Abstract
This research presents the results of different periods of T6 heat treatment (homogenization and artificial aging) for A356 aluminum alloy used in the fabrication of motorcycles. The samples were cast using gravity die casting, and industrial furnaces for T6 were used in the experiment. Two heat treatment conditions were used, with a total time of 7 h and 12 h, and the results were compared with the alloy without heat treatment. The effects of the reduction of treatment time on mechanical behavior were evaluated in terms of hardness, Charpy and tensile tests, as well as morphological analysis of fractures and microstructural behavior via optical microscopy, SEM-EDS, measurement of eutectic Si evolution, and XRD. Excellent mechanical properties were achieved with a treatment period of 7 h, which achieved a yield strength of 226.58 (±3.76) MPa, tensile strength limit of 264.78 (±4.27) MPa and elongation of 3.41 (±0.47) %. This is competitive with other cast alloys subjected to T6 heat treatment in longer treatment cycles. The peak of hardness and highest impact resistance was recorded for the sample treated for 12 h; however, in the impact test, there was no significant difference between the two experiments.
1. Introduction
Global concerns regarding the environment have increased, particularly in relation to minimizing the use of energy resources and reducing pollution. Many governments have created strategies involving incentives to industries and the elaboration of global targets in order to make better use of existing resources [1,2].
Faced with a scenario that requires increasing environmental responsibility, aluminum alloys and, in particular, cast aluminum components subjected to high mechanical stress have attracted great attention from industries since they can be recycled [3]. Industries in the aluminum foundry sector adopt process optimizations in order to achieve better operational efficiency, reducing energy use and costs via the use of recycled aluminum alloys, and this difference represents a saving of ~95% of energy [4].
Due to their high strength-to-weight ratio, low cost and possibility of being recycled, aluminum alloys are environmentally friendly, since they improve energy consumption and reduce the greenhouse effect. This reduction is due to its use in various industrial segments, such as the manufacture of passenger vehicles, in which it is used in wheels, steering joints, suspension, engine blocks and transmissions [3]. One of the aluminum alloys that can be highlighted is the alloy Al-7% Si-0.35% Mg (A356), which is used in structural components, both in the aviation and automotive industries. A356 has good castability, specifically in GDC (gravity die casting) processes, and has excellent corrosion resistance and significant mechanical strength [4].
Magnesium, together with the silicon in A356, allows the formation of the phase (Mg2Si), which is responsible for promoting hardening via precipitation and, consequently, the increase in the mechanical strength of these alloys [5]. Other chemical elements, such as Cu, Mn, Zn, Ti and B, are added to aluminum-silicon alloys in order to improve resistance to fracture, which widens the range of use of these alloys [6]. Some elements, such as iron, are incorporated involuntarily as a result of the process used to obtain primary aluminum or due to contamination during the recycling process [7]. The mechanical properties of Al-Si-Mg alloys can be improved through appropriate heat treatment, which allows the alloy to be used in structural components that are subjected to high mechanical stress [8].
Homogenization and aging (T6) is a type of heat treatment applied to certain types of aluminum alloys. If performed properly, T6 generates a uniform distribution of precipitates in the microstructure of the alloy Al-Si-Mg, which increases the mechanical strength and hardness of the alloy [9]. The improvement of the mechanical properties of the Al-Si-Mg alloy is also influenced by the average grain size and morphology and the dispersion of the Mg2Si phase obtained through the nucleation of the precipitates after treatment [10].
A number of studies have been carried out seeking to improve the mechanical properties of A356 using T6 heat treatment. Dias [8] studied the behavior of the mechanical properties of A356 after different T6 heat treatment cycles. In this study, he incrementally varied the total T6 heat treatment time from 6 to 19 h with homogenization temperatures between 520 and 540 °C and aging between 155 and 185 °C, and observed a 2.5% reduction in tensile strength for the heat treatment using homogenization temperatures of 540 °C and aging at 185 °C. Lu et al. [11] carried out a study of a T6 heat treatment process for alloy A356 with a short duration of 2 h and another with a conventional duration of 15 h. The authors observed a small increase in mechanical strength (1.4%) for the reduced heat treatment of 2 h. Another study [12] evaluated the T6 heat treatment with different temperatures and increasing homogenization treatment times of 1 h, 2.5 h and 4.5 h, and artificial aging of 1.5 h, 4 h and 6 h, on the mechanical properties of AlSi11(Fe) alloy. The result of this study was a 52% increase in tensile strength for the alloy with treatment times of 1 h, 2.5 h and 4.5 h. Previous work with A356 aluminum alloy relates the effect of different heat treatment conditions on obtaining new properties or maintaining properties with a reduction in treatment time. Reducing the heat treatment time for alloy A356 in the industry is very important to promote an increase in productivity and a reduction in energy costs, which makes the process environmentally friendly. However, studies carried out in an industrial environment in which all the conditions and variables of the foundry production process for the production of motorcycle parts are used are very rare.
Therefore, this study was carried out in the industrial environment of the casting process and aimed to study the relationship between the mechanical and microstructural behavior of aluminum alloy Al-7% Si-0.35 Mg (A356) after reducing the time of T6 heat treatment from 12 h to 7 h.
2. Materials and Methods
This topic presents a general description of the research developed that began with the preliminary study of the mechanical behavior of aluminum alloy Al-7% Si-0.35 Mg (A356) used in the manufacture of front suspension for low, medium and high displacement motorcycles.
2.1. Materials
The A356 aluminum alloy used in this work was analyzed with an optical emission spectrometer (Shimadzu, PDA-7000, Kyoto, Japan) to confirm its chemical composition. In this test, five readings were performed according to the procedures in ASTM E 1251-17 [13]. The recommended chemical composition for the alloy to be used in the casting process is specified by Al Castings GDC/LPDC-28109-AAZ-10 [14].
2.2. Casting Process
The production of the components involved gravity die casting in a permanent mold with a sand core. After the solidification of the piece, the homogenization and artificial aging (T6 heat treatment) were carried out. For the melting of the aluminum alloy, we used an electric arc furnace (CNG, Shine Grand Industrial, Taipei City, Taiwan), which has a capacity of 2 t and production of between 400 and 500 kg/h, and melting, conservation and crucible zones. The furnace is also equipped with a rotary degasser for aluminum (ECO-Des fixed tower, DJ Fornos, Ferraz de Vasconcelos, São Paulo, Brazil), which has the function of removing dissolved hydrogen in the metal bath to avoid defects such as microbubbles, by means of the Foundry Degassing Unit method [14]. Table 1 shows the process parameters of the melting furnace during the process.
A grain refiner, Al-5Ti-B (Fengyue, Qinhuangdao, Hebei, China), was added to the molten alloy, with proportions of between 0.3 and 0.5% by weight of the alloy. Slagging fluxes (NaCl-KCl) (Pyrotek, Cortland, NY, USA) were also added to the surface of the liquid alloy to form a slag of impurities that rise to the surface of the metal bath. Subsequently, there was the addition of Sr (supplier: Joinville, Santa Catarina, AGM Brasil) at a proportion of 200 ppm, used with eutectic modifier to improve mechanical properties [15]. Then, the aluminum alloy was cast in a metal mold (die), without cooling, as shown in Figure 1a,b. Figure 1a shows the design of the die for the GDC process, and the green part represents the feed channels and feeder, the pink is the right-side damper, and the blue is the left-side damper. Figure 1b shows the part obtained after the casting process and the removal location for the manufacturing of the sample specimens. Table 1 shows the process parameters during the aluminum alloy casting of front suspension units for a 300 cc motorcycle.
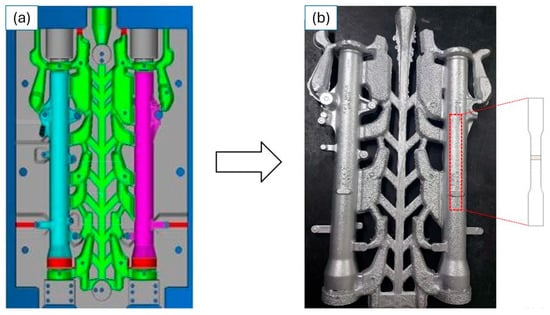
Figure 1.
(a) Diagram of the metal mold used; (b) part obtained from which the samples for the mechanical tests were made. Photo credit: Reinaldo Almeida. “(a) Colors in the mold: green represents the feeding channels. Blue and pink are the motorcycle parts. Red: gas vent channel”.

Table 1.
Parameters of the gravity die casting process.
Table 1.
Parameters of the gravity die casting process.
| Equipment | Parameters | Specification |
|---|---|---|
| Melting furnace | Melting chamber temperature | 1200 °C (max.) |
| Conservation chamber temperature | 1200 °C (max.) | |
| Temperature in the crucible (at the pouring point) | 710–750 °C | |
| Nitrogen pressure (N) for degassing | 3–10 bar | |
| Nitrogen (N) flow rate for degassing | 5–15 L/min | |
| Rotary degasser | 260–320 rpm | |
| Metal mold | Mold temperature | 360–420 °C |
| Molding time | 30–40 s | |
| Solidification time | 130–150 s |
Source: Specification Al castings GDC/LPDC-28109-AAZ-10 [16].
2.3. Heat Treatment Process
The heat treatment of the samples was carried out in an electric resistance furnace (109/07, Sauder, Embu-Guaçu, São Paulo, Brazil) with air circulation and a power rating of 718 kW, with automatic processing being used.
Temperature monitoring was performed via 16 K-type thermocouple sensors with an accuracy of ±1 °C (CS-13, Ecil, São Paulo, São Paulo, Brazil), which were installed directly in the aluminum parts, and a temperature controller for data storage (6100-A, ECIL, Brazil).
