Effect of Hydrogen and Hydrogen-Blended Natural Gas on Additive-Manufactured 316L Stainless Steel in Ambient Oil and Gas Environments
Abstract
1. Introduction
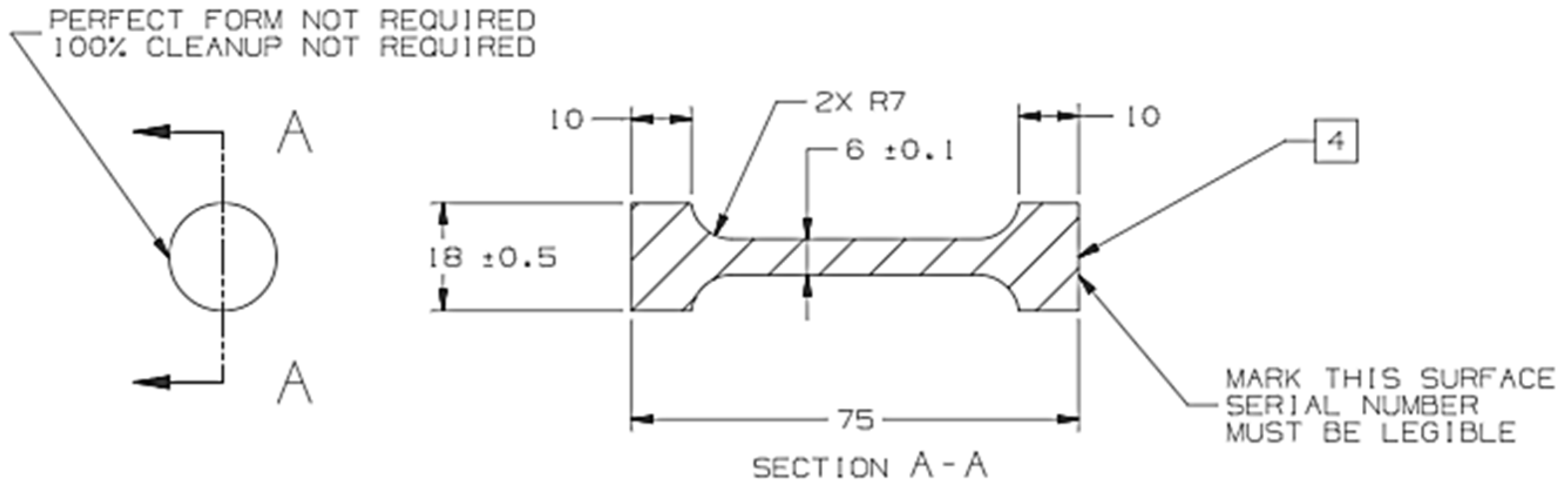
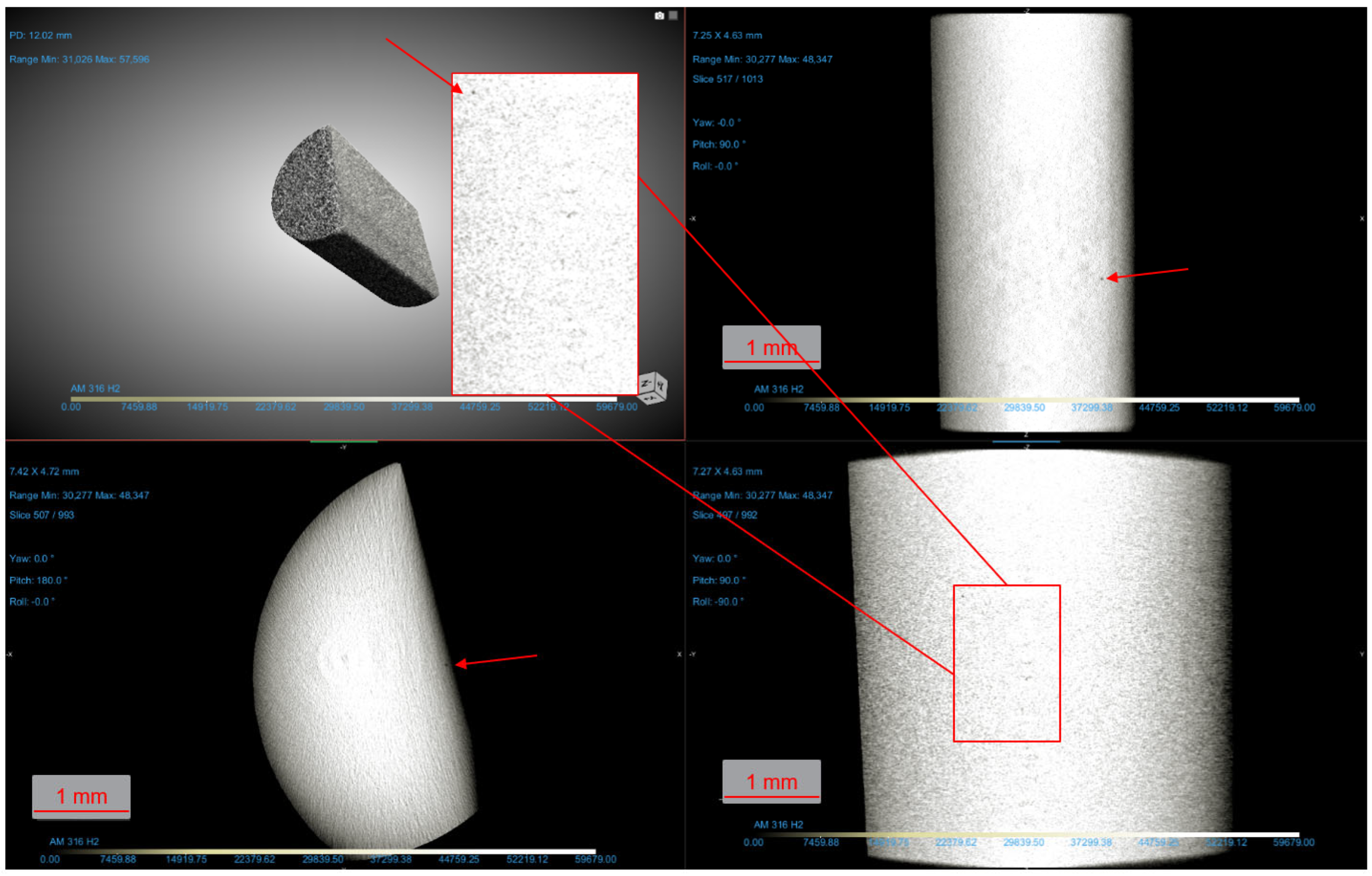
2. Materials and Methods
3. Results and Discussions
