Abstract
This study investigates the optimization of an ammonia-based leaching process for the recovery of lithium and cobalt from spent LiCoO2 cathodes, coupled with an energy-efficient ammonia stripping approach. Kinetic analysis revealed that both lithium and cobalt extraction follow pseudo-first-order kinetics, with activation energies of 76.54 kJ/mol and 97.22 kJ/mol, respectively, indicating a chemically controlled process. Optimal leaching conditions were established at 6 M NH3, 1.5 M (NH4)2CO3, liquid-to-solid ratio of 10:1, and 70 °C for 5 h, achieving 82.5% lithium and 96.1% cobalt recovery. The ammonia stripping process was optimized for energy efficiency, with operations at 95–98 °C providing the best balance between rapid NH3 removal and energy consumption. At 98 °C, energy demand was reduced to ~282 kJ/mol, a sevenfold improvement over lower temperature operations. A stepwise separation strategy was developed, involving selective lithium precipitation at pH 10.7–10.8, followed by controlled ammonia stripping to precipitate cobalt at pH 8.8–9.0. This integrated approach offers a promising alternative to conventional acid-based recycling methods, combining high metal recovery with improved energy efficiency and reagent recyclability.
1. Introduction
The rapid expansion of lithium-ion battery (LIB) production, driven by the demand for electric vehicles and renewable energy storage, has intensified concerns regarding the sustainable management of spent LIBs. These batteries contain critical metals such as lithium and cobalt, which are essential for battery performance but are at risk of supply shortages due to limited natural reserves and geopolitical constraints [1,2,3]. Efficient recovery of these metals from end-of-life LIBs is therefore crucial for reducing environmental impact and ensuring a stable supply chain.
Current industrial recycling technologies primarily rely on pyrometallurgical and hydrometallurgical approaches [4,5,6]. Pyrometallurgical methods, such as high-temperature smelting (above 1400 °C), enable the recovery of cobalt and nickel in metallic form while lithium is typically lost in slag [7,8,9,10]. Although effective, these processes require high energy consumption, significant CO2 emissions, and the need for further refining steps to recover lithium. In contrast, hydrometallurgical methods, particularly acid leaching, have gained prominence due to their ability to extract lithium, cobalt, and other valuable metals with high efficiency at relatively lower temperatures [11,12,13,14]. However, acid leaching generates large volumes of sulfate- or chloride-containing waste solutions, which need to be utilized [15,16,17].
As an alternative to acid leaching, ammoniacal leaching has been explored as a selective hydrometallurgical approach that forms thermodynamically stable metal–ammonia complexes, particularly with cobalt, while limiting the dissolution of impurities such as aluminum, manganese, and iron [18,19,20,21].
Several studies have demonstrated its efficiency and selectivity. Qi et al. [22] achieved 91.16% Co and 97.57% Li recovery using an ammonia–ammonium bicarbonate system, identifying interfacial chemical reaction control as the rate-limiting step. Ku et al. [23] used ammonium sulfite as a reductant to enhance Co and Cu leaching while minimizing Mn and Al dissolution. Wu et al. [24] optimized a ternary NH3–(NH4)2SO3–NH4HCO3 system to improve Ni and Cu extraction. Chen et al. [25] showed that thermal pretreatment enhances Co-leaching by inducing phase transformations, while Li et al. [26] accelerated leaching kinetics using high-energy ball milling. Wang et al. [27] revealed that Co3(PO4)2 and Co3O4 layers hinder dissolution and developed a multistage approach with nearly complete metal recovery [28]. Ou et al. [29] demonstrated selective Mn recovery and successful ammonia reuse. Table 1 summarizes representative examples from the literature, highlighting the range of parameters used (leaching system, temperature, liquid-to-solid ratio, and time) and the corresponding extraction efficiencies for target metals.

Table 1.
Representative literature data on ammonia leaching of Li-ion batteries cathodes.
At the same time, challenges remain in optimizing reagent recovery and energy efficiency in ammonia stripping, as conventional distillation methods require high energy input for ammonia recycling. Moreover, most previous studies focused primarily on cobalt recovery and process optimization, while comprehensive kinetic analysis of both cobalt and lithium leaching, as well as systematic evaluation of process parameters (NH3 concentration, temperature, L/S ratio), remains limited in the literature. Unlike previous studies that mainly focused on cobalt recovery or process optimization, this work provides a comprehensive kinetic analysis of both lithium and cobalt leaching from LiCoO2 cathodes in ammonia–carbonate media under varying process conditions. The novelty of this study lies in the combined kinetic analysis of lithium and cobalt leaching from LiCoO2 cathodes in ammonia-based solutions, along with the demonstration of a practical approach for partial ammonia recovery via controlled distillation. This enables reduced reagent loss and supports improved process sustainability compared to once-through reagent use, although further optimization would be required for large-scale industrial implementation. The work applies pseudo-first-order and pseudo-second-order kinetic models to elucidate the rate-limiting steps of metal dissolution, since such models are widely used to distinguish between surface chemical reaction control and diffusion-controlled processes in hydrometallurgical leaching systems [30,31,32,33]. Additionally, a refined ammonia distillation approach is proposed to enhance process sustainability by reducing reagent loss and operational costs.
