Glass-Forming Ability and Crystallization Behavior of Mo-Added Fe82−xSi4B12Nb1MoxCu1 (x = 0–2) Nanocrystalline Alloy
Abstract
1. Introduction
2. Experiments
3. Results and Discussions
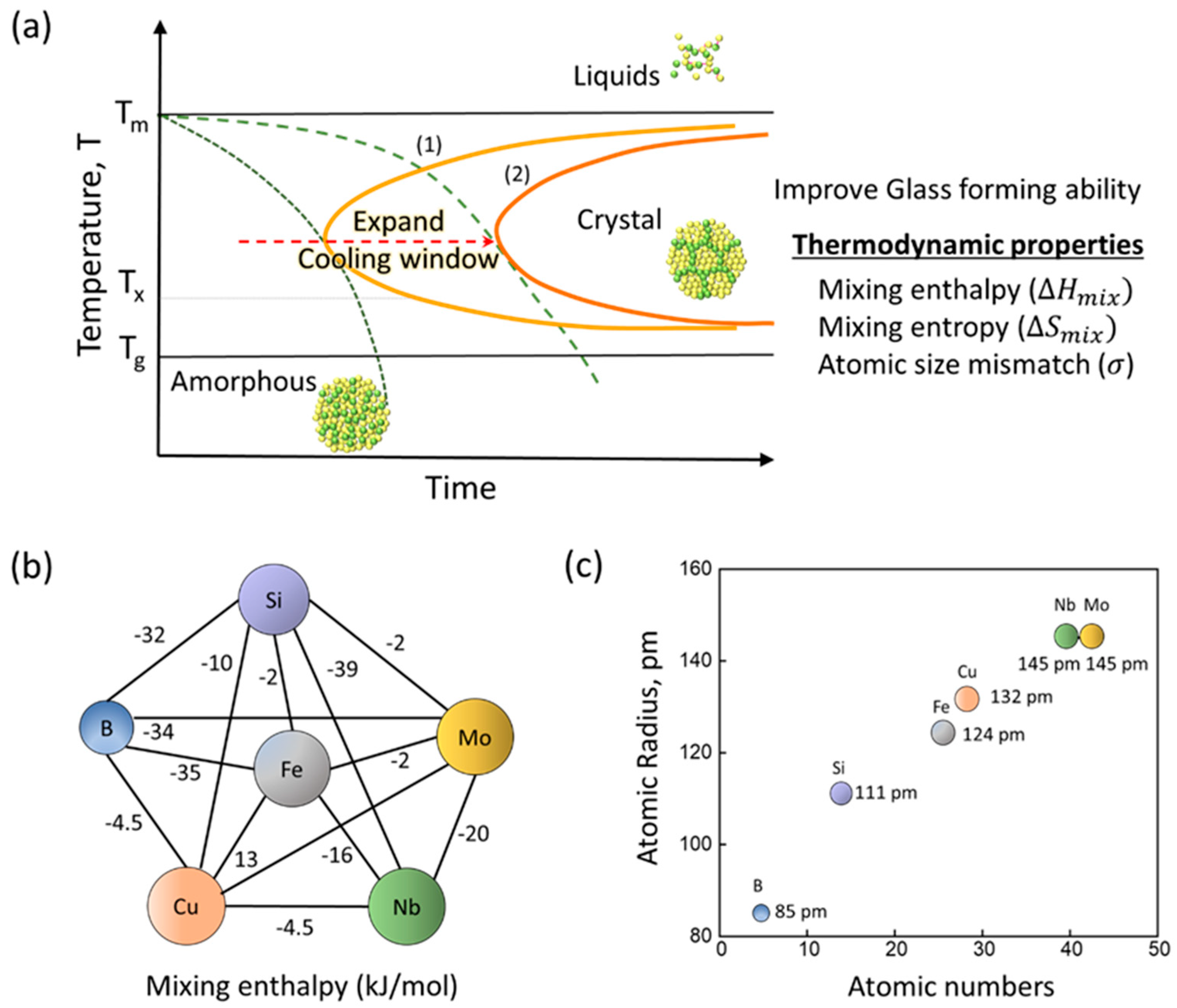
3.1. Effect of Mo Addition on Thermodynamic Factors and GFA in Fe82−xSi4B12Nb1MoxCu1 (x = 0–2) Nanocrystalline Ribbons
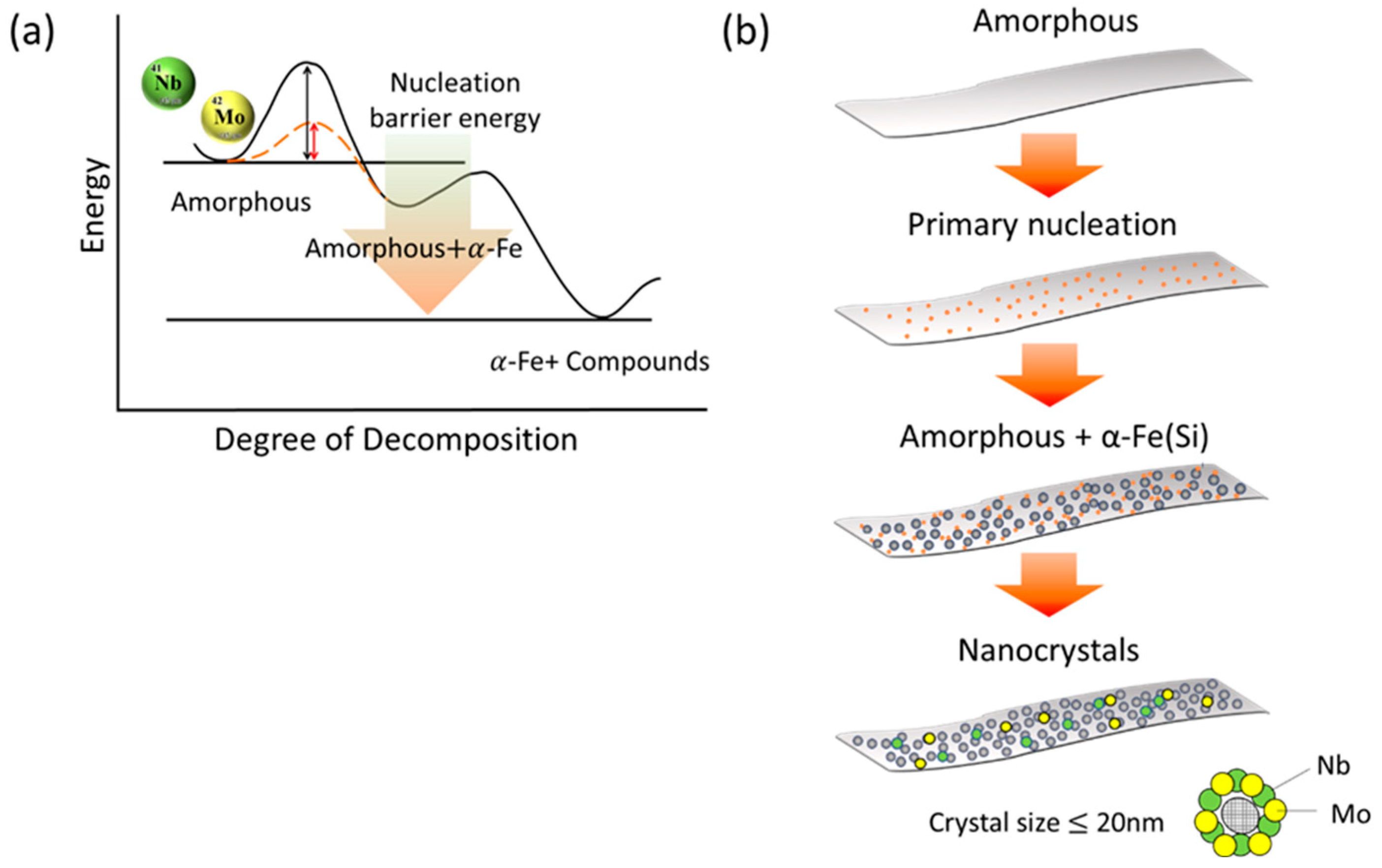
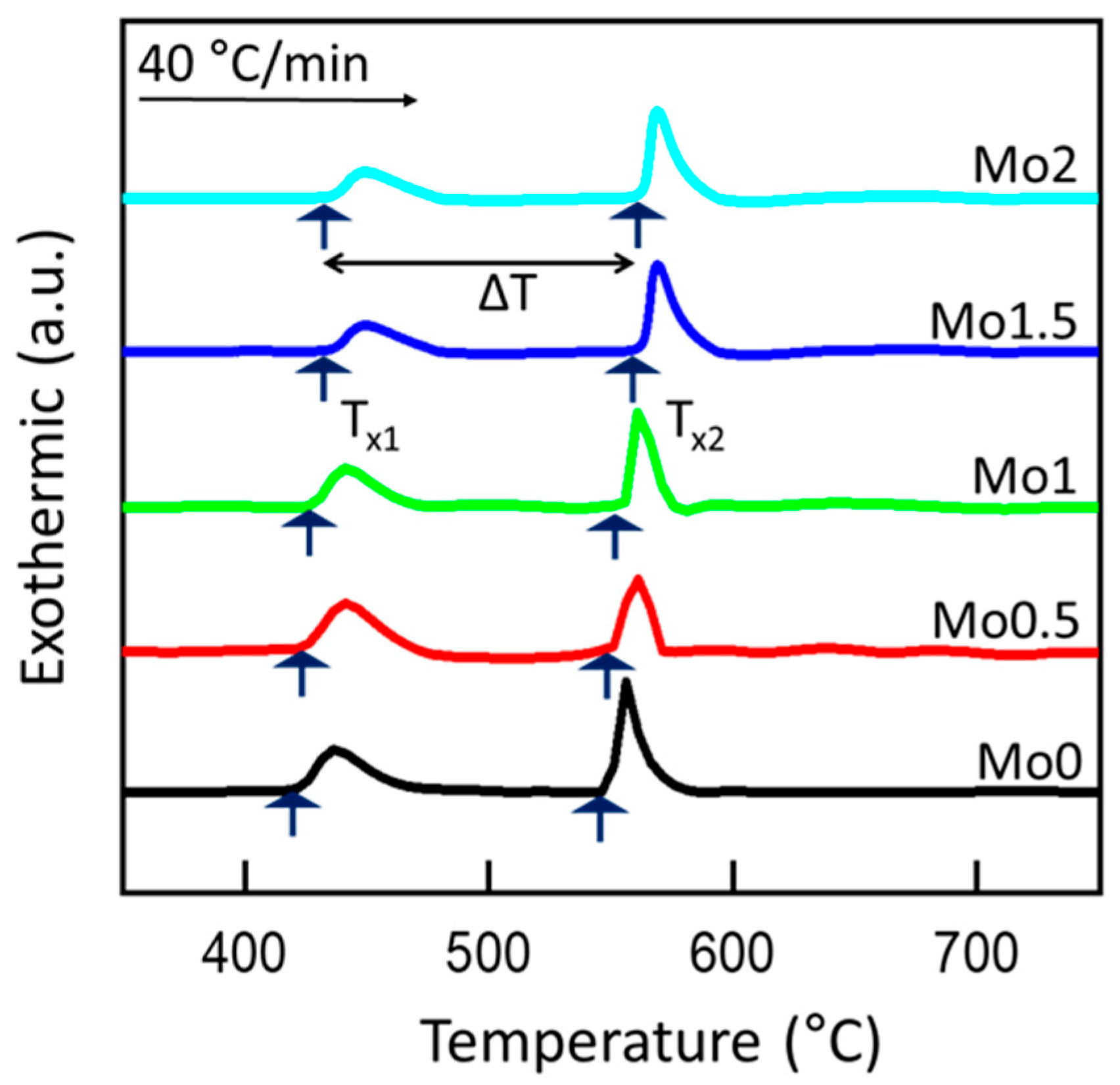
3.2. Influence of Mo Addition on Thermal Stability and Nanocrystalline Structure Formation
3.3. Optimization of Soft Magnetic Properties in Fe82−xSi4B12Nb1MoxCu1 (x = 0–2) Nanocrystalline Ribbons via Mo Additions
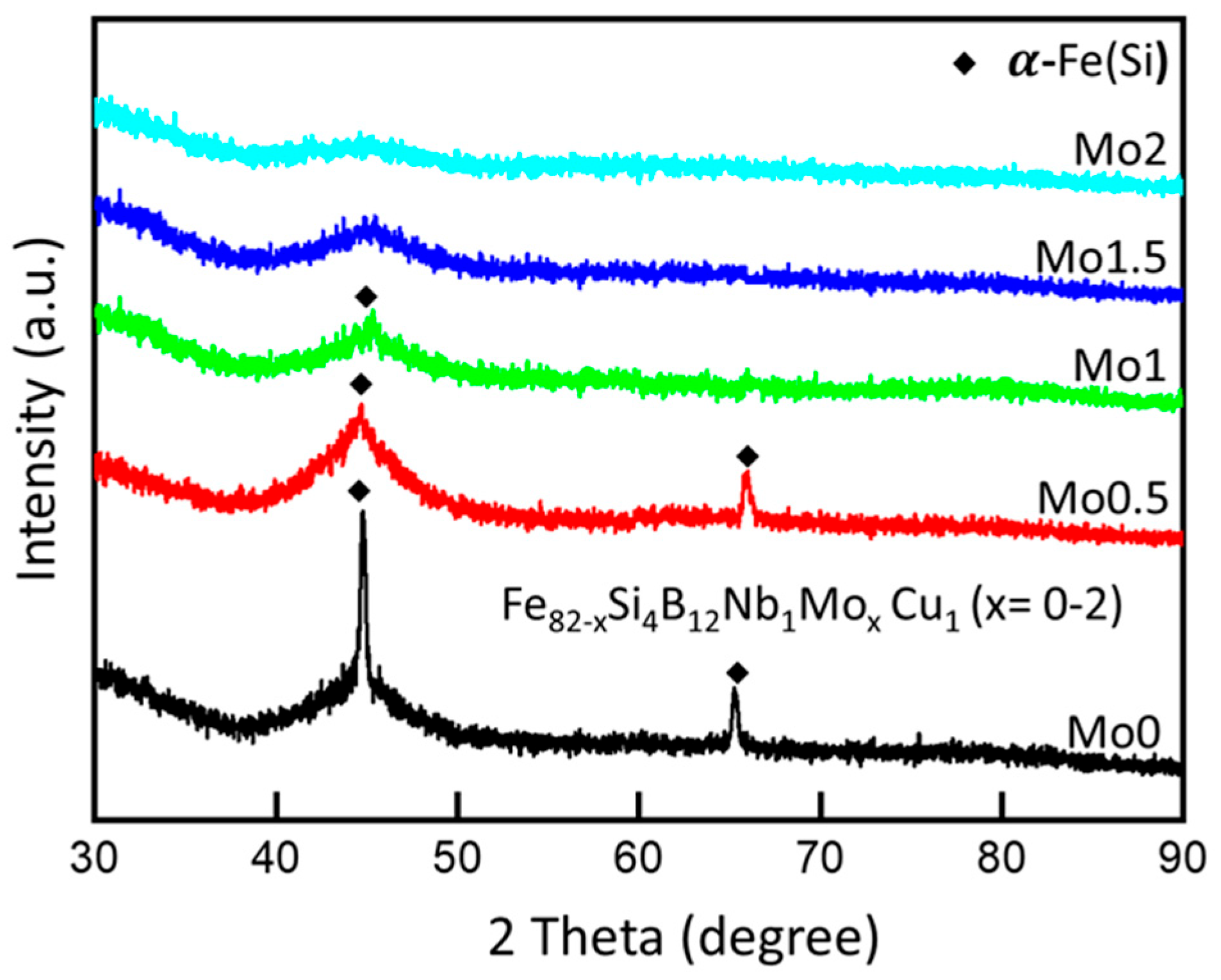
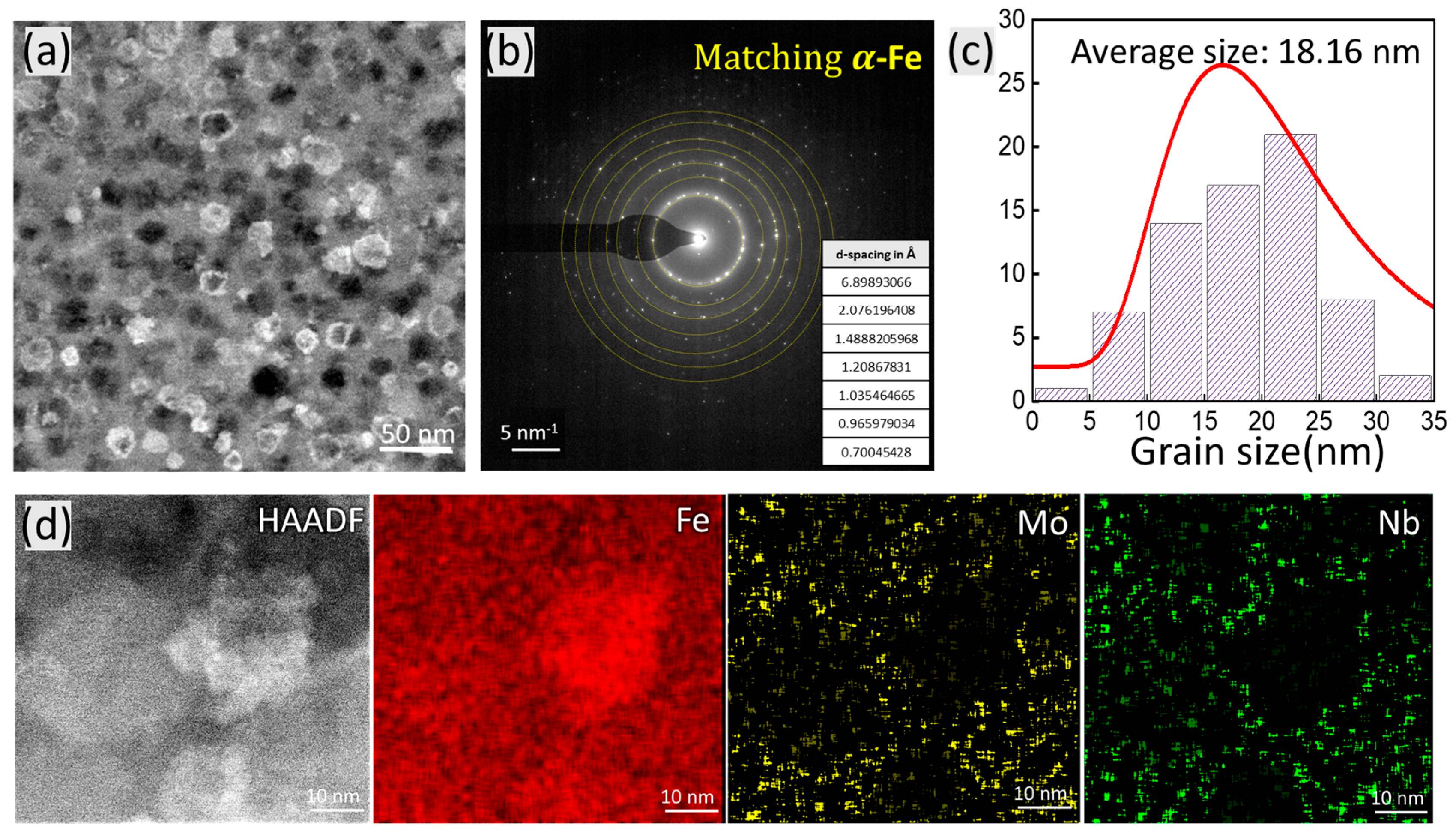
3.4. Structural Evolution in Fe82−xSi4B12Nb1MoxCu1 (x = 0–2) Nanocrystalline Ribbons
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Herzer, G. Nanocrystalline soft magnetic alloys. Handb. Magn. Mater. 1997, 10, 415–462. [Google Scholar]
- Raja, M.M.; Chattopadhyay, K.; Majumdar, B.; Narayanasamy, A. Structure and soft magnetic properties of Finemet alloys. J. Alloys Compd. 2000, 297, 199–205. [Google Scholar] [CrossRef]
- Blázquez, J.S.; Borrego, J.M.; Conde, C.F.; Conde, A.; Greneche, J.M. On the effects of partial substitution of Co for Fe in FINEMET and Nb-containing HITPERM alloys. J. Phys. Condens. Matter 2003, 15, 3957–3968. [Google Scholar] [CrossRef]
- Ohta, M.; Yoshizawa, Y. Effect of heating rate on soft magnetic properties in nanocrystalline Fe80 5Cu1 5Si4B14 and Fe82Cu1Nb1Si4B12 alloys. Appl. Phys. Express 2009, 2, 023005. [Google Scholar] [CrossRef]
- Setyawan, A.D.; Takenaka, K.; Sharma, P.; Nishijima, M.; Nishiyama, N.; Makino, A. Magnetic properties of 120-mm wide ribbons of high Bs and low core-loss NANOMET® alloy. J. Appl. Phys. 2015, 117, 17B715. [Google Scholar] [CrossRef]
- Matsuura, M.; Nishijima, M.; Takenaka, K.; Takeuchi, A.; Ofuchi, H.; Makino, A. Evolution of fcc Cu clusters and their structure changes in the soft magnetic Fe85.2Si1B9P4Cu0.8 (NANOMET) and FINEMET alloys observed by X-ray absorption fine structure. J. Appl. Phys. 2015, 117, 17D124. [Google Scholar] [CrossRef]
