Activity and Operational Loss of IrO2-Ta2O5/Ti Anodes During Oxygen Evolution in Acidic Solutions
Abstract
1. Introduction
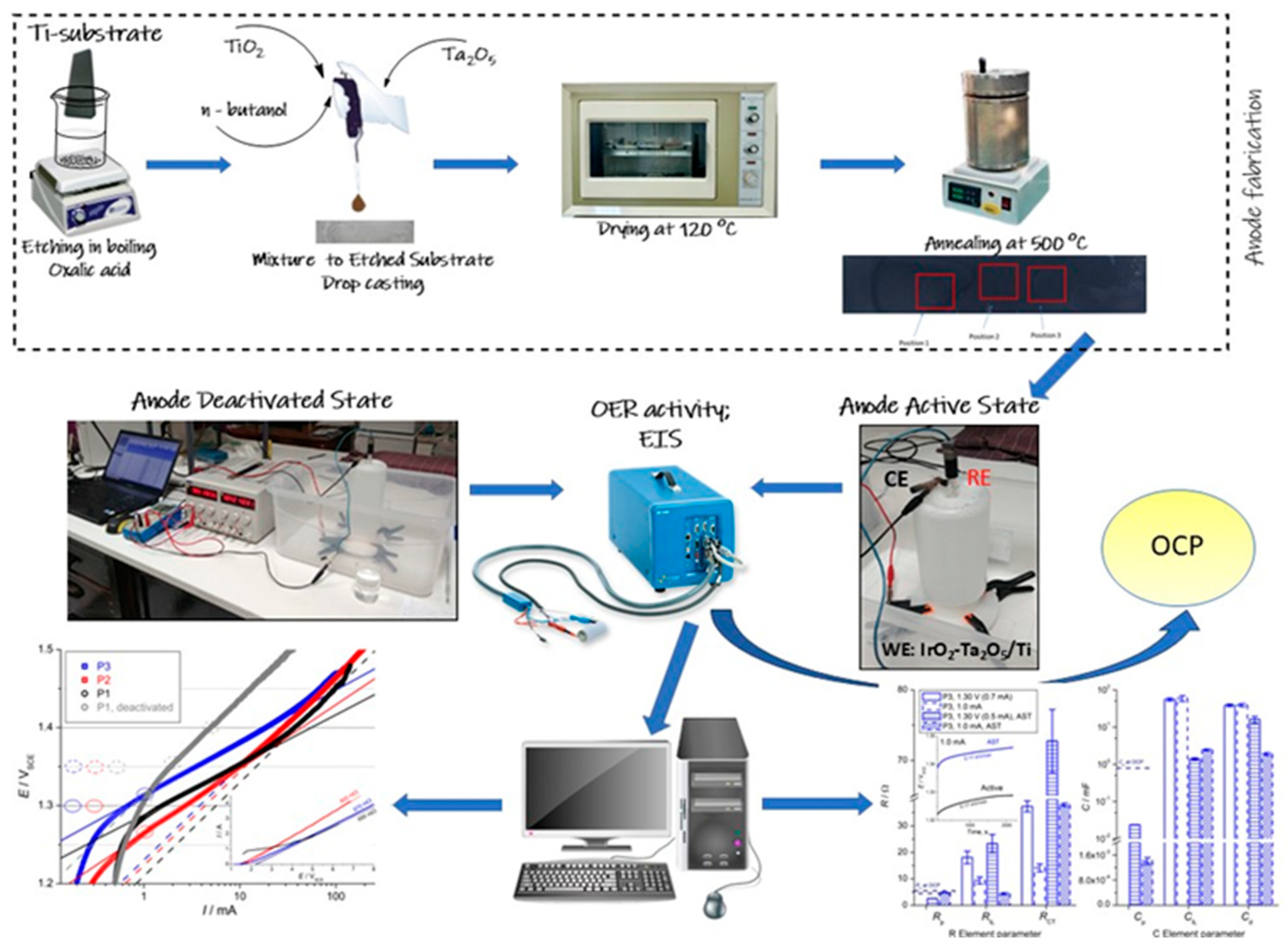
2. Materials and Methods
2.1. Electrode Preparation
2.2. Electrochemical Measurements
2.3. Taffel Slope
3. Results and Discussion
- Active sites for OER: IrO2 provides the necessary active sites where the electrochemical oxidation of water to oxygen occurs. The number and accessibility of these active sites directly correlate with the anode’s OER activity, as evidenced by our voltammetry capacitance measurements (as discussed in relation to our previous work [19]), which are proportional to the electrochemically active surface area (ECSA).
- Pseudocapacitive behavior: The IrO2 component is responsible for the characteristic pseudocapacitive behavior observed in our cyclic voltammetry (CV) and EIS measurements. The associated redox transitions (e.g., Ir(III)/Ir(IV)) are inherent to iridium oxide and contribute to its ability to participate in charge transfer processes. Roles 1 and 2 are intrinsically caused by a specific interaction between hydrated oxide moieties at the electrode surface and OH adsorbed from the water molecule. The interaction is also pH dependent.