Different T6 heat treatments designated by indications T6—12 (indicates that the heat treatment performed was the standard T6) and T6—7 (indicates a reduction in heat treatment time based on ASTM B917) were performed within the parameters shown in Table 2. Figure 2 shows the graphs referring to the treatment conditions used with increase in the homogenization temperature, but there is a reduction in time, and this will provide greater productivity.

Table 2.
Specifications of the different heat treatment times (T6) performed.
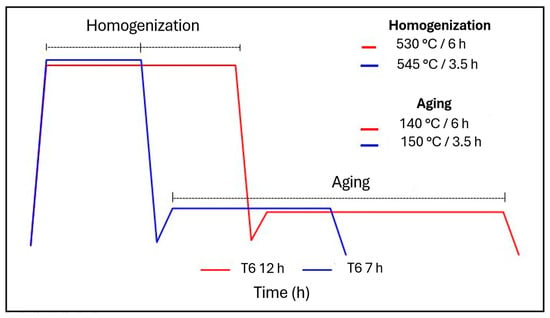
Figure 2.
T6 heat treatment conditions applied in this study.
According to ASTM B917 (Heat Treatment of Aluminum-Alloy Castings of All Processes) [17], the time intervals for heat treatments are quite wide. Typically, modified alloys and structures with low thicknesses require shorter heat treatment times and a small increase in temperature, but always within the specification (540 °C ± 5 °C for homogenization and 155 °C ± 5 °C for artificial aging).
2.4. Microstructural Characterization
The evolution of the microstructure and microhardness as a function of the heat treatment processes was investigated. The metallography of the samples and chemical etching in Keller’s solution (HF + HCl + HNO3) was performed for 30 s, both in the sample without treatment (F) and in the samples with the two periods of thermal treatments.
The micrographic analyses were performed according to ASTM E 3-17 [18]. The samples were used to obtain the morphology and microconstituents (metallography) that make up each alloy and the effects of the heat treatment on their microstructure. An optical microscope (Cx31, Olympus, Shinjuku-ku, Tokyo, Japan) was used. Subsequently, the samples were observed using a scanning electron microscope (SEM) with energy dispersive spectroscopy—EDS (Jeol JSM IT 200, Jeol, Akishima, Tokyo, Japan). SEM investigations were conducted in order to analyze the effect on the microstructure after the reduction in the T6 heat treatment period.
After tensile testing, the specimens were adapted for SEM observation along their cross-sections in order to study fracture behavior. This was performed using a scanning electron microscope (Jeol JSM IT 200, Jeol, Akishima, Tokyo, Japan) with an acceleration voltage of 20 kV and equipped with an EDS detector.
For the investigation of the phases present in the samples without treatment and those with different treatments, X-ray diffraction (XRD) was used for the aforementioned study. The X-ray diffractometer (XRD-7000, Shimadzu, Kyoto, Japan.) used K-alpha CU radiation (λ = 512.8 nm), current and voltage standards of 30 kV and 40 mA, respectively, and 2θ angles between 20 and 90 degrees.
2.5. Mechanical Characterization
A Vickers microhardness testing machine, automatic hardness testing system AUTOVICK (HM-200, Mitutoyo, Japan) with a 50× objective was used. The indenter was a square pyramid with an angle of 136° between the opposite faces. Measurements were carried out under a force of 0.5 kgf according to ISO 6507-1 [17]. The measurements obtained in HV were converted to HRB by the AVPAK-10/20 equipment software version 3.0 (Mitutoyo Corporation, Kawasaki, Japan).
Tensile tests were performed at room temperature according to JIS Z 2241:2020 [19]. A universal electromechanical testing machine (5582, Instron, Norwood, MA, USA), properly calibrated according to ISO 7500-1 [20], load cell capacity: 10 kN, class 0.5, and an electronic extensometer (Type C, Instron, USA) with a capacity of 2.5 mm, class C, were used.
The tests were performed at a temperature of 23 ± 5 °C and a speed of 20 MPa·s−1. The test results were submitted to calculations using Bluehill Universal software version 3.0 (Instron, Norwood, MA, USA) and the estimation of measurement uncertainty according to JCGM 100:2008 was adopted. The samples were machined according to the dimensions of Figure 3. The figure shows the following dimensions: width W2: 12 mm, clamping region on the test machine, R: 15 ± 5 mm is the radius so that no stress concentration occurs, useful length L0: 45 mm, width W: 8 ± 0.5 mm and thickness t: 4 mm, which can be down to a minimum thickness of 2 mm.
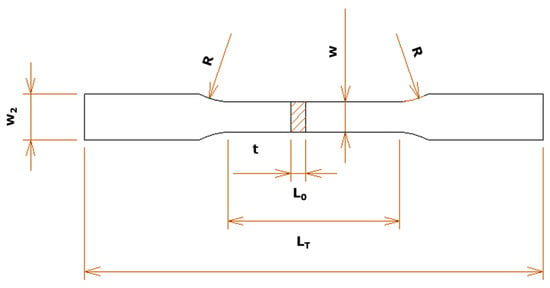
Figure 3.
Tensile test specimen, according to JIS Z 2241:2020 [21].
To study the effects of reducing the heat treatment time T6 on the impact properties, the impact strength test of the alloy was performed using Charpy without notching, according to JIS Z 2242:2018 [22]. Five samples were obtained for each heat treatment condition (T6) as well as an untreated sample (F), in the dimensions of 4 × 10 × 55 mm. The test was performed using a pendulum machine (CEAST 9050, Instron, USA) with a maximum capacity of 50 J and an impact speed of 5.6 m/s.
2.6. Statistical Analysis
A statistical analysis was performed on the results of the tensile properties, hardness, Charpy and the measurement of precipitates. The analysis of variance (ANOVA), at a 5% level of significance, was used to evaluate the statements about the results using the Minitab 19.1 software, in order to verify whether there was a significant difference between the means and whether the factors exert influence on a dependent variable. The Tukey test was used to assess the magnitude of these differences, as it allows all possible treatment comparisons to be made pair by pair. The Tukey method specifies that the entire comparison set must have an error rate of 0.05, equivalent to a simultaneous confidence level of 95%.
In a second step, via the Minitab 19.1 software, cluster analysis was used with the objective of clustering the variables according to their proximity and common characteristics, seeking to show homogeneity within the groups and heterogeneity between the clusters. This consists of transforming a set of original variables into a small number of linear combinations, the so-called principal components, of equivalent dimensions, and with important properties.
A multivariate classification was adopted, which aimed to group the data according to the similarities between them, both in variables and by observations (between experiments). It is important to highlight that, in cluster analysis, the input of the information is quantitative variables, and the output of the clusters represents qualitative variables.
A fundamental concept is the choice of a criterion that measures the distance between two objects, or that quantifies how similar they are. This measurement is called the dissimilarity coefficient (the higher the value, the greater the difference between the objects); while, in the similarity criteria, the closer to 100, the greater the similarity.
For the clustering analysis of the observations (0 h; 12 h; 7 h of T6 treatment), as the linking method, the mean of the normalized results was adopted, and the quadratic Euclidean distance was adopted for distance measurement. For the cluster analysis of the variables, the mean of the normalized results and distance measurements by correlation were adopted as the linking method [23].
3. Results and Discussion
3.1. Chemical Analysis of the A356 Alloy
Emission spectroscopy was performed on the two alloys used in this work to verify whether they are compatible with the chemical composition of the A356 aluminum alloy standard (ASTM E 1251-17).
Table 3 presents the results of the chemical composition of the alloy obtained during the analysis by optical emission spectrometry. It was observed that the chemical elements responsible for hardening the Si-Mg alloy [24] were within the standard. Although the standard alloy A356 does not have a strontium (Sr) specification, the alloys used in this research used this eutectic modifier [25].

Table 3.
Chemical composition of the alloy obtained.
3.2. Microstructural Characterization
3.2.1. Analysis of the Phases
Figure 4a shows the micrographs obtained via optical microscopy and SEM in the as-cast state and in the different heat treatment conditions, T6 (12 h and 7 h). The lighter primary phase (Al α-dendrites), in addition to the eutectic Si phase, has darker, fibrous forms that are evenly distributed around the primary α-Al dendrites. It is also possible to observe small intermetallic phases.

Figure 4.
Microstructures of A356 alloy: (a–c) SEM images. (a) Images in the as-cast state (ST), (b) Represent samples treated for 12 h, and (c) Represent samples treated for 7 h.
Figure 4a shows, in SEM, the phases present in the raw melt structure, without any change caused by heat treatment. Figure 4 shows a change in the morphology of the Si-eutectic precipitate from coarse acicular particles (Figure 4a) to refined and spheroidized particles (Figure 4b,c). This behavior occurred due to the T6 heat treatment [26,27,28]. The microstructures obtained with the treatment performed for 12 h are seen in Figure 4b, and those treated for 7 h are seen in Figure 4c.
According to Paray [29], the spheroidization of the branches of the eutectic Si occurs in two phases. In the first phase of fragmentation, initially, a strangulation of the Si particles occurs, and, in the second phase, they separate into fragmented segments and are subsequently converted into spheres. From the microstructures, it is evident that a large number of Si particles are more or less spheroidized, and a considerable number of particles are rod-shaped.
As shown in Figure 4b,c, the presence of needle-shaped Fe-rich intermetallics in all heat treatment conditions is confirmed, which can affect the mechanical properties, especially ductility, but can be reduced [25].
