Effect of Electrolytic-Plasma Hardening on the Microstructure and Tribological Properties of Low-Alloy Steels
Abstract
1. Introduction
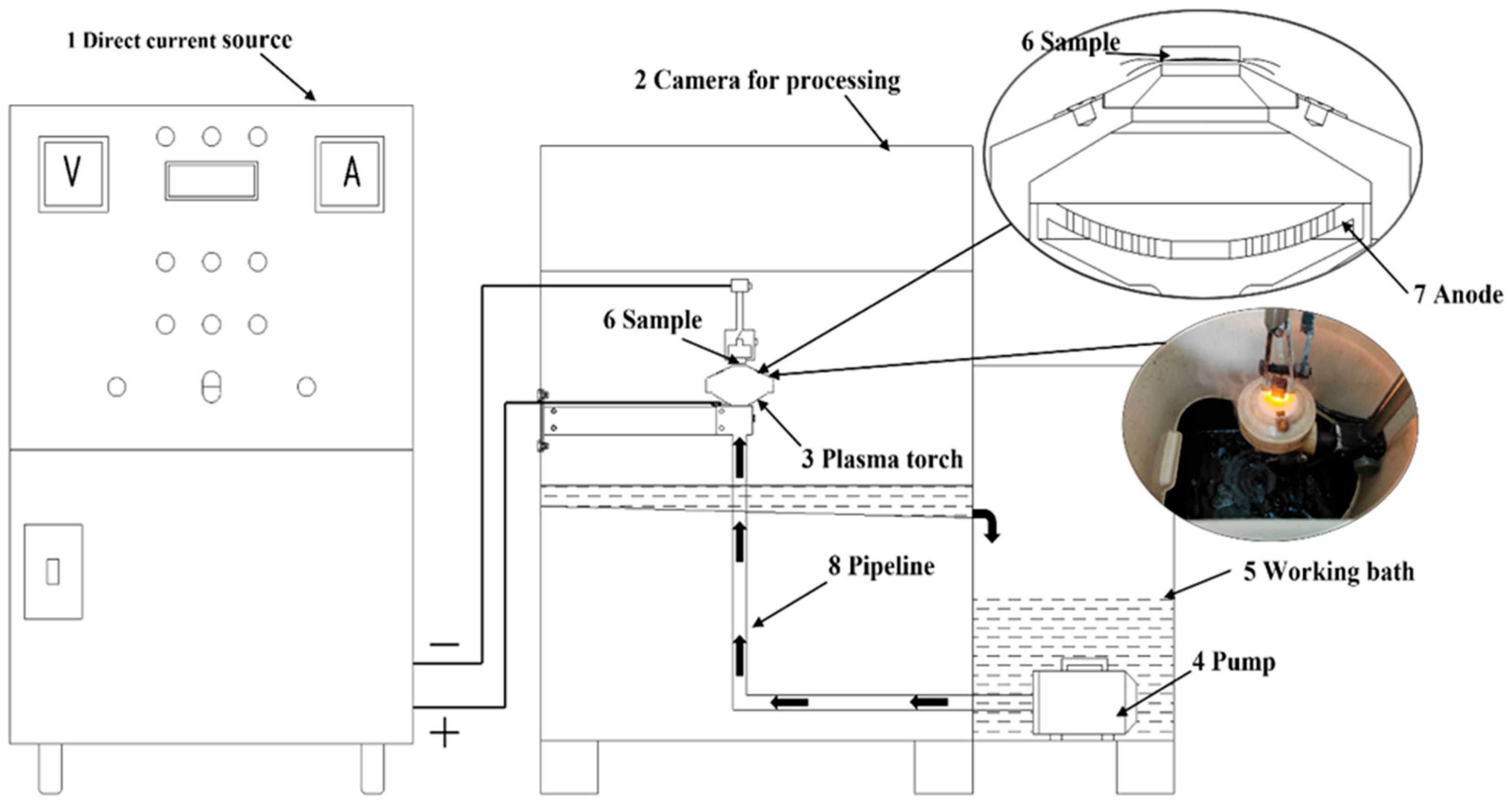
2. Materials and Methods
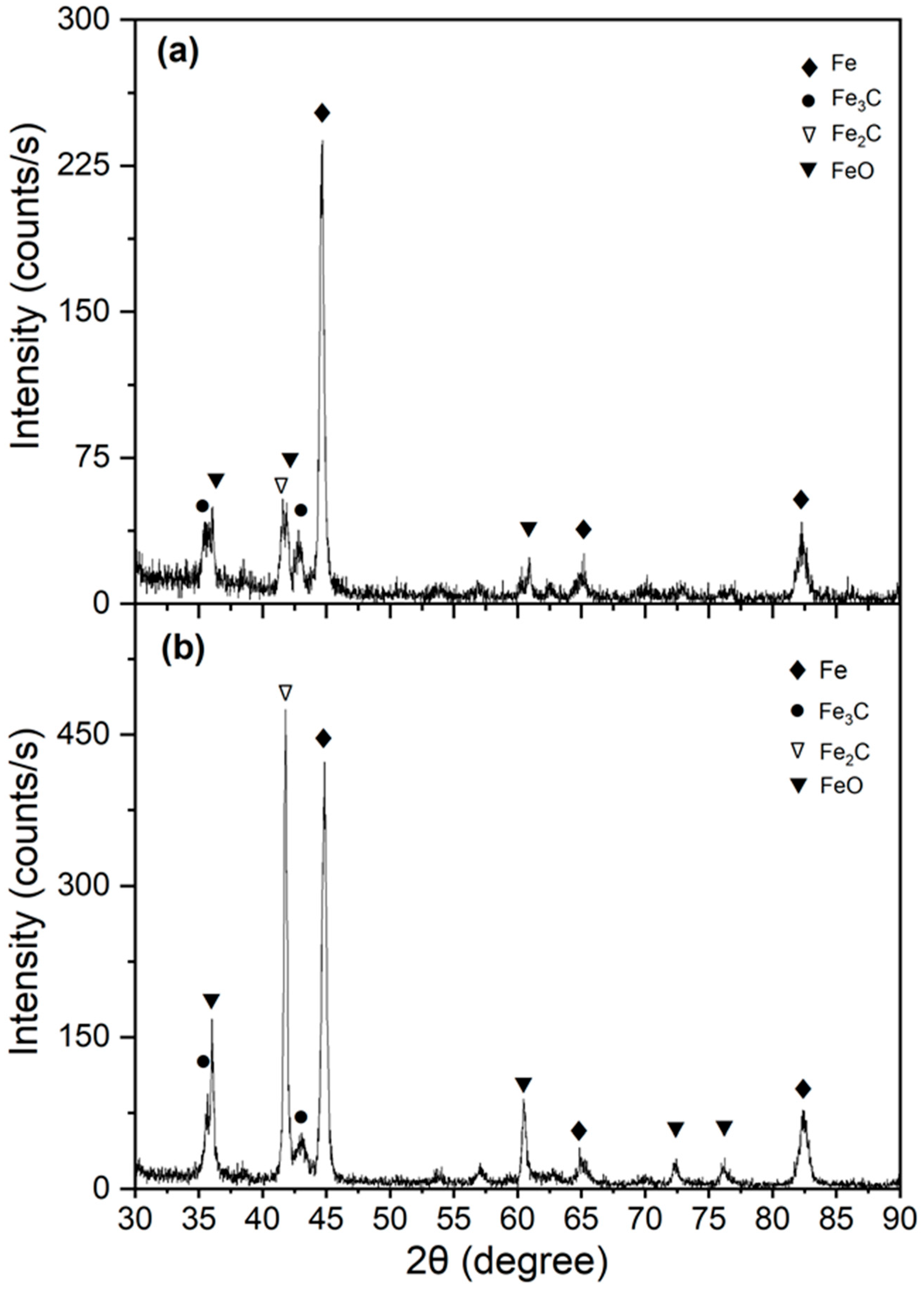
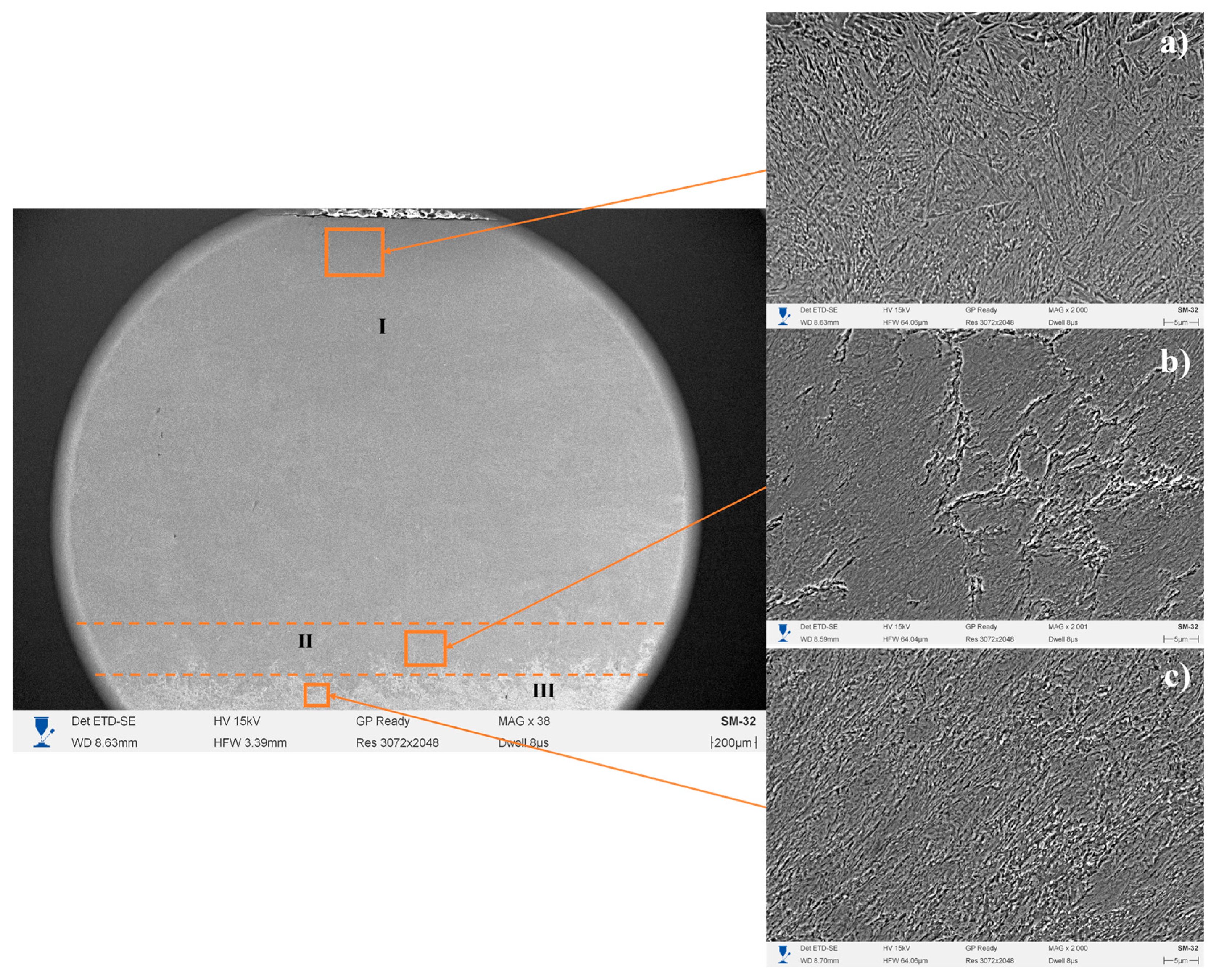
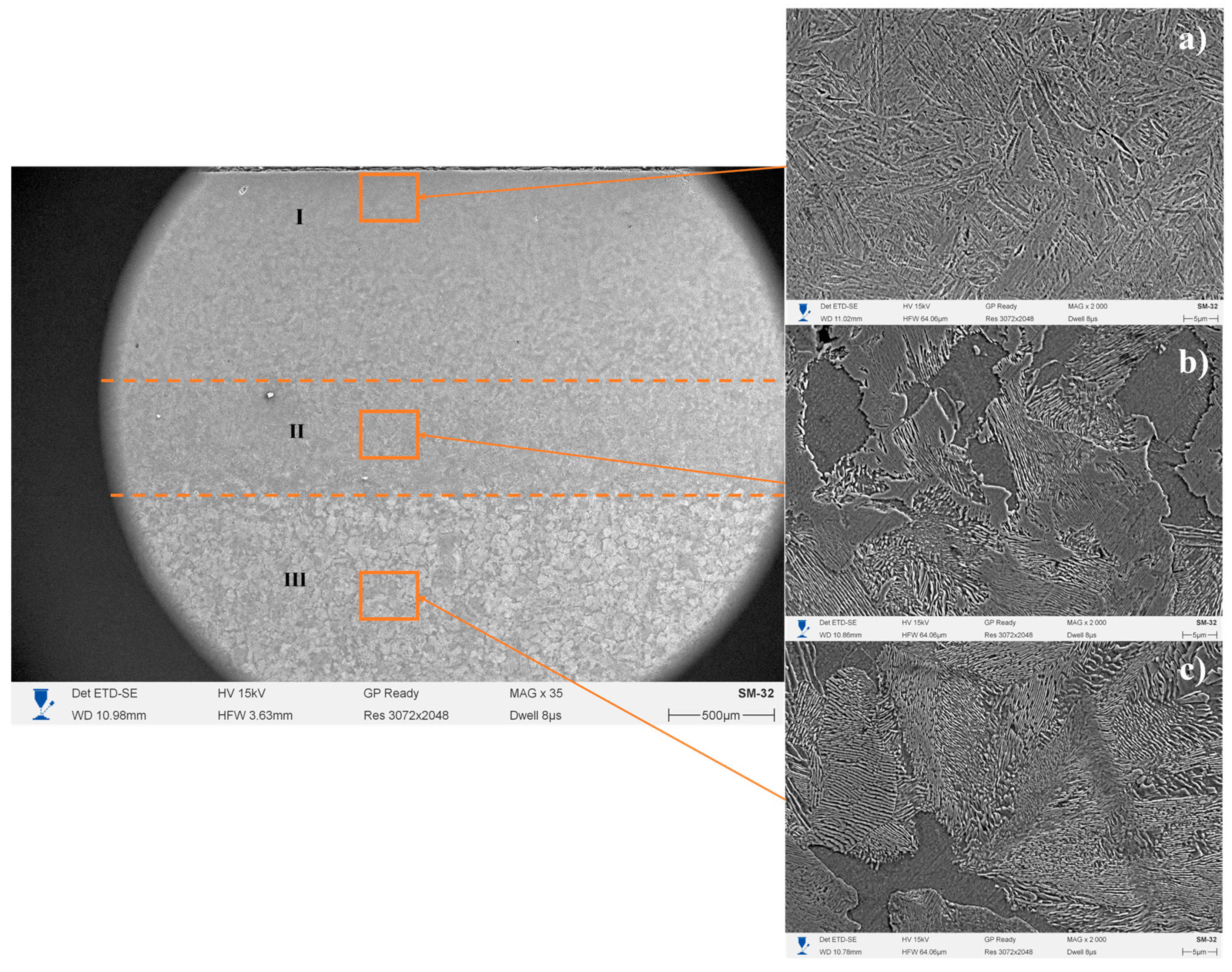
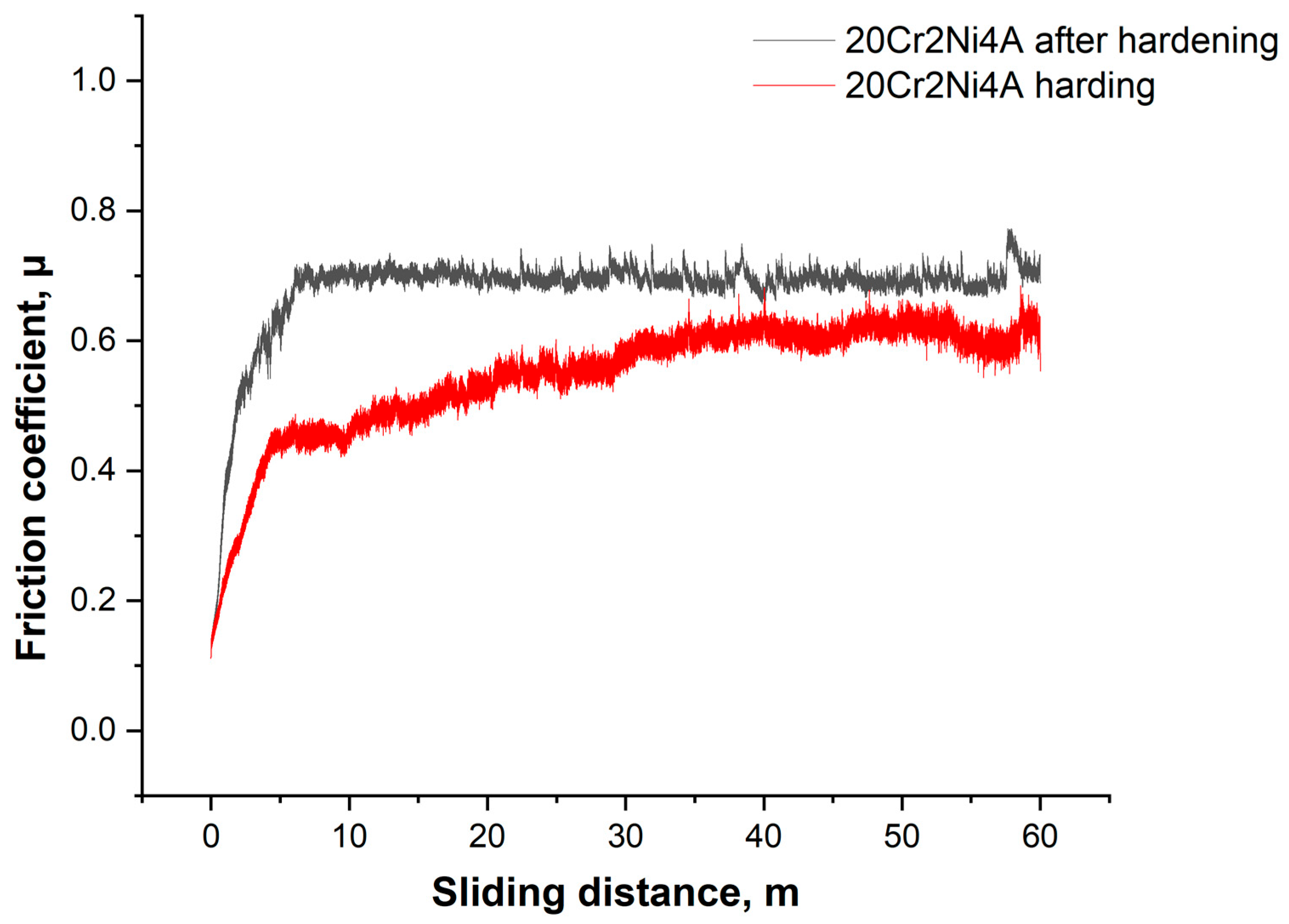
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Belkin, V.P.; Belkin, P.N.; Krit, B.; Filatova, E.A. Increasing Wear Resistance of Low-Carbon Steel by Anodic Plasma-Electrolytic Nitroboriding. J. Mater. Eng. Perform. 2020, 29, 564–572. [Google Scholar] [CrossRef]
- Łach, Ł. Recent Advances in Laser Surface Hardening: Techniques, Modeling Approaches, and Industrial Applications. Crystals 2024, 14, 726. [Google Scholar] [CrossRef]
- Pantelis, D.I.; Bouyiouri, E.; Kouloumbi, N.; Vassiliou, P.; Koutsomichalis, A. Wear and Corrosion Resistance of Laser Surface Hardened Structural Steel. Surf. Coat. Technol. 2002, 161, 125–134. [Google Scholar] [CrossRef]
- Kulka, M.; Pertek, A. Laser Surface Modification of Carburized and Borocarburized 15CrNi6 Steel. Mater. Charact. 2007, 58, 461–470. [Google Scholar] [CrossRef]