- Influence on morphology: Although we do not explicitly focus on the morphology of discrete IrO2 particles in this study, the overall structure of the mixed oxide coating is critical. The distribution, porosity, and connectivity of the IrO2 within the Ta2O5 matrix influence the effective surface area and mass transport properties, impacting overall OER efficiency.
- Role in anode deactivation (iridium dissolution): Crucially, while being the active component, IrO2 is also susceptible to electrochemical dissolution, particularly regarding the high anodic potentials required for OER. This iridium dissolution takes place in parallel to OER.
- Synergy with Ta2O5: The presence of Ta2O5 within the mixed oxide plays a vital role in stabilizing the IrO2 component, improving its dispersion, and mitigating the rate of iridium dissolution, but it also possibly affects the “spil-over” effect related to OER/Ir dissolution intermediates. This synergistic effect is essential for achieving the desired long-term durability of the anode.
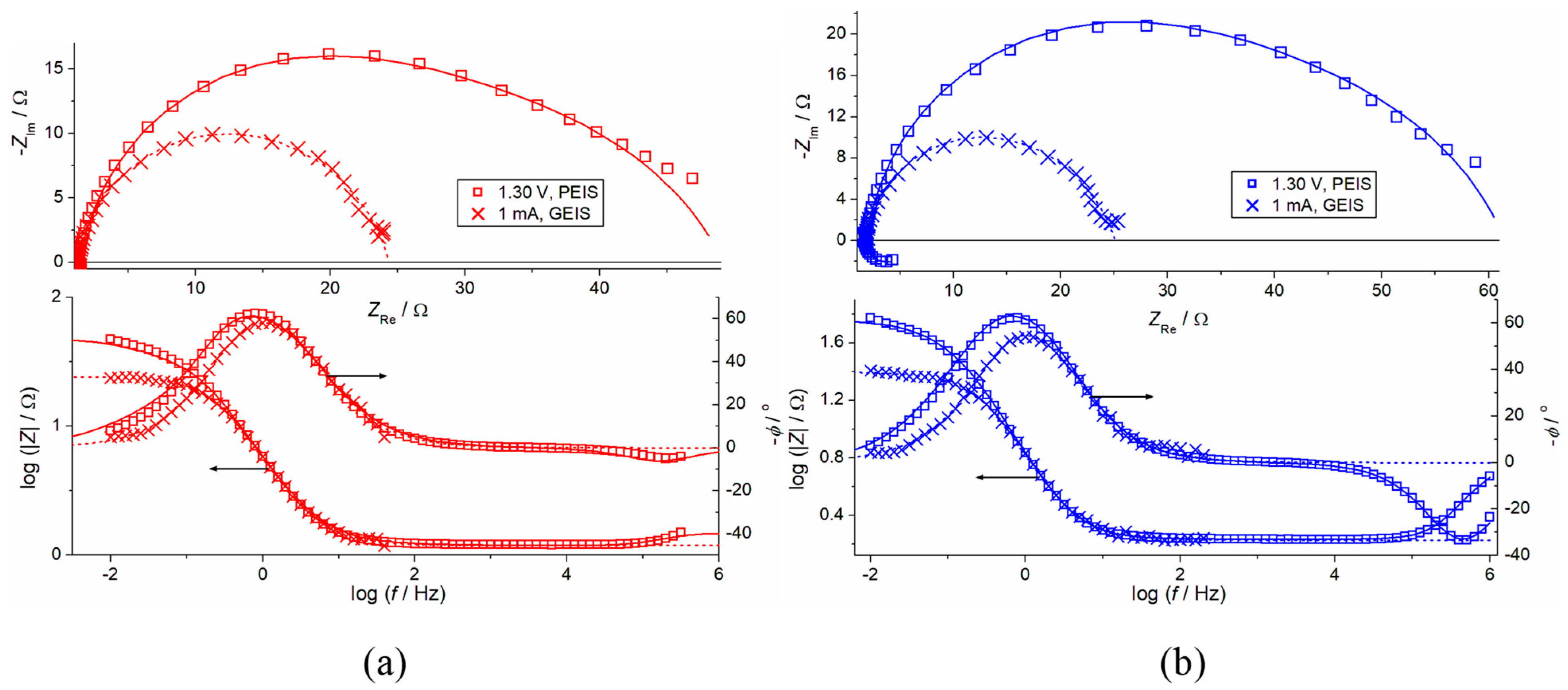
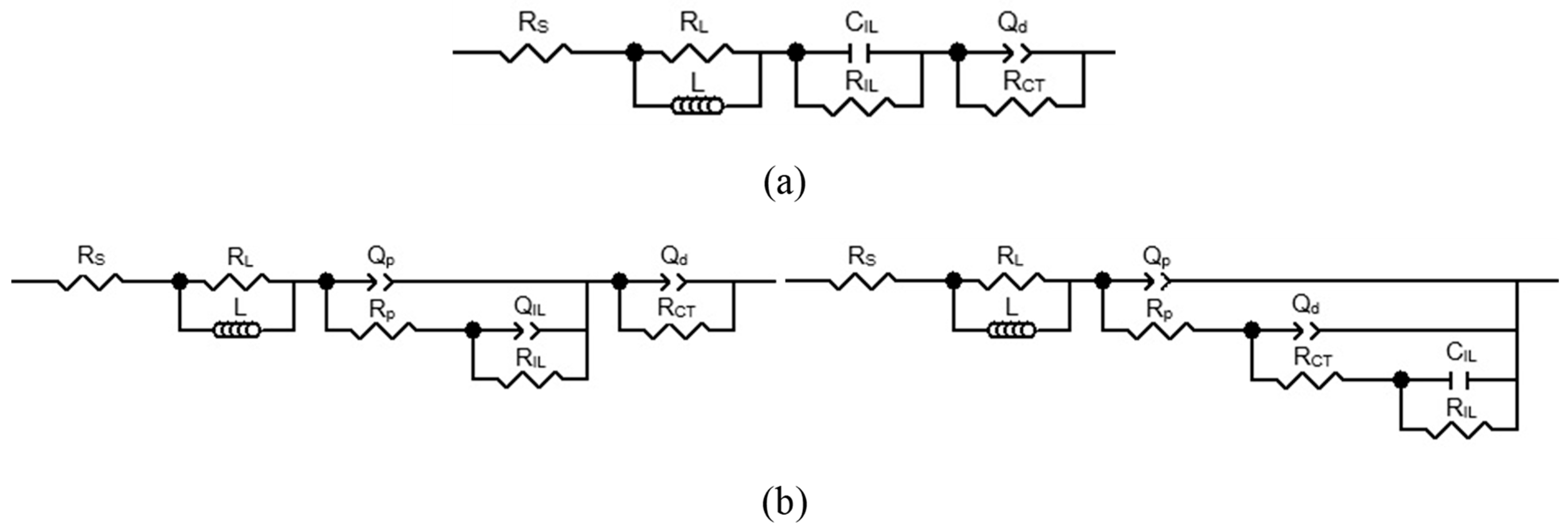
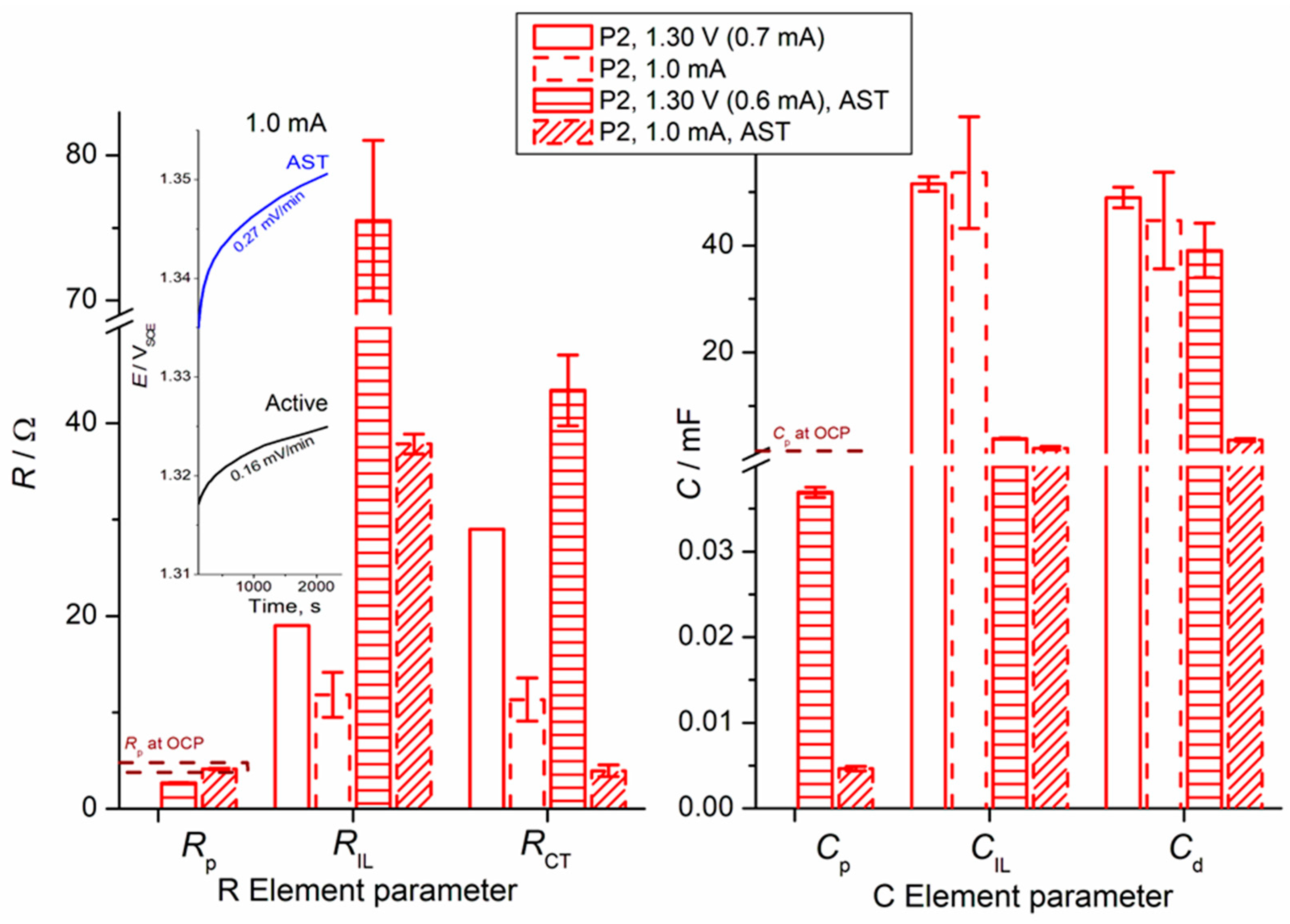
- Charge Transfer (CT) Processes: The primary charge transfer arc (represented by RCT and Cd elements in our equivalent electrical circuit, Figure 4) is directly associated with the oxygen evolution reaction (OER). The diameter of this arc in the Nyquist plot is proportional to the charge transfer resistance, reflecting the ease with which electrons are transferred across the interface during the OER.
