Effect of Oxygen and Zirconium on Oxidation and Mechanical Behavior of Fully γ Ti52AlxZr Alloys
Abstract
1. Introduction
2. Materials and Methods
3. Results
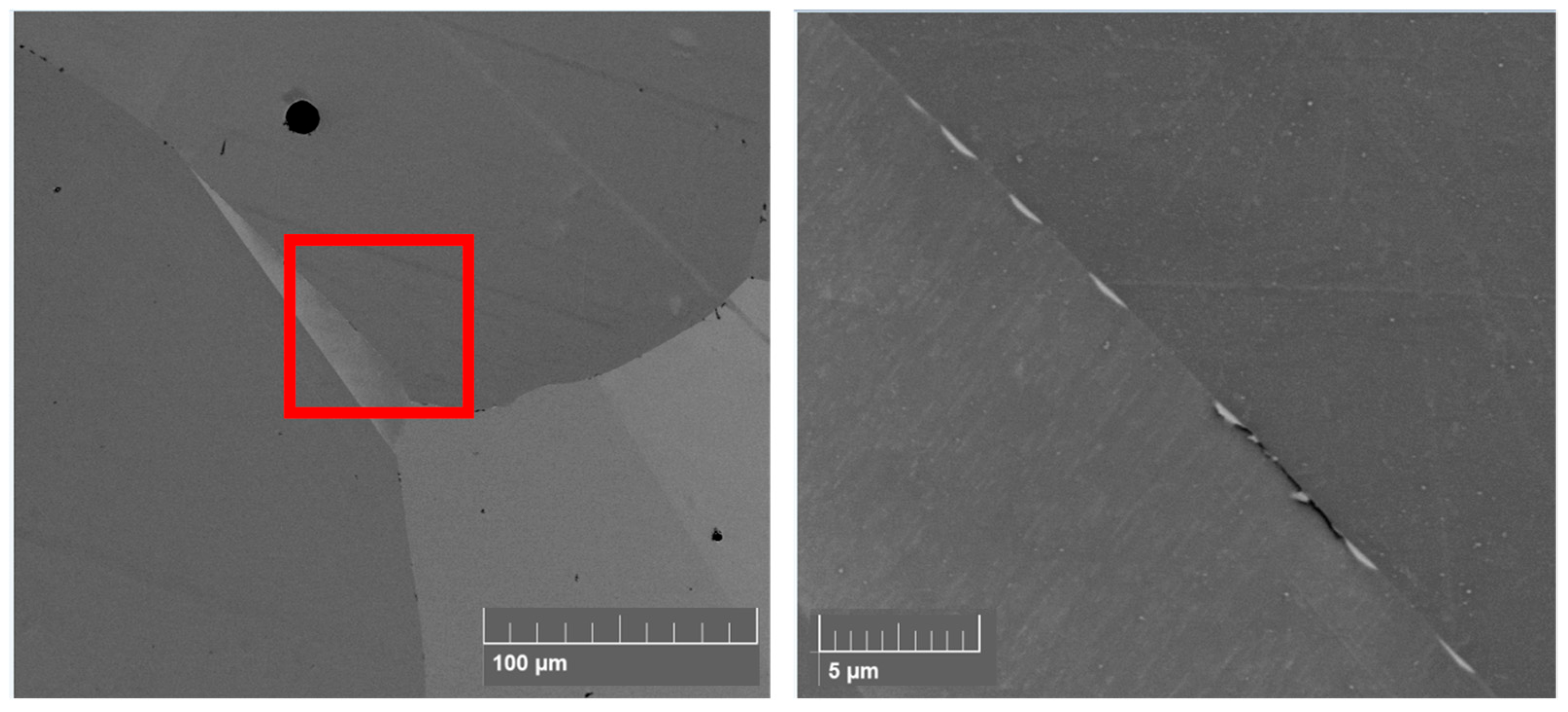
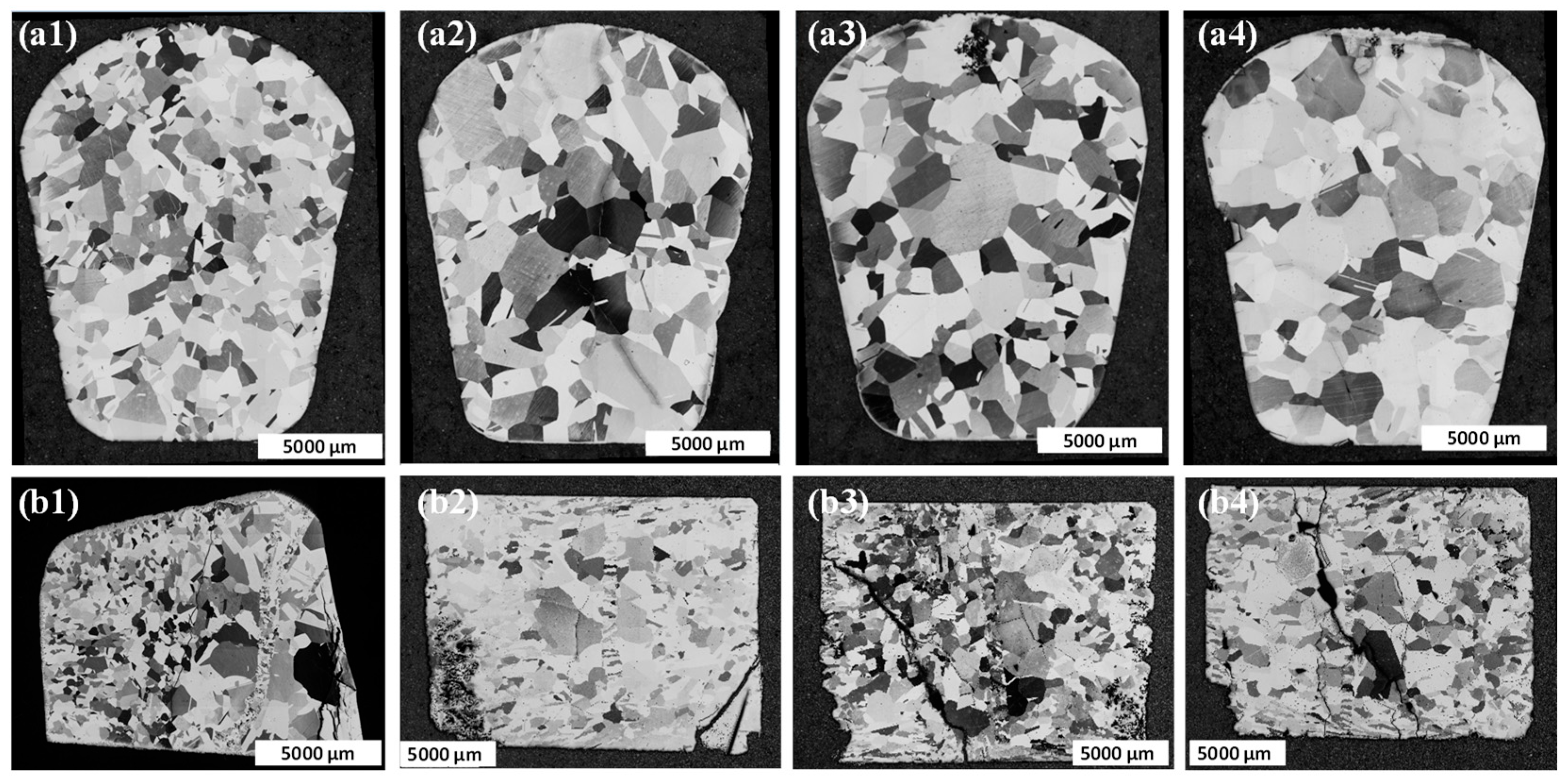
3.1. Microstructural Analysis of LOx and HOx Ti52AlxZr Alloys
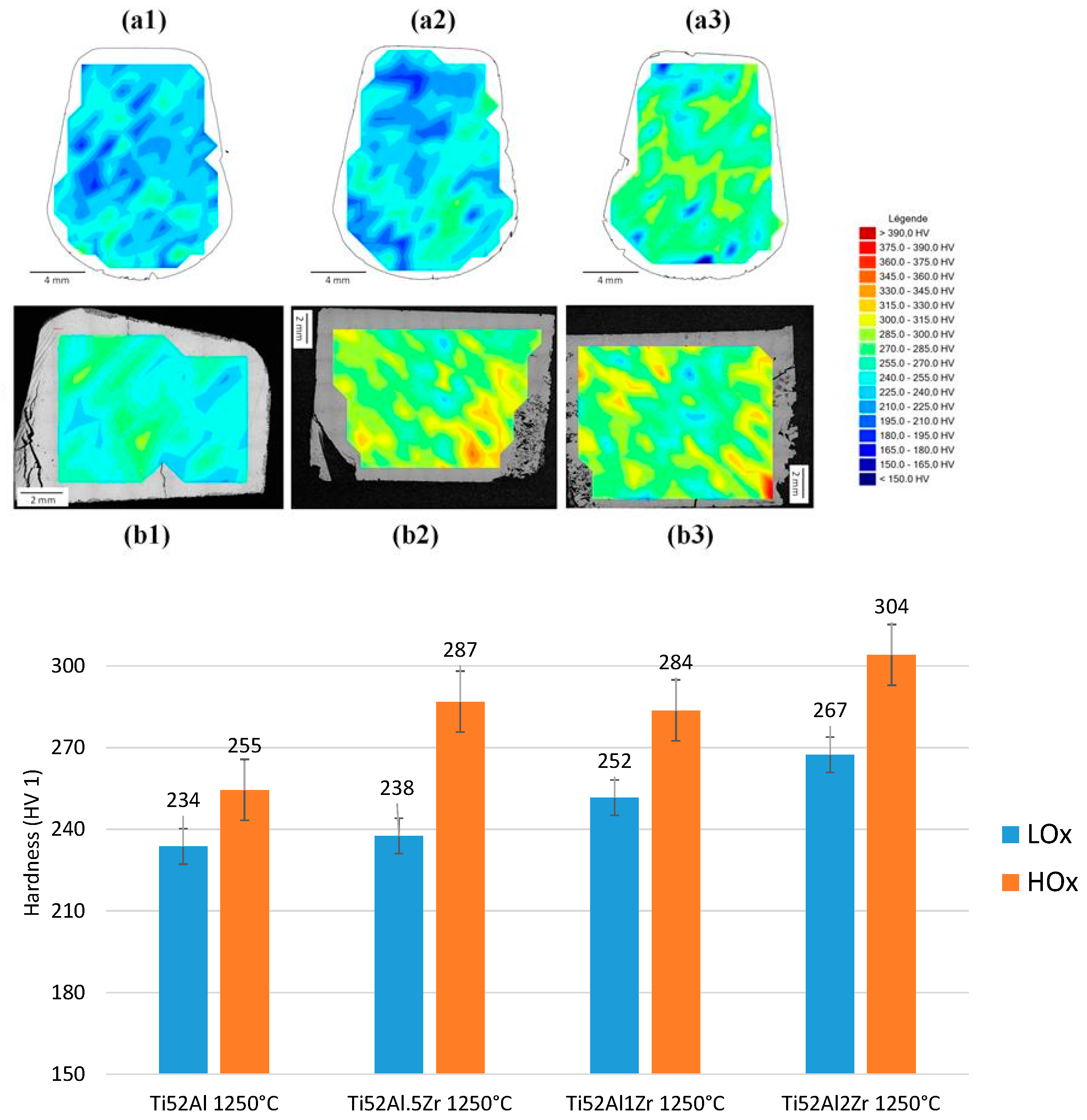
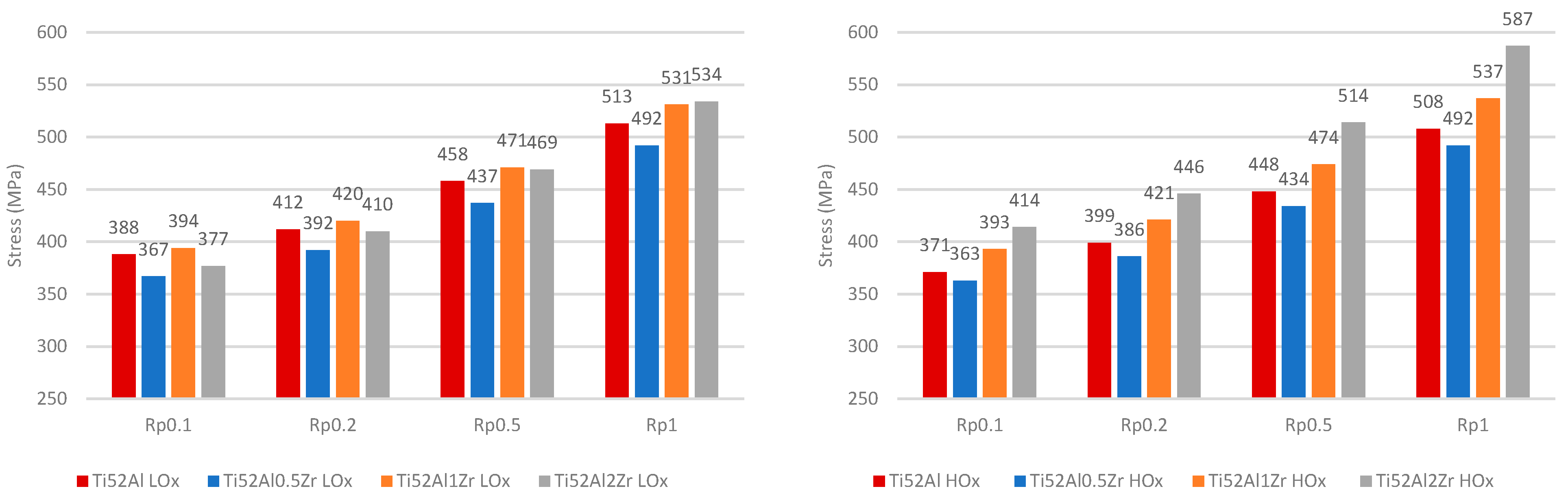
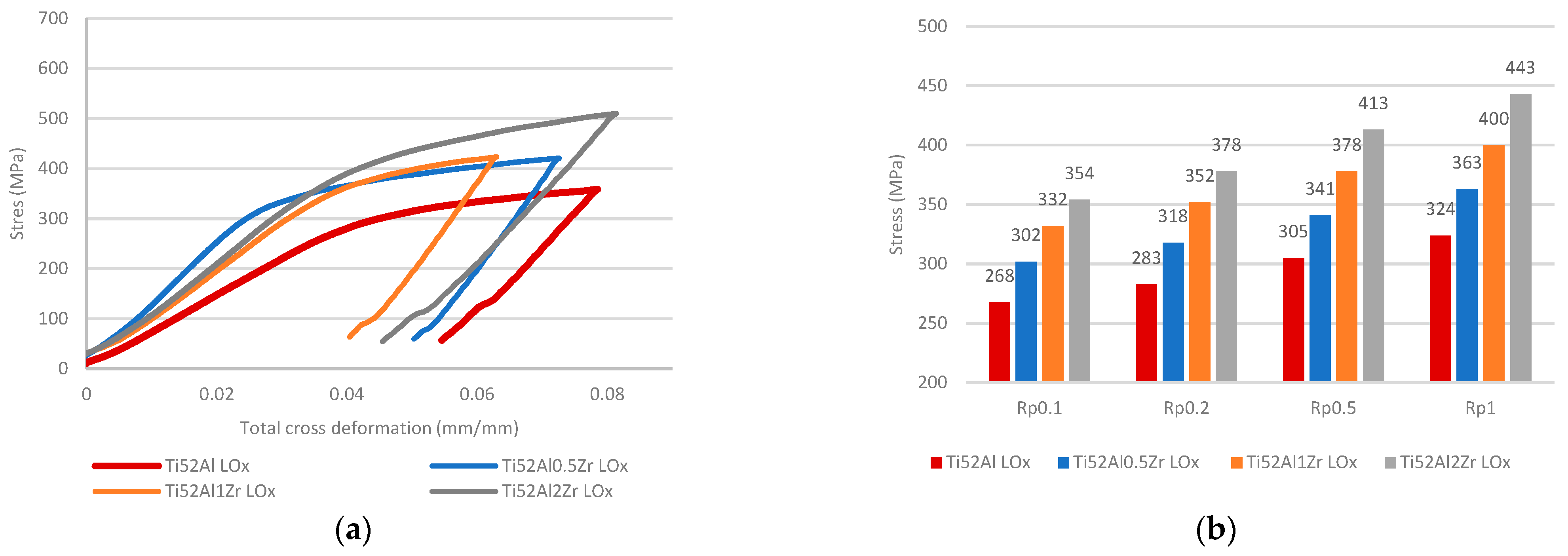
3.2. Mechanical Analysis of LOx and HOx Ti52AlxZr Alloys
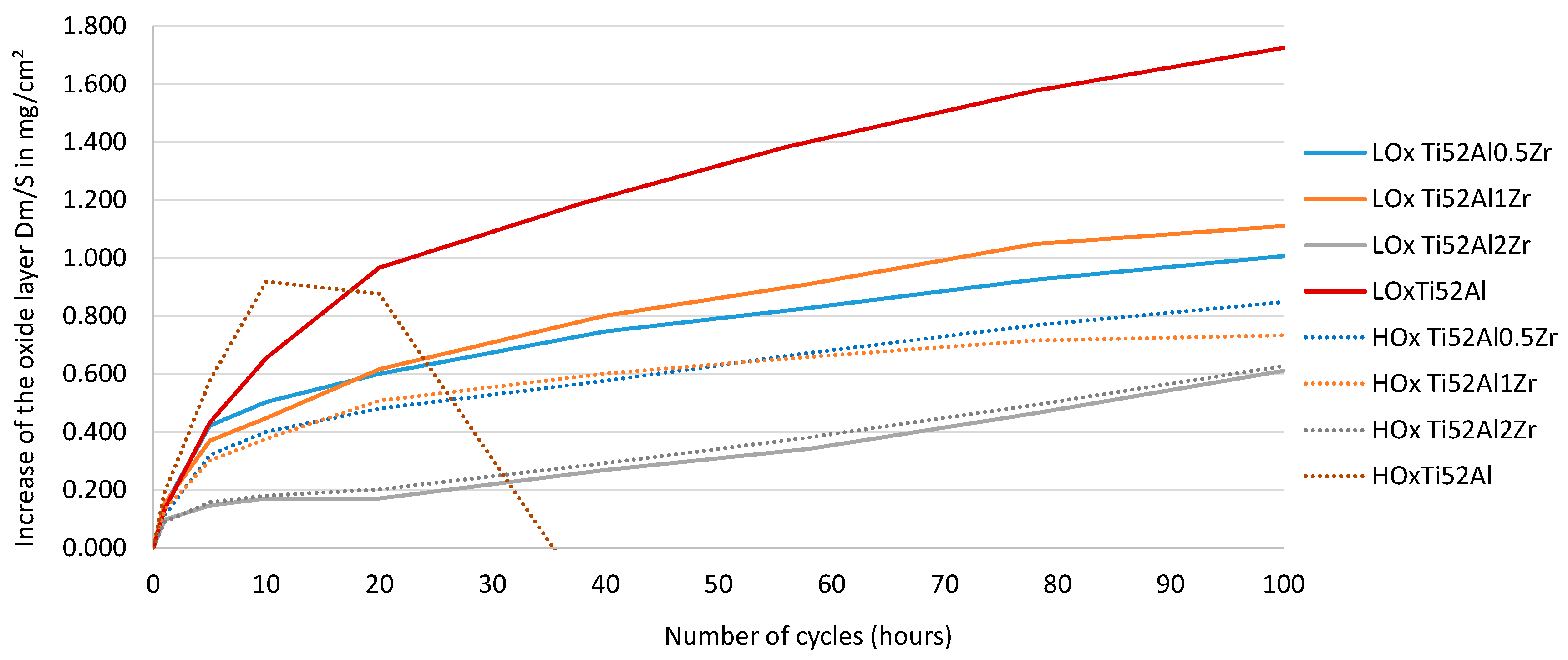
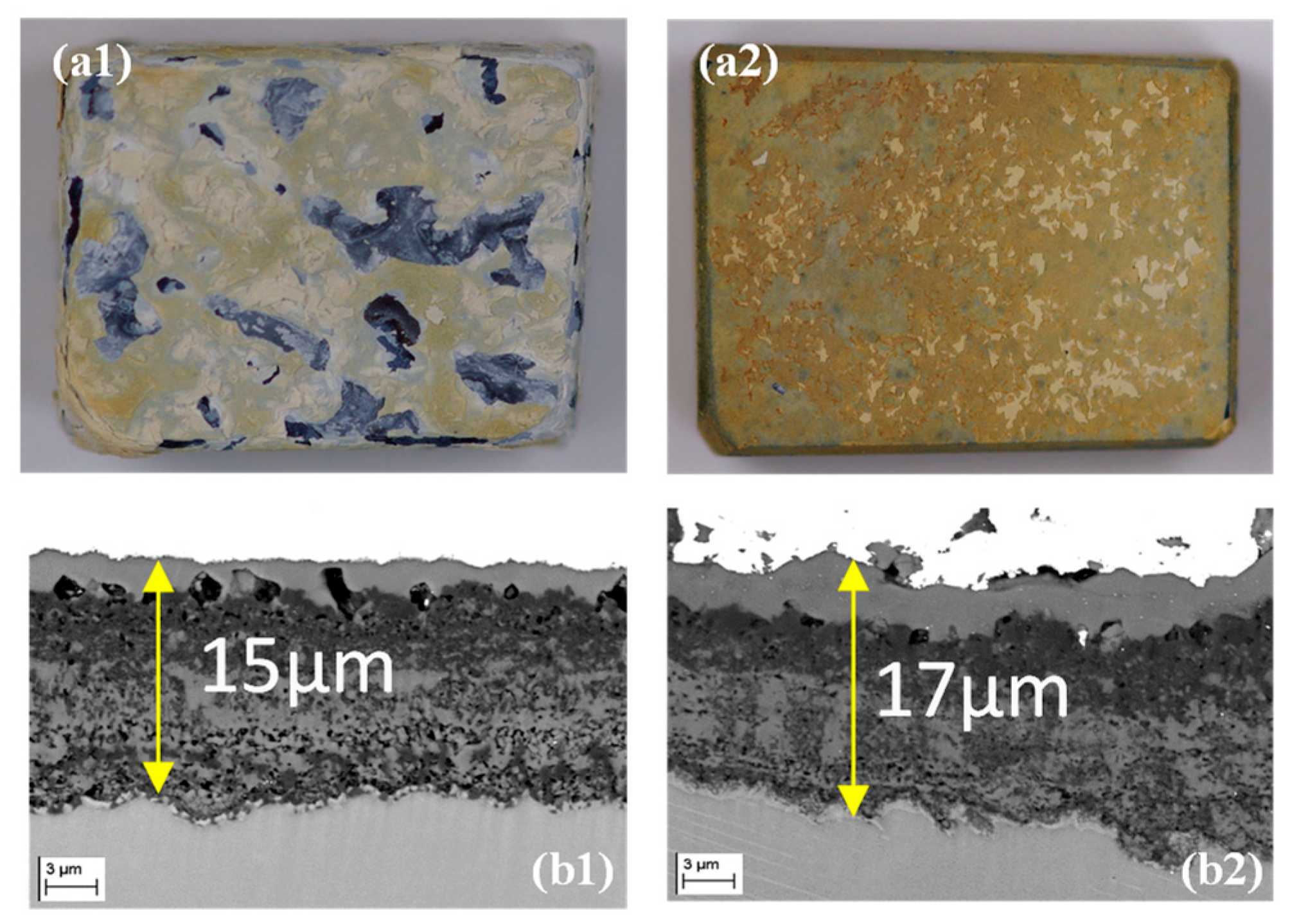
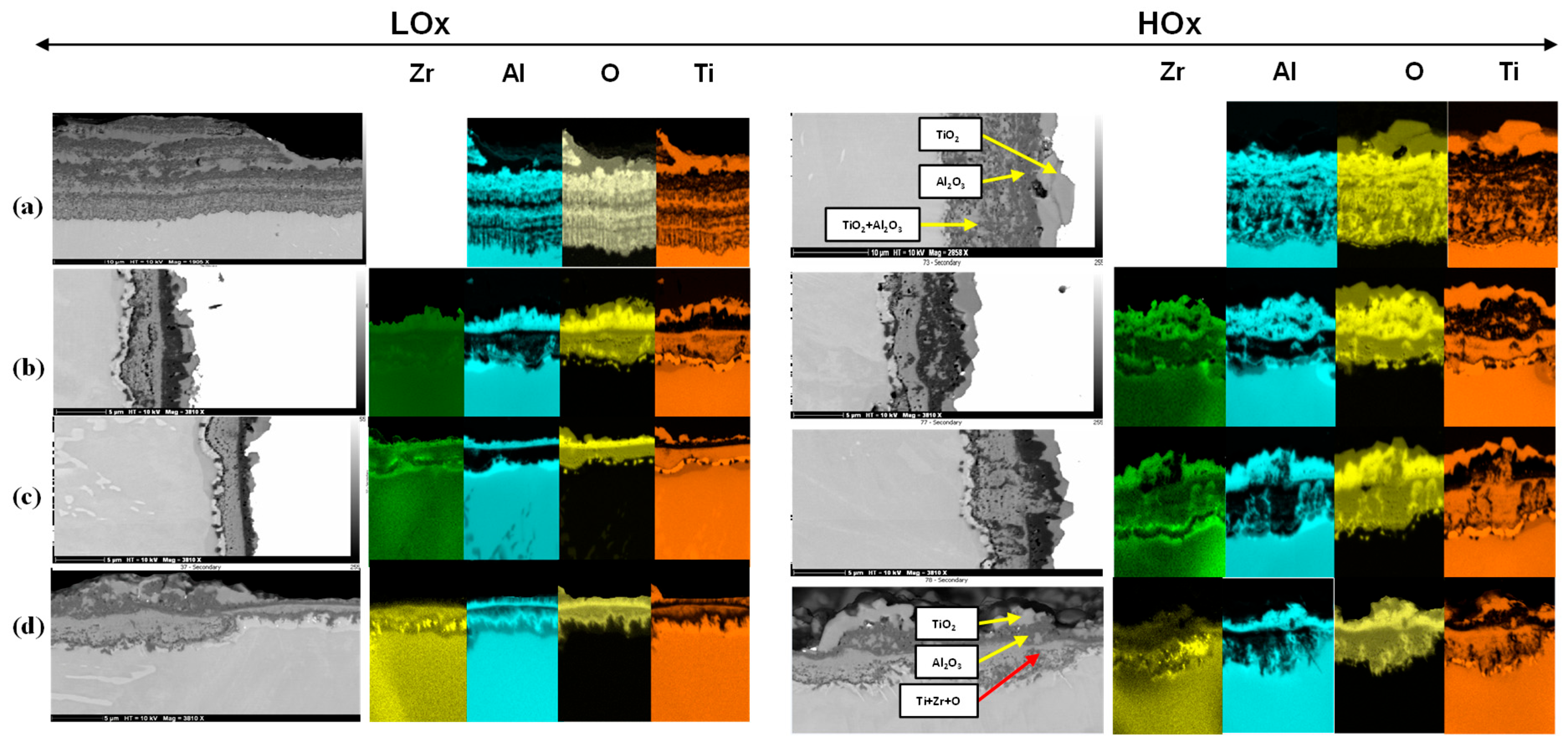
3.3. Oxidation Resistance of Ti52Al and Ti52AlxZr Alloys
4. Discussion
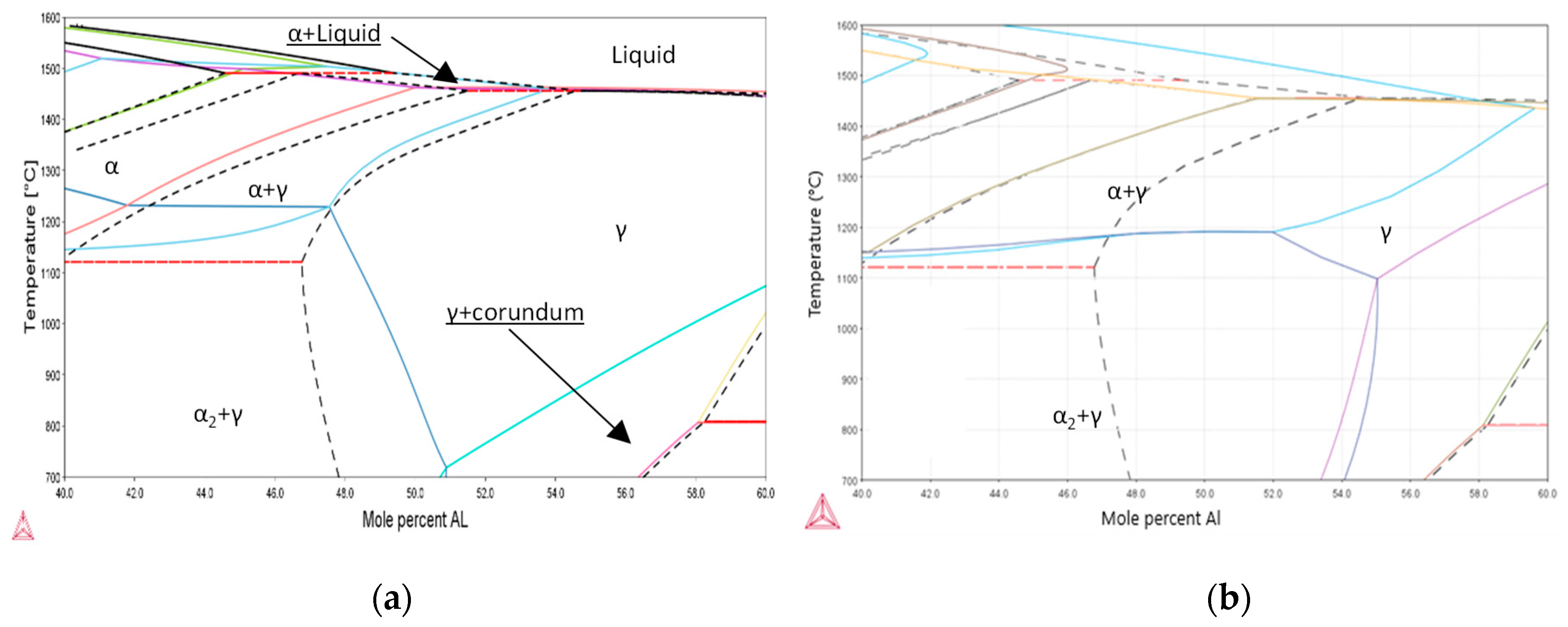
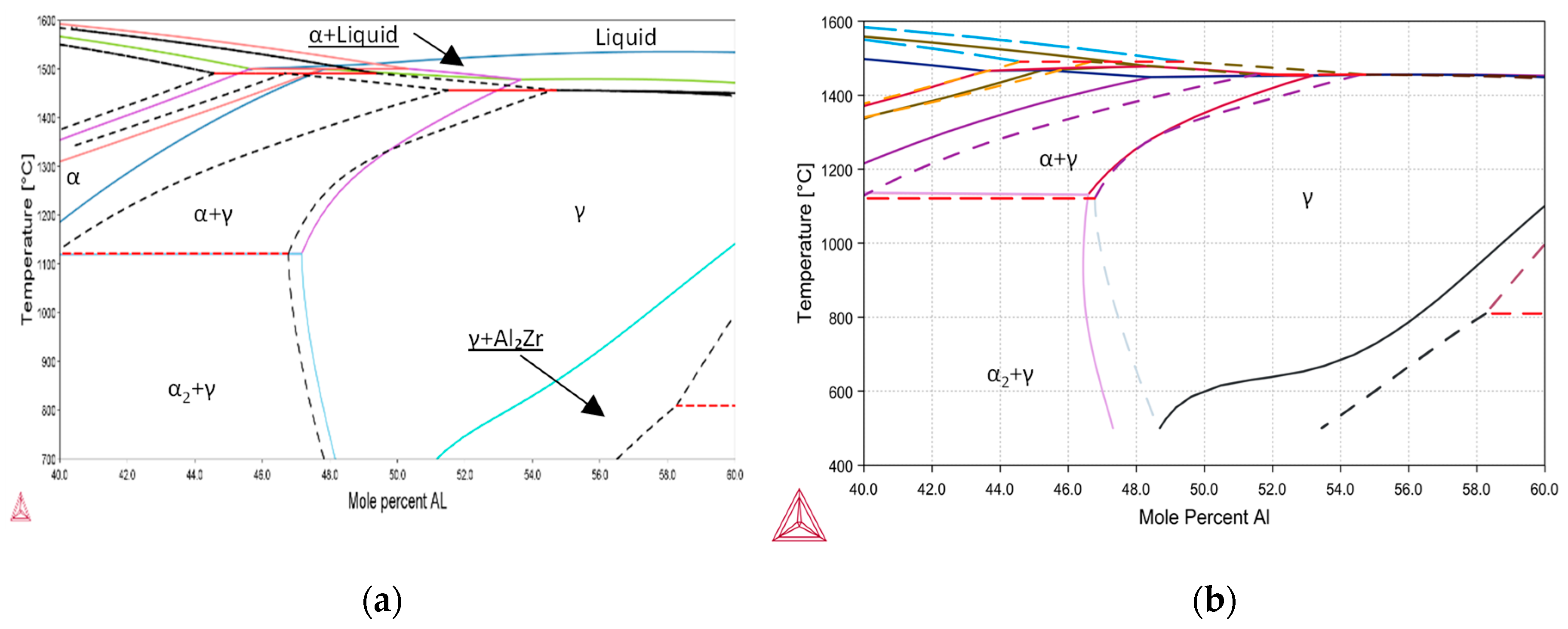
