Abstract
Aluminum–air batteries are highly promising energy storage systems due to their high theoretical energy density, environmental friendliness, and cost-effectiveness. However, the self-corrosion of aluminum anodes in alkaline electrolytes remains a critical issue that significantly limits their practical application and commercialization. This review paper comprehensively examined various advanced strategies aimed at suppressing the self-corrosion of anodes in Al–air batteries. We summarized the fundamental principles of these approaches, their advantages and disadvantages, and provided an in-depth analysis of their effectiveness, supported by experimental and theoretical evidence. Specifically, this review systematically analyzes six major strategies for suppressing anode self-corrosion: anode alloying, electrolyte additives, novel electrolytes, anode surface treatment, battery structural design, and computer-aided investigation. Furthermore, we proposed the challenges and future research directions in this field. Overall, this review aimed to offer valuable insights and guidance for the development of high-performance, long-lasting Al–air batteries.
1. Introduction
Metal–air batteries (lithium, aluminum, zinc, magnesium, etc.) have garnered extensive attention due to their advantages, such as high energy density and low cost [1,2,3,4,5,6,7,8]. They are regarded as candidates for the most promising safe, green, and clean energy sources [9,10,11,12,13,14,15,16]. Al–air batteries exhibit distinct advantages, including the highest capacity density and energy density of 2.98 Ah g−1 and 8.1 Wh g−1 among aqueous metal–air batteries, demonstrating superior competitiveness compared with other metal–air batteries, as shown in Figure 1a [17]. Mostly, Al–air cells consist of an aluminum anode, a porous air cathode, and an alkaline electrolyte [18,19]. During operation, the aluminum anode undergoes oxidation to release electrons, while oxygen reduction occurs at the cathode, generating electrical energy. Researchers have been focusing on such high-energy–density systems since Zaromb proposed the concept of an Al–air battery in 1962 [20]. The conventional Al–air battery configuration comprises an aluminum anode, an air cathode, and compatible electrolytes (neutral saline or alkaline solutions) [21]. During discharge, the aluminum anode supplies electrons through oxidation reactions in the electrolyte, while the air cathode undergoes reduction reactions, efficiently converting aluminum’s chemical energy into electrical power [22]. Al–air batteries demonstrate unique advantages across critical applications: (1) marine power systems (submarines, AUVs) utilize seawater electrolytes for exceptional energy density and eco-friendly operation without toxic emissions [23]; (2) electric vehicles (including cold chain logistics) leverage lightweight design and high specific energy to extend driving range while reducing operational energy consumption [24]; (3) backup power sources (substations, base stations) provide compact, long-life solutions with stable performance under high-load conditions [25]. These diverse applications highlight their market potential in advancing clean energy transitions [26,27], but anode self-corrosion remains the primary barrier to commercialization.
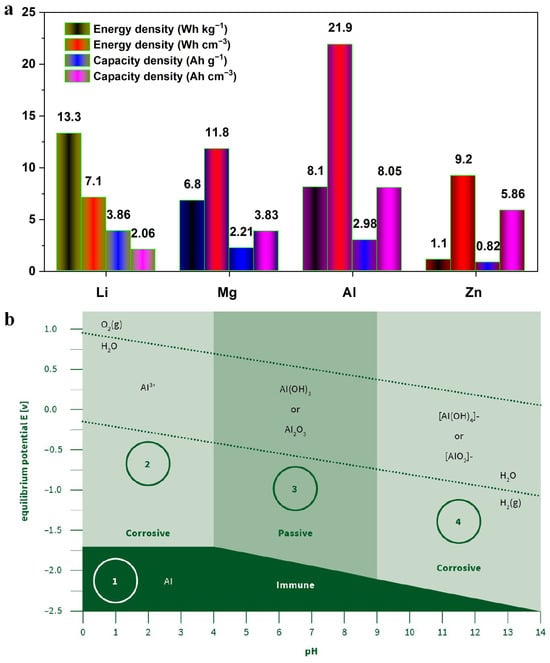
Figure 1.
(a) Specific capacity and energy density of metal anodes (Adapted from Ref. [17]); (b) Pourbaix diagram of aluminum (Al) showing four different regions as Region 1 indicating immunity, Region 2 indicating acidic corrosion, Region 3 indicating passivation, and Region 4 indicating alkaline corrosion. (Adapted from Ref. [28]).
Equilibrium potential E–pH diagram of Al was provided in Figure 1b [28]. During battery operation in neutral saline solutions, the underlying electrochemical reaction mechanisms [29] are governed by Equations (1)–(3):
The overall reaction of Al–air batteries in neutral saline solutions
The reactions in alkaline electrolytes are governed by Equations (4)–(7) [29]:
Overall cell reaction
Self-corrosion reactions exist in both of the above solutions:
The Al–air battery using aqueous acidic electrolytes are described by the following Equations (8)–(11) [30]:
Overall cell reaction
Self-corrosion reactions exist in both of the above solutions:
In neutral electrolytes, aluminum corrosion predominantly manifests as localized pitting corrosion, driven by chloride ion (Cl−)-induced depassivation of the native oxide film (Al2O3). While the oxide layer retains partial stability, its breakdown at structural defects (e.g., grain boundaries or vacancies) initiates metastable pitting, subsequently transitioning to stable pit growth. This process generates insulating passivation layers which progressively impede ionic diffusion and electron transfer at the electrode–electrolyte interface [31]. Although the corrosion rate of the aluminum anode in neutral electrolyte is significantly reduced, the formation of attached passivation film on the aluminum surface reduces the anode activity and reduces the possibility of high-power operation. On the contrary, the polarization caused by the surface passivation layer in the alkaline electrolyte is significantly reduced, resulting in excellent electrochemical performance.
Conversely, in alkaline electrolytes (pH > 12), aluminum undergoes uniform electrochemical dissolution with negligible oxide film stability. The anodic is kinetically coupled with a severe parasitic hydrogen evolution reaction (HER), which operates concurrently at cathodic sites on the anode surface. This HER-driven self-corrosion accelerates mass loss, reducing anode utilization efficiency to <50% and generating gaseous hydrogen that further destabilizes the interface [32,33]. Notably, the transition between localized pitting (pH 4–8) and uniform dissolution (pH > 12) occurs within a critical pH window (pH 8–12), wherein mixed corrosion modes coexist. In this regime, competitive adsorption of OH− and Cl− at the oxide–electrolyte interface leads to stochastic fluctuations in corrosion current density, with both metastable pitting and HER contributing to cumulative anode degradation.
However, the aluminum anode exhibits rapid self-corrosion in the alkaline electrolyte, accompanied by an intense hydrogen evolution reaction [34]. The self-corrosion of aluminum electrodes seriously damages the utilization efficiency of the anode [31]. The generated hydrogen poses inherent flammability and explosion hazards, with its explosive range in air spanning 4–75% by volume [35]. This corrosion-induced capacity loss and hydrogen evolution hazard directly compromise the safety and longevity of Al–air batteries in critical applications such as submarine power systems, electric vehicles, and backup power stations. Thus, suppressing anode self-corrosion is imperative for commercialization. To address these challenges, researchers have developed mitigation strategies including alloying aluminum anodes [36,37,38], electrolyte additives [39,40], novel electrolytes [41,42,43,44], anode surface treatment [45,46], battery structural design [46,47,48], and comprehensive application of multiple strategies [49]. Beyond neutral and alkaline systems, acidic gel polymer electrolytes (GPEs) represent an emerging alternative to mitigate anode corrosion challenges. Notably, recent work has demonstrated the feasibility of solid, strongly acidic electrolytes for Al–air batteries using concentrated HCl hydrogels [30,50]. As emerging materials and advanced technologies continue to evolve alongside increasingly stringent environmental regulations, this field will present unprecedented opportunities for suppressing the self-corrosion of the anode in Al–air batteries.
2. Alloying Strategy and Anodic Coatings
From a thermodynamic perspective, an aluminum anode ought to demonstrate a potential of −1.47 V in saline electrolytes and −2.35 V in alkaline electrolytes. However, in practice, aluminum electrodes function at a markedly lower potential [51,52,53]. The reasons are as follows: (1) aluminum is typically coated with an oxide film. This film induces a time delay in achieving a steady-state voltage, attributed to internal resistance. (2) Aluminum experiences a parasitic corrosion reaction. This leads to a metal utilization rate of less than 100% and results in the evolution of hydrogen [54]. The intrinsic corrosion characteristic of the aluminum anode substantially degrades the performance of aluminum–air batteries, thereby significantly reducing their discharge efficiency. Aluminum alloying involves adding specific elements to aluminum to modify its surface properties and electrochemical behavior, thereby suppressing self-corrosion. Common alloying elements include gallium (Ga), tin (Sn), indium (In), and zinc (Zn) [55]. For instance, adding Ga to aluminum can disrupt the oxide film on the aluminum surface, reduce the formation of anodic polarization, and inhibit self-corrosion. Studies have shown that an Al-Ga alloy exhibited significantly lower self-corrosion rates compared to pure aluminum anodes. Similarly, Sn and In can also enhance the corrosion resistance of aluminum anodes. Alloying elements can alter the electronic structure of aluminum, reduce its intrinsic activity, and form more stable oxide or hydroxide layers on the surface, acting as barriers to prevent direct contact between aluminum and the electrolyte, thus suppressing self-corrosion.