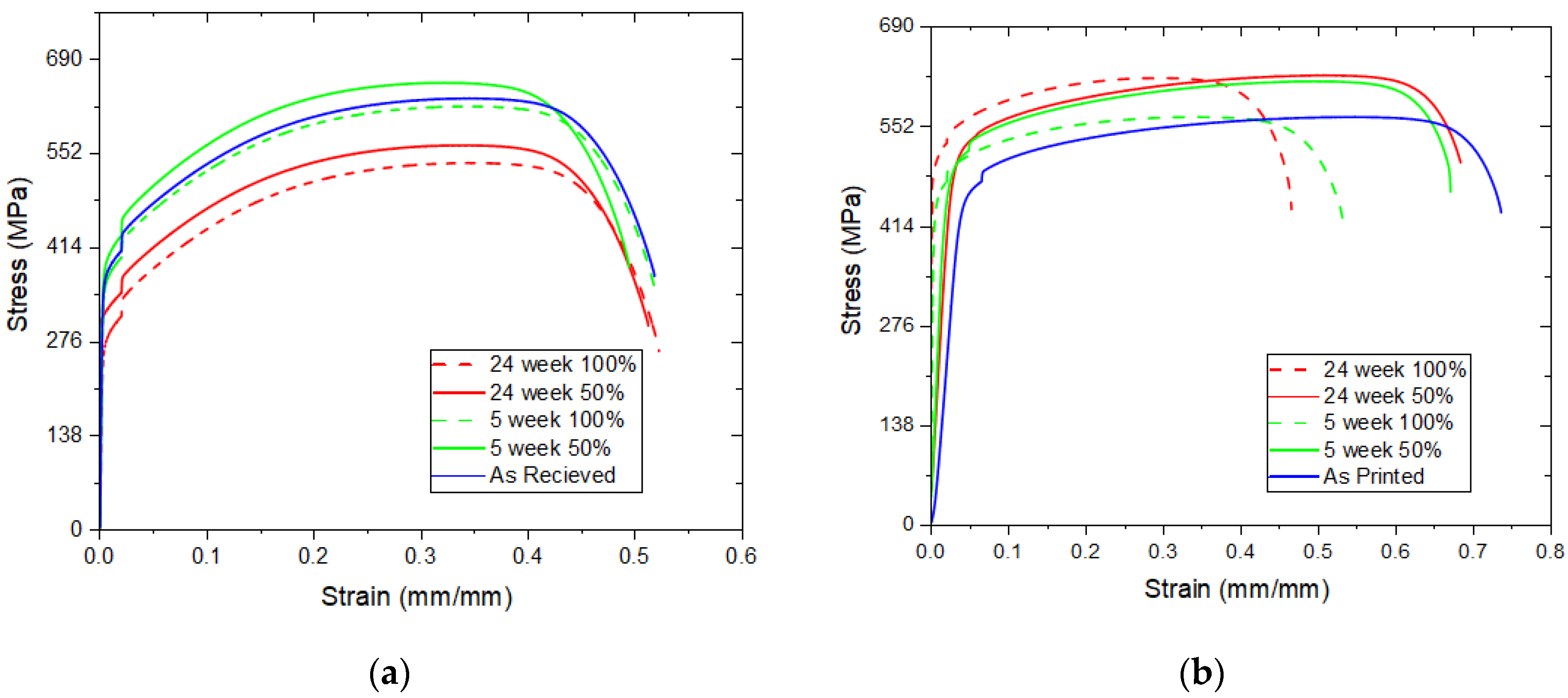
3.1. Mechanical Response
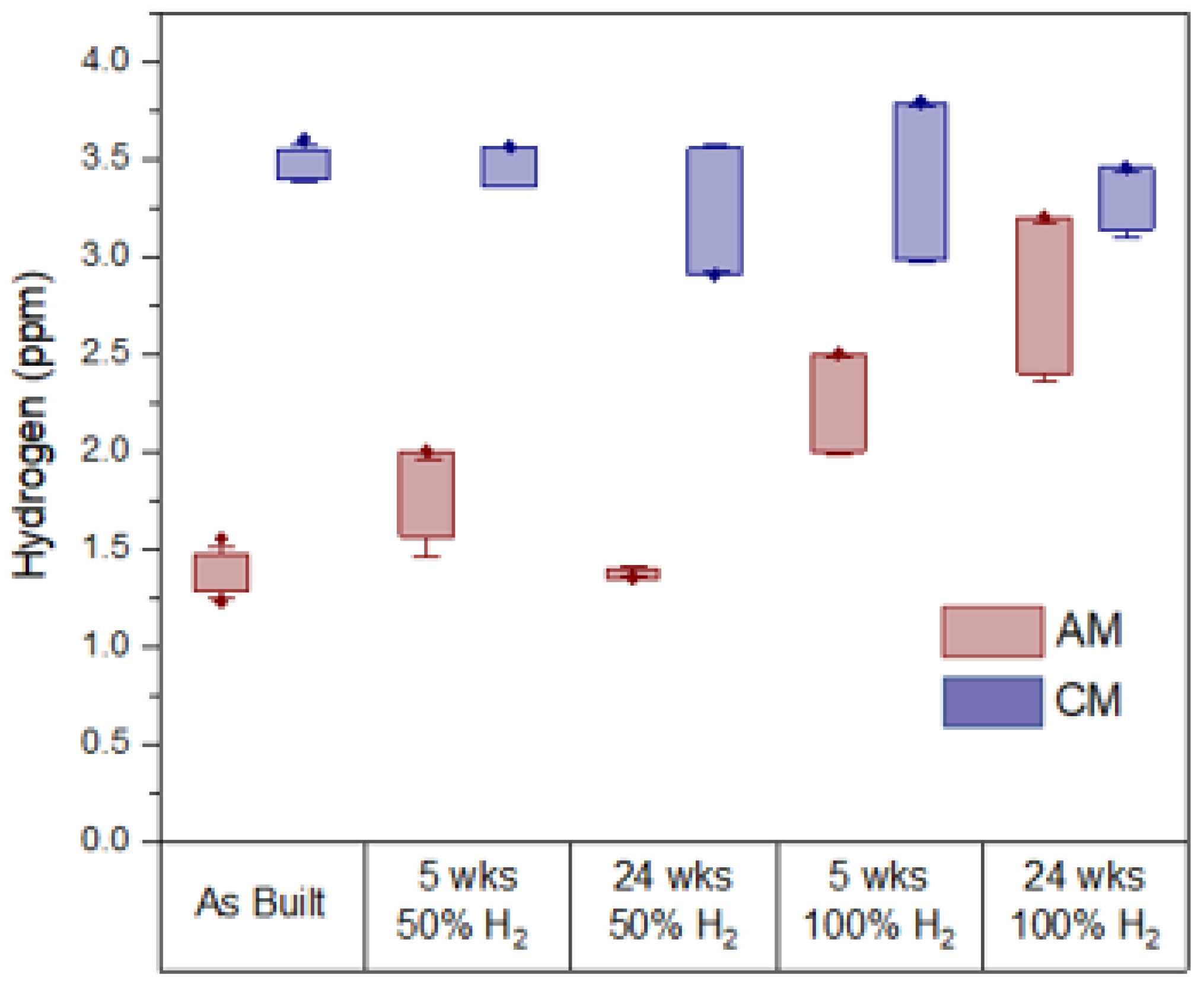
3.2. Chemical Analysis
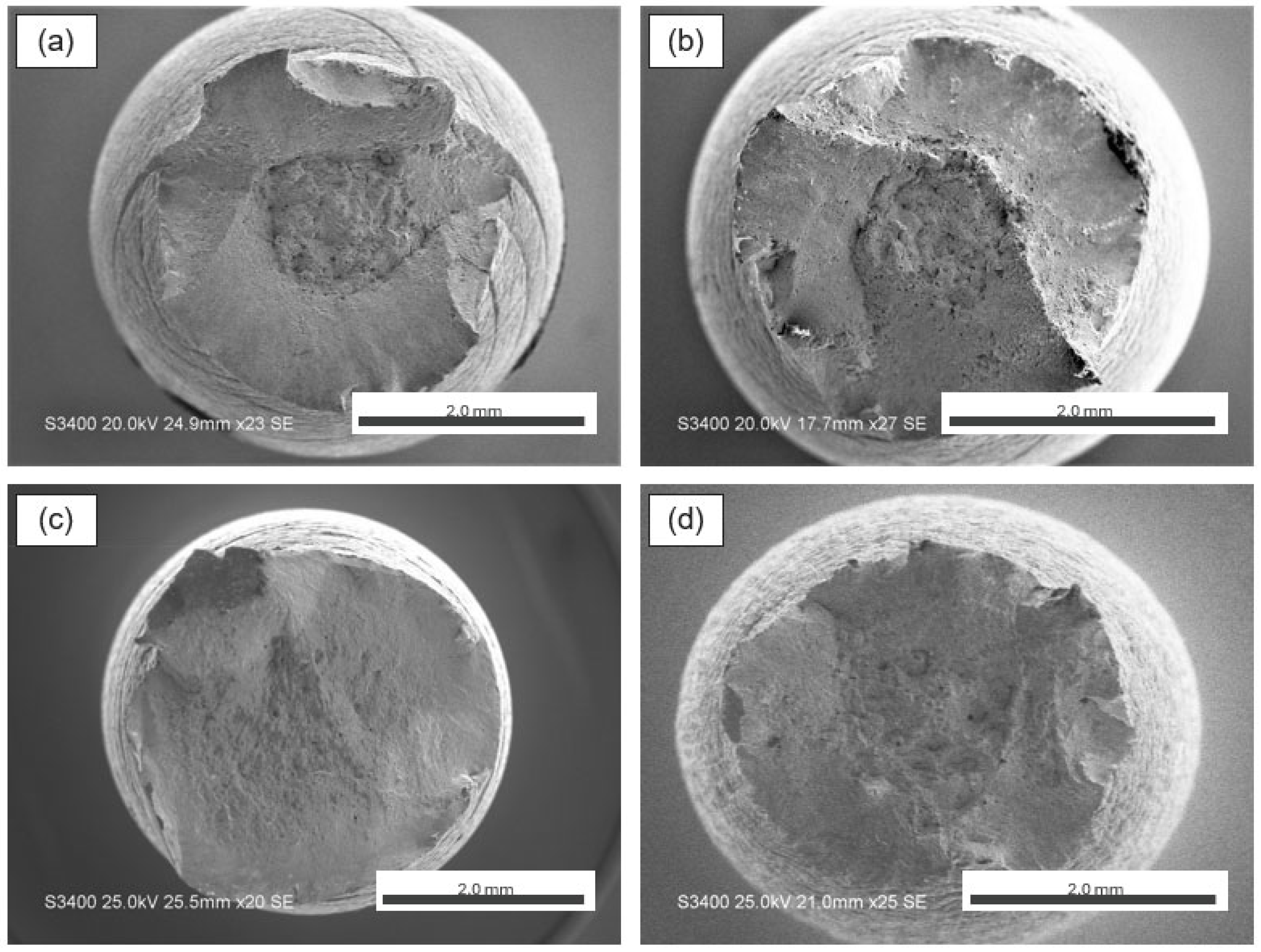
