thin films at different flux contents. The results indicate an evident variation of the electrochemical behavior as a function of the nitrogen concentration in the thin films. Regarding the corrosion potential (Ecorr) values, a tendency towards more positive values with increasing nitrogen flux was evident. The Ti6Al4V substrate presented a corrosion potential of −0.4348 mV vs. SCE, while the samples coated with 120 mTorr, 140 mTorr, 160 mTorr, and 180 mTorr of nitrogen presented corrosion potentials of −0.2023 mV vs. SCE, −0.1805 mV vs. SCE, −0.1655 mV vs. SCE, and −0.1428 mV vs. SCE, respectively. This progression towards more positive Ecorr indicates excellent corrosion resistance at higher nitrogen flows [
32].
The uncoated Ti6Al4V substrates exhibited corrosion current density (Icorr) values of 2.3771 × 10
−7 A/cm
2. These Icorr values allow the efficiency of the coatings to be assessed. At a pressure of 120 mTorr, the Icorr was reduced to 5.0187 × 10
−8 A/cm
2. At a pressure of 140 mTorr, a further reduction to 3.9418 × 10
−8 A/cm
2 was observed. At 160 mTorr, the Icorr decreased further to 1.3662 × 10
−8 A/cm
2, and at 180 mTorr, the corrosion current density was 2.5128 × 10
−9 A/cm
2. The results revealed a significant decrease in Icorr with increasing N
2 pressure. A 78.88% reduction in Icorr was observed for the coating deposited at 120 mTorr compared to the uncoated substrate. This percentage reduction corresponds to 83.42%, 94.25%, and 98.94% for the coatings deposited at 140 mTorr, 160 mTorr, and 180 mTorr, respectively. Electrochemical characterization results demonstrate the effectiveness of ReN coatings in improving the corrosion resistance of Ti6Al4V. The positive influence of N
2 pressure is attributed to forming a dense layer, which reduces the permeability of oxygen and other corrosive agents. Furthermore, higher N
2 pressure favors the incorporation of nitrogen into the ReN structure [
33,
34].
The corrosion rate, measured in micrometers per year (µm/year), was directly correlated with the corrosion current density. For the Ti6Al4V substrate, the corrosion rate was 1.364 µm/year. This value is consistent with that reported in the literature for this biocompatible alloy when exposed to simulated corrosive environments [
35,
36]. This feature may indicate a significant susceptibility to degradation in aggressive environments, which underlines the need to apply protective coatings to extend its useful life in biocompatible applications. The corrosion rates for the samples coated with 120 mTorr, 140 mTorr, 160 mTorr, and 180 mTorr of nitrogen were 0.290 µm/year, 0.226 µm/year, 0.079 µm/year, and 0.0145 µm/year, respectively. With increasing nitrogen pressure, the coatings improve their adhesion to the substrate. The main reason for selecting the nitrogen flow range is that at amounts lower than 120 mTorr, the coatings present low adhesion due to the high level of residual stress between the coating and the substrate [
37,
38]. The remarkable reduction in the corrosion rate in the coatings concerning the increase in nitrogen content suggests a superior protective performance, which is essential for applications in highly corrosive environments, such as the human body. The results obtained in the development of this research are consistent with the information recorded by previous studies, which indicate that rhenium nitride and other nitride-based coatings can offer excellent corrosion resistance due to chemical stability and the ability to form practical protective barriers [
39]. It is possible to conclude that applying ReN coatings to devices used in biocompatibility can significantly extend their useful life.