This alloying methodology effectively enhances electrochemical efficiency by tailoring the electronic structure and corrosion resistance through controlled composition design [36,37,38]. The corrosion resistance of aluminum alloys is significantly affected by particular alloying elements, each demonstrating unique mechanisms and compromises. Magnesium (Mg) increases the density of passive films. However, its content must be restricted to less than 3 wt% to prevent the formation of the detrimental β-phase (Al3Mg2), which can initiate intergranular corrosion [56]. Manganese (Mn) forms protective Mn-rich oxide layers (e.g., MnAl6) and mitigates the cathodic effect of iron impurities by forming (Fe,Mn)Al6 phases [57]. Chromium (Cr) stabilizes the passive film through the incorporation of Cr2O3. Nevertheless, due to its environmental toxicity, research is being carried out to find Cr-free substitutes [58,59]. Copper (Cu) raises the corrosion potential when in solid solution. However, it promotes severe pitting corrosion through the cathodic Al2Cu precipitates [56]. Strategic combinations like AlMgSi or AlZnMgCu alloys offer synergistic advantages, while unfavorable interactions should be avoided [56,60]. To achieve optimal corrosion performance, precise compositional control, micro-alloying for grain refinement, and environment-specific selections are essential to balance electrochemical protection and mechanical properties. The effects of the doping of different chemical elements on the self-corrosion of aluminum anodes are shown in Table 1.

Table 1.
The self-corrosion performance of aluminum and Al alloy in Al–air batteries.
However, alloying may compromise the ductility and processability of aluminum and increase material costs. Therefore, optimizing alloy compositions and preparation processes to achieve a balance between corrosion resistance and mechanical properties is a key challenge in anode alloying research.
The aluminum anodic coating strategy is indeed fascinating. Recent efforts to mitigate aluminum anode self-corrosion mainly concentrate on engineered surface coatings, which serve as physical and electrochemical barriers against parasitic hydrogen evolution reactions and oxide passivation. Metal oxide coatings, such as electrospun Al2O3 films, can form dense ceramic interlayers that effectively prevent electrolyte penetration. Research has shown that a 6 μm Al2O3 coating can increase the interfacial resistance to 1480 Ω·cm2, compared to 38.6 Ω·cm2 for bare aluminum, while reducing the double-layer capacitance by over 60%, indicating that charge transfer at the electrode–electrolyte interface has been suppressed [68]. Similarly, sol–gel-derived SiO2-TiO2-ZrO2 composite coatings create multilayer structures. The TiO2/ZrO2 underlayer improves adhesion, and the SiO2 top layer offers hydrophobicity. In chloride-containing electrolytes, this reduces the corrosion current density by two to three orders of magnitude compared to uncoated anodes [69]. Polymer-based coatings take advantage of their adjustable conductivity and barrier properties. Conductive polymers like polyaniline (PANI) form charge-transfer complexes that stabilize the oxide layer. Hydrophobic polymers (e.g., fluorinated acrylics) physically isolate the anode from aqueous electrolytes [70]. Composite coatings combine these approaches. For example, zinc electrodeposition on Al alloys (e.g., Al6061) followed by hydrophobic polymer sealing achieves a specific capacity of 414.6 mAh·g−1 and 207 h stability in 3.5% NaCl by integrating galvanic activation with corrosion suppression [71]. Rare-earth-enhanced coatings (e.g., CeO2/La2O3) precipitate hydroxide deposits at cathodic intermetallic sites, locally neutralizing pH spikes that cause pitting corrosion [72]. Novel lithium-incorporated silica coatings further showcase ion-exchange capabilities. The immobilized Li+ ions selectively repel corrosive anions while allowing OH− transport necessary for battery operation [73].
Overall, these diverse coating strategies, ranging from metal oxides and polymers to composites and rare-earth elements, offer multifunctional protection through physical barriers, electrochemical stabilization, and selective ion regulation. Crucially, synergistic effects in bilayer and composite architectures have proven to be the most effective in achieving corrosion suppression and the electrochemical functionality needed for durable aluminum batteries.
3. Corrosion Inhibitor Strategies in Single Electrolyte
Adding specific chemicals to the electrolyte is an effective method to suppress anode self-corrosion. These additives can adsorb onto the aluminum anode surface, forming a protective layer that inhibits corrosion reactions or reduces the activity of free water molecules in the electrolyte, thereby slowing down self-corrosion. The following are some common types of electrolyte additives, such as inorganic salts, organic solvents, organic inhibitors, and their hybrids.
3.1. Inorganic Corrosion Inhibitors
Inorganic corrosion inhibitors primarily consist of metal salts, oxides, and hydroxides, including stannates, chromates, nitrites, silicates, molybdates, tungstates, polyphosphates, and zinc salts. Stannates serve as critical additives in alkaline electrolytes for Al–air batteries. In alkaline environments, SnO32− ions undergo electrochemical reduction to metallic tin (Sn), forming a porous deposited layer on the aluminum electrode surface. This metallic tin deposit exhibits a high hydrogen evolution overpotential, thereby elevating the energy barrier for the hydrogen evolution reaction and significantly suppressing the hydrogen evolution corrosion process on aluminum electrodes [74]. Some Researchers indicate that the addition of Na2SnO3 to KOH can reduce the self-corrosion of PAS (commercial pure aluminum sheet). The following reaction (12) may occur in an SST-containing solution [75].
However, despite the demonstrated efficacy of stannates as corrosion inhibitors, their practical implementation faces multifaceted challenges. A critical limitation lies in the compromised stability of stannate-derived protective films, where heterogeneous deposition on metal surfaces creates localized micro-galvanic cells, leading to substantial variations in localized protection efficiency and ultimately undermining the uniformity and reliability of corrosion protection [76]. Furthermore, environmental and health considerations cannot be overlooked. Under specific operating conditions, stannates may undergo chemical transformations, yielding hazardous byproducts, which pose risks to ecological integrity and human health. Moreover, although inorganic stannate-based corrosion inhibitors can mitigate aluminum corrosion to some extent, their inhibition efficiency remains insufficient to fully meet the operational demands of Al–air batteries under extreme conditions, particularly in scenarios involving high humidity, elevated temperatures, or strongly alkaline environments [77].
Other metallic species or their ionic counterparts, such as zinc oxide (ZnO) and related inorganic compounds, have demonstrated considerable promise in mitigating aluminum corrosion by effectively suppressing corrosion progression under specific environmental conditions. Wang Xiaoyan employed a gasometric method to measure the corrosion rate of pure aluminum in 4 mol L−1 KOH solutions containing varying ZnO concentrations. The study revealed a striking reduction in corrosion current density by one to two orders of magnitude with ZnO addition. At a moderate ZnO concentration of 0.2 mol L−1, a peak inhibition efficiency of 97.5% was achieved through the formation of a zincate-stabilized passivation layer [78]. In the context of Al–air battery applications, while zinc oxide demonstrates certain corrosion inhibition efficacy, a critical limitation lies in the porous and structurally compromised protective layers formed on aluminum electrodes. These layers exhibit adhesion deficiencies and are prone to delamination, resulting in transient protection that fails to ensure long-term corrosion suppression. Furthermore, under harsh operating conditions, particularly at high current densities or elevated temperatures, the inhibition efficiency of ZnO typically declines to below 70%, falling short of industrial application requirements. Furthermore, ZnO may undergo complex phase transformations under specific electrochemical environments, yielding deleterious byproducts such as zinc hydroxide hydrate or zincate complexes. These byproducts not only compromise corrosion inhibition efficacy but also induce pore occlusion within electrode architectures, severely impeding electrolyte ion transport kinetics. This dual detrimental effect ultimately diminishes both the power output and energy density of the battery system [79,80].
The aforementioned analysis demonstrates that while the utilization of inorganic corrosion inhibitors in Al–air batteries exhibits appreciable efficacy in corrosion suppression, critical limitations persist, particularly regarding the structural instability of protective films and compromised electrochemical performance due to interfacial polarization losses. These inherent constraints necessitate the development of optimized inhibitor formulations through molecular engineering and interfacial design strategies to achieve synergistic improvements in film adhesion strength, charge transfer kinetics, and long-term durability.
3.2. Organic Corrosion Inhibitors
Organic corrosion inhibitors primarily consist of organic acids, amines, thiols, and heterocyclic compounds through adsorption onto Al surfaces, modulating interfacial charge distribution, or forming hydrophobic barrier films to impede corrosion initiation. Furthermore, certain organic inhibitors chemically interact with aggressive ions in corrosive media to yield insoluble complexes, thereby suppressing charge transfer and mass transport processes essential for corrosion propagation [81,82].
Carboxylate-based corrosion inhibitors have garnered significant attention due to their environmental benignity and superior inhibition efficacy. Carboxylate-based corrosion inhibitors encompass a diverse array of chemical species, including straight-chain carboxylic acids, polycyclic aromatic hydrocarbon carboxylates, and metal carboxylate soaps [83]. These compounds form protective films through chemical bonding or physical adsorption at metal–electrolyte interfaces, effectively suppressing corrosion rates by 70–90% as quantified via electrochemical impedance spectroscopy (EIS) and polarization resistance measurements [84,85].
Furthermore, researchers have reported the utilization of organic corrosion inhibitors derived from natural products, which exhibit exceptional corrosion inhibition performance while maintaining environmental sustainability [86]. Representative natural inhibitors include amino acids (e.g., histidine and lysine) and plant-extracted polyphenols (e.g., catechin and tannic acid). Their functional groups (–NH2, –COOH, phenolic –OH) adsorb onto Al surfaces via coordination bonds with Al3+, forming hydrophobic films that block active sites for hydrogen evolution [33]. These techniques facilitate the selective extraction of effective corrosion-inhibiting phytochemicals from plant matrices through optimized process parameters, enabling targeted isolation of bioactive constituents [87,88].