2. Theoretical Background
The ammonia leaching of LiCoO2 involves complex physicochemical interactions, including lattice degradation and coordination chemistry. Lithium occupies interlayer octahedral sites between CoO2 slabs, while cobalt resides in the layered framework. The ammonia leaching of LiCoO2 involves complex physicochemical interactions, including lattice degradation and coordination chemistry. Lithium occupies interlayer octahedral sites between CoO2 slabs, while cobalt resides in the layered framework. Upon contact with ammoniacal solution, cobalt is released via disruption of Co–O bonds and subsequent complexation in solution. Under the alkaline ammonia conditions applied in this study, cobalt predominantly dissolves as divalent Co2+ ions, forming stable Co(NH3)62+ ammine complexes. The formation of Co3+ species and Co(NH3)63+ complex is not expected in the absence of a dedicated oxidant or reducing agent, as confirmed by recent studies on LiCoO2 leaching in ammoniacal media [26,34]. The dissolution of LiCoO2 can be represented by the following simplified ionic reactions:
LiCoO2 + 2H2O ⇌ Li+ + Co2+ + 4OH−
Cobalt ions in solution form stable hexammine complexes as follows:
Co2+ + 6NH3 ⇌ Co(NH3)62+
This complex is thermodynamically stable in alkaline ammonia solutions (log β6 ≈ 8.0–9.0), facilitating efficient cobalt dissolution at moderate temperatures.
Lithium, on the other hand, does not form stable ammine complexes and remains as Li+ in solution until it reacts with carbonate ions to form lithium carbonate [35,36,37]:
2Li+ + CO32− ⇌ Li2CO3↓
In the present process conditions, lithium ions do not precipitate as lithium carbonate during the leaching stage. Although carbonate ions are present in solution, the pH (~9–9.6) and carbonate concentration are insufficient to induce significant Li2CO3 precipitation during leaching. Therefore, lithium is leached into solution and remains as solvated Li+ ions. Selective precipitation of lithium as Li2CO3 is performed in a separate step after leaching by controlled pH adjustment (pH 10.7–10.8) using NaOH addition. This clarification has been added to the revised manuscript to avoid misunderstanding.
After leaching, ammonia is gradually distilled from solution. As ammonia is removed, the stability of Co(NH3)62+ complex decreases, and cobalt precipitates as CoCO3:
Co(NH3)62+ + CO32− ⇌ CoCO3↓ + 6NH3
This approach enables selective cobalt precipitation and closed-loop ammonia recycling. The volatilized ammonia is recovered in an absorption unit and reused in leaching, improving process sustainability.
Part of lithium may remain associated with residual graphite via intercalation or surface adsorption, making it inaccessible to ammonia leaching without prior oxidation or thermal treatment. This limits the achievable lithium recovery in the present process.
In real black mass, additional elements such as Cu, Ni, and Mn are typically present. Under ammoniacal leaching, Cu remains largely insoluble, while Ni2+ forms stable ammine complexes and can co-leach with Co. Mn dissolution is generally low in the absence of reducing agents. These behaviors should be considered when extending the process to mixed cathode materials.
3. Materials and Methods
3.1. Source and Pretreatment of Spent LIB Cathodes
Spent LIBs were collected from consumer electronics and were first manually disassembled to separate the cathodes from other components. The cathode sheets were then subjected to thermal treatment at 500 °C for 2 h in an inert atmosphere to remove residual organic binders and electrolyte components. After thermal pretreatment, the active cathode material was mechanically detached from the aluminum current collectors [38]. The recovered material was then ground using a planetary ball mill (300 rpm, 2 h) and sieved to obtain a fine powder (<100 µm; corresponding to 150 mesh) for leaching experiments.
3.2. Characterization of Cathode Material
The chemical composition of the cathode material was determined using atomic absorption spectrometry (AAS) (GBC Scientific Equipment Pty Ltd., Keysborough, Australia) following complete acid digestion (HCl/H2O2 mixture, 80 °C, 2 h). The accuracy of AAS measurements was verified by calibration with certified standard solutions, with an analytical error below 3% relative.
The phase composition and crystallinity of the cathode material were analyzed by X-ray diffraction (XRD) using Cu-Kα radiation in the 2θ range of 10°–80°, with a step size of 0.02° and a scan rate of 2°/min. The surface morphology of the cathode material was examined using Quanta 200i 3D (FEI Company, Hillsboro, OR, USA) electron microscopy.
3.3. Ammonia Leaching of Cathode Material
Ammonia leaching experiments were conducted to selectively dissolve lithium and cobalt from pretreated LiCoO2 cathode material. The process took place in a 1 L sealed glass reactor with mechanical stirring (600 rpm) and temperature control at ambient pressure. The leaching solution, comprising aqueous ammonia (NH3·H2O) and ammonium carbonate ((NH4)2CO3), was selected for its selective complexation with transition metals and minimal impurity dissolution. Ammonia concentrations ranged from 4 to 8 mol/L, and ammonium carbonate from 1 to 2 mol/L. The liquid-to-solid ratio was varied (5:1, 10:1, and 15:1 mL/g). Leaching durations of 2–6 h and temperatures between 50 °C and 80 °C were tested to optimize metal dissolution kinetics. The selection of leaching parameters and their levels (NH3 concentration, carbonate concentration, temperature, liquid-to-solid ratio, and leaching time) was based on a combination of relevant literature data (as summarized in Table 1) and preliminary optimization tests conducted in this study to identify suitable ranges for systematic kinetic analysis.
Solution samples were collected at set intervals, filtered, and analyzed for lithium and cobalt concentrations using atomic absorption spectrometry (AAS, GBC Scientific Equipment Pty Ltd., Australia) with appropriate calibration standards. Metal recovery rates were calculated based on the initial metal content in the cathode material (determined by complete digestion) and the concentration of metals in the filtered leachate after leaching.
All leaching experiments were conducted in triplicate under identical conditions. The results presented in this study correspond to the average values obtained from three independent experiments. The standard deviation of the measured extraction efficiencies did not exceed 5% for both lithium and cobalt, ensuring the reproducibility and reliability of the reported data.
