Selective Reduction of Iron from Iron–Manganese Ore of the Keregetas Deposit Using Hydrogen
Abstract
1. Introduction
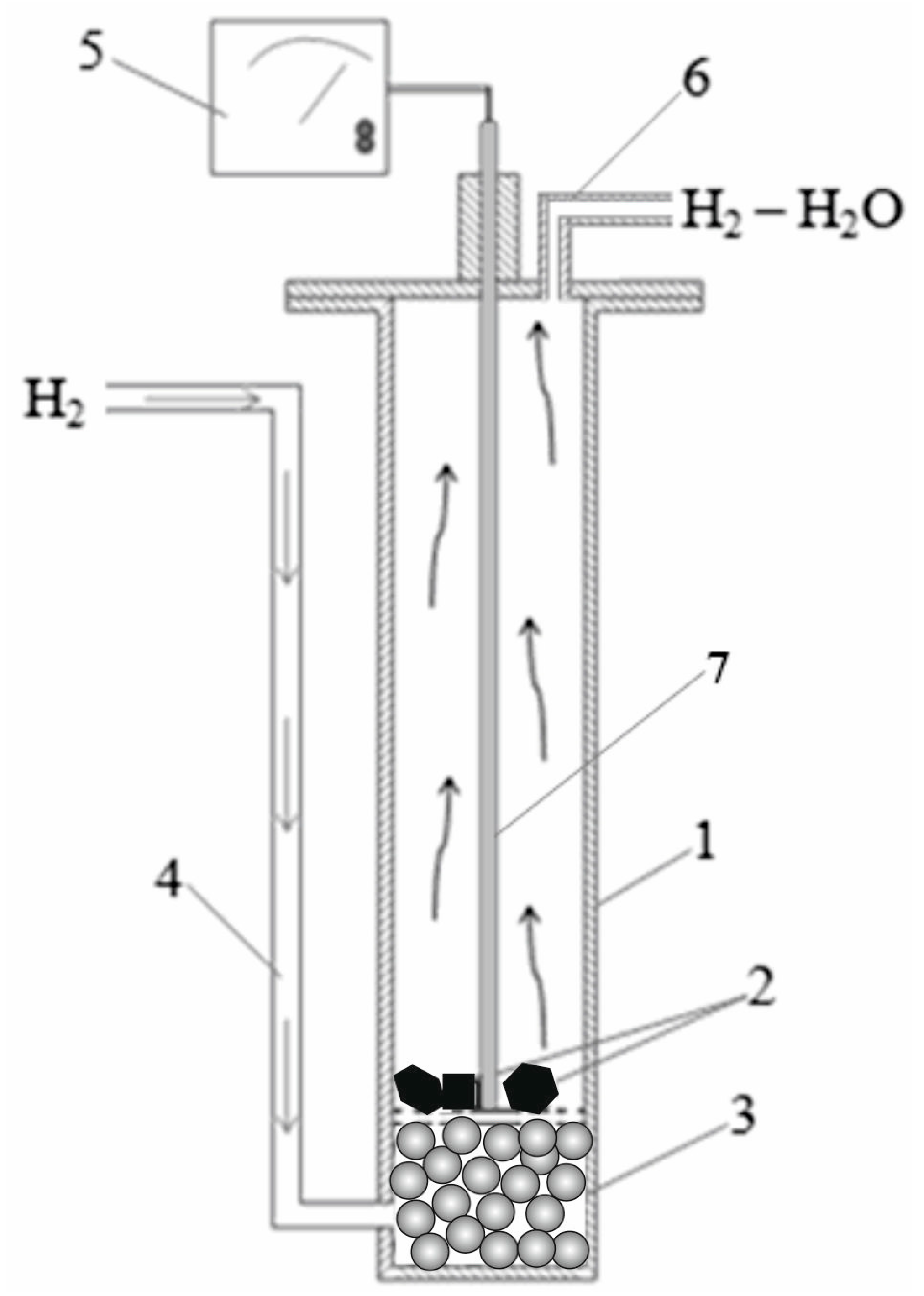
2. Materials and Methods
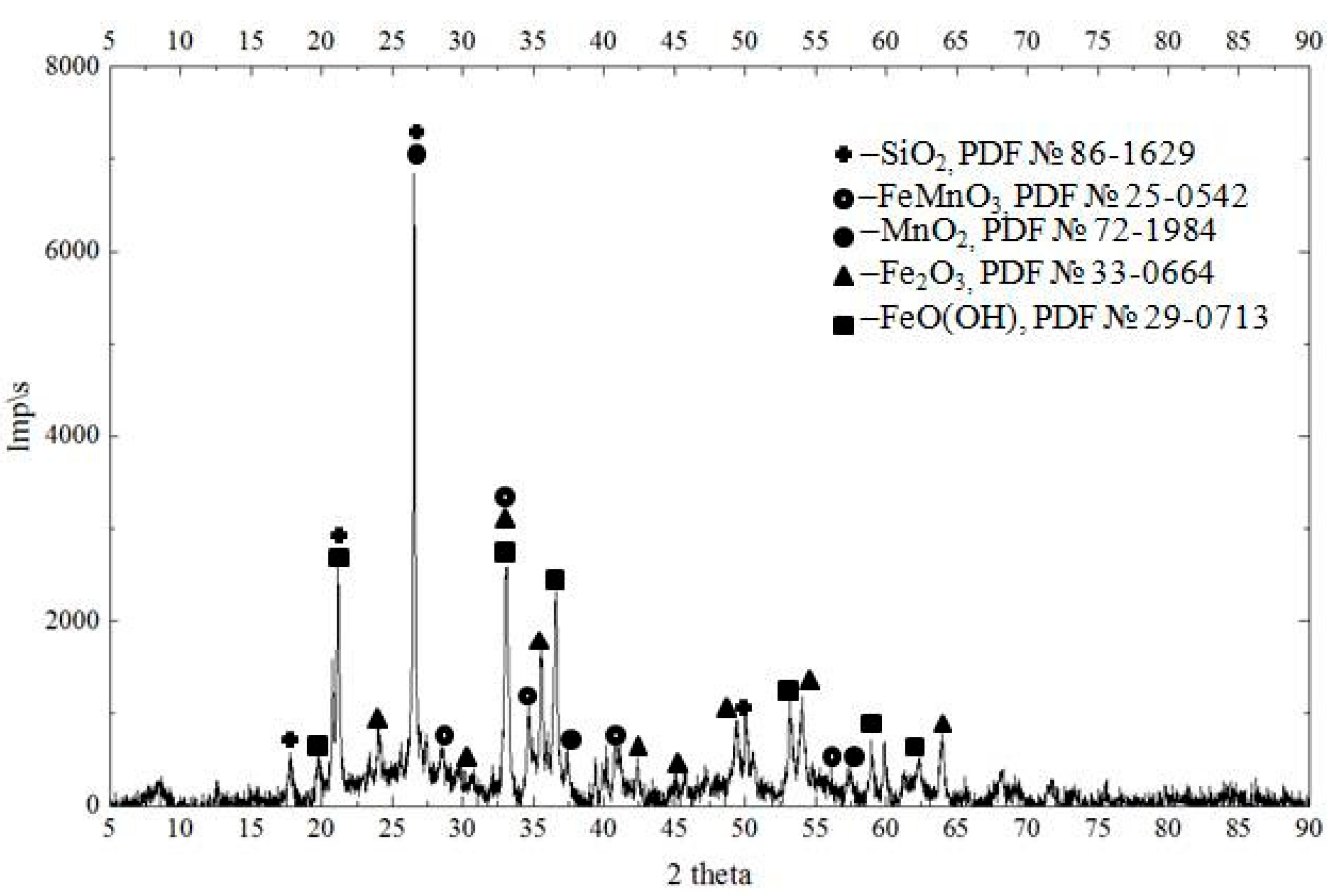