- Skeeba, V.Y.; Ivancivsky, V.V.; Martyushev, N.V. Peculiarities of High-Energy Induction Heating during Surface Hardening in Hybrid Processing Conditions. Metals 2021, 11, 1354. [Google Scholar] [CrossRef]
- Mazhyn, S.; Laila, Z.; Michael, S. Influence of Regimes Electrolytic-Plasma Processing on Phase Structure and Hardening of Steel 30CrMnSi. Adv. Mater. Res. 2013, 601, 79–83. [Google Scholar]
- Ramanathan, T.; Sekar, K.; Shanmugam, N. Microstructural Evaluation and Effect of Heat Generation in FSW of AA1100. Chiang Mai J. Sci. 2022, 49, 487–495. [Google Scholar] [CrossRef]
- Kombayev, K.; Nedobitkov, A.; Gridunov, I.; Kozhakhmetov, Y.; Khoshnaw, F.; Aibar, K. Surface Crack Analysis and Quality Enhancement of 30X13 (AISI 420) Martensitic Stainless Steel Gate Valve Shutters via Electrolytic Plasma Hardening. Results Eng. 2025, 26, 104966. [Google Scholar] [CrossRef]
- Kombayev, K.; Khoshnaw, F.; Kozhakhmetov, Y.; Tleuzhanova, G.; Azamatov, B.; Tabiyeva, Y. Microplasma Sprayed Tantalum Coatings on Ti Grade 5 Extra-Low Interstitials: Investigation of Thickness and Porosity Control. Coatings 2025, 15, 464. [Google Scholar] [CrossRef]
- Korotkov, V.S. Wear Resistance of Plasma-Hardened Materials. J. Frict. Wear 2011, 32, 17–22. [Google Scholar] [CrossRef]