- Liu, L.; Zhou, B.; Zhang, Y.; He, A.; Zhang, T.; Li, F.; Dong, Y.; Wang, X. FeSiBPNbCu bulk nanocrystalline alloys with high GFA and excellent soft-magnetic properties. Metals 2019, 9, 219. [Google Scholar] [CrossRef]
- Yoshizawa, Y.; Oguma, S.; Yamauchi, K. New Fe-based soft magnetic alloys composed of ultrafine grain structure. J. Appl. Phys. 1988, 64, 6044–6046. [Google Scholar] [CrossRef]
- Makino, A.; Kubota, T.; Makabe, M.; Chang, C.T.; Inoue, A. Fe-metalloid metallic glasses with high magnetic flux density and high glass-forming ability. Mater. Sci. Forum 2007, 539–543, 1361–1366. [Google Scholar] [CrossRef]
- Karataş, M.M. Synthesis and Characterization of Bulk Amorphous/Nanocrystalline Soft Magnetic Materials. Ph.D. Thesis, Middle East Technical University, Ankara, Türkiye, 2016. [Google Scholar]
- Su, Y.-G.; Chen, F.; Wu, C.-Y.; Chang, M.-H.; Chung, C.-A. Effects of manufacturing parameters in planar flow casting process on ribbon formation and puddle evolution of Fe–Si–B alloy. ISIJ Int. 2015, 55, 2383–2390. [Google Scholar] [CrossRef]
- Lashgari, H.; Chu, D.; Xie, S.; Sun, H.; Ferry, M.; Li, S. Composition dependence of the microstructure and soft magnetic properties of Fe-based amorphous/nanocrystalline alloys: A review study. J. Non-Cryst. Solids 2014, 391, 61–82. [Google Scholar] [CrossRef]
- Herzer, G. Nanocrystalline soft magnetic materials. J. Magn. Magn. Mater. 1992, 112, 258–262. [Google Scholar] [CrossRef]
- Yoshizawa, Y.; Yamauchi, K. Magnetic properties of Fe–Cu–M–Si–B (M = Cr, V, Mo, Nb, Ta, W) alloys. Mater. Sci. Eng. A 1991, 133, 176–179. [Google Scholar] [CrossRef]
- Wang, A.D.; Zhao, C.L.; He, A.N.; Men, H.; Chang, C.T.; Wang, X.M. Composition design of high Bs Fe-based amorphous alloys with good amorphous-forming ability. J. Alloys Compd. 2016, 656, 729–734. [Google Scholar] [CrossRef]
- Shi, L.; Yao, K. Composition design for Fe-based soft magnetic amorphous and nanocrystalline alloys with high Fe content. Mater. Des. 2020, 189, 108511. [Google Scholar] [CrossRef]
- Herzer, G. Modern soft magnets: Amorphous and nanocrystalline materials. Acta Mater. 2013, 61, 718–734. [Google Scholar] [CrossRef]
