Mass and Heat Balance Model and Its Engineering Application for the Oxygen Blast Furnace Smelting Process of Vanadium–Titanium Magnetite
Abstract
1. Introduction
2. Mathematical Model Development
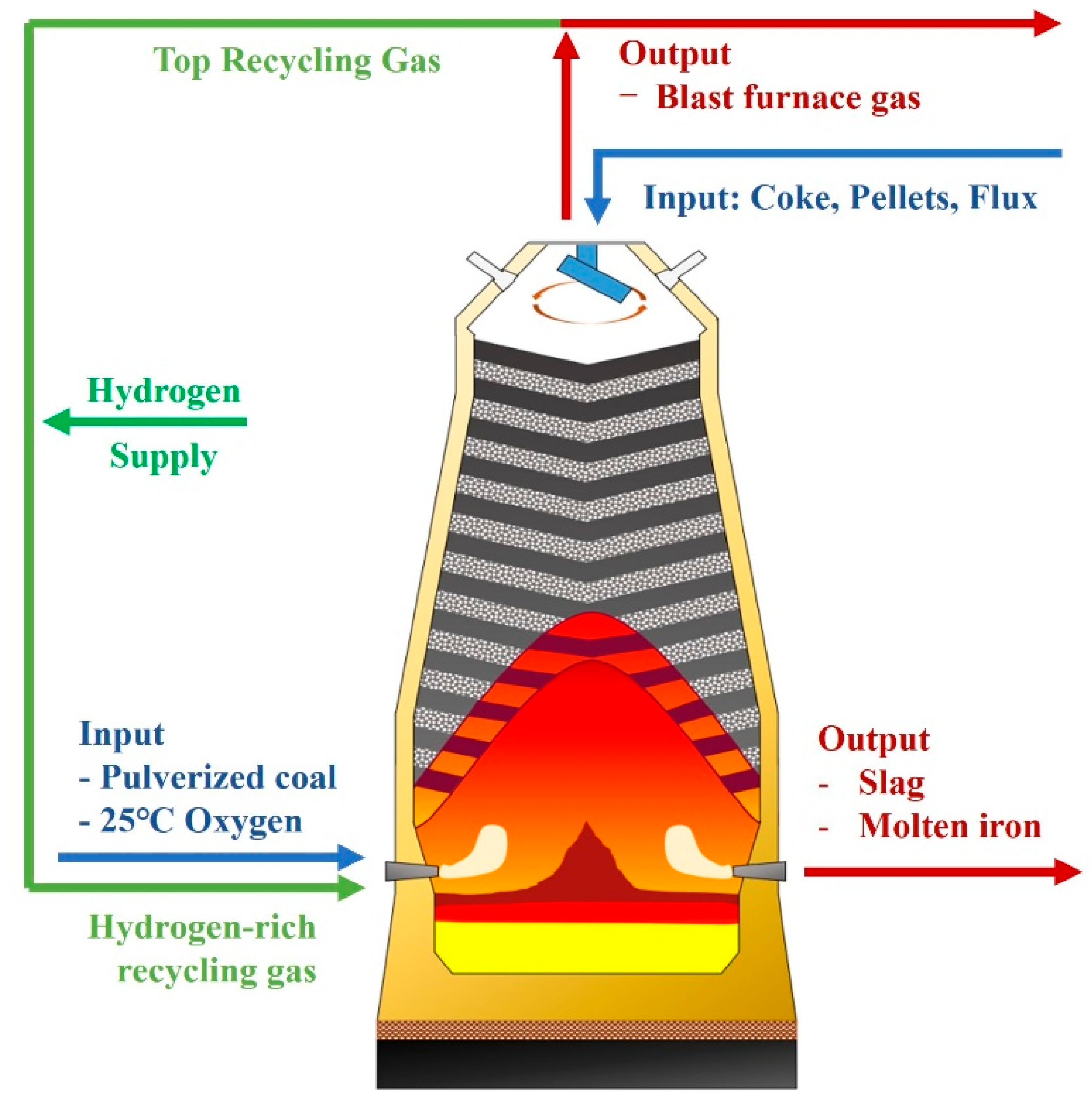
2.1. Process Characteristics of the Oxygen Blast Furnace
2.2. Methodology and Calculation Conditions
2.2.1. Assumptions and Boundary Conditions
2.2.2. Calculation Method of Key Parameters
- Utilization of CO and H2
- 2.
- Hot Metal Productivity
- 3.
- Blast Kinetic Energy
- 4.
