Effect of Heat Treatment on the Corrosion Behavior of Selective Laser Melted CX Stainless Steel
Abstract
1. Introduction
2. Experimental Conditions
2.1. Experimental Materials
2.2. Preparation Process
2.3. Test Methods
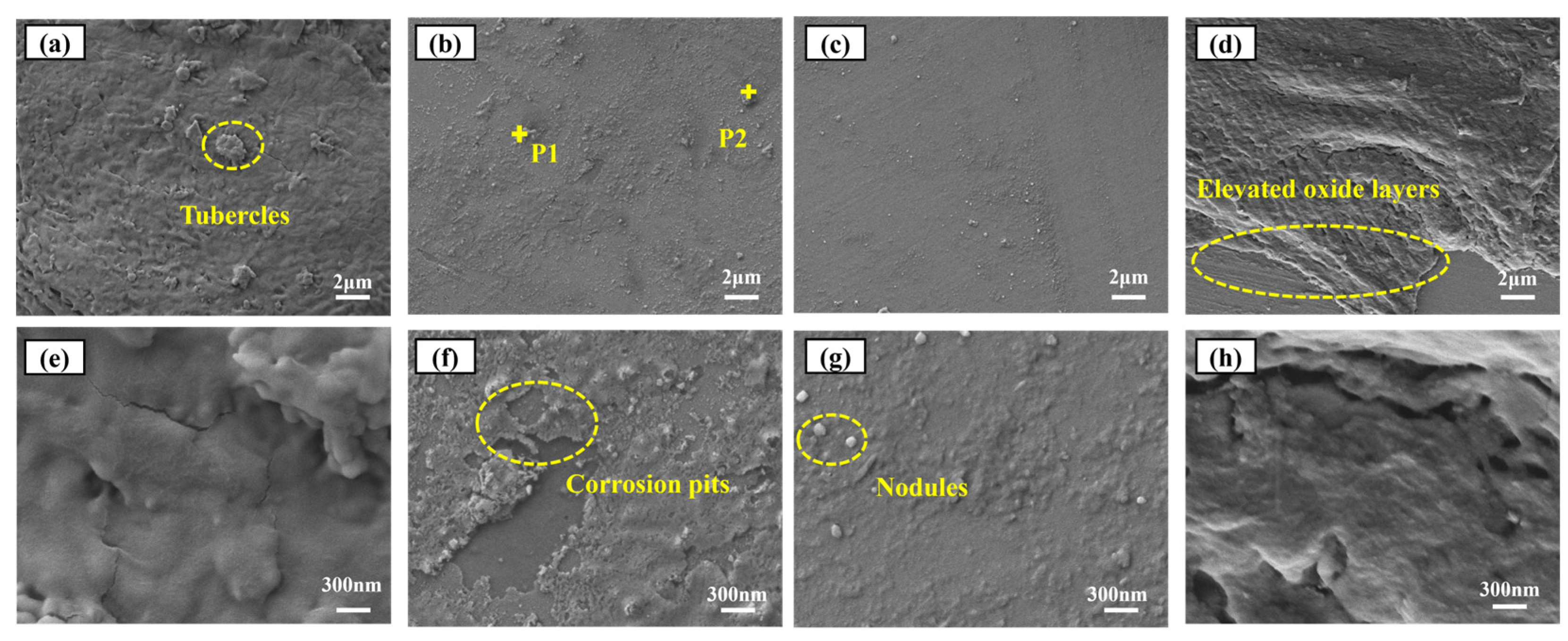
3. Results and Discussions
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yang, Y.; Bai, Y.; Wang, Y.; Zhang, Y.; Weng, C.; Lu, W.F.; Wang, H. Compressive mechanical response and microstructures in low strain rate plastic deformation of stainless steel 316L fabricated by selective laser melting. J. Mater. Res. Technol. 2024, 29, 4327–4344. [Google Scholar] [CrossRef]
- Zhang, C.; Li, Z.; Zhang, J.; Tang, H.; Wang, H. Additive manufacturing of magnesium matrix composites: Comprehensive review of recent progress and research perspectives. J. Magnes. Alloys 2023, 11, 425–461. [Google Scholar] [CrossRef]
- Qi, X.; Gao, X.; Ma, C.; Huang, R.; Huang, F.; Liu, J.; Zhang, S. Effect of heat treatment on the intergranular corrosion of 316 L stainless steel fabricated by selective laser melting. Mater. Charact. 2025, 220, 114648. [Google Scholar] [CrossRef]
- Wei, J.; Wang, J.; Yang, J.; Zeng, Y.; Guan, Y. Effect of internal defects on the compression behavior of stainless steel lattice structure fabricated by selective laser melting. J. Manuf. Process. 2024, 120, 809–826. [Google Scholar] [CrossRef]