As shown in Figure 5 and Figure 6, through the analysis via energy dispersive spectroscopy (EDS), the microstructure is observed in the as-cast state and is confirmed as eutectic Si in the acicular form in the matrix that is predominantly composed of Al-α (dendrites). Small amounts of titanium (Ti) were also observed. It is seen that the Ti-rich aggregate element in the EDS analysis regions is due to the samples containing a titanium-based grain refiner. The possible intermetallic phase may be TiAl3, which is the main compound of the grain refiners for aluminum alloys [26].
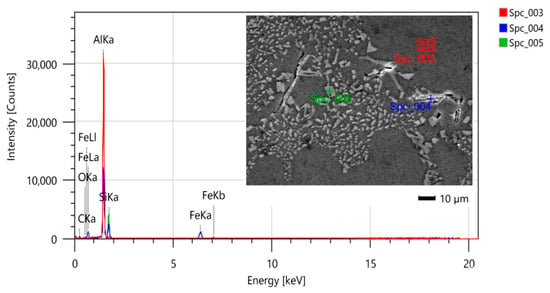
Figure 5.
SEM/EDS micrograph of the sample without heat treatment for identification of the phases.
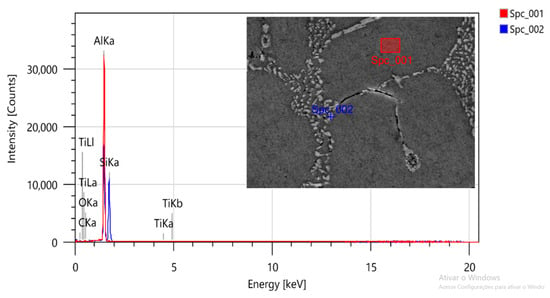
Figure 6.
SEM/EDS micrograph of the sample without heat treatment for identification of phases.
Figure 7 and Figure 8 show the samples after they have been subjected to T6 heat treatment for 12 h and 7 h, respectively. The phases present in the micrographs are the same as those found in the sample shown in Figure 8, i.e., a matrix composed of Al-α (dendrites) and eutectic Si, but with morphological modifications due to the heat treatment [27].
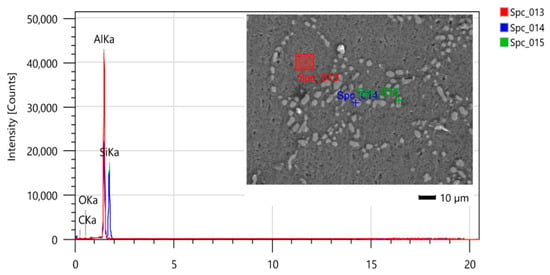
Figure 7.
SEM/EDS micrograph for identification of phases present in the sample after 12 h of T6 heat treatment.
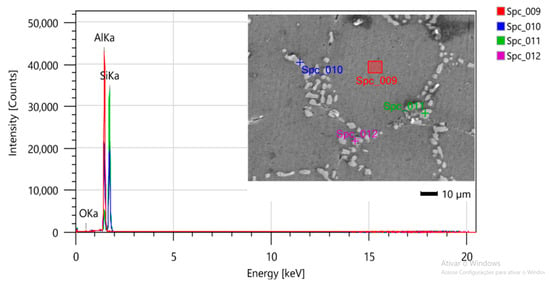
Figure 8.
SEM/EDS micrograph for identification of phases present in the sample after 7 h of T6 heat treatment.
In Figure 7, the heat treatment performed was homogenization at 530 °C/6 h and artificial aging at 140 °C/6 h, totaling 12 h. In Figure 8, there was a reduction in the total heat treatment time, whereby homogenization was performed at 545 °C/3.5 h and artificial aging at 150 °C/3.5 h.
The EDS analyses performed on the eutectic particles show the presence of a non-negligible amount of iron, in addition to Al and Si. It has in role in increasing the mechanical properties of the heat-treated samples [28]. It was evident that a little Al2O3 exists on the surface of the samples after metallographic chemical attack [29], as indicated in Figure 5, Figure 6, Figure 7 and Figure 8.
3.2.2. Effect of T6 Heat Treatment on the Microstructure
Figure 9 shows the mean diameter of the eutectic Si and the evolution of spheroidization of the sample in the as-cast state (0 h) and the two different heat treatment conditions (12 h and 7 h).
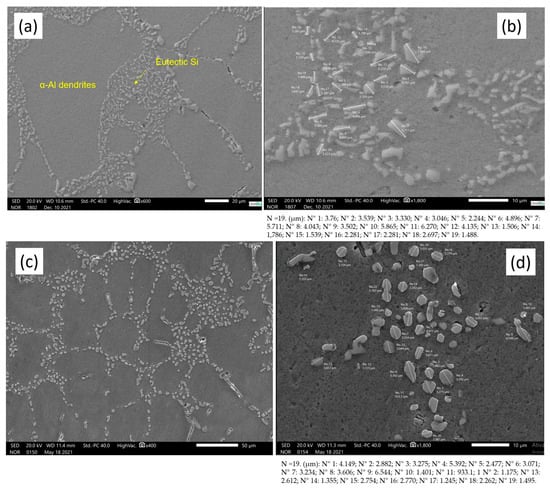
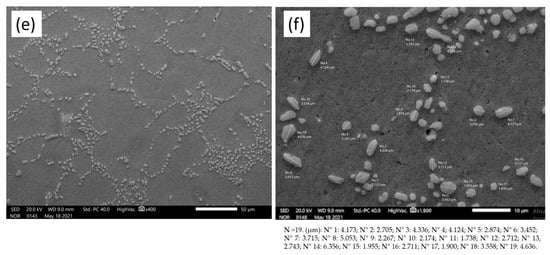
Figure 9.
SEM images with eutectic Si measurement. (a,b) As-cast state, (c,d) 12 h T6 heat treatment and (e,f) 7 h T6 heat treatment. The figure shows the 19 measurements (N) of the precipitates.
The cast sample with 0 h of T6 heat treatment shows the largest modified eutectic diameters of Si (4.02 µm). The samples with the two heat treatment conditions (12 h and 7 h) showed 2.79 µm and 3.32 µm, respectively. The measurement data can be checked via Table 4.

Table 4.
Results of the measurements of the eutectic Si.
In Figure 9a,b, the structure in its as-cast state is shown, without heat treatment, and with the eutectic Si in an acicular form. This characteristic of a non-coarse or fibrous form comes from the modifying agents added to the alloy, specifically strontium (Sr) in the proportion of 200 ppm [30]. In Figure 9c–f, it is also possible to see the microstructures from the two different heat treatment times, T6, 12 h and 7 h, respectively, and the spheroidization of the eutectic Si can also be observed.
According to a study conducted by Carneiro, Puga and Meireles [31], the homogenization and aging treatment is able to successfully agglomerate the eutectic Si particles due to the self-diffusion and interdiffusion of Si at the Si-Al interface. Paray [27] claim that the spheroidization of Si particles has been reported to occur in two consecutive stages. In the first phase of fragmentation, the particles are initially strangled and then separated into segments. The second results in particle size refinement and, subsequently, fragmented segments are converted into spheres.
Via the results found in Table 5, it can be seen that the analysis of variance (ANOVA) performed shows a significant difference in the means of the eutectic Si measurements. The p-value found is equal to 0.040; in other words, a p-value of ≤0.05 (significance level).

Table 5.
Analysis of variance for eutectic Si measurement.
The Tukey test was performed, with a 95% confidence level, on the measurements of the eutectic Si precipitate to evaluate the magnitude of these differences in pairs of clusters. Table 6 shows the difference in the measurements of the samples with and without treatment.

Table 6.
Tukey’s simultaneous tests for the differences in means of the measurement of precipitates.
The data shown in Table 5 and Table 6 indicate that there is a difference in the dimensions of the precipitates between the samples and this was reflected in the hardness of the material, that is, the sample with 7 h of heat aging treatment presented a lower hardness than the sample that was subjected to 12 h of aging treatment and which is in agreement with the literature [32].
Figure 10 shows the statistical differences found for the eutectic Si measurement using the Tukey test. When comparing the 7 h and 0 h samples, the difference between the means was −0.696 with p = 0.308. Ibrahim et al. [33] stated that fragmentation of acicular silicon is facilitated by prior treatment with Sr, so that the resulting eutectic fibrous silicon would be spheroidized in less time.
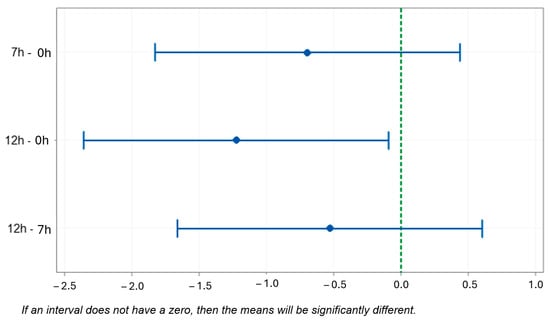
Figure 10.
Tukey test. Difference in the means of the measurement of eutectic Si (95% confidence interval).
In the comparison of the results for 12 h and 0 h of T6 treatment, the largest difference (−1.227) is observed with p = 0.031. Studies found in the literature [34,35,36] corroborate the explanation of the statistically significant difference between the dimensions of the eutectic Si of the sample without treatment (NT—no treatment) and with treatment (12 h), since the refinement or spheroidization of the magnesium silicide phase occurs (Mg2Si).