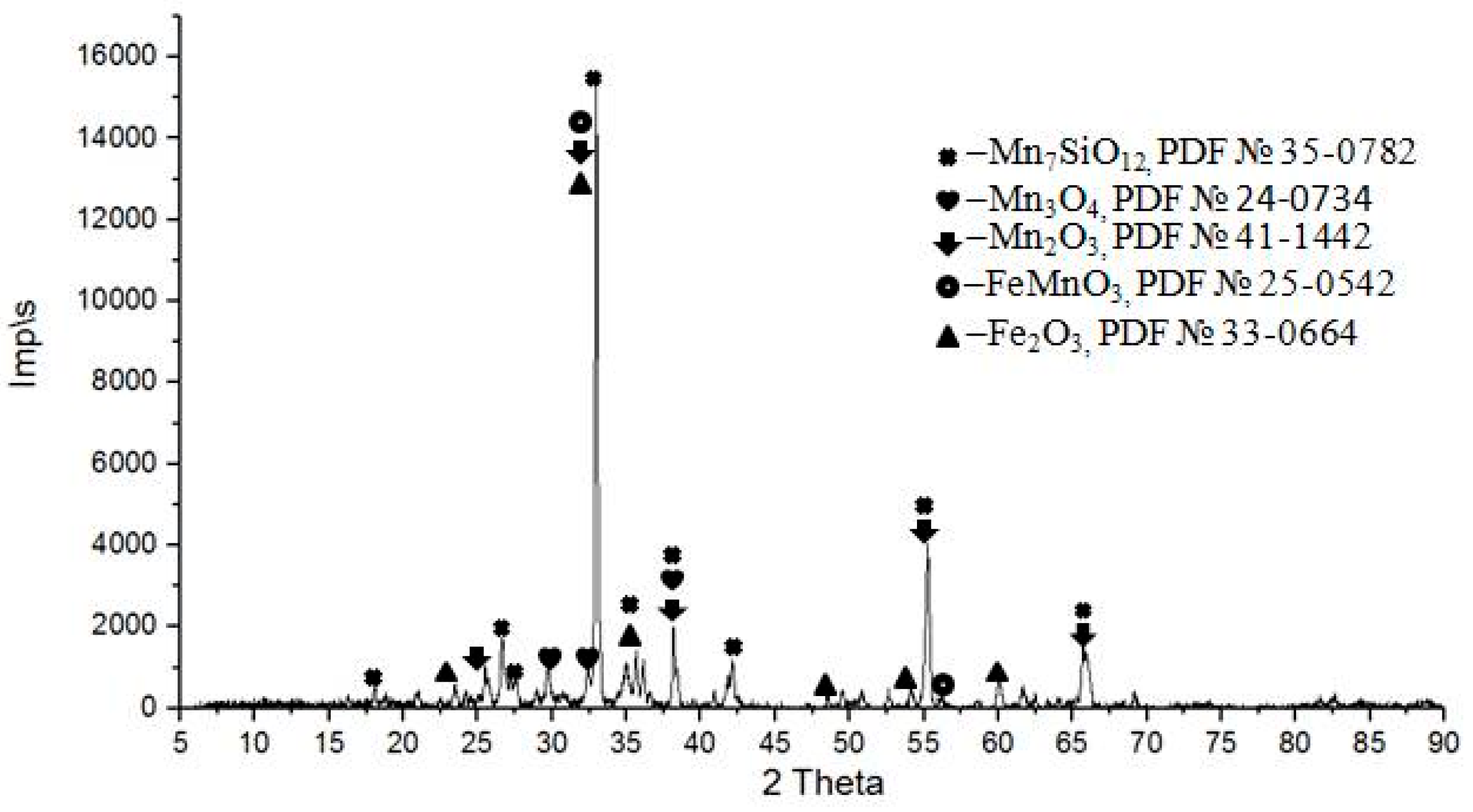
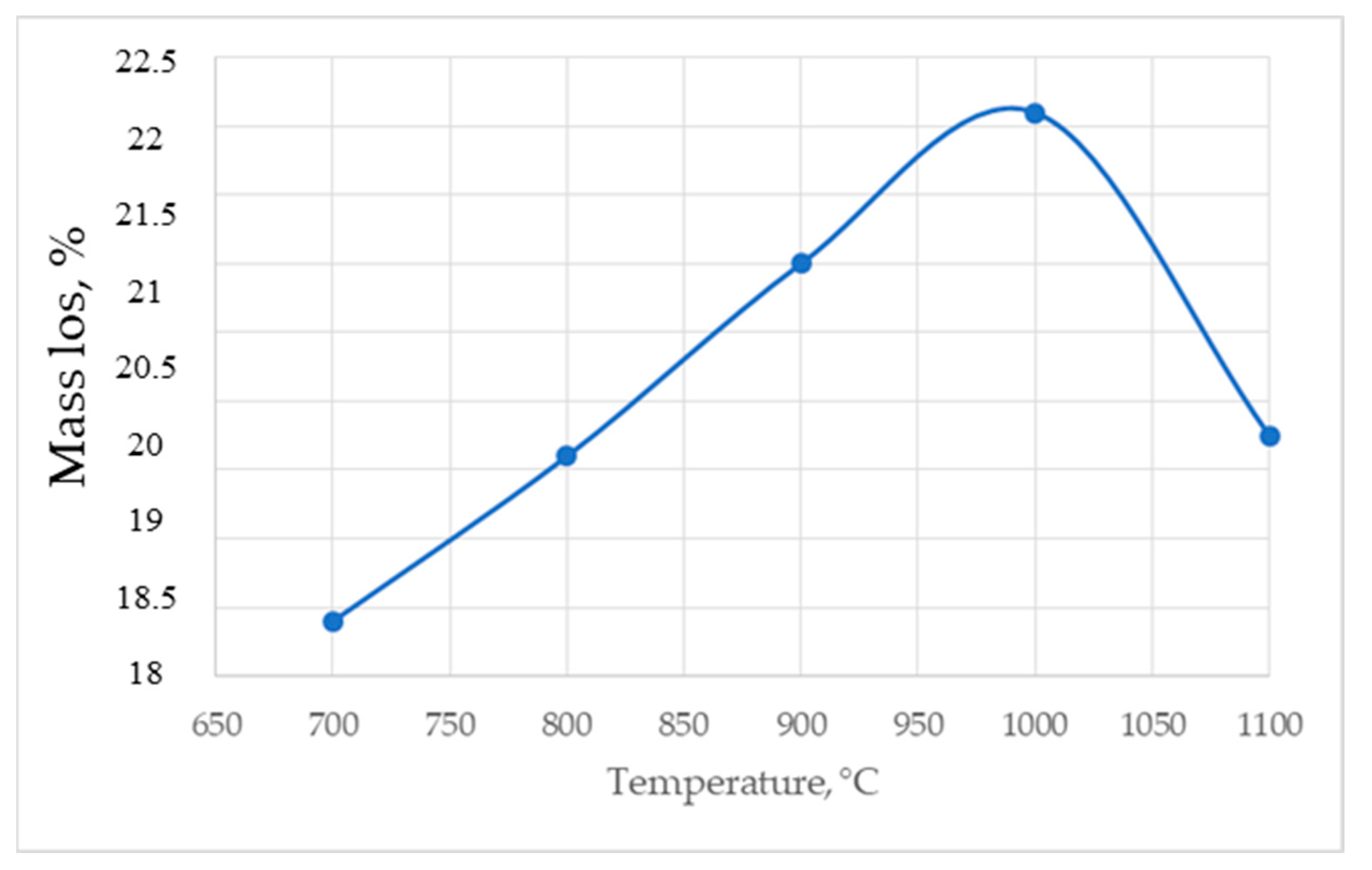
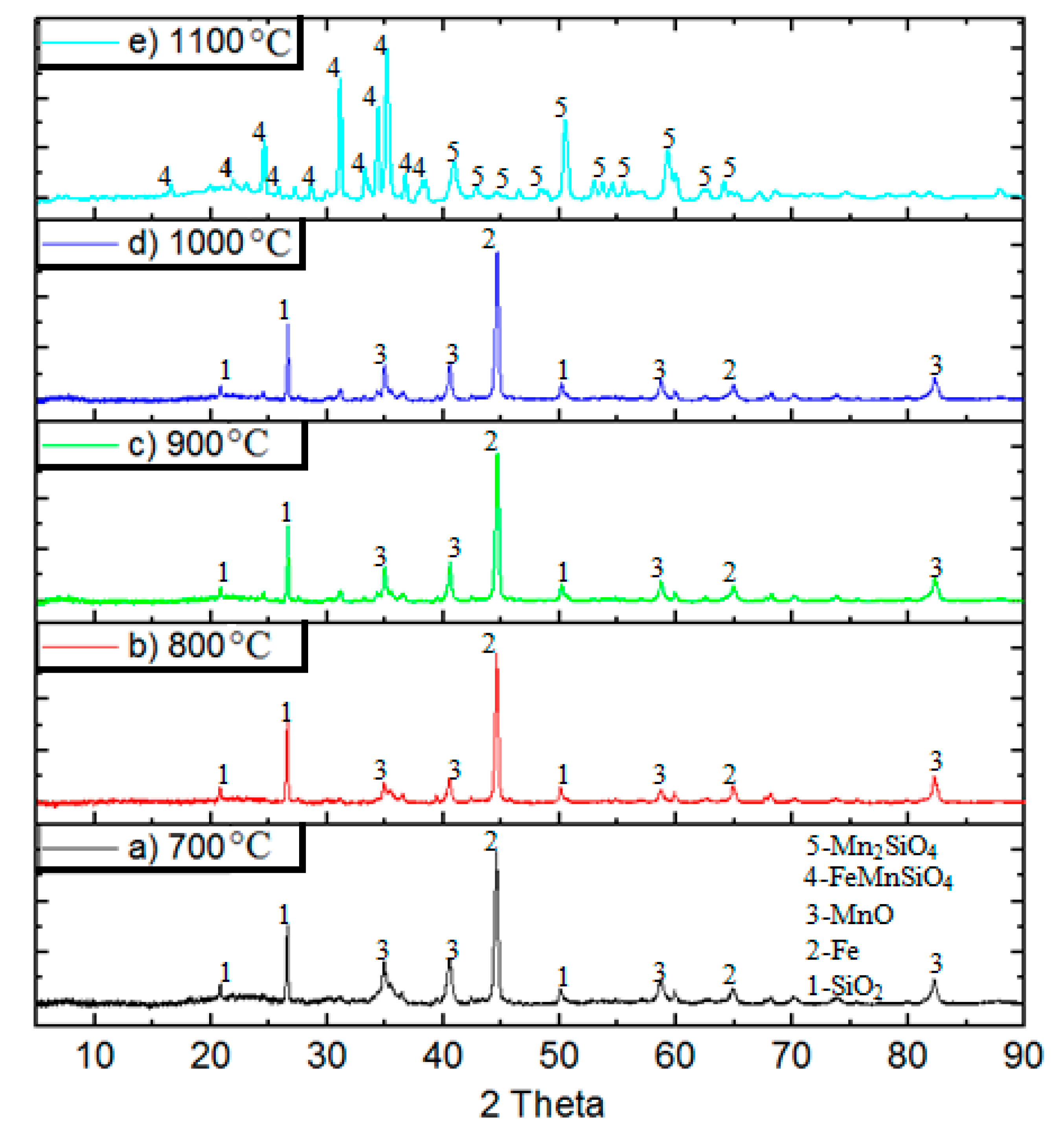
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ministry of Energy of the Republic of Kazakhstan. News Release. Available online: https://www.gov.kz/memleket/entities/energo/press/news/details/862876?lang=ru (accessed on 13 January 2025).
- Forbes Kazakhstan. How a $50 Billion Green Hydrogen Project Is Being Implemented in Kazakhstan. Available online: https://forbes.kz/articles/kak_v_kazahstane_realizuyut_proekt_po_proizvodstvu_zelenogo_vodoroda_stoimostyu_50_mlrd (accessed on 13 January 2025).