- Saunders, N.; Miodownik, A.P. Thermodynamic aspects of amorphous phase formation. J. Mater. Res. 1986, 1, 38–46. [Google Scholar] [CrossRef]
- Lee, S.-W.; Huh, M.-Y.; Chae, S.-W.; Lee, J.-C. Mechanism of the deformation-induced nanocrystallization in a Cu-based bulk amorphous alloy under uniaxial compression. Scr. Mater. 2006, 54, 1439–1444. [Google Scholar] [CrossRef]
- Hono, K.; Ping, D.; Ohnuma, M.; Onodera, H. Cu clustering and Si partitioning in the early crystallization stage of an Fe73.5Si13.5B9Nb3Cu1 amorphous alloy. Acta Mater. 1999, 47, 997–1006. [Google Scholar] [CrossRef]
- Ayers, J.D.; Harris, V.G.; Sprague, J.A.; Elam, W.T.; Jones, H. On the formation of nanocrystals in the soft magnetic alloy Fe73.5Nb3Cu1Si13.5B9. Acta Mater. 1998, 46, 1861–1874. [Google Scholar] [CrossRef]
- Ohta, M.; Yoshizawa, Y. Recent progress in high Bs Fe-based nanocrystalline soft magnetic alloys. J. Phys. D Appl. Phys. 2011, 44, 064004. [Google Scholar] [CrossRef]
- Wu, Y.; Bitoh, T.; Hono, K.; Makino, A.; Inoue, A. Microstructure and properties of nanocrystalline Fe–Zr–Nb–B soft magnetic alloys with low magnetostriction. Acta Mater. 2001, 49, 4069–4077. [Google Scholar] [CrossRef]
- Ohta, M.; Yoshizawa, Y.; Takezawa, M.; Yamasaki, J. Effect of surface microstructure on magnetization process in Fe80.5Cu1.5Si4B14 nanocrystalline alloys. IEEE Trans. Magn. 2010, 46, 203–206. [Google Scholar] [CrossRef]
- Takeuchi, A.; Inoue, A. Analyses of characteristics of atomic pairs in ferrous bulk metallic glasses using classification of bulk metallic glasses and Pettifor map. J. Optoelectron. Adv. Mater. 2006, 8, 1679–1684. [Google Scholar]
- Liu, F.; Yang, Q.; Pang, S.; Zhang, T. Effect of Mo element on the properties of Fe–Mo–P–C–B bulk metallic glasses. J. Non-Cryst. Solids 2009, 355, 1444–1447. [Google Scholar] [CrossRef]
- Takeuchi, A.; Inoue, A. Classification of bulk metallic glasses by atomic size difference, heat of mixing and period of constituent elements and its application to characterization of the main alloying element. Mater. Trans. 2005, 46, 2817–2829. [Google Scholar] [CrossRef]