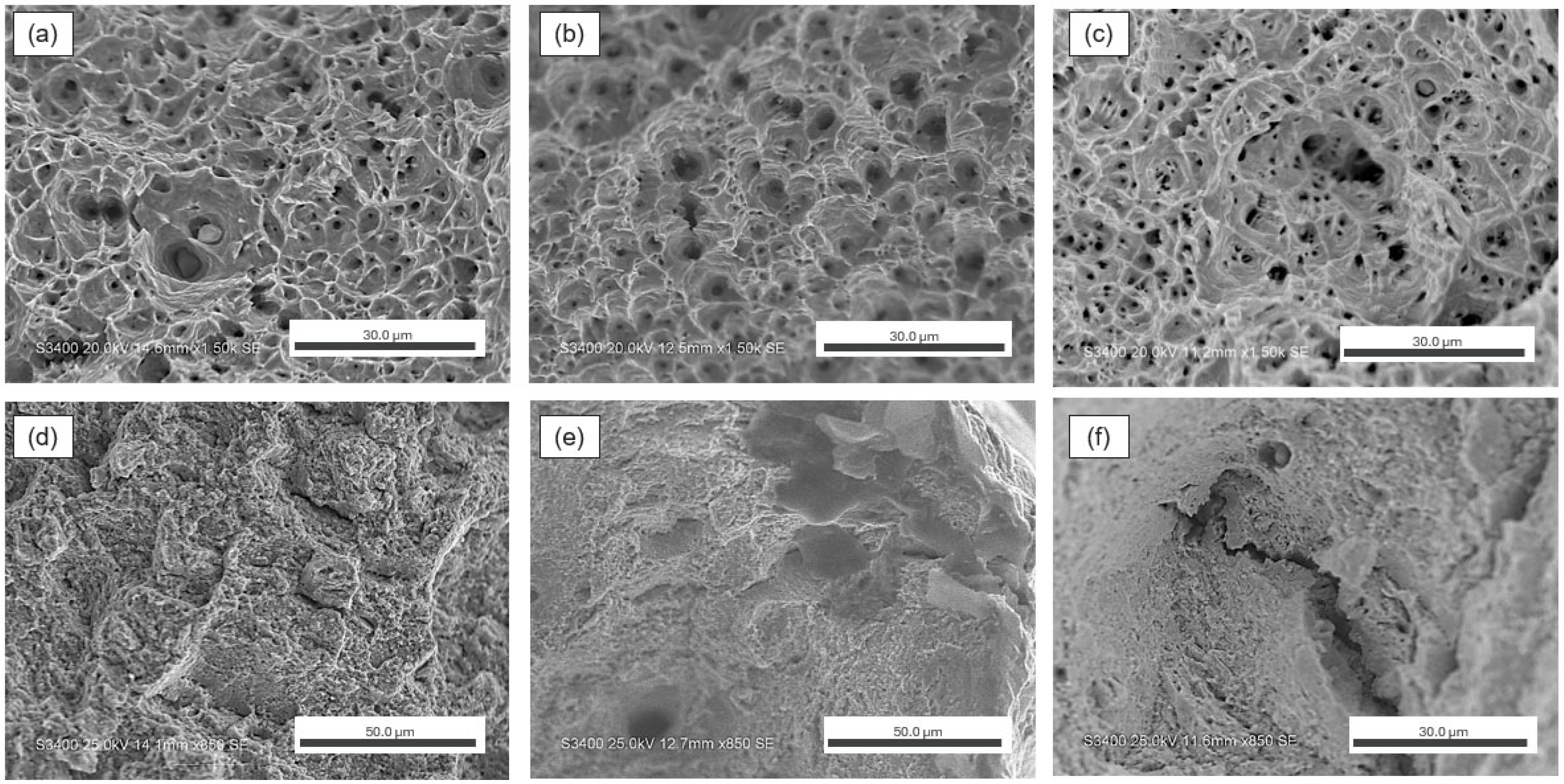
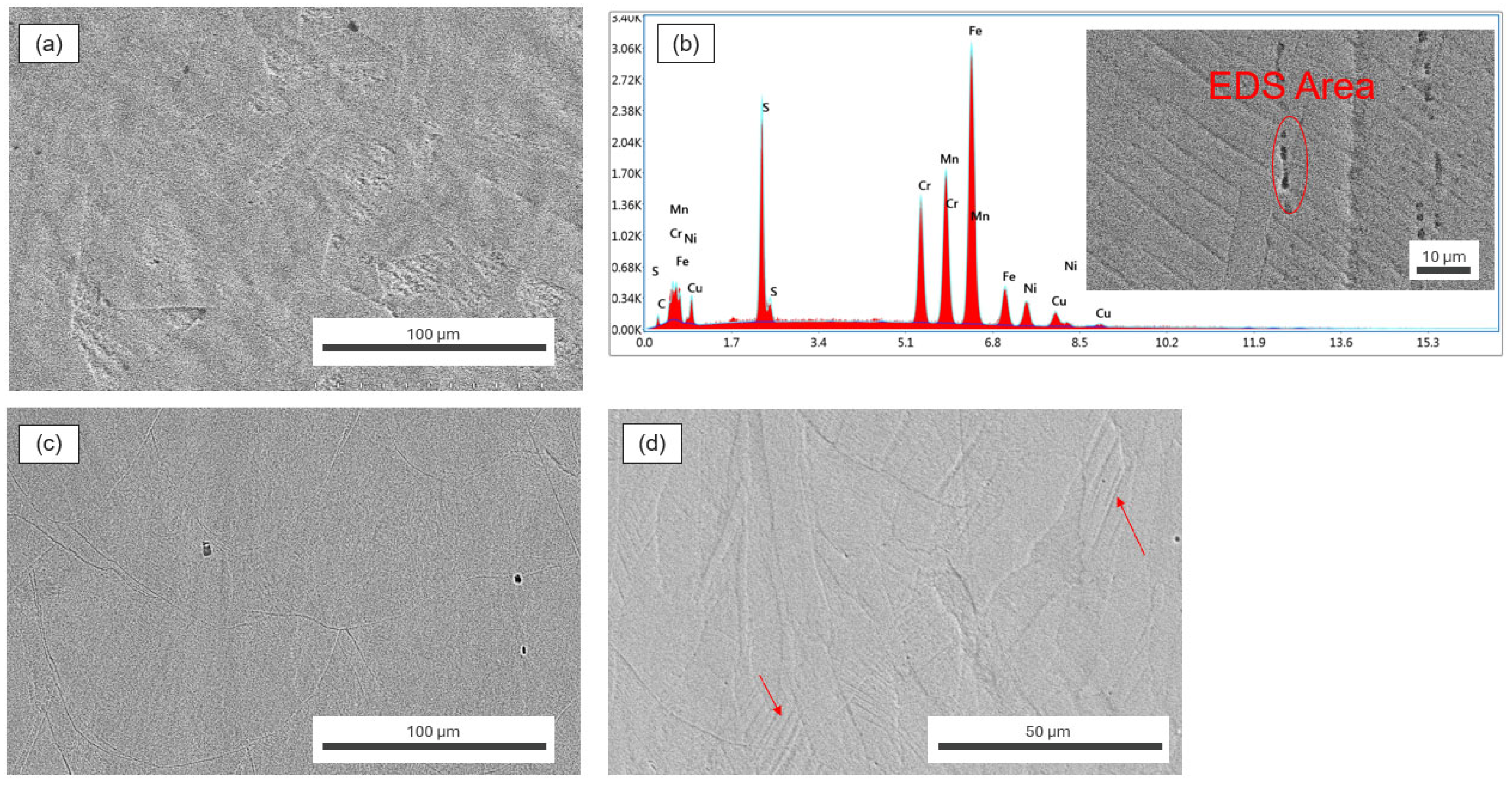
3.3. Fractography and Microstructure Analyses
4. Conclusions
- CM 316L did not show any changes in hydrogen ppm in all conditions, but consistently showed a higher hydrogen content than AM 316L due to MnS inclusions.