- Raceway Zone
3. Results and Discussion
3.1. Effect of OBF Conditions on Productivity
3.1.1. Effect of Pulverized Coal Ratio on Productivity
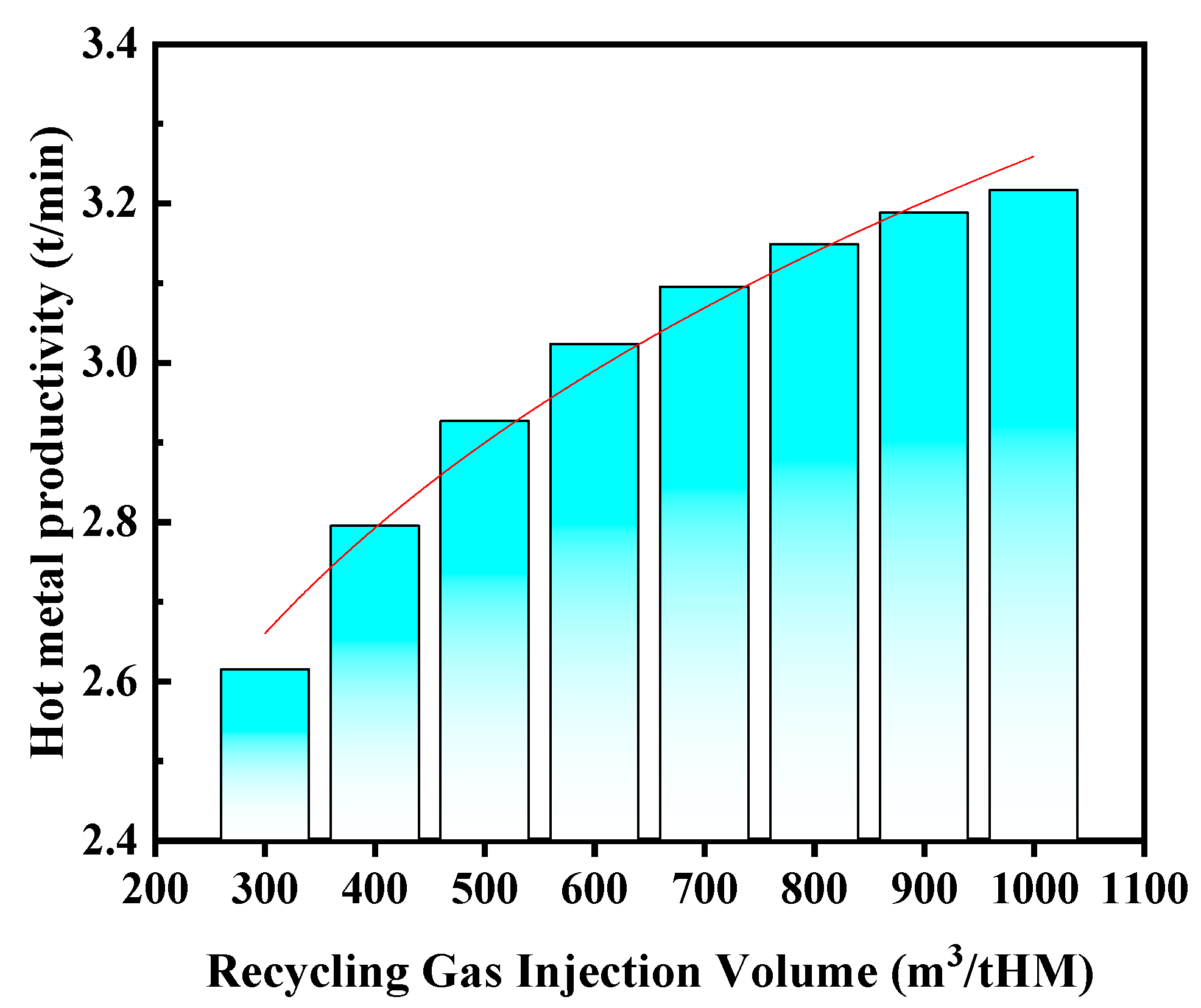
3.1.2. Effect of Recycling Gas Injection Volume on Productivity
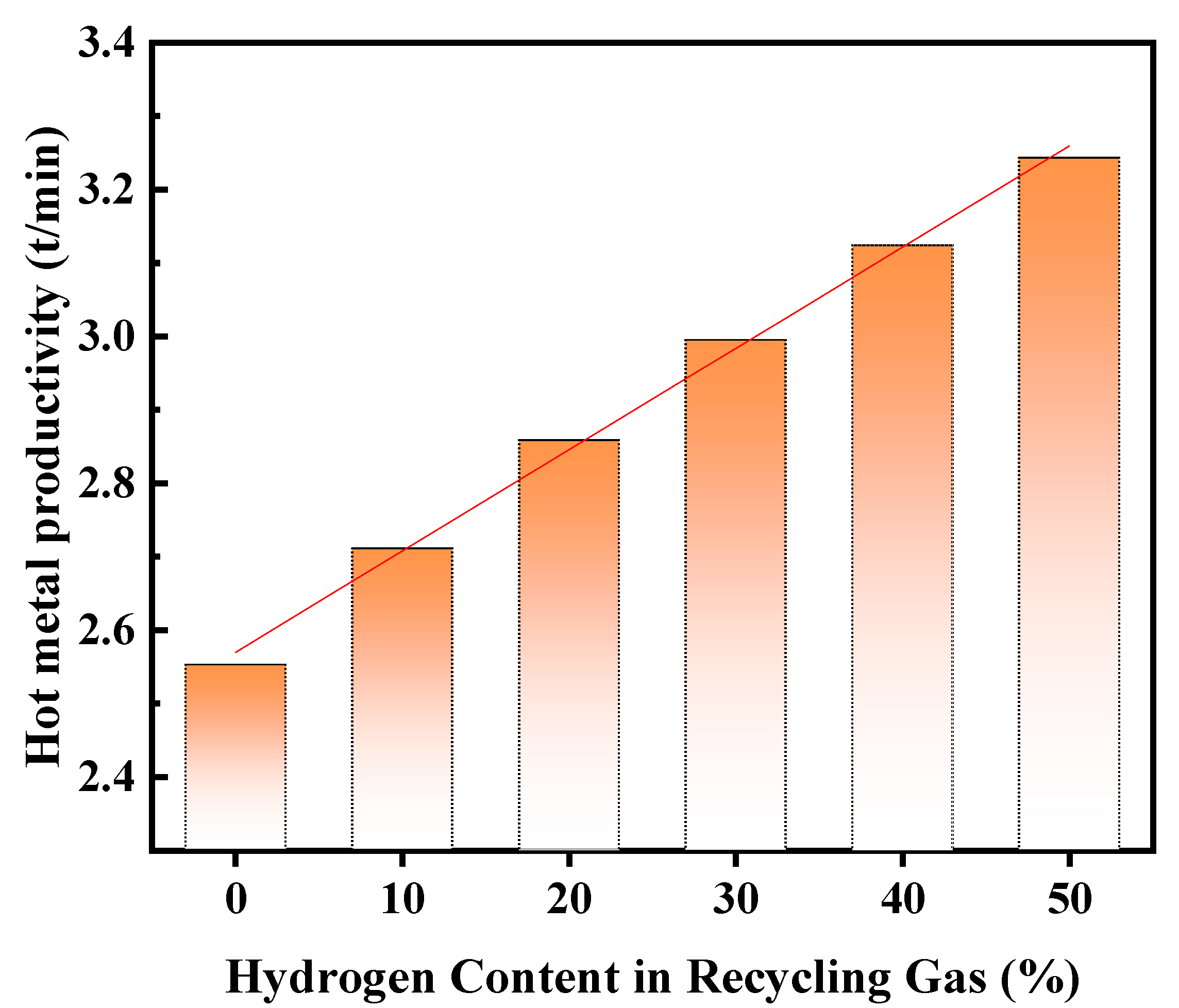
3.1.3. Effect of Hydrogen Content in Recycling Gas on Productivity
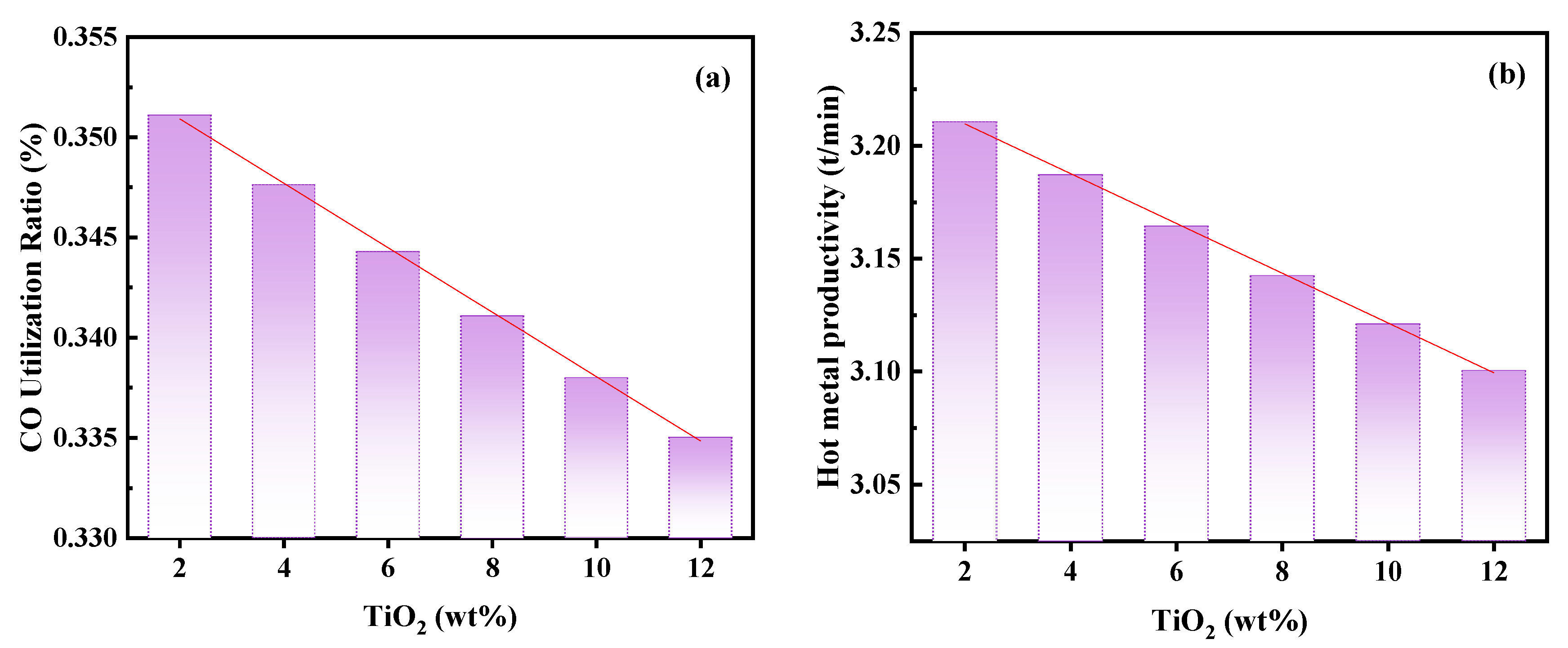
3.1.4. Effect of Titanium Content in Charge on Productivity
3.2. Effect of Injection Parameters on Theoretical Flame Temperature and Heat Distribution
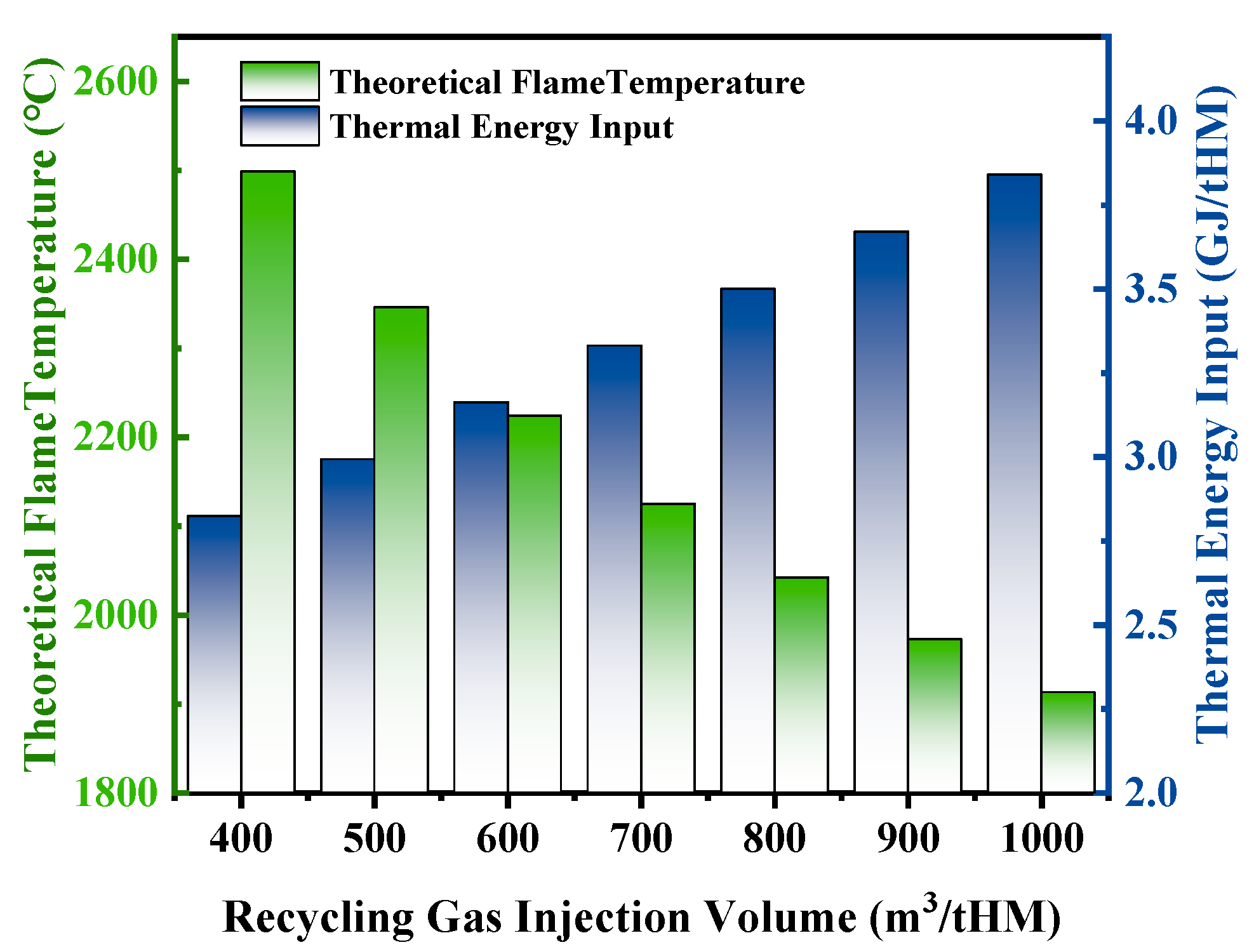
3.2.1. Effect of Recycling Gas Injection Volume on Theoretical Flame Temperature and Heat Distribution
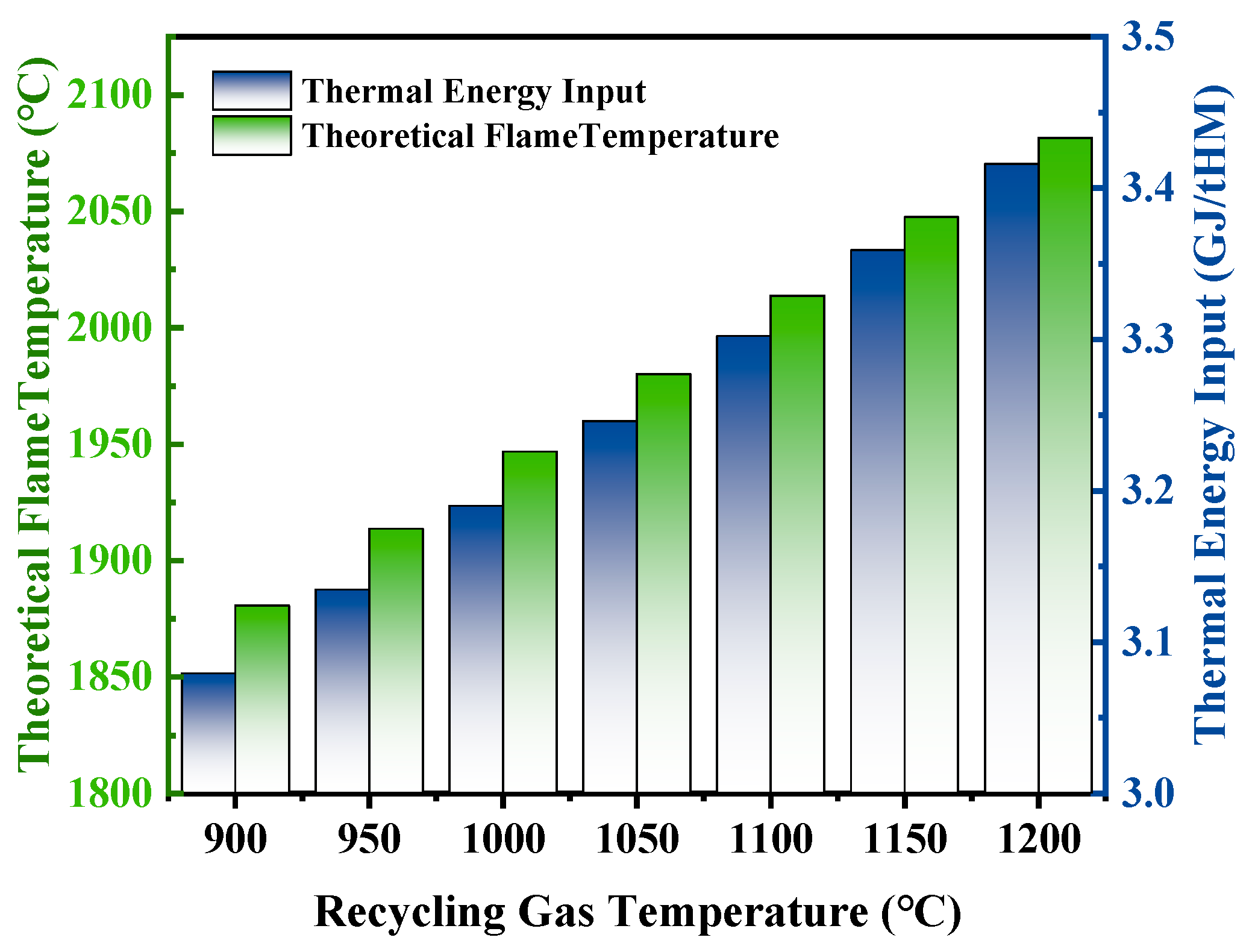
3.2.2. Effect of Recycling Gas Temperature on Theoretical Flame Temperature and Heat Distribution
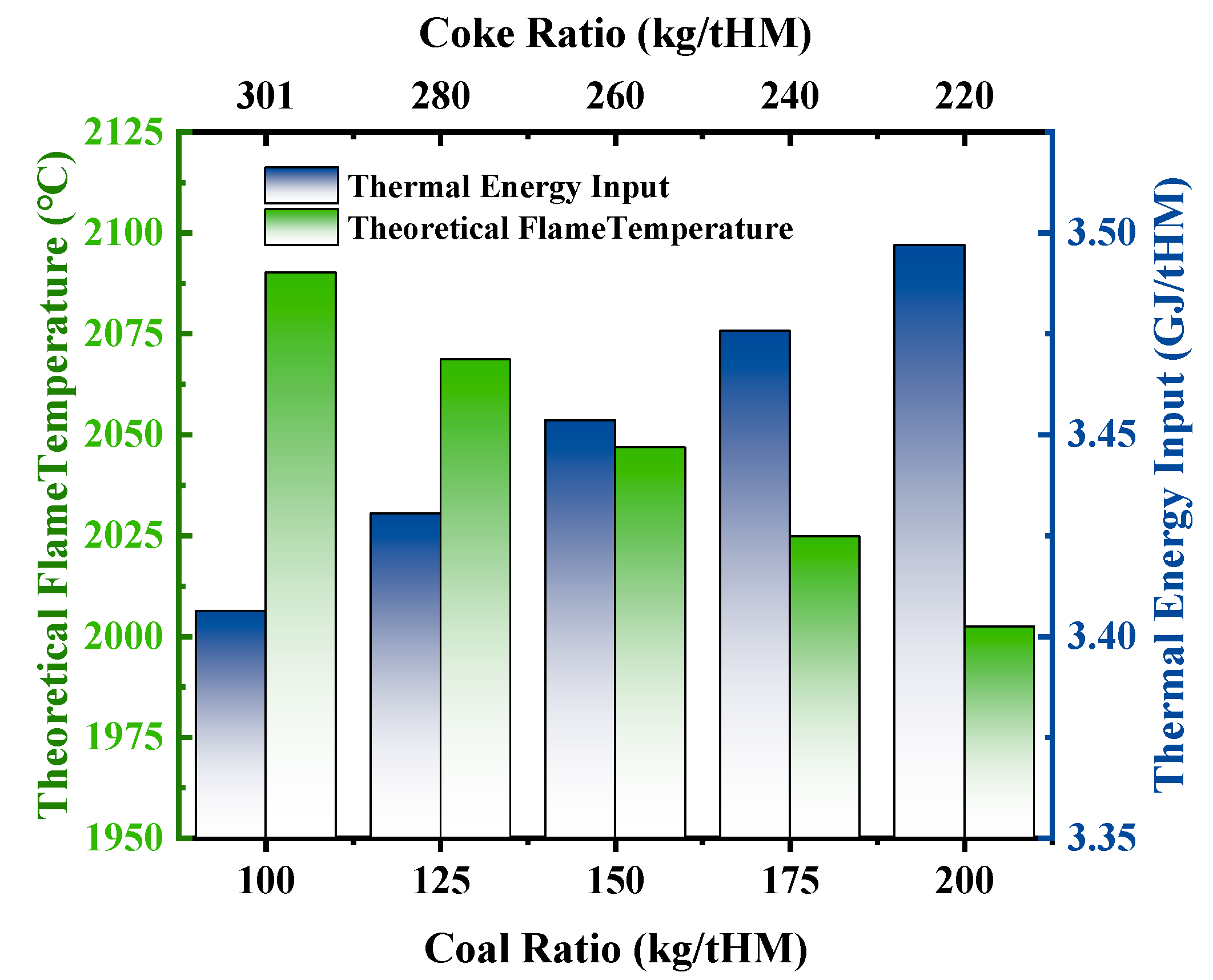
3.2.3. Effect of Pulverized Coal Ratio and Coke Ratio on Theoretical Flame Temperature and Heat Distribution
3.3. Effect of Injection Parameters on Blast Kinetic Energy and Raceway Zone Structure
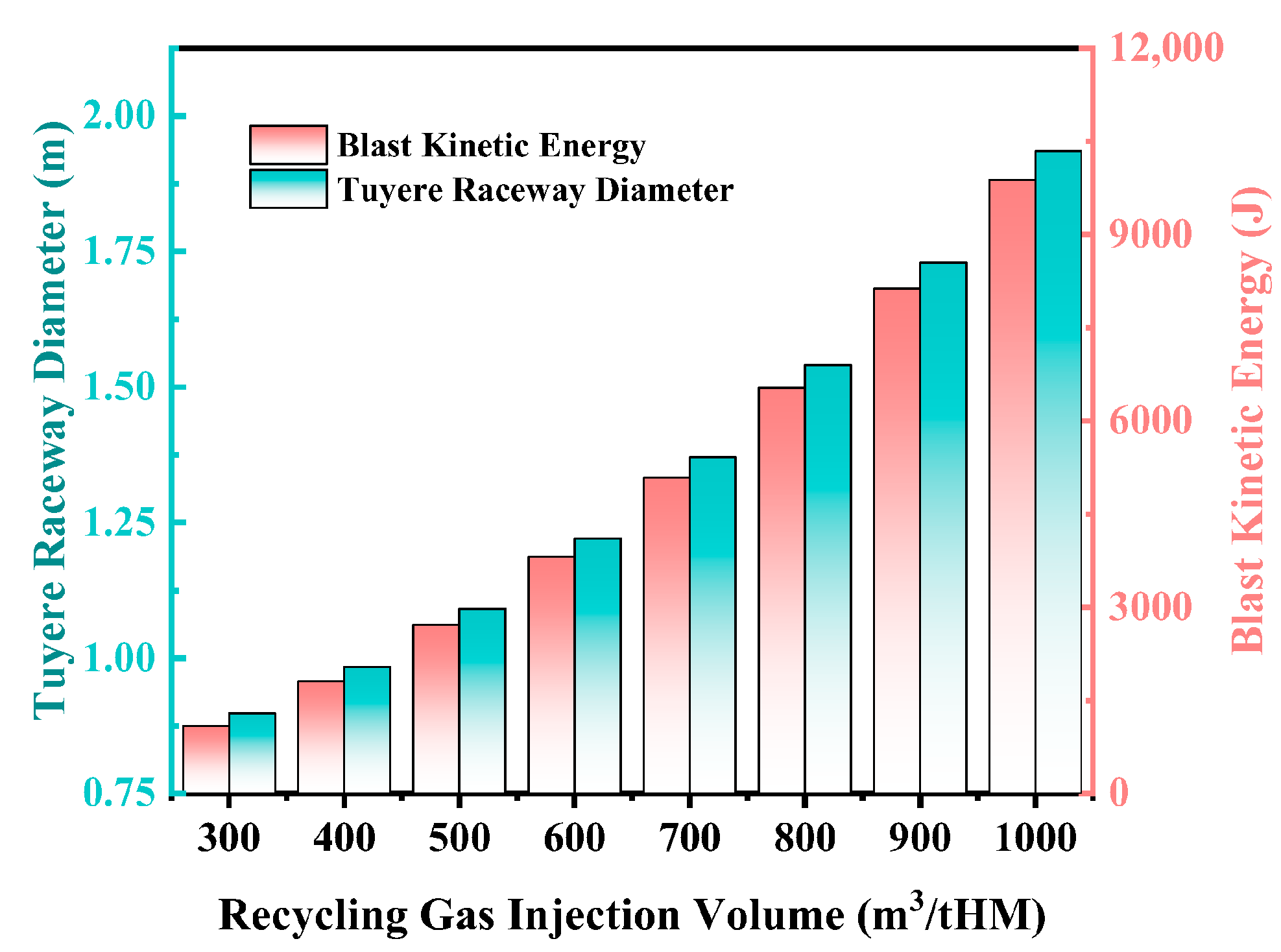
3.3.1. Effect of Recycling Gas Injection Volume on Blast Kinetic Energy and Raceway Zone Structure
3.3.2. Effect of Hydrogen Content in Recycling Gas on Blast Kinetic Energy and Raceway Zone Structure
3.3.3. Effect of Tuyere Diameter on Blast Kinetic Energy and Raceway Zone Structure
4. Conclusions
- As the coal ratio increases, blast furnace productivity decreases slightly, but the overall benefits are significant. On the one hand, it effectively conserves coking resources and reduces environmental pollution. On the other hand, the increased gas volume in the hearth promotes indirect reduction and significantly improves the “cold upper and hot lower” condition in OBF. However, for every 25 kg/tHM increase in the coal ratio, the theoretical flame temperature decreases by 21.95 °C, while the heat in the high-temperature zone increases.