- Ngoy, D.; Sukhomlinov, D.; Tangstad, M. Pre-reduction behaviour of manganese ores in H2 and CO containing gases. ISIJ Int. 2020, 60, 2325–2331. [Google Scholar] [CrossRef]
- Ernst, M.S.; Tangstad, M.; Du Preez, S.P. Pre-reduction of Nchwaning manganese ore in CO/CO2, H2/H2O, and H2 atmospheres. Miner. Eng. 2024, 216, 108854. [Google Scholar] [CrossRef]
- Naseri Seftejani, M.; Schenk, J. Thermodynamic of liquid iron ore reduction by hydrogen thermal plasma. Metals 2018, 8, 1051. [Google Scholar] [CrossRef]
- Spreitzer, D.; Schenk, J. Reduction of iron oxides with hydrogen—A review. Steel Res. Int. 2019, 90, 1900108. [Google Scholar] [CrossRef]
- John, D.H.S.; Hayes, P.C. Microstructural features produced by the reduction of wustite in H2/H2O gas mixtures. Metall. Trans. B 1982, 13, 117–124. [Google Scholar] [CrossRef]
- Matthew, S.P.; Cho, T.R.; Hayes, P.C. Mechanisms of porous iron growth on wustite and magnetite during gaseous reduction. Metall. Trans. B 1990, 21, 733–741. [Google Scholar] [CrossRef]
- Matthew, S.P.; Hayes, P.C. Microstructural changes occurring during the gaseous reduction of magnetite. Metall. Trans. B 1990, 21, 153–172. [Google Scholar] [CrossRef]
- Matthew, S.P.; Hayes, P.C. In situ observations of the gaseous reduction of magnetite. Metall. Trans. B 1990, 21, 141–151. [Google Scholar] [CrossRef]
- Farren, M.; Matthew, S.P.; Hayes, P.C. Reduction of solid wustite in H2/H2O/CO/CO2 gas mixtures. Metall. Trans. B 1990, 21, 135–139. [Google Scholar] [CrossRef]
- Suleimen, B. Selective Reduction of Iron in High-Phosphorus Oolitic Ore from the Lisakovsk Deposit. Materials 2024, 17, 5271. [Google Scholar] [CrossRef]
- Roshchin, V.E.; Gamov, P.A.; Roshchin, A.V.; Salikhov, S.P. Prospects for the Development of Hydrogen Technologies in Domestic Metallurgy. Ferrous Metallurgy. Bull. Sci. Tech. Econ. Inf. 2023, 79, 144–153. [Google Scholar] [CrossRef]
- Sarkar, A.; Schanche, T.L.; Safarian, J. Isothermal Pre-Reduction Behavior of Nchwaning Manganese Ore in H2 Atmosphere. Mater. Proc. 2023, 15, 58. [Google Scholar] [CrossRef]
- Schanche, T.L.; Tangstad, M. Prereduction of Nchwaning Ore in CO/CO2/H2 Gas Mixtures. Minerals 2021, 11, 1097. [Google Scholar] [CrossRef]
- Suleimen, B.; Salikhov, S.P. Behavior of extrusion briquettes (Brex) and pellets from oolite iron ore in solid-phase metallization. AIP Conf. Proc. 2022, 2456, 020054. [Google Scholar]
- Salikhov, S.P.; Suleimen, B.; Roshchin, V.E. Selective reduction of iron and phosphorus from oolitic ore. Izv. Ferrous Metall. 2020, 63, 560–567. (In Russian) [Google Scholar] [CrossRef]