- EIS Response and Interlayer Processes: Given the nature of noble metal oxides like IrO2, the coating exhibits additional behavior, arising from Ir dissolution upon polarization. The observed elements (including CIL associated with RIL) reflect the charge transfer characteristics within the distributed morphology of an internal interface of the active IrO2-Ta2O5 coating. The assignment of the ‘interlayer’ (IL) element refers to the response within this complex porous oxide structure, not a separate physical layer.
- Parallel Processes: As discussed in our manuscript, the OER occurs in parallel with the electrochemical dissolution of iridium (Ir dissolution), particularly at higher potentials. Both these faradaic processes contribute to the overall charge transfer resistance and thus influence the shape and magnitude of the observed arcs. The interplay between these processes defines the overall electrochemical response observed in EIS.
- Surface Heterogeneity and Porosity: The multi-arc or overlapping arc structures often observed in our EIS data (Figure 2 and Figure 3) can also indicate the presence of multiple time constants, reflecting the heterogeneity of the electrode surface, the porous nature of the coating, and varied accessibilities of active sites within the coating bulk.
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, B.; Ma, B.; Chen, Y.; Wang, C. Corrosion mechanism of Ti/IrO2-RuO2-SiO2 anode for oxygen evolution in sulfuric acid solution. Corros. Sci. 2020, 170, 108662. [Google Scholar] [CrossRef]
- Hoseinieh, S.M.; Ashrafizadeh, F.; Maddahi, M.H. A Comparative Investigation of the Corrosion Behavior of RuO2 – IrO2 – TiO2 Coated Titanium Anodes in Chloride Solutions. J. Electrochem. Soc. 2010, 157, 50–56. [Google Scholar] [CrossRef]
- Wei, Q.; Ren, X.; Du, J.; Wei, S.; Hu, S.R. Study of the electrodeposition conditions of metallic manganese in an electrolytic membrane reactor. Miner. Eng. 2010, 23, 578–586. [Google Scholar] [CrossRef]
- Marcela, G.T.; Lozele, V.A.; Aline, M.S.S.; Josealdo, T.; Carlos, A.M.H.; Carmem, L.P.S.Z. Electrochemical oxidation of Methyl Red using Ti/Ru0.3Ti0.7O2 and Ti/Pt anodes. Chem. Eng. J. 2012, 204–206, 141–150. [Google Scholar]
- Eloy, I.C.; Baró, M.D.; Rossinyol, E.; Morales-Ortiz, U.; Sort, J.; Brillas, E.; Pellicer, E. Comparative electrochemical oxidation of methyl orange azo dye using Ti/Ir-Pb, Ti/Ir-Sn, Ti/Ru-Pb, Ti/Pt-Pd and Ti/RuO2 anodes. Electrochim. Acta 2017, 244, 199–208. [Google Scholar]