The difference between the dimensions of the precipitates of the samples with heat treatment times of 12 h and 7 h is shown by the difference in means, with a value of −0.531 and p = 0.500. The studies conducted by Abdulwahab [37] and Mørtsell [38] explain the statistically significant difference between the samples. As the aging time increases, the hardness increases until the peak hardness.
3.2.3. Microstructural Analysis Using XRD
The analysis using X-ray diffraction (XRD) had the purpose of evaluating whether or not there were structural changes in a qualitative way and how these changes can influence the mechanical properties of materials. In Figure 11, it can be seen that the samples without heat treatment, those heat treated for 7 h and those heat treated for 12 h obtained similar peak aspects of Al, Si, Sr, AlSr, SiAl and Mg2Si [39].
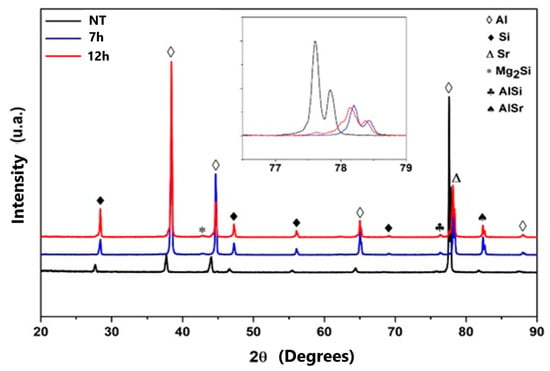
Figure 11.
X-ray diffraction patterns of samples without treatment, and with 12 h and 7 h of T6 heat treatment. NT—no treatment.
The solubility of Sr in Al primarily exists in the form of Al4Sr, forming an intermetallic compound in the Al alloy, which easily decomposes due to the diffusion of Sr atoms. The energy of Sr is compatible with Al solubility in terms of chemical activity, resulting in a greater microstructural modification of the alloy. The microstructural modification allows for a reduction in microstresses, which may lead to improved mechanical performance. This phenomenon occurs due to the thermal treatment dwell time, and such considerations are also reported in the literature by Tunçay, Bayoğlu [40] and Yuan et al. [41].
The samples that underwent heat treatment obtained a slight displacement to the right when compared with the untreated sample. According to Mishra et al. and Lu [42,43], these changes are related to microtensions in the structure, and this phenomenon occurs due to the thermal condition to which the material was subjected. Such characteristics are common in aluminum alloys, since they indicate that there are phase formations [44].
Via the analysis of Figure 11, it can be observed that the peaks of Al and Si in the heat-treated samples become more intense, and these intensities are attributed to the intermetallic forms formed from the heat treatment [45]. These observations are also corroborated by the analyses already performed in this study via optical microscopy and SEM.
Figure 11 shows the formation of the peak referring to Mg2Si. We note that this can only be formed via heat treatment, and the intensity of the peaks for the samples of 7 and 12 h is similar. These formations allow improvements in the hardness of the materials [46,47]. This property was also seen in the present work, and we emphasize that in the 7 h sample, the peak of 2 Ɵ = 65 º has greater intensity when compared with the 12 h sample.
The intermetallic SiAl was more evident in the treated samples, confirming that diffusion occurred in the thermally treated materials and generated an improvement in mechanical properties [45,48,49]. In the diffractograms of the peaks 2 Ɵ = 77.5 and 78.5 º of the materials that correspond to the displacements of the Al and Sr, in that order, we noted that their intensities decreased with the treatment times used in this study [48,49,50].
3.3. Mechanical Characterization
3.3.1. Hardness Tests
The results shown in Figure 12 reveal the mean values and distribution of hardness values found in samples without heat treatment (0 h), with 12 h of T6 treatment and with 7 h of T6 treatment. A significant increase in this property can be noted due to the thermal treatments performed. For Yildinm and Ozyurek [51], the hardness of Al-Si-Mg alloys, after application of T6 heat treatment, can be 2.5 times higher than the hardness presented by the same material in the as-cast state. According to ASTM B108 (Standard Specification for Aluminum-Alloy Permanent Mold Castings) [52], after T6 heat treatment, a minimum hardness of approximately 37 HRB is recommended for A356 alloy (commercial Al-Si-Mg alloy).
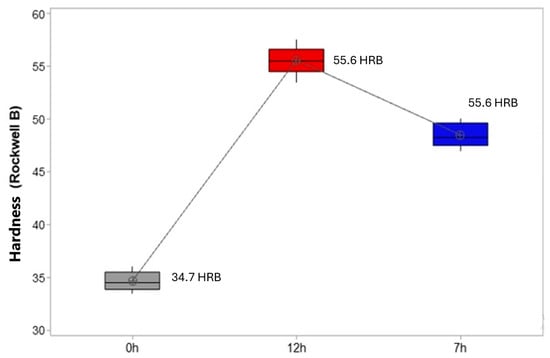
Figure 12.
Boxplot for hardness behavior of A356 alloy according to treatment time.
Through the results found in Table 7, the analysis of variance (ANOVA) performed shows a significant difference in the means of HRB hardness measurements. The p-value found is equal to 0.000, in other words, ≤0.05 (significance level).

Table 7.
Analysis of variance for hardness measurement.
The Tukey test, with 95% confidence, statistically proves the difference in hardness of the samples with treatment and without treatment, as observed in Table 8. When we compared the samples of 0 h and 12 h, the difference between the means was 20.95, and, when we compared the samples of 0 h and 7 h, the difference was 13.85.

Table 8.
Tukey’s simultaneous tests for mean differences for hardness.
The hardness behavior and other mechanical properties of Mg–Si, Mg–Si–Al, and Al–Mg–Si alloys are due to the precipitation and decomposition behavior of a supersaturated solid fine particle solution during the diffusion process that occurs during precipitation heat treatment (T6) [51].
The studies conducted by Abdulwahab [37] and Mørtsell [52] explain the statistically significant difference between the 12 h and 7 h samples of the T6 treatment, as can be seen in Figure 13. As the aging time increases, the hardness increases to that of peak hardness for the alloy, which is characterized by super aging.
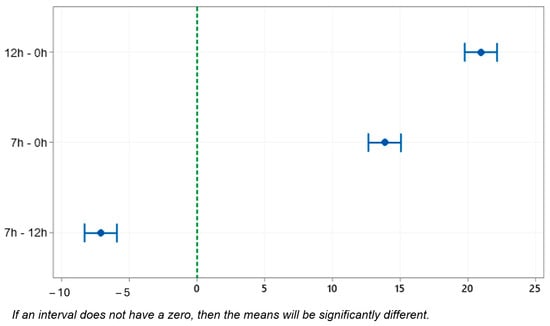
Figure 13.
Tukey test. Mean difference in hardness measurement (HRB); 95% confidence interval.
An important point observed through XRD in this research is the formation of the compound Mg2Si, which allows improvements in hardness, and the 7 h sample had greater hardness compared to the 12 h sample. According to one author [53], the increase in hardness after artificial aging occurs as a result of a microstructure that is refined by heat treatment in a fine dispersion of Mg2Si strengthening phases.
The increased hardness after treatment indicates a spheroidized structure of silicon particles in the aluminum matrix, and that the grain sizes are smaller and consistent with the matrix. Solute atoms form clusters that serve as a barrier against displacement, and, therefore, the greatest hardness was obtained [54,55].
In Figure 14, a correlation between the hardness and dimensional behavior of the eutectic Si can be seen in the samples with the different heat treatment conditions (0 h; 12 h; 7 h) studied.
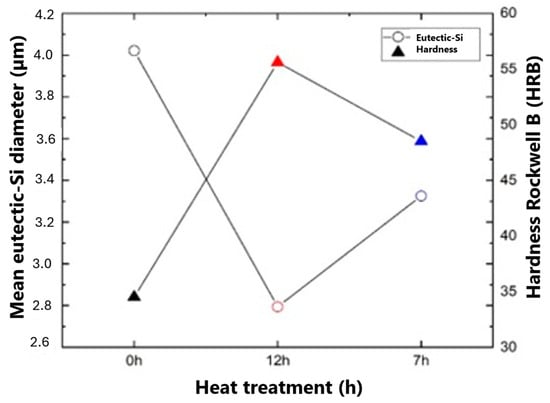
Figure 14.
Correlation between eutectic Si diameter and hardness.
3.3.2. Impact Test
Figure 15 shows the impact strength of the samples according to the absorbed energy (J). The sample without heat treatment T6 (0 h) has the lowest value of energy absorbed in the test, with an average of 4.9 J.
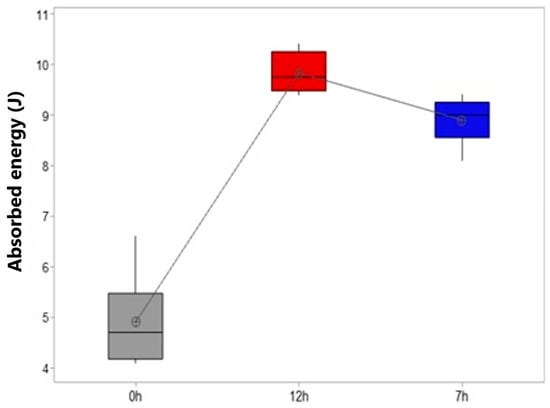
Figure 15.
Boxplot showing the impact resistance of the samples.
When compared with the samples in the different T6 heat treatment conditions, for the 12 h T6 treatment, the mean value was 9.8 J and, for the samples with 7 h of T6 treatment, the absorbed energy value was 8.9 J.
It can be seen that the T6 heat treatment process results in a considerable increase in the total energy absorbed for the two analyzed experiments compared to the sample in the as-cast state (0 h). It was observed that, after heat treatment, there was an increase in impact resistance of approximately 53% when compared with the untreated sample.