- Liang, J.; Hu, L.; Yerokhin, A.L.; Matthews, A. Electrolytic Plasma Technology: Science and Engineering—An Overview. Surf. Rev. Lett. 2016, 23, 1630002. [Google Scholar]
- Magdaleno, C.; Silva, R. Oxy-Nitriding AISI 304 Stainless Steel by Plasma Electrolytic Surface Saturation to Increase Wear Resistance. Metals 2023, 13, 309. [Google Scholar] [CrossRef]
- Karimi Zarchi, M.; Shariat, M.H.; Dehghan, S.A.; Solhjoo, S. Characterization of Nitrocarburized Surface Layer on AISI 1020 Steel by Electrolytic Plasma Processing in a Urea Electrolyte. J. Mater. Res. Technol. 2013, 2, 213–220. [Google Scholar] [CrossRef]
- Stepanova, T.Y. Technologies of Surface Hardening of Machine Parts: Tutorial; Ivanovo State Chemical-Technological University: Ivanovo, Russia, 2009; p. 64. [Google Scholar]
- Skakov, M.; Rakhadilov, B.; Sheffler, M. Influence of Electrolyte Plasma Treatment on Structure, Phase Composition and Microhardness of Steel P6M5. Key Eng. Mater. 2013, 531, 627–631. [Google Scholar] [CrossRef]
- Tabieva, E.; Zhurerova, L.; Baizhan, D. Enhancing the Wear Resistance of 45# Steel. Mater. Today Commun. 2024, 38, 108327. [Google Scholar]