- Dineshraj, S.; Gupta, R.K.; Govind. Development, Post Processing and Characterization of Additive Manufactured Stainless Maraging Steel. Trans. Indian Inst. Met. 2024, 77, 3729–3738. [Google Scholar] [CrossRef]
- Yan, X.; Chen, C.; Chang, C.; Dong, D.; Zhao, R.; Jenkins, R.; Wang, J.; Ren, Z.; Liu, M.; Liao, H.; et al. Study of the microstructure and mechanical performance of C-X stainless steel processed by selective laser melting (SLM). Mater. Sci. Eng. A 2020, 781, 139227. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, M.; Niu, L.; Liu, J.; Wang, J.; Liu, Y.; Shi, Z. Effect of process parameters and heat treatment on the properties of stainless steel CX fabricated by selective laser melting. J. Alloys Compd. 2021, 877, 160062. [Google Scholar] [CrossRef]
- Sun, Y.; Moroz, A.; Alrbaey, K. Sliding Wear Characteristics and Corrosion Behaviour of Selective Laser Melted 316L Stainless Steel. J. Mater. Eng. Perform. 2013, 23, 518–526. [Google Scholar] [CrossRef]
- Asgari, H.; Mohammadi, M. Microstructure and mechanical properties of stainless steel CX manufactured by Direct Metal Laser Sintering. Mater. Sci. Eng. A 2018, 709, 82–89. [Google Scholar] [CrossRef]
- Schaller, R.F.; Taylor, J.M.; Rodelas, J.; Schindelholz, E.J. Corrosion Properties of Powder Bed Fusion Additively Manufactured 17-4 PH Stainless Steel. Corrosion 2017, 73, 796–807. [Google Scholar] [CrossRef]