- AM 316L showed no changes in 50% hydrogen, likely due to an extremely low dissociation rate and the shielding effect of the natural gas.
- AM 316L experienced HE in 100% hydrogen due to a significant number of micro-voids from the AM processing conditions.
- CM 316L showed no changes in mechanical properties, but AM 316L showed a reduction of 20% ductility and an increase of about 15% yield stress for the 100% hydrogen condition.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HE | Hydrogen Embrittlement |
| LBPF | Laser-Bed Powder Fusion |
| AM | Additive-Manufactured |
| CM | Conventionally Manufactured |
References
- Carey, J. The other benefit of electric vehicles. Proc. Natl. Acad. Sci. USA 2023, 120, e2220923120. [Google Scholar] [CrossRef]
- Drennen, T.E.; Erickson, J.D.; Chapman, D. Solar power and climate change policy in developing countries. Energy Policy 1996, 24, 9–16. [Google Scholar] [CrossRef]
- Siqueira, D.S.; de Almeida Meystre, J.; Hilário, M.Q.; Rocha, D.H.D.; Menon, G.J.; da Silva, R.J. Current perspectives on nuclear energy as a global climate change mitigation option. Mitig. Adapt. Strat. Glob. Change. 2019, 24, 749–777. [Google Scholar] [CrossRef]
- Susskind, L.; Chun, J.; Gant, A.; Hodgkins, C.; Cohen, J.; Lohmar, S. Sources of opposition to renewable energy projects in the United States. Energy Policy 2022, 165, 112922. [Google Scholar] [CrossRef]
- Baron, J.; Herzog, S. Public opinion on nuclear energy and nuclear weapons: The attitudinal nexus in the United States. Energy Res. Soc. Sci. 2020, 68, 101567. [Google Scholar] [CrossRef]
- Road map to a US hydrogen economy. Fuel Cells Bull. 2020, 2020, 12. [CrossRef]
- Ekhtiari, A.; Flynn, D.; Syron, E. Green Hydrogen Blends with Natural Gas and Its Impact on the Gas Network. Hydrogen 2022, 3, 402–417. [Google Scholar] [CrossRef]
- Raju, A.; Martinez-Morales, A. Hydrogen Blending Impacts Study; California Public Utilities Commission: Riverside, CA, USA, 2022. [Google Scholar]
- Kappes, M.A.; Perez, T.E. Blending hydrogen in existing natural gas pipelines: Integrity consequences from a fitness for service perspective. J. Pipeline Sci. Eng. 2023, 3, 100141. [Google Scholar] [CrossRef]
- Cristello, J.B.; Yang, J.M.; Hugo, R.; Lee, Y.; Park, S.S. Feasibility analysis of blending hydrogen into natural gas networks. Int. J. Hydrogen Energy 2023, 48, 17605–17629. [Google Scholar] [CrossRef]
- Alami, A.H.; Olabi, A.G.; Alashkar, A.; Alasad, S.; Aljaghoub, H.; Rezk, H.; Abdelkareem, M.A. Additive manufacturing in the aerospace and automotive industries: Recent trends and role in achieving sustainable development goals. Ain Shams Eng. J. 2023, 14, 102516. [Google Scholar] [CrossRef]
- Vendra, L. Metal additive manufacturing in the oil and gas industry. In Proceedings of the 2018 International Solid Freeform Fabrication Symposium, Austin, TX, USA, 13–15 August 2018. [Google Scholar]
- Bell, B.; Speese, D. Additive Manufacturing the Fisher Cavitrol Hex Trim—The Cool Parts Show; Technologies, Valves, Actuators & Regulators, 2023. Available online: https://www.emersonautomationexperts.com/2023/valves-actuators-regulators/additive-manufacturing-fisher-cavitrol-hex-trim-cool-parts-show/ (accessed on 16 May 2025).
