Advanced Strategies for Suppressing the Self-Corrosion of the Anode in Al–Air Batteries
Abstract
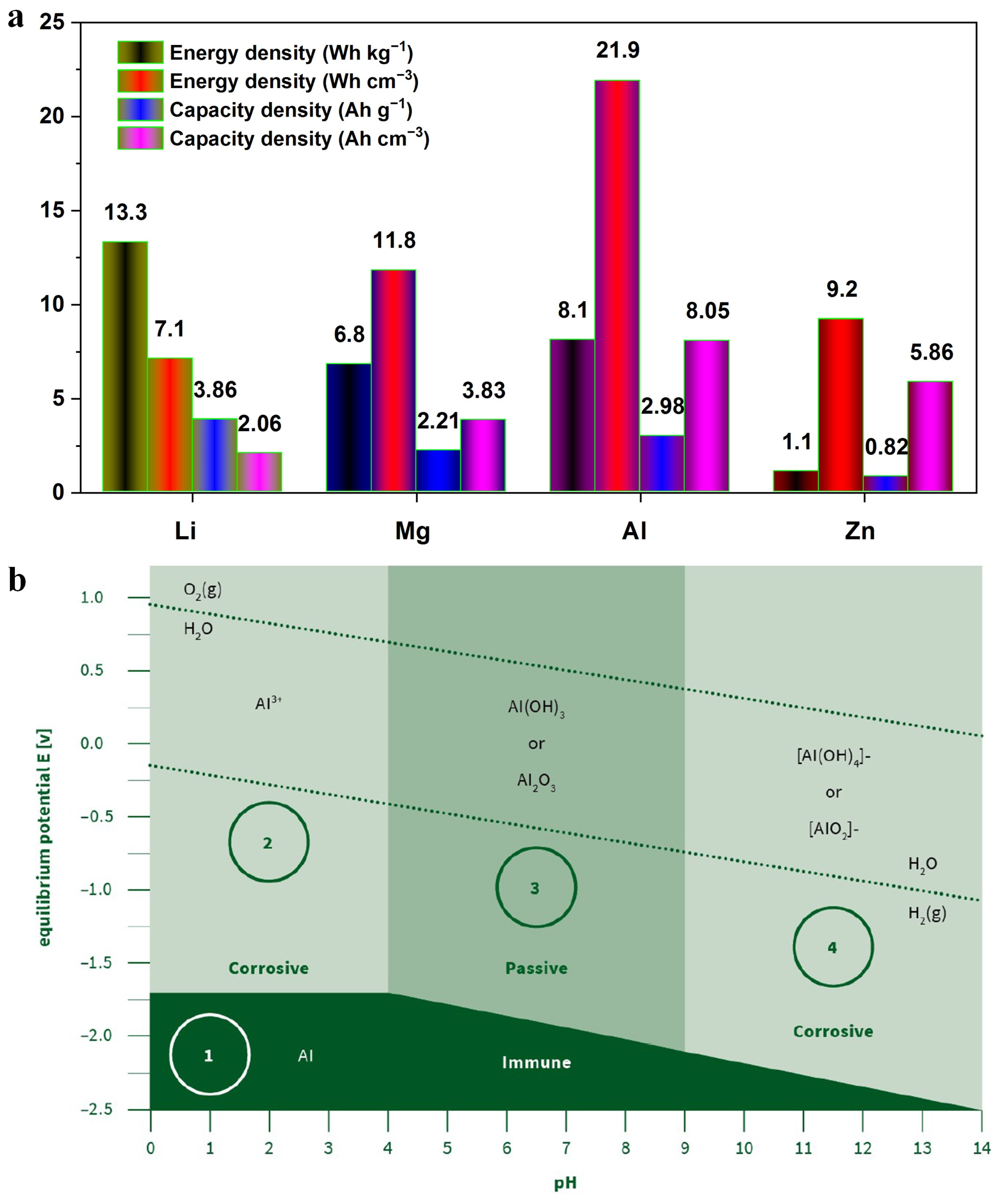
1. Introduction
2. Alloying Strategy and Anodic Coatings
3. Corrosion Inhibitor Strategies in Single Electrolyte
3.1. Inorganic Corrosion Inhibitors
3.2. Organic Corrosion Inhibitors
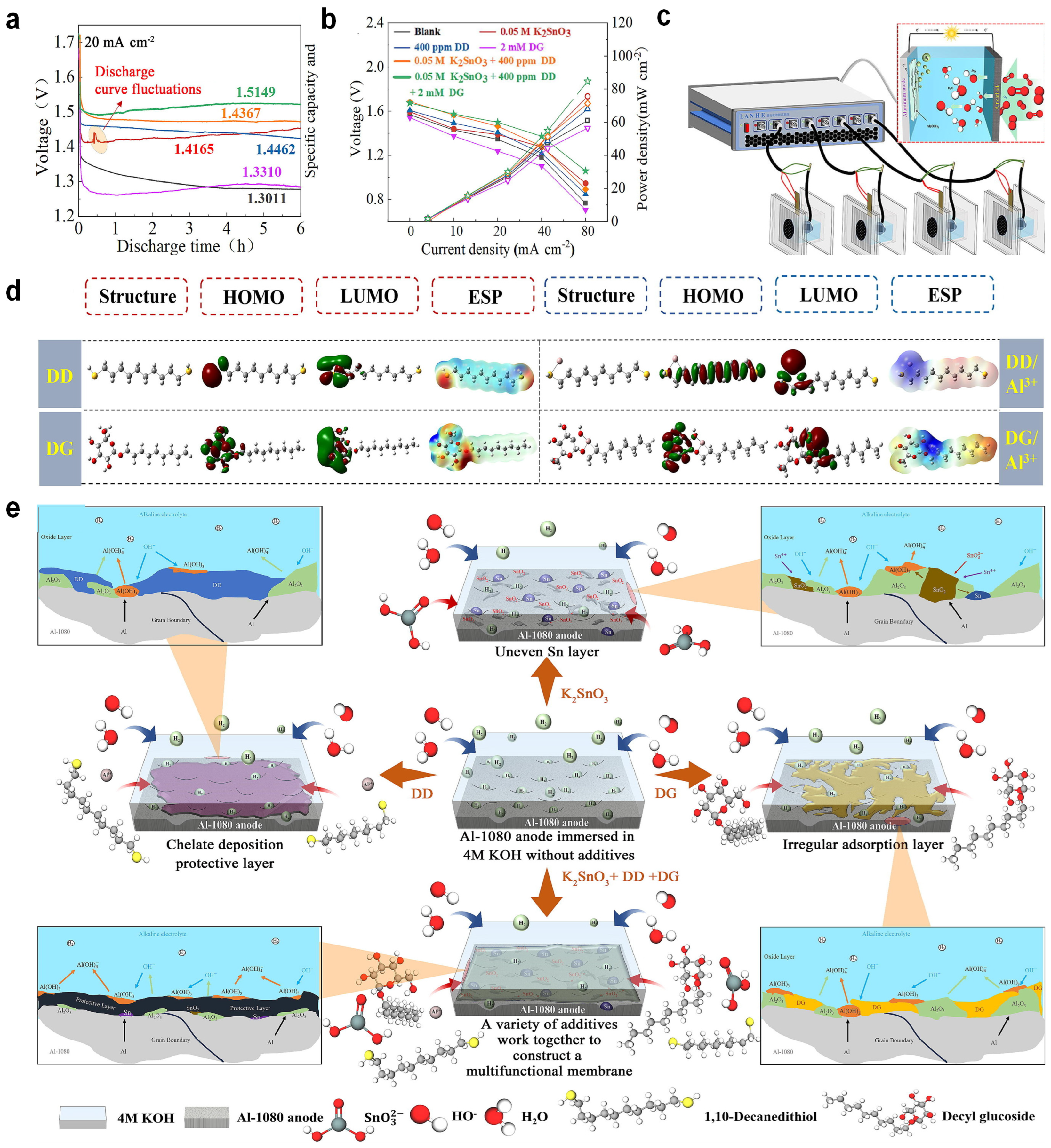
3.3. Hybrid Corrosion Inhibitors
| Additives | Anode | Inhibition Efficiency | Anode Utilization | Ref. |
|---|---|---|---|---|
| K2SnO3 | Al-Mg-Ga-Sn-Zn-Fe-Cu-Si alloy | 86.86% | 63.78% | [80] |
| ZnO | AA5052 alloy | 44.12% | 66.5% | [96] |
| Nonoxynol-9 | 99.99% pure Al | 85.6% | 58.8% | [97] |
| Thiourea | Al-0.01Mg-0.002In-0.001Mn | 57% | 86.1% | [98] |
| Urea | Al-0.01Mg-0.002In-0.001Mn | 51.5% | 85.4% | [98] |
| K2SnO3 + APG | Al-Mg-Ga-Sn-Zn-Fe-Cu-Si alloy | 94.14% | 73.87% | [80] |
| ZnO + CMC | AA5052 alloy | 70.32% | 91.4% | [99] |
| ZnO + AQ | AA5052 alloy | 71.59% | 70.3% | [100] |
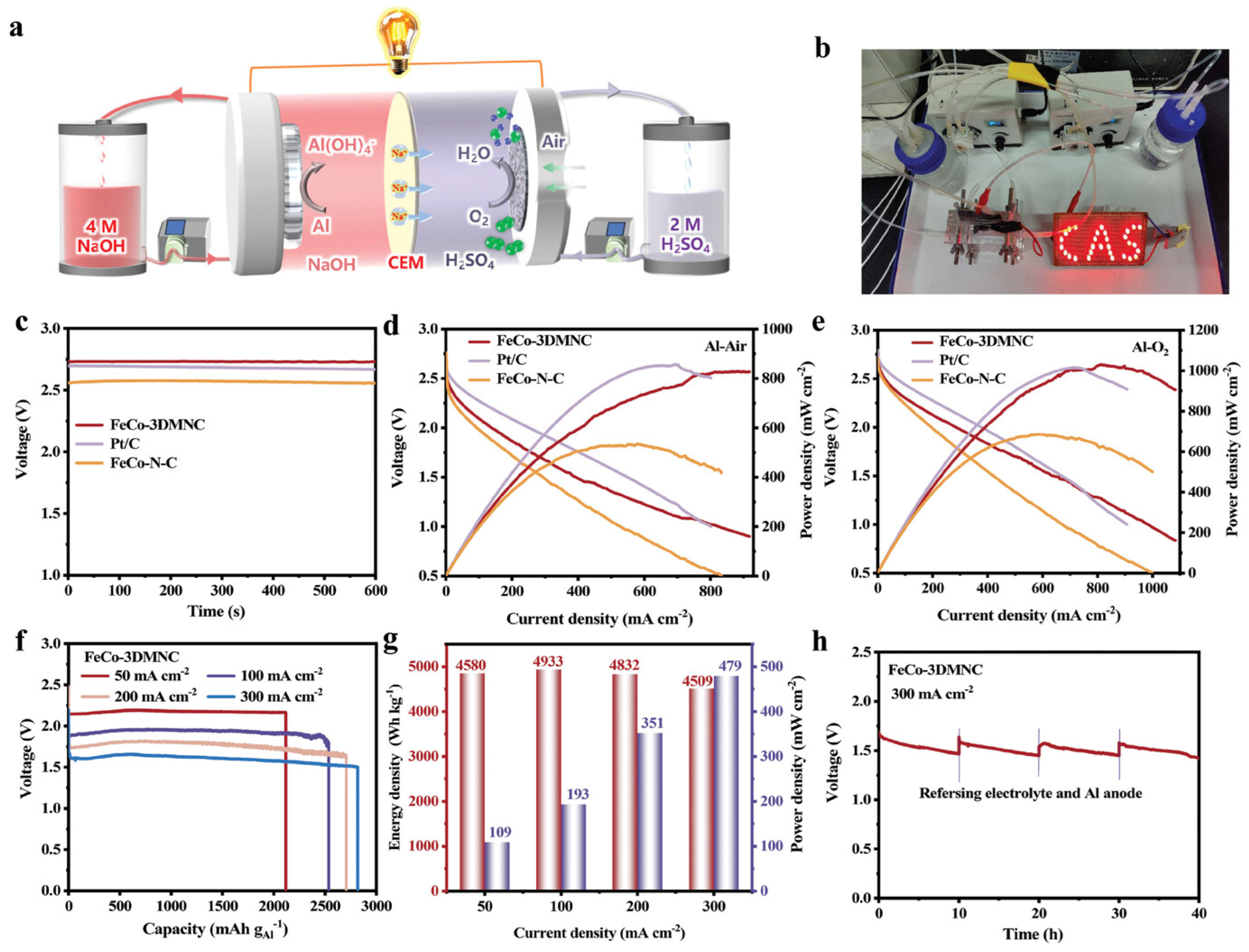
4. Corrosion Inhibitor Strategies in Double Electrolytes
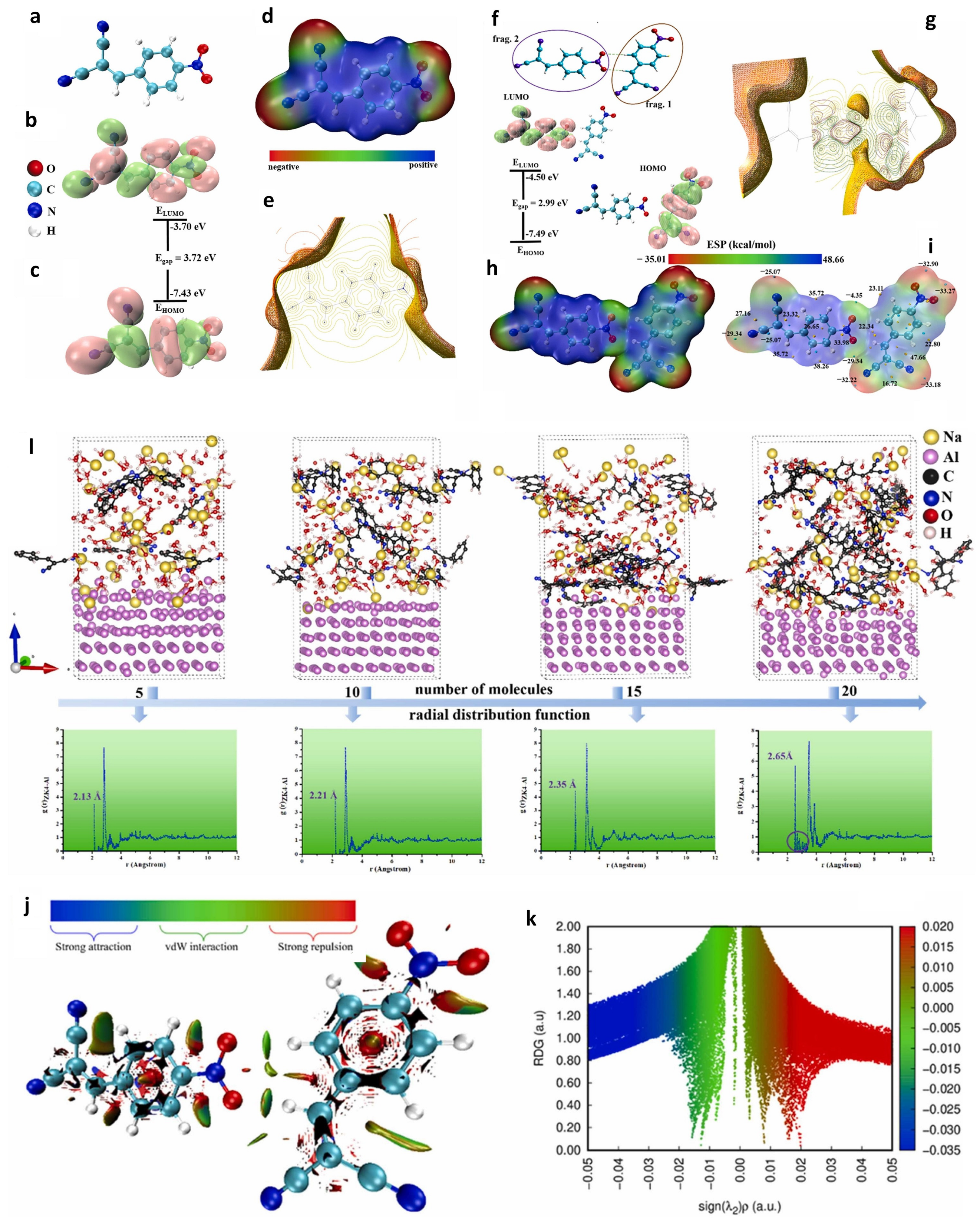
5. Computer-Aided Investigation for Al–Air Batteries
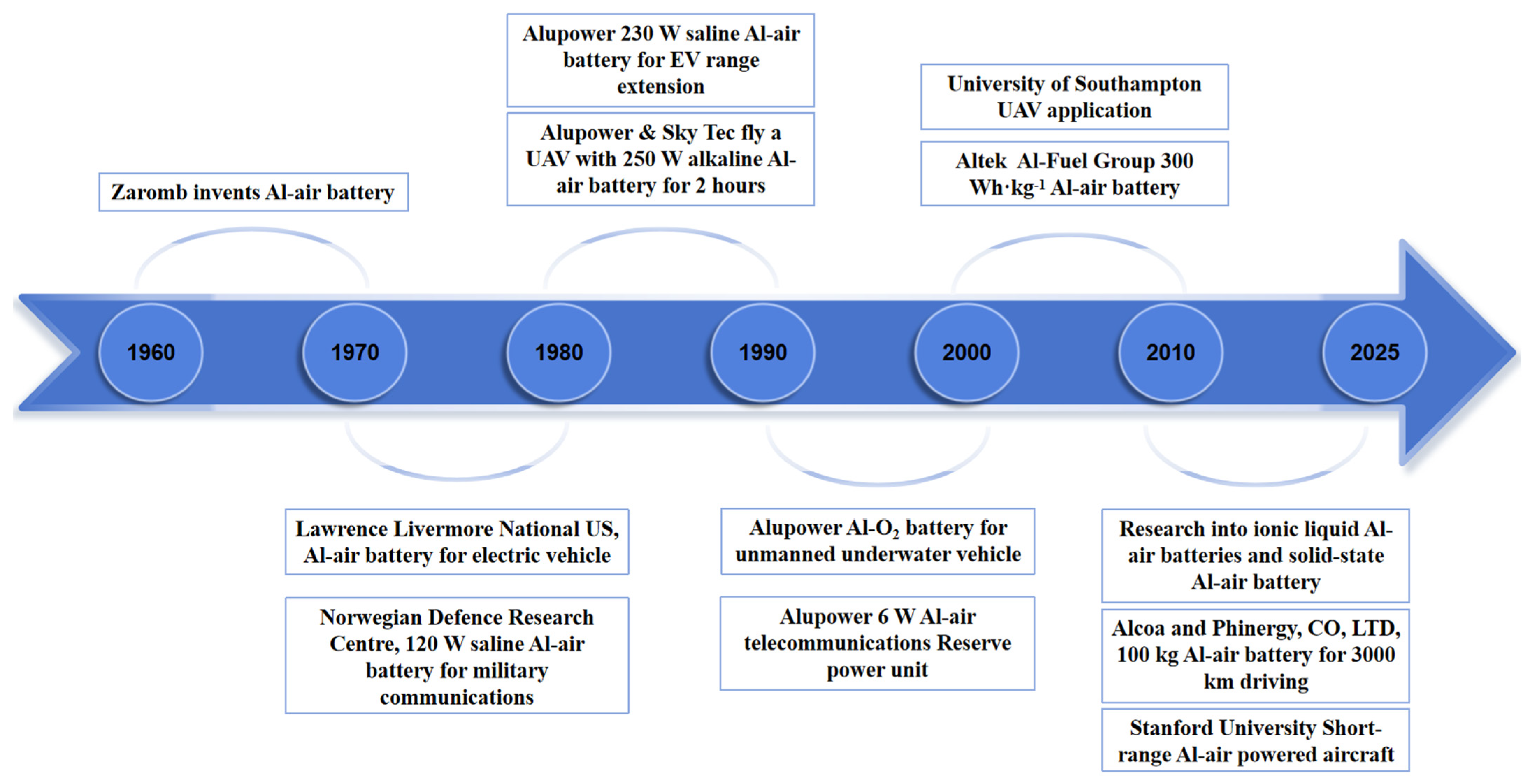
6. Practical Applications of Al–Air Batteries
7. Conclusions and Prospects
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hao, M.-D.; Li, Q.; Sun, J.-H.; Liu, D.; Yu, H.-L.; Liu, R. Polyphenol-metal coordination derived high-entropy alloy as bifunctional oxygen electrocatalyst for Zn-air batteries. Rare Met. 2025, 44, 2836–2844. [Google Scholar] [CrossRef]
- Wu, X.-G.; Wang, R.; Ma, F.; Liu, X.-L.; Jia, D.-L.; Yang, H.-C.; Liu, Y.-P.; Wang, Z.-X.; Zheng, H.-Z.; Zhang, Y.-N.; et al. FeCo-N encapsuled in nitrogen-doped carbon nanotubes as bifunctional electrocatalysts with a high stability for zinc air batteries. Rare Met. 2023, 42, 1526–1534. [Google Scholar] [CrossRef]
- Xu, Y.; Tang, J.-Y.; Li, S.-D.; Hu, H.-L.; He, Y.-J.; Wang, S.-F.; Wu, Z.-M.; Jeong, S.; Cai, Z.-Y.; Lin, X.; et al. Co-doping-induced electronic reconfiguration of nanosized ZnS for facilitating oxygen reduction reaction in flexible aluminum-air batteries. Rare Met. 2025, 44, 2352–2365. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, Y.; Peng, X.; Lin, T.; Huang, X.; Alghamdi, N.S.; Rana, M.; Chen, P.; Zhang, C.; Whittaker, A.K.; et al. Enhancing performance and longevity of solid-state zinc-iodine batteries with fluorine-rich solid electrolyte interphase. Mater. Futures 2024, 3, 035102. [Google Scholar] [CrossRef]
- Yu, F.; Mu, Y.; Han, M.; Liu, J.; Zheng, K.; Zou, Z.; Hu, H.; Man, Q.; Li, W.; Wei, L.; et al. Electrochemically stable and ultrathin polymer-based solid electrolytes for dendrite-free all-solid-state lithium-metal batteries. Mater. Futures 2025, 4, 015101. [Google Scholar] [CrossRef]
- Zhou, M.; Fu, K.; Xing, Y.; Liu, J.; Meng, F.; Wei, X.; Liu, J. 500-mW cm−2 underwater Zn-H2O2 batteries with ultrafine edge-enriched electrocatalysts. Sci. China Mater. 2024, 67, 2908–2914. [Google Scholar] [CrossRef]
- Yue, C.; Zhang, N.; Zhu, Z.; Chen, P.; Meng, F.; Liu, X.; Wei, X.; Liu, J. Multi-Strategy Architecture of High-Efficiency Electrocatalysts for Underwater Zn-H2O2 Batteries with Superior Power Density of 442 mW cm-2. Small 2022, 18, 2106532. [Google Scholar] [CrossRef]
- Xu, L.; Liu, J.; Chen, P.; Wang, Z.; Tang, D.; Liu, X.; Meng, F.; Wei, X. High-power aqueous Zn-H2O2 batteries for multiple applications. Cell Rep. Phys. Sci. 2020, 1, 100027. [Google Scholar] [CrossRef]