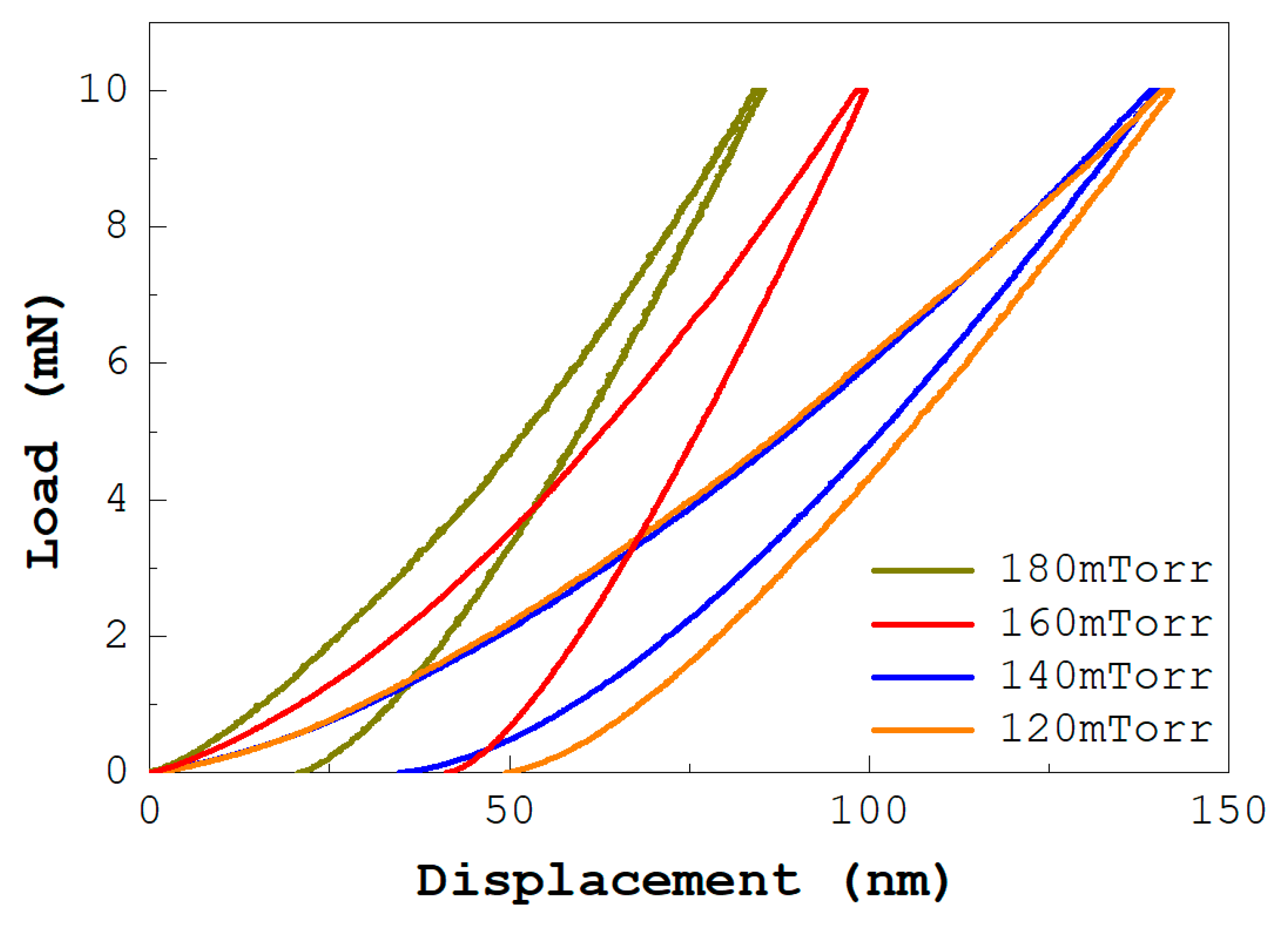
Typical load versus displacement curves of the ReN coatings are shown in
Figure 5. The films were evaluated for their mechanical properties by nanoindentation [
40]. The hardness measurement is representative of the entire layer because it uses a 16-point indentation array. It is important to note that the hardness value obtained reaches approximately 34 GPa at the maximum nitrogen flow rate (180 mTorr), 28 GPa for 160 mTorr, 17 GPa for 140 mTorr, and 10 GPa for 120 mTorr. The hardness values of 34 GPa and 10 GPa, obtained for the highest and lowest nitrogen flow rates, respectively, are consistent with the information reported by other authors [
20].
The variation of the nano hardness of the multilayers as a function of the nitrogen content shows a proportional trend as the nitrogen content flow decreases. However, when this parameter falls below 120 mTorr, a tendency to increase the hardness values is evident, as shown in
Figure 6. The research results indicate that the hardness values of the coatings produced tend to be high, which allows them to be classified as hard and resistant coatings [
38].