4.1. Alloys’ Thermodynamic Prediction
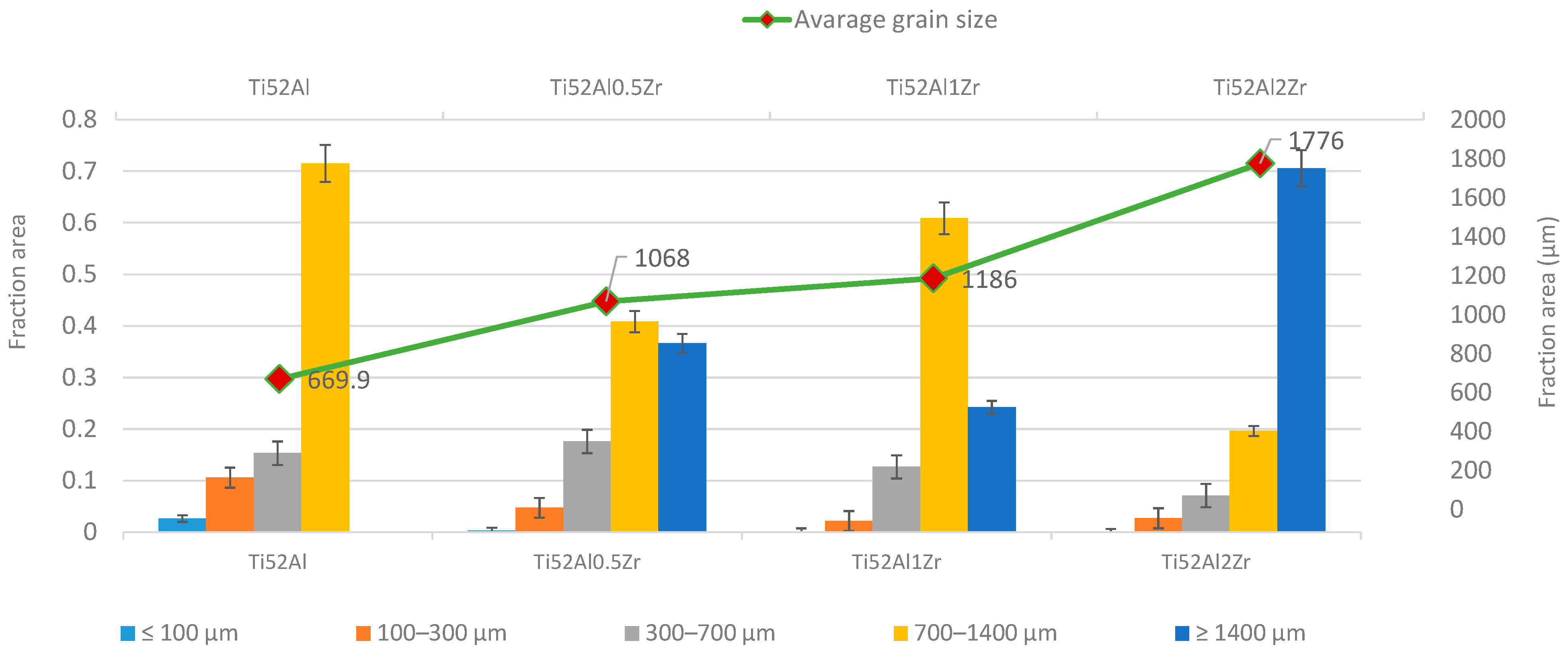
4.2. Microstructure Analysis
4.3. Oxidation Resistance Analysis
- Formation of stable ZrO2 oxide: Zr readily oxidizes at high temperatures to form ZrO2, a thermodynamically stable oxide with low O diffusivity. The presence of ZrO2 at the metal–oxide interface acts as an effective barrier that limits inward O ingress and outward diffusion of metal cations. This suppresses the growth of TiO2 and Al2O3 layers.
- Improved oxide scale adherence: Zr tends to segregate at grain boundaries and at the metal–oxide interface, where it enhances adhesion between the oxide scale and the γ-TiAl substrate. It reduces scale spallation, particularly during thermal cycling, by relieving internal stresses and minimizing thermal expansion mismatch.