- Hossain, M.I.; Tareq, F.K.; Rudra, S. Light-driven photocathodes in Li/Zn-O2 (air) batteries: An analytical review, technological breakthroughs and future challenges. Energy Storage Mater. 2025, 75, 104025. [Google Scholar] [CrossRef]
- Jiang, P.; Li, D.; Hou, R.; Yang, H.; Yang, J.; Zhu, S.; Wang, L.; Guan, S. A micro-alloyed Mg-Zn-Ge alloy as promising anode for primary Mg-air batteries. J. Magnes. Alloys 2024, 12, 4157–4173. [Google Scholar] [CrossRef]
- Zhou, K.; Liu, S.; Li, L. Recent advances of high-entropy materials as electrocatalysts for rechargeable Zn-air batteries. J. Alloys Compd. 2025, 1011, 178435. [Google Scholar] [CrossRef]
- Meng, F.; Chen, P.; Li, S.; Cao, N.; Cheng, S.; Wei, X.; Di, J.; Liu, J. Fish-Gill-Inspired Underwater Self-Breathing Zinc-Air Batteries. ACS Appl. Energy Mater. 2024, 7, 9442–9450. [Google Scholar] [CrossRef]
- Dai, J.; Chen, P.; Meng, F.; Han, F.; Zhu, C.; Wei, X.; Zheng, L.; Liu, J. Starch-reinforced adhesive hydrogel electrolyte enables high-performance flexible zinc-air batteries. J. Energy Storage 2024, 102, 114035. [Google Scholar] [CrossRef]
- Zhu, Z.; Jin, L.; Zhou, M.; Fu, K.; Meng, F.; Wei, X.; Liu, J. Single-cell-array biomass-templated architecture of hierarchical porous electrocatalysts for Zn–air and Zn–H2O2 batteries. Chem. Commun. 2023, 59, 4356–4359. [Google Scholar] [CrossRef]
- Jin, L.; Xing, A.; Zhu, Z.; Fu, K.; Zhou, M.; Meng, F.; Wei, X.; Liu, J. In situ potential-regulated architecture of an ultrafine Ru-based electrocatalyst for ultralow overpotential lithium–oxygen batteries. Chem. Commun. 2023, 59, 5926–5929. [Google Scholar] [CrossRef]
- Liu, J.; Chen, W.; Xing, A.; Wei, X.; Xue, D. Lithium cell-assisted low-overpotential Li-O2 batteries by in situ discharge activation. Chem. Commun. 2017, 53, 10568–10571. [Google Scholar] [CrossRef]
- Liu, J.; Zhou, M.; Jin, K.; Li, J.; Meng, F.; Wei, X. Beyond metal–air battery, emerging aqueous metal–hydrogen peroxide batteries with improved performance. Battery Energy 2024, 3, 20230049. [Google Scholar] [CrossRef]
- Fei, H.; Liu, R.; Zhang, Y.; Wang, H.; Wang, M.; Wang, S.; Ni, M.; Wu, Z.; Wang, J. Extending MoS2-based materials into the catalysis of non-acidic hydrogen evolution: Challenges, progress, and perspectives. Mater. Futures 2023, 2, 022103. [Google Scholar] [CrossRef]
- Dilshad, M.; Li, T.; Lee, S.-L.; Qin, L. Next-Generation Aluminum-Air Batteries: Integrating New Materials and Technologies for Superior Performance. ACS Appl. Energy Mater. 2025, 8, 3248–3275. [Google Scholar] [CrossRef]
- Zaromb, S. The use and behavior of aluminum anodes in alkaline primary batteries. J. Electrochem. Soc. 1962, 109, 1125. [Google Scholar] [CrossRef]
- Zhang, Z.; Zuo, C.; Liu, Z.; Yu, Y.; Zuo, Y.; Song, Y. All-solid-state Al–air batteries with polymer alkaline gel electrolyte. J. Power Sources 2014, 251, 470–475. [Google Scholar] [CrossRef]
- Goel, P.; Dobhal, D.; Sharma, R.C. Aluminum–air batteries: A viability review. J. Energy Storage 2020, 28, 101287. [Google Scholar] [CrossRef]
- Rasul, M.J.M.A.; Kim, J. Comprehensive review and comparison on battery technologies as electric-powered source in marine applications. J. Energy Storage 2024, 88, 111509. [Google Scholar] [CrossRef]
- Yang, S.; Knickle, H. Design and analysis of aluminum/air battery system for electric vehicles. J. Power Sources 2002, 112, 162–173. [Google Scholar] [CrossRef]