- As the recycling gas volume increases, blast furnace productivity shows an upward trend, though at a gradually slowing rate. Additionally, an increase in recycling gas volume leads to a significant decrease in theoretical flame temperature, while the heat in the hearth zone rises markedly, and both blast kinetic energy and the diameter of the tuyere raceway zone increase significantly, which is conducive to maintaining the stability of the raceway zone and improving permeability. It is recommended that in industrial production, the recycling gas volume should be ≥600 m3/tHM.
- As the hydrogen content increases, blast furnace productivity rises significantly. However, an increase in hydrogen content in the recycling gas leads to a decrease in blast kinetic energy and the size of the tuyere raceway zone, which may adversely affect the stability of the blast furnace smelting process. Therefore, it is necessary to optimize the hydrogen content in the recycling gas. It is suggested that the hydrogen content be controlled at ≤20%. In addition, with the increase in titanium content in the charge, both CO utilization and blast furnace productivity show a downward trend.
- In the future, research should focus on further verifying the feasibility of smelting VTM in OBF through laboratory investigations and industrial-scale trials.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Xu, C.; Zhang, Y.; Liu, T.; Huang, J. Characterization and Pre-Concentration of Low-Grade Vanadium-Titanium Magnetite Ore. Minerals 2017, 7, 137. [Google Scholar] [CrossRef]
- Zhou, M.; Jiang, T.; Ding, X.; Ma, S.; Wei, G.; Xue, X. Thermodynamic Study of Direct Reduction of High-Chromium Vanadium-Titanium Magnetite (HCVTM) Based on Phase Equilibrium Calculation Model. J. Therm. Anal. Calorim. 2019, 136, 885–892. [Google Scholar] [CrossRef]
