Evaluation of the Corrosion Behavior of Inconel 718 Alloy Processed by SLM Additive Manufacturing Method After 5000 h of Immersion in Natural Seawater
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials Preparation
2.2. Electrochemical Measurements
2.3. Surface Examination Methods
3. Results and Discussion
3.1. Electrochemical Results
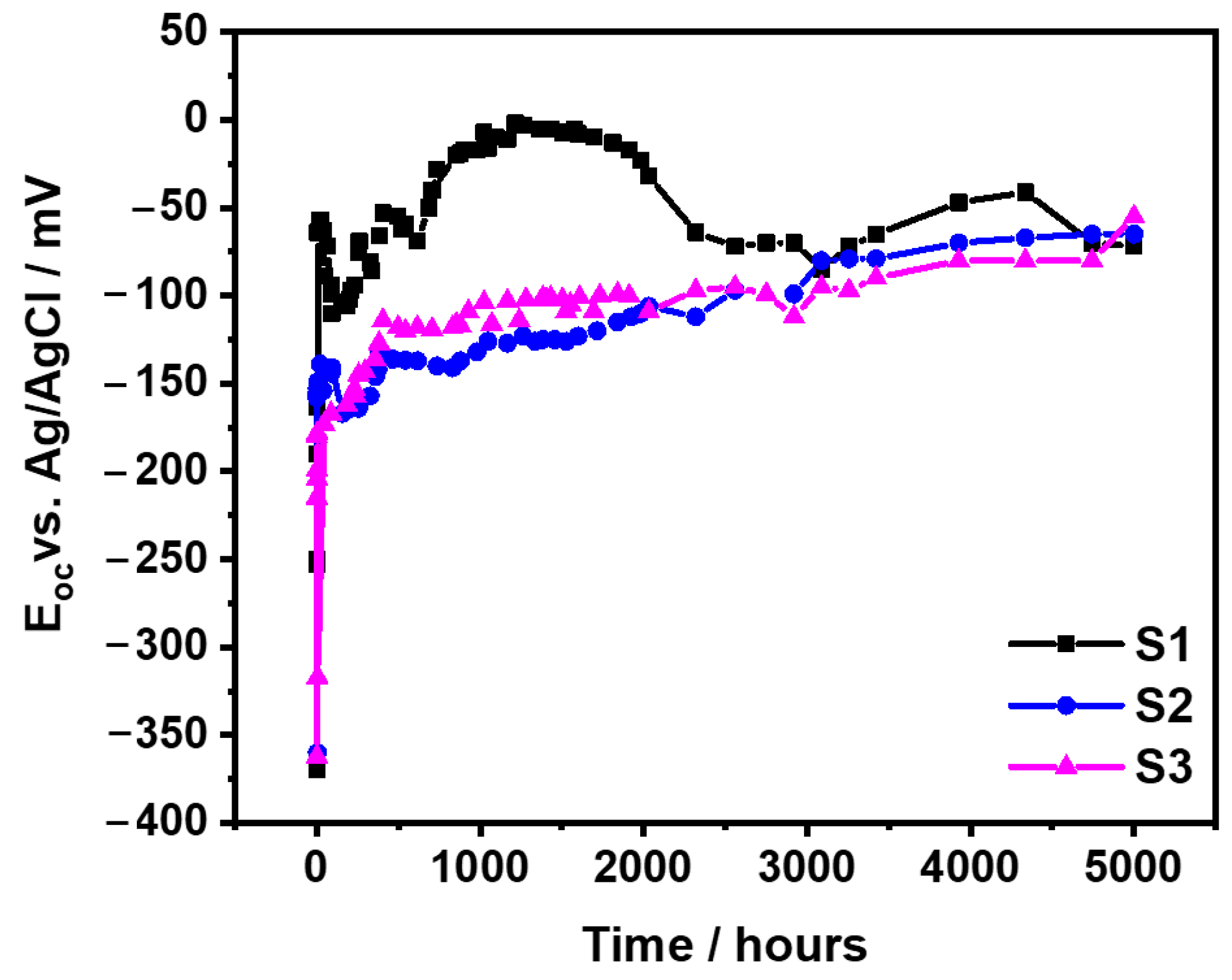
3.1.1. OCP Evolution
3.1.2. EIS Measurements
3.1.3. Potentiodynamic Polarization
3.2. Surface Characterization
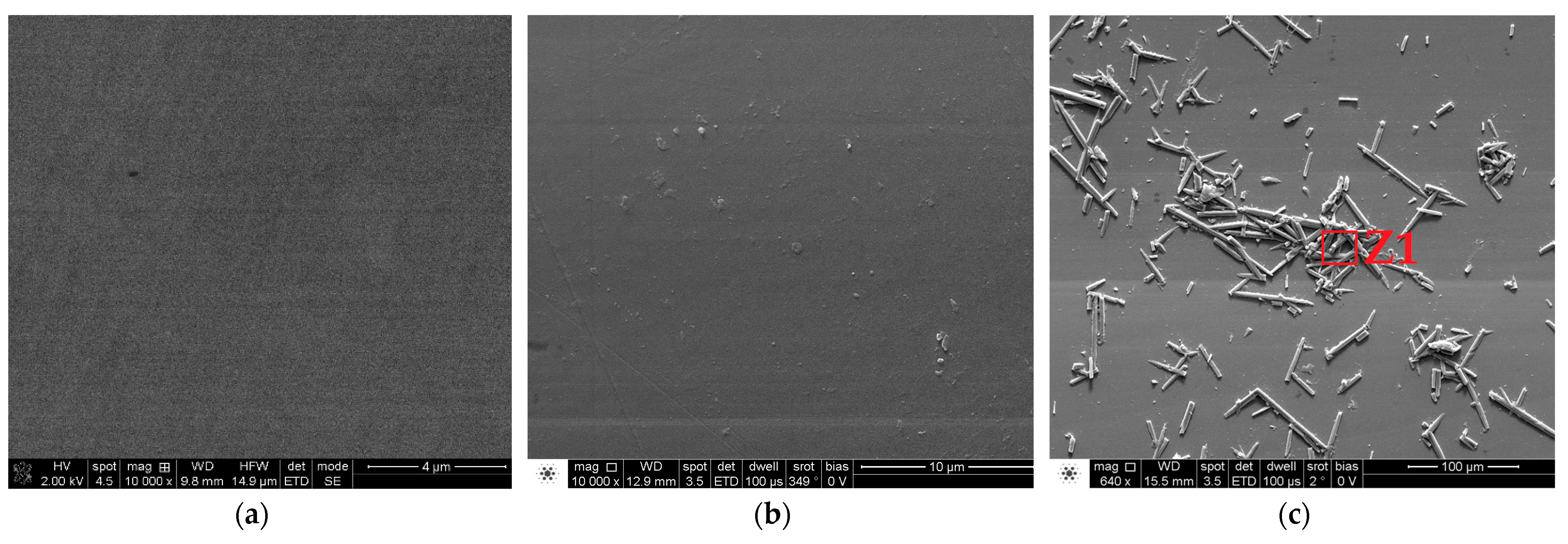
Surface Topography and Morphology
4. Conclusions
- (1)
- This study demonstrates that the corrosion resistance of Ni-based alloys exposed to seawater is significantly influenced by the manufacturing process, which governs the structure and chemistry of the protective film formed.
- (2)
- The wrought Inconel 718 alloy (S1 sample) developed a dense, continuous, and compositionally stable protective film enriched in Cr and Mo, which provided the most effective protection against the corrosion process. In contrast, the additively manufactured SLM Inconel 718 alloy (S2 sample) exhibited a porous and chemically heterogeneous layer, disrupted by salt entrapment and surface defects, leading to lower corrosion resistance. The findings align well with the corrosion parameters estimated from Tafel curves during the immersion period, showing that the corrosion rate (Rcorr) of the wrought 718 alloy is significantly lower (thirty times) than that of the SLS 718 alloy. This suggests that the wrought 718 alloy exhibits superior corrosion resistance. Additionally, these results are further supported by electrochemical impedance spectroscopy (EIS) data, which indicate a higher polarization resistance (Rp) for the protective film of the wrought 718 alloy, measuring 1.622 MΩ cm−2, compared to just 8.6 kΩ cm−2 for the SLM 718 alloy.