- Modification of oxide growth kinetics: In the presence of Zr, the parabolic oxidation rate constant (kp) decreases, indicating slower, diffusion-controlled oxide growth. This effect is especially prominent in Ti-rich regions where rutile-type TiO2 typically dominates. A fine and homogeneously distributed microstructure containing Zr promotes uniform oxide growth and reduces overall scale thickening.
- Suppression of volatile Ti–O species: At elevated temperatures, Ti tends to form volatile oxides such as TiO2. Zr helps stabilize the oxide layer and suppresses TiO2 volatilization, thereby indirectly protecting the underlying TiAl matrix from rapid oxidation.
- Synergistic effects with other elements: In combination with elements such as yttrium (Y), Zr exhibits synergistic effects that further improve oxidation resistance. The joint addition of Y and Zr promotes grain refinement, inhibits cationic diffusion, and significantly increases oxide scale stability under cyclic oxidation conditions.
4.4. Mechanical Analysis
- With increasing Zr content, solidification-induced segregation becomes more pronounced, promoting the formation and volumetric dominance of massive γ grains. This structural evolution is particularly relevant under high-temperature exposure, where Zr acts as a γ-phase stabilizer. Through solid solution strengthening, Zr increases resistance to plastic deformation, leading to a noticeable improvement in high-temperature strength.
- Moreover, in fully γ-TiAl systems, the high solubility of Zr in the γ lattice facilitates the stabilization of a single-phase microstructure, though excessive Zr addition may induce microscale segregation. Such segregation can locally reduce ductility and negatively impact fracture toughness. Despite this trade-off, Zr additions up to moderate levels have been shown to refine microstructure and delay grain boundary diffusion, which is beneficial not only for creep resistance but also for long-term structural stability. In this context, Zr serves as an effective alternative to traditional alloying elements such as niobium (Nb) in β-free TiAl systems.
