Exothermic and Slag Formation Behavior of Aluminothermic Reduction of Mo and V Oxides
Abstract
1. Introduction
2. Research Methodology
2.1. Raw Materials and Experimental Plan
- (i)
- Chemical reactions: Reduction of V2O5 and MoO3 by Al (Equation (1)), supported by stoichiometry and exothermicity;
- (ii)
- Physical process: Alloy formation of V, Mo, and excess Al (Equation (2)). This distinction ensures accurate representation of both the redox chemistry and solid-state alloying. The V:Mo = 1:1 ratio and the role of excess Al in controlling (V + Mo) content are retained.
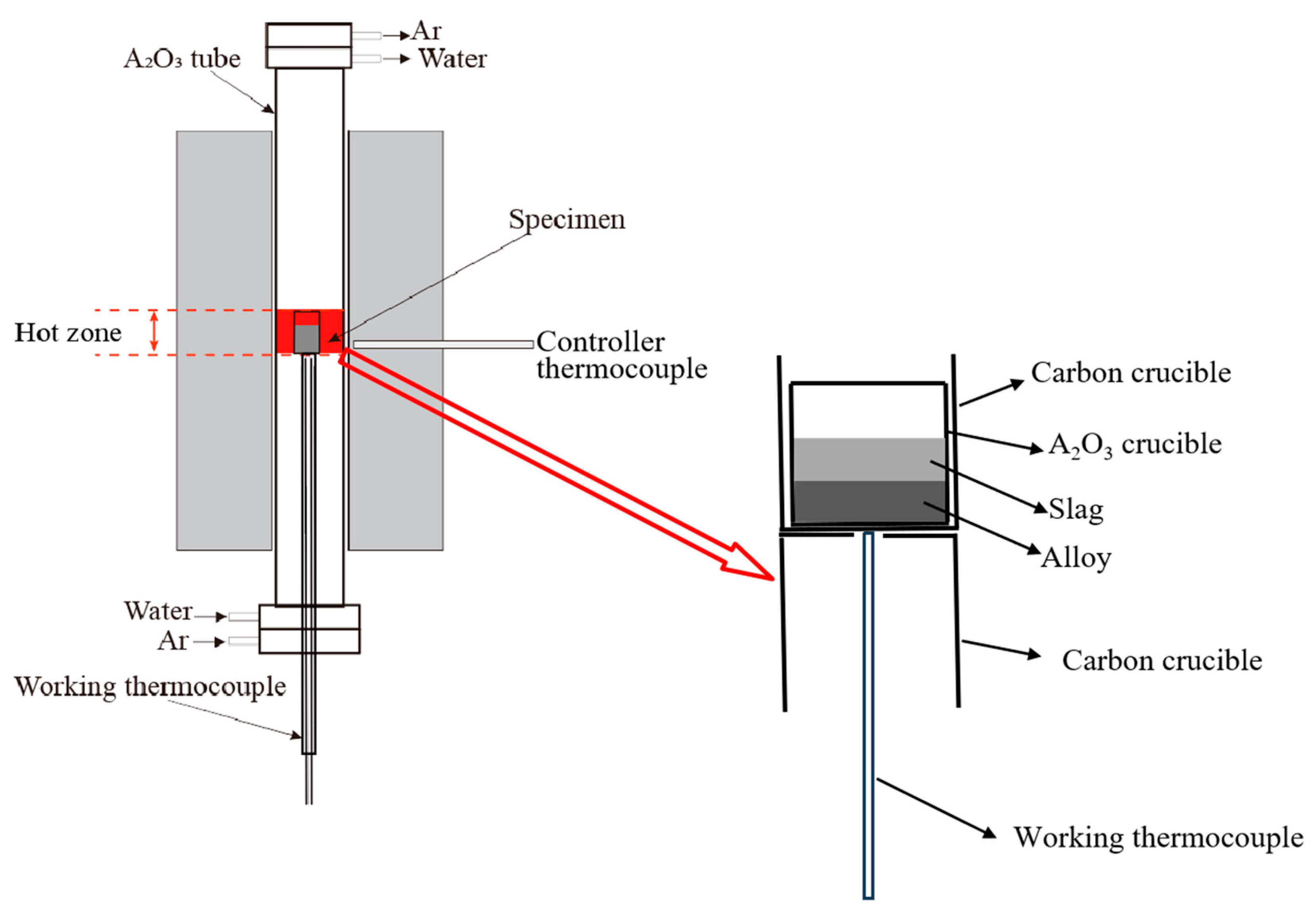
2.2. Experimental Procedure
2.3. Sample Characterization
2.4. Thermodynamic Predictions
3. Thermodynamic Analysis of Aluminothermic Reactions
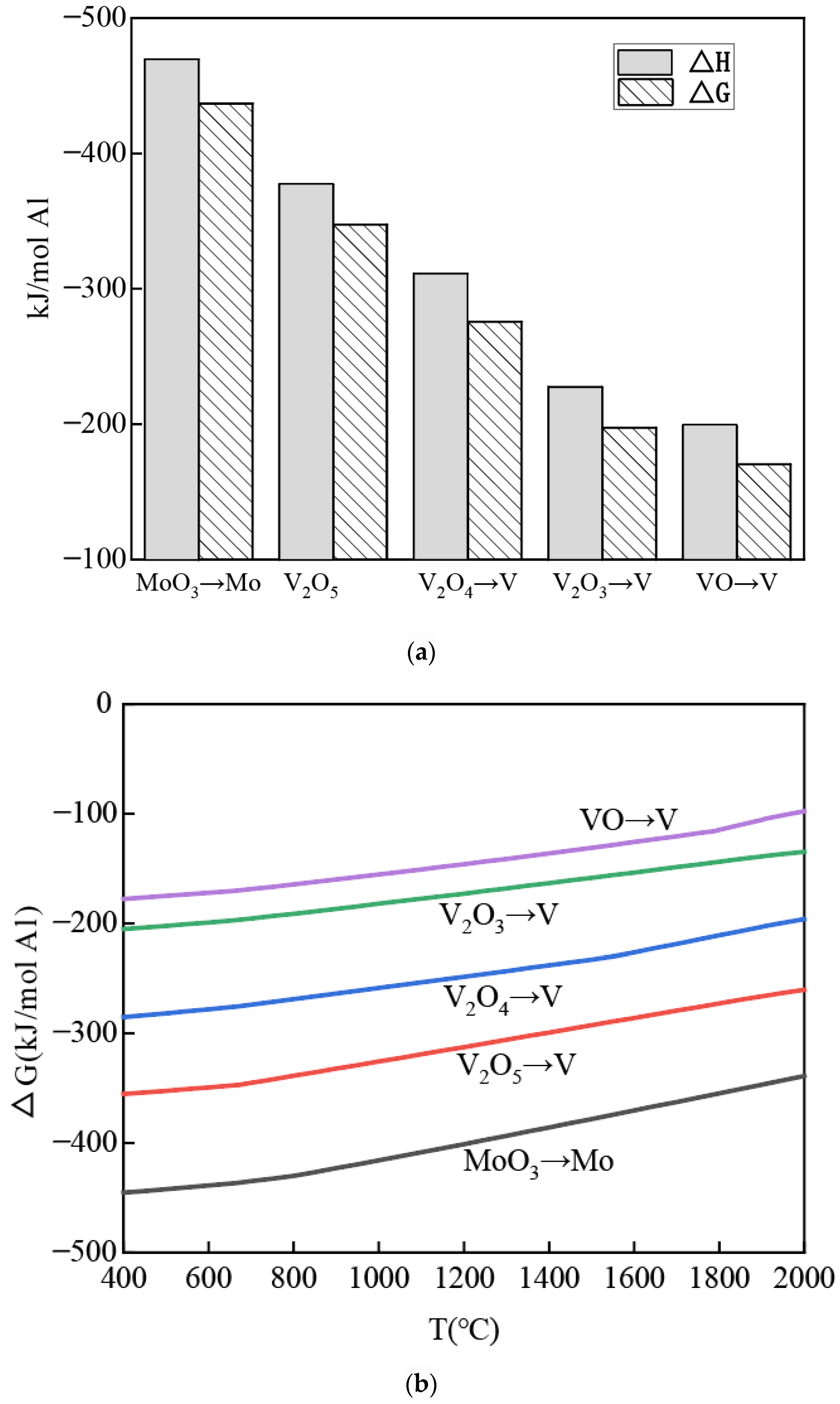
3.1. Gibbs Free Energy and Enthalpy Changes in Aluminothermic Reactions
- (1)
- All reactions exhibit negative values for both Gibbs free energy (ΔG) and enthalpy changes (ΔH), confirming that these reactions are thermodynamically spontaneous and exothermic at 660 °C.
- (2)
- Comparing the free energy and enthalpy changes for the reduction of vanadium oxides from different oxidation states to metallic vanadium, higher oxidation states (e.g., V5+ in V2O5) demonstrate greater thermodynamic favorability and release more heat. Notably, the reduction of MoO3 to metallic Mo is thermodynamically more favorable and releases significantly more heat compared to the reduction of V2O5 to metallic V.