- Ozawa, T. Non-isothermal kinetics of diffusion and its application to thermal analysis. J. Therm. Anal. 1973, 5, 563–576. [Google Scholar] [CrossRef]
- Takemoto, S.; Nitta, H.; Iijima, Y.; Yamazaki, Y. Diffusion of tungsten in α-iron. Philos. Mag. 2007, 87, 1619–1629. [Google Scholar] [CrossRef]
- Oono, N.; Nitta, H.; Iijima, Y. Diffusion of niobium in α-iron. Mater. Trans. 2003, 44, 2078–2083. [Google Scholar] [CrossRef]
- Perez, R.A.; Nakajima, H.; Dyment, F. Diffusion in α-Ti and Zr. Mater. Trans. 2003, 44, 2–13. [Google Scholar] [CrossRef]
- Li, X.; Qin, C.L.; Kato, H.; Makino, A.; Inoue, A. Mo microalloying effect on the glass-forming ability, magnetic, mechanical and corrosion properties of (Fe0.76Si0.096B0.084P0.06)100-xMox bulk glassy alloys. J. Alloys Compd. 2011, 509, 7688–7691. [Google Scholar] [CrossRef]
- Sohrabi, S.; Arabi, H.; Beitollahi, A.; Gholamipour, R. Planar flow casting of Fe71Si13.5B9Nb3Cu1 Al1.5Ge1 ribbons. J. Mater. Eng. Perform. 2013, 22, 2185–2190. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, Y.; Zhang, T. Application of 3D balanced growth theory to the formation of bulk amorphous alloys. Front. Mater. 2021, 8, 694920. [Google Scholar] [CrossRef]
- Jayalakshmi, S.; Gupta, M. Amorphous alloys/bulk metallic glasses (BMG). In Metallic Amorphous Alloy Reinforcements in Light Metal Matrices; Springer: Singapore, 2015; pp. 59–83. [Google Scholar]
- Gheiratmand, T.; Hosseini, H.M. Finemet nanocrystalline soft magnetic alloy: Investigation of glass forming ability, crystallization mechanism, production techniques, magnetic softness and the effect of replacing the main constituents by other elements. J. Magn. Magn. Mater. 2016, 408, 177–192. [Google Scholar] [CrossRef]
- Liu, T.; Wang, A.; Zhao, C.; Yue, S.; Wang, X.; Liu, C. Compositional design and crystallization mechanism of high Bs nanocrystalline alloys. Mater. Res. Bull. 2019, 112, 323–330. [Google Scholar] [CrossRef]
- Cheng, Y.; Ma, E. Atomic-level structure and structure–property relationship in metallic glasses. Prog. Mater. Sci. 2011, 56, 379–473. [Google Scholar] [CrossRef]