Polymeric corrosion inhibitors have emerged as a central research thrust in recent years. Leveraging their high-molecular weight characteristics, these inhibitors form compact mono- or multi-layer protective films on substrate surfaces. Compared to low-molecular-weight counterparts, polymeric systems demonstrate superior efficiency, enhanced durability, and improved environmental compatibility, solidifying their position as a pivotal innovation frontier in corrosion mitigation science. In an investigation, the corrosion inhibition performance of polyelectrolyte poly(diallyldimethylammonium chloride) (PDDA) as an electrolyte additive for alkaline Al–air batteries has been systematically investigated, with comparative evaluation against its monomeric counterpart dimethyldiallylammonium chloride (DMDAAC). Experimental results demonstrate that the polymeric additive exhibits superior corrosion inhibition efficacy compared to the monomer in suppressing the degradation processes of aluminum electrodes. This mechanistic understanding bridges macroscopic electrochemical behavior with molecular-level insights into adsorption energetics and orbital interactions [89]. Also, PVA plays the same role in 4 M NaOH electrolytes [90].
Very recently, the innovative concept of using ultrashort tripod-linked cyanuric acid (CA) as a molecular armor has been proposed to inhibit the self-corrosion of aluminum anodes [91]. This strategy leverages the unique planar triacid structure of cyanuric acid, which effectively adsorbs onto the aluminum surface in a manner resembling armor. This forms a robust protective layer that significantly mitigates the self-corrosion of the aluminum anode in Figure 2. The closed face-to-face structure of cyanuric acid in contact with aluminum, characterized by a minimized interfacial distance, offers several advantages: it reduces the overpotential by maintaining efficient ion pathways, minimizes water contact with the aluminum surface, facilitates the detachment of hydrogen bubbles, and reduces bubble retention. Electrochemical characterization confirmed that this approach enabled the aluminum anode to achieve a high utilization efficiency of 89.9%, delivering a capacity of 2680 mAh g−1 at a current density of 50 mA cm−2.
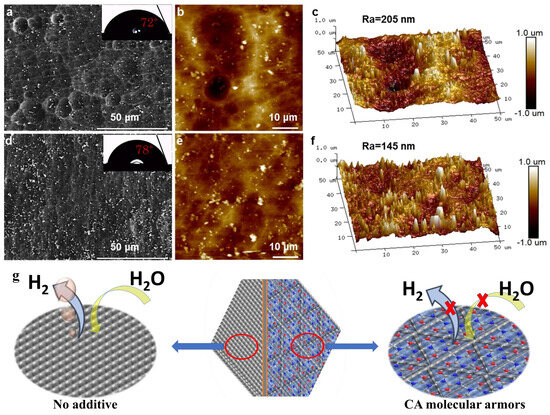
Figure 2.
(a) FESEM image and contact angle (inset) and (b) 2D and (c) 3D AFM topography of Al anode immersed in 4 M KOH solution. (d) FESEM image and contact angle (inset) and (e) 2D and (f) 3D AFM topography of Al anode immersed in 4 M KOH solution with CA additive. (g) Proposed corrosion mechanism of the Al anodes with/without the CA in 4 M KOH. Adapt from Ref. [91].
However, single-component organic corrosion inhibitors in Al–air batteries often fail to effectively suppress both anode corrosion and the hydrogen evolution reaction (HER), as demonstrated by mechanistic studies [92]. Furthermore, the environmental compatibility of organic corrosion inhibitors requires critical evaluation as certain compounds may contain ecotoxic constituents posing potential risks to ecosystems. Practical implementation faces additional challenges in precise dosage control and environmental adaptability. To enhance the performance and safety of Al–air batteries, current research focuses on developing advanced inhibitor systems, including organic–inorganic hybrid inhibitors, alongside innovations in novel battery configurations and materials engineering.
3.3. Hybrid Corrosion Inhibitors
Hybrid corrosion inhibitors are rationally designed systems comprising optimally proportioned inorganic and organic components, synergistically combining the merits of both constituents to enhance corrosion mitigation efficacy. These advanced formulations operate through dual protection mechanisms: establishing stable passivation films on metal surfaces while concurrently mitigating corrosion progression via concerted adsorption and interfacial chemical reaction pathways [93]. In Table 2, the additives demonstrate promising potential to emerge as dominant candidates in next-generation corrosion inhibition systems for Al–air batteries, given their demonstrated technical superiority in balancing electrochemical activity modulation with long-term stability requirements.
Liu et al. demonstrated that the PAAS/ZnO hybrid inhibitor system significantly enhances corrosion inhibition performance for 6061 aluminum alloy in 4 mol/L NaOH electrolyte compared to single-component counterparts. Mechanistic analysis reveals that this hybrid system optimizes anodic activation kinetics while constructing more stable protective films through synergistic zincate–aluminate coordination, effectively suppressing hydrogen evolution-induced self-corrosion. The formulation elevates the average discharge voltage to −1.681 V and yields a 13.5% increase in anode utilization efficiency, corresponding to a 28% enhancement in specific capacity, thereby significantly improving Al–air battery discharge performance [39]. Furthermore, Ma et al. investigated the corrosion behavior and discharge performance of 3102 Al alloy in 4 M NaOH electrolyte using L-cysteine/ZnO hybrid inhibitors. The composite system effectively reduced the alloy’s self-corrosion rate by 58% compared to the blank electrolyte. Electrochemical mechanistic studies revealed distinct dissolution pathways: dissolution kinetics transitioned from charge transfer-controlled regimes in pure NaOH and NaOH/L-cysteine systems to diffusion-limited processes mediated by zincate/corrosion product layers when ZnO was introduced [94]. Potassium stannate, decyl glucoside, and 1,10-decanedithiol were used to regulate solid–liquid interface reactions in an alkaline Al–air battery. The optimal hybrid corrosion inhibitor could reduce the hydrogen evolution rate from 0.2095 to 0.0406 mL cm−2 min−1, achieving an inhibition efficiency of 80.62%. The surface analysis discussed in detail the evolution process of the solid–liquid interface after the introduction of the hybrid corrosion inhibitor into the battery. Experiments and theoretical calculations revealed that decyl glucoside enhanced the adsorption and coverage efficiency of the hybrid corrosion inhibitor through the “micelle solubilization” effect and optimized the structure and properties of the solid–liquid interface. This study also contributed valuable insights into the corrosion inhibition mechanism at the solid–liquid interface of alkaline Al–air batteries (Figure 3) [95].
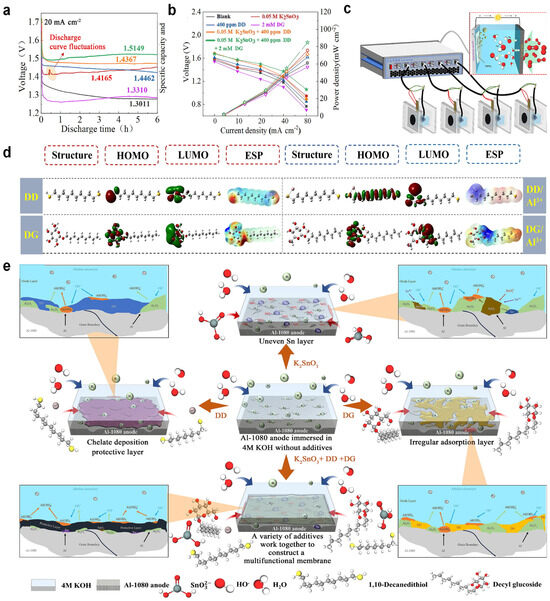
Figure 3.
(a) Discharge parameters of the Al-1080 anode in 4 M KOH with and without additives of continuous discharging for 6 h; (b) summary of energy density; (c) operating principle, discharge setup, and battery model of the Al–air battery system; (d) schematic diagram of the corrosion inhibition mechanism of the Al-1080 anode in 4 M KOH with and without additive; (e) optimized structures of LUMO (Lowest Unoccupied Molecular Orbital), HOMO (Highest Occupied Molecular Orbital), and ESP(electrostatic potential) for DD, DG, DD/Al3+, and DG/Al3+. Reprinted with permission from ref. [95], 2025, Elsevier.

Table 2.
The inhibition efficiency of different additives on the self-corrosion of Al–air.
Table 2.
The inhibition efficiency of different additives on the self-corrosion of Al–air.
| Additives | Anode | Inhibition Efficiency | Anode Utilization | Ref. |
|---|---|---|---|---|
| K2SnO3 | Al-Mg-Ga-Sn-Zn-Fe-Cu-Si alloy | 86.86% | 63.78% | [80] |
| ZnO | AA5052 alloy | 44.12% | 66.5% | [96] |
| Nonoxynol-9 | 99.99% pure Al | 85.6% | 58.8% | [97] |
| Thiourea | Al-0.01Mg-0.002In-0.001Mn | 57% | 86.1% | [98] |
| Urea | Al-0.01Mg-0.002In-0.001Mn | 51.5% | 85.4% | [98] |
| K2SnO3 + APG | Al-Mg-Ga-Sn-Zn-Fe-Cu-Si alloy | 94.14% | 73.87% | [80] |
| ZnO + CMC | AA5052 alloy | 70.32% | 91.4% | [99] |
| ZnO + AQ | AA5052 alloy | 71.59% | 70.3% | [100] |
4. Corrosion Inhibitor Strategies in Double Electrolytes
Double electrolyte-based rational battery structural design and optimization of operational conditions can also effectively suppress anode self-corrosion. A suitable separator can prevent direct contact between the anode and cathode with different electrolytes while controlling ion transport. Using a separator with appropriate porosity and hydrophilicity can reduce the contact area between the electrolyte and the aluminum anode, thereby decreasing the self-corrosion rate. Additionally, functional separators between double electrolytes with corrosion inhibition properties can further enhance the suppression of self-corrosion.