- Kombayev, K.; Sypainova, G.; Khoshnaw, F.; Kozhakhmetov, Y.; Kurbanbekov, S.; Tabiyeva, Y. Practical and Theoretical Optimization of Plasma Cutting Parameters for Enhanced Quality and Efficiency in Steel Alloy Processing. Multidiscip. Model. Mater. Struct. 2024, in press.
- Kombayev, K.; Khoshnaw, F.; Uazyrkhanova, G.; Moldabayeva, G. Experimental and Mathematical Modelling Investigation of Plasma Electrolytic Oxidation (PEO) for Surface Hardening of 20Ch Steel. Materials 2024, 17, 6043. [Google Scholar] [CrossRef]
- Rakhadilov, B.K.; Buranich, V.V.; Satbayeva, Z.A.; Sagdoldina, Z.B.; Kozhanova, R.S.; Pogrebnjak, A.D. The Cathodic Electrolytic Plasma Hardening of the 20Cr2Ni4A Chromium-Nickel Steel. J. Mater. Res. Technol. 2020, 9, 6969–6976. [Google Scholar] [CrossRef]
- Tyurin, Y.N.; Pogrebnjak, A.D. Electric Heating Using a Liquid Electrode. Surf. Coat. Technol. 2001, 142, 293–299. [Google Scholar] [CrossRef]
- Rakhadilov, B.; Kussainov, R.; Kalitova, A.; Satbayeva, Z.; Shynarbek, A. The Impact of Technological Parameters of Electrolytic-Plasma Treatment on the Changes in the Mechano-Tribological Properties of Steel 45. AIMS Mater. Sci. 2024, 11, 4. [Google Scholar] [CrossRef]