- Zhou, M.; Jiang, T.; Yang, S.; Xue, X. Vanadium–Titanium Magnetite Ore Blend Optimization for Sinter Strength Based on Iron Ore Basic Sintering Characteristics. Int. J. Miner. Process. 2015, 142, 125–133. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, J.; Liu, Z.; Wang, G.; Jiao, K.; Li, K.; Yang, T. Production of Ferrotitanium Alloy from Titania Slag Based on Aluminothermic Reduction. J. Alloys Compd. 2019, 810, 151969. [Google Scholar] [CrossRef]
- Yang, S.; Zhou, M.; Jiang, T.; Xue, X. Study on Sintering Characteristics of Ultra-Poor Vanadium-Titanium Magnetite. Minerals 2021, 11, 515. [Google Scholar] [CrossRef]
- Devaraju, T.; Jayaraj, K.; Sudhakara, T.; Alapieti, T.; Spiering, B.; Kaukonen, R. Mineralogy, Geochemistry and Petrogenesis of the V-Ti-Bearing and Chromiferous Magnetite Deposits Hosted by Neoarchaean Channagiri Mafic-Ultramafic Complex, Western Dharwar Craton, India: Implications for Emplacement in Differentiated Pulses. Open Geosci. 2014, 6, 518–548. [Google Scholar] [CrossRef]
- Liang, X.; Zhong, Y.; Tan, W.; Zhu, J.; Yuan, P.; He, H.; Jiang, Z. The Influence of Substituting Metals (Ti, V, Cr, Mn, Co and Ni) on the Thermal Stability of Magnetite. J. Therm. Anal. Calorim. 2013, 111, 1317–1324. [Google Scholar] [CrossRef]
- Surmeneva, M.A.; Koptyug, A.; Khrapov, D.; Ivanov, Y.F.; Mishurova, T.; Evsevleev, S.; Prymak, O.; Loza, K.; Epple, M.; Bruno, G.; et al. In Situ Synthesis of a Binary Ti–10at% Nb Alloy by Electron Beam Melting Using a Mixture of Elemental Niobium and Titanium Powders. J. Mater. Process. Technol. 2020, 282, 116646. [Google Scholar] [CrossRef]
- Yan, L.; Yuan, Y.; Ouyang, L.; Li, H.; Mirzasadeghi, A.; Li, L. Improved Mechanical Properties of the New Ti-15Ta-xZr Alloys Fabricated by Selective Laser Melting for Biomedical Application. J. Alloys Compd. 2016, 688, 156–162. [Google Scholar] [CrossRef]