- Chen, Q.; Jin, Z. The Fe–Cu system: A thermodynamic evaluation. Metall. Mater. Trans. A 1995, 26, 417–426. [Google Scholar] [CrossRef]
- An, Z.; Li, A.; Mao, S.; Yang, T.; Zhu, L.; Wang, R.; Wu, Z.; Zhang, B.; Shao, R.; Jiang, C. Negative mixing enthalpy solid solutions deliver high strength and ductility. Nature 2024, 625, 697–702. [Google Scholar] [CrossRef]
- Suzuki, K.; Makino, A.; Inoue, A.; Masumoto, T. Soft magnetic properties of bcc Fe–M–B–Cu (M = Ti, Nb or Ta) alloys with nanoscale grain size. Jpn. J. Appl. Phys. 1991, 30, L1729. [Google Scholar] [CrossRef]
- Takeuchi, A.; Inoue, A. Calculations of mixing enthalpy and mismatch entropy for ternary amorphous alloys. Mater. Trans. JIM 2000, 41, 1372–1378. [Google Scholar] [CrossRef]
- Bhatt, J.; Jiang, W.; Junhai, X.; Qing, W.; Dong, C.; Murty, B.S. Optimization of bulk metallic glass forming compositions in Zr–Cu–Al system by thermodynamic modeling. Intermetallics 2007, 15, 716–721. [Google Scholar] [CrossRef]
- Inoue, A. High strength bulk amorphous alloys with low critical cooling rates (overview). Mater. Trans. JIM 1995, 36, 866–875. [Google Scholar] [CrossRef]
- Louzguine-Luzgin, D.V. Bulk metallic glasses and glassy/crystalline materials. In Novel Functional Magnetic Materials: Fundamentals and Applications; Springer: Berlin/Heidelberg, Germany, 2016; Volume 231, pp. 397–440. [Google Scholar]
- Takeuchi, A.; Amiya, K.; Wada, T.; Yubuta, K.; Zhang, W.; Makino, A. Entropies in alloy design for high-entropy and bulk glassy alloys. Entropy 2013, 15, 3810–3821. [Google Scholar] [CrossRef]
- Xing, Q.-W.; Zhang, Y. Amorphous phase formation rules in high-entropy alloys. Chin. Phys. B 2017, 26, 018104. [Google Scholar] [CrossRef]
- Feng, R.; Gao, M.C.; Lee, C.; Mathes, M.; Zuo, T.; Chen, S.; Hawk, J.A.; Zhang, Y.; Liaw, P.K. Design of light-weight high-entropy alloys. Entropy 2016, 18, 333. [Google Scholar] [CrossRef]
- Li, W.; Xie, C.; Liu, H.; Wang, K.; Liao, Z.; Yang, Y. Minor-metalloid substitution for Fe on glass formation and soft magnetic properties of Fe–Co–Si–B–P–Cu alloys. J. Non-Cryst. Solids 2020, 533, 119937. [Google Scholar] [CrossRef]