Through the results found in Table 9, the analysis of variance (ANOVA) shows a significant difference in the means of impact resistance measurements. The p-value found is equal to 0.000, in other words, a value of ≤ 0.05 (significance level).

Table 9.
Analysis of variance for impact resistance measurement.
The Tukey test was performed at 95% confidence and shows the statistical difference between the samples of the Charpy test, as observed in Table 10. When comparing the samples of 12 h–0 h, i.e., 12 h of T6 treatment and a sample without treatment, the difference between the means was 4.93, and the p-value was 0.000, evidencing a statistically significant difference. Between the samples with 7 h–0 h, the difference between the means was 4.00, and the p-value was 0.000, again demonstrating a statistically significant difference between the means, since the p-value was < significance level (0.05). When we analyzed the means between the samples 7 h–12 h, the difference was -0.93 and the p-value was 0.061, demonstrating that there was no statistically significant difference between the means, because the p-value was greater than the significance level (0.05). In Figure 16, it can be noted that the two experiments, 7 h–12 h, were the only ones in which the confidence interval contained zero.

Table 10.
Tukey’s simultaneous tests for mean differences for Charpy.
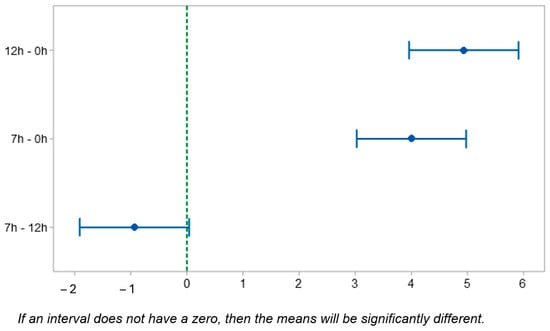
Figure 16.
Tukey test. Difference in means in the Charpy test; 95% confidence interval.
In Figure 17, we can observe a correlation between the behavior of the absorbed energy and the size of the eutectic Si precipitates in the samples with the different heat treatment times (0 h; 12 h; 7 h). It was found that microstructural changes, such as spheroidization of eutectic Si, have an inversely proportional relationship to absorbed energy (J).
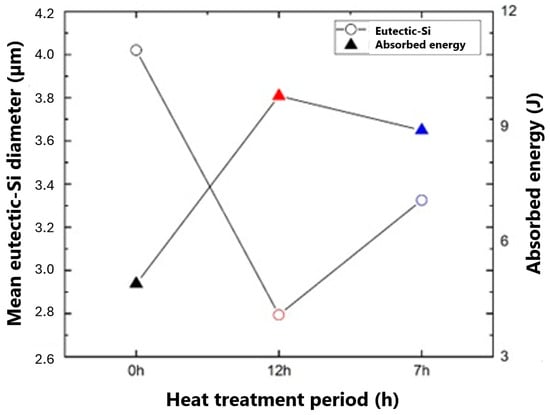
Figure 17.
Correlation between eutectic Si diameter and absorbed energy.
Impact resistance suffers direct influence when eutectic Si particles undergo modification, either by modifying agents or by heat treatments [56,57].
3.3.3. Tensile Test
In order to evaluate the tensile properties of the 12 h, 7 h and 0 h experiments with T6 heat treatment, tensile tests were performed using previously described test parameters, as observed in Figure 18.
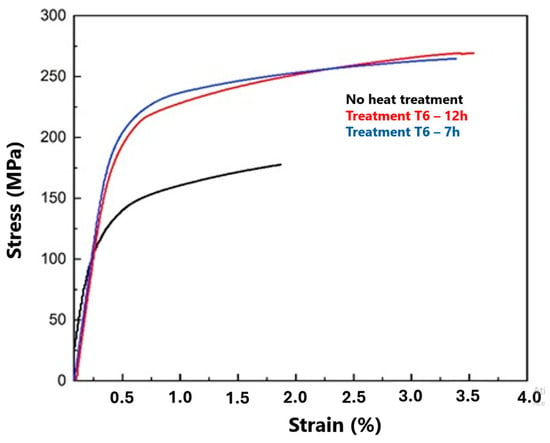
Figure 18.
Engineering stress vs. strain curves for the aluminium studied.
There was an increase in yield strength, tensile strength and elongation properties (%) when compared with the untreated sample, even with different treatment times (12 h and 7 h). The studies by Filizzolab et al. [58], Ram, Chattopadhyay and Chakrabarty [27], and Hu et al. [59] explain that the objective of heat treatments, specifically T6, is to improve the tensile mechanical properties.
In the results found in Table 11, the p-value calculated via the analysis of variance (ANOVA) performed for the three experiments shows a significant difference in the means of the measurements of the evaluated properties. The p-value found is equal to 0.000 for all three conditions.

Table 11.
Tensile test results and p-value (ANOVA).
A comparison was made between the properties evaluated in the tensile test and the behavior in the different conditions of T6 heat treatment performed, and the difference of means was found using the Tukey test.
Figure 19 shows, by means of the Tukey test, the pair-by-pair clustering referring to the mean stress yield of 12 h–0 h and 7 h–0 h. The two experiments were significantly different since the confidence interval did not include zero (Figure 19a).
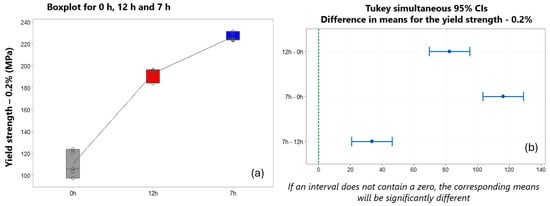
Figure 19.
(a) Behavior of the yield limit in the different treatment conditions. (b) Tukey test for analysis of the means for the yield limit.
This significant difference is also evident in the means of tensile strength or maximum engineering stress for the experiments of 12 h–0 h and 7 h–0 h (Figure 19b). In both cases (yield strength and tensile strength), the difference is due to the effect of the T6 heat treatment on these properties, in which there is a significant increase in stress in the material [60,61,62,63,64]. When comparing the yield stress between the two different T6 treatment conditions, 7 h–12 h, there was a higher yield stress for the 7 h T6 sample (226.58 MPa) compared to the sample yield of the 12 h T6 treatment (192.92 MPa).
Research conducted by Cai et al. [65] and Lu et al. [66] shows that the mechanical properties of yield (0.2%) have a better performance with the short T6 treatment and may have potential in industrial applications. This improvement may be related to the level of Sr, since the 7 h sample presented a higher intensity when compared to the 12 h sample, according to the results found in the XRD.
Figure 20b also shows, through the Tukey test, the comparison of the clustering of the tensile strength means for the 7 h–12 h experiments, in which no significant difference was evidenced, even with the reduction in T6 treatment time, ranging between 269.71 MPa for 12 h and 264.78 MPa for 7 h. Some studies that have been conducted explain a behavior of tensile strength even after the reduction in T6 time, thus showing a possible industrial application [65,66,67,68].
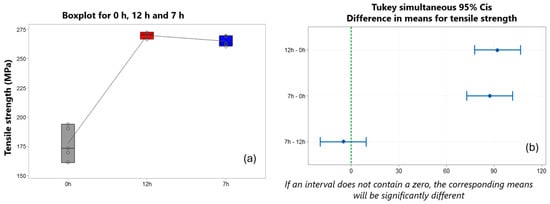
Figure 20.
(a) Behavior of tensile strength under different treatment conditions. (b) Tukey test for analysis of the means for tensile strength.
It is important to note, in Figure 21, that, unlike the studies conducted by Hu et al. [59], the elongation value (%) in the heat-treated samples did not reduce when compared to the untreated sample. There was an increase from 1.86% to 3.46% when comparing the sample without treatment with the sample from the 12 h experiment, and from 1.86% to 3.38% in the comparison of the sample without T6 treatment.
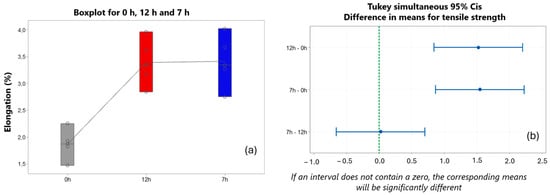
Figure 21.
(a) Elongation behavior (%) for the samples of the different treatment conditions. (b) Tukey test for analysis of elongation means (%).
When comparing the means of the results of the experiments of 7 h–12 h, in Figure 21b, it can be observed that there was no significant difference, and the values were 3.41% for the 7 h sample and 3.38% for the 12 h sample. According to a study conducted by Xu et al. [60], the mechanical elongation property showed better performance with the short T6 treatment. This significant improvement is due to the homogenization of the α-Al dendrite and improved equilibrium levels of eutectic Si, even with a shorter treatment time.
According to a study by Chang, et al. [55], the variation in the measurements of eutectic Si is highly correlated with the improvement in mechanical properties, and it also correlates with the increased level of modification of eutectic Si compared to unmodified alloy. Based on the study, the plotting in Figure 22a–c was carried out, and it relates the variation in the mean size of the eutectic Si to the mechanical properties of tensile strength evaluated in the three experiments.