- Soro, N.; Attar, H.; Wu, X.; Dargusch, M.S. Investigation of the Structure and Mechanical Properties of Additively Manufactured Ti-6Al-4V Biomedical Scaffolds Designed with a Schwartz Primitive Unit-Cell. Mater. Sci. Eng. A 2019, 745, 195–202. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, J.; Wang, Z.; Zong, Y.; Guo, P.; Jiang, D.; Zhang, S. Advancements in Oxygen Blast Furnace Technology and Its Application in the Smelting of Vanadium-Titanium Magnetite: A Comprehensive Review. Miner. Eng. 2024, 212, 108732. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, Z.; Zhang, J.; Guo, P.; Jiang, D.; Si, R. Effect of TiO2 and FeO on Viscosity and Structure of HIsmelt Titanium-Containing Slag. Ceram. Int. 2024, 50, 791–798. [Google Scholar] [CrossRef]
- Zhang, S.S.; Wang, Z.Y.; Zhang, J.L.; Guo, P.M.; Jiang, D.W.; Zhang, S. Effect of Basicity and MgO/Al2 O3 Ratio on Viscosity and Crystallization Behavior of HIsmelt Titanium-Containing Slag. Steel Res. Int. 2024, 95, 2300835. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, J.; Wang, Z.; Jiang, D.; Liang, Z.; Zhang, S. Effect of Basicity on the Structure and Viscosity Properties of HIsmelt Slag: A Molecular Dynamics Simulation. Can. Metall. Q. 2024, 64, 799–807. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, M.; Li, G.; Sui, Z. Recovery of Titanium Compounds from Molten Ti-Bearing Blast Furnace Slag under the Dynamic Oxidation Condition. Miner. Eng. 2007, 20, 684–693. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, L.; Qi, T.; Chen, D.; Zhao, H.; Liu, Y. A Novel Method to Extract Iron, Titanium, Vanadium, and Chromium from High-Chromium Vanadium-Bearing Titanomagnetite Concentrates. Hydrometallurgy 2014, 149, 106–109. [Google Scholar] [CrossRef]
- Li, R.; Liu, T.; Zhang, Y.; Huang, J.; Xu, C. Efficient Extraction of Vanadium from Vanadium–Titanium Magnetite Concentrate by Potassium Salt Roasting Additives. Minerals 2018, 8, 25. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, Z.; Hu, P.; Rao, J.; Zhang, J.; Pang, J. Distribution Behavior of Vanadium and Titanium between Hot Metal and High Titanium Slag Relevant to HIsmelt Smelting Condition. J. Mater. Res. Technol. 