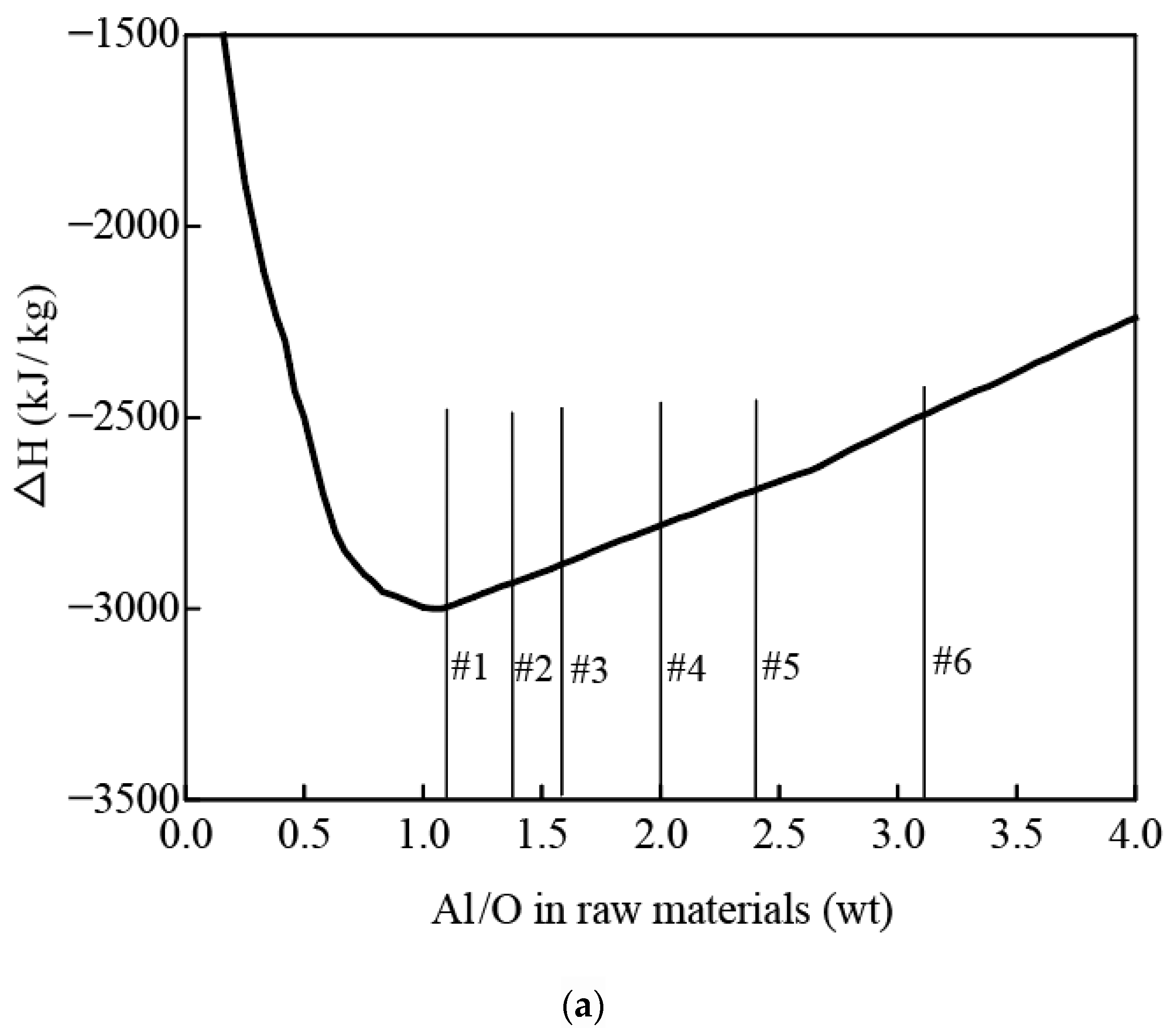
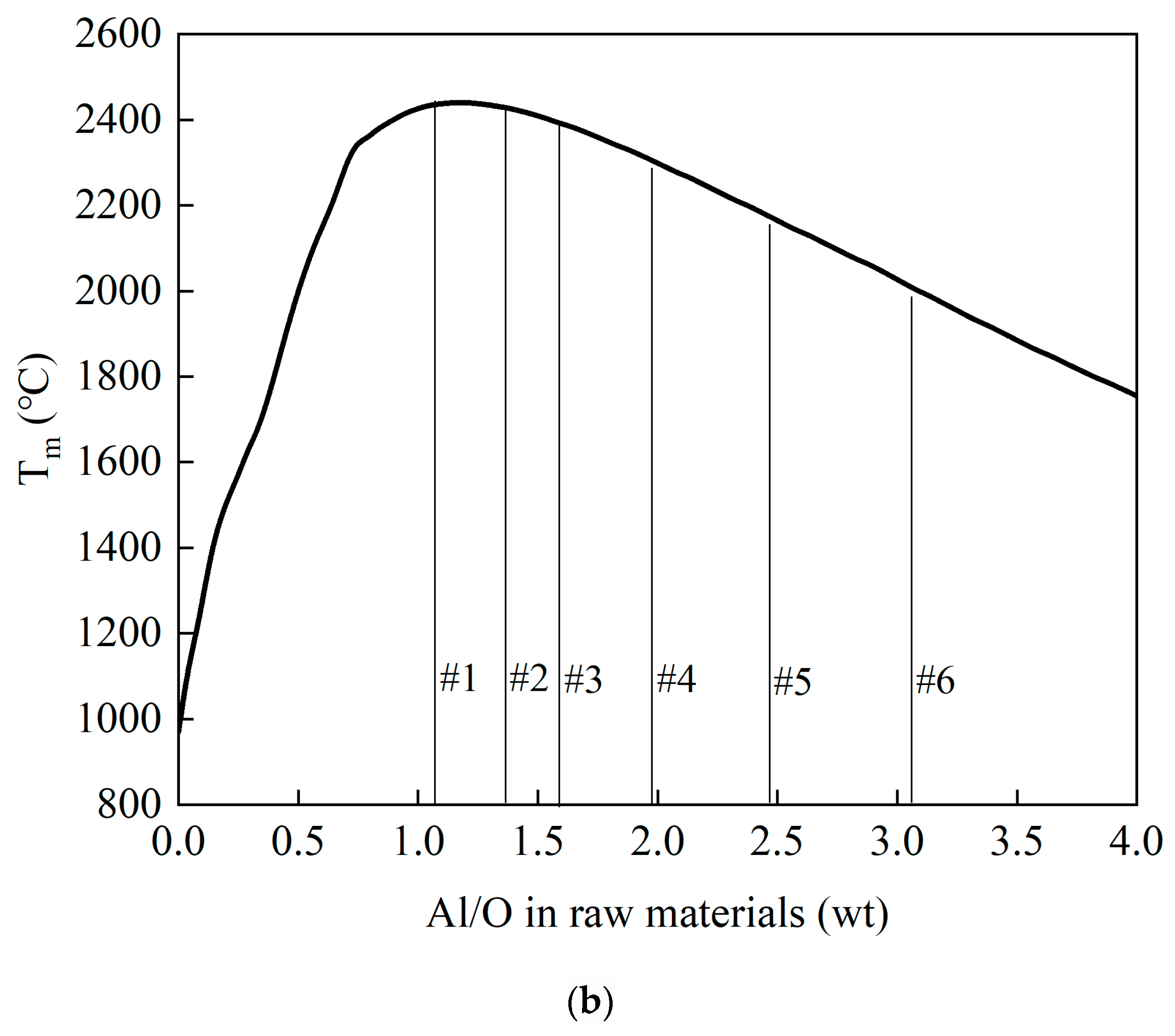
3.2. Thermodynamic Analysis of the Reaction System
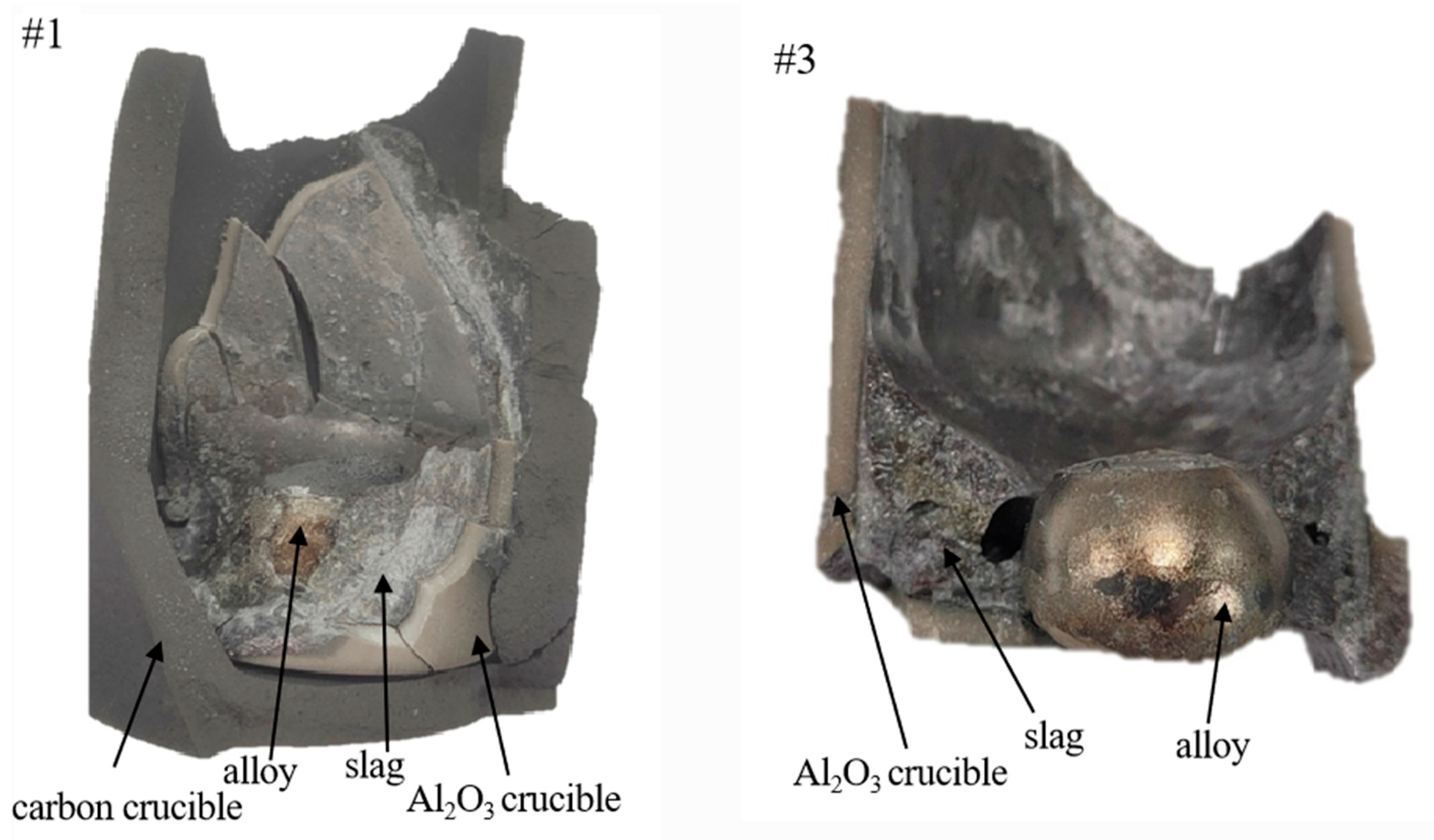
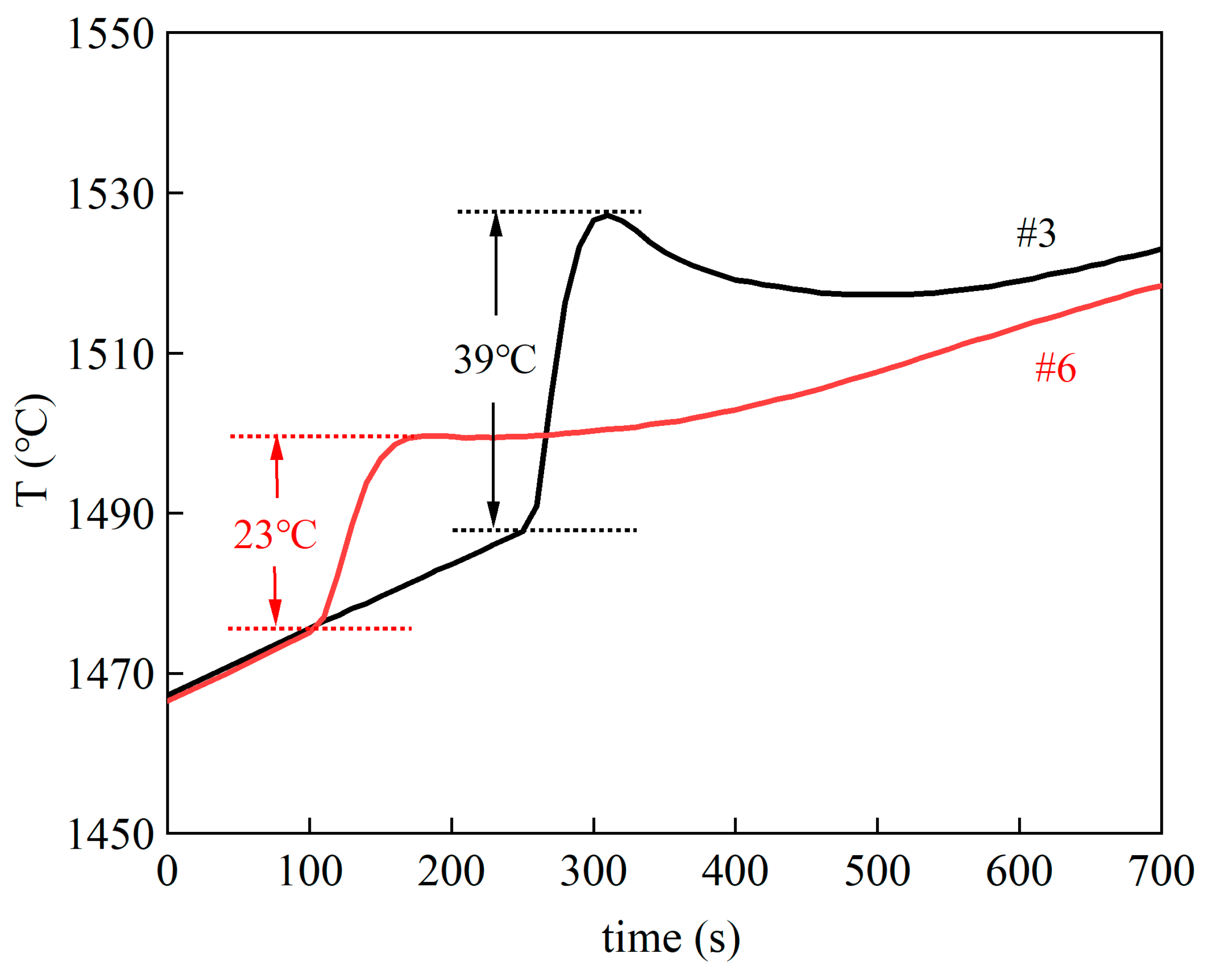
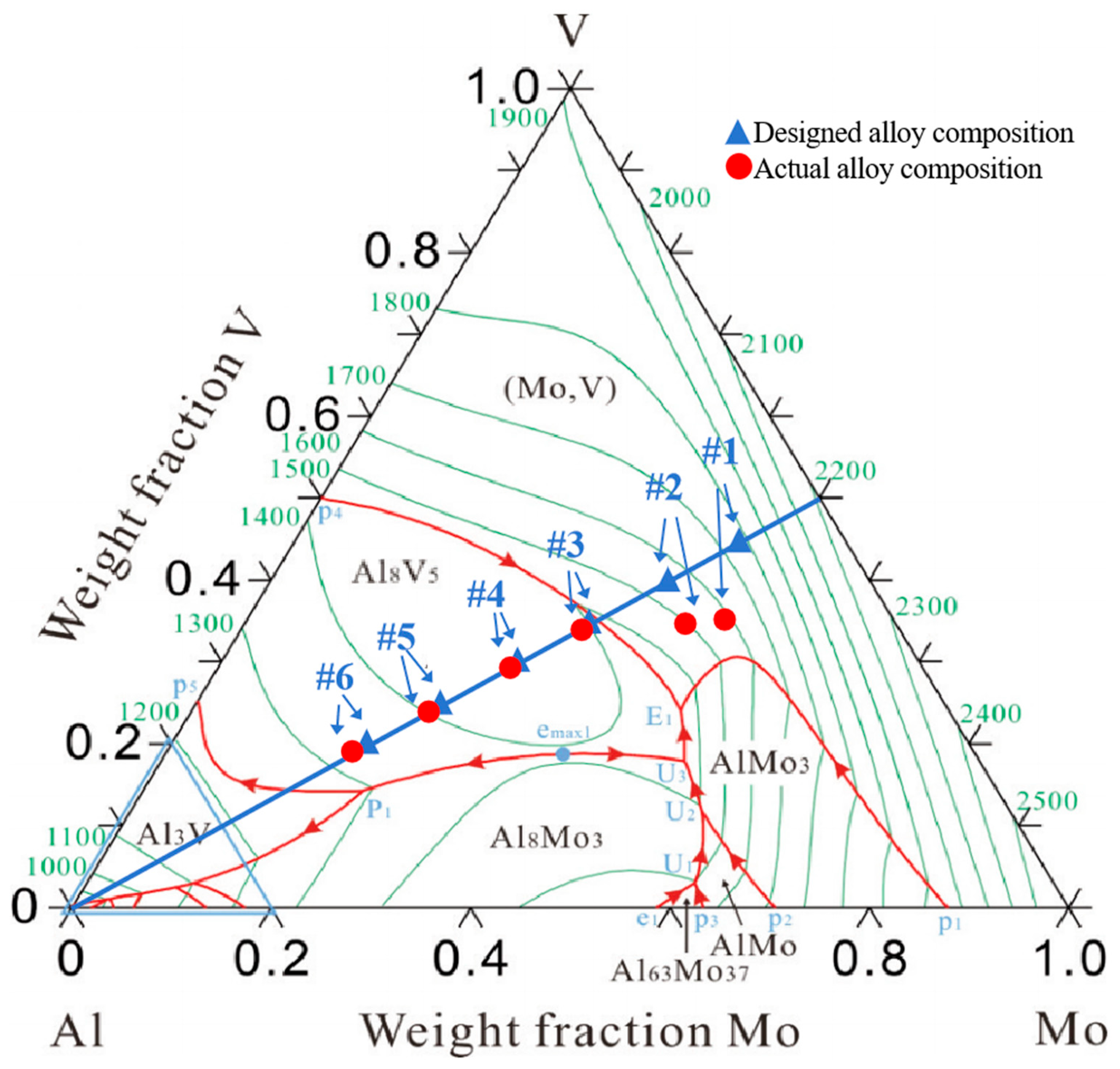
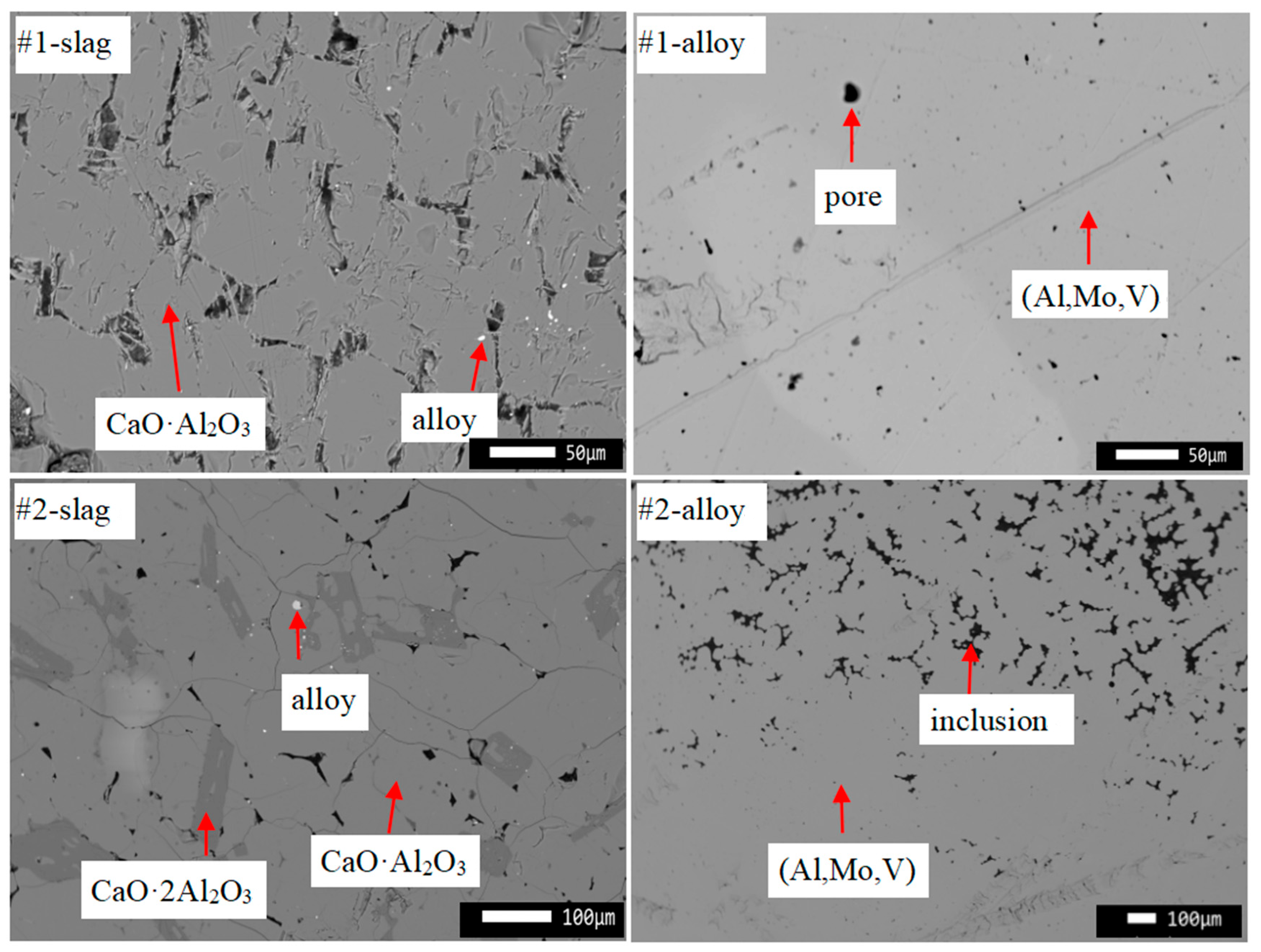
4. Experimental Results and Discussion
5. Conclusions
- (1)