- Hejazi, D.; Calka, A.; Dunne, D.; Pereloma, E. Effect of gaseous hydrogen charging on the tensile properties of standard and medium Mn X70 pipeline steels. Mater. Sci. Technol. 2016, 32, 675–683. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Heo, H.M.; Park, J.; Nahm, S.H.; Beak, U.B. Stress concentration affecting hydrogen-assisted crack in API X70 pipeline base and weld steel under hydrogen/natural gas mixture. Eng. Fail. Anal. 2021, 122, 105242. [Google Scholar] [CrossRef]
- Martin, M.L.; Connolly, M.J.; DelRio, F.W.; Slifka, A.J. Hydrogen embrittlement in ferritic steels. Appl. Phys. Rev. 2020, 7, 041301. [Google Scholar] [CrossRef]
- Bhadeshia, H.K.D.H. Prevention of Hydrogen Embrittlement in Steels. ISIJ Int. 2016, 56, 24–36. [Google Scholar] [CrossRef]
- Michler, T.; Marchi, C.S.; Naumann, J.; Weber, S.; Martin, M. Hydrogen environment embrittlement of stable austenitic steels. Int. J. Hydrogen Energy 2012, 37, 16231–16246. [Google Scholar] [CrossRef]
- Lee, J.S.; Kim, S.R.; Shin, J.; Yun, K.S.; Kim, C.H.; Je, G.J.; Choi, H.I.; Chen, L. Development of a regulator for high-pressure hydrogen charging and storage system. Int. J. Air-Cond. Refrig. 2024, 32, 10. [Google Scholar] [CrossRef]
- Álvarez, G.; Harris, Z.; Wada, K.; Rodríguez, C.; Martínez-Pañeda, E. Hydrogen embrittlement susceptibility of additively manufactured 316L stainless steel: Influence of post-processing, printing direction, temperature and pre-straining. Addit. Manuf. 2023, 78, 103834. [Google Scholar] [CrossRef]
- Bertsch, K.M.; Nagao, A.; Rankouhi, B.; Kuehl, B.; Thoma, D.J. Hydrogen embrittlement of additively manufactured austenitic stainless steel 316 L. Corros. Sci. 2021, 192, 109790. [Google Scholar] [CrossRef]
- Yao, J.; Tan, Q.; Venezuela, J.; Atrens, A.; Zhang, M.-X. Recent research progress in hydrogen embrittlement of additively manufactured metals—A review. Curr. Opin. Solid. State Mater. Sci. 2023, 27, 101106. [Google Scholar] [CrossRef]
- Metalnikov, P.; Ben-Hamu, G.; Eliezer, D. Hydrogen Trapping in Laser Powder Bed Fusion 316L Stainless Steel. Metals 2022, 12, 1748. [Google Scholar] [CrossRef]
- Khedr, M.; Hamada, A.; Abd-Elaziem, W.; Jaskari, M.; Elsamanty, M.; Kömi, J.; Järvenpää, A. Effects of Wall Thickness Variation on Hydrogen Embrittlement Susceptibility of Additively Manufactured 316L Stainless Steel with Lattice Auxetic Structures. Materials 2023, 16, 2523. [Google Scholar] [CrossRef]
- Zhang, P.; Laleh, M.; Hughes, A.E.; Marceau, R.K.W.; Hilditch, T.; Tan, M.Y. A systematic study on the influence of electrochemical charging conditions on the hydrogen embrittlement behaviour of a pipeline steel. Int. J. Hydrogen Energy 2023, 48, 16501–16516. [Google Scholar] [CrossRef]
- Koren, E.; Hagen, C.M.H.; Wang, D.; Lu, X.; Johnsen, R.; Yamabe, J. Experimental comparison of gaseous and electrochemical hydrogen charging in X65 pipeline steel using the permeation technique. Corros. Sci. 2023, 215, 111025. [Google Scholar] [CrossRef]
- Yang, R.; Schell, C.A.; Rayasam, D.; Groth, K.M. Hydrogen impact on transmission pipeline risk: Probabilistic analysis of failure causes. Reliab. Eng. Syst. Saf. 2025, 257 Pt A, 110825. [Google Scholar] [CrossRef]
- Sofia, D.; Lotrecchiano, N.; Giuliano, A.; Barletta, D.; Poletto, M. Optimization of Number and Location of Sampling Points of an Air Quality Monitoring Network in an Urban Contest. Chem. Eng. Trans. 2019, 74, 277–282. [Google Scholar]
- Shim, D.S.; Lee, H.; Son, Y.; Oh, W.J. Effects of pre- and post-repair heat treatments on microstructure and tensile behaviors of 630 stainless steel repaired by metal additive manufacturing. J. Mater. Res. Technol. 2021, 13, 980–999. [Google Scholar] [CrossRef]
- Lynch, S. Hydrogen embrittlement phenomena and mechanisms. Corros. Rev. 2012, 30, 105–123. [Google Scholar] [CrossRef]
- Babakr, A.; Gamboa, G. Optimizing Additive Manufacturing in the Chemical Industry. Chemical Engineering, 15 March 2024. [Google Scholar]
- Emerson, P.R.M. MR95 Series Industrial Pressure Regulators; Emerson: St. Louis, MO, USA, 2022. [Google Scholar]
- ASTM E8/E8M-22; Test Methods for Tension Testing of Metallic Materials. ASTM International: West Conshohocken, PA, USA, 2022. [CrossRef]
- Hatano, M.; Fujinami, M.; Arai, K.; Fujii, H.; Nagumo, M. Hydrogen embrittlement of austenitic stainless steels revealed by deformation microstructures and strain-induced creation of vacancies. Acta Mater. 2014, 67, 342–353. [Google Scholar] [CrossRef]