2022, 19, 4517–4524. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, Z.; Hu, P.; Rao, J.; Zong, Y.; Pang, J. Influence of Composition and Temperature on Distribution Behavior of V, Ti and Si in HIsmelt. Trans. Nonferrous Met. Soc. China 2023, 33, 3835–3846. [Google Scholar] [CrossRef]
- Ma, J. The Balanced Oxygen Blast Furnace (BOBF) Ironmaking Process. Scand. J. Metall. 1992, 21, 104–111. [Google Scholar]
- Edstroem, J.O.; Scheele, J. The Balanced Oxygen Blast Furnace Compared with Other Alternatives for Hot Metal Production. Scand. J. Metall. 1993, 22, 2–16. [Google Scholar]
- Fink, F. Suspension Smelting Reduction—A New Method of Hot Iron Production. Steel Times 1996, 224, 398–399. [Google Scholar]
- Tovarovskiy, I.G. Substitution of Coke and Energy Saving in Blast Furnaces. Part 4. System Analysis of the Processes under Influence of the Complex of Parameters. Energy Sci. Technol. 2013, 6, 44. [Google Scholar]
- Lu, W.K.; Kumar, R.V. The Feasibility of Nitrogen Free Blast Furnace Operation. ISS Trans. 1984, 5, 25. [Google Scholar]
- Zhang, W.; Dai, J.; Li, C.; Yu, X.; Xue, Z.; Saxén, H. A Review on Explorations of the Oxygen Blast Furnace Process. Steel Res. Int. 2021, 92, 2000326. [Google Scholar] [CrossRef]
- Zhang, X.; Jiao, K.; Zhang, J.; Guo, Z. A Review on Low Carbon Emissions Projects of Steel Industry in the World. J. Clean. Prod. 2021, 306, 127259. [Google Scholar] [CrossRef]
- Shahabuddin, M.; Brooks, G.; Rhamdhani, M.A. Decarbonisation and Hydrogen Integration of Steel Industries: Recent Development, Challenges and Technoeconomic Analysis. J. Clean. Prod. 2023, 395, 136391. [Google Scholar] [CrossRef]
- Lan, C.; Hao, Y.; Shao, J.; Zhang, S.; Liu, R.; Lyu, Q. Effect of H2 on Blast Furnace Ironmaking: A Review. Metals 2022, 12, 1864. [Google Scholar] [CrossRef]
- Ariyama, T.; Sato, M.; Nouchi, T.; Takahashi, K. Evolution of Blast Furnace Process toward Reductant Flexibility and Carbon Dioxide Mitigation in Steel Works. ISIJ Int. 2016, 56, 1681–1696. [Google Scholar] [CrossRef]