- Thermodynamic analysis confirms the viability of synthesizing V/Al/Mo alloys via aluminothermic reduction. Specifically, MoO3 exhibits higher reducibility to metallic Mo compared to V2O5 reduction to metallic V, with higher-valence vanadium oxides releasing greater exothermic energy during reduction.
- (2)
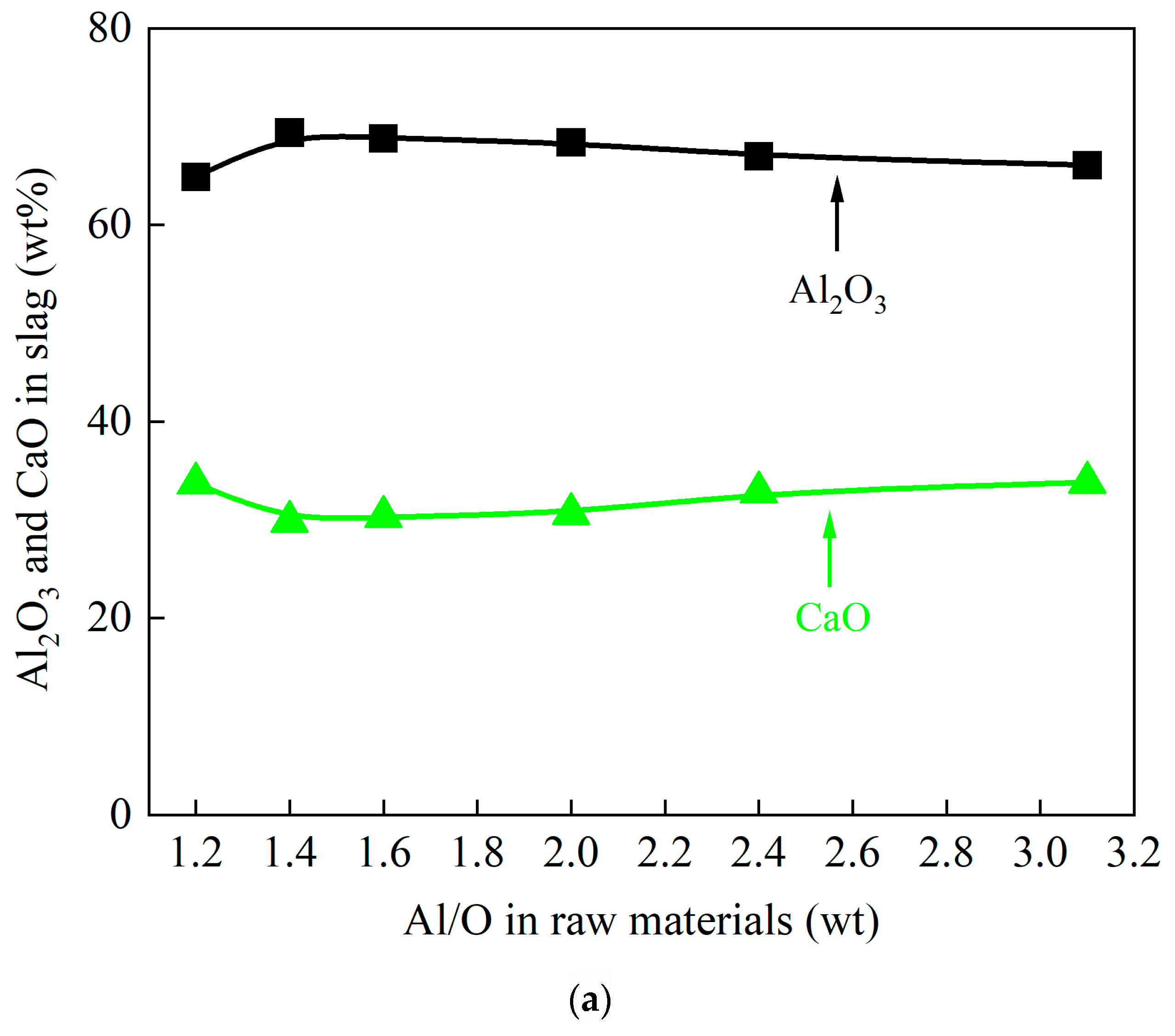
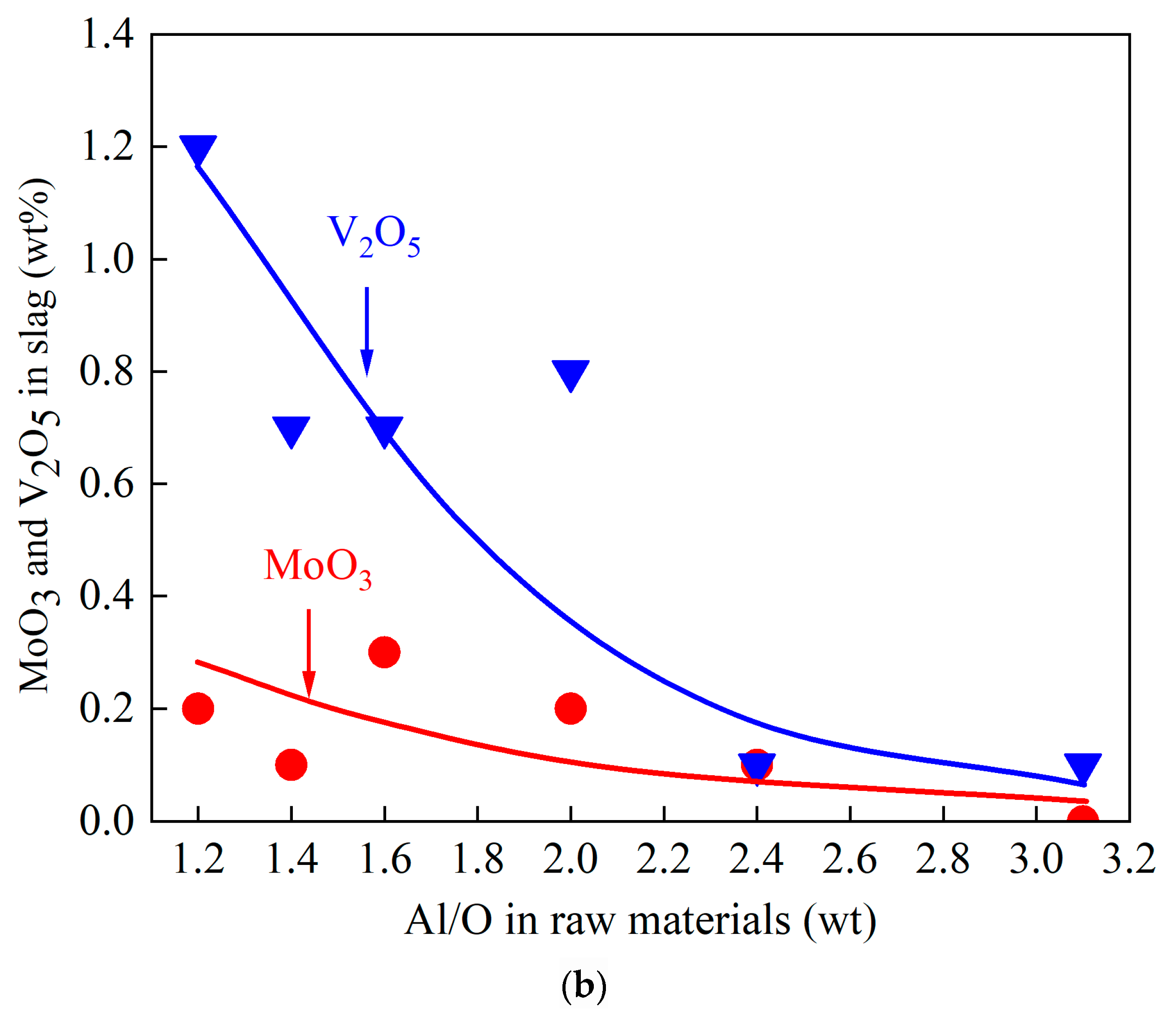