- (3)
- In conclusion, polarization curve analysis reveals that wrought Inconel 718 and Incoloy 825 alloys display pseudopassivation and activation dissolution behavior. In contrast, the SLM Inconel 718 alloy shows increased sensitivity, indicated by a continuous rise in current density within the tens of microamperes range, suggesting a high dissolution rate linked to surface film heterogeneity. Notably, surface analysis of all samples after 5000 h of immersion indicated no localized corrosion, confirming the alloys’ resistance to marine corrosion.
- (4)
- The Incoloy 825 commercial alloy (S3 sample) showed an intermediate response. Although its corrosion performance was superior to the AM-produced Inconel 718 (S2 sample), it did not achieve the same level of uniformity and chemical stability in the protective film as identified in the S1 alloy. The presence of widespread acicular deposits rich in calcium (Ca), oxygen (O), and chromium (Cr) indicates the accumulation of some products, which interfere with the formation of a homogeneous protective film on the alloy’s surface.
- (5)
- Compared to the corrosion performance of Inconel 718 alloys reported in NaCl solution, the examined alloy in seawater displayed superior corrosion resistance for wrought Inconel 718, while SLM Inconel 718 exhibited comparable corrosion performance, making them suitable for a broader range of applications in marine environments.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pavapootanont, G.; Wongpanya, P.; Viyanit, E.; Lothongkum, G. Corrosion Behavior of Ni Steels in Aerated 3.5-wt.% NaCl Solution at 25 °C by Potentiodynamic Method. Eng. J. 2018, 22, 1–12. [Google Scholar] [CrossRef]
- Zang, L.N.; Ojo, O.A. Corrosion behavior of wire arc additive manufactured. J. Alloys Compd. 2020, 829, 154455. [Google Scholar] [CrossRef]
- Yuan, S.Y.; Pehkonen, S.O. Surface characterization and corrosion behavior of 70/30 Cu-Ni alloy in pristine and sulfite-containing simulated seawater. Corros. Sci. 2007, 49, 1276–1304. [Google Scholar] [CrossRef]
- Wang, Q.-Y.; Zhang, X.-S.; Zheng, H.-B.; Liu, T.-Y.; Dong, L.-J.; Zhang, J.; Xi, Y.-C.; Zeng, D.-Z.; Lin, Y.-H.; Luo, H. Intergranular corrosion mechanism of sub-grain in laser additive manufactured Hastelloy C22 induced by heat treatment. Appl. Surf. Sci. 2023, 608, 155140. [Google Scholar] [CrossRef]
- Mehta, S.; Jha, S.; Liang, H. Corrosion of nickel-based alloys fabricated through additive manufacturing: A review. Prog. Addit. Manuf. 2022, 7, 1257–1273. [Google Scholar] [CrossRef]
- Thuneman, T.; Raja, K.S.; Charit, I. Room Temperature Corrosion Behavior of Selective Laser Melting (SLM)-Processed Ni-Fe Superalloy (Inconel 718) in 3.5% NaCl Solution at Different pH Conditions: Role of Microstructures. Crystals 2024, 14, 89. [Google Scholar] [CrossRef]
- Galvele, J.R. Tafel’s law in pitting corrosion and crevice corrosion susceptibility. Corros. Sci. 2005, 47, 3053–3067. [Google Scholar] [CrossRef]
- Henderson, J.D.; Almusned, B.; Momeni, M.; Anderson, S.; Dehnavi, V.; Zagidulin, D.; Shoesmith, D.W.; Noël, J.J. Investigating the influence of Cr and Mo Additions to Commercial Ni-Based Alloys Exposed to Neutral and Acidic Chloride Solution. J. Electrochim. Soc. 2020, 167, 131512. [Google Scholar] [CrossRef]