- Tang, G.; Guo, D.; Wu, K. Optimal Backup Power Allocation for 5G Base Stations. In GreenEdge: New Perspectives to Energy Management and Supply in Mobile Edge Computing; Tang, G., Guo, D., Wu, K., Eds.; Springer Nature: Singapore, 2022; pp. 51–65. [Google Scholar]
- Suresh, T.; Samuel Ratna Kumar, P.S.; Nithyadharseni, P. Aluminium air batteries for sustainable environment: A review. J. Alloys Compd. Commun. 2025, 6, 100048. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, Q.; Li, W.; Adair, K.R.; Li, J.; Sun, X. A comprehensive review on recent progress in aluminum–air batteries. Green Energy Environ. 2017, 2, 246–277. [Google Scholar] [CrossRef]
- Hasannaeimi, V.; Sadeghilaridjani, M.; Mukherjee, S. Electrochemical and Corrosion Behavior of Metallic Glasses; MDPI-Multidisciplinary Digital Publishing Institute: Basel, Switzerland, 2021. [Google Scholar]
- Chen, L.D.; Nørskov, J.K.; Luntz, A.C. Al–air batteries: Fundamental thermodynamic limitations from first-principles theory. J. Phys. Chem. Lett. 2015, 6, 175–179. [Google Scholar] [CrossRef]
- Gaele, M.F.; Gargiulo, P.; Di Palma, T.M. Aluminum-air batteries with acidic bio-polymer gel electrolytes and wood-derived metal-free cathodes. J. Power Sources 2025, 626, 235784. [Google Scholar] [CrossRef]
- Li, X.; Ma, Z.-Q.; Teng, Z. Study on a New Kind of Al Alloy Anode Material for Aluminum-Air Battery. Mater. Sci. 2012, 02, 52–57. [Google Scholar]
- Zhu, C.; Han, Y.; Luo, L.; Yan, L.; Xiang, B.; Zhou, Y.; Zou, X.; Guo, L. Dual modulation of electrolyte inner solvent structure and anode interface for high performance alkaline Al-air battery. Chem. Eng. J. 2024, 496, 153814. [Google Scholar] [CrossRef]
- Wu, G.-X.; Wei, Z.-S.; Li, S.-Q.; Cui, L.-Y.; Zhang, G.-X.; Zeng, R.-C. Corrosion inhibition of polyelectrolytes to the Al anode in Al-air battery: A comparative study of functional group effect. J. Power Sources 2024, 592, 233907. [Google Scholar] [CrossRef]
- Fan, L.; Lu, H.; Leng, J. Performance of fine structured aluminum anodes in neutral and alkaline electrolytes for Al-air batteries. Electrochim. Acta 2015, 165, 22–28. [Google Scholar] [CrossRef]
- Seyam, S.; Dincer, I.; Agelin-Chaab, M. Exergetic, exergoeconomic and exergoenvironmental analyses of a hybrid combined locomotive powering system for rail transportation. Energy Convers. Manag. 2021, 245, 114619. [Google Scholar] [CrossRef]
- Zhao, R.; He, P.; Yu, F.; Yang, J.; Sun, Z.; Hu, W. Performance improvement for aluminum-air battery by using alloying anodes prepared from commercially pure aluminum. J. Energy Storage 2023, 73, 108985. [Google Scholar] [CrossRef]
- Tong, F.; Zhuang, W.; Song, M.; Kim, J.; Gao, W.; Wei, S. Micro-alloyed aluminium alloys as anodes for aluminium-air batteries with a neutral electrolyte. Mater. Today Commun. 2024, 39, 108518. [Google Scholar] [CrossRef]
- Ren, J.; Liu, T.; Zhang, J.; Jiang, M.; Dong, Q.; Fu, C. Spray-formed commercial aluminum alloy anodes with suppressed self-corrosion for Al-Air batteries. J. Power Sources 2022, 524, 231082. [Google Scholar] [CrossRef]
- Liu, H.; Wu, M.; Duan, W.; Ma, Y.; Li, C.; Mou, X. Influences of Sodium Polyacrylate/ZnO Composite Corrosion Inhibitor on the Electrochemical Activity and Discharge Performance of 6061 Aluminum Alloy in Alkaline Electrolyte. Mater. Prot. 2023, 56, 96–103. [Google Scholar] [CrossRef]
- Lin, X.Z.; Gong, M. Research progress of environmentally friendly corrosion inhibitors for copper. Corros. Prot. 2009, 30, 328–331+342. [Google Scholar]