3.4. Ammonia Distillation and Recovery of Li and Co
Ammonia distillation was performed on the leachate obtained after the ammonia leaching process to recover lithium and cobalt while enabling ammonia recycling. The distillation process was conducted in a three-necked flask equipped with a reflux condenser, thermometer, and ammonia absorption unit. The flask was placed in an oil bath for precise temperature control, and continuous stirring at 600 rpm was maintained. The leachate was gradually heated, with the distillation temperature varied between 80 °C and 98 °C to study its impact on ammonia recovery and metal precipitation. The distillation process continued until a predetermined volume of the solution (30–60% of the initial volume) was evaporated. The residual ammonia vapors were passed through an ammonia absorption system containing a dilute sulfuric acid solution. Following distillation, the precipitated solids were collected by filtration, washed with deionized water to remove impurities, and dried at 80 °C for 12 h. The recovered solids were subjected to further characterization. The remaining solution, containing residual ammonia, was stored for reuse in the ammonia leaching stage.
4. Results and Discussion
4.1. Characterization of Spent Cathode Sample
The XRD pattern of the spent cathode sample is presented in Figure 1.
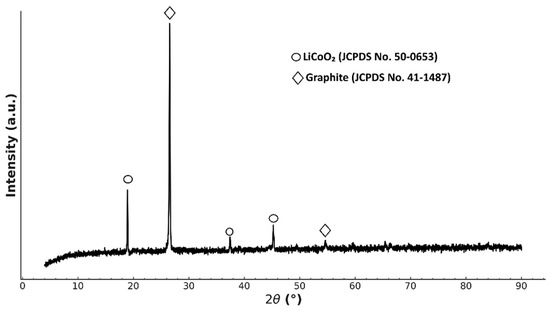
Figure 1.
XRD pattern of cathode material.
The diffraction peaks correspond to layered LiCoO2 (JCPDS No. 50-0653), indicating that the primary cathode material remained structurally intact after battery cycling and pretreatment. In addition, peaks of crystalline graphite, which is part of lithium batteries as an anode material, were detected, which may indicate its transfer or contamination during operation and pre-treatment.
Chemical composition of cathode sample is presented in Table 2.

Table 2.
Chemical composition of the spent cathode material determined by AAS after acid digestion.
The chemical composition analysis shows that cobalt is the predominant component (58.3 wt%), corresponding to the LiCoO2 structure. Lithium content (7.6 wt%) is lower than the theoretical value for stoichiometric LiCoO2, which can be explained by irreversible lithium intercalation into the graphite anode during battery cycling and by the fact that end-of-life batteries are typically not fully discharged, leaving part of the lithium migrated to the anode. Aluminum (0.9 wt%) and copper (0.4 wt%) are present as residuals from current collectors. Minor amounts of nickel and manganese (<50 ppm) detected by AAS in the initial LiCoO2 cathode are likely due to trace impurities from precursor salts.
SEM image of the powder is presented in Figure 2.

Figure 2.
SEM Image of cathode material powder from a LIB.
The SEM image reveals particles of different sizes and morphologies. Small spherical and irregular particles (~0.1–0.3 µm) likely correspond to primary LiCoO2 grains, while larger agglomerates (up to 50 µm) indicate particle clustering. As it was demonstrated earlier, XRD analysis confirmed a content of LiCoO2 with a fraction of graphite. The observed large agglomerates may include graphite phases, while the predominant submicron particles align with LiCoO2.
4.2. Kinetic Analysis of Ammonia Leaching Process
The kinetic behavior of lithium and cobalt extraction during ammonia leaching of LiCoO2 cathode material was analyzed using two widely adopted models: the pseudo-first-order (PFO) and pseudo-second-order (PSO) kinetic models [35,39,40]. The experimental data for lithium and cobalt extraction were fitted to both models to determine the controlling mechanism of the leaching process and evaluate the kinetic parameters, including rate constants and equilibrium extraction capacities.
The PFO model assumes that the rate of occupation of active sites is proportional to the number of unoccupied sites, typically indicating a surface reaction-controlled process. The linearized form of this model is expressed as:
where α is the fraction of target metal recovered at time t, k1 is the pseudo-first-order rate constant.
ln(1 − α) = 1 − k1t
The PSO model, on the other hand, assumes that the rate-limiting step may be related to chemical sorption or chemisorption, involving valence forces through sharing or exchange of electrons. The corresponding equation is given by:
where k2 represents the pseudo-second-order rate constant.
t/α = 1/k2 + t
The first series of experiments were carried out using 6 M NH4OH + 1.5 M (NH4)2CO3 aqueous solution at L:S = 10 mL/g and 70 °C. The corresponding kinetic plots for lithium and cobalt extraction, fitted to the PFO and PSO models, are presented in Figure 3a,b. These plots illustrate the relationship between experimental data and the predicted values from both models, allowing for a visual comparison of the fitting accuracy and evaluation of the reaction kinetics.
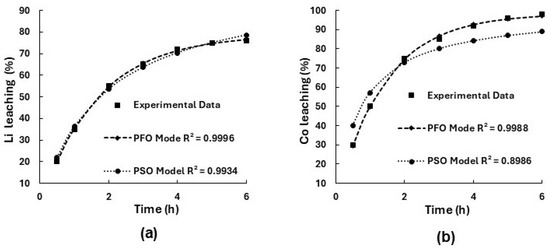
Figure 3.
Kinetic models for Li (a) and Co (b) ammonia extraction from LiCoO2 fitted with pseudo-first-order and pseudo-second-order models, showing experimental data, model predictions, and corresponding R2 values (6 M NH4OH, 1.5 M (NH4)2CO3, L:S = 10 mL/g, 70 °C).
The kinetic analysis demonstrated that lithium and cobalt extractions during ammonia leaching of LiCoO2 follow pseudo-first-order kinetics, indicating a chemically controlled process. For lithium, the pseudo-first-order model provided the best fit with an R2 of 0.9996. Although the pseudo-second-order model showed a high correlation (R2 = 0.9934), it was lower than that of the pseudo-first-order model, confirming reaction control. The maximum lithium recovery achieved was 76% after 6 h of leaching. For cobalt, the pseudo-first-order model also showed superior fitting (R2 = 0.9988). The rapid formation of the stable hexammine cobalt complex (Co(NH3)63+) accounts for the efficient cobalt dissolution (98% cobalt recovery in 6 h of leaching). However, it should be noted that the observed activation energy reflects the rate-determining step of the entire leaching process, and the role of complex formation is to shift equilibrium rather than directly control reaction kinetics.