- Suryanarayana, C.; Inoue, A. Bulk Metallic Glasses; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Scott, M. Crystallization. In Amorphous Metallic Alloys; Elsevier: Amsterdam, The Netherlands, 1983; pp. 144–168. [Google Scholar]
- Lu, H.-J.; Zou, N.; Zhao, X.-S.; Shen, J.-Y.; Lu, X.-G.; He, Y.-L. Thermodynamic investigation of the Zr–Fe–Nb system and its applications. Intermetallics 2017, 88, 91–100. [Google Scholar] [CrossRef]
- Descamps, M.; Dudognon, E. Crystallization from the amorphous state: Nucleation–growth decoupling, polymorphism interplay, and the role of interfaces. J. Pharm. Sci. 2014, 103, 2615–2628. [Google Scholar] [CrossRef]
- Mattern, N.; Danzig, A.; Müller, M. Effect of Cu and Nb on crystallization and magnetic properties of amorphous Fe77.5Si15.5B7 alloys. Mater. Sci. Eng. A 1995, 194, 77–85. [Google Scholar] [CrossRef]
- Clavaguera-Mora, M.T. Glass formation in metallic systems. Ber. Bunsenges. Phys. Chem. 1998, 102, 1291–1297. [Google Scholar] [CrossRef]
- Suzuki, K. Nanocrystalline soft magnetic materials: A decade of alloy development. J. Metastable Nanocryst. Mater. 1999, 2, 521–530. [Google Scholar]
- Yeo, J.-G.; Kim, D.H.; Choi, Y.J.; Lee, B.W. Improving power-inductor performance by mixing sub-micro Fe powder with amorphous soft magnetic composites. J. Electron. Mater. 2019, 48, 6018–6023. [Google Scholar] [CrossRef]
- Schmitt, E.A.; Law, D.; Zhang, G.G.Z. Nucleation and crystallization kinetics of hydrated amorphous lactose above the glass transition temperature. J. Pharm. Sci. 1999, 88, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Kong, F.; Xie, L.; Wang, A.; Chang, C.; Wang, X.; Liu, C.-T. Fe(Co)SiBPCCu nanocrystalline alloys with high Bs above 1.83 T. J. Magn. Magn. Mater. 2017, 441, 174–179. [Google Scholar] [CrossRef]
- Zhao, C.; Wang, A.; He, A.; Yue, S.; Chang, C.; Wang, X.; Li, R.-W. Correlation between soft-magnetic properties and Tx1–Tc in high Bs FeCoSiBPC amorphous alloys. J. Alloys Compd. 2016, 659, 193–197. [Google Scholar] [CrossRef]
- Protasova, S.G.; Straumal, B.B.; Dobatkin, S.V.; Goll, D.; Schütz, G.; Baretzky, B.; Mazilkin, A.A.; Nekrasov, A.N. Coercivity and domain structure of nanograined Fe–C alloys after high-pressure torsion. J. Mater. Sci. 2008, 43, 3775–3781. [Google Scholar] [CrossRef][Green Version]