- Yerbolat, M.; Yesmurat, M.; Asyylbek, A. Modeling the ferrosilicomanganese smelting process using manganese-rich slag. Acta Metall. Slovaca 2024, 30, 29–33. [Google Scholar]
- Pavlov, M.V.; Shabanov, V.F.; Pavlov, V.F. Comprehensive Processing of High-Phosphorus Manganese Ores from the Novonikolaevskoye Deposit. Chem. Sustain. Dev. 2012, 20, 443–448. [Google Scholar]
- Gasik, M.; Dashevskii, V.; Bizhanov, A. Ferroalloys: Theory and Technology, 3rd ed.; Infra-Engineering: Moscow, Russia; Vologda, Russia, 2021; 288p, ISBN 978-5-0729-0566-9. [Google Scholar]
- Ding, P.; Liu, Q.J.; Pang, W.H. A review of manganese ore beneficiation situation and development. Appl. Mech. Mater. 2013, 380–384, 4431–4433. [Google Scholar] [CrossRef]
- Tripathy, S.K.; Banerjee, P.K.; Suresh, N. Effect of desliming on the magnetic separation of low-grade ferruginous manganese ore. Int. J. Miner. Metall. Mater. 2015, 22, 661–673. [Google Scholar] [CrossRef]
- Grieco, G.; Kastrati, S.; Pedrotti, M. Magnetic enrichment of braunite-rich manganese ore at different grain sizes. Miner. Process. Extr. Metall. Rev. 2014, 35, 257–265. [Google Scholar] [CrossRef]
- Mpho, M.; Samson, B.; Ayo, A. Evaluation of reduction roasting and magnetic separation for upgrading Mn/Fe ratio of fine ferromanganese. Int. J. Min. Sci. Technol. 2013, 23, 537–541. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, Y.; Lu, M.; Su, Z.; Li, G.; Jiang, T. Extraction and separation of manganese and iron from ferruginous manganese ores: A review. Miner. Eng. 2019, 131, 286–303. [Google Scholar] [CrossRef]
- Zhou, F.; Chen, T.; Yan, C.; Liang, H.; Chen, T.; Li, D.; Wang, Q. The flotation of low-grade manganese ore using a novel linoleate hydroxamic acid. Colloids Surf. A Physicochem. Eng. Asp. 2015, 466, 1–9. [Google Scholar] [CrossRef]
- Zhu, D.; Xu, J.; Guo, Z.; Pan, J.; Li, S.; Pan, L.; Yang, C. Synergetic utilization of copper slag and ferruginous manganese ore via co-reduction followed by magnetic separation process. J. Clean. Prod. 2020, 250, 119462. [Google Scholar] [CrossRef]
- Yi, L.; Huang, Z.; Jiang, T.; Zhao, P.; Zhong, R.; Liang, Z. Carbothermic reduction of ferruginous manganese ore for Mn/Fe beneficiation: Morphology evolution and separation characteristic. Minerals 2017, 7, 167. [Google Scholar] [CrossRef]
- Gao, L.; Liu, Z.; Pan, Y.; Feng, C.; Ge, Y.; Chu, M. A study on separation of Mn and Fe from high-alumina ferruginous manganese ores by the carbothermal roasting reduction process. Adv. Powder Technol. 2020, 31, 51–60. [Google Scholar] [CrossRef]