- Man, C.; Dong, C.; Liu, T.; Kong, D.; Wang, D.; Li, X. The enhancement of microstructure on the passive and pitting behaviors of selective laser melting 316L SS in simulated body fluid. Appl. Surf. Sci. 2019, 467–468, 193–205. [Google Scholar] [CrossRef]
- Zhao, X.; Jia, D.; Wei, S.; Gao, Y.; Liu, H. Investigation of tribo-corrosion behaviors of SLM-printed CX stainless steel under different loads. J. Mater. Res. Technol. 2024, 30, 5266–5277. [Google Scholar] [CrossRef]
- Zhao, X.; Zhao, K.; Gao, Y.; Wang, D. Influence of Microdefect on Mechanical Behaviors of CX Stainless Steel Produced by Selective Laser Melting. Met. Mater. Int. 2024, 31, 70–83. [Google Scholar] [CrossRef]
- Gao, P.; Jing, G.; Lan, X.; Li, S.; Zhou, Y.; Wang, Y.; Yang, H.; Wei, K.; Wang, Z. Effect of heat treatment on microstructure and mechanical properties of Fe–Cr–Ni–Co–Mo maraging stainless steel produced by selective laser melting. Mater. Sci. Eng. A 2021, 814, 141149. [Google Scholar] [CrossRef]
- Huang, C.A.; Lin, W.; Lin, S.C. The electrochemical polishing behaviour of P/M high-speed steel (ASP 23) in perchloric–acetic mixed acids. Corros. Sci. 2003, 45, 2627–2638. [Google Scholar] [CrossRef]