In Figure 4, the high-voltage Al–air batteries were reported with a NaOH/CEM/H2SO4 double-electrolyte structure [101]. This approach enables the efficient evaluation of a vast number of diverse diatomic combinations, ultimately identifying Fe–Co dual-atomic sites as optimal ORR electrocatalysts. The enhanced electrocatalytic performance of Fe/Co diatomic sites supported on three-dimensional interconnected ordered macroporous carbon (FeCo-3DMNC) has been experimentally confirmed. Additionally, innovative hybrid acid/alkali aluminum–air fuel cells (hA/A-AAFCs), utilizing FeCo-3DMNC as the cathode catalyst, demonstrate a remarkably high open-circuit voltage of 2.72 V and an unprecedented power density of 827 mW cm−2. These performance metrics significantly surpass those of conventional alkaline Al–air fuel cells. This research represents a substantial advancement by combining state-of-the-art computational screening with rigorous experimental validation to develop highly efficient electrocatalysts, potentially paving the way for advanced energy storage and conversion technologies.
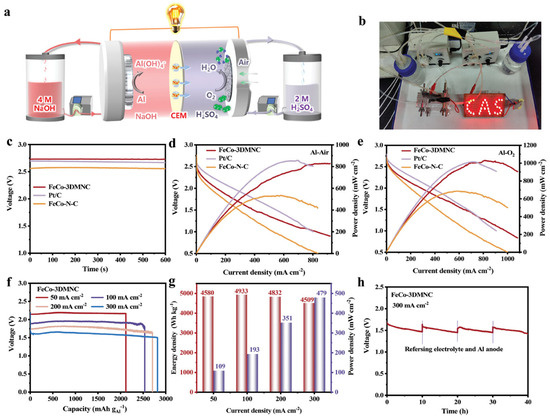
Figure 4.
Electrochemical performance of hybrid acid/alkali Al–air fuel cells. (a) Schematic illustration of the hA/A-AAFCs. (b) Actual photo of an hA/A-AAFC device using the target catalyst as the air cathode to light up the LED array. (c) Open-circuit voltage of the hA/A-AAFCs based on FeCo-3DMNC, Pt/C, and FeCo-N-C. (d) Polarization curves and corresponding power density plots of the hA/A-AAFCs based on FeCo-3DMNC, Pt/C, and FeCo-N-C. (e) Polarization curves and corresponding power density plots of the hA/A-AAFCs in O2 based on FeCo-3DMNC, Pt/C, and FeCo-N-C. (f) Plot of voltage versus specific capacity for the FeCo-3DMNC-based hA/A-AAFCs. (g) Energy density and power density of the FeCo-3DMNC-based hA/A-AAFCs at various current densities. (h) Long-term discharge curves at 300 mA cm−2 of the hA/A-AAFCs based on FeCo-3DMNC. Reprinted with permission from ref. [101] 2025, John Wiley and Sons.
5. Computer-Aided Investigation for Al–Air Batteries
The utilization of computational software for molecular modeling of organic compounds has become a highly effective method for theoretically assessing the corrosion inhibition potential of these compounds. In theoretical studies of corrosion inhibition, several commonly employed techniques include density functional theory (DFT), molecular dynamics (MD), and Monte Carlo (MC) simulations, as well as artificial neural networks (ANN) and quantitative structure–activity relationship (QSAR) modeling. Computational modeling enables the elucidation of the chemical reactivity and corrosion inhibition mechanisms of organic compounds [102]. This approach is particularly advantageous as it represents a time-efficient and environmentally friendly method for preliminary screening of organic compounds with potential corrosion inhibition capabilities. Such screening can be conducted before undertaking wet laboratory synthesis, thereby reducing resource expenditure. Additionally, computational modeling facilitates the identification of molecular sites responsible for interactions with metallic surfaces (i.e., active or adsorption sites) and enables the prediction of the orientation of organic compounds on these surfaces. Through the use of various theoretical descriptors and parameters, the effectiveness of corrosion inhibition and the nature of the metal–inhibitor interactions can also be accurately predicted. This section compiles significant advancements in the field of computational modeling, specifically in the design and evaluation of the corrosion inhibition efficacy of organic corrosion inhibitors [102,103,104,105].
In the study of corrosion inhibitors for Al–air batteries, computational simulation technologies play a pivotal role. Through molecular simulations and quantum chemical calculations, researchers achieve an atomic-level understanding of the interactions between inhibitor molecules and aluminum surfaces, thereby elucidating the fundamental corrosion inhibition mechanisms [106]. The increased capabilities of machine learning (ML) methods and a better understanding of mechanisms of inhibition provide the potential to discover new corrosion inhibitors faster and cheaper than ever before [107]. Machine learning methods have also been applied to investigate structure–property relationships of aluminum alloy corrosion inhibitors. By developing predictive models, the potential efficacy of inhibitors can be evaluated before experimental validation, significantly accelerating the screening process for novel inhibitors [108]. Furthermore, computational simulation enables parameter sensitivity analysis by adjusting model parameters to investigate the sensitivity of battery performance, thereby identifying critical factors influencing battery characteristics. This methodology guides experimental design, reduces experimental iterations, and accelerates research progress.
Computational simulation technologies have not only enhanced research efficiency and accuracy but also provided theoretical guidance for the design and development of corrosion inhibitors. These advancements have facilitated the commercialization of Al–air battery technology. Computer-aided design plays a crucial role in the development of new corrosion inhibitor materials [109]. Building upon known structural and performance data of corrosion inhibitors, researchers can utilize computer-aided design software to predict the structures and properties of novel inhibitors. This approach not only accelerates the development cycle of new materials but also reduces experimental costs and enhances research efficiency. Through rational design of inhibitor molecular structures, more efficient protection of aluminum surfaces can be achieved, thereby improving the overall performance of Al–air batteries.
To delve deeper into the nature of the interaction and elucidate the hybridization mechanism of Z4 molecules on the aluminum (Al) surface from a quantum chemical perspective, El-Alouani and coworkers conducted a comprehensive evaluation of the charge transfer behavior, physicochemical properties, and bonding mechanisms within the molecular network of Z4 molecules using density functional theory (DFT)-based calculations. This analysis encompassed the investigation of molecular orbital (MO) energy level distributions, electrostatic potential (ESP)-colored van der Waals (vdW) surfaces, molecular electrostatic potential (MESP) surfaces, total density of states (TDOS), projected density of states (PDOS), and reduced density gradient (RDG) analysis. These methodologies were specifically utilized to examine the noncovalent interactions of Z4 molecules and their intrinsic reactivity. The MD method was also utilized to examine the adsorption configurations of the Z4 inhibitor on the aluminum (Al) surface and to estimate its favorable interactions with the surface by systematically varying the number of Z4 molecules from 5 to 20. The calculated results are illustrated in Figure 5 [110].
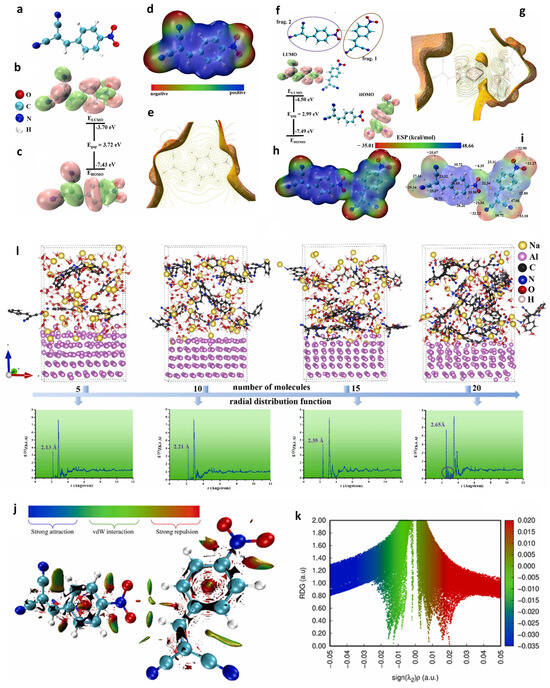
Figure 5.
Electronic structure analysis of the Z4 monomer showing optimized geometry (a), LUMO (b) and HOMO (c) orbital distributions, ESP (d), and MESP visualization (e). Molecular electronic properties of the ZK4 molecule dimer: PDOS for s, p atomic orbitals (f), MESP (g), ESP (h), ESP mapped vdW surface (i), and RDG visualization and scatter plot (j,k). (l) Dynamic adsorption configurations of Z4 inhibitor adsorbed onto Al(111) surfaces in an alkaline medium, with the number of molecules varying from 5 to 20, along with their RDF analyses. Reprinted with permission from ref. [110] 2025, Elsevier.
They also examined the distribution of electron density in LUMO and HOMO orbitals of these compounds within the (Na+, OH−) environment to characterize the mechanism of organic molecule attack [111]. This analysis enables the prediction of the spatial localization of these orbitals and infers their reactivity based on the predominant atom types involved. The results illustrated in Figure 5 indicate that the HOMO and LUMO density distributions for these two sugar molecules are similar in both the gas and aqueous phases. The HOMO and LUMO densities are predominantly localized on the O–H and −C–C– groups, identifying these as the primary adsorption centers for these compounds. Therefore, molecules with more adsorption centers are more likely to induce flat-direction adsorption on the aluminum surface.