- Furthermore, the role of O must be considered, as it acts as an interstitial solute solution strengthening in the γ phase. While low concentrations of O contribute to solid solution strengthening and enhanced hardness, they simultaneously reduce elongation and fracture toughness. The interaction between Zr and O is critical—at suboptimal Zr levels (<1 at.%), the strengthening effect of O is not effectively supported, resulting in an overall reduction in mechanical performance. Therefore, a synergistic balance between Zr and O content is essential for optimizing both strength and ductility in fully γ-TiAl alloys.
5. Conclusions
- An increased O content does not significantly increase the α2-phase fraction in Ti52AlxZr alloys after high-temperature treatment at 1250 °C when compared to the reference Ti52Al alloy.
- Increasing Zr content does not lead to α2-phase stabilization, suggesting that, unlike O, Zr does not act as a stabilizer of the α2 phase but may mitigate the negative structural effects caused by O.
- The microstructure of LOx samples contains fine α2 colonies located at grain boundaries.
- With increasing Zr content, a noticeable grain coarsening is observed in Ti52AlxZr alloys in comparison to the binary Ti52Al alloy.
- The presence of both O and Zr, particularly at Zr concentrations above 2 at.%, reduces the oxidation layer growth rate, and alloys with higher O content show a comparable mass gain for both LOx and HOx samples.
- Zr modifies the chemical composition of the oxide layers by decreasing the homogeneity of TiO2 and increasing the homogeneity of Al2O3, leading to changes in the characteristics of the oxide layers and a reduction in its overall thickness.
- The combined effect of O and Zr increases hardness and strength. In Ti52AlxZr alloys, Zr contributes to mechanical stability under HTT conditions when compared to the reference Ti52Al alloy.
- Optimize Zr content in the range of 1–2 at.% to improve both strength and oxidation resistance.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| α2 | Alpha-2 phase (Ti3Al), an ordered phase in titanium aluminides |
| β(B2) | Beta phase with B2 crystal structure in titanium aluminides |
| γ | Gamma phase of titanium aluminide (γ-TiAl) |
| γ-TiAl | Gamma titanium aluminides |
| Al2O3 | Aluminum oxide (alumina) |
| DFT | Density functional theory |
| HOx | High oxygen content |
| HTT | High-temperature testing |
| HT | Heat treatment |
| ICP-OES | Inductively coupled plasma optical emission spectrometry |
| LOx | Low oxygen content |
| OM | Optical microscopy |
| RTT | Room temperature testing |
| TCTI1 | Thermo-Calc TiAl database, version 1 |
| TCTI5 | Thermo-Calc TiAl database, version 5 |
| Ti52Al | Specific designation of titanium aluminides where the value 52 corresponds to at.% Al |
| Ti52Al0.5Zr | Specific designation of titanium aluminides, where the value 52 corresponds to at.% Al and 0.5 to at.% Zr |
| TiO2 | Titanium dioxide |
| TNM | Titanium aluminide alloy (43–47% Ti, 43–47% Al, 8–10% Nb, ~1% Mo) |
| VAM | Vacuum arc melting |
References
- Mallikarjuna, B.; Reutzel, E.W. Reclamation of Intermetallic Titanium Aluminide Aero-Engine Components Using Directed Energy Deposition Technology. Manuf. Rev. 2022, 9, 27. [Google Scholar] [CrossRef]
- Knippscheer, S.; Frommeyer, G. Neu Entwickelte TiAl-Basislegierungen für den Leichtbau von Triebwerks- und Motorkomponenten—Eigenschaften, Herstellung, Anwendung. Mater. Werkst. Tech. 2006, 37, 724–730. [Google Scholar] [CrossRef]
- Ganji, D.K.; Gajjela, R. Selection of Elemental Composition of a Nickel-Based Super Alloy Through Neural Networks and Multi-Criteria Decision Making. Aust. J. Mech. Eng. 2023, 21, 1287–1299. [Google Scholar] [CrossRef]
- Gudivada, G.; Pandey, A.K. Recent Developments in Nickel-Based Superalloys for Gas Turbine Applications: Review. J. Alloys Compd. 2023, 963, 171128. [Google Scholar] [CrossRef]
- Caldwell, E.C.; Fela, F.J.; Fuchs, G.E. The Segregation of Elements in High-Refractory-Content Single-Crystal Nickel-Based Superalloys. JOM 2004, 56, 44–48. [Google Scholar] [CrossRef]
- Thomas, M.; Berteaux, O.; Popoff, F.; Bacos, M.-P.; Morel, A.; Passilly, B.; Ji, V. Effects of Exposure at 700 °C on RT Tensile Properties in a PM γ-TiAl Alloy. Intermetallics 2006, 14, 1143–1150. [Google Scholar] [CrossRef]
- Pyczak, F.; Appel, F.; Limberg, W.; Lorenz, U.; Oehring, M.; Paul, J.; Schimansky, F.-P. Novel Processing Techniques for γ-TiAl Alloys. Mater. Sci. Forum 2011, 690, 145–148. [Google Scholar] [CrossRef]
- Sallot, P.; Monchoux, J.-P.; Joulié, S.; Couret, A.; Thomas, M. Impact of β-Phase in TiAl Alloys on Mechanical Properties After High Temperature Air Exposure. Intermetallics 2020, 119, 106729. [Google Scholar] [CrossRef]
- Stangl, C.; Kollmannsberger, E.; Krüger, M.; Huber, O.; Klaus, H.; Saage, H. Reducing the Environmental Embrittlement Effect of TiAl Alloys Exposed to Air at High Temperatures by a Fine-Grained Surface Structure. Intermetallics 2024, 175, 108479. [Google Scholar] [CrossRef]
- Musi, M.; Graf, G.; Clemens, H.; Spoerk-Erdely, P. Alloying Elements in Intermetallic γ-TiAl-Based Alloys—A Review on Their Influence on Phase Equilibria and Phase Transformations. Adv. Eng. Mater. 2024, 26, 2300610. [Google Scholar] [CrossRef]
- Peters, M.; Kumpfert, J.; Ward, C.; Leyens, C. Titanium Alloys for Aerospace Applications. Adv. Eng. Mater. 2003, 5, 419–427. [Google Scholar] [CrossRef]
- Xu, X.; Liang, H.; Ding, H.; Davidson, K.P.; Ramanujan, R.V.; Chen, R.; Guo, J.; Fu, H. Oxidation Behavior and Mechanical Properties of a Directionally Solidified High Nb TiAl-Based Alloy Between 800 °C and 900 °C. Intermetallics 2025, 176, 108538. [Google Scholar] [CrossRef]
- Connétable, D.; Prillieux, A.; Thenot, C.; Monchoux, J.-P. Theoretical Study of Oxygen Insertion and Diffusivity in the γ-TiAl L10 System. J. Phys. Condens. Matter 2020, 32, 175702. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Zhang, L.; Li, J. The Effect of Refined Coherent Grain Boundaries on High-Temperature Oxidation Behavior of TiAl-Based Alloys Through Cyclic Heat Treatment. Metals 2024, 14, 521. [Google Scholar] [CrossRef]