- Li, Z.; Qiu, C.; Liu, C.; Bai, Y.; Meng, H. The mechanoelectrochemical effect on the electrochemical corrosion of austenitic stainless steel. J. Mater. Res. Technol. 2023, 24, 1203–1215. [Google Scholar] [CrossRef]
- Mutua, J.; Nakata, S.; Onda, T.; Chen, Z.-C. Optimization of selective laser melting parameters and influence of post heat treatment on microstructure and mechanical properties of maraging steel. Mater. Des. 2018, 139, 486–497. [Google Scholar] [CrossRef]
- Li, J.; Jiang, W.; Zhang, Y.; Liu, L.; Yu, Y.; Luan, J.; Jiao, Z.; Liu, C.T.; Zhang, Z. Evolution and strengthening of nanoprecipitates in a high strength maraging stainless steel. Mater. Sci. Eng. A 2024, 915, 147198. [Google Scholar] [CrossRef]
- Bi, J.; Lei, Z.; Chen, Y.; Chen, X.; Lu, N.; Tian, Z.; Qin, X. An additively manufactured Al-14.1Mg-0.47Si-0.31Sc-0.17Zr alloy with high specific strength, good thermal stability and excellent corrosion resistance. J. Mater. Sci. Technol. 2021, 67, 23–35. [Google Scholar] [CrossRef]
- Ralston, K.D.; Birbilis, N.; Davies, C.H.J. Revealing the relationship between grain size and corrosion rate of metals. Scr. Mater. 2010, 63, 1201–1204. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, D. Effect of heat treatment on precipitation behavior of second phase and property evolution of martensitic stainless steel. Mater. Today Commun. 2023, 37, 107267. [Google Scholar] [CrossRef]