- Wei, Y.; Shi, Y.; Chen, Y.; Xiao, C.; Ding, S. Development of solid electrolytes in Zn-air and Al-air batteries: From material selection to performance improvement strategies. J. Mater. Chem. A 2021, 9, 4415–4453. [Google Scholar] [CrossRef]
- Maria, F.G.; Di Palma, T.M. Polymer Electrolytes for Al-Air Batteries: Current State and Future Perspectives. Energy Fuels 2022, 36, 12875–12895. [Google Scholar] [CrossRef]
- Buckingham, R.; Asset, T.; Atanassov, P. Aluminum-air batteries: A review of alloys, electrolytes and design. J. Power Sources 2021, 498, 229762. [Google Scholar] [CrossRef]
- Lu, J.; Jaumaux, P.; Wang, T.; Wang, C.; Wang, G. Recent progress in quasi-solid and solid polymer electrolytes for multivalent metal-ion batteries. J. Mater. Chem. A 2021, 9, 24175–24194. [Google Scholar] [CrossRef]
- Yu, H.; Lv, C.; Yan, C.; Yu, G. Interface Engineering for Aqueous Aluminum Metal Batteries: Current Progresses and Future Prospects. Small Methods 2024, 8, 2300758. [Google Scholar] [CrossRef]
- Wang, H.-F.; Xu, Q. Materials Design for Rechargeable Metal-Air Batteries. Matter 2019, 1, 565–595. [Google Scholar] [CrossRef]
- Yu, H.; Liu, D.; Feng, X.; Zhang, Y. Mini Review: Recent Advances on Flexible Rechargeable Li-Air Batteries. Energy Fuels 2021, 35, 4751–4761. [Google Scholar] [CrossRef]
- Zhang, Z.; Xiong, L.; Wang, S.; Xie, Y.; You, W.; Du, X. A review of the Al-gas batteries and perspectives for a “Real” Al-air battery. J. Power Sources 2023, 580, 233375. [Google Scholar] [CrossRef]
- Verma, C. Chapter5-Classification of corrosion inhibitors. In Handbook of Science & Engineering of Green Corrosion Inhibitors; Verma, C., Ed.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 41–48. [Google Scholar]
- Migliardini, F.; Palma, T.; Gaele, M.; Corbo, P. Solid and acid electrolytes for Al-air batteries based on xanthan-HCl hydrogels. J. Solid State Electrochem. 2018, 22, 2901–2916. [Google Scholar] [CrossRef]
- Cai, S.; Pan, C.; Zhang, D. Effect of multifunctional composite additives on the anodic hydrogen evolution reaction in alkaline Al-air batteries. J. Power Sources 2024, 608, 234610. [Google Scholar] [CrossRef]
- Ren, J.; Fu, C.; Dong, Q.; Jiang, M.; Dong, A.; Zhu, G.; Zhang, J.; Sun, B. Evaluation of Impurities in Aluminum Anodes for Al-Air Batteries. ACS Sustain. Chem. Eng. 2021, 9, 2300–2308. [Google Scholar] [CrossRef]
- Wu, S.; Hu, S.; Zhang, Q.; Sun, D.; Wu, P.; Tang, Y.; Wang, H. Hybrid high-concentration electrolyte significantly strengthens the practicability of alkaline aluminum-air battery. Energy Storage Mater. 2020, 31, 310–317. [Google Scholar] [CrossRef]
- Li, Q.; Bjerrum, N.J. Aluminum as anode for energy storage and conversion: A review. J. Power Sources 2002, 110, 1–10. [Google Scholar] [CrossRef]
- Sun, Z.; Lu, H.; Fan, L.; Hong, Q.; Leng, J.; Chen, C. Performance of Al-Air Batteries Based on Al–Ga, Al–In and Al–Sn Alloy Electrodes. J. Electrochem. Soc. 2015, 162, A2116. [Google Scholar] [CrossRef]
- Li, J.; Dang, J. A Summary of Corrosion Properties of Al-Rich Solid Solution and Secondary Phase Particles in Al Alloys. Metals 2017, 7, 84. [Google Scholar] [CrossRef]
- del Olmo, R.; Mohedano, M.; Matykina, E.; Arrabal, R. Permanganate loaded Ca-Al-LDH coating for active corrosion protection of 2024-T3 alloy. Corros. Sci. 2022, 198, 110144. [Google Scholar] [CrossRef]
- Wang, Z.; Yan, Y.; Wu, Y.; Zhang, Y.; Zhao, X.; Su, Y.; Qiao, L. Recent research progress on the passivation and selective oxidation for the 3d-transition-metal and refractory multi-principal element alloys. npj Mater. Degrad. 2023, 7, 86. [Google Scholar] [CrossRef]