- Zhang, Y.; Cao, M.; Lv, H.; Wei, J.; Gu, Y.; Liu, D.; Zhang, W.; Ryan, M.P.; Wu, X. Electrodeposited nanometer-size IrO2/Ti electrodes with 0.3 mg IrO2 cm−2 for sludge dewatering electrolysers. Electrochim. Acta 2018, 265, 507–513. [Google Scholar] [CrossRef]
- Escalera-López, D.; Czioska, S.; Geppert, J.; Boubnov, A.; Röse, P.; Saraçi, E.; Krewer, U.; Grunwaldt, J.D.; Cherevko, S. Phase- and Surface Composition-Dependent Electrochemical Stability of Ir-Ru Nanoparticles during Oxygen Evolution Reaction. ASC Catal. 2021, 11, 8968–10094. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, X.; Ou, C.; Zhang, Y.; Wang, W.; Dong, S.; Dong, X. Transition metal-based self-supported anode for electrocatalytic water splitting at a large current density. Coord. Chem. Rev. 2023, 495, 215381. [Google Scholar] [CrossRef]
- Bhattacharyya, K.; Poidevin, C.; Auer, A.A. Structure and Reactivity of IrOx Nanoparticles for the Oxygen Evolution Reaction in Electrocatalysis: An Electronic Structure Theory Study. J. Phys. Chem. C 2021, 125, 4331–4926. [Google Scholar] [CrossRef]
- Liu, B.; Wang, C.; Chen, Y.; Ma, B. Electrochemical behavior and corrosion mechanism of Ti/IrO2-RuO2 anodes in sulphuric acid solution. J. Electroanal. Chem 2019, 837, 175–183. [Google Scholar] [CrossRef]
- Mehdipour, M.; Tabaian, S.H.; Firoozi, S. Electrochemical evaluation of IrO2–Ta2O5-MWCNT-coated anodes with different pretreatments of titanium substrate. J. Iran. Chem. Soc. 2021, 18, 233–243. [Google Scholar] [CrossRef]
- Kasian, O.; Li, T.; Mingers, M.A.; Schweinar, K.; Savan, A.; Ludwig, A.; Mayrhofer, K. Stabilization of an iridium oxygen evolution catalyst by titanium oxides. J. Phys. Energy 2021, 3, 034006. [Google Scholar] [CrossRef]
- Chen, X.; Chen, G. Stable Ti/RuO2–Sb2O5–SnO2 electrodes for O2 evolution. Electrochim. Acta 2005, 50, 4155–4159. [Google Scholar] [CrossRef]
- Iwakura, C.; Sakamoto, K. Effect of active layer composition on the service life of (SnO2 and RuO2)-coated Ti electrodes in sulfuric acid solution. J. Electrochem. Soc. 1985, 132, 2420–2423. [Google Scholar] [CrossRef]
- Cao, H.; Zhang, L.; Chen, M. Characteristics of anodic TiO2 nanotube arrays mediated IrO2 Active Anode in the Oxygen Evolution Reaction. Int. J. Electrochem. Sci. 2022, 17, 220461. [Google Scholar] [CrossRef]