- Gamboa, G.; Babakr, A.; Griffin, J.; Young, M. Susceptibility Study of Common Regulator Alloys to Hydrogen Embrittlement. In Proceedings of the AMPP Annual Conference, New Orleans, LA, USA, 3–7 March 2024; Volume 1149. [Google Scholar]
- Lee, J.-Y.; Lee, S.M. Hydrogen trapping phenomena in metals with B.C.C. and F.C.C. crystals structures by the desorption thermal analysis technique. Surf. Coat. Technol. 1986, 28, 301–314. [Google Scholar] [CrossRef]
- Bandyopadhyay, A.; Traxel, K.D.; Lang, M.; Juhasz, M.; Eliaz, N.; Bose, S. Alloy design via additive manufacturing: Advantages, challenges, applications and perspectives. Mater. Today 2022, 52, 207–224. [Google Scholar] [CrossRef]
- Norouzi, E.; Miresmaeili, R.; Shahverdi, H.R.; Askari-Paykani, M.; Vergani, L.M. Hydrogen embrittlement behavior in FeCCrNiBSi TRIP steel. J. Mater. Res. Technol. 2023, 23, 859–868. [Google Scholar] [CrossRef]
- Gamboa, G.; Babakr, A.; Young, M. Effect of Hydrogen-Blended Natural Gas on Additive Manufactured 316L Stainless Steel in Pressure Regulator Environments. In TMS Annual Meeting & Exhibition; Springer: Cham, Switzerland, 2025; pp. 200–210. [Google Scholar] [CrossRef]
- Claeys, L.; Deconinck, L.; Verbeken, K.; Depover, T. Effect of additive manufacturing and subsequent heat and/or surface treatment on the hydrogen embrittlement sensitivity of 316L austenitic stainless steel. Int. J. Hydrogen Energy 2023, 48, 36142–36157. [Google Scholar] [CrossRef]
- Hong, Y.; Zhou, C.; Wagner, S.; Schlabach, S.; Pundt, A.; Zhang, L.; Zheng, J. Strain-induced twins and martensite: Effects on hydrogen embrittlement of selective laser melted (SLM) 316 L stainless steel. Corros. Sci. 2022, 208, 110669. [Google Scholar] [CrossRef]
- Liu, C.; Yang, H.; Wang, C.; Zhang, H.; Ding, R.; Ai, L.; Fan, X.; Zhang, R.; Xu, X.; Ning, Y.; et al. Effects of CH4 and CO on hydrogen embrittlement susceptibility of X80 pipeline steel in hydrogen blended natural gas. Int. J. Hydrogen Energy 2023, 48, 27766–27777. [Google Scholar] [CrossRef]
- Chen, Y.-S.; Huang, C.; Liu, P.-Y.; Yen, H.-W.; Niu, R.; Burr, P.; Moore, K.L.; Martínez-Pañeda, E.; Atrens, A.; Cairney, J.M. Hydrogen trapping and embrittlement in metals—A review. Int. J. Hydrogen Energy 2025, 136, 789–821. [Google Scholar] [CrossRef]
- Lynch, S.P. Hydrogen embrittlement (HE) phenomena and mechanisms. In Stress Corrosion Cracking; Elsevier: Amsterdam, The Netherlands, 2011; pp. 90–130. [Google Scholar] [CrossRef]
- Yu, H.; He, J.; Morin, D.D.; Ortiz, M.; Zhang, Z. A self-consistent void-based rationale for hydrogen embrittlement. Scr. Mater. 2025, 255, 116403. [Google Scholar] [CrossRef]
- Yusuf, S.M.; Gao, N. Influence of energy density on metallurgy and properties in metal additive manufacturing. Mater. Sci. Technol. 2017, 33, 1269–1289. [Google Scholar] [CrossRef]






| Alloy | C | Mn | Si | S | P | Ni | Cr | Mo | Cu | Condition |
|---|---|---|---|---|---|---|---|---|---|---|
| S316L (CM) | 0.020 | 1.72 | 0.43 | 0.026 | 0.03 | 10.1 | 16.9 | 2.03 | 0.580 | Annealed |
| S316L (AM) | 0.004 | 0.63 | 0.47 | 0.020 | 0.01 | 12.9 | 17.9 | 2.16 | 0.004 | Stress-Relieved |
| Pressure | Atmosphere | 10 MPa | |
|---|---|---|---|
| H2 Concentration | 0% (As-Received) | 50% | 100% |
| Test Intervals | N/A | 5 weeks | 5 weeks |
| 24 weeks | 24 weeks | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gamboa, G.; Babakr, A.; Young, M.L. Effect of Hydrogen and Hydrogen-Blended Natural Gas on Additive-Manufactured 316L Stainless Steel in Ambient Oil and Gas Environments. Metals 2025, 15, 689. https://doi.org/10.3390/met15070689
Gamboa G, Babakr A, Young ML. Effect of Hydrogen and Hydrogen-Blended Natural Gas on Additive-Manufactured 316L Stainless Steel in Ambient Oil and Gas Environments. Metals. 2025; 15(7):689. https://doi.org/10.3390/met15070689
Chicago/Turabian StyleGamboa, Gerardo, Ali Babakr, and Marcus L. Young. 2025. "Effect of Hydrogen and Hydrogen-Blended Natural Gas on Additive-Manufactured 316L Stainless Steel in Ambient Oil and Gas Environments" Metals 15, no. 7: 689. https://doi.org/10.3390/met15070689
APA StyleGamboa, G., Babakr, A., & Young, M. L. (2025). Effect of Hydrogen and Hydrogen-Blended Natural Gas on Additive-Manufactured 316L Stainless Steel in Ambient Oil and Gas Environments. Metals, 15(7), 689. https://doi.org/10.3390/met15070689