- Peltier, F.; Thierry, D. Review of Cr-Free Coatings for the Corrosion Protection of Aluminum Aerospace Alloys. Coatings 2022, 12, 518. [Google Scholar] [CrossRef]
- Zhao, H.; Yin, Y.; Wu, Y.; Zhang, S.; Mingers, A.M.; Ponge, D.; Gault, B.; Rohwerder, M.; Raabe, D. How solute atoms control aqueous corrosion of Al-alloys. Nat. Commun. 2024, 15, 561. [Google Scholar] [CrossRef]
- Yu, S.; Yang, X.; Liu, Y.; Zhan, F.; Wen, Q.; Li, J.; Li, W. High power density Al-air batteries with commercial three-dimensional aluminum foam anode. Ionics 2020, 26, 5045–5054. [Google Scholar] [CrossRef]
- Qin, J.; Li, L.; Tu, Y.; Cao, F.; Suo, Y.; Wang, X.; Cui, J. Electrochemical behavior and discharge performance of Al-1Zn-0.4Mn-0.1Sn-xBi as anode alloys for Al-air battery in KOH solution. Mater. Chem. Phys. 2025, 331, 130200. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, P.; Xue, J.; Zhu, C.; Li, X.; Wang, Z. High energy efficiency of Al-based anodes for Al-air battery by simultaneous addition of Mn and Sb. Chem. Eng. J. 2021, 417, 128006. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, Y.; Wei, X.; Feng, J.; Xin, Y.; Wang, X.; Wei, Y. High energy efficiency and high discharge voltage of 2N-purity Al-based anodes for Al-air battery by simultaneous addition of Mn, Zn and Ga. J. Power Sources 2023, 563, 232845. [Google Scholar] [CrossRef]
- Ma, K.; Zhang, W.; Liu, Y.; Huang, Y.; Shao, H.; Chen, C. Effect of indium content on the discharge properties of Al-Mg-Ga based anodes in neutral Al-air batteries. J. Power Sources 2025, 636, 236489. [Google Scholar] [CrossRef]
- Huang, Y.; Shi, W.; Guo, L.; Zhang, Q.; Wang, K.; Zheng, X.; Verma, C.; Qiang, Y. Corrosion inhibition of L-tryptophan on Al-5052 anode for Al-air battery with alkaline electrolyte. J. Power Sources 2023, 564, 232866. [Google Scholar] [CrossRef]
- Meng, A.; Sun, Y.; Cheng, W.; Huang, L.; Chen, Y. Discharge performance of Al-0.1Sn-0.1In-0.05Ga alloy for Al–air battery anodes. J. Energy Storage 2024, 81, 110414. [Google Scholar] [CrossRef]
- Zuo, Y.; Yu, Y.; Liu, H.; Zhiqing, G.; Cao, Q.; Zuo, C. Electrospun Al2O3 Film as Inhibiting Corrosion Interlayer of Anode for Solid Aluminum–Air Batteries. Batteries 2020, 6, 19. [Google Scholar] [CrossRef]
- Bautista-Ruiz, J.; Bautista-Ruiz, W.A.; Aperador, W. Anti-corrosive characterization of silicon, titanium, and zirconium oxide coatings deposited on aeronautical aluminum substrates via sol-gel. J. Phys. Conf. Ser. 2020, 1708, 012003. [Google Scholar] [CrossRef]
- Namsheer, K.; Rout, C.S. Conducting polymers: A comprehensive review on recent advances in synthesis, properties and applications. RSC Adv. 2021, 11, 5659–5697. [Google Scholar] [CrossRef]
- Sitanggang, R.; Nur’aini, S.; Susanto, S.; Widiyastuti, W.; Setyawan, H. The Enhancement Discharge Performance by Zinc-Coated Aluminum Anode for Aluminum–Air Battery in Sodium Chloride Solution. Appl. Sci. 2024, 14, 6263. [Google Scholar] [CrossRef]
- Yasakau, K.A.; Zheludkevich, M.L.; Lamaka, S.V.; Ferreira, M.G.S. Mechanism of Corrosion Inhibition of AA2024 by Rare-Earth Compounds. J. Phys. Chem. B 2006, 110, 5515–5528. [Google Scholar] [CrossRef]
- Thuy, T.; Anh Truc, T.; Pham, T.; Nguyen Xuan, H.; Vu, P.; Xuan Hang, T. Synthesis of Lithium-exchange Silica Particles for Corrosion Protection of Aluminum Alloys. J. Surf. Sci. Nanotechnol. 2020, 18, 239–248. [Google Scholar] [CrossRef]
- Lv, C.; Zhang, Y.; Li, Y.; Zhu, Y.; Kuang, J.; Tang, Y.; Wang, H. Regulating solvation and interface chemistry enables advanced aluminum-air batteries. Chem. Commun. 2023, 59, 2588–2591. [Google Scholar] [CrossRef] [PubMed]