- Herrada, R.A.; Rodil, S.E.; Sepúlveda-Guzmán, S.; Manriquez, J.; Exner, K.S.; Bustos, E. Characterization of Ti electrodes electrophoretically coated with IrO2-Ta2O5 films with different Ir:Ta molar ratios. J. Alloys Compd. 2021, 862, 158015. [Google Scholar] [CrossRef]
- Yan, Z.; Li, G.; Wang, J.; Zhang, Z.; Feng, Z.; Tang, M.; Zhang, R. Electro-catalytic study of IrO2single bondTa2O5 coated anodes with pretreated titanium substrates. J. Alloys Compd. 2016, 680, 60–66. [Google Scholar] [CrossRef]
- Tolba, R.; Tian, M.; Wen, J.; Jiang, Z.H.; Chen, A. Electrochemical oxidation of lignin at IrO2- based oxide electrodes. J. Electroanal. Chem. 2010, 649, 9–15. [Google Scholar] [CrossRef]
- Bošnjaković, J.; Panić, V.; Stevanović, M.; Stopic, S.; Stevanović, J.; Grgur, B.; Šekularac, G. Revealing the Surface and In-Depth Operational Performances of Oxygen-Evolving Anode Coatings: A Guideline for the Synthesis of Inert Durable Anodes in Metal Electrowinning from Acid Solutions. Metals 2024, 14, 1339. [Google Scholar] [CrossRef]
- Lee, Y.; Suntivich, J.; May, K.J.; Perry, E.E.; Shao-Horn, Y. Synthesis and Activities of Rutile IrO2 and RuO2 Nanoparticles for Oxygen Evolution in Acid and Alkaline Solutions. J. Phys. Chem. Lett. 2012, 3, 399–404. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, L.; Xuan, J.; Xin, Y.; Li, Y.; Duan, T.; Liu, F. A comparative study on Ti/IrO2–Ta2O5 anodes prepared by microwave plasma-assisted sintering and conventional thermal decomposition methods. J. Mater. Res. Technol. 2023, 23, 1447–1457. [Google Scholar] [CrossRef]
- Heijden, O.; Park, S.; Vos, R.E.; Eggebeen, J.J.J.; Koper, M.T.T. Tafel Slope Plot as a Tool to Analyze Electrocatalytic Reactions. ACS Energy Lett. 2024, 9, 1871–1879. [Google Scholar] [CrossRef]
- Mefford, J.T.; Zhao, Z.; Bajdich, M.; Chueh, W.C. Interpreting Tafel Behavior of Consecutive Electrochemical Reactions through Combined Thermodynamic and Steady State Microkinetic Approaches. Energy Environ. Sci. 2020, 13, 622–634. [Google Scholar] [CrossRef]
- Fletcher, S. Tafel Slopes from First Principles. J. Solid State Electrochem. 2009, 13, 537–549. [Google Scholar] [CrossRef]
- Nong, H.N.; Falling, L.J.; Bergmann, A.; Klingenhof, M.; Tran, H.P.; Spöri, C.; Mom, R.; Timoshenko, J.; Zichittella, G.; KnopGericke, A.; et al. Key Role of Chemistry versus Bias in Electrocatalytic Oxygen Evolution. Nature 2020, 587, 408–413. [Google Scholar] [CrossRef]