Figure 6 also illustrates the relationship between the elastic modulus and deposition conditions for ReN coatings. After removing the extreme values from the data set, the values plotted in
Figure 6 correspond to the statistical averages of the experimental data obtained for each sample. This analysis methodology allows for obtaining a more accurate and reliable representation of the mechanical behavior of the material, eliminating the influence of outliers that could distort the results. The error bars correspond to the respective standard deviations. The elastic modulus values have been incorporated into the mechanical characterization of the ReN films. This parameter determines the most favorable combination of hardness and reduced elastic modulus to obtain hard coatings with optimal protective properties.
The highest hardness and elastic modulus values were determined for ReN films deposited with a nitrogen flow of 180 mTorr with 268 GPa. Values of 110 GPa were observed in the ReN coatings synthesized at 120 mTorr. According to these results, it is possible to conclude from the investigation that the hardness of the ReN coatings varies proportionally with the nitrogen flow. This behavior in hardness can be attributed to the contribution of the metal–nitrogen (M-N) bond. ReN films deposited at 180 mTorr can be considered in potential high-hardness coating applications. It is also important to highlight that the hardness values obtained in the development of this study coincide with the findings published by M. Grisales et al. (2022) [
39].
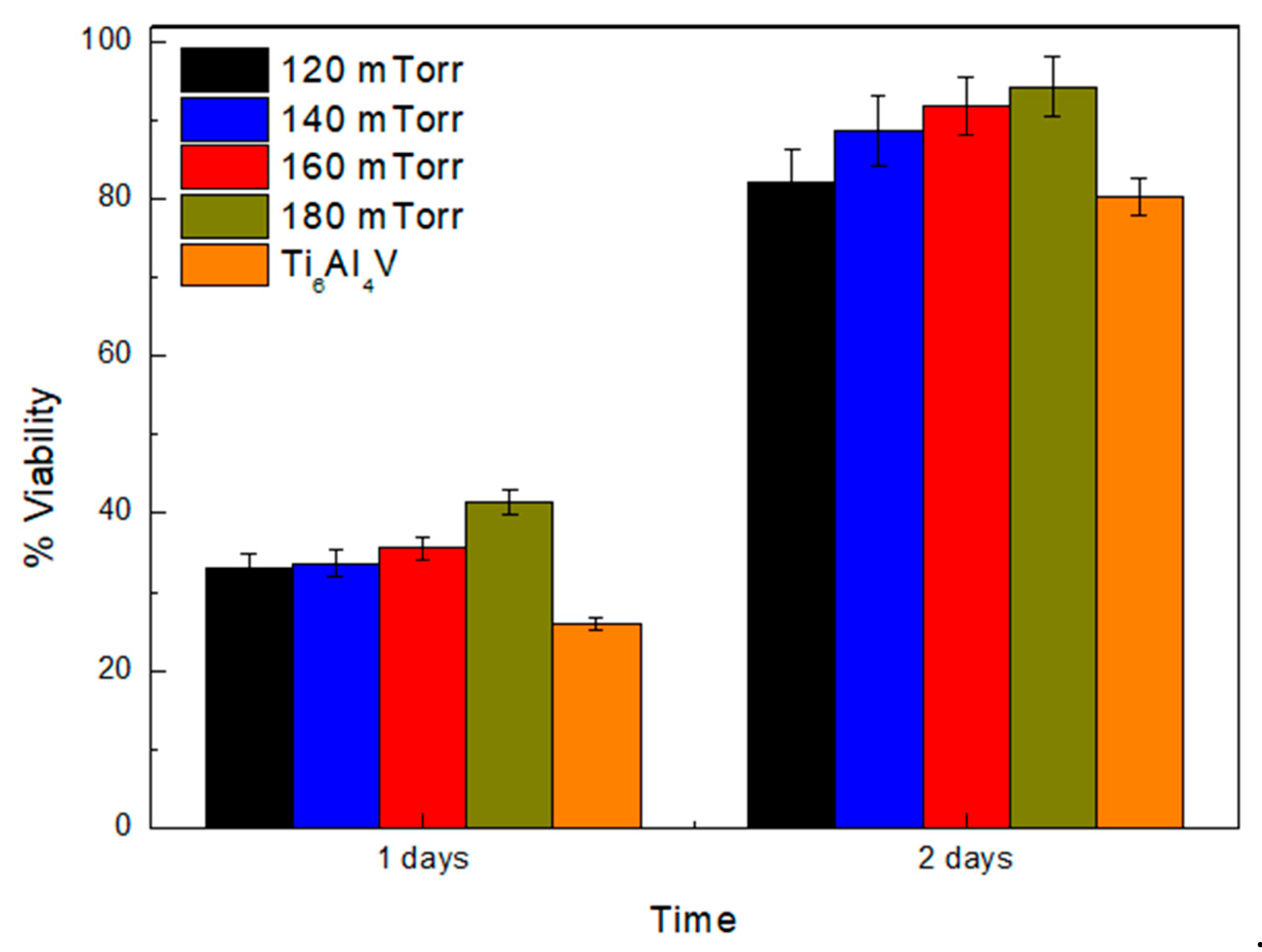
Figure 7 shows the results obtained for cell viability after incubation for 24 h and 48 h on uncoated Ti6Al4V substrates and coatings synthesized with different nitrogen flows. It is evident that the percentage of cell viability improves in the ReN coatings compared to that observed for the uncoated substrates. The graph in
Figure 7 shows the cell viability of the ReN coatings obtained at a nitrogen flow of 180 mTorr. Statistical analysis using a one-way ANOVA indicated significant differences among the groups (