- Pirgazi, H.; Sanjari, M.; Tamimi, S.; Shalchi Amirkhiz, B.; Kestens, L.A.I.; Mohammadi, M. Texture evolution in selective laser melted maraging stainless steel CX with martensitic transformation. J. Mater. Sci. 2020, 56, 844–853. [Google Scholar] [CrossRef]
- Dong, D.; Chang, C.; Wang, H.; Yan, X.; Ma, W.; Liu, M.; Deng, S.; Gardan, J.; Bolot, R.; Liao, H. Selective laser melting (SLM) of CX stainless steel: Theoretical calculation, process optimization and strengthening mechanism. J. Mater. Sci. Technol. 2021, 73, 151–164. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, W.; Fan, Y.; Zhang, T.; Dong, B.; Chen, L.; Wang, Y. Influence of microstructure on the corrosion behavior of super 13Cr martensitic stainless steel under heat treatment. Mater. Charact. 2021, 175, 111066. [Google Scholar] [CrossRef]
- Shahriari, A.; Sanjari, M.; Mahmoudiniya, M.; Pirgazi, H.; Shalchi Amirkhiz, B.; Kestens, L.A.I.; Mohammadi, M. Quasi In-Situ Study of Microstructure in a Laser Powder Bed Fusion Martensitic Stainless Steel. Metall. Mater. Trans. A 2024, 55, 1302–1310. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhao, Z.; Jiang, S.; Duan, D. Effect of heat treatment on the tribocorrosion behavior of 20Cr13 martensitic stainless steel. Tribol. Int. 2024, 197, 109768. [Google Scholar] [CrossRef]