- Marshall, A.T.; Vaisson-Béthune, L. Avoid the Quasi Equilibrium Assumption When Evaluating the Electrocatalytic Oxygen Evolution Reaction Mechanism by Tafel Slope Analysis. Electrochem. Commun. 2015, 61, 23–26. [Google Scholar] [CrossRef]
- Chizmadzhevand, Y.A.; Chirkov, Y.G. Porous Electrodes. In Comprehensive Treatise of Electrchemistry, 1st ed.; Yeager, E., Bockris, J.O.M., Conway, B.E., Sarangapani, S., Eds.; Springer Science + Business Media: New York, NY, USA, 1983; Volume 6, pp. 317–385. [Google Scholar]
- Damjanovic, A.; Dey, A.; Bockris, I.J. O’M. Electrode Kinetics of Oxygen Evolution and Dissolution on Rh, Ir, and Pt-Rh Alloy Electrodes. J. Electrochem. Soc. 1966, 113, 741–746. [Google Scholar] [CrossRef]
- Jovanovič, P.; Hodnik, N.; Ruiz-Zepeda, F.; Arčon, I.; Jozinović, B.; Zorko, M.; Bele, M.; Šala, M.; Šelih, V.S.; Hočeva, S. Electrochemical Dissolution of Iridium and Iridium Oxide Particles in Acidic Media: Transmission Electron Microscopy, Electrochemical Flow Cell Coupled to Inductively Coupled Plasma Mass Spectrometry, and X-ray Absorption Spectroscopy Study. J. Am. Chem. Soc. 2017, 139, 12837–12846. [Google Scholar] [CrossRef]
- Zlatar, M.; Escalera-Lopez, D.; Cornelius, S.; Briega-Martos, V.; Stojanovski, K.; Cherevko, S. pH dependence of noble metals dissolution: Iridium. Electrochim. Acta. 2025, 513, 145450. [Google Scholar] [CrossRef]
- Buckley, D.N.; Burke, L.D. The oxygen electrode. Part 6. Oxygen evolution and corrosion at iridium anodes. J. Chem. Soc. Faraday Trans. 1 Phys. Chem. Condens. Phases 1975, 12, 1–77. [Google Scholar] [CrossRef]
- Kasian, O.; Grote, J.P.; Geiger, S.; Cherevko, S.; Mayrhofer, K.J.J. The common Intermediates of Oxygen Evolution and Dissolution Reactions during Water Electrolysis on Iridium. J. Prakt. Chem. 2018, 57, 2488–2491. [Google Scholar] [CrossRef] [PubMed]
- Gajic-Krstajic, L.M.; Trisovic, T.L.; Krstajic, N.V. Spectrophotometric study of the anodic corrosion of Ti/RuO2 electrode in acid sulfate solution. Corros. Sci. 2004, 46, 65–74. [Google Scholar] [CrossRef]
- Jovic, V.D. Determination of the Correct Value of Cdl from the Impedance Results Fitted by the Commercially Available Software; Gamry Instruments, Inc.: Warminster, PA, USA, 2003; pp. 1–3. [Google Scholar]