- Optimizing reductant dosage (Al/O ratio) is essential for temperature regulation. When Al/O > 1, higher aluminum content reduces both reaction exothermicity and theoretical reaction temperature.
- (3)
- Adjusting the Al/O ratio effectively governs the composition and phase distribution of the ternary alloy. Aluminum–vanadium-based phases dominate the alloy matrix, while the slag primarily consists of a low melting point CaO·Al2O3 phase.
- (4)
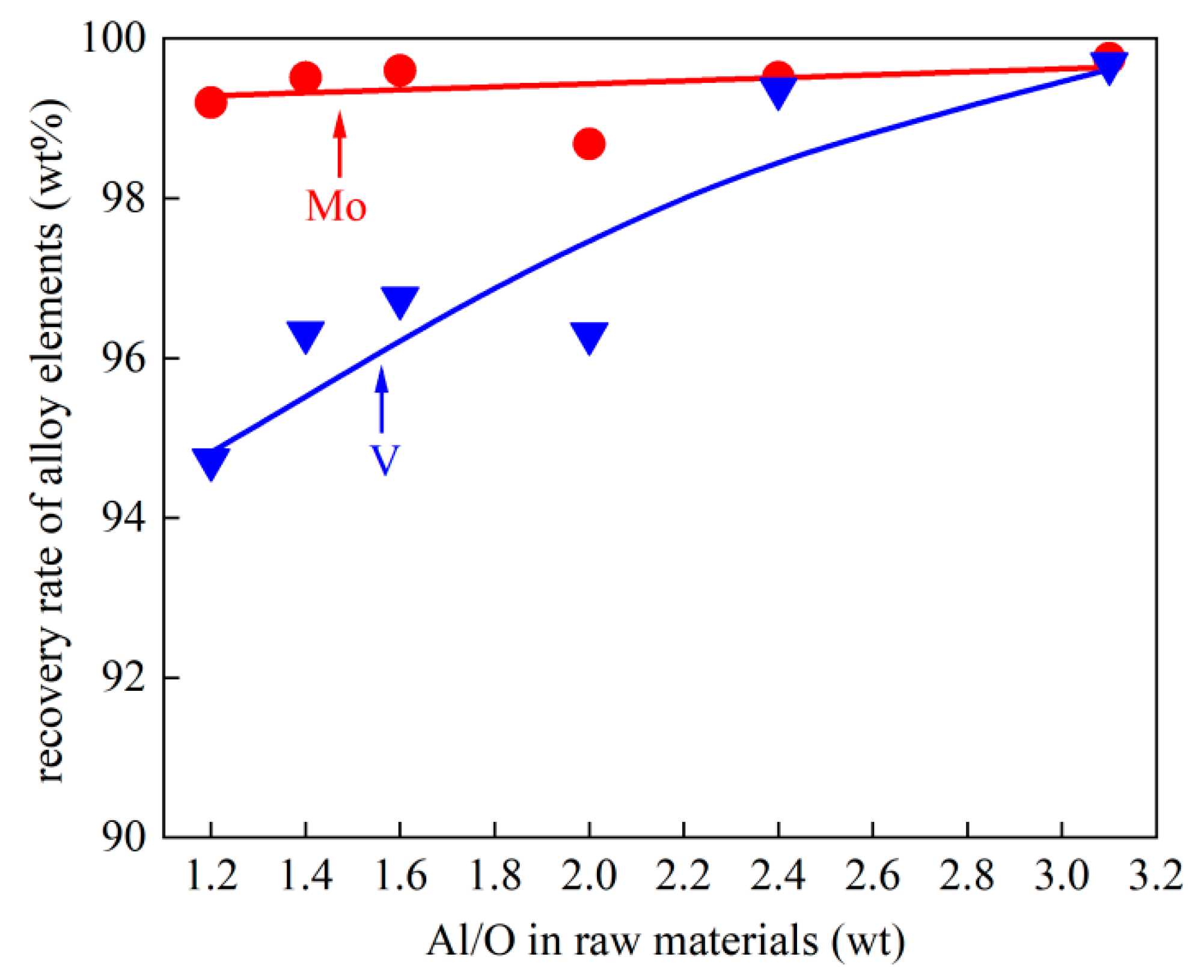
- High-quality V/Al/Mo alloys can be synthesized in a single-step aluminothermic reduction process at 1550 °C with a high recovery rate. When the aluminum content in the alloy is controlled above 30%, the recovery rates of Mo and V exceed 99% and 95%, respectively.