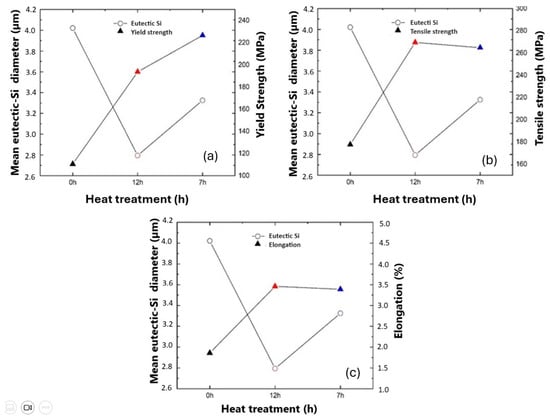
Figure 22.
Mean diameter of eutectic Si as a function of tensile mechanical properties. (a) Eutectic Si X yield (MPa), (b) Eutectic Si X tensile strength (MPa), (c) Eutectic Si X elongation (%).
The yield stress (Figure 22) showed a different behavior in the treatment time of 7 h because, even with a small increase in the diameter of the eutectic Si (3.32 µm), it obtained a tensile strength of 226.58 MPa against eutectic Si of 2.79 µm and tensile strength of 192.92 MPa in the treatment of 12 h. The study conducted by Kang et al. [56] corroborates this increase in yield stress, even with the reduced treatment time. Kang’s study showed that, in one of the experiments, there was an increase in yield stress from 235.2 MPa to 239.92 MPa with a reduction in treatment time by 40%. In Figure 22b,c, an inversely proportional behavior is observed when the mean diameter of the eutectic Si is compared with the properties of tensile strength and elongation (%). The study by Hu [59] showed that the yield strength and tensile strength values remained almost identical for several T6 heat treatment conditions, and that elongation values (%) vary according to the size of the eutectic Si.
Analysis of Fracture Behavior
The fracture surfaces of A356 alloys, after tensile testing, treated with different conditions of T6 (0 h, 12 h, and 7 h), are shown in Figure 23. It is possible to note the presence of microcavities in the fractures, which may contribute to variations during the characterization of the mechanical properties [69,70]. In all the images, large and small undulations are observed, which are characteristic of ductile fracture [71].
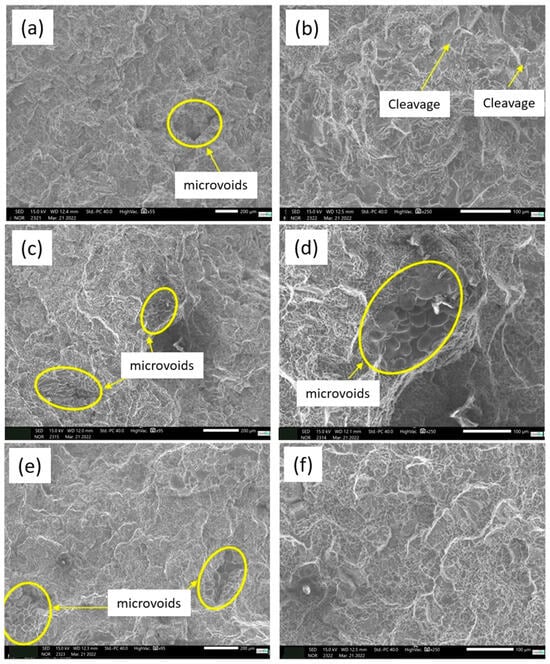
Figure 23.
Fracture behavior of A356 alloy under different treatment conditions, T6. (a,b) No heat treatment—0 h, (c,d) 12 h of T6 heat treatment and (e,f) 7 h of T6 heat treatment.
In Figure 23a,b, which is the sample without heat treatment, the fracture surface shows that the eutectic silicon has an acicular and almost spherical morphology as a result of the modifying agents. It is noted that the fracture surfaces of the A356 are mixed with small cleavage planes and a large number of corrugations [25,72].
In Figure 23c,d, we can see the morphology of the fractures for the two different T6 heat treatment conditions for the 12 h experiment, and in Figure 23e,f, for the 7 h experiment. As shown in Figure 23c,d, (treated for 12 h), the average size of the dimples on the fracture surface of the alloy is smaller when compared with the fracture of the sample treated for 7 h (Figure 23e,f). This is possible due to a greater fragmentation and spheroidization of the eutectic Si in the 12 h treatment [73].
For the two treatment conditions, the fractures show a cone shape, and obvious plastic deformation can be observed on the outer surface of the specimens after tensile fracture. This means that it undergoes a greater amount of plastic deformation before fracture [74]. It is possible to observe a fine and uniform distribution of equiaxial dimples, which means a greater plastic deformation compared to the untreated sample [65,75]. In the two heat treatment conditions performed, the ripples are larger due to the higher state of triaxial stress. The fracture mechanisms found are similar in the two treatment conditions and consist of nucleation, growth and coalescence of cavities around the constituent particles [76].
4. Conclusions
This study evaluated the influence of reducing the T6 heat treatment time from 12 h to 7 h on the Al-7%Si-0.35%Mg (A356) aluminum alloy, applied to structural motorcycle components. The results indicated that reducing the heat treatment time maintained the suitability of the alloy’s mechanical and microstructural properties, enabling a more efficient and sustainable approach in the industry.
The measured properties demonstrated competitive performance for the T6—7 h condition. The yield strength was 226.58 ± 3.76 MPa, while the tensile strength reached 264.78 ± 4.27 MPa, with an elongation of 3.41 ± 0.47%. The hardness showed values above the commercial standard, with statistically significant differences compared to the untreated sample. Impact resistance also benefited from heat treatment, reaching an absorbed energy of 8.9 J for the T6—7 h condition.
Microstructural characterization revealed the spheroidization and refinement of eutectic Si precipitates, with an average diameter of 3.32 µm for the 7 h treatment. X-ray diffraction (XRD) analysis confirmed the formation of the Mg2Si phase only after heat treatment, with similar peak intensities observed for both treatment conditions.
Therefore, considering the efficiency of reducing processing time without significantly compromising mechanical and microstructural properties, applying the T6—7 h treatment is feasible for the industrial sector, providing gains in productivity and reductions in energy costs. For future studies, it is recommended to evaluate fatigue behavior to expand the understanding of the durability of treated components.
Author Contributions
Conceptualization, R.A.R. and J.C.d.M.N.; methodology, J.C.M.d.C., A.C.K. and M.V.F.; software, J.C.M.d.C. and A.C.K.; validation, N.R.d.N., J.S.d.O. and J.S.d.C.; formal analysis, J.C.M.d.C., A.C.K., J.S.d.O. and S.C.P.; investigation, R.A.R., J.C.M.d.C., J.S.d.O., G.G.d.P., J.L.V.R. and M.V.F.; resources, A.C.K. and J.L.V.R.; data curation, N.R.d.N. and M.V.F.; writing—original draft, R.A.R., G.G.d.P. and J.C.d.M.N.; writing—review and editing, R.A.R., J.S.d.C., S.C.P., G.G.d.P. and M.V.F.; visualization, N.R.d.N., J.S.d.O., S.C.P. and J.L.V.R.; supervision, J.S.d.C., S.C.P. and J.C.d.M.N.; project administration, J.C.d.M.N.; funding acquisition, J.L.V.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The original contributions presented in this study. Further inquiries can be directed to the corresponding author.
Acknowledgments
This article was carried out with the technical and financial contribution of the Escuela de Ingeniería Mecánica de la Pontificia Unicersidad Católica de Valparaiso, Chile.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Wijethilake, C. Proactive Sustainability Strategy and Corporate Sustainability Performance: The Mediating Effect of Sustainability Control Systems. J. Environ. Manag. 2017, 196, 569–582. [Google Scholar] [CrossRef] [PubMed]
- Ram, S.C.; Chattopadhyay, K.; Chakrabarty, I. Microstructures and High Temperature Mechanical Properties of A356-Mg2Si Functionally Graded Composites in as-Cast and Artificially Aged (T6) Conditions. J. Alloys Compd. 2019, 805, 454–470. [Google Scholar] [CrossRef]
- Rivera, J.L.V.; Gonçalves, E.; Soares, P.V.; Milito, G.; Perez, J.O.R.; Roque, G.F.P.; Fernández, M.V.; Losada, H.F.; Pereira, F.A.; del Pino, G.G.; et al. The Restored Premolars Biomechanical Behavior: FEM and Experimental Moiré Analyses. Appl. Sci. 2022, 12, 6768. [Google Scholar] [CrossRef]
- Das, S.K.; Green, J.A.S.; Kaufman, J.G. Aluminum recycling: Economic and environmental benefits. Light Metal Age 2010, 68, 22–24. [Google Scholar]
- Dutta, S.; Narala, S.K.R. Experimental Investigation to Study the Effects of Processing Parameters on Developed Novel AM(Al-Mn) Series Alloy. Mater. Manuf. Process. 2020, 35, 1842–1851. [Google Scholar] [CrossRef]
- Gecu, R.; Acar, S.; Kisasoz, A.; Altug GULER, K.; Karaaslan, A. Influence of T6 Heat Treatment on A356 and A380 Aluminium Alloys Manufactured by Thixoforging Combined with Low Superheat Casting. Trans. Nonferrous Met. Soc. China 2018, 28, 385–392. [Google Scholar] [CrossRef]
- De Vasconcelos, J.P.A. Otimização Do Tratamento Térmico T6 Para Ligas de Alumínio A356; University of Porto: Porto, Portugal, 2020. [Google Scholar]
- Rana, R.S.; Purohit, R.; Das, S. Reviews on the Influences of Alloying Elements on the Microstructure and Mechanical Properties of Aluminum Alloys and Aluminum Alloy Composites. Int. J. Sci. Res. Publ. 2012, 2, 1–7. [Google Scholar]
- Taylor, J.A. Iron-Containing Intermetallic Phases in Al-Si Based Casting Alloys. Procedia Mater. Sci. 2012, 1, 19–33. [Google Scholar] [CrossRef]
- Padovano, E.; Badini, C.; Pantarelli, A.; Gili, F.; D’Aiuto, F. A Comparative Study of the Effects of Thermal Treatments on AlSi10Mg Produced by Laser Powder Bed Fusion. J. Alloys Compd. 2020, 831, 154822. [Google Scholar] [CrossRef]
- Shrivastava, V.; Gupta, G.K.; Singh, I.B. Heat Treatment Effect on the Microstructure and Corrosion Behavior of Al-6061 Alloy with Influence of α-Nanoalumina Reinforcement in 3.5% NaCl Solution. J. Alloys Compd. 2019, 775, 628–638. [Google Scholar] [CrossRef]
- Seth, P.P.; Parkash, O.; Kumar, D. Structure and Mechanical Behavior of in Situ Developed Mg 2 Si Phase in Magnesium and Aluminum Alloys—A Review. RSC Adv. 2020, 10, 37327–37345. [Google Scholar] [CrossRef] [PubMed]
- ASTM E1251; Standard Test Method for Analysis of Aluminum and Aluminum Alloys by Spark Atomic Emission Spectrometry. ASTM International: West Conshohocken, PA, USA, 2017.