- Mirsha, A.K.; Shoesmith, D.W. The activation/depassivation of nickel–chromium–molybdenum alloys: An oxyanion or a pH effect—Part II. Electrochim. Acta 2013, 102, 328–335. [Google Scholar] [CrossRef]
- Lloyd, A.C.; Noël, J.J.; McIntyre, S.D.W.; Shoesmith, D.W. Cr, Mo and W alloying additions in Ni and their effect on passivity. Electrochim. Acta 2004, 49, 3015–3027. [Google Scholar] [CrossRef]
- Klapper, H.S.; Zadorozne, N.S.; Rebak, R.B. Localized corrosion characteristics of nickel alloys: A review. Acta Metall. Sin. 2017, 30, 296–305. [Google Scholar] [CrossRef]
- Gerretsen, J.H.; De Wit, J.H.W. The role of molybdenum in the active-passive transition of iron-chromium alloys. Electrochim. Acta 1991, 36, 1465–1467. [Google Scholar] [CrossRef]
- Stanciu, E.M.; Pascu, A.; Ţierean, M.H.; Voiculescu, I.; Roată, I.C.; Croitoru, C.; Hulka, I. Dual Coating Laser Cladding of NiCrBSi and Inconel 718. Mater. Manuf. Process. 2016, 31, 1556–1564. [Google Scholar] [CrossRef]
- Choudhury, I.A.; El-Baradie, M.A. Machinability of nickel-base super alloys: A general review. J. Mater. Res. Technol. 1998, 77, 278–284. [Google Scholar] [CrossRef]
- Amigo, F.J.; Urbikain, G.; Pereira, O.; Fernández-Lucio, P.; Fernández-Valdivielso, A.; López de Lacalle, L.N. Combination of high feed turning with cryogenic cooling on Inconel 718. J. Manuf. Process. 2020, 58, 208–222. [Google Scholar] [CrossRef]
- Mostafaei, A.; Ghiaasiaan, R.; Ho, I.-T.; Strayer, S.; Chang, K.-C.; Shamsaei, N.; Shao, S.; Paul, S.; Cho Yeh, A.G.; Tin, S.; et al. Additive manufacturing of nickel-based superalloys: A state-of-the-art review on process-structure-defect-property relationship. Prog. Mater. Sci. 2023, 136, 101108. [Google Scholar] [CrossRef]
- Pereira, R.B.D.; Gómez-Escudero, G.; Calleja-Ochoa, A.; Pereira, O.; González-Barrio, H.; Brandão, L.C.; López de Lacalle, L.N. Hybrid cryogenic/MQL helical milling for hole-making of Inconel 718. Results Eng. 2025, 26, 104776. [Google Scholar] [CrossRef]
- González, H.; Pereira, O.; López de Lacalle, L.N.; Calleja, A.; Ayesta, I.; Muñoa, J. Flank-milling of integral blade rotors made in Ti6Al4V using cryo CO₂ and minimum quantity lubrication (MQL). J. Manuf. Sci. Eng. 2021, 143, 091011. [Google Scholar] [CrossRef]
- Shahwaz, M.; Nath, P.; Sen, I. Recent advances in additive manufacturing technologies for Ni-Based Inconel superalloys—A comprehensive review. J. Alloys Compd. 2025, 1010, 177654. [Google Scholar] [CrossRef]
- Tang, Y.; Shen, X.; Liu, Z.; Xiao, Y.; Yang, L.; Lu, D.; Zhou, J.; Xu, J. Corrosion Behaviors of Selective Laser Melted Inconel 718 Alloy in NaOH Solution. Acta Metall. Sin. 2022, 58, 324–333. [Google Scholar] [CrossRef]
- Jeyaprakash, N.; Yang, C.H.; Karuppasamy, S.S.; Radhika, N. Microstructural evolution and anti-corrosion characteristics of as-cast and LPBF printed Inconel plates in seawater environment. Surf. Rev. Lett. 2023, 31, 2450032. [Google Scholar] [CrossRef]
- ISO 8044/2020; Corrosion of Metals and Alloys—Basic Terms and Definitions. ISO: Geneva, Switzerland, 2020.