- Wada, Y.; Ishida, K.; Tachibana, M.; Shimizu, R. High temperature electrochemical reaction parameters affecting elecrochemical corrosion potential of type 316L stainless steel. J. Nucl. Sci. Technol. 2021, 59, 491–498. [Google Scholar] [CrossRef]
- Ramadas, H.; Kumar Nath, A.; Madapana, D.; Dutta Majumdar, J. Role of heat treatment and laser shock peening on the electrochemical corrosion properties of 15–5 precipitation hardening stainless steel manufactured by laser powder bed fusion process. Appl. Surf. Sci. 2024, 676, 160969. [Google Scholar] [CrossRef]
- Jia, J.; Zhuang, W.; Li, J.; Cao, Q.; Liu, J. Corrosion behaviors of La doped in-situ synthesized nano TiB2/6061 composites. Mater. Lett. 2024, 377, 137564. [Google Scholar] [CrossRef]
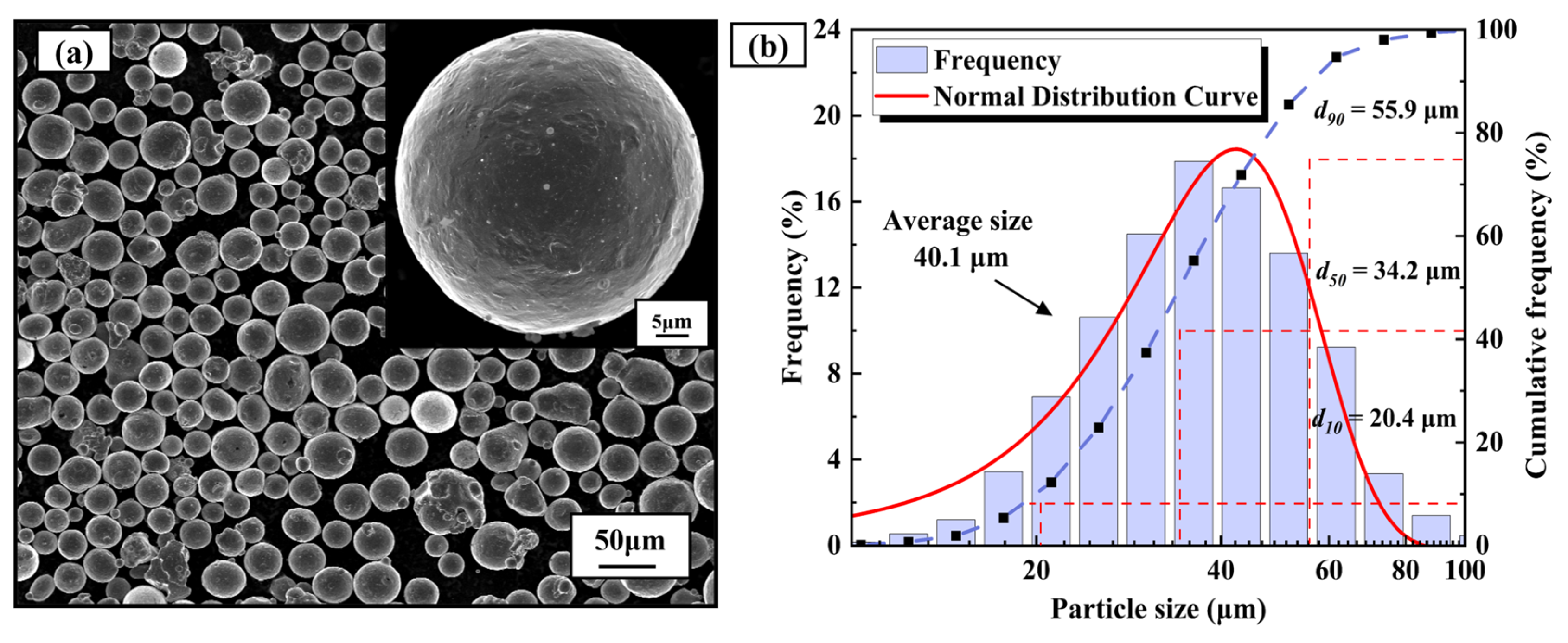

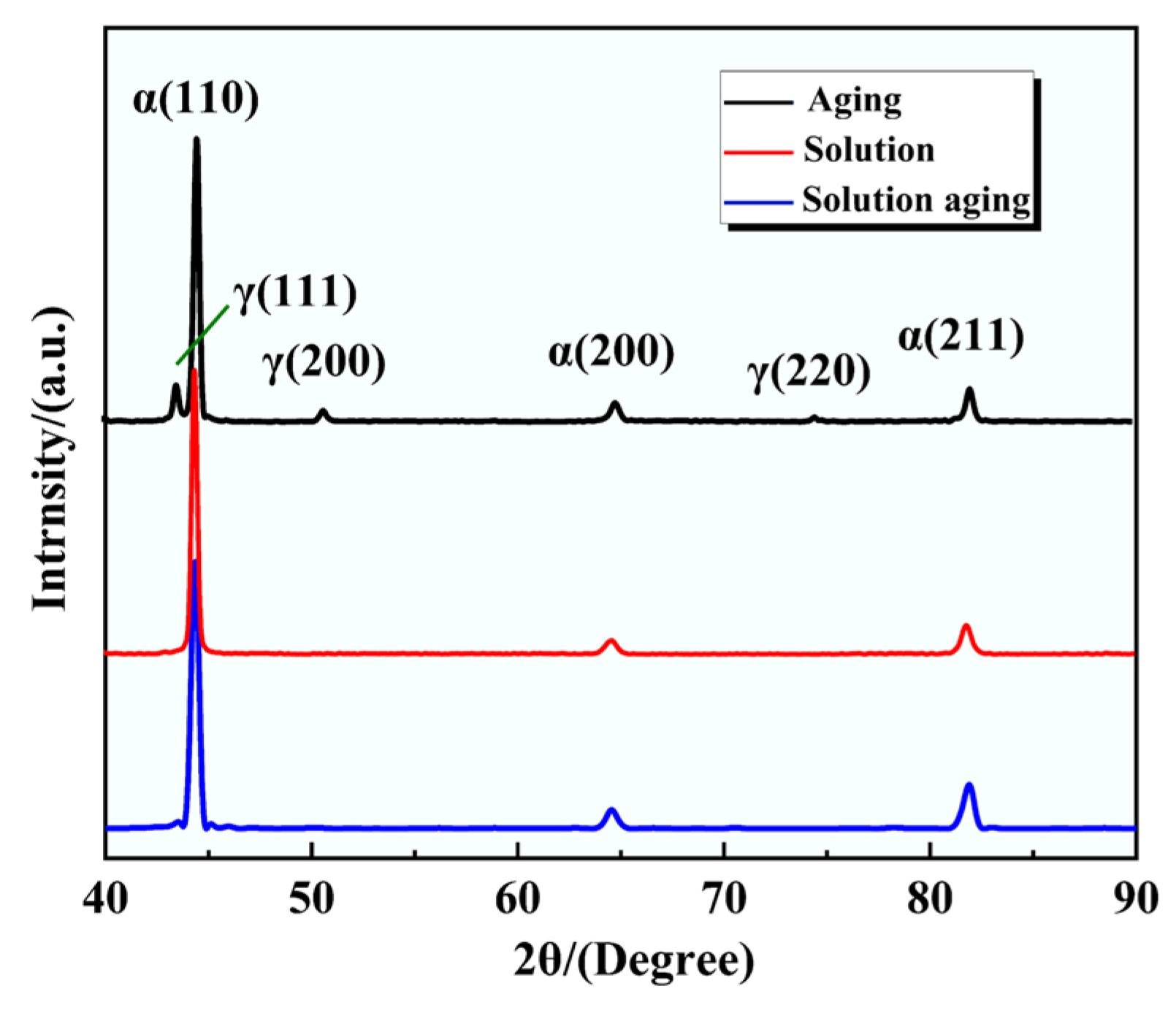
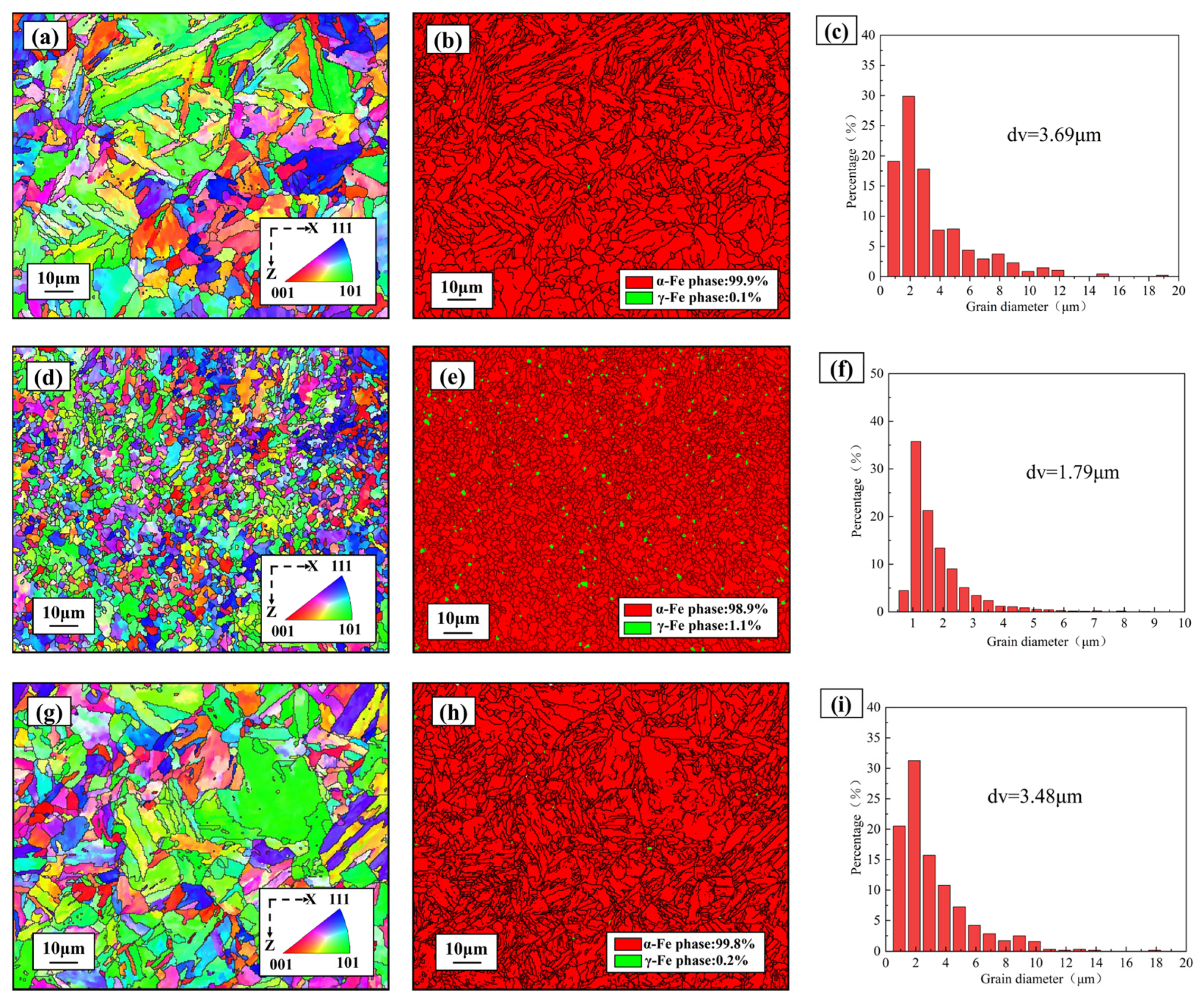
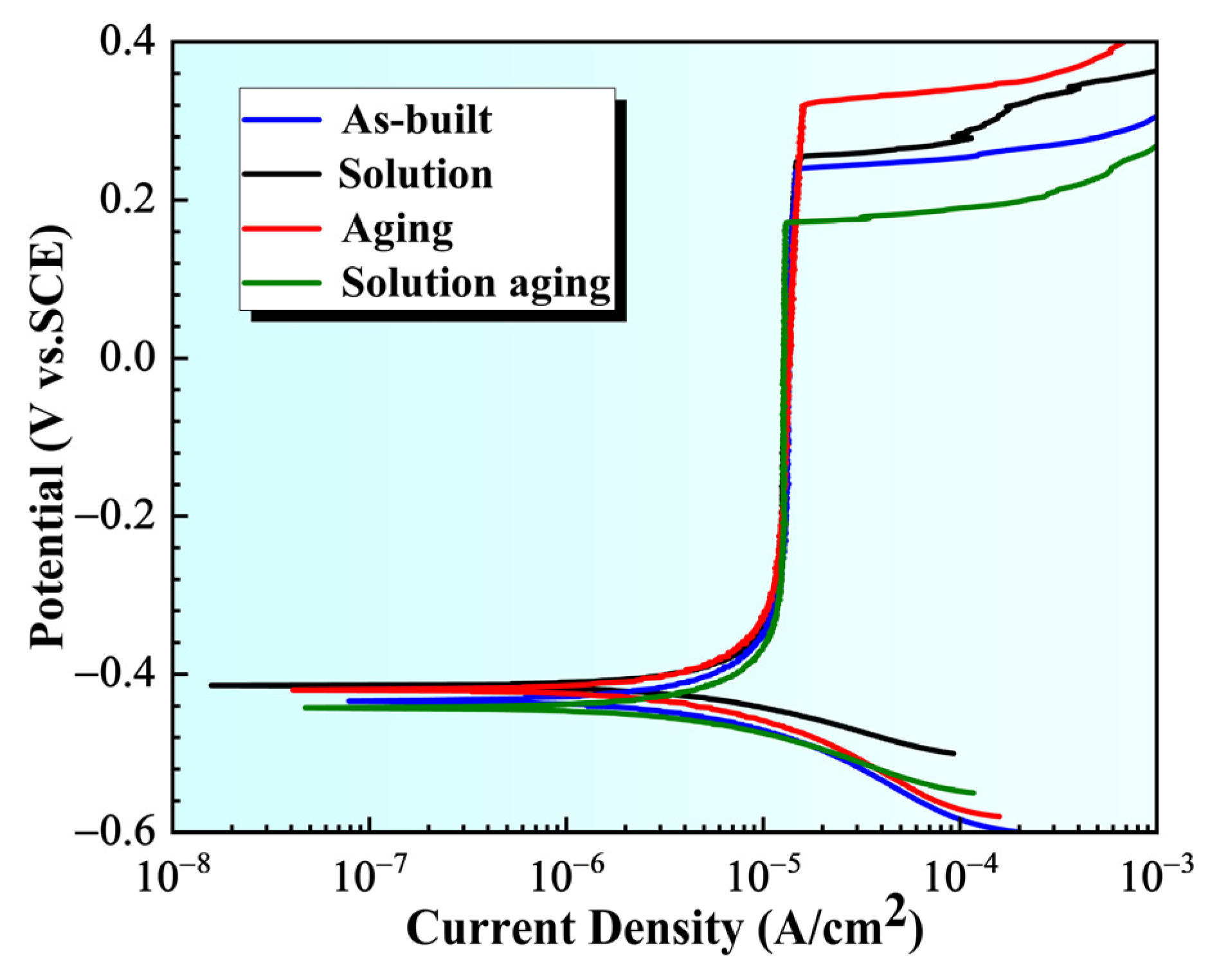


| Element | Cr | Ni | Al | Mo | Mn | Si | P | C | Fe |
|---|---|---|---|---|---|---|---|---|---|
| wt% | 11.75 | 9.42 | 1.5 | 1.38 | 0.16 | 0.16 | 0.007 | 0.009 | Bal. |
| Samples | Ecorr (mV) | Icorr (μA·cm−2) | Epit (mV) | ΔE |
|---|---|---|---|---|
| As-built | −434 | 0.536 | 239 | 673 |
| ST | −420 | 0.497 | 252 | 666 |
| AT | −414 | 0.405 | 322 | 742 |
| ST + AT | −442 | 0.588 | 172 | 614 |