- (5)
- In actual production, it is essential to obtain a master alloy to meet the requirements of the high-melting-point elements V and Mo in the titanium alloy. The proper control of the maximum temperature and compositions of alloy and slag ensures a high alloy yield, which can be achieved by an optimum Al/O ratio in the raw materials. This work provides a theoretical foundation for aluminothermic reduction-based preparation of V/Al/Mo alloys used in titanium alloys and offers practical guidelines for industrial-scale production.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Harun, W.S.W.; Manam, N.S.; Kamariah, M.S.I.N.; Sharif, S.; Zulkifly, A.H.; Ahmad, I.; Miura, H. A review of powdered additive manufacturing techniques for Ti-6Al-4V biomedical applications. Powder Technol. 2018, 331, 74–97. [Google Scholar] [CrossRef]
- Hamidi, M.F.F.A.; Harun, W.S.W.; Samykano, M. A review of biocompatible metal injection moulding process parameters for biomedical applications. Mater. Sci. Eng. C 2017, 78, 1263–1276. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Ge, J.B. Application of laser forming technology in the manufacturing of large titanium alloy components for aircraft. Appl. Laser 2018, 38, 202–206. [Google Scholar] [CrossRef]
- Zhao, Q.Y.; Chen, Y.N.; Xu, Y.K.; Zhao, Y.Q. Progress and prospects of low-cost preparation technologies for titanium alloy materials. Chin. J. Nonferrous Met. 2021, 31, 3127–3140. [Google Scholar] [CrossRef]
- Wang, Z.J.; Jia, L.; Miao, Q.D.; Li, M.Y.; Zhao, P.; Liu, W.; Yang, S.F. Research progress on the preparation technology of titanium alloys for ships. China Metall. Ind. 2024, 34, 14–25. [Google Scholar] [CrossRef]
- Li, Z.H. Discussion on the application of titanium alloys in the aerospace field. Dual-Use Technol. Prod. 2017, 12, 126. [Google Scholar] [CrossRef]
- Winstone, M.R.; Partridge, A.; Brooks, J.W. The contribution of advanced high-temperature materials to future aero-engines. Proc. Inst. Mech. Eng. Part L J. Mater. Des. Appl. 2001, 215, 63–73. [Google Scholar] [CrossRef]
- Wu, M.Y.; Mi, G.B.; Li, P.J.; Huang, X. Evolution and mechanism of combustion microstructure of 600 °C high temperature titanium alloy. Acta Phys. Sin. 2023, 72, 166102. [Google Scholar] [CrossRef]
- Ma, F.C.; Lu, S.Y.; Liu, P.; Li, W.; Liu, X.K.; Chen, X.H.; Zhang, K.; Pan, D. Evolution of strength and fiber orientation of a short-fibers reinforced Ti-matrix composite after extrusion. Mater. Des. 2017, 126, 297–304. [Google Scholar] [CrossRef]
- Ma, F.C.; Lu, S.Y.; Liu, P.; Li, W.; Liu, X.K.; Chen, X.H.; Zhang, K.; Pan, D. Microstructure and mechanical properties variation of TiB/Ti matrix composite by thermo-mechanical processing in beta phase field. J. Alloys Compd. 2017, 695, 1515–1522. [Google Scholar] [CrossRef]
- Ma, F.C.; Zhou, J.J.; Liu, P.; Li, W.; Liu, X.K.; Pan, D.; Lu, W.J.; Zhang, D.; Wu, L.Z. Strengthening effects of TiC particles and microstructure refinement in in situ TiC-reinforced Ti matrix composites. Mater. Charact. 2017, 127, 27–34. [Google Scholar] [CrossRef]
- Ma, F.C.; Liu, P.; Li, W.; Liu, X.K.; Chen, X.D.; Zhang, K.; Pan, P.; Lu, W.J. The mechanical behavior dependence on the TiB whisker realignment during hot-working in titanium matrix composites. Sci. Rep. 2016, 6, 36126. [Google Scholar] [CrossRef] [PubMed]
- Ma, F.C.; Wang, T.R.; Liu, P.; Li, W.; Liu, X.K.; Chen, X.H.; Pan, D.; Lu, W.J. Mechanical properties and strengthening effects of in situ (TiB+TiC)/Ti-1100 composite at elevated temperatures. Mater. Sci. Eng. A 2016, 654, 352–358. [Google Scholar] [CrossRef]
- Wang, Z.J.; Liu, Q.; He, J.C.; Sun, X.; Liu, Z.; Duan, S.; Ji, H.; Wang, D.; Zhang, J. A Method for Preparing an Aluminum-Molybdenum-Vanadium-Titanium Intermediate Alloy and Its Preparation Method. Chinese Patent CN113981294A, 28 January 2022. [Google Scholar]
- Zhang, Z.S.; Guo, S.J.; Tian, F.L. An Al-Mo-V Intermediate Alloy and Process for Preparing Same. Chinese Patent CN1629346A, 22 June 2005. [Google Scholar]
- Wang, Z.J.; Li, X.R.; Liu, Q.; Wang, W.; Sun, X.; He, J.; Zhang, J.; Zhu, J.; Meng, X. A Method for Preparing an Aluminum-Molybdenum-Vanadium Intermediate Alloy and Its Preparation Method. Chinese Patent CN116005043A, 25 April 2023. [Google Scholar]