- Tang, Z.; Yang, C.; Duan, Y.; Ma, L.; Zheng, S.; Li, M. Corrosion and wear behaviors of Inconel 718 nickel-based alloy by boroaluminizing. Surf. Coat. Technol. 2024, 478, 130500. [Google Scholar] [CrossRef]
- Huang, X.; Qiu, W.; Niu, B.; Zou, X.; Pan, L.; Kwok, C.T. Role of complex nonmetallic inclusions on the localized corrosion resistance of wire arc additively manufactured super duplex stainless steel. J. Mater. Res. Technol. 2024, 28, 799–813. [Google Scholar] [CrossRef]
- Pourbaix, M. Atlas of Electrochemical Equilibria. In Aqueous Solutions, 2nd ed.; National Association of Corrosion: Houston, TX, USA, 1974. [Google Scholar]
- Dobri, G.; Banu, A.; Donath, C.; Marcu, M. The Influence of the Tantalum Content on the Main Properties of the TixTa9Nb8Zr2Ag Alloy. Metals 2023, 13, 1294. [Google Scholar] [CrossRef]
- Xie, F.X.; He, X.B.; Lu, S.L.; Qu, X.H. Structural characterization and electrochemical behavior of a laser-sintered porous Ti–10Mo alloy. Corros. Sci. 2013, 67, 217–224. [Google Scholar] [CrossRef]
- Orezam, M.E.; Tribolet, B. Electrochemical Impedance Spectroscopy; Wiley: New York, NY, USA, 2017. [Google Scholar]
- Jiang, X.; Di, X.; Li, C.; Wang, D.; Hu, W. Improvement of mechanical properties and corrosion resistance for wire arc additive manufactured nickel alloy 690 by adding TiC particles. J. Alloys Compd. 2022, 928, 167198. [Google Scholar] [CrossRef]
- Banu, A.; Preda, L.; Marcu, M.; Dinca, L.L.; Maxim, M.E.; Dobri, G. Electrochemical Behavior of SLM Ti–6Al–4V Alloy After Long Time of Immersion in Lactic Acid. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2022, 53, 2060–2070. [Google Scholar] [CrossRef]
- Tang, Y.; Shen, X.; Qiao, Y.; Yang, L.; Chen, J.; Lu, D.; Zhang, Z. Corrosion behavior of a Selective Laser Melted Inconel 718 Alloy in a 3.5% NaCl Solution. J. Mater. Eng. Perform. 2021, 30, 5506–5514. [Google Scholar] [CrossRef]
- Klapper, H.S.; Stevens, J. Susceptibility to Pitting Corrosion of Nickel-Based Alloy 718 Exposed to Simulated Drilling Environments. Corrosion 2014, 70, 899–906. [Google Scholar] [CrossRef]
- Markovic, T. The passivity of nickel in neutral and alkaline solutions. Werkst. Korros. 1965, 16, 570. [Google Scholar] [CrossRef]
- Macdonald, D.D. Passivity-The key to our metals-based civilization. Pure Appl. Chem. 1999, 71, 951–978. [Google Scholar] [CrossRef]
- Zhu, J.; Li, D.; Chang, W.; Wang, Z.; Hu, L.; Zhang, Y.; Wang, M.; Yang, Z.; Song, J.; Chen, S.; et al. In situ marine exposure study on corrosion behaviors of five alloys in coastal waters of western Pacific Ocean. J. Mater. Res. Technol. 2020, 9, 8104–8116. [Google Scholar] [CrossRef]
- Li, X.; Ogle, K. The Passivation of Ni-Cr-Mo Alloys: Time Resolved Enrichment and Dissolution of Cr and Mo during Passive-Active Cycles. J. Electrochem. Soc. 2019, 166, C3179–C3185. [Google Scholar] [CrossRef]