| Samples | R1 (Ω·cm2) | Q (×10−6F·cm−2) | n | R2 (Ω·cm2) | C (×10−6F·cm−2) | R3 (Ω·cm2) |
|---|---|---|---|---|---|---|
| As-built | 6.04 | 81.1 | 0.867 | 47.4 | 5.28 | 591,000 |
| ST | 4.99 | 70.8 | 0.885 | 46.9 | 6.21 | 47,500 |
| AT | 5.34 | 67.3 | 0.858 | 14.5 | 9.81 | 248,000 |
| ST + AT | 5.72 | 47.6 | 0.921 | 46.6 | 9.86 | 23,900 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, S.; Wu, S.; Xing, S.; Wang, T.; Hou, J.; Zhao, Y.; Li, Z.; Liu, Y. Effect of Heat Treatment on the Corrosion Behavior of Selective Laser Melted CX Stainless Steel. Metals 2025, 15, 517. https://doi.org/10.3390/met15050517
Wu S, Wu S, Xing S, Wang T, Hou J, Zhao Y, Li Z, Liu Y. Effect of Heat Treatment on the Corrosion Behavior of Selective Laser Melted CX Stainless Steel. Metals. 2025; 15(5):517. https://doi.org/10.3390/met15050517
Chicago/Turabian StyleWu, Shaoqian, Shuo Wu, Shilong Xing, Tianshu Wang, Jiabin Hou, Yuantao Zhao, Zongan Li, and Yanbo Liu. 2025. "Effect of Heat Treatment on the Corrosion Behavior of Selective Laser Melted CX Stainless Steel" Metals 15, no. 5: 517. https://doi.org/10.3390/met15050517
APA StyleWu, S., Wu, S., Xing, S., Wang, T., Hou, J., Zhao, Y., Li, Z., & Liu, Y. (2025). Effect of Heat Treatment on the Corrosion Behavior of Selective Laser Melted CX Stainless Steel. Metals, 15(5), 517. https://doi.org/10.3390/met15050517