- Kusmanov, S.A.; Kusmanova, Y.V.; Naumov, A.R.; Belkin, P.N. Features of Anode Plasma Electrolytic Nitrocarburising of Low-Carbon Steel. Surf. Coat. Technol. 2015, 272, 149–157. [Google Scholar] [CrossRef]
- Tabieva, E.; Zhurerova, L.; Baizhan, D. Influence of Electrolyte-Plasma Hardening Parameters on Structure and Properties of Banding Steel. Key Eng. Mater. 2020, 839, 57–62. [Google Scholar] [CrossRef]
- Belkin, P.N. Anode Electrochemical Thermal Modification of Metals and Alloys. Surf. Eng. Appl. Electrochem. 2010, 46, 558–569. [Google Scholar] [CrossRef]
- Sagdoldina, Z.; Zhurerova, L.; Tyurin, Y.; Baizhan, D.; Kuykabayeba, A.; Abildinova, S.; Kozhanova, R. Modification of the Surface of 40Kh Steel by Electrolytic Plasma Hardening. Metals 2022, 12, 2071. [Google Scholar] [CrossRef]
- Rakhadilov, B.K.; Seitkhanova, A.; Satbayeva, Z.A.; Yerbolatova, G.; Icheva, Y.; Sagdoldina, Z. Investigation of the Structural, Mechanical and Tribological Properties of Plasma Electrolytic Hardened Chromium–Nickel Steel. Lubricants 2021, 9, 108. [Google Scholar] [CrossRef]
- Mukhacheva, T.; Kusmanov, S.; Suminov, I.; Podrabinnik, P.; Khmyrov, R.; Grigoriev, S. Increasing Wear Resistance of Low-Carbon Steel by Anodic Plasma Electrolytic Sulfiding. Metals 2022, 12, 1641. [Google Scholar] [CrossRef]