p < 0.05). Tukey’s post hoc test confirmed that the cell viability in the ReN coatings obtained at 180 mTorr was significantly higher than that of the uncoated substrate (
p < 0.01). However, no statistically significant differences were observed between the coatings deposited at 120 mTorr and 140 mTorr (
p > 0.05), suggesting similar behavior in terms of cell adhesion and proliferation. These results highlight the potential application of ReN coatings in biocompatibility applications [
40]. Additionally, the results in
Figure 7 indicate that a longer incubation time significantly improves cell viability percentages. From the Icorr results obtained for the uncoated Ti6Al4V substrate, a high corrosion rate was evident, reflecting a greater susceptibility to degradation. Consequently, these substrates do not provide a suitable medium for cell growth, as observed in
Figure 4. However, the corrosion rate decreases significantly when ReN coatings are applied at different nitrogen pressures.
This reduction in the corrosion rate suggests that the material’s surface becomes more passive and less prone to releasing metal ions into the medium, minimizing the inflammatory and toxic response in the surrounding tissues while enhancing cell viability. Additionally, coatings with a higher nitrogen content form more effective and chemically stable barriers, limiting the interaction between the material and the biological environment, which translates into better cell acceptance. Greater nitrogen flow during the coating synthesis improves film adhesion to the substrate, preventing detachment of the base material that could hinder cell growth. In conclusion, the decrease in corrosion rate with increasing nitrogen content in coatings on Ti6Al4V is positively correlated with cell viability. This correlation suggests that the chemical and physical stability of the coated surface contributes to improved biocompatibility, making ReN coatings promising candidates for medical applications [
41].