- Taeri, O.; Hassanzadeh, A.; Ravari, F. Synergistic Inhibitory Effect of Potassium Sodium Tartrate Tetrahydrate and Sodium Stannate Trihydrate on Self-Corrosion of Aluminum in Alkaline Aluminum-Air Batteries. ChemElectroChem 2020, 7, 2123–2135. [Google Scholar] [CrossRef]
- Luo, L.; Zhu, C.; Yan, L.; Guo, L.; Zhou, Y.; Xiang, B. Synergistic construction of bifunctional interface film on anode via a novel hybrid additive for enhanced alkaline Al-air battery performance. Chem. Eng. J. 2022, 450, 138175. [Google Scholar] [CrossRef]
- Nie, Y.; Gao, J.; Wang, E.; Jiang, L.; An, L.; Wang, X. An effective hybrid organic/inorganic inhibitor for alkaline aluminum-air fuel cells. Electrochim. Acta 2017, 248, 478–485. [Google Scholar] [CrossRef]
- Wang, X.-Y. Study on electrochemical behaviors for aluminum in alkaline solution with two different inhibitors. Chem. Reag. 2010, 32, 639–642. [Google Scholar]
- Hao, C.; Yuan, Z.; Zhifan, H.; Zheng, L.; Tao, W.; Yao, L.; Zhongliang, T.; Ke, P. Effects of corrosion inhibitors on composition and properties of electrolyte after discharging for aluminum-air batteries. J. Cent. South Univ. (Sci. Technol.) 2023, 54, 702–709. [Google Scholar] [CrossRef]
- Wu, S.; Zhang, Q.; Sun, D.; Luan, J.; Shi, H.; Hu, S.; Tang, Y.; Wang, H. Understanding the synergistic effect of alkyl polyglucoside and potassium stannate as advanced hybrid corrosion inhibitor for alkaline aluminum-air battery. Chem. Eng. J. 2020, 383, 123162. [Google Scholar] [CrossRef]
- Ahmed, M.A.; Amin, S.; Mohamed, A.A. Current and emerging trends of inorganic, organic and eco-friendly corrosion inhibitors. RSC Adv. 2024, 14, 31877–31920. [Google Scholar] [CrossRef]
- Shehnazdeep; Pradhan, B. A study on effectiveness of inorganic and organic corrosion inhibitors on rebar corrosion in concrete: A review. Mater. Today Proc. 2022, 65, 1360–1366. [Google Scholar] [CrossRef]
- Zhou, Y.; Lu, X.; Würger, T.; Höche, D.; Zheludkevich, M.L.; Wang, F. New insights into the inhibition mechanism of carboxylate species on magnesium surface. Corros. Sci. 2024, 232, 112009. [Google Scholar] [CrossRef]
- Wysocka, J.; Cieslik, M.; Krakowiak, S.; Ryl, J. Carboxylic acids as efficient corrosion inhibitors of aluminium alloys in alkaline media. Electrochim. Acta 2018, 289, 175–192. [Google Scholar] [CrossRef]
- Gnedenkov, A.S.; Sinebryukhov, S.L.; Nomerovskii, A.D.; Marchenko, V.S.; Ustinov, A.Y.; Gnedenkov, S.V. Carboxylates as green corrosion inhibitors of magnesium alloy for biomedical application. J. Magnes. Alloys 2024, 12, 2909–2936. [Google Scholar] [CrossRef]
- Thacker, H.; Ram, V. Green corrosion inhibitors derived from plant extracts and drugs for mild steel in acid media: A review. Results Surf. Interfaces 2025, 18, 100364. [Google Scholar] [CrossRef]
- Wang, X.; Ren, S.; Jiang, H.; Hou, L.; Zhou, W. Preparation method and research direction of plant corrosion inhibitors. Surf. Technol. 2018, 47, 196. [Google Scholar]
- Bitwell, C.; Indra, S.S.; Luke, C.; Kakoma, M.K. A review of modern and conventional extraction techniques and their applications for extracting phytochemicals from plants. Sci. Afr. 2023, 19, e01585. [Google Scholar] [CrossRef]
- Zhu, J.; Xu, S.; Wu, J.; Yin, Y.; Cheng, S.; Zhang, C.; Qiang, Y.; Wang, W. Probing corrosion protective mechanism of an amide derivative additive on anode for enhanced alkaline Al-air battery performance. J. Power Sources 2024, 593, 233957. [Google Scholar] [CrossRef]