- Distl, B.; Dehm, G.; Stein, F. Effect of Oxygen on High-Temperature Phase Equilibria in Ternary Ti–Al–Nb Alloys. J. Inorg. Gen. Chem. 2020, 646, 1151–1156. [Google Scholar] [CrossRef]
- Fröbel, U.; Stark, A.; Paul, J.; Pyczak, F. Assessment of the Capability of Zr, Y, La, Gd, Dy, C, and Si to Enhance the Creep Strength of Gamma Titanium Aluminide Alloys Based on Their Effect on Flow Stress and Thermal Activation Parameters. Intermetallics 2023, 162, 108018. [Google Scholar] [CrossRef]
- Voisin, T.; Monchoux, J.-P.; Thomas, M.; Deshayes, C.; Couret, A. Mechanical Properties of the TiAl IRIS Alloy. Metall. Mater. Trans. A 2016, 47, 6097–6108. [Google Scholar] [CrossRef]
- Tian, S.; Zhang, T.; Zeng, S.; Zhang, Y.; Song, D.; Chen, Y.; Kang, Q.; Jiang, H. Cyclic Oxidation Kinetics and Thermal Stress Evolution of TiAl Alloys at High Temperature. Metals 2024, 14, 28. [Google Scholar] [CrossRef]
- Liang, H.; Ding, H.; Xu, X.; Zhang, X.; Chen, R.; Guo, J.; Fu, H. Effect of Variation in Zr Content on Microstructure and High-Temperature Tensile Properties of a γ-TiAl Alloy. Mater. Sci. Eng. A 2024, 893, 1460. [Google Scholar] [CrossRef]
- Imayev, V.; Ganeev, A.; Trofimov, D.; Parkhimovich, N.; Imayev, R. The Effect of Initial Microstructure on Hot Deformation Behavior of TiAl–Nb Intermetallic Alloy. Mater. Sci. Eng. A 2021, 817, 141388. [Google Scholar] [CrossRef]
- Chen, R.; Zhao, X.; Yang, Y.; Guo, J.; Ding, H.; Su, Y.; Fu, H. Effect of Zr on Microstructure and Mechanical Properties of Binary TiAl Alloys. Trans. Nonferrous Met. Soc. China 2018, 28, 1724–1734. [Google Scholar] [CrossRef]
- Chepak-Gizbrekht, M.V.; Knyazeva, A.G. Oxidation of TiAl Alloy by Oxygen Grain Boundary Diffusion. Intermetallics 2023, 162, 107993. [Google Scholar] [CrossRef]
- Haußmann, L.; Bresler, J.; Neumeier, S.; Pyczak, F.; Göken, M. Interdiffusion Coefficients and Strengthening Effects of Nb, Ta, and Zr in the α2-Ti3Al Phase. J. Phase Equilib. Diffus. 2024, 45, 764–771. [Google Scholar] [CrossRef]
- Matsunaga, A.; Seriizawa, A.; Yamabe-Mitarai, Y. Effect of Zr on Microstructure and Oxidation Behavior of α and α + α2 Ti–Al–Nb Alloys. Mater. Trans. 2016, 57, 1902–1907. [Google Scholar] [CrossRef]
- Distl, B.; Dehm, G.; Pyczak, F. Phase Equilibria and Phase Transformations of Ti–Al–X (X = Nb, Mo, W) Alloys for High-Temperature Structural Applications Between 700 and 1300 °C. Dissertation Thesis, Ruhr University Bochum, Bochum, Germany, 2022. Available online: https://web.archive.org/web/20220827180640id_/https://hss-opus.ub.ruhr-uni-bochum.de/opus4/frontdoor/deliver/index/docId/9130/file/diss.pdf (accessed on 11 December 2024).
- Kulkova, S.E.; Bakulin, A.V.; Kulkov, S.S. First-Principles Calculations of Oxygen Diffusion in Ti–Al Alloys. Latv. J. Phys. Tech. Sci. 2018, 6, 20–29. [Google Scholar] [CrossRef]
- Bolibruchová, D.; Kuriš, M.; Matejka, M.; Kasińska, J. Study of the Influence of Zirconium, Titanium and Strontium on the Properties and Microstructure of AlSi7Mg0.3Cu0.5 Alloy. Materials 2022, 15, 3709. [Google Scholar] [CrossRef]
- Bolibruchová, D.; Matejka, M.; Brůna, M.; Kuriš, M.; Michalcová, A. Investigation of Microstructure and Mechanical Properties of AlSi7Mg0.3Cu0.5 Alloy with Addition of Zr, Ti and Sr. Int. J. Met. Cast. 2023, 17, 2584–2597. [Google Scholar] [CrossRef]
- Molénat, G.; Monchoux, J.-P.; Musi, M.; Spoerk-Erdely, P.; Clemens, H.; Couret, A. A TEM Study of a<001> Dislocations in the β₀ Phase of an Intermetallic TNM-TiAl Alloy. Scr. Mater. 2023, 226, 115247. [Google Scholar] [CrossRef]
- Murugesan, J.; Ping, D.; Yamabe-Mitarai, Y. Effect of Zr Addition on High Temperature Mechanical Properties of Near Alpha Ti–Al–Zr–Sn-Based Alloy. Mater. Sci. Forum 2014, 783–786, 580–583. [Google Scholar] [CrossRef]
- Kahrobaee, Z.; Palm, M. Critical Assessment of the Al–Ti–Zr System. J. Phase Equilib. Diffus. 2020, 41, 687–701. [Google Scholar] [CrossRef]
- Firstov, S.; Kulak, L.; Kuzmenko, M.; Shevchenko, O. Alloys of the Ti–Al–Zr–Si System Intended for Operation at High Temperatures. Mater. Sci. 2019, 54, 783–788. [Google Scholar] [CrossRef]
- Morris, D.G.; Naka, S.; Caron, P. High-Temperature Ordered Intermetallic Alloys; Wiley-VCH Verlag GmbH: Weinheim, Germany, 2000; ISBN 3-527-30192-5. [Google Scholar]
- Belov, N.A.; Akopyan, T.K.; Belov, V.D.; Gershman, J.S.; Gorshenkov, M.V. The Effect of Cr and Zr on the Structure and Phase Composition of TNM Gamma Titanium Aluminide Alloy. Intermetallics 2017, 84, 121–129. [Google Scholar] [CrossRef]
- Bresler, J.; Neumeier, S.; Ziener, M.; Pyczak, F.; Göken, M. The Influence of Niobium, Tantalum and Zirconium on the Microstructure and Creep Strength of Fully Lamellar γ/α2 Titanium Aluminides. Mater. Sci. Eng. A 2019, 744, 46–53. [Google Scholar] [CrossRef]
- Wang, Y.; Li, S.; Ma, T.; Wang, X.; Dong, D.; Zhu, D.; Fang, H.; Chen, R. Improving High Temperature Oxidation Resistance of TiAl Alloy via Hierarchical Ti₅Si3–Ti2AlC Precipitation Strategy. Corros. Sci. 2024, 228, 111834. [Google Scholar] [CrossRef]
- Yang, H.; Xu, S.; Lan, J.; Gao, J.; Cao, H.; Zheng, G.; Zhang, H.; Feng, W. Effect of Rhenium Addition on the High-Temperature Oxidation Resistance of Anodized γ-TiAl Alloy. J. Alloys Compd. 2025, 1010, 178087. [Google Scholar] [CrossRef]
- Feng, L.; Li, B.; Li, Q.; Gao, Y.; Pei, Z.; Liang, C. Enhancement of Mechanical Properties and Oxidation Resistance of TiAl Alloy with Addition of Nb and Mo Alloying Elements. Mater. Chem. Phys. 2024, 316, 129148. [Google Scholar] [CrossRef]
- Wang, H.; Liu, Z.; Liang, J. High-Temperature Oxidation Behavior of Ti–48Al–2Cr–2Nb Alloy by Laser Additive Manufacturing. Mater. Chem. Phys. 2025, 333, 130365. [Google Scholar] [CrossRef]
- Gao, Z.; Hu, R.; Zou, H.; Zhou, M.; Luo, X. Insight into the Ta Alloying Effects on the Oxidation Behavior and Mechanism of Cast TiAl Alloy. Mater. Des. 2024, 241, 112941. [Google Scholar] [CrossRef]