- Mehta, K.D.; Das, C.; Pandey, B.D. Leaching of copper, nickel and cobalt from Indian Ocean manganese nodules by Aspergillus niger. Hydrometallurgy 2010, 105, 89–95. [Google Scholar] [CrossRef]
- Tian, X.; Wen, X.; Yang, C.; Liang, Y.; Pi, Z.; Wang, Y. Reductive leaching of manganese from low-grade manganese dioxide ores using corncob as reductant in sulfuric acid solution. Hydrometallurgy 2010, 100, 157–160. [Google Scholar] [CrossRef]
- Liu, Y.; Lin, Q.; Li, L.; Fu, J.; Zhu, Z.; Wang, C.; Qian, D. Study on hydrometallurgical process and kinetics of manganese extraction from low-grade manganese carbonate ores. Int. J. Min. Sci. Technol. 2014, 24, 567–571. [Google Scholar] [CrossRef]
- Cheraghi, A.; Becker, H.; Eftekhari, H.; Yoozbashizadeh, H.; Safarian, J. Characterization and calcination behavior of a low-grade manganese ore. Mater. Today Commun. 2020, 25, 101382. [Google Scholar] [CrossRef]
- Sinha, M.K.; Purcell, W. Reducing agents in the leaching of manganese ores: A comprehensive review. Hydrometallurgy 2019, 187, 168–186. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, Y.; Wang, J.; Wang, J.; Su, Z.; Li, G.; Jiang, T. New understanding on separation of Mn and Fe from ferruginous manganese ores by the magnetic reduction roasting process. Appl. Surf. Sci. 2018, 444, 133–144. [Google Scholar] [CrossRef]
- Kosdauletov, N.; Mukhambetgaliev, E.K.; Roshchin, V.E. Separation of ferromanganese ore components by non-contact and contact carbothermic reduction. Izv. Ferrous Metall. 2021, 64, 761–767. (In Russian) [Google Scholar] [CrossRef]
- Kosdauletov, N.; Mukambetgaliyev, E.K.; Roshchin, V.E. Separation of Ferromanganese Ore Components by Reduction with Carbon and Carbon Monoxide. Steel Transl. 2022, 52, 416–421. [Google Scholar] [CrossRef]
- Kosdauletov, N.Y.; Roshchin, V.E. Estimation of selective reduction of iron and phosphorus from manganese ores of different genesis. IOP Conf. Ser. Mater. Sci. Eng. 2020, 966, 012036. [Google Scholar] [CrossRef]
- Kosdauletov, N.K.; Roshchin, V.R. Solid-phase reduction and separation of iron and phosphorus from manganese oxides in ferromanganese ore. Defect Diffus. Forum 2021, 410, 281–286. [Google Scholar] [CrossRef]
- GOST 3022-80; Metals. Methods for Determination of Density. Izdatelstvo Standartov: Moscow, Russia, 1980. (In Russian)
- GOST 10157–2016; Fireclay Refractories. Specifications. Standartinform: Moscow, Russia, 2016. (In Russian)


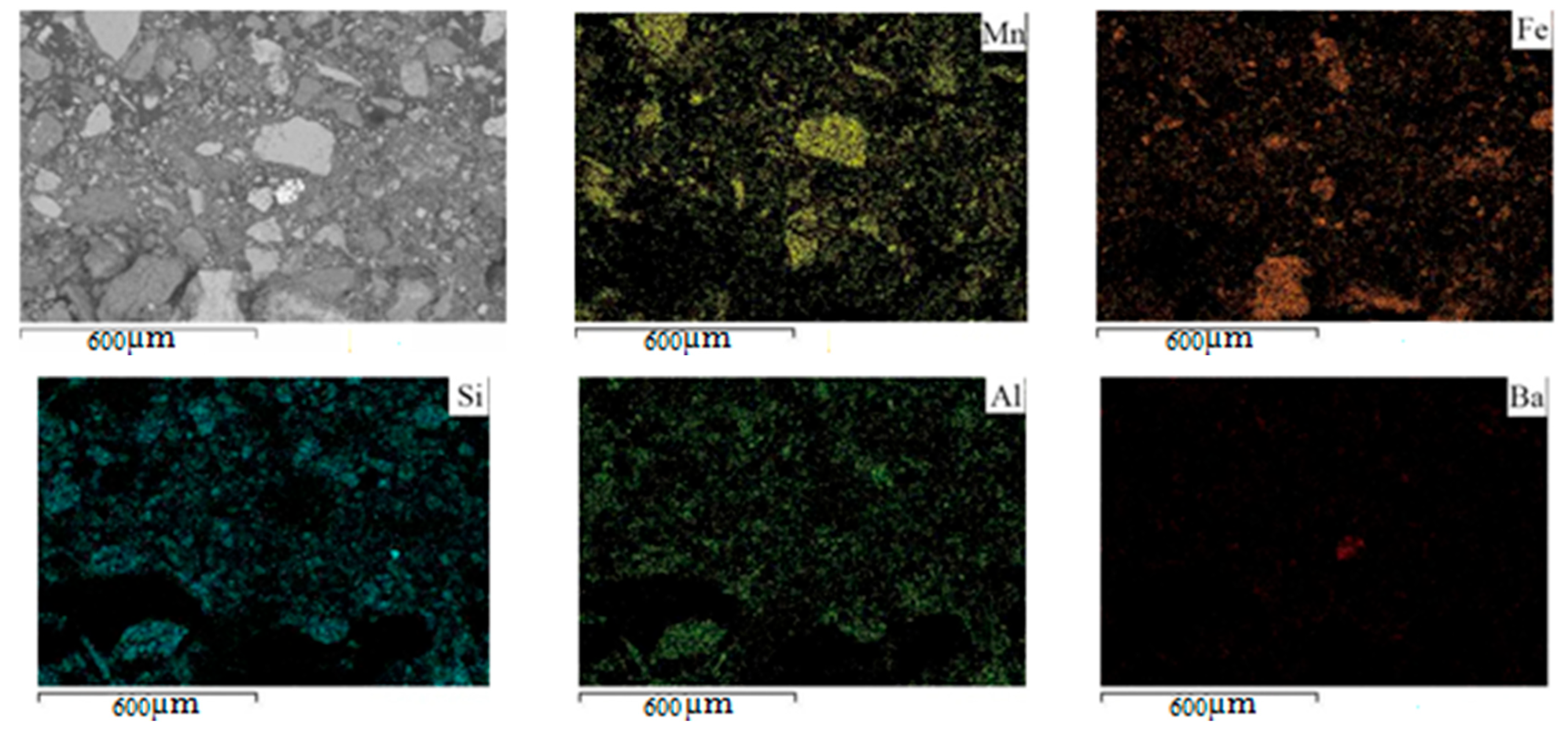
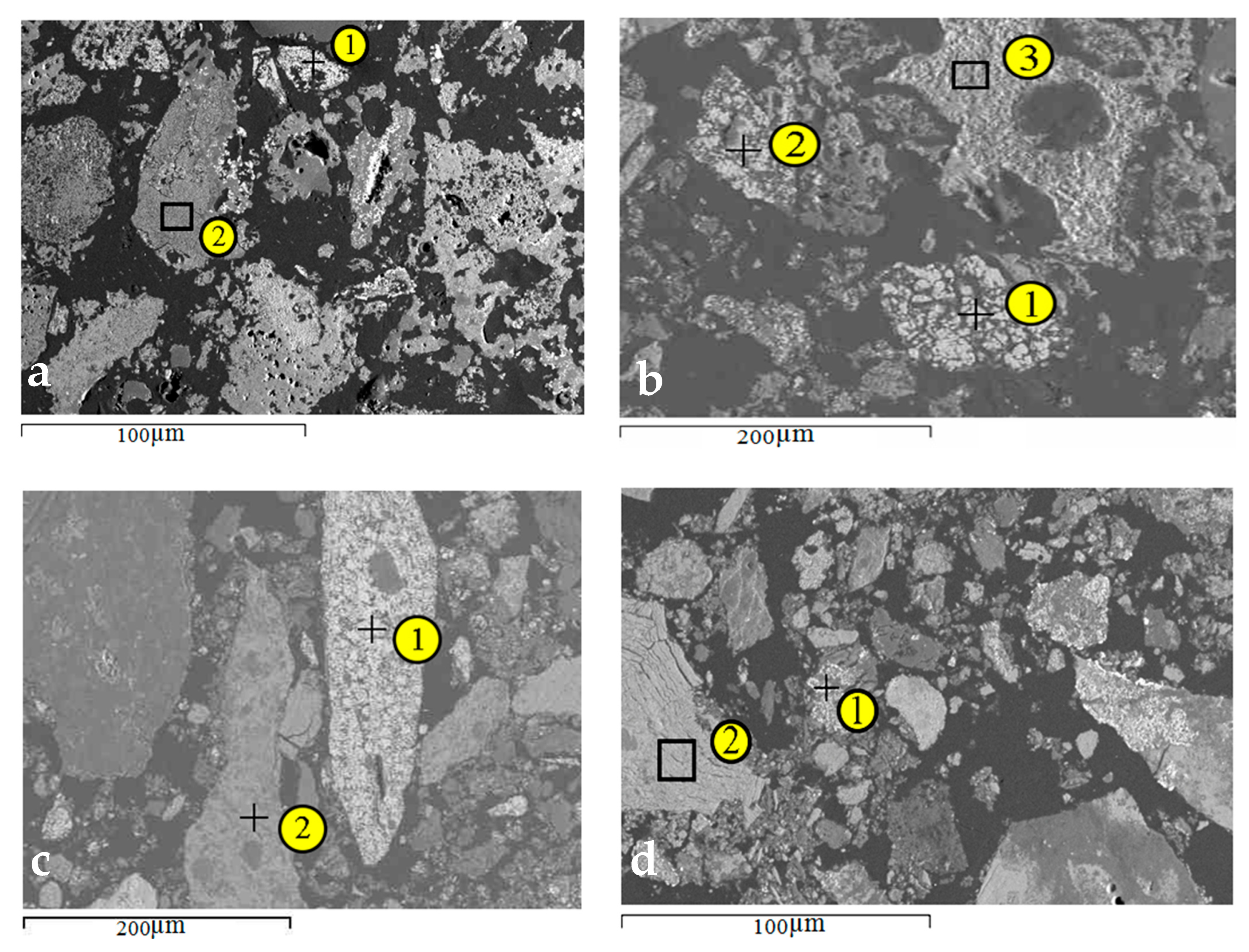