Computational simulations and computer-aided design are playing an increasingly vital role in corrosion inhibitor research for Al–air batteries. By enabling in-depth exploration of inhibition mechanisms, optimization of inhibitor performance, and guidance for synthesizing novel inhibitor materials, these computational approaches have revitalized advancements in Al–air battery technology. With continuous progress in computational capabilities and refinement of simulation methodologies, it is anticipated that computational methods will unlock even broader application prospects in corrosion inhibitor research.
6. Practical Applications of Al–Air Batteries
Al–air batteries have demonstrated extensive applicability across multiple critical domains, spanning submarine power systems, electric vehicles, power stations, and telecommunication base stations. These energy systems exhibit remarkable application potential and promising commercial prospects in next-generation energy storage technologies (Figure 6) [26,27].
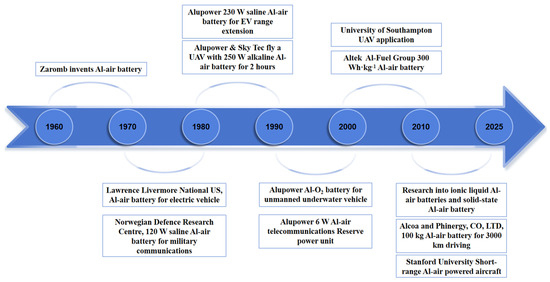
Figure 6.
Timeline of the development of Al–air batteries.
Especially in marine power applications, Al–air batteries demonstrate unique advantages through their utilization of seawater electrolytes, offering exceptional energy density and extended operational range. Furthermore, their inherent environmental compatibility ensures marine ecosystem preservation during operation as no corrosive byproducts or toxic emissions are generated. This combination of high-performance characteristics and ecological safety positions these batteries as an optimal power source for submarine systems, autonomous underwater vehicles, and various subsea instrumentation [23].
Al–air batteries are increasingly positioned as preferred power systems for pure electric vehicles and cold chain logistics vehicles, owing to their lightweight configuration and superior specific energy characteristics. These systems demonstrate dual technical merits: driving range enhancement that addresses escalating demands for extended-range electric mobility, and systematic mass reduction strategies that synergistically contribute to improved energy utilization efficiency and operational energy consumption reduction [22,24,27].
Additionally, Al–air batteries demonstrate significant advantages as backup power sources in electrical substations and telecommunication base stations due to their compact size, lightweight design, extended service life, and low operational noise. These characteristics effectively address the performance degradation issues of conventional batteries under high-load and prolonged operation, ensuring a stable and reliable power supply. This makes them particularly valuable for emergency power systems and off-grid energy supply applications [25].
Al–air batteries demonstrate promising market potential owing to their superior performance, environmental compatibility, and versatile application scenarios. These systems are poised to play a pivotal role in advancing clean energy transitions and sustainable development initiatives. With continuous technological refinement and industrial chain optimization, their commercialization is expected to progressively expand, delivering more efficient and sustainable energy solutions across diverse sectors [112].
7. Conclusions and Prospects
In summary, inhibiting the self-corrosion of aluminum anodes in Al–air batteries is crucial for their application and commercialization. Various strategies have been developed, such as anode alloying with elements like Mg and Sn to optimize surface and electrochemical properties, single-electrolyte corrosion inhibitors (inorganic, organic, or composite) to suppress self-corrosion via physical or chemical interactions with the aluminum surface, a dual-electrolyte system with different electrolyte properties at anode and cathode to reduce self-corrosion, and computer-aided methods (DFT calculations, molecular dynamics simulations, and machine learning algorithms) to explore corrosion-inhibition mechanisms and improve battery performance. These strategies act synergistically to create efficient and stable Al–air battery systems, surmounting technical hurdles in mass- production and practical use, thus advancing battery commercialization and addressing energy and environmental issues.
However, challenges persist. The self-corrosion mechanism, especially the dynamic surface evolution of the anode and interactions between aluminum and the electrolyte, is not fully understood. Also, new materials and technologies like ionic liquid electrolytes, gel electrolytes, anode alloys, and surface-treatment methods need better compatibility and stability. Most studies focus on single-strategy performance, with limited research on the combined effects of multiple strategies. Moreover, real-world performance testing and evaluation of Al–air batteries are insufficient. Future research should strengthen the basic understanding of self-corrosion mechanisms, enhance the compatibility and stability of new materials, new cell structure, and novel technologies, explore the synergistic effects of multiple strategies, and establish comprehensive testing and evaluation standards and methods to facilitate the commercialization of Al–air batteries.
Author Contributions
Conceptualization, S.L., Z.L. and J.L.; methodology, S.L. and J.L.; validation, S.L., Z.L., H.W. and H.M.; formal analysis, S.L.; investigation, J.L.; resources, H.W. and H.M.; data curation, S.L.; writing—original draft preparation, S.L., Z.L., X.W. and J.L.; writing—review and editing, S.L., Z.L., X.W. and J.L.; visualization, S.L., Z.L., X.W. and J.L.; supervision, X.W. and J.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (U1832136), the Natural Science Foundation of Anhui Province (305067828053), the Fundamental Research Funds for the Central Universities (PA2024GDGP0042 and PA2025GDGP0025), and the College Students Innovation and Entrepreneurship Training Program (202510359016 and S202410359046).
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Hao, M.-D.; Li, Q.; Sun, J.-H.; Liu, D.; Yu, H.-L.; Liu, R. Polyphenol-metal coordination derived high-entropy alloy as bifunctional oxygen electrocatalyst for Zn-air batteries. Rare Met. 2025, 44, 2836–2844. [Google Scholar] [CrossRef]
- Wu, X.-G.; Wang, R.; Ma, F.; Liu, X.-L.; Jia, D.-L.; Yang, H.-C.; Liu, Y.-P.; Wang, Z.-X.; Zheng, H.-Z.; Zhang, Y.-N.; et al. FeCo-N encapsuled in nitrogen-doped carbon nanotubes as bifunctional electrocatalysts with a high stability for zinc air batteries. Rare Met. 2023, 42, 1526–1534. [Google Scholar] [CrossRef]
- Xu, Y.; Tang, J.-Y.; Li, S.-D.; Hu, H.-L.; He, Y.-J.; Wang, S.-F.; Wu, Z.-M.; Jeong, S.; Cai, Z.-Y.; Lin, X.; et al. Co-doping-induced electronic reconfiguration of nanosized ZnS for facilitating oxygen reduction reaction in flexible aluminum-air batteries. Rare Met. 2025, 44, 2352–2365. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, Y.; Peng, X.; Lin, T.; Huang, X.; Alghamdi, N.S.; Rana, M.; Chen, P.; Zhang, C.; Whittaker, A.K.; et al. Enhancing performance and longevity of solid-state zinc-iodine batteries with fluorine-rich solid electrolyte interphase. Mater. Futures 2024, 3, 035102. [Google Scholar] [CrossRef]
- Yu, F.; Mu, Y.; Han, M.; Liu, J.; Zheng, K.; Zou, Z.; Hu, H.; Man, Q.; Li, W.; Wei, L.; et al. Electrochemically stable and ultrathin polymer-based solid electrolytes for dendrite-free all-solid-state lithium-metal batteries. Mater. Futures 2025, 4, 015101. [Google Scholar] [CrossRef]
- Zhou, M.; Fu, K.; Xing, Y.; Liu, J.; Meng, F.; Wei, X.; Liu, J. 500-mW cm−2 underwater Zn-H2O2 batteries with ultrafine edge-enriched electrocatalysts. Sci. China Mater. 2024, 67, 2908–2914. [Google Scholar] [CrossRef]