- Butvinová, B.; Švec Sr, P.; Janotová, I.G.; Dias, L.V.; Janičkovič, D.; Maťko, I. Magnetic properties and structure of short-term annealed FeCuBPSi nanocrystalline alloys. J. Magn. Magn. Mater. 2024, 590, 171662. [Google Scholar] [CrossRef]
- Herzer, G. Grain size dependence of coercivity and permeability in nanocrystalline ferromagnets. IEEE Trans. Magn. 1990, 26, 1397–1402. [Google Scholar] [CrossRef]
- Li, H.; Wang, A.; Liu, T.; Chen, P.; He, A.; Li, Q.; Luan, J.; Liu, C.-T. Design of Fe-based nanocrystalline alloys with superior magnetization and manufacturability. Mater. Today 2021, 42, 49–56. [Google Scholar] [CrossRef]
- Varela, M.; Lupini, A.R.; Benthem, K.v.; Borisevich, A.Y.; Chisholm, M.F.; Shibata, N.; Abe, E.; Pennycook, S.J. Materials characterization in the aberration-corrected scanning transmission electron microscope. Annu. Rev. Mater. Res. 2005, 35, 539–569. [Google Scholar] [CrossRef]
- Lu, W.; Fan, J.; Wang, Y.; Yan, B. Microstructure and magnetic properties of Fe72.5Cu1M2V2Si13.5B9 (M = Nb, Mo, (NbMo), (MoW)) nanocrystalline alloys. J. Magn. Magn. Mater. 2010, 322, 2935–2937. [Google Scholar] [CrossRef]
- Fiorillo, F.; Bertotti, G.; Appino, C.; Pasquale, M. Soft magnetic materials. In Wiley Encyclopedia of Electrical and Electronics Engineering; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2016; pp. 1–42. [Google Scholar]


| Alloy ID | Compositions |
|---|---|
| Mo 2 | Fe80Si4B12Nb1Mo2Cu1 |
| Mo 1.5 | Fe80.5Si4B12Nb1Mo1.5Cu1 |
| Mo 1 | Fe81Si4B12Nb1Mo1Cu1 |
| Mo 0.5 | Fe81.5Si4B12Nb1Mo0.5Cu1 |
| Mo 0 | Fe82Si4B12Nb1Cu1 |
| (%) | Fe | Si | B | Cu | Nb | Mo | |
|---|---|---|---|---|---|---|---|
| Fe | - | 5.8 | 31.8 | 0.79 | 14.2 | 9.2 | |
| Si | −2 | - | 26.1 | 4.49 | 20.0 | 15.0 | |
| B | −35 | −32 | - | 19.07 | 45.5 | 40.7 | |
| Cu | 13 | −10 | −4.5 | - | 5.54 | 4.13 | |
| Nb | −16 | −39 | −54 | −4.5 | - | 5.0 | |
| Mo | −2 | −34 | −34 | −6.7 | −20 | - | |
| Alloy ID | Mixing Enthalpy, (J∙mol−1) | Mixing Entropy, (J∙K−1∙mol−1) | (%) |