| Area Analysis | O | Al | Si | K | Ca | Mn | Fe | As | Ba |
|---|---|---|---|---|---|---|---|---|---|
| At. % | 66.6 | 4.5 | 9.9 | 0.9 | 0.5 | 11.8 | 5.4 | 0.4 | 0.1 |
| Mass. % | 42.5 | 4.8 | 11.1 | 1.5 | 0.7 | 25.8 | 12.0 | 1.1 | 0.5 |
| at.% | O | Si | K | Ca | Mn | Fe | As | Minerals |
|---|---|---|---|---|---|---|---|---|
| Area 1 | 70.6 | 1.8 | 0.6 | 26.2 | 0.7 | 0.1 | MnO2 | |
| Area 2 | 66.1 | 1.4 | 0.4 | 32.0 | 0.1 | Fe2O3 | ||
| Area 3 | 67.9 | 1.2 | 1.6 | 0.2 | 16.4 | 12.6 | MnFeO3 |
| at.% | O | Al | Si | K | Mn | Fe | Ba | As |
|---|---|---|---|---|---|---|---|---|
| Area | 68.5 | 3.6 | 11.5 | 1.6 | 8.2 | 5.6 | 0.7 | 0.2 |
| Sample | Element Content, at.% | ||||||
|---|---|---|---|---|---|---|---|
| Analysis Points | O | Al | Si | Mn | Fe | As | |
| 700 °C | 1 | 0.0 | 0.0 | 0.0 | 0.0 | 98.7 | 1.3 |
| 2 | 39.5 | 0.0 | 2.4 | 52.1 | 6.1 | 0.0 | |
| 800 °C | 1 | 0.0 | 0.0 | 0.0 | 0.0 | 99.5 | 0.5 |
| 2 | 0.0 | 0.0 | 0.0 | 0.0 | 98.9 | 1.1 | |
| 3 | 42.0 | 2.0 | 0.7 | 53.0 | 2.2 | 0.0 | |
| 900 °C | 1 | 0.0 | 0.0 | 0.0 | 2.8 | 95.3 | 1.9 |
| 2 | 38.5 | 0.0 | 1.3 | 57.9 | 2.4 | 0.0 | |
| 1000 °C | 1 | 0.0 | 0.0 | 0.0 | 4.4 | 93.6 | 2.0 |
| 2 | 39.8 | 0.0 | 1.9 | 55.6 | 2.7 | 0.0 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kosdauletov, N.; Nurumgaliyev, A.; Zhautikov, B.; Suleimen, B.; Adilov, G.; Kelamanov, B.; Smirnov, K.; Zhuniskaliyev, T.; Kuatbay, Y.; Bulekova, G.; et al. Selective Reduction of Iron from Iron–Manganese Ore of the Keregetas Deposit Using Hydrogen. Metals 2025, 15, 691. https://doi.org/10.3390/met15070691
Kosdauletov N, Nurumgaliyev A, Zhautikov B, Suleimen B, Adilov G, Kelamanov B, Smirnov K, Zhuniskaliyev T, Kuatbay Y, Bulekova G, et al. Selective Reduction of Iron from Iron–Manganese Ore of the Keregetas Deposit Using Hydrogen. Metals. 2025; 15(7):691. https://doi.org/10.3390/met15070691
Chicago/Turabian StyleKosdauletov, Nurlybai, Assylbek Nurumgaliyev, Bakyt Zhautikov, Bakyt Suleimen, Galymzhan Adilov, Bauyrzhan Kelamanov, Konstantin Smirnov, Talgat Zhuniskaliyev, Yerbol Kuatbay, Gulzat Bulekova, and et al. 2025. "Selective Reduction of Iron from Iron–Manganese Ore of the Keregetas Deposit Using Hydrogen" Metals 15, no. 7: 691. https://doi.org/10.3390/met15070691
APA StyleKosdauletov, N., Nurumgaliyev, A., Zhautikov, B., Suleimen, B., Adilov, G., Kelamanov, B., Smirnov, K., Zhuniskaliyev, T., Kuatbay, Y., Bulekova, G., & Abdirashit, A. (2025). Selective Reduction of Iron from Iron–Manganese Ore of the Keregetas Deposit Using Hydrogen. Metals, 15(7), 691. https://doi.org/10.3390/met15070691