- Yue, C.; Zhang, N.; Zhu, Z.; Chen, P.; Meng, F.; Liu, X.; Wei, X.; Liu, J. Multi-Strategy Architecture of High-Efficiency Electrocatalysts for Underwater Zn-H2O2 Batteries with Superior Power Density of 442 mW cm-2. Small 2022, 18, 2106532. [Google Scholar] [CrossRef]
- Xu, L.; Liu, J.; Chen, P.; Wang, Z.; Tang, D.; Liu, X.; Meng, F.; Wei, X. High-power aqueous Zn-H2O2 batteries for multiple applications. Cell Rep. Phys. Sci. 2020, 1, 100027. [Google Scholar] [CrossRef]
- Hossain, M.I.; Tareq, F.K.; Rudra, S. Light-driven photocathodes in Li/Zn-O2 (air) batteries: An analytical review, technological breakthroughs and future challenges. Energy Storage Mater. 2025, 75, 104025. [Google Scholar] [CrossRef]
- Jiang, P.; Li, D.; Hou, R.; Yang, H.; Yang, J.; Zhu, S.; Wang, L.; Guan, S. A micro-alloyed Mg-Zn-Ge alloy as promising anode for primary Mg-air batteries. J. Magnes. Alloys 2024, 12, 4157–4173. [Google Scholar] [CrossRef]
- Zhou, K.; Liu, S.; Li, L. Recent advances of high-entropy materials as electrocatalysts for rechargeable Zn-air batteries. J. Alloys Compd. 2025, 1011, 178435. [Google Scholar] [CrossRef]
- Meng, F.; Chen, P.; Li, S.; Cao, N.; Cheng, S.; Wei, X.; Di, J.; Liu, J. Fish-Gill-Inspired Underwater Self-Breathing Zinc-Air Batteries. ACS Appl. Energy Mater. 2024, 7, 9442–9450. [Google Scholar] [CrossRef]
- Dai, J.; Chen, P.; Meng, F.; Han, F.; Zhu, C.; Wei, X.; Zheng, L.; Liu, J. Starch-reinforced adhesive hydrogel electrolyte enables high-performance flexible zinc-air batteries. J. Energy Storage 2024, 102, 114035. [Google Scholar] [CrossRef]
- Zhu, Z.; Jin, L.; Zhou, M.; Fu, K.; Meng, F.; Wei, X.; Liu, J. Single-cell-array biomass-templated architecture of hierarchical porous electrocatalysts for Zn–air and Zn–H2O2 batteries. Chem. Commun. 2023, 59, 4356–4359. [Google Scholar] [CrossRef]
- Jin, L.; Xing, A.; Zhu, Z.; Fu, K.; Zhou, M.; Meng, F.; Wei, X.; Liu, J. In situ potential-regulated architecture of an ultrafine Ru-based electrocatalyst for ultralow overpotential lithium–oxygen batteries. Chem. Commun. 2023, 59, 5926–5929. [Google Scholar] [CrossRef]
- Liu, J.; Chen, W.; Xing, A.; Wei, X.; Xue, D. Lithium cell-assisted low-overpotential Li-O2 batteries by in situ discharge activation. Chem. Commun. 2017, 53, 10568–10571. [Google Scholar] [CrossRef]
- Liu, J.; Zhou, M.; Jin, K.; Li, J.; Meng, F.; Wei, X. Beyond metal–air battery, emerging aqueous metal–hydrogen peroxide batteries with improved performance. Battery Energy 2024, 3, 20230049. [Google Scholar] [CrossRef]
- Fei, H.; Liu, R.; Zhang, Y.; Wang, H.; Wang, M.; Wang, S.; Ni, M.; Wu, Z.; Wang, J. Extending MoS2-based materials into the catalysis of non-acidic hydrogen evolution: Challenges, progress, and perspectives. Mater. Futures 2023, 2, 022103. [Google Scholar] [CrossRef]
- Dilshad, M.; Li, T.; Lee, S.-L.; Qin, L. Next-Generation Aluminum-Air Batteries: Integrating New Materials and Technologies for Superior Performance. ACS Appl. Energy Mater. 2025, 8, 3248–3275. [Google Scholar] [CrossRef]
- Zaromb, S. The use and behavior of aluminum anodes in alkaline primary batteries. J. Electrochem. Soc. 1962, 109, 1125. [Google Scholar] [CrossRef]
- Zhang, Z.; Zuo, C.; Liu, Z.; Yu, Y.; Zuo, Y.; Song, Y. All-solid-state Al–air batteries with polymer alkaline gel electrolyte. J. Power Sources 2014, 251, 470–475. [Google Scholar] [CrossRef]
- Goel, P.; Dobhal, D.; Sharma, R.C. Aluminum–air batteries: A viability review. J. Energy Storage 2020, 28, 101287. [Google Scholar] [CrossRef]
- Rasul, M.J.M.A.; Kim, J. Comprehensive review and comparison on battery technologies as electric-powered source in marine applications. J. Energy Storage 2024, 88, 111509. [Google Scholar] [CrossRef]
- Yang, S.; Knickle, H. Design and analysis of aluminum/air battery system for electric vehicles. J. Power Sources 2002, 112, 162–173. [Google Scholar] [CrossRef]
- Tang, G.; Guo, D.; Wu, K. Optimal Backup Power Allocation for 5G Base Stations. In GreenEdge: New Perspectives to Energy Management and Supply in Mobile Edge Computing; Tang, G., Guo, D., Wu, K., Eds.; Springer Nature: Singapore, 2022; pp. 51–65. [Google Scholar]
- Suresh, T.; Samuel Ratna Kumar, P.S.; Nithyadharseni, P. Aluminium air batteries for sustainable environment: A review. J. Alloys Compd. Commun. 2025, 6, 100048. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, Q.; Li, W.; Adair, K.R.; Li, J.; Sun, X. A comprehensive review on recent progress in aluminum–air batteries. Green Energy Environ. 2017, 2, 246–277. [Google Scholar] [CrossRef]
- Hasannaeimi, V.; Sadeghilaridjani, M.; Mukherjee, S. Electrochemical and Corrosion Behavior of Metallic Glasses; MDPI-Multidisciplinary Digital Publishing Institute: Basel, Switzerland, 2021. [Google Scholar]
- Chen, L.D.; Nørskov, J.K.; Luntz, A.C. Al–air batteries: Fundamental thermodynamic limitations from first-principles theory. J. Phys. Chem. Lett. 2015, 6, 175–179. [Google Scholar] [CrossRef]
- Gaele, M.F.; Gargiulo, P.; Di Palma, T.M. Aluminum-air batteries with acidic bio-polymer gel electrolytes and wood-derived metal-free cathodes. J. Power Sources 2025, 626, 235784. [Google Scholar] [CrossRef]
- Li, X.; Ma, Z.-Q.; Teng, Z. Study on a New Kind of Al Alloy Anode Material for Aluminum-Air Battery. Mater. Sci. 2012, 02, 52–57. [Google Scholar]
- Zhu, C.; Han, Y.; Luo, L.; Yan, L.; Xiang, B.; Zhou, Y.; Zou, X.; Guo, L. Dual modulation of electrolyte inner solvent structure and anode interface for high performance alkaline Al-air battery. Chem. Eng. J. 2024, 496, 153814. [Google Scholar] [CrossRef]
- Wu, G.-X.; Wei, Z.-S.; Li, S.-Q.; Cui, L.-Y.; Zhang, G.-X.; Zeng, R.-C. Corrosion inhibition of polyelectrolytes to the Al anode in Al-air battery: A comparative study of functional group effect. J. Power Sources 2024, 592, 233907. [Google Scholar] [CrossRef]
- Fan, L.; Lu, H.; Leng, J. Performance of fine structured aluminum anodes in neutral and alkaline electrolytes for Al-air batteries. Electrochim. Acta 2015, 165, 22–28. [Google Scholar] [CrossRef]
- Seyam, S.; Dincer, I.; Agelin-Chaab, M. Exergetic, exergoeconomic and exergoenvironmental analyses of a hybrid combined locomotive powering system for rail transportation. Energy Convers. Manag. 2021, 245, 114619. [Google Scholar] [CrossRef]
- Zhao, R.; He, P.; Yu, F.; Yang, J.; Sun, Z.; Hu, W. Performance improvement for aluminum-air battery by using alloying anodes prepared from commercially pure aluminum. J. Energy Storage 2023, 73, 108985. [Google Scholar] [CrossRef]
- Tong, F.; Zhuang, W.; Song, M.; Kim, J.; Gao, W.; Wei, S. Micro-alloyed aluminium alloys as anodes for aluminium-air batteries with a neutral electrolyte. Mater. Today Commun. 2024, 39, 108518. [Google Scholar] [CrossRef]
- Ren, J.; Liu, T.; Zhang, J.; Jiang, M.; Dong, Q.; Fu, C. Spray-formed commercial aluminum alloy anodes with suppressed self-corrosion for Al-Air batteries. J. Power Sources 2022, 524, 231082. [Google Scholar] [CrossRef]
- Liu, H.; Wu, M.; Duan, W.; Ma, Y.; Li, C.; Mou, X. Influences of Sodium Polyacrylate/ZnO Composite Corrosion Inhibitor on the Electrochemical Activity and Discharge Performance of 6061 Aluminum Alloy in Alkaline Electrolyte. Mater. Prot. 2023, 56, 96–103. [Google Scholar] [CrossRef]
- Lin, X.Z.; Gong, M. Research progress of environmentally friendly corrosion inhibitors for copper. Corros. Prot. 2009, 30, 328–331+342. [Google Scholar]
- Wei, Y.; Shi, Y.; Chen, Y.; Xiao, C.; Ding, S. Development of solid electrolytes in Zn-air and Al-air batteries: From material selection to performance improvement strategies. J. Mater. Chem. A 2021, 9, 4415–4453. [Google Scholar] [CrossRef]
- Maria, F.G.; Di Palma, T.M. Polymer Electrolytes for Al-Air Batteries: Current State and Future Perspectives. Energy Fuels 2022, 36, 12875–12895. [Google Scholar] [CrossRef]