- Xu, Z.; Qin, X.; Liu, X.; Su, L.; Sun, Z.; Li, W.; Ma, X.; Guo, W.; Cheng, W. Effect of surface morphology and microstructure on the corrosion behavior of Inconel 718 superalloy prepared by laser additive and subtractive hybrid manufacturing. Mater. Today Commun. 2025, 44, 112176. [Google Scholar] [CrossRef]
- Zhao, B.; Hutt, K.; Yamaguchi, H.; Pan, S. Surface corrosion in laser powder bed fusion-fabricated Inconel 718 with magnetic field-assisted post-processing. J. Manuf. Process. 2025, 143, 277–292. [Google Scholar] [CrossRef]
- Li, X.; Wang, J.; Han, E.-H.; Ke, W. Corrosion behavior for Alloy 690 and Alloy 800 tubes in simulated primary water. Corros. Sci. 2013, 67, 169–178. [Google Scholar] [CrossRef]










| Sample | Ni | Cr | Mo | Fe | Nb | Mn | Cu | Ti |
|---|---|---|---|---|---|---|---|---|
| Inconel 718 (S1) | 55 | 22 | 3 | 13 | 3 | 0.5 | - | 1 |
| SLM Inconel 718 (S2) | 55 | 22 | 3 | 13 | 3 | 0.5 | - | 1 |
| Incoloy 825 (S3) | 40 | 21 | 3 | 31 | - | 1 | 1.8 | 1.2 |
| Ions | Na+ | K+ | Ca2+ | Mg2+ | Cl− | SO42− | NO3− | PO43− |
|---|---|---|---|---|---|---|---|---|
| mg L−1 | 14,110 | 652 | 712 | 1350 | 23,250 | 3689 | 0.8 | 0.11 |
| Medium | pH | Conductivity (mS cm−1) | Salinity (ppt) | Dissolved Oxygen (mg L−1) |
|---|---|---|---|---|
| Seawater | 6.5 | 55.25 | 35.2 | 7.1 |
| Sample | Rs (ohm cm2) | CPEf sn Ω−1 cm−2 | n | Rf (ohm cm2) | CPEdl sn Ω−1 cm−2 | n | Rct (ohm cm2) | Rp (ohm cm2) |
|---|---|---|---|---|---|---|---|---|
| S1 | 5.46 ± 0.1 | 1.40 × 10−5 | 0.91 | 761,835 ± 20 | 2.53 × 10−7 | 0.9 | 859,695 ± 20 | 1,621,535 ± 51 |
| S2 | 6.26 ± 0.2 | 2.50 × 10−5 | 0.89 | 1350 ± 12 | 3.52 × 10−5 | 0.51 | 7290 ± 70 | 8646 ± 7 |
| S3 | 3.07 ± 0.1 | 4.90 × 10−5 | 0.82 | 11,331 ± 15 | 1.41 × 10−5 | 0.88 | 213,561 ± 10 | 224,895 ± 12 |
| Exposure Time, h | S1 | S2 | S3 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Ecorr (V) | jcor (µA cm−2) | Rcorr (µm y−1) | Ecorr (V) | jcor (µA cm−2) | Rcorr (µm y−1) | Ecorr (V) | jcor (µA cm−2) | Rcorr (µm y−1) | |
| 336 | −0.158 ± 0.05 | 0.055 ± 0.01 | 0.39 ± 0.03 | −0.202 ± 0.03 | 6.7 ± 0.2 | 70.6 ± 0.3 | −0.284 ± 0.01 | 1.8 ± 0.2 | 19.3 ± 0.3 |
| 500 | −0.103 ± 0.01 | 0.031 ± 0.01 | 0.33 ± 0.04 | −0.156 ± 0.01 | 7.62 ± 0.15 | 80.4 ± 0.5 | −0.165 ± 0.01 | 2.3 ± 0.1 | 25.3 ± 0.5 |
| 1000 | −0.095 ± 0.02 | 0.019 ± 0.02 | 0.20 ± 0.03 | −0.090 ± 0.01 | 11.8 ±0.1 | 86.5 ± 0.7 | −0.153 ± 0.01 | 6.5 ± 0.2 | 69.8 ± 0.2 |
| 2000 | −0.097 ± 0.01 | 0.117 ± 0.01 | 0.82 ± 0.04 | −0.079 ± 0.02 | 15.36 ± 0.1 | 113.0 ± 0.5 | −0.135 ± 0.01 | 10.1 ± 0.2 | 129.0 ± 0.3 |
| 5000 | −0.164 ± 0.05 | 0.173 ± 0.02 | 1.21 ± 0.02 | −0.058 ± 0.01 | 2.33 ± 0.2 | 35.1 ± 0.6 | −0.104 ± 0.01 | 0.348 ± 0.01 | 4.1 ± 0.2 |
| Alloy | icorr (A cm−2) | Ecorr (mV) | Rcorr (µm y−1) | Time Immersion, h | References |
|---|---|---|---|---|---|