- Gui, W.; Lin, J.; Hao, G.; Qu, Y.; Liang, Y.; Zhang, H. Electrolytic plasma processing-an innovative treatment for surface modification of 304 stainless steel. Sci. Rep. 2017, 7, 308. [Google Scholar] [CrossRef]
- Kusmanov, S.A.; Dyakov, I.G.; Kusmanova, Y.V.; Belkin, P.N. Surface modification of low-carbon steels by plasma electrolytic nitrocarburising. Plasma Chem. Plasma Process. 2016, 36, 1271–1286. [Google Scholar] [CrossRef]
- Satbayeva, Z.; Maulit, A.; Ispulov, N.; Baizhan, D.; Rakhadilov, B.; Kusainov, R. Electrolytic Plasma Nitriding of Medium-Carbon Steel 45 for Performance Enhancement. Crystals 2024, 14, 895. [Google Scholar] [CrossRef]
- GOST R ISO 6507-1-2007; Metallic Materials—Vickers Hardness Test—Part 1: Test Method. Standartinform (Federal Agency on Technical Regulating and Metrology): Moscow, Russia, 2008.
- ASTM Standard G133-05; Standard Test Method for Linearly Reciprocating Ballon-Flat Sliding Wear. ASTM International: West Conshohocken, PA, USA, 2016.


| Steel | C | Si | Mn | Ni | Cr | Cu | P | S |
|---|---|---|---|---|---|---|---|---|
| 20Cr2Ni4A | 0.16–0.22% | 0.17–0.37% | 0.3–0.6% | 3.25–3.65% | 1.25–1.65% | up to 0.3% | up to 0.025% | up to 0.025% |