|---|---|---|---|
| Mo 2 | −15.99 | 6.09 | 12.56 |
| Mo 1.5 | −15.89 | 5.93 | 12.35 |
| Mo 1 | −15.78 | 5.75 | 12.13 |
| Mo 0.5 | −15.68 | 5.56 | 11.9 |
| Mo 0 | −15.57 | 5.3 | 11.67 |
| Alloy ID | Tx1 (°C) | Tx2 (°C) | (Tx2 − Tx1) (°C) |
|---|---|---|---|
| Mo 2 | 437 | 569 | 131 |
| Mo 1.5 | 435 | 561 | 126 |
| Mo 1 | 430 | 556 | 126 |
| Mo 0.5 | 426 | 548 | 122 |
| Mo 0 | 422 | 544 | 120 |
| Annealing Temperature (°C) | Alloy ID | 430 °C | 470 °C | 510 °C | 550 °C |
|---|---|---|---|---|---|
| Coercivity, Hc (A/m) | Mo 0 | 121 | 21 | 81.4 | 212 |
| Mo 0.5 | 91.5 | 20.8 | 303 | 368 | |
| Mo 1 | 74.7 | 24.3 | 51.1 | 48.3 | |
| Mo 1.5 | 26.8 | 23.8 | 16.4 | 8.09 | |
| Mo 2 | 15.8 | 4.54 | 8.09 | 13.1 | |
| Relative permeability, | Mo 0 | 1875 | 26,000 | 2416 | 1025 |
| Mo 0.5 | 1871 | 33,150 | 3251 | 1125 | |
| Mo 1 | 3213 | 36,440 | 5604 | 1860 | |
| Mo 1.5 | 5437 | 35,420 | 6604 | 3798 | |
| Mo 2 | 4662 | 48,410 | 7923 | 4504 | |
| Saturation magnetization, Ms (emu/g) | Mo 1.5 | 179.84 | 182.08 | 181.34 | 185.55 |
| Mo 2 | 172.27 | 175.24 | 177.53 | 179.98 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Im, H.A.; An, S.; Kim, K.-b.; Yang, S.; Lee, J.w.; Jeong, J.W. Glass-Forming Ability and Crystallization Behavior of Mo-Added Fe82−xSi4B12Nb1MoxCu1 (x = 0–2) Nanocrystalline Alloy. Metals 2025, 15, 744. https://doi.org/10.3390/met15070744
Im HA, An S, Kim K-b, Yang S, Lee Jw, Jeong JW. Glass-Forming Ability and Crystallization Behavior of Mo-Added Fe82−xSi4B12Nb1MoxCu1 (x = 0–2) Nanocrystalline Alloy. Metals. 2025; 15(7):744. https://doi.org/10.3390/met15070744
Chicago/Turabian StyleIm, Hyun Ah, Subong An, Ki-bong Kim, Sangsun Yang, Jung woo Lee, and Jae Won Jeong. 2025. "Glass-Forming Ability and Crystallization Behavior of Mo-Added Fe82−xSi4B12Nb1MoxCu1 (x = 0–2) Nanocrystalline Alloy" Metals 15, no. 7: 744. https://doi.org/10.3390/met15070744
APA StyleIm, H. A., An, S., Kim, K.-b., Yang, S., Lee, J. w., & Jeong, J. W. (2025). Glass-Forming Ability and Crystallization Behavior of Mo-Added Fe82−xSi4B12Nb1MoxCu1 (x = 0–2) Nanocrystalline Alloy. Metals, 15(7), 744. https://doi.org/10.3390/met15070744