| Inconel 718 | 6.67 × 10−6 | −930 vs. SCE | 1 | [23] | |
| Inconel 718 SLM | 2.1 × 10−7 | −200 vs. SCE | 3.4 | 1 | [31] |
| Inconel 718 LDED * | 3.43 × 10−5 | −382 | [35] | ||
| Inconel 718- LPBF ** | 24.61 × 10−6 | −850 vs. Ag/AgCl | 0.5 | [36] | |
| Inconel 718 SLM | 2.33 × 10−6 | −58 vs. Ag/AgCl | 35 | 5000 | This paper *** |
| Inconel 718 wrought | 1.73 × 10−7 | −164 vs. Ag/AgCl | 1.21 | 5000 | This paper *** |
| Areas | O K | NaK | MgK | MoL | TiK | S K | ClK | K K | CaK | CrK | FeK | NiK |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Global | 3.3 | 2.15 | 1.75 | 2.7 | 1.37 | 5.67 | 1.02 | 0.95 | 1.17 | 19.48 | 4.11 | 56.33 |
| Z1 | 3.26 | 1.98 | 1.88 | 2.32 | 5.34 | 6.24 | 0.98 | 1.09 | 1.26 | 19.43 | 4.13 | 52.09 |
| Areas | O K | NaK | MgK | MoL | TiK | S K | ClK | K K | CaK | CrK | FeK | NiK |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Global | 2.84 | 1.79 | 1.54 | 2.31 | 1.17 | 5.73 | 1.05 | 0.94 | 1.13 | 19.46 | 4.9 | 57.14 |
| Z1 | 3.37 | 5.53 | 3.49 | 3.49 | 1.64 | 7.68 | 0.87 | 1.35 | 1.69 | 16.72 | 4.88 | 49.29 |
| Z2 | 4.01 | 6.93 | 4.82 | 5.31 | 2.23 | 9.62 | 0.94 | 1.6 | 2.27 | 14.47 | 5.55 | 42.25 |
| Areas | O K | NaK | MgK | MoL | S K | K K | ClK | CaK | TiK | CrK | FeK | NiK |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Global | 4.24 | 1.35 | 1.27 | 1.66 | 2.22 | 0.85 | 1.37 | 2.6 | 1.89 | 19.34 | 25.9 | 37.31 |
| Z1 | 25.43 | 2.02 | 1.21 | 1.74 | 1.71 | 0.78 | 1.41 | 44.17 | 1.33 | 5.14 | 5.89 | 9.17 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neacsu, E.I.; Donath, C.; Preda, L.; Anastasescu, M.; Banu, A.; Paraschiv, A.; Bibis, A.; Marcu, M. Evaluation of the Corrosion Behavior of Inconel 718 Alloy Processed by SLM Additive Manufacturing Method After 5000 h of Immersion in Natural Seawater. Metals 2025, 15, 713. https://doi.org/10.3390/met15070713
Neacsu EI, Donath C, Preda L, Anastasescu M, Banu A, Paraschiv A, Bibis A, Marcu M. Evaluation of the Corrosion Behavior of Inconel 718 Alloy Processed by SLM Additive Manufacturing Method After 5000 h of Immersion in Natural Seawater. Metals. 2025; 15(7):713. https://doi.org/10.3390/met15070713
Chicago/Turabian StyleNeacsu, Elena Ionela, Cristina Donath, Loredana Preda, Mihai Anastasescu, Alexandra Banu, Alexandru Paraschiv, Adrian Bibis, and Maria Marcu. 2025. "Evaluation of the Corrosion Behavior of Inconel 718 Alloy Processed by SLM Additive Manufacturing Method After 5000 h of Immersion in Natural Seawater" Metals 15, no. 7: 713. https://doi.org/10.3390/met15070713
APA StyleNeacsu, E. I., Donath, C., Preda, L., Anastasescu, M., Banu, A., Paraschiv, A., Bibis, A., & Marcu, M. (2025). Evaluation of the Corrosion Behavior of Inconel 718 Alloy Processed by SLM Additive Manufacturing Method After 5000 h of Immersion in Natural Seawater. Metals, 15(7), 713. https://doi.org/10.3390/met15070713