- Liu, X.N. Research on the electrothermal production technology of vanadium-aluminum alloys. Pet. Petrochem. Mater. Procure 2021, 1, 78. [Google Scholar]
- Li, J.B.; Chen, Y.X.; Li, X.D. Control of Impurities such as Fe, Si, O, and C in the Preparation of Vanadium-Aluminum Alloys by the Thermite Process. Hunan Nonferrous Met. 2023, 39, 68–71. [Google Scholar]
- Li, L.J.; Li, B.J.; Wan, H.L.; Chen, M.; Yee, S. Study of Controlled Aluminum-Vanadium Alloy Prepared by Vacuum Resistance Furnace. Int. J. Photoenergy 2021, 2021, 7055415. [Google Scholar] [CrossRef]
- Sun, Z.H.; Chen, H.J.; Liu, F.Q.; Xian, Y.; Li, Q.; Wang, Y.; Zhou, F.; Meng, W. Preparation Method of Vanadium-Aluminum Alloy. Chinese Patent CN102031402A, 27 April 2011. [Google Scholar]
- Yin, D.F.; Sun, Z.H.; Wang, Y.G.; Chen, H.; Du, G.; Zhou, F.; Jing, H.; Sun, D. Method for Preparing Low Impurity Vanadium-Aluminum Alloy. Chinese Patent CN105886869B, 1 December 2017. [Google Scholar]
- Yu, J.Y.; Li, D.M.; Li, L.J.; Lu, M.L. Method for Preparing Vanadium-Aluminum Alloys Using Vanadium Pentoxide. Chinese Patent CN112779447A, 11 May 2021. [Google Scholar]
- Yee, S.L.; Wan, H.L.; Chen, M.; Wan, H.L.; Ma, X.D. Development of a Cleaner Route for Aluminum–Vanadium Alloy Production. J. Mater. Res. Technol. 2022, 16, 187–193. [Google Scholar] [CrossRef]
- Zhang, N.; Li, L.J.; Zhang, S.X.; Xu, F. Study on the Effect of Fluxing Agents on the Quality of Vanadium-Aluminum Alloys. Steel Vanadium Titan. 2020, 41, 24–28. [Google Scholar] [CrossRef]
- Deng, B. Experimental Study on the Preparation of Ferro-Vanadium by Thermite Process. Steel Vanadium Titan. 2012, 33, 26–29. [Google Scholar]
- Li, J.B.; Li, X.D. Discussion on the Factors Affecting Vanadium Yield in the Production Process of Vanadium-Aluminum Alloys. Hunan Nonferrous Met. 2021, 37, 49–51. [Google Scholar]
- Zhang, S.X.; Li, L.J.; Li, J.Q. Research on the Electric Thermite Process for Producing Vanadium-Aluminum Alloys. Hebei Metall. 2018, 273, 23–25. [Google Scholar] [CrossRef]
- Li, J.J.; Li, L.J.; Zhang, N. Source Analysis of Iron Impurity in Vanadium-Aluminum Alloys Produced by Electric Thermite Process. Min. Metall. Eng. 2019, 28, 94–122. [Google Scholar]
- Li, S.; Lü, C. Application of FactSage in Metallurgical and Materials Research; Northeastern University Press: Shenyang, China, 2020. [Google Scholar]
- Cao, Z.M.; Song, X.Y.; Qiao, Z.Y. Thermodynamic Simulation Software FactSage and Its Application. Rare Met. 2008, 32, 216–219. [Google Scholar] [CrossRef]
- Jing, H. Kinetic Study on the Preparation of Vanadium-Aluminum Alloys by the Thermite Process. Iron Steel Vanadium Titan. 2017, 38, 40–43. [Google Scholar] [CrossRef]
- Duan, S.C.; Wang, Z.Q.; Guo, H.J.; Guo, J.; Shi, J.; Liu, S. Thermodynamic and Kinetic Studies on the Preparation of Vanadium-Aluminum Alloys by the Thermite Process. Steel Vanadium Titan. 2017, 38, 47–54. [Google Scholar] [CrossRef]
- Yin, D.F.; Chen, H.J.; Xian, Y.; Li, H.F. Thermodynamic Study on the Preparation of Vanadium-Aluminum Alloys by the Thermite Process. In Proceedings of the Second Advanced Technology Exchange Conference on the Vanadium Industry, Kunming, China, 26–28 August 2013; pp. 119–121. [Google Scholar]
- Lu, Y.F.; Huang, H. Principles of Metallurgy, 2nd ed.; Metallurgical Industry Press: Beijing, China, 2018. [Google Scholar]
- Hu, B.; Yao, B.; Wang, J.; Zhao, J.R.; Min, F.F.; Du, Y. Thermodynamic Assessment of the Al-Mo-V Ternary System. J. Min. Metall. Sect. B Metall. 2017, 53, 95–106. [Google Scholar] [CrossRef]
- Zhang, Q.Y. Phase Diagram Atlas of Ternary Alloys; Chemical Industry Press: Beijing, China, 2022; p. 191. [Google Scholar]
| Material | Form | Purity (%) | Manufacturer |
|---|---|---|---|
| V2O5 | Powder | ≥99.7 | Chengde Vanadium Titanium Co., Ltd. (Chengdu, China) |
| Al | Granules | ≥99.9 | Beijing Kerry New Materials Co., Ltd. (Beijing, China) |
| CaO | Powder | ≥99.5 | Xilong Scientific Co., Ltd. (Shantou, China) |
| MoO3 | Powder | ≥99.5 | Shanghai Macklin Biochemical Co., Ltd. (Shanghai, China) |
| Exp No. | Al (g) | V2O5 (g) | MoO3 (g) | CaO (g) | Al:V:Mo in Alloy | Al in Alloy (wt%) | Al/O (wt) |