It is important to note that the PFO and PSO models used in this study are empirical in nature and serve to compare leaching behavior under the tested conditions. The calculated rate constants and apparent reaction orders do not imply mechanistic conclusions. The heterogeneous nature of the system, involving solid–liquid interfaces and possible diffusion or product-layer effects, limits the applicability of simplified kinetic interpretations. Therefore, the observed kinetic fits should be regarded as approximate descriptions rather than definitive indicators of the rate-determining steps.
The next series of experiments was carried out to determine the effect of temperature on the extraction of lithium and cobalt into solution; the corresponding dependences are shown in Figure 4a,b.
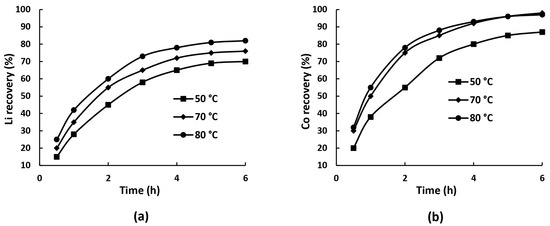
Figure 4.
Dependence of lithium (a) and cobalt (b) recovery on temperature during ammonia leaching (6 M NH4OH, 1.5 M (NH4)2CO3, L:S = 10 mL/g,).
Higher temperatures noticeably accelerated dissolution. Lithium extraction increased from 70% at 50 °C to 76% at 70 °C and 79% at 80 °C, while cobalt extraction rose from 87% at 50 °C to 96% at 70 °C, with little further improvement at 80 °C. At 50 °C, lithium leaching was slow, presumably due to limited Li+ mobility and the formation of surface Li2CO3, which hindered diffusion. At 70 °C, dissolution was significantly faster, likely due to enhanced lithium extraction from the interlayer structure, which weakens the integrity of the LiCoO2 lattice and facilitates cobalt release into solution. At 80 °C, the extraction rate showed only a slight increase. Cobalt followed a similar trend but dissolved more efficiently due to the stability of the Co(NH3)62+ complex. The sharp rise between 50 °C and 70 °C shows a strong temperature dependence, with a minor difference between 70 °C and 80 °C. Overall, 70 °C appears to be the optimal temperature.
To determine the kinetic parameters of lithium and cobalt extraction, the experimental data were fitted to the PFO and PSO models using the previously introduced Equations (5) and (6). The linear dependencies were plotted to evaluate the correlation between experimental results and theoretical models. For the PFO, a plot of (1 − α) vs. t was constructed, where the slope corresponds to −k1. For the PSO, a plot of t/α vs. t was used, with the slope representing 1/k2. By comparing the correlation coefficients (R2) obtained from these linear relationships, the most suitable model for describing the leaching kinetics was identified. The resulting linear plots for lithium and cobalt extraction at 70 °C are presented in Figure 5a,b.
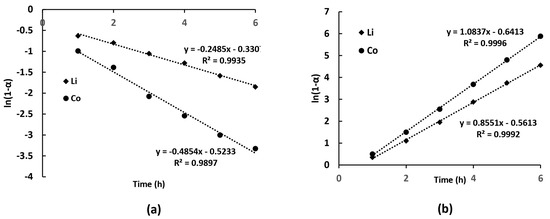
Figure 5.
Linearized plots for PFO (a) and PSO (b) kinetic models describing the leaching process of Li and Co (6 M NH4OH, 1.5 M (NH4)2CO3, L:S = 10 mL/g, 70 °C).
The calculated values of pseudo-first-order rate constant for lithium and cobalt recovery were 0.2485 and 0.4854 h−1, respectively, while the values of pseudo-second-order rate constant were 1.0837 (for Li) and 0.9228 (for Co) h−1, respectively. The kinetic analysis of lithium and cobalt extraction using PFO and PSO models showed excellent agreement with experimental data. It should be emphasized that these models provide only an empirical fit to the observed leaching behavior; due to the coupled processes of dissolution and complexation, they do not allow definitive conclusions regarding the true rate-determining step of the system.
The PFO model indicates that cobalt leaches faster than lithium, as reflected by the more negative ln(1 − α) values for cobalt. This suggests a surface-controlled dissolution mechanism, with calculated rate constants of 0.4854 h−1 for cobalt and 0.2485 h−1 for lithium. The PSO model, based on t/α vs. time, suggests a chemisorption-controlled process, with rate constants of 1.0837 h−1 for lithium and 0.9228 h−1 for cobalt. While both models fit well, PFO provides a better description of cobalt leaching, indicating a reaction-limited process, whereas PSO does not fully capture lithium kinetics, suggesting possible diffusion limitations. The difference between the apparent rate constants in the PFO and PSO models reflects the different aspects captured by these models. The PFO model emphasizes initial dissolution dynamics dominated by surface reactions, where cobalt leaching is faster due to rapid formation of Co(NH3)62+ complexes. The PSO model, which is sensitive to the cumulative effect of slower stages, shows a higher apparent rate constant for lithium, as lithium remains predominantly in solution without complexation limitations. This shows that the models are empirical and should be interpreted with caution in such heterogeneous systems.
The faster leaching of cobalt is attributed to the rapid formation of the Co(NH3)63+ complex. In contrast, lithium does not form stable ammine complexes and part of it may remain trapped in residual solid phases or associated with graphite, limiting its complete extraction.
While this position allows lithium mobility, its release is governed by precipitation of Li2CO3 and possible diffusion limitations, leading to slower leaching under the same conditions. The slowdown in lithium extraction over time may also be related to a gradual decrease in the available surface area of the solid. Possible additional factors include surface deposition of Li2CO3 and lithium retention in residual graphite.
Additionally, it is possible that part of the lithium remains retained in the residual graphite originating from the anode material. SEM and XRD results (Figure 1 and Figure 2) indicate the presence of carbonaceous phases, and lithium may be partially intercalated or adsorbed on graphite surfaces. This may contribute to the lower observed lithium recovery, despite the near-complete dissolution of LiCoO2.
The calculated values of pseudo-first-order rate constants for lithium and cobalt recovery for temperatures of 50 and 80 °C, as well as the previously described constants at 70 °C, are given in Table 3; the corresponding coefficients of determination (R2) are also given there. These k-values were obtained from the kinetic model fitting shown in Figure 5.

Table 3.
Pseudo-first-order rate constants and corresponding coefficients of determination for lithium and cobalt recovery.
To assess the temperature dependence of lithium and cobalt leaching, the activation energy (Ea) was determined using the Arrhenius equation [41]:
where A is the pre-exponential factor, k is the rate constant (h−1), R = 8.314 J/(mol·K), and T is the absolute temperature (K).
lnk = lnA − Ea/(RT)
By plotting lnk vs. 1/T, the activation energy is obtained from the slope −Ea/R.
The corresponding plots are presented in Figure 6.
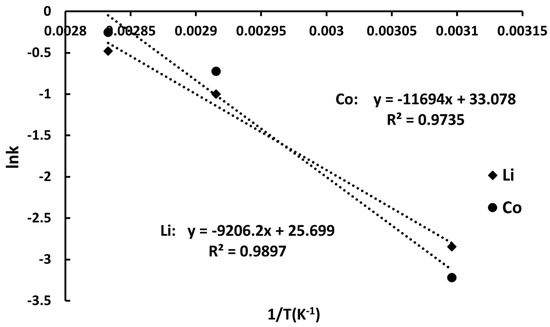
Figure 6.
Arrhenius plot for Li and Co recovery into ammonia solutions (6 M NH4OH, 1.5 M (NH4)2CO3, L:S = 10 mL/g).
The higher activation energy for cobalt (97.22 kJ/mol), compared to lithium (76.54 kJ/mol), suggests a reaction-controlled process for both metals. This difference reflects the distinct structural roles in LiCoO2: lithium is interlayered and relatively mobile, whereas cobalt is embedded within the CoO2 lattice. Cobalt dissolution requires disruption of Co–O bonds followed by the formation of the stable Co(NH3)62+ complex, which governs the kinetics of extraction. These results align with data from the literature for ammoniacal systems, where ligand formation steps can limit reaction rates [42,43]. It should be noted that the applicability of a given kinetic model may vary with process conditions. At low carbonate concentrations, lithium precipitation as Li2CO3 may become rate-limiting, while insufficient NH3 can hinder Co(NH3)62+ complexation. Such shifts in the rate-determining step can lead to deviations from pseudo-first-order kinetics observed under optimized conditions. Moreover, the rate-determining step may shift depending on reagent concentrations and the solid-to-liquid ratio. Under certain conditions, different elementary steps—such as Li+ release (reaction 1), complexation of Co3+ with NH3 (reaction 2), or Li2CO3 precipitation (reaction 3)—can become limiting, thus affecting the overall kinetics.
Our results are consistent with trends reported in the literature. Ku et al. demonstrated that raising the temperature from 30 °C to 70 °C in an NH3–(NH4)2CO3–(NH4)2SO3 system cuts the time required for complete Co dissolution to <30 min [23]. Zheng et al. found that increasing the temperature of reductive ammoniacal leaching of NCM scrap (NH3–(NH4)2SO4–Na2SO3) from 50 °C to 80 °C boosts Ni, Co, and Li extraction to > 98% while shortening the leach time [44]. For LiCoO2, Li et al. reported an increase in Co recovery from ~70% at 25 °C to > 95% at 80 °C and calculated an activation energy of 36 kJ mol−1, indicating chemical-reaction control [45]. Liu et al. observed that mixed NCM cathodes dissolve completely in about one hour at 90 °C, whereas more than six hours are needed at 50 °C [46]. These findings corroborate our own data, which reveal a pronounced acceleration of dissolution as temperature rises.
The observed difference in cobalt and lithium recovery can be explained by their distinct extraction mechanisms. Cobalt is efficiently leached through the formation of stable Co(NH3)62+ complexes, which allows for rapid and nearly complete extraction. In contrast, lithium extraction is partially limited by diffusion constraints within the residual LixCo1−yO2 structure that forms as cobalt is removed. As the layered lattice becomes disrupted, the remaining lithium ions experience reduced mobility, which limits their release into solution under standard leaching conditions. Similar behavior has been reported in previous studies [24,26].
XRD pattern of the solid residue after leaching (Figure 7) shows that the original layered LiCoO2 structure has been largely destroyed, as evidenced by the absence of sharp LiCoO2 reflections. Only a weak and broadened feature near 18.7° remains, corresponding to a residual Li-deficient LixCo1−yO2 phase. The diffraction pattern also displays a prominent graphite peak at ~26°, consistent with the presence of residual graphite from the original cathode material.
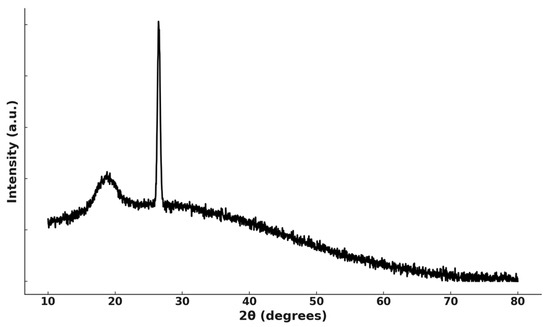
Figure 7.
XRD pattern of solid residue after leaching. The broad peak at ~18.7° is assigned to a residual Li-deficient layered oxide phase, LixCo1−yO2, and the sharp reflection at ~26° corresponds to graphite.
5. Ammonia Leaching Process Optimization
A systematic investigation of the effects of NH3 concentration, (NH4)2CO3 concentration, liquid-to-solid (L:S) ratio, temperature, and leaching time was conducted to identify the optimal leaching conditions for lithium and cobalt extraction.
In previous experiments, it was established that 70 °C and 5 h were the optimal conditions for lithium and cobalt leaching, as extending the leaching time beyond this point provided minimal additional metal recovery. Therefore, the present study focused on the influence of NH3 and (NH4)2CO3 concentrations as well as the liquid-to-solid ratio (L:S) on extraction efficiency.
Ammonia plays a crucial role in cobalt dissolution by forming Co(NH3)63+ complexes, while Ammonium carbonate facilitates the precipitation of lithium as Li2CO3, maintaining a low Li+ concentration in solution and thus promoting further dissolution of LiCoO2. The L:S ratio affects mass transfer and reagent availability, where excessively low values result in incomplete metal extraction due to reagent depletion, whereas very high ratios may cause dilution effects, limiting efficiency.
A limited factorial design was implemented to evaluate the combined effects of NH3 concentration (4–8 mol/L), (NH4)2CO3 concentration (1.0–2.0 mol/L), and L:S ratio (5:1–15:1 mL/g). The results of the experiments are shown in Table 4.

Table 4.
Effect of NH3 and (NH4)2CO3 concentrations and L:S ratio on lithium and cobalt recovery.
The experimental results demonstrate the influence of NH3 and (NH4)2CO3 concentrations and the liquid-to-solid (L:S) ratio on lithium and cobalt extraction efficiencies. Cobalt recovery is strongly influenced by NH3 concentration, increasing from 85.5% at 4 M NH3 to 96.1% at 6 M, with a slight further increase to 96.7% at 8 M, indicating that cobalt extraction approaches saturation above 6 M NH3. Lithium recovery shows greater dependence on (NH4)2CO3 concentration, increasing from 65.4% at 1.0 M to 82.5% at 2.0 M, which confirms the role of carbonate ions in promoting lithium recovery.
The L:S ratio also affects metal recovery. An increase from 5:1 to 10:1 mL/g results in significant improvement in lithium extraction, while further increase to 15:1 yields only marginal benefits, indicating diminishing returns. Among the tested combinations, the condition of 6 M NH3, 1.5 M (NH4)2CO3, and L:S = 10:1 at 70 °C provided the highest lithium and cobalt recoveries with efficient reagent utilization.
As shown in Table 3, increasing the liquid-to-solid (L:S) ratio improves the final recovery of both lithium and cobalt. This outcome is likely due to more favorable dissolution conditions at higher solution volumes, which help shift the equilibrium and reduce the risk of local saturation as the leaching progresses. At the same time, these recovery values reflect the overall process efficiency after several hours of leaching and do not necessarily tell us how fast the reaction proceeds at the initial stage. To gain a better understanding of whether the dissolution kinetics are affected by solution saturation or determined by the surface reaction, we also looked at how the L:S ratio influences the extraction performance in the very beginning of the process. Instead of calculating leaching rates directly, we used the metal recoveries after the first hour as a simple and practical indicator of the initial dissolution behavior, before any significant passivation or product-layer buildup could take place. The results of this analysis are presented in Table 5.

Table 5.
Initial leaching rates of Li and Co at different L:S ratios in 1 h of leaching at 70 °C.
The data show that changes in the L:S ratio (5, 10, 15 mL/g) had no significant impact on the initial dissolution rates of either Li or Co. This finding supports the conclusion that the leaching process is not limited by the bulk concentration of dissolved species.
In addition to the L:S ratio, the availability of ammonia and carbonate may also affect the leaching mechanism by influencing complexation and precipitation equilibria. To verify whether changes in these reagent concentrations could alter the initial dissolution behavior, we examined the lithium and cobalt recoveries after the first hour of leaching under varying ammonia and carbonate levels. The results are summarized in Table 6.

Table 6.
Effect of ammonia and carbonate concentrations on Li and Co recovery after 1 h of leaching (70 °C, L:S = 10:1).
The data show that within the tested concentration range, variations in ammonia and carbonate did not significantly affect the initial recovery of lithium and cobalt. This suggests that the rate-determining step remains stable under these conditions, supporting the applicability of the proposed kinetic model.
6. Lithium and Cobalt Precipitation: Optimization of Ammonia Stripping for Energy Efficiency
After leaching the cathode material under optimal conditions, a productive solution of the following composition was obtained: Li—6.2 g/L, Co—56.0 g/L, pH = 9.6. The above metals can be isolated from the solution by precipitating them in the form of poorly soluble compounds by adjusting the pH.
Pourbaix diagram of the Co–NH3–H2O–CO32− system was constructed to better illustrate the stability domains of cobalt species under the studied leaching conditions (Figure 8). The diagram reflects the combined effects of complexation with ammonia and carbonate as well as hydrolysis equilibria. The stable phases include metallic cobalt (Co0), cobalt hydroxide (Co(OH)2↓), cobalt carbonate (CoCO3↓), and dissolved species Co2+ and Co(NH3)62+. The Co(NH3)62+ complex is stabilized in a moderately alkaline region (pH ≈ 8.2–11.2) due to the high ammonia concentration used in this study (6 M NH3), which is consistent with previous research [47]. At higher pH values, precipitation of Co(OH)2↓ and CoCO3↓ becomes thermodynamically favored, while at lower pH, Co2+ dominates in solution as free ions. Co3+-related species were not included in the diagram, in line with evidence from the literature that Co3+ is not stabilized in ammonia–carbonate systems without a suitable oxidizing agent. The H2 and O2 equilibrium lines are also shown as reference boundaries for water stability.
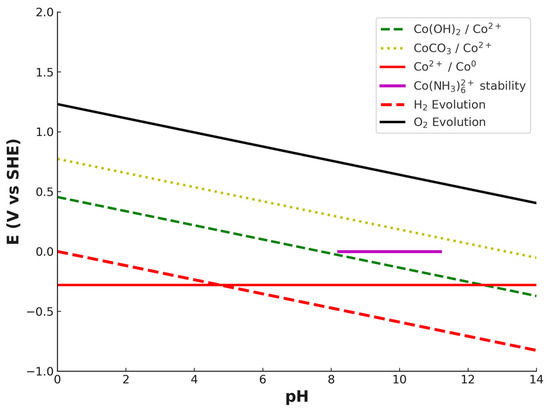
Figure 8.
Pourbaix diagram of the Co–NH3–H2O–CO32− system, illustrating stability domains of solid phases (Co(OH)2↓, CoCO3↓, Co0) and dissolved species (Co2+, Co(NH3)62+) under leaching conditions. The Co(NH3)62+ stability region is limited to pH ≈ 8.2–11.2 due to complexation equilibria at high ammonia concentration.
Lithium does not form stable ammine complexes and remains in solution across a wide pH range [48]. Unlike cobalt, lithium ions exhibit weak interaction with ammonia ligands and are not retained in complex form. Therefore, to achieve selective separation, lithium should be precipitated prior to ammonia stripping. This is accomplished by increasing the pH of the leachate to approximately 10.7–10.8 using sodium hydroxide, which facilitates the formation of lithium carbonate in the presence of carbonate ions. Under these conditions, lithium precipitates as a Li2CO3 as the main phase.
This stepwise separation strategy minimizes co-precipitation and enables efficient and selective recovery of both lithium and cobalt from the ammoniacal leachate. Experimental results confirmed that increasing the pH to 10.7–10.8 with NaOH led to lithium precipitation with a lithium content of 16.4% and an extraction efficiency of 98.5%. The diffraction pattern of the dried precipitate is shown in Figure 9, from which it follows that the crystalline phase of the precipitate consists only of lithium carbonate.
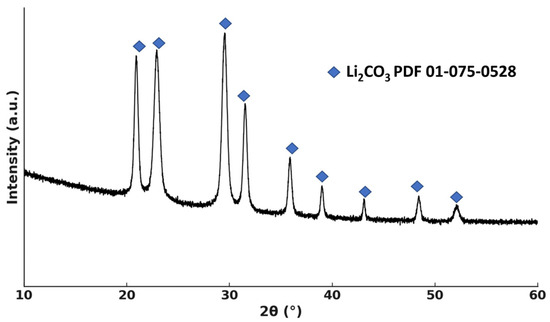
Figure 9.
XRD pattern of Li2CO3 precipitate.
After separation of the lithium solid, ammonia stripping was conducted to gradually reduce the pH to ~9.0, leading to the destabilization of the Co(NH3)62+ complex and subsequent cobalt precipitation as CoCO3.
Ammonia stripping is a crucial step in the recovery process, allowing for the removal of excess NH3 from the pregnant leach solution while enabling its recycling to reduce reagent consumption. The fundamental challenge in ammonia evaporation lies in the diminishing removal rate at lower residual concentrations—as the NH3 concentration decreases, its vapor pressure declines, making further removal increasingly energy-intensive. This effect is exacerbated at lower temperatures, where NH3 remains highly soluble in water.
To achieve efficient ammonia removal with minimal energy input, two key factors must be optimized:
- temperature control—higher temperatures enhance ammonia volatilization but increase energy consumption;
- pH adjustment—since NH3 exists in equilibrium with NH4+, raising the pH shifts the equilibrium towards volatile NH3 gas, facilitating its removal.
In this process, NaOH is added to raise the pH above 10.5 and precipitate Li2CO3. Simultaneously, this pH increase promotes the conversion of NH4+ to free NH3, which is subsequently stripped from the solution. According to a stoichiometry, approximately one mole of NaOH facilitates the release of up to one mole of NH3, suggesting a near-equivalent molar exchange. This indicates that the NH3 recycling stage can partially offset the alkali input required for lithium recovery.
This study aims to determine the optimal combination of temperature and pH for maximizing ammonia removal while minimizing energy requirements.
The series of experiments were conducted to evaluate the effects of temperature and pH on NH3 volatilization. The objective was to establish conditions that facilitate rapid ammonia removal without excessive energy input, ensuring process sustainability and reagent recycling. Since ammonia volatilization is an endothermic process, increasing temperature reduces NH3 solubility and raises its vapor pressure, thereby enhancing its release.
To completely precipitate cobalt as cobalt carbonate from an ammoniacal solution (6 M NH3, 1.5 M ammonium carbonate, NaOH to pH 10.8), ammonia must be evaporated to lower the pH. At pH 10.8, cobalt exists as stable ammine complexes and does not precipitate. Precipitation starts at pH ~9.5 and completes at pH 8.8 [49]. To reach this pH, ammonia concentration must decrease from 6 M to ~1.55 M, requiring the removal of 4.45 moles (75.6 g) of NH3 per liter of solution.
Ammonia stripping was carried out in the temperature range of 80–98 °C. At temperatures below 80 °C, the process of ammonia evaporation is extremely slow, and at temperatures above 98 °C, intense boiling of the solution occurs, which leads to uncontrolled losses of water and ammonia.
Figure 10 shows a diagram of the effect of the solution heating temperature on the time required to achieve pH = 8.8.
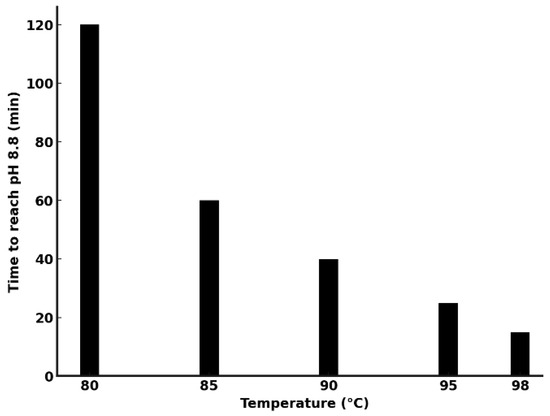
Figure 10.
Time required to reach pH 8.8 in ammoniacal solution during ammonia evaporation at different temperatures.
As the temperature increases, the time required to lower the pH decreases markedly due to enhanced NH3 release. At 80 °C, the process requires 120 min, while at 98 °C, it completes in merely 15 min.
Figure 11 presents an approximate estimation of energy consumption for ammonia removal to achieve pH 8.8 (per mole of NH3). The estimation includes two components: (1) the heat required to raise the solution temperature from 25 °C to the operating value, using the specific heat capacity of water (4.18 kJ/kg·°C) as an approximation, and (2) the energy required for ammonia phase transition, based on the vaporization enthalpy of pure NH3 (23.35 kJ/mol). Heating duration was considered to assess cumulative energy input under batch conditions.
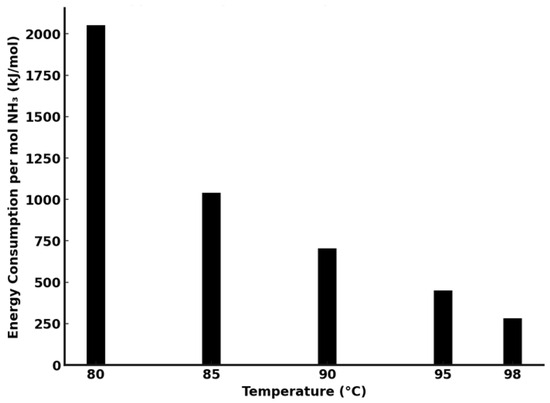
Figure 11.
Energy consumption per mole of NH3 evaporated to reach pH 8.8 in an ammoniacal solution.
It should be noted that these estimations are based on simplified assumptions. The specific heat of water and vaporization enthalpy of pure ammonia do not fully reflect the actual energy involved in removing ammonia from aqueous ammonium solutions, where solvation and equilibrium interactions play a significant role. Literature data suggest that the enthalpy of ammonia evaporation from solution may be in the range of 30–35 kJ/mol. Therefore, the calculated values are intended only for relative comparison of process behavior at different temperatures, rather than precise thermodynamic evaluation.
Despite the simplified nature of the energy model, the estimations provide useful insight into the influence of temperature on ammonia removal efficiency. At 80 °C, limited NH3 release leads to prolonged processing and higher calculated energy demand (~1980 kJ/mol). Increasing the temperature to 90 °C reduces the estimated value to ~700 kJ/mol, and further improvement is observed at 95 °C (~450 kJ/mol). The most favorable conditions are achieved at 98 °C, with a minimum estimated energy input of 282 kJ/mol.
Although 98 °C requires the highest initial temperature input, it enables the fastest ammonia release and shortest process time. As total energy consumption depends on both temperature and duration, the energy input was assessed by integrating heating over time. The reduced stripping time at 98 °C minimizes cumulative thermal losses, resulting in the lowest overall energy demand per mole of NH3. This justifies the conclusion that 98 °C offers the most energy-efficient conditions within the tested range.
The ammonia evaporation method applied here is suitable for industrial-scale processing, particularly in semi-batch or batch operations, where controlled ammonia stripping facilitates cobalt separation and ammonia recovery. As the pH decreases to ~9 during ammonia removal, Co(NH3)62+ complexes destabilize, leading to the selective precipitation of cobalt, predominantly as cobalt carbonate (CoCO3), as predicted by the constructed Pourbaix diagram (Figure 8) and process chemistry. The obtained cobalt precipitate exhibited a characteristic pale-pink color consistent with CoCO3, and chemical analysis showed a cobalt content of approximately 49.5 wt%, in agreement with the theoretical value for cobalt carbonate.
In addition to improving the process sustainability, reducing the energy demand for ammonia stripping provides clear economic benefits. By selecting 98 °C as the optimal evaporation temperature, the energy input required per mole of ammonia recovered is minimized, resulting in lower operational costs for industrial-scale processing.
A schematic representation of the overall leaching and recovery process is provided in Figure 12. The scheme summarizes the main process steps developed in this study, including thermal pretreatment of the cathode material, ammonia leaching, lithium and cobalt precipitation, ammonia evaporation, and ammonia recycling.
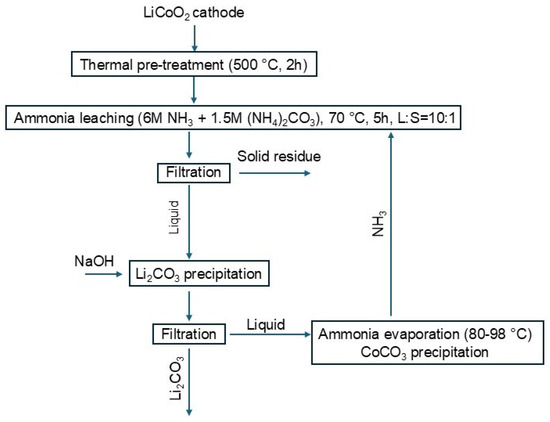
Figure 12.
Schematic flow diagram of the overall ammonia leaching and metal recovery process.
7. Conclusions
This study has provided comprehensive insights into the ammonia leaching process for recovering lithium and cobalt from spent LiCoO2 cathodes, coupled with an optimized ammonia stripping approach. The key findings and implications are as follows:
- Kinetic analysis revealed that both lithium and cobalt extraction follow pseudo-first