- Yang, Y.; Han, Y.; Ran, X.; Liu, Y. Improving the High-Temperature Ductility of γ-TiAl Matrix Composites by Incorporation of AlCoCrFeNi High Entropy Alloy Particles. J. Alloys Compd. 2025, 1012, 178515. [Google Scholar] [CrossRef]
- Li, L.; Zhao, Z.; Feng, Z.-X.; Sun, J.; Li, X.-Q. Microstructure and Shear Strength of γ-TiAl/GH536 Joints Brazed with Ti–Zr–Cu–Ni–Fe–Co–Mo Filler Alloy. Trans. Nonferrous Met. Soc. China 2020, 30, 2143–2155. [Google Scholar] [CrossRef]
- Bacos, M.-P.; Morel, A.; Naveos, S.; Bachelier-Locq, A.; Josso, P.; Thomas, M. The Effect of Long-Term Exposure in Oxidising and Corroding Environments on the Tensile Properties of Two Gamma-TiAl Alloys. Intermetallics 2006, 14, 102–113. [Google Scholar] [CrossRef]
- Liu, Z.; Xin, S.; Zhao, Y. Research Progress on the Creep Resistance of High-Temperature Titanium Alloys: A Review. Metals 2023, 13, 1975. [Google Scholar] [CrossRef]
- Shaaban, A.; Nakashima, H.; Ueda, M.; Takeyama, M. Effects of Oxygen Addition on the Microstructure, Phase Transformations, and Oxidation Resistance of TiAl Alloys. J. Alloys Compd. 2022, 918, 165716. [Google Scholar] [CrossRef]
- Neumeier, S.; Bresler, J.; Zenk, C.; Haußmann, L.; Stark, A.; Pyczak, F.; Göken, M. Partitioning Behavior of Nb, Ta, and Zr in Fully Lamellar γ/α2 Titanium Aluminides and Its Effect on the Lattice Misfit and Creep Behavior. Adv. Eng. Mater. 2021, 23, 2100156. [Google Scholar] [CrossRef]
- Mitoraj-Królikowska, M.; Drożdż, E. Some Aspects of Oxidation and Reduction Processes in Ti–Al and Ti–Al–Nb Systems. Materials 2022, 15, 1640. [Google Scholar] [CrossRef]
- Soyama, J.; Oehring, M.; Limberg, W.; Ebel, T.; Kainer, K.U.; Pyczak, F. The Effect of Zirconium Addition on Sintering Behaviour, Microstructure and Creep Resistance of the Powder Metallurgy Processed Alloy Ti–45Al–5Nb–0.2B–0.2C. Mater. Des. 2015, 84, 87–94. [Google Scholar] [CrossRef]
- Musi, M.; Kardos, S.; Hatzenbichler, L.; Holec, D.; Stark, A.; Allen, M.; Güther, V.; Clemens, H.; Spoerk-Erdely, P. The Effect of Zirconium on the Ti-(42–46 at.%)Al System. Acta Mater. 2022, 241, 118414. [Google Scholar] [CrossRef]
- Illarionov, A.G.; Stepanov, S.I.; Naschetnikova, I.A.; Popov, A.A.; Soundappan, P.; Raman, K.H.T.; Suwas, S. A Review—Additive Manufacturing of Intermetallic Alloys Based on Orthorhombic Titanium Aluminide Ti2AlNb. Materials 2023, 16, 991. [Google Scholar] [CrossRef]
- Lindwall, G.; Wang, P.; Kattner, U.; Campbell, C. The Effect of Oxygen on Phase Equilibria in the Ti–V System: Impacts on the AM Processing of Ti Alloys. JOM 2018, 70, 1692–1705. [Google Scholar] [CrossRef]
- Perdrix, F.; Trichet, M.-F.; Bonnentien, J.-L.; Cornet, M.; Bigot, J.-P. Variations in the α2/γ Lamellar Microstructure in Ti–48Al Alloys as a Function of Cooling Rate and Oxygen Content. J. Phys. IV France 2000, PR6, 15–20. [Google Scholar] [CrossRef]
- Lamirand, M.; Bonnentien, J.-L.; Ferrière, G.; Guérin, S.; Chevalier, J.-P. Influence of a Pre-Oxidation Treatment on the Oxidation Resistance of TiAl Alloys. Metall. Mater. Trans. A 2006, 37A, 2369–2376. [Google Scholar] [CrossRef]
- Perdrix, F.; Trichet, M.-F.; Bonnentien, J.-L.; Cornet, M.; Bigot, J. Influence of Oxygen on the Microstructure and Mechanical Properties of Ti–52Al–48 Alloys. Intermet. Superalloys 2000, 10, 289–293. [Google Scholar]
- Bacos, M.-P.; Thomas, M.; Raviart, J.-L.; Morel, A.; Navéos, S.; Josso, P. Influence of Surface Modification and Long-Term Exposure on Mechanical Creep Properties of γ-TiAl G4. Mater. High Temp. 2010, 27, 339–346. [Google Scholar] [CrossRef]
- Braun, J.; Ellner, M. Phase Equilibria Investigations on the Aluminum-Rich Part of the Binary System Ti–Al. Metall. Mater. Trans. A 2001, 32A, 1037–1047. [Google Scholar] [CrossRef]
- Couret, D.; Reyes, D.; Thomas, M.; Ratel-Ramond, N.; Deshayes, C.; Monchoux, J.-P. Effect of Ageing on the Properties of the W-Containing IRIS-TiAl Alloy. Acta Mater. 2020, 199, 169–180. [Google Scholar] [CrossRef]




| Experimental Alloys | Chemical Composition (at.%) | ||
|---|---|---|---|
| Al | Zr | O | |
| LOx samples | |||
| Ti52Al0.5Zr | 51.92 | 0.50 | 0.137 |
| Ti52Al1Zr | 52.07 | 0.97 | 0.137 |
| Ti52Al2Zr | 51.90 | 2.03 | 0.148 |
| Ti52Al | 51.98 | - | 0.079 |
| HOx samples | |||
| Ti52Al0.5Zr | 51.51 | 0.50 | 0.488 |
| Ti52Al1Zr | 51.79 | 1.00 | 0.446 |
| Ti52Al2Zr | 51.65 | 2.00 | 0.425 |
| Ti52Al | 51.86 | - | 0.313 |
| Evaluation Method | Ti52Al 1250 °C | Ti52Al0.5Zr 1250 °C | Ti52Al1Zr 1250 °C | Ti52Al2Zr 1250 °C |
|---|---|---|---|---|
| LOx samples | ||||
| TCTI1 LOx | 0.0 | 0.0 | 1.2 | 1.6 |
| TCTI5 LOx | 0.6 | 1.0 | 1.2 | 1.3 |
| Real image analyses LOx | 0.0 | 0.0 | 0.0 | 0.0 |
| XRD analyses LOx | 0.0 | 0.0 | 0.0 | 0.0 |
| HOx samples | ||||
| TCTI1 HOx | 0.0 | 0.0 | 1.1 | 4.7 |
| TCTI5 HOx | 2.0 | 4.1 | 3.6 | 3.9 |
| Real image analyses HOx | 1.1 | 1.9 | 2.1 | 2.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuris, M.; Tsoutsouva, M.; Thomas, M.; Vaubois, T.; Sallot, P.; Habiyaremye, F.; Monchoux, J.-P. Effect of Oxygen and Zirconium on Oxidation and Mechanical Behavior of Fully γ Ti52AlxZr Alloys. Metals 2025, 15, 745. https://doi.org/10.3390/met15070745
Kuris M, Tsoutsouva M, Thomas M, Vaubois T, Sallot P, Habiyaremye F, Monchoux J-P. Effect of Oxygen and Zirconium on Oxidation and Mechanical Behavior of Fully γ Ti52AlxZr Alloys. Metals. 2025; 15(7):745. https://doi.org/10.3390/met15070745
Chicago/Turabian StyleKuris, Michal, Maria Tsoutsouva, Marc Thomas, Thomas Vaubois, Pierre Sallot, Frederic Habiyaremye, and Jean-Philippe Monchoux. 2025. "Effect of Oxygen and Zirconium on Oxidation and Mechanical Behavior of Fully γ Ti52AlxZr Alloys" Metals 15, no. 7: 745. https://doi.org/10.3390/met15070745
APA StyleKuris, M., Tsoutsouva, M., Thomas, M., Vaubois, T., Sallot, P., Habiyaremye, F., & Monchoux, J.-P. (2025). Effect of Oxygen and Zirconium on Oxidation and Mechanical Behavior of Fully γ Ti52AlxZr Alloys. Metals, 15(7), 745. https://doi.org/10.3390/met15070745