|---|---|---|---|---|---|---|---|
| #1 | 2.8 | 3.0 | 2.5 | 4.5 | 2:9:9 | 10 | 1.2 |
| #2 | 3.3 | 3.0 | 2.5 | 4.5 | 1:2:2 | 20 | 1.4 |
| #3 | 3.9 | 3.0 | 2.5 | 4.5 | 6:7:7 | 30 | 1.6 |
| #4 | 4.7 | 3.0 | 2.5 | 4.5 | 4:3:3 | 40 | 2.0 |
| #5 | 5.8 | 3.0 | 2.5 | 4.5 | 2:1:1 | 50 | 2.4 |
| #6 | 7.4 | 3.0 | 2.5 | 4.5 | 3:1:1 | 60 | 3.1 |
| No. | Phases | wt% | mol% | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Al2O3 | CaO | “V2O5” | MoO3 | Al2O3 | CaO | “V2O5” | MoO3 | ||
| #1 | CaO·Al2O3 | 65.2 | 34.7 | 0.1 | 0.0 | 50.8 | 49.2 | 0.0 | 0.0 |
| Bulk | 64.9 | 33.7 | 1.2 | 0.2 | 51.1 | 48.3 | 0.5 | 0.1 | |
| #2 | Inclusion in alloy | 65.1 | 34.7 | 0.2 | 0.0 | 50.8 | 49.2 | 0.0 | 0.0 |
| CaO·Al2O3 | 65.2 | 34.8 | 0.0 | 0.0 | 50.5 | 49.5 | 0.0 | 0.0 | |
| CaO·2Al2O3 | 78.9 | 21.1 | 0.0 | 0.0 | 67.3 | 32.7 | 0.0 | 0.0 | |
| Bulk | 69.4 | 29.8 | 0.7 | 0.1 | 56.1 | 43.6 | 0.3 | 0.0 | |
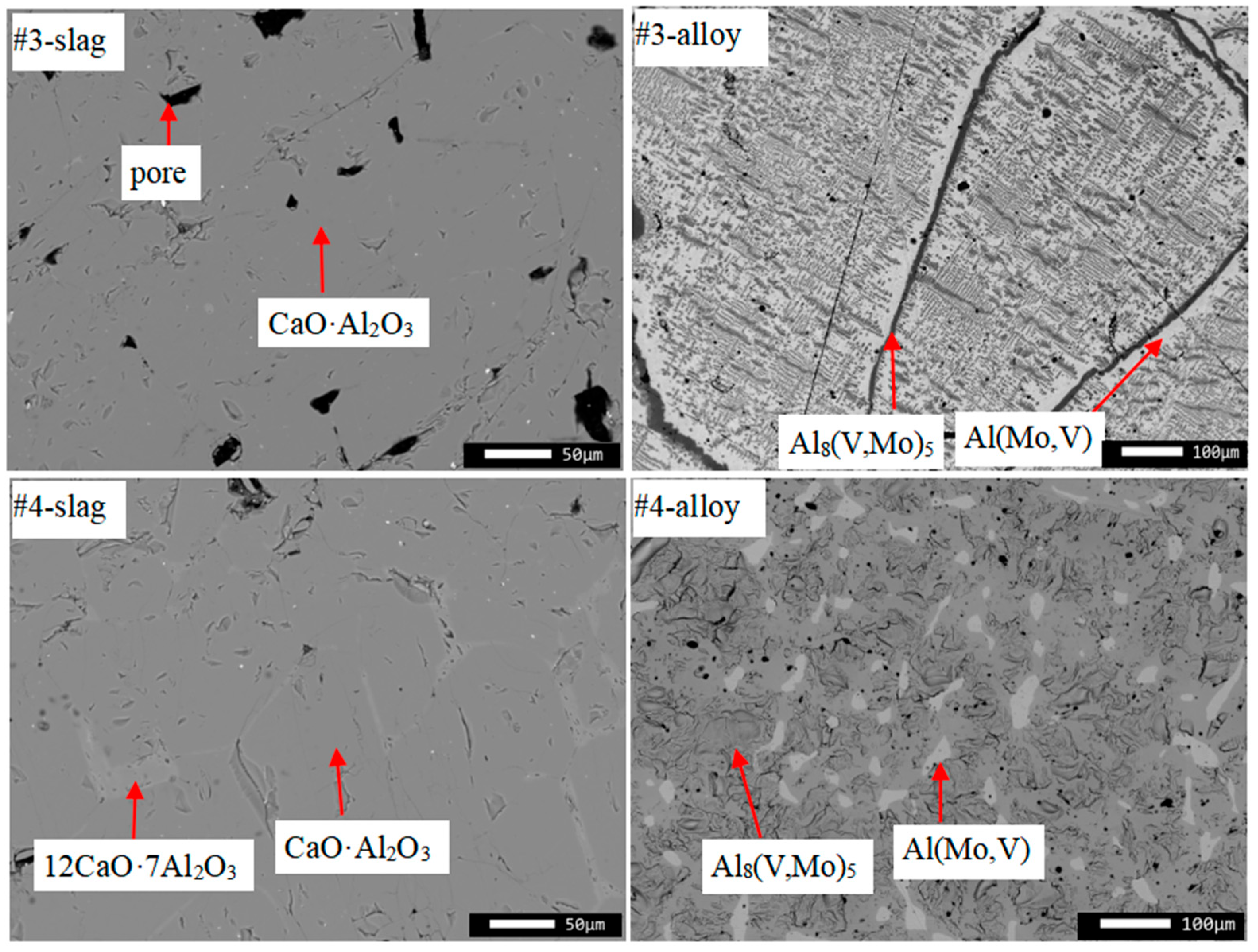
| #3 | CaO·Al2O3 | 64.8 | 35.2 | 0.0 | 0.0 | 50.3 | 49.7 | 0.0 | 0.0 |
| Bulk | 68.9 | 30.3 | 0.7 | 0.1 | 55.4 | 44.3 | 0.3 | 0.0 | |
| #4 | CaO·Al2O3 | 63.3 | 36.7 | 0.0 | 0.0 | 48.5 | 51.5 | 0.0 | 0.0 |
| 12CaO·7Al2O3 | 54.2 | 45.8 | 0.0 | 0.0 | 39.3 | 60.7 | 0.0 | 0.0 | |
| Bulk | 68.4 | 30.6 | 0.8 | 0.2 | 55.0 | 44.6 | 0.3 | 0.1 | |
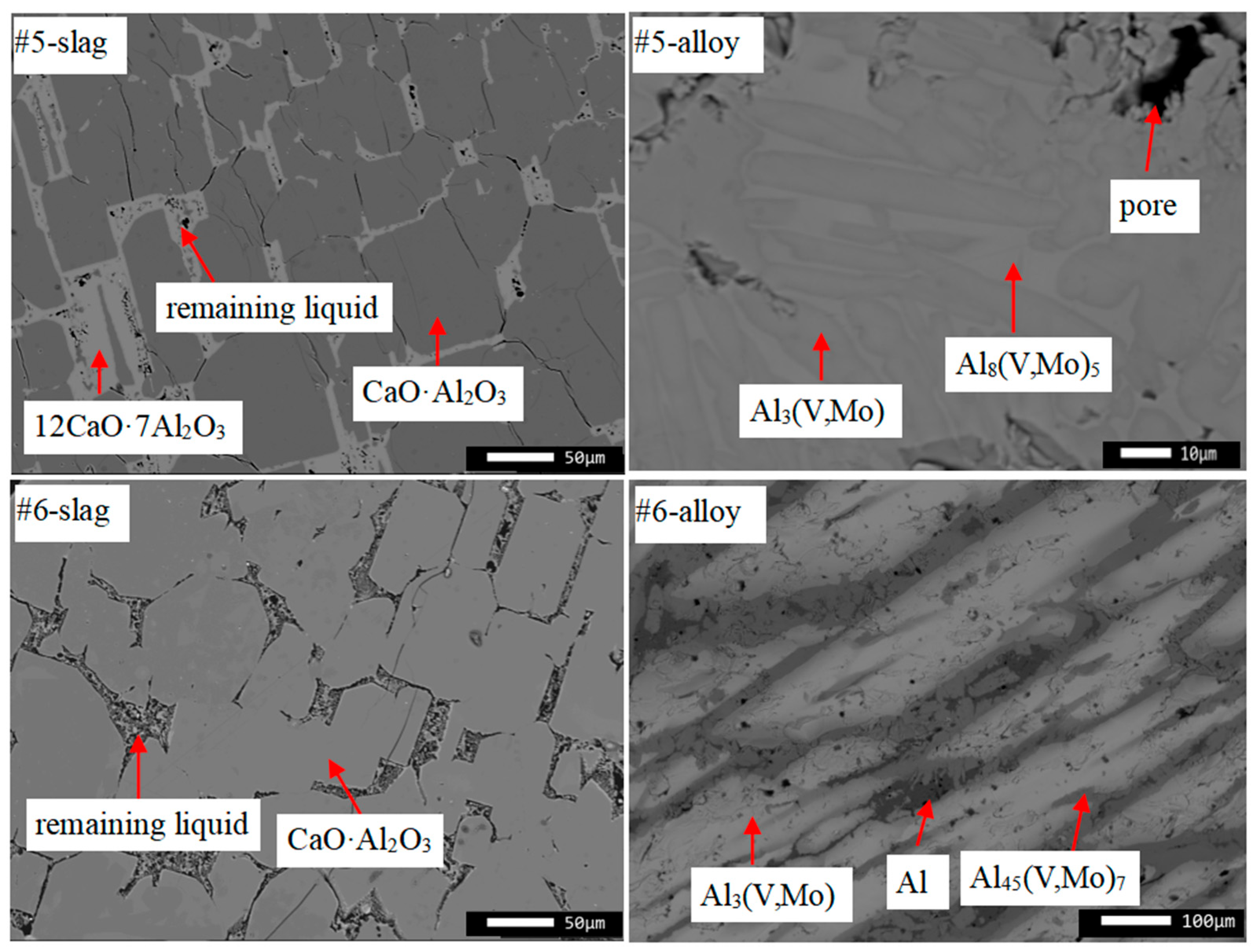
| #5 | Inclusion in alloy | 64.7 | 35.3 | 0.0 | 0.0 | 50.3 | 49.7 | 0.0 | 0.0 |
| CaO·Al2O3 | 64.8 | 35.2 | 0.0 | 0.0 | 50.3 | 49.7 | 0.0 | 0.0 | |
| 12CaO·7Al2O3 | 51.7 | 48.3 | 0.0 | 0.0 | 37.0 | 63.0 | 0.0 | 0.0 | |
| Bulk | 67.0 | 32.8 | 0.1 | 0.1 | 52.7 | 47.1 | 0.1 | 0.1 | |
| #6 | Inclusion in alloy | 65.4 | 34.4 | 0.0 | 0.0 | 51.2 | 48.8 | 0.0 | 0.0 |
| CaO·Al2O3 | 64.8 | 35.2 | 0.0 | 0.0 | 50.3 | 49.7 | 0.0 | 0.0 | |
| Bulk | 66.1 | 33.8 | 0.1 | 0.0 | 51.9 | 48.1 | 0.0 | 0.0 | |
| No. | Phases | wt% | mol% | ||||||
|---|---|---|---|---|---|---|---|---|---|
| V | Al | Mo | Ca | V | Al | Mo | Ca | ||
| #1 | Alloy in slag | 93.9 | 2.6 | 0.0 | 3.5 | 90.9 | 4.8 | 0.0 | 4.3 |
| (Al,Mo,V) | 36.3 | 16.0 | 47.5 | 0.2 | 39.5 | 32.9 | 27.4 | 0.2 | |
| Bulk | 35.9 | 17.2 | 46.6 | 0.3 | 38.4 | 34.7 | 26.5 | 0.4 | |
| #2 | Alloy in slag | 93.2 | 0.4 | 6.4 | 0.0 | 95.4 | 0.9 | 3.7 | 0.0 |
| (Al,Mo,V) | 35.6 | 19.5 | 44.9 | 0.0 | 36.9 | 38.4 | 24.7 | 0.0 | |
| Bulk | 35.6 | 21.1 | 42.7 | 0.6 | 36.0 | 40.4 | 22.9 | 0.7 | |
| #3 | Alloy in slag | 37.3 | 62.3 | 0.1 | 0.3 | 24.0 | 75.6 | 0.1 | 0.3 |
| Al(Mo,V) | 37.1 | 26.5 | 36.4 | 0.0 | 34.7 | 47.2 | 18.1 | 0.0 | |
| Al8(V,Mo)5 | 27.4 | 38.8 | 33.8 | 0.0 | 23.1 | 61.8 | 15.1 | 0.0 | |
| Bulk | 33.9 | 31.6 | 34.3 | 0.2 | 30.2 | 53.4 | 16.2 | 0.2 | |
| #4 | Alloy in slag | 37.5 | 62.0 | 0.1 | 0.4 | 24.1 | 75.5 | 0.1 | 0.3 |
| Al(Mo,V) | 39.9 | 27.1 | 33.0 | 0.0 | 36.8 | 47.0 | 16.2 | 0.0 | |
| Al8(V,Mo)5 | 28.9 | 39.8 | 31.3 | 0.0 | 23.9 | 62.4 | 13.7 | 0.0 | |
| Bulk | 29.8 | 40.8 | 29.3 | 0.1 | 24.3 | 62.9 | 12.7 | 0.1 | |
| #5 | Al8(V,Mo)5 | 36.6 | 42.5 | 20.9 | 0.0 | 28.7 | 62.7 | 8.6 | 0.0 |
| Al3(V,Mo) | 19.3 | 53.0 | 27.7 | 0.0 | 14.4 | 74.7 | 10.9 | 0.0 | |
| Bulk | 24.2 | 52.2 | 23.2 | 0.4 | 17.9 | 72.6 | 9.1 | 0.4 | |
| #6 | Al3(V,Mo) | 15.9 | 54.1 | 30.0 | 0.0 | 11.9 | 76.2 | 11.9 | 0.0 |
| Al45(V,Mo)7 | 16.0 | 76.6 | 7.4 | 0.0 | 9.8 | 87.8 | 2.4 | 0.0 | |
| Al | 0.5 | 99.3 | 0.2 | 0.0 | 0.2 | 99.7 | 0.1 | 0.0 | |
| Bulk | 18.0 | 62.6 | 18.7 | 0.7 | 12.2 | 80.5 | 6.7 | 0.6 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Liao, J.; Ma, X.; Zhao, B. Exothermic and Slag Formation Behavior of Aluminothermic Reduction of Mo and V Oxides. Metals 2025, 15, 704. https://doi.org/10.3390/met15070704
Wang X, Liao J, Ma X, Zhao B. Exothermic and Slag Formation Behavior of Aluminothermic Reduction of Mo and V Oxides. Metals. 2025; 15(7):704. https://doi.org/10.3390/met15070704
Chicago/Turabian StyleWang, Xiaoshu, Jinfa Liao, Xiaodong Ma, and Baojun Zhao. 2025. "Exothermic and Slag Formation Behavior of Aluminothermic Reduction of Mo and V Oxides" Metals 15, no. 7: 704. https://doi.org/10.3390/met15070704
APA StyleWang, X., Liao, J., Ma, X., & Zhao, B. (2025). Exothermic and Slag Formation Behavior of Aluminothermic Reduction of Mo and V Oxides. Metals, 15(7), 704. https://doi.org/10.3390/met15070704