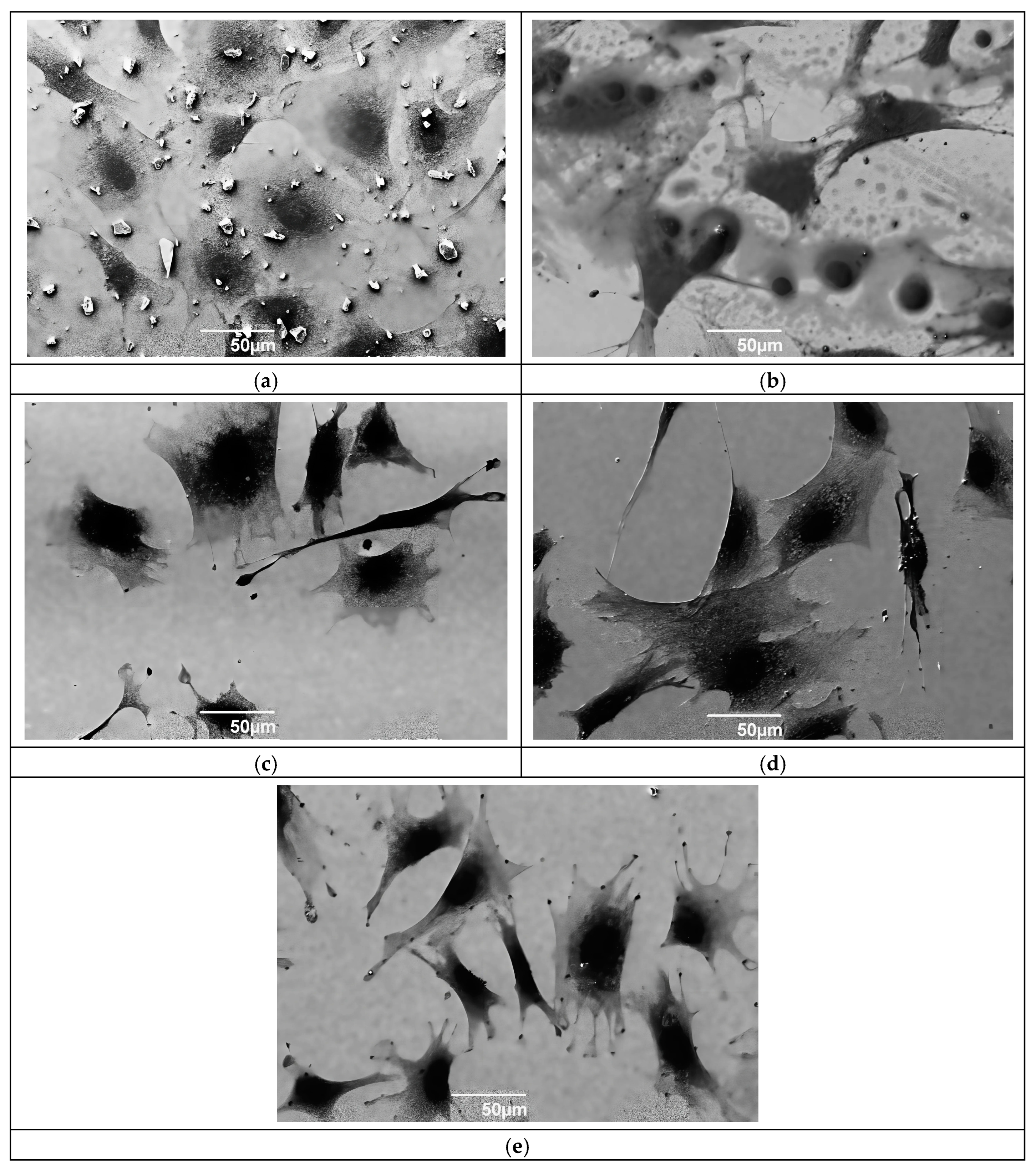
Figure 8 presents SEM micrographs showing cellular activity related to the protein–surface interaction on Ti6Al4V substrates and ReN coatings synthesized at different nitrogen flows. A higher amount of particulate material is observed in the case of ReN coatings with different nitrogen concentrations. The increase in nitrogen percentage helps stabilize the ReN coating, reducing the interaction with proteins and therefore cell toxicity. Ti6Al4V substrates favor cell adhesion, as evidenced by the presence of cells (
Figure 8a), although incubation in a cell medium degrades the surface of the alloy. The surface irregularities in
Figure 8b, related to ReN coatings at 120 mTorr, establish a favorable environment for cell adhesion and growth, characterized by a higher number of cells than those found for the uncoated substrate. There is a favorable effect on cell adhesion and growth due to the increased nitrogen flow in the synthesis of ReN films, as observed in
Figure 8c–e. Furthermore,
Figure 8e confirms the passivity of the coating and minimal damage. However, protein activity has a significant effect on the ReN coating in the form of degradation.