- Pang, Z.; Bu, J.; Yuan, Y.; Zheng, J.; Xue, Q.; Wang, J.; Guo, H.; Zuo, H. The Low-Carbon Production of Iron and Steel Industry Transition Process in China. Steel Res. Int. 2024, 95, 2300500. [Google Scholar] [CrossRef]
- van der Stel, J.; Louwerse, G.; Sert, D.; Hirsch, A.; Eklund, N.; Pettersson, M. Top Gas Recycling Blast Furnace Developments for ‘Green’ and Sustainable Ironmaking. Ironmak. Steelmak. 2013, 40, 483–489. [Google Scholar] [CrossRef]
- Ohno, Y.; Hotta, H.; Matsuura, M.; Mitsufuji, H.; Saito, H. Development of Oxygen Blast Furnace Process with Preheating Gas Injection into Upper Shaft. Tetsu-to-Hagané 1989, 75, 1278–1285. [Google Scholar] [CrossRef]
- Ohno, Y.; Matsuura, M.; Mitsufuji, H.; Furukawa, T. Process Characteristics of a Commercial-Scale Oxygen Blast Furnace Process with Shaft Gas Injection. ISIJ Int. 1992, 32, 838–847. [Google Scholar] [CrossRef]
- Tseitlin, M.A.; Lazutkin, S.E.; Styopin, G.M. A Folw-Chart for Iron Making on the Basis of 100% Usage of Process Oxygen and Hot Reducing Gases Injection. ISIJ Int. 1994, 34, 570–573. [Google Scholar] [CrossRef]
- Quader, M.A.; Ahmed, S.; Dawal, S.; Nukman, Y. Present Needs, Recent Progress and Future Trends of Energy-Efficient Ultra-Low Carbon Dioxide (CO2) Steelmaking (ULCOS) Program. Renew. Sustain. Energy Rev. 2016, 55, 537–549. [Google Scholar] [CrossRef]
- Junjie, Y. Progress and Future of Breakthrough Low-Carbon Steelmaking Technology (ULCOS) of EU. Int. J. Miner. Process. Extr. Met. 2018, 3, 15. [Google Scholar] [CrossRef]
- Zuo, G.; Hirsch, A. The Trial of the Top Gas Recycling Blast Furnace at LKAB’s EBF and Scale-Up. Metall. Res. Technol. 2009, 106, 387–392. [Google Scholar] [CrossRef]