- Buckingham, R.; Asset, T.; Atanassov, P. Aluminum-air batteries: A review of alloys, electrolytes and design. J. Power Sources 2021, 498, 229762. [Google Scholar] [CrossRef]
- Lu, J.; Jaumaux, P.; Wang, T.; Wang, C.; Wang, G. Recent progress in quasi-solid and solid polymer electrolytes for multivalent metal-ion batteries. J. Mater. Chem. A 2021, 9, 24175–24194. [Google Scholar] [CrossRef]
- Yu, H.; Lv, C.; Yan, C.; Yu, G. Interface Engineering for Aqueous Aluminum Metal Batteries: Current Progresses and Future Prospects. Small Methods 2024, 8, 2300758. [Google Scholar] [CrossRef]
- Wang, H.-F.; Xu, Q. Materials Design for Rechargeable Metal-Air Batteries. Matter 2019, 1, 565–595. [Google Scholar] [CrossRef]
- Yu, H.; Liu, D.; Feng, X.; Zhang, Y. Mini Review: Recent Advances on Flexible Rechargeable Li-Air Batteries. Energy Fuels 2021, 35, 4751–4761. [Google Scholar] [CrossRef]
- Zhang, Z.; Xiong, L.; Wang, S.; Xie, Y.; You, W.; Du, X. A review of the Al-gas batteries and perspectives for a “Real” Al-air battery. J. Power Sources 2023, 580, 233375. [Google Scholar] [CrossRef]
- Verma, C. Chapter5-Classification of corrosion inhibitors. In Handbook of Science & Engineering of Green Corrosion Inhibitors; Verma, C., Ed.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 41–48. [Google Scholar]
- Migliardini, F.; Palma, T.; Gaele, M.; Corbo, P. Solid and acid electrolytes for Al-air batteries based on xanthan-HCl hydrogels. J. Solid State Electrochem. 2018, 22, 2901–2916. [Google Scholar] [CrossRef]
- Cai, S.; Pan, C.; Zhang, D. Effect of multifunctional composite additives on the anodic hydrogen evolution reaction in alkaline Al-air batteries. J. Power Sources 2024, 608, 234610. [Google Scholar] [CrossRef]
- Ren, J.; Fu, C.; Dong, Q.; Jiang, M.; Dong, A.; Zhu, G.; Zhang, J.; Sun, B. Evaluation of Impurities in Aluminum Anodes for Al-Air Batteries. ACS Sustain. Chem. Eng. 2021, 9, 2300–2308. [Google Scholar] [CrossRef]
- Wu, S.; Hu, S.; Zhang, Q.; Sun, D.; Wu, P.; Tang, Y.; Wang, H. Hybrid high-concentration electrolyte significantly strengthens the practicability of alkaline aluminum-air battery. Energy Storage Mater. 2020, 31, 310–317. [Google Scholar] [CrossRef]
- Li, Q.; Bjerrum, N.J. Aluminum as anode for energy storage and conversion: A review. J. Power Sources 2002, 110, 1–10. [Google Scholar] [CrossRef]
- Sun, Z.; Lu, H.; Fan, L.; Hong, Q.; Leng, J.; Chen, C. Performance of Al-Air Batteries Based on Al–Ga, Al–In and Al–Sn Alloy Electrodes. J. Electrochem. Soc. 2015, 162, A2116. [Google Scholar] [CrossRef]
- Li, J.; Dang, J. A Summary of Corrosion Properties of Al-Rich Solid Solution and Secondary Phase Particles in Al Alloys. Metals 2017, 7, 84. [Google Scholar] [CrossRef]
- del Olmo, R.; Mohedano, M.; Matykina, E.; Arrabal, R. Permanganate loaded Ca-Al-LDH coating for active corrosion protection of 2024-T3 alloy. Corros. Sci. 2022, 198, 110144. [Google Scholar] [CrossRef]
- Wang, Z.; Yan, Y.; Wu, Y.; Zhang, Y.; Zhao, X.; Su, Y.; Qiao, L. Recent research progress on the passivation and selective oxidation for the 3d-transition-metal and refractory multi-principal element alloys. npj Mater. Degrad. 2023, 7, 86. [Google Scholar] [CrossRef]
- Peltier, F.; Thierry, D. Review of Cr-Free Coatings for the Corrosion Protection of Aluminum Aerospace Alloys. Coatings 2022, 12, 518. [Google Scholar] [CrossRef]
- Zhao, H.; Yin, Y.; Wu, Y.; Zhang, S.; Mingers, A.M.; Ponge, D.; Gault, B.; Rohwerder, M.; Raabe, D. How solute atoms control aqueous corrosion of Al-alloys. Nat. Commun. 2024, 15, 561. [Google Scholar] [CrossRef]
- Yu, S.; Yang, X.; Liu, Y.; Zhan, F.; Wen, Q.; Li, J.; Li, W. High power density Al-air batteries with commercial three-dimensional aluminum foam anode. Ionics 2020, 26, 5045–5054. [Google Scholar] [CrossRef]
- Qin, J.; Li, L.; Tu, Y.; Cao, F.; Suo, Y.; Wang, X.; Cui, J. Electrochemical behavior and discharge performance of Al-1Zn-0.4Mn-0.1Sn-xBi as anode alloys for Al-air battery in KOH solution. Mater. Chem. Phys. 2025, 331, 130200. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, P.; Xue, J.; Zhu, C.; Li, X.; Wang, Z. High energy efficiency of Al-based anodes for Al-air battery by simultaneous addition of Mn and Sb. Chem. Eng. J. 2021, 417, 128006. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, Y.; Wei, X.; Feng, J.; Xin, Y.; Wang, X.; Wei, Y. High energy efficiency and high discharge voltage of 2N-purity Al-based anodes for Al-air battery by simultaneous addition of Mn, Zn and Ga. J. Power Sources 2023, 563, 232845. [Google Scholar] [CrossRef]
- Ma, K.; Zhang, W.; Liu, Y.; Huang, Y.; Shao, H.; Chen, C. Effect of indium content on the discharge properties of Al-Mg-Ga based anodes in neutral Al-air batteries. J. Power Sources 2025, 636, 236489. [Google Scholar] [CrossRef]
- Huang, Y.; Shi, W.; Guo, L.; Zhang, Q.; Wang, K.; Zheng, X.; Verma, C.; Qiang, Y. Corrosion inhibition of L-tryptophan on Al-5052 anode for Al-air battery with alkaline electrolyte. J. Power Sources 2023, 564, 232866. [Google Scholar] [CrossRef]
- Meng, A.; Sun, Y.; Cheng, W.; Huang, L.; Chen, Y. Discharge performance of Al-0.1Sn-0.1In-0.05Ga alloy for Al–air battery anodes. J. Energy Storage 2024, 81, 110414. [Google Scholar] [CrossRef]
- Zuo, Y.; Yu, Y.; Liu, H.; Zhiqing, G.; Cao, Q.; Zuo, C. Electrospun Al2O3 Film as Inhibiting Corrosion Interlayer of Anode for Solid Aluminum–Air Batteries. Batteries 2020, 6, 19. [Google Scholar] [CrossRef]
- Bautista-Ruiz, J.; Bautista-Ruiz, W.A.; Aperador, W. Anti-corrosive characterization of silicon, titanium, and zirconium oxide coatings deposited on aeronautical aluminum substrates via sol-gel. J. Phys. Conf. Ser. 2020, 1708, 012003. [Google Scholar] [CrossRef]
- Namsheer, K.; Rout, C.S. Conducting polymers: A comprehensive review on recent advances in synthesis, properties and applications. RSC Adv. 2021, 11, 5659–5697. [Google Scholar] [CrossRef]
- Sitanggang, R.; Nur’aini, S.; Susanto, S.; Widiyastuti, W.; Setyawan, H. The Enhancement Discharge Performance by Zinc-Coated Aluminum Anode for Aluminum–Air Battery in Sodium Chloride Solution. Appl. Sci. 2024, 14, 6263. [Google Scholar] [CrossRef]
- Yasakau, K.A.; Zheludkevich, M.L.; Lamaka, S.V.; Ferreira, M.G.S. Mechanism of Corrosion Inhibition of AA2024 by Rare-Earth Compounds. J. Phys. Chem. B 2006, 110, 5515–5528. [Google Scholar] [CrossRef]
- Thuy, T.; Anh Truc, T.; Pham, T.; Nguyen Xuan, H.; Vu, P.; Xuan Hang, T. Synthesis of Lithium-exchange Silica Particles for Corrosion Protection of Aluminum Alloys. J. Surf. Sci. Nanotechnol. 2020, 18, 239–248. [Google Scholar] [CrossRef]
- Lv, C.; Zhang, Y.; Li, Y.; Zhu, Y.; Kuang, J.; Tang, Y.; Wang, H. Regulating solvation and interface chemistry enables advanced aluminum-air batteries. Chem. Commun. 2023, 59, 2588–2591. [Google Scholar] [CrossRef] [PubMed]
- Taeri, O.; Hassanzadeh, A.; Ravari, F. Synergistic Inhibitory Effect of Potassium Sodium Tartrate Tetrahydrate and Sodium Stannate Trihydrate on Self-Corrosion of Aluminum in Alkaline Aluminum-Air Batteries. ChemElectroChem 2020, 7, 2123–2135. [Google Scholar] [CrossRef]
- Luo, L.; Zhu, C.; Yan, L.; Guo, L.; Zhou, Y.; Xiang, B. Synergistic construction of bifunctional interface film on anode via a novel hybrid additive for enhanced alkaline Al-air battery performance. Chem. Eng. J. 2022, 450, 138175. [Google Scholar] [CrossRef]
- Nie, Y.; Gao, J.; Wang, E.; Jiang, L.; An, L.; Wang, X. An effective hybrid organic/inorganic inhibitor for alkaline aluminum-air fuel cells. Electrochim. Acta 2017, 248, 478–485. [Google Scholar] [CrossRef]
- Wang, X.-Y. Study on electrochemical behaviors for aluminum in alkaline solution with two different inhibitors. Chem. Reag. 2010, 32, 639–642. [Google Scholar]