| 37Cr4 (1.7034) (EN) | 0.36–0.44% | 0.17–0.37% | 0.5–0.8% | up to 0.3% | 0.8–1.1% | up to 0.3% | up to 0.035% | up to 0.035% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rakhadilov, B.; Satbayeva, Z.; Maulit, A.; Kurmangaliyev, R.; Rustemov, A. Effect of Electrolytic-Plasma Hardening on the Microstructure and Tribological Properties of Low-Alloy Steels. Metals 2025, 15, 698. https://doi.org/10.3390/met15070698
Rakhadilov B, Satbayeva Z, Maulit A, Kurmangaliyev R, Rustemov A. Effect of Electrolytic-Plasma Hardening on the Microstructure and Tribological Properties of Low-Alloy Steels. Metals. 2025; 15(7):698. https://doi.org/10.3390/met15070698
Chicago/Turabian StyleRakhadilov, Bauyrzhan, Zarina Satbayeva, Almasbek Maulit, Rinat Kurmangaliyev, and Anuar Rustemov. 2025. "Effect of Electrolytic-Plasma Hardening on the Microstructure and Tribological Properties of Low-Alloy Steels" Metals 15, no. 7: 698. https://doi.org/10.3390/met15070698
APA StyleRakhadilov, B., Satbayeva, Z., Maulit, A., Kurmangaliyev, R., & Rustemov, A. (2025). Effect of Electrolytic-Plasma Hardening on the Microstructure and Tribological Properties of Low-Alloy Steels. Metals, 15(7), 698. https://doi.org/10.3390/met15070698