- Danloy, G.; Berthelemot, A.; Grant, M.; Borlée, J.; Sert, D.; van der Stel, J.; Jak, H.; Dimastromatteo, V.; Hallin, M.; Eklund, N.; et al. ULCOS—Pilot Testing of the Low-CO2 Blast Furnace Process at the Experimental BF in Luleå. Metall. Res. Technol. 2009, 106, 1–8. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, J.; Xue, Z. Exergy Analyses of the Oxygen Blast Furnace with Top Gas Recycling Process. Energy 2017, 121, 135–146. [Google Scholar] [CrossRef]



| Component | TFe | FeO | SiO2 | CaO | MgO | Al2O3 | TiO2 | V2O5 | MnO | S | P |
|---|---|---|---|---|---|---|---|---|---|---|---|
| wt% | 54.97 | 1.06 | 3.54 | 0.67 | 2.97 | 3.41 | 9.73 | 0.69 | 0.42 | 0.01 | 0.01 |
| Component | C | S | CaO | SiO2 | MgO | Fe2O3 | Al2O3 | H2O | Volatile |
|---|---|---|---|---|---|---|---|---|---|
| Coke | 84.84 | 0.77 | 0.61 | 7.00 | 0.16 | 0.66 | 3.94 | 0.88 | 1.14 |
| Coal | 67.98 | 0.16 | 1.75 | 4.35 | 0.23 | 0.48 | 2.21 | 1.27 | 21.56 |
| Flux | — | — | 47.94 | 1.69 | 2.48 | — | — | — | 47.89 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Y.; Chu, M.; Gan, X.; Zhang, S.; Wang, Z.; Zhang, J. Mass and Heat Balance Model and Its Engineering Application for the Oxygen Blast Furnace Smelting Process of Vanadium–Titanium Magnetite. Metals 2025, 15, 805. https://doi.org/10.3390/met15070805
Huang Y, Chu M, Gan X, Zhang S, Wang Z, Zhang J. Mass and Heat Balance Model and Its Engineering Application for the Oxygen Blast Furnace Smelting Process of Vanadium–Titanium Magnetite. Metals. 2025; 15(7):805. https://doi.org/10.3390/met15070805
Chicago/Turabian StyleHuang, Yun, Mansheng Chu, Xian Gan, Shushi Zhang, Zhenyang Wang, and Jianliang Zhang. 2025. "Mass and Heat Balance Model and Its Engineering Application for the Oxygen Blast Furnace Smelting Process of Vanadium–Titanium Magnetite" Metals 15, no. 7: 805. https://doi.org/10.3390/met15070805
APA StyleHuang, Y., Chu, M., Gan, X., Zhang, S., Wang, Z., & Zhang, J. (2025). Mass and Heat Balance Model and Its Engineering Application for the Oxygen Blast Furnace Smelting Process of Vanadium–Titanium Magnetite. Metals, 15(7), 805. https://doi.org/10.3390/met15070805