- Lu, S.; Du, R.; Liu, J.; Chen, L.; Wu, S. A New Fast Heat Treatment Process for Cast A356 Alloy Motorcycle Wheel Hubs. China Foundry 2018, 15, 11–16. [Google Scholar] [CrossRef]
- Jarco, A.; Pezda, J. Effect of Heat Treatment Process and Optimization of Its Parameters on Mechanical Properties and Microstructure of the AlSi11(Fe) Alloy. Materials 2021, 14, 2391. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, R.A. Efeito de Diferentes Condições do Tratamento Térmico T6 Na Microestrurura e Propriedades Mecânicas Da Liga de Aluminio A356 Visanado a Utilização Em Motocicletas; Universidade Federal do Amazonas—UFAM: Manaus, Brazil, 2022. [Google Scholar]
- ASTM B917/B917M-12(2020); Standard Practice for Heat Treatment of Aluminum-Alloy Castings From All Processes. ASTM: West Conshohocken, PA, USA, 2020.
- Xia, X.; Zhao, Q.; Peng, Y.; Zhang, P.; Liu, L.; Ding, J.; Luo, X.; Wang, L.; Huang, L.; Zhang, H.; et al. Precipitation Behavior and Mechanical Performances of A356.2 Alloy Treated by Al–Sr–La Composite Refinement-Modification Agent. J. Alloys Compd. 2020, 818, 153370. [Google Scholar] [CrossRef]
- ASTM E3-11; Standard Guide for Preparation of Metallographic Specimens. ASTM International: West Conshohocken, PA, USA, 2017.
- ISO 6507-1; Metallic Materials—Vickers Hardness Test—Part 1: Test Method. International Organization for Standardization: Geneva, Switzerland, 2018.
- IS Z 2241; Metallic Materials—Tensile Testing—Method of Test at Room Temperature. Japanese Industrial Standards Committee: Tokyo, Japan, 2011.
- DIN EN ISO 7500-1; Metallic Materials—Calibration and Verification of Static Uniaxial Testing Machines—Part 1: Tension/Compression Testing Machines—Calibration and Verification of the Force-Measuring System. International Organization for Standardization: Geneva, Switzerland, 2018.
- IS Z 2242; Method for Charpy Pendulum Impact Test of Metallic Materials. Japanese Industrial Standards Committee: Tokyo, Japan, 2018.
- Fávero, L.P.; Belfiore, P. Manual de Análise de Dados: Estatística e Modelagem Multivariada Com Excel®, SPSS® e Stata®; Elsevier: Rio de Janeiro, Brazil, 2017. [Google Scholar]
- Smithson, C.; Tang, N.; Sammons, S.; Frace, M.; Batra, D.; Li, Y.; Emerson, G.L.; Carroll, D.S.; Upton, C. The Genomes of Three North American Orthopoxviruses. Virus Genes 2017, 53, 21–34. [Google Scholar] [CrossRef]
- Cheng, W.; Liu, C.Y.; Huang, H.F.; Zhang, L.; Zhang, B.; Shi, L. High Strength and Ductility of Al–Si–Mg Alloys Fabricated by Deformation and Heat Treatment. Mater. Charact. 2021, 178, 111278. [Google Scholar] [CrossRef]
- Paray, F.; Gruzleski, J.E. Modification—A Parameter to Consider in the Heat Treatment of Al-Si Alloys. Cast Met. 1992, 5, 187–198. [Google Scholar] [CrossRef]
- El-Sabbagh, A.M.; Samuel, A.M.; Samuel, F.H.; Doty, H.W.; Valtierra, S. Effect of Heat Treatment and Alloying Elements on the Tensile and Impact Properties of A356 Alloy. Mater. Sci. Eng. A 2014, 610, 100–115. [Google Scholar] [CrossRef]
- Carneiro, V.H.; Puga, H.; Meireles, J. Heat Treatment as a Route to Tailor the Yield-Damping Properties in A356 Alloys. Mater. Sci. Eng. A 2018, 729, 1–8. [Google Scholar] [CrossRef]
- Liu, G.; Gao, J.; Che, C.; Lu, Z.; Yi, W.; Zhang, L. Optimization of Casting Means and Heat Treatment Routines for Improving Mechanical and Corrosion Resistance Properties of A356-0.54Sc Casting Alloy. Mater. Today Commun. 2020, 24, 101227. [Google Scholar] [CrossRef]
- Macêdo Neto, J.C.; Miranda, A.G.; Verçosa, L.A.; García del Pino, G.; Rodrigues, R.A.; Nascimento, D.A.; Vieira, A.R.C. Influência do Tratamento Térmico de Normalização na Microestrutura e Propriedades Mecânicas do aço sae 1035 Utilizado em Motocicletas in Engenharia de Materiais e Metalúrgica: Tudo à sua Volta 2, 2nd ed.; Atena Editora: Curitiba, Paraná, Brazil, 2021; Volume 3, pp. 29–39. [Google Scholar] [CrossRef]
- Ibrahim, M.; Abdelaziz, M.; Samuel, A.; Doty, H.; Samuel, F. Spheroidization and Coarsening of Eutectic Si Particles in Al-Si-Based Alloys. Adv. Mater. Sci. Eng. 2021, 2021, 6678280. [Google Scholar] [CrossRef]
- Santos, J. Al-7Si-Mg Semi-Solid Castings–Microstructure and Mechanical Properties; Jönköping University: Jönköping, Sweden, 2018. [Google Scholar]
- Mota, L.N.; Garcia del Pino, G.; Kieling, A.; Mendes, C.S.A.; De Oliveira, J.B.; De Mendonça, K.P. Characterization of 5052 Aluminum Alloy under Different Heat Treatments. Eur. Acad. Res. 2020, 8, 164–178. [Google Scholar] [CrossRef]
- Jiang, B.; Ji, Z.; Hu, M.; Xu, H.; Xu, S. A Novel Modifier on Eutectic Si and Mechanical Properties of Al-Si Alloy. Mater. Lett. 2019, 239, 13–16. [Google Scholar] [CrossRef]
- Mørtsell, E.A.; Qian, F.; Marioara, C.D.; Li, Y. Precipitation in an A356 Foundry Alloy with Cu Additions—A Transmission Electron Microscopy Study. J. Alloys Compd. 2019, 785, 1106–1114. [Google Scholar] [CrossRef]
- Abdulwahab, M.; Madugu, I.A.; Yaro, S.A.; Hassan, S.B.; Popoola, A.P.I. Effects of Multiple-Step Thermal Ageing Treatment on the Hardness Characteristics of A356.0-Type Al–Si–Mg Alloy. Mater. Des. 2011, 32, 1159–1166. [Google Scholar] [CrossRef]
- Gupta, M.K.; Singh, R.; Kumar, S.; Singh, H.; Kumar, N. Microstructure and Mechanical Properties of A356 Aluminum Alloy Processed by Different Casting Techniques. Mater. Today Proc. 2020, 26, 2062–2066. [Google Scholar] [CrossRef]
- Mishra, S.K.; Roy, H.; Lohar, A.K.; Samanta, S.K.; Tiwari, S.; Dutta, K. A Comparative Assessment of Crystallite Size and Lattice Strain in Differently Cast A356 Aluminium Alloy. IOP Conf. Ser. Mater. Sci. Eng. 2015, 75, 012001. [Google Scholar] [CrossRef]
- Society for Experimental Mechanics (US). Handbook of Measurement of Residual Stresses; Lu, J., Ed.; Fairmont Press: Lilburn, GA, USA, 1996. [Google Scholar]
- Ordoñez, S.; Palominos, P.; Martínez, F.; Fernández, H.; Bustos, O.; Lisboa, J. Evolución Microestructural de Una Aleación de Aluminio A356 Con y Sin Reforzamiento. de SiC Sometida a Molienda Mecánica. Matéria (Rio Janeiro) 2018, 23, 12144. [Google Scholar] [CrossRef]
- Seo, Y.S.; Nasir, L.M.M.; Zuhailawati, H.; Anasyida, A.S. Microstructure Evolution of Conventional and Semi-Solid A356 Alloy with Addition of Strontium. Adv. Mater. Res. 2015, 1087, 488–492. [Google Scholar] [CrossRef]
- Tunçay, T.; Bayoğlu, S. The Effect of Iron Content on Microstructure and Mechanical Properties of A356 Cast Alloy. Metall. Mater. Trans. B 2017, 48, 794–804. [Google Scholar] [CrossRef]
- Haghshenas, M.; Zarei-Hanzaki, A.; Fatemi-Varzaneh, S.M. A Study of the Si-Phase Growth Mechanism in Thixocast (A356) Alloy during Hot Deformation. Int. J. Mater. Res. 2008, 99, 208–211. [Google Scholar] [CrossRef]
- Leo, P.; Cerri, E. Silicon Particle Damage in a Thixocast A356 Aluminium Alloy. Metall. Sci. Tecnol. 2003, 21. [Google Scholar]