- Hao, C.; Yuan, Z.; Zhifan, H.; Zheng, L.; Tao, W.; Yao, L.; Zhongliang, T.; Ke, P. Effects of corrosion inhibitors on composition and properties of electrolyte after discharging for aluminum-air batteries. J. Cent. South Univ. (Sci. Technol.) 2023, 54, 702–709. [Google Scholar] [CrossRef]
- Wu, S.; Zhang, Q.; Sun, D.; Luan, J.; Shi, H.; Hu, S.; Tang, Y.; Wang, H. Understanding the synergistic effect of alkyl polyglucoside and potassium stannate as advanced hybrid corrosion inhibitor for alkaline aluminum-air battery. Chem. Eng. J. 2020, 383, 123162. [Google Scholar] [CrossRef]
- Ahmed, M.A.; Amin, S.; Mohamed, A.A. Current and emerging trends of inorganic, organic and eco-friendly corrosion inhibitors. RSC Adv. 2024, 14, 31877–31920. [Google Scholar] [CrossRef]
- Shehnazdeep; Pradhan, B. A study on effectiveness of inorganic and organic corrosion inhibitors on rebar corrosion in concrete: A review. Mater. Today Proc. 2022, 65, 1360–1366. [Google Scholar] [CrossRef]
- Zhou, Y.; Lu, X.; Würger, T.; Höche, D.; Zheludkevich, M.L.; Wang, F. New insights into the inhibition mechanism of carboxylate species on magnesium surface. Corros. Sci. 2024, 232, 112009. [Google Scholar] [CrossRef]
- Wysocka, J.; Cieslik, M.; Krakowiak, S.; Ryl, J. Carboxylic acids as efficient corrosion inhibitors of aluminium alloys in alkaline media. Electrochim. Acta 2018, 289, 175–192. [Google Scholar] [CrossRef]
- Gnedenkov, A.S.; Sinebryukhov, S.L.; Nomerovskii, A.D.; Marchenko, V.S.; Ustinov, A.Y.; Gnedenkov, S.V. Carboxylates as green corrosion inhibitors of magnesium alloy for biomedical application. J. Magnes. Alloys 2024, 12, 2909–2936. [Google Scholar] [CrossRef]
- Thacker, H.; Ram, V. Green corrosion inhibitors derived from plant extracts and drugs for mild steel in acid media: A review. Results Surf. Interfaces 2025, 18, 100364. [Google Scholar] [CrossRef]
- Wang, X.; Ren, S.; Jiang, H.; Hou, L.; Zhou, W. Preparation method and research direction of plant corrosion inhibitors. Surf. Technol. 2018, 47, 196. [Google Scholar]
- Bitwell, C.; Indra, S.S.; Luke, C.; Kakoma, M.K. A review of modern and conventional extraction techniques and their applications for extracting phytochemicals from plants. Sci. Afr. 2023, 19, e01585. [Google Scholar] [CrossRef]
- Zhu, J.; Xu, S.; Wu, J.; Yin, Y.; Cheng, S.; Zhang, C.; Qiang, Y.; Wang, W. Probing corrosion protective mechanism of an amide derivative additive on anode for enhanced alkaline Al-air battery performance. J. Power Sources 2024, 593, 233957. [Google Scholar] [CrossRef]
- Choi, S.-R.; Kim, K.-M.; Kim, J.-G. Organic corrosion inhibitor without discharge retardation of aluminum-air batteries. J. Mol. Liq. 2022, 365, 120104. [Google Scholar] [CrossRef]
- Liu, J.; Xing, Y.; Ma, B.; Wei, X.; Li, S.; Li, D.; Meng, F.; Deng, W.; Liu, J. Tripod-Linked Molecular Armor for Low-Overpotential Al–Air Batteries. ACS Electrochem. 2025, 1, 774–1002. [Google Scholar] [CrossRef]
- Zhao, Q.; Wu, P.; Sun, D.; Wang, H.; Tang, Y. A dual-electrolyte system for highly efficient Al–air batteries. Chem. Commun. 2022, 58, 3282–3285. [Google Scholar] [CrossRef]
- Liu, C.; Chen, X.; Yang, J. Corrosion inhibitors and its application in CO2 corrosion. Chem. Ind. Eng. Prog. 2021, 40, 6305. [Google Scholar]
- Ma, J.; Tong, S.; Ren, F.; Wang, G.; Li, Y.; Wen, J. Influences of Inhibitor L-Cysteine/zinc Oxide on Electrochemical Performance of 3102 Al-alloy in Alkaline Solution. J. Chin. Soc. Corros. Prot. 2018, 38, 351–357. [Google Scholar]
- Zhang, P.; Peng, W.; Miao, J.; Ren, G.; Wang, Y.; Li, Y.; Zhang, P. Evolution of the solid-liquid interface using a novel hybrid corrosion inhibitor to improve Al-air battery performance. J. Energy Chem. 2025, 104, 69–78. [Google Scholar] [CrossRef]
- Zhu, C.; Yang, H.; Wu, A.; Zhang, D.; Gao, L.; Lin, T. Modified alkaline electrolyte with 8-hydroxyquinoline and ZnO complex additives to improve Al-air battery. J. Power Sources 2019, 432, 55–64. [Google Scholar] [CrossRef]
- Deyab, M.A. Effect of nonionic surfactant as an electrolyte additive on the performance of aluminum-air battery. J. Power Sources 2019, 412, 520–526. [Google Scholar] [CrossRef]
- Moghadam, Z.; Shabani-Nooshabadi, M.; Behpour, M. Electrochemical performance of aluminium alloy in strong alkaline media by urea and thiourea as inhibitor for aluminium-air batteries. J. Mol. Liq. 2017, 242, 971–978. [Google Scholar] [CrossRef]
- Liu, J.; Wang, D.; Zhang, D.; Gao, L.; Lin, T. Synergistic effects of carboxymethyl cellulose and ZnO as alkaline electrolyte additives for aluminium anodes with a view towards Al-air batteries. J. Power Sources 2016, 335, 1–11. [Google Scholar] [CrossRef]
- Li, X.; Li, J.; Zhang, D.; Gao, L.; Qu, J.; Lin, T. Synergistic effect of 8-aminoquinoline and ZnO as hybrid additives in alkaline electrolyte for Al-air battery. J. Mol. Liq. 2021, 322, 114946. [Google Scholar] [CrossRef]
- Chen, K.; Liang, Y.; Pan, D.; Huang, J.; Gao, J.; Lu, Z.; Liu, X.; Chen, J.; Zhang, H.; Hu, X.; et al. Mass Transportation Facilitated Porous Fe/Co Dual-Site Catalytic Cathodes for Ultrahigh-Power-Density Al–Air Fuel Cells. Adv. Energy Mater. 2025, 15, 2404140. [Google Scholar] [CrossRef]
- Ebenso, E.E.; Verma, C.; Olasunkanmi, L.O.; Akpan, E.D.; Verma, D.K.; Lgaz, H.; Guo, L.; Kaya, S.; Quraishi, M.A. Molecular modelling of compounds used for corrosion inhibition studies: A review. Phys. Chem. Chem. Phys. 2021, 23, 19987–20027. [Google Scholar] [CrossRef]
- Xiao, Z.; Chi, Z.; Song, L.; Cao, Z.; Li, A. Progress of the simulation model for power lithium ion battery. Chem. Ind. Eng. Prog. 2019, 38, 3604. [Google Scholar]
- Duda, Y.; Govea-Rueda, R.; Galicia, M.; Beltran, H.I.; Zamudio-Rivera, L.S. Corrosion inhibitors: Design, performance, and computer simulations. J. Phys. Chem. B 2005, 109, 22674–22684. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Dai, J.; Guo, X.; Fu, D.; Ma, L.; Keil, P.; Mol, A.; Zhang, D. Data-driven corrosion inhibition efficiency prediction model incorporating 2D–3D molecular graphs and inhibitor concentration. Corros. Sci. 2023, 222, 111420. [Google Scholar] [CrossRef]
- Liu, Z.; Ran, B.; Pei, H.; Luo, K.; Zhao, Z.; Pan, P.; Qiang, Y. Synergistic corrosion inhibition effect of a compound inhibitor for aluminum. J. Chin. Soc. Corros. Prot. 2023, 44, 312–322. [Google Scholar]
- Winkler, D.A.; Hughes, A.E.; Özkan, C.; Mol, A.; Würger, T.; Feiler, C.; Zhang, D.; Lamaka, S.V. Impact of inhibition mechanisms, automation, and computational models on the discovery of organic corrosion inhibitors. Prog. Mater. Sci. 2025, 149, 101392. [Google Scholar] [CrossRef]
- Xie, J.; Su, Y.; Xue, D.; Jiang, X.; Fu, H.; Huang, H. Machine learning for materials research and development. Acta Metall. Sin. 2021, 57, 1343–1361. [Google Scholar]
- Numin, M.S.; Hassan, A.; Jumbri, K.; Eng, K.K.; Borhan, N.; Daud, N.M.R.N.M.; M Nor A, A.; Suhor, F.; Abdul Wahab, R. A recent review on theoretical studies of Gemini surfactant corrosion inhibitors. J. Mol. Liq. 2022, 368, 120649. [Google Scholar] [CrossRef]
- El-Alouani, M.; Kharbouch, O.; Dahmani, K.; Galai, M.; Dkhireche, N.; Benzekri, Z.; Boukhris, S.; Ebn Touhami, M.; Almeer, R.; Chafiq, M.; et al. Probing corrosion protective mechanism of 2-(4-nitrobenzylidene) malononitrile on anode for enhanced alkaline (4 M NaOH) Aluminum-air battery performance. J. Alloys Compd. 2025, 1010, 178239. [Google Scholar] [CrossRef]
- El-Alouani, M.; Kharbouch, O.; Dahmani, K.; Errahmany, N.; Saber, I.; Galai, M.; Benzekri, Z.; Boukhris, S.; Ebn Touhami, M.; Al-Sadoon, M.K.; et al. Enhancing Al–Air Battery Performance with Beta-d-Glucose and Adonite Additives: A Combined Electrochemical and Theoretical Study. Langmuir 2025, 41, 431–449. [Google Scholar] [CrossRef]
- Li, X.; Zhong, S.; Wei, X.; Liu, Z.; Zhan, P.; Liu, J. Carbon Dioxide: From the Past to the Future. Univ. Chem. 2025, 40, 2503013. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).