- Choi, S.-R.; Kim, K.-M.; Kim, J.-G. Organic corrosion inhibitor without discharge retardation of aluminum-air batteries. J. Mol. Liq. 2022, 365, 120104. [Google Scholar] [CrossRef]
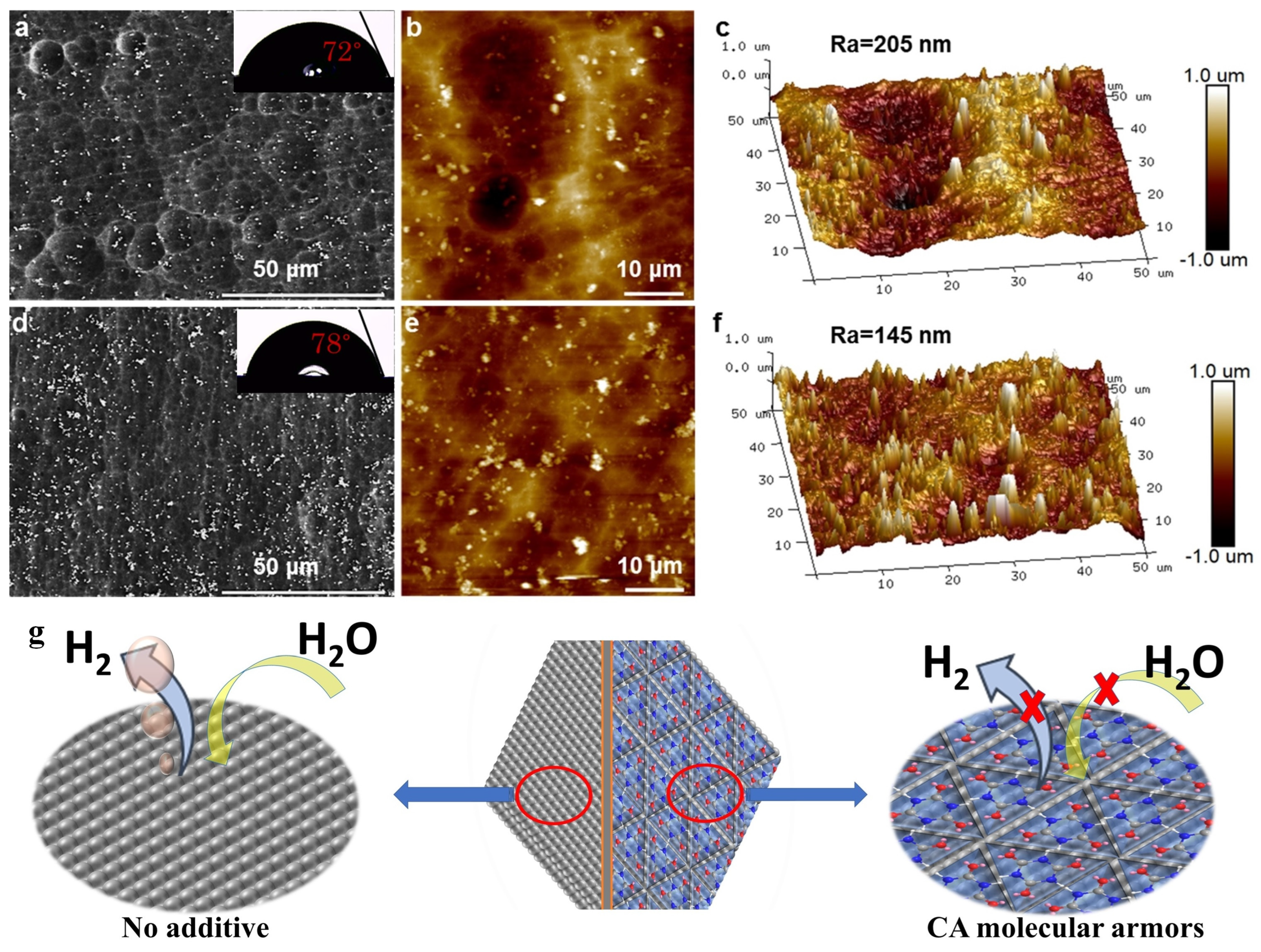
- Liu, J.; Xing, Y.; Ma, B.; Wei, X.; Li, S.; Li, D.; Meng, F.; Deng, W.; Liu, J. Tripod-Linked Molecular Armor for Low-Overpotential Al–Air Batteries. ACS Electrochem. 2025, 1, 774–1002. [Google Scholar] [CrossRef]
- Zhao, Q.; Wu, P.; Sun, D.; Wang, H.; Tang, Y. A dual-electrolyte system for highly efficient Al–air batteries. Chem. Commun. 2022, 58, 3282–3285. [Google Scholar] [CrossRef]
- Liu, C.; Chen, X.; Yang, J. Corrosion inhibitors and its application in CO2 corrosion. Chem. Ind. Eng. Prog. 2021, 40, 6305. [Google Scholar]
- Ma, J.; Tong, S.; Ren, F.; Wang, G.; Li, Y.; Wen, J. Influences of Inhibitor L-Cysteine/zinc Oxide on Electrochemical Performance of 3102 Al-alloy in Alkaline Solution. J. Chin. Soc. Corros. Prot. 2018, 38, 351–357. [Google Scholar]
- Zhang, P.; Peng, W.; Miao, J.; Ren, G.; Wang, Y.; Li, Y.; Zhang, P. Evolution of the solid-liquid interface using a novel hybrid corrosion inhibitor to improve Al-air battery performance. J. Energy Chem. 2025, 104, 69–78. [Google Scholar] [CrossRef]
- Zhu, C.; Yang, H.; Wu, A.; Zhang, D.; Gao, L.; Lin, T. Modified alkaline electrolyte with 8-hydroxyquinoline and ZnO complex additives to improve Al-air battery. J. Power Sources 2019, 432, 55–64. [Google Scholar] [CrossRef]
- Deyab, M.A. Effect of nonionic surfactant as an electrolyte additive on the performance of aluminum-air battery. J. Power Sources 2019, 412, 520–526. [Google Scholar] [CrossRef]
- Moghadam, Z.; Shabani-Nooshabadi, M.; Behpour, M. Electrochemical performance of aluminium alloy in strong alkaline media by urea and thiourea as inhibitor for aluminium-air batteries. J. Mol. Liq. 2017, 242, 971–978. [Google Scholar] [CrossRef]
- Liu, J.; Wang, D.; Zhang, D.; Gao, L.; Lin, T. Synergistic effects of carboxymethyl cellulose and ZnO as alkaline electrolyte additives for aluminium anodes with a view towards Al-air batteries. J. Power Sources 2016, 335, 1–11. [Google Scholar] [CrossRef]
- Li, X.; Li, J.; Zhang, D.; Gao, L.; Qu, J.; Lin, T. Synergistic effect of 8-aminoquinoline and ZnO as hybrid additives in alkaline electrolyte for Al-air battery. J. Mol. Liq. 2021, 322, 114946. [Google Scholar] [CrossRef]
- Chen, K.; Liang, Y.; Pan, D.; Huang, J.; Gao, J.; Lu, Z.; Liu, X.; Chen, J.; Zhang, H.; Hu, X.; et al. Mass Transportation Facilitated Porous Fe/Co Dual-Site Catalytic Cathodes for Ultrahigh-Power-Density Al–Air Fuel Cells. Adv. Energy Mater. 2025, 15, 2404140. [Google Scholar] [CrossRef]
- Ebenso, E.E.; Verma, C.; Olasunkanmi, L.O.; Akpan, E.D.; Verma, D.K.; Lgaz, H.; Guo, L.; Kaya, S.; Quraishi, M.A. Molecular modelling of compounds used for corrosion inhibition studies: A review. Phys. Chem. Chem. Phys. 2021, 23, 19987–20027. [Google Scholar] [CrossRef]
- Xiao, Z.; Chi, Z.; Song, L.; Cao, Z.; Li, A. Progress of the simulation model for power lithium ion battery. Chem. Ind. Eng. Prog. 2019, 38, 3604. [Google Scholar]
- Duda, Y.; Govea-Rueda, R.; Galicia, M.; Beltran, H.I.; Zamudio-Rivera, L.S. Corrosion inhibitors: Design, performance, and computer simulations. J. Phys. Chem. B 2005, 109, 22674–22684. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Dai, J.; Guo, X.; Fu, D.; Ma, L.; Keil, P.; Mol, A.; Zhang, D. Data-driven corrosion inhibition efficiency prediction model incorporating 2D–3D molecular graphs and inhibitor concentration. Corros. Sci. 2023, 222, 111420. [Google Scholar] [CrossRef]
- Liu, Z.; Ran, B.; Pei, H.; Luo, K.; Zhao, Z.; Pan, P.; Qiang, Y. Synergistic corrosion inhibition effect of a compound inhibitor for aluminum. J. Chin. Soc. Corros. Prot. 2023, 44, 312–322. [Google Scholar]
- Winkler, D.A.; Hughes, A.E.; Özkan, C.; Mol, A.; Würger, T.; Feiler, C.; Zhang, D.; Lamaka, S.V. Impact of inhibition mechanisms, automation, and computational models on the discovery of organic corrosion inhibitors. Prog. Mater. Sci. 2025, 149, 101392. [Google Scholar] [CrossRef]
- Xie, J.; Su, Y.; Xue, D.; Jiang, X.; Fu, H.; Huang, H. Machine learning for materials research and development. Acta Metall. Sin. 2021, 57, 1343–1361. [Google Scholar]
- Numin, M.S.; Hassan, A.; Jumbri, K.; Eng, K.K.; Borhan, N.; Daud, N.M.R.N.M.; M Nor A, A.; Suhor, F.; Abdul Wahab, R. A recent review on theoretical studies of Gemini surfactant corrosion inhibitors. J. Mol. Liq. 2022, 368, 120649. [Google Scholar] [CrossRef]
- El-Alouani, M.; Kharbouch, O.; Dahmani, K.; Galai, M.; Dkhireche, N.; Benzekri, Z.; Boukhris, S.; Ebn Touhami, M.; Almeer, R.; Chafiq, M.; et al. Probing corrosion protective mechanism of 2-(4-nitrobenzylidene) malononitrile on anode for enhanced alkaline (4 M NaOH) Aluminum-air battery performance. J. Alloys Compd. 2025, 1010, 178239. [Google Scholar] [CrossRef]
- El-Alouani, M.; Kharbouch, O.; Dahmani, K.; Errahmany, N.; Saber, I.; Galai, M.; Benzekri, Z.; Boukhris, S.; Ebn Touhami, M.; Al-Sadoon, M.K.; et al. Enhancing Al–Air Battery Performance with Beta-d-Glucose and Adonite Additives: A Combined Electrochemical and Theoretical Study. Langmuir 2025, 41, 431–449. [Google Scholar] [CrossRef]
- Li, X.; Zhong, S.; Wei, X.; Liu, Z.; Zhan, P.; Liu, J. Carbon Dioxide: From the Past to the Future. Univ. Chem. 2025, 40, 2503013. [Google Scholar] [CrossRef]
| Anode | Anode Efficiency @Current Density (mA cm−2) | Discharge Capacity (mAh g−1) | Electrolyte | Ref. |
|---|---|---|---|---|
| 3D Al Foam | 62.5%@100 | 1983 | 4 M NaOH | [61] |
| 4N6 Al alloy | 57.9%@100 | 1870 | 4 M KOH | [62] |
| Al-0.1In | 84%@40 | 2506 | 1 M KOH | [55] |
| Al-0.1Sn | 90%@40 | 2694 | 1 M KOH | [55] |
| Al-0.05Mn | 87.4%@80 | 2605 | 4 M KOH | [63] |
| 2N Al-0.1Ga-1Zn-0.5Mn | 73%@80 | ~1800 | 4 M NaOH | [64] |
| Al-Mg-In-Ga | 83.9%@20 | 2500 | 2 M NaCl | [65] |
| Al-1Zn-0.4Mn-0.1Sn-xBi | 89.2%@90 | 2656 | 4 M KOH | [62] |
| 5052 alloy (Al-Mg alloy) | 33.2%@20 | 990 | 4 M NaOH | [66] |
| Al-0.1Sn-0.1In-0.05Ga | 74.3%@50 | 2090 | 4 M NaOH | [67] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Liu, Z.; Wei, X.; Wu, H.; Mei, H.; Liu, J. Advanced Strategies for Suppressing the Self-Corrosion of the Anode in Al–Air Batteries. Metals 2025, 15, 760. https://doi.org/10.3390/met15070760
Li S, Liu Z, Wei X, Wu H, Mei H, Liu J. Advanced Strategies for Suppressing the Self-Corrosion of the Anode in Al–Air Batteries. Metals. 2025; 15(7):760. https://doi.org/10.3390/met15070760
Chicago/Turabian StyleLi, Shenjia, Zhiqiang Liu, Xiangfeng Wei, Hao Wu, Haoyu Mei, and Jiehua Liu. 2025. "Advanced Strategies for Suppressing the Self-Corrosion of the Anode in Al–Air Batteries" Metals 15, no. 7: 760. https://doi.org/10.3390/met15070760
APA StyleLi, S., Liu, Z., Wei, X., Wu, H., Mei, H., & Liu, J. (2025). Advanced Strategies for Suppressing the Self-Corrosion of the Anode in Al–Air Batteries. Metals, 15(7), 760. https://doi.org/10.3390/met15070760