- Haripriya, M.; Manimekala, T.; Dharmalingam, G.; Minakshi, M.; Sivasubramanian, R. Asymmetric Supercapacitors Based on ZnCo2O4 Nanohexagons and Orange Peel Derived Activated Carbon Electrodes. Chem. Asian J. 2024, 19, e202400202. [Google Scholar] [CrossRef] [PubMed]
- Vasudevan, S.; Tharani, D.S.; Manickam, M.; Sivasubramanian, R. A sol–gel derived LaCoO3 perovskite as an electrocatalyst for Al–air batteries. Dalton Trans. 2024, 53, 3713–3721. [Google Scholar] [CrossRef]
- Moradi, F.; Dehghanian, C. Addition of IrO2 to RuO2+TiO2 coated anodes and its effect on electrochemical performance of anodes in acid media. Prog. Nat. Sci. Mater. Int. 2014, 24, 134–141. [Google Scholar] [CrossRef]
- Liu, B.; Wang, C.; Chen, Y. Surface determination and electrochemical behavior of IrO2-RuO2-SiO2 ternary oxide coatings in oxygen evolution reaction application. Electrochim. Acta 2018, 264, 350–357. [Google Scholar] [CrossRef]


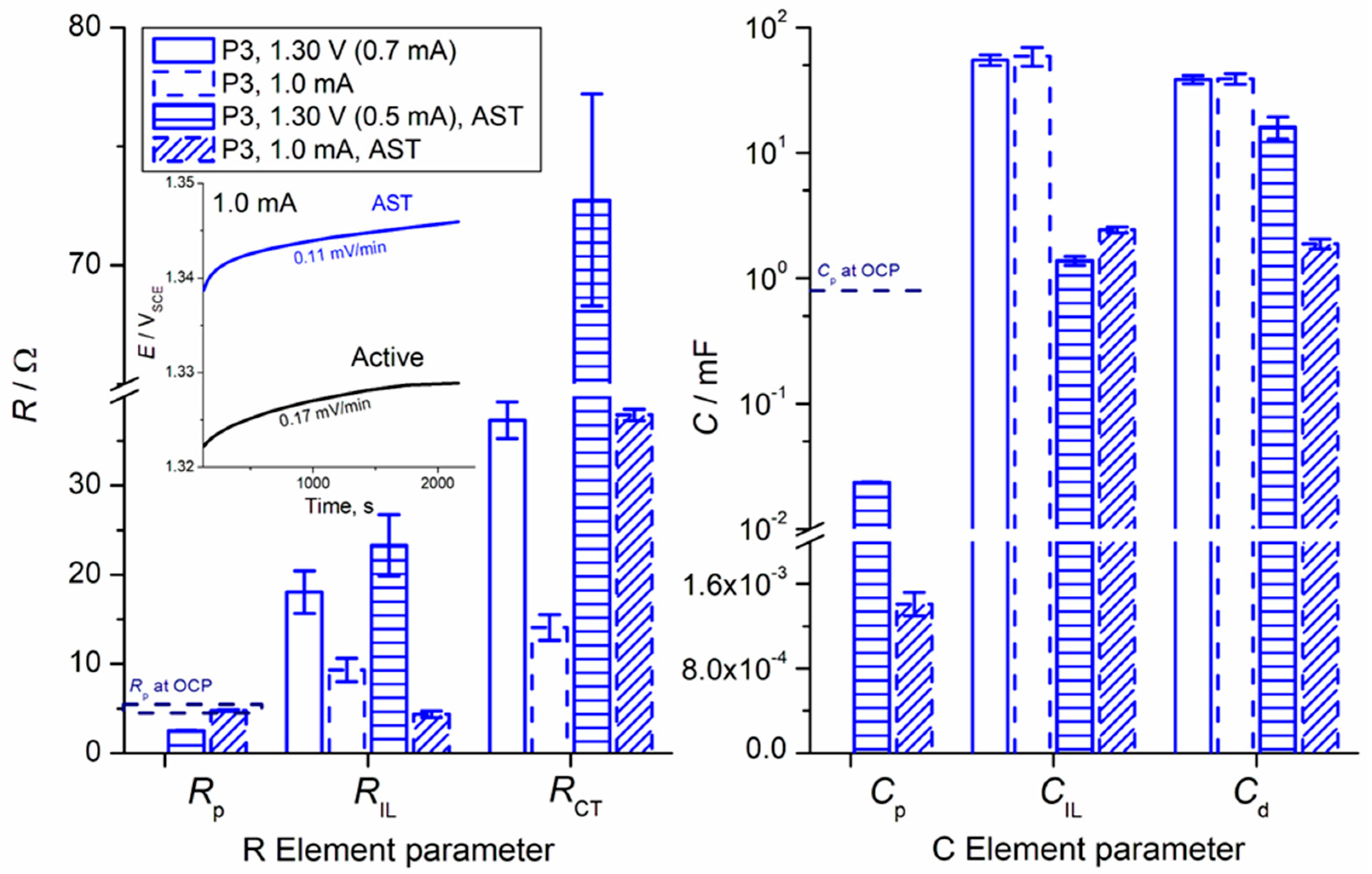
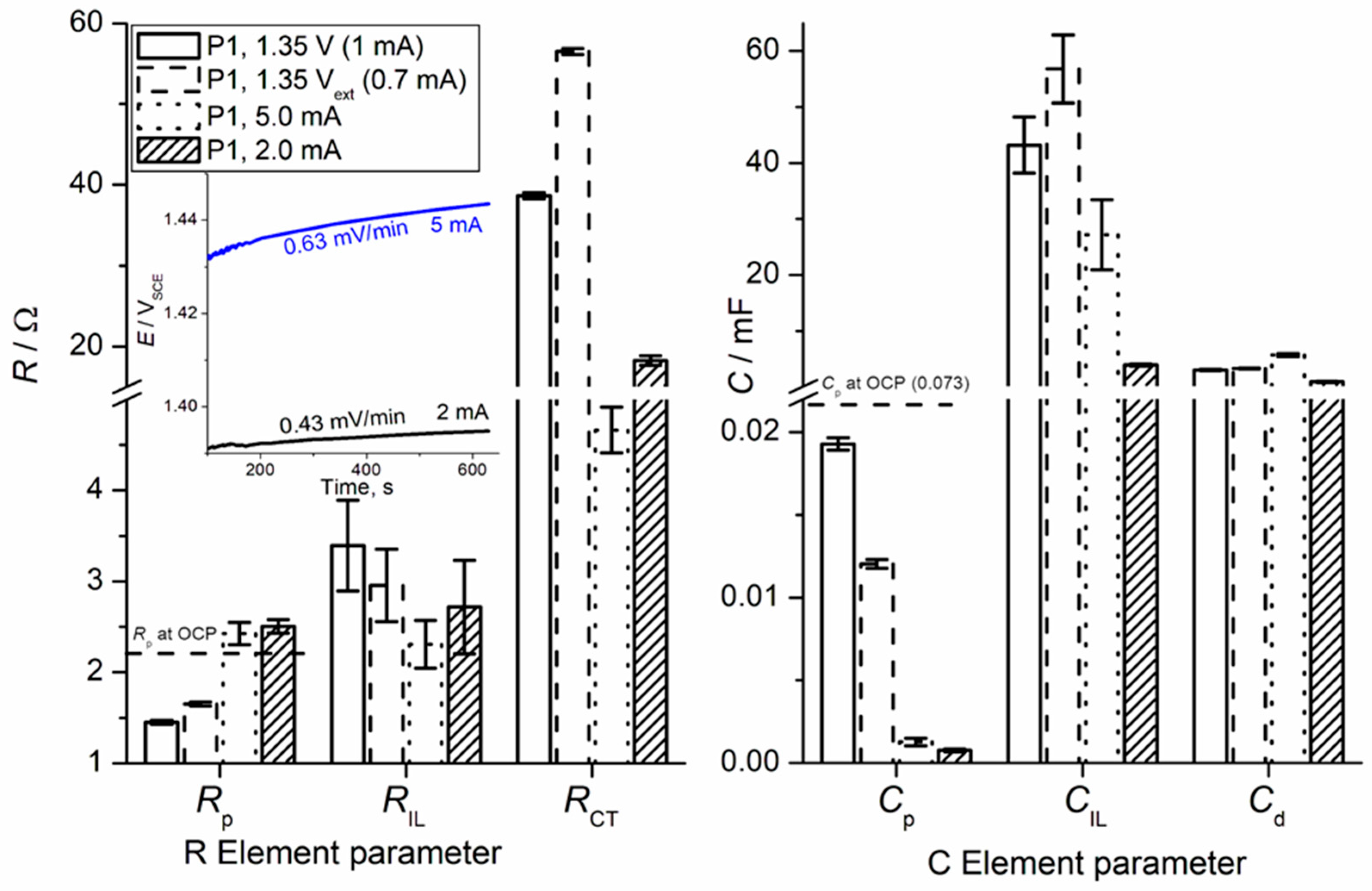
| Surface Position | RS/Ω | Tafel Slope, mV (Low Overpotentials, <1.42 V) | RS,PEIS/Ω from PEIS (±SD of the Fitting) | |
|---|---|---|---|---|
| 1.30 V | OCP | |||
| P1 | 1.56 | 64 | – | – |
| P2 | 1.32 | 82 | 1.19 ± 0.01 | 1.16 ± 0.01 |
| P3 | 3.12 | 67 | 1.71 ± 0.01 | 1.67 ± 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bošnjaković, J.; Stevanović, M.; Mihailović, M.; Tadić, V.M.; Stevanović, J.; Panić, V.; Šekularac, G. Activity and Operational Loss of IrO2-Ta2O5/Ti Anodes During Oxygen Evolution in Acidic Solutions. Metals 2025, 15, 721. https://doi.org/10.3390/met15070721
Bošnjaković J, Stevanović M, Mihailović M, Tadić VM, Stevanović J, Panić V, Šekularac G. Activity and Operational Loss of IrO2-Ta2O5/Ti Anodes During Oxygen Evolution in Acidic Solutions. Metals. 2025; 15(7):721. https://doi.org/10.3390/met15070721
Chicago/Turabian StyleBošnjaković, Jovana, Maja Stevanović, Marija Mihailović, Vojin M. Tadić, Jasmina Stevanović, Vladimir Panić, and Gavrilo Šekularac. 2025. "Activity and Operational Loss of IrO2-Ta2O5/Ti Anodes During Oxygen Evolution in Acidic Solutions" Metals 15, no. 7: 721. https://doi.org/10.3390/met15070721
APA StyleBošnjaković, J., Stevanović, M., Mihailović, M., Tadić, V. M., Stevanović, J., Panić, V., & Šekularac, G. (2025). Activity and Operational Loss of IrO2-Ta2O5/Ti Anodes During Oxygen Evolution in Acidic Solutions. Metals, 15(7), 721. https://doi.org/10.3390/met15070721