- Tavitas-Medrano, F.J.; Gruzleski, J.E.; Samuel, F.H.; Valtierra, S.; Doty, H.W. Effect of Mg and Sr-Modification on the Mechanical Properties of 319-Type Aluminum Cast Alloys Subjected to Artificial Aging. Mater. Sci. Eng. A 2008, 480, 356–364. [Google Scholar] [CrossRef]
- AMNE ELAHI, M.; SHABESTARI, S.G. Effect of Various Melt and Heat Treatment Conditions on Impact Toughness of A356 Aluminum Alloy. Trans. Nonferrous Met. Soc. China 2016, 26, 956–965. [Google Scholar] [CrossRef]
- Merlin, M.; Timelli, G.; Bonollo, F.; Garagnani, G.L. Impact Behaviour of A356 Alloy for Low-Pressure Die Casting Automotive Wheels. J. Mater. Process. Technol. 2009, 209, 1060–1073. [Google Scholar] [CrossRef]
- Asghar, G.; Peng, L.; Fu, P.; Yuan, L.; Liu, Y. Role of Mg2Si Precipitates Size in Determining the Ductility of A357 Cast Alloy. Mater. Des. 2020, 186, 108280. [Google Scholar] [CrossRef]
- Azimi, H.; Nourouzi, S.; Jamaati, R. Materials Science & Engineering A Effects of Ti Particles and T6 Heat Treatment on the Microstructure and Mechanical Properties of A356 Alloy Fabricated by Compocasting. Mater. Sci. Eng. A 2021, 818, 141443. [Google Scholar] [CrossRef]
- Akhyar, H.; Iswanto, P.T.; Malau, V. Non Treatment, T4 and T6 on Tensile Strength of Al-5.9cu-1.9mg Alloy Investigated by Variation of Casting Temperature. Mater. Sci. Forum 2018, 929, 56–62. [Google Scholar] [CrossRef]
- So, T.I.; Jung, H.C.; Lee, C.D.; Shin, K.S. Effects of T6-Treatment on the Defect Susceptibility of Tensile Strength to Microporosity Variation in Low Pressure Die-Cast A356 Alloy. Met. Mater. Int. 2015, 21, 842–849. [Google Scholar] [CrossRef]
- Lima, A.M.; García del Pino, G.; Valin Rivera, J.L.; Chong, K.B.; Bezazi, A.; Costa de Macêdo Neto, J.C.; da Silva Valenzuela, M.G.; Dehaini, J.; Valenzuela Díaz, F. Characterization of Polyester Resin Nanocomposite with Curauá, Fibers and Graphene Oxide. Rev. Cienc. Técnicas Agropecu. 2019, 28, 1–10. [Google Scholar] [CrossRef]
- Cai, Q.; Mendis, C.L.; Chang, I.T.H.; Fan, Z. Effect of Short T6 Heat Treatment on the Microstructure and the Mechanical Properties of Newly Developed Die-Cast Al–Si–Mg–Mn Alloys. Mater. Sci. Eng. A 2020, 788, 139610. [Google Scholar] [CrossRef]
- Chang, L.; Ding, Y.; Guo, B.; Ding, J.; Xia, X.; Tang, Y.; Li, C.; Sun, X.; Guo, J.; Song, K.; et al. Modification Mechanism and Tensile Property of Al-9Si-0.4Mg-0.1Cu Alloy. Mater. Charact. 2022, 184, 111693. [Google Scholar] [CrossRef]
- Kang, H.; Jang, H.; Oh, S.; Yoon, P.; Lee, G.; Park, J.; Kim, E.; Choi, Y. Effects of Solution Treatment Temperature and Time on the Porosities and Mechanical Properties of Vacuum Die-Casted and T6 Heat-Treated Al–Si–Mg Alloy. Vacuum 2021, 193, 110536. [Google Scholar] [CrossRef]
- Tocci, M.; Pola, A.; Gelfi, M.; La Vecchia, G.M. Effect of a New High-Pressure Heat Treatment on Additively Manufactured AlSi10Mg Alloy. Metall. Mater. Trans. A 2020, 51, 4799–4811. [Google Scholar] [CrossRef]
- Filizzolab, D.M.; Santosa, T.S.; Mirandaa, A.G.; Costaa, J.C.M.; Nascimentoc, N.R.; Santosb, M.D.; Belloa, R.H.; Pinob, G.G.; Netoa, J.C.M. Annealing Effect on the Microstructure and Mechanical Properties of AA 5182 Aluminum Alloy. Mater. Res. 2021, 24, e20200545. [Google Scholar] [CrossRef]
- Hu, X.; Zhao, Y.; Wang, Q.; Zhang, X.; Li, R.; Zhang, B. Effect of Pouring and Cooling Temperatures on Microstructures and Mechanical Properties of As-Cast and T6 Treated A356 Alloy. China Foundry 2019, 16, 380–385. [Google Scholar] [CrossRef]
- Xu, C.; Xiao, W.; Zheng, R.; Hanada, S.; Yamagata, H.; Ma, C. The Synergic Effects of Sc and Zr on the Microstructure and Mechanical Properties of Al-Si-Mg Alloy. Mater. Des. 2015, 88, 485–492. [Google Scholar] [CrossRef]
- Rashnoo, K.; Jafar, M.; Azadi, M.; Azadi, M. Influences of Reinforcement and Displacement Rate on Microstructure, Mechanical Properties and Fracture Behaviors of Cylinder-Head Aluminum Alloy. Mater. Chem. Phys. 2020, 255, 123441. [Google Scholar] [CrossRef]
- Zhu, M.; Jian, Z.; Yang, G.; Zhou, Y. Effects of T6 Heat Treatment on the Microstructure, Tensile Properties, and Fracture Behavior of the Modified A356 Alloys. Mater. Des. 2012, 36, 243–249. [Google Scholar] [CrossRef]
- Wang, Q.G.; Apelian, D.; Lados, D.A. Fatigue behavior of A356-T6 aluminum cast alloys. Part I: Effect of casting defects. J. Light Met. 2001, 1, 73–84. [Google Scholar] [CrossRef]
- Shivkumar, S.; Keller, C.; Apelian, D. Porosity in aluminum castings: A review. Int. Mater. Rev. 1990, 35, 245–264. [Google Scholar]
- Cáceres, C.H.; Selling, B.I. Casting defects and the tensile properties of an Al–Si–Mg alloy. Mater. Sci. Eng. A 1996, 220, 109–116. [Google Scholar] [CrossRef]
- Nogita, K.; Dahle, A.K. Phase formation in hypoeutectic and eutectic Al–Si alloys. Mater. Sci. Eng. A 2001, A335, 108–116. [Google Scholar]
- Moustafa, M.A.; Samuel, A.M.; Doty, H.W. Effect of solution heat treatment and aging on the microstructure of A356.2 aluminum alloy. J. Mater. Sci. 2002, 37, 4211–4224. [Google Scholar]
- Djurdjevic, M.B.; Sokolowski, J.H. Design of optimal heat treatment properties for 319 aluminum alloy using quality index methodology. J. Mater. Process. Technol. 2004, 148, 268–278. [Google Scholar] [CrossRef]
- Lee, S.; Backerud, L.; Chai, G. Effect of solution treatment on the microstructure and tensile properties of A356 alloys. Metall. Mater. Trans. A 2001, 32, 1931–1941. [Google Scholar]
- Samuel, F.H.; Doty, H.W.; Samuel, A.M. Microstructure-fracture characteristics in A356 alloys. Mater. Charact. 1997, 39, 511–527. [Google Scholar]
- Liu, Z.Y.; Samuel, A.M.; Samuel, F.H.; Doty, H.W.; Valtierra, S. Microstructural features and tensile properties of A356 aluminum alloy. J. Mater. Sci. 2003, 38, 455–463. [Google Scholar]
- Srivatsan, T.S.; Sudarshan, T.S.; Lavernia, E.J. The fatigue and fracture behavior of cast aluminum alloy A356. Mater. Sci. Eng. A 1996, A197, 1–9. [Google Scholar]
- Marquis, F.D.S.; Darrieulat, M.; Baroux, B. Effect of microstructure and environment on the fatigue crack initiation of A356 aluminum alloy. Int. J. Fatigue 2003, 25, 109–118. [Google Scholar]
- Wang, Q.G. Microstructural effects on the tensile and fracture behavior of aluminum casting alloys. Mater. Sci. Eng. A 2003, A356, 107–116. [Google Scholar] [CrossRef]
- Górka, J.; Stawarz, M.; Głowacka, M.; Kowalczyk-Gajewska, K. Fracture toughness and fatigue behavior of A356 aluminum alloy after different heat treatment cycles. Metals 2020, 10, 1354. [Google Scholar] [CrossRef]
- Rao, A.G.; Reddy, G.M.; Rao, K.P. Effect of aging treatment on microstructure and mechanical properties of A356 aluminum alloy. Trans. Indian Inst. Met. 2009, 62, 469–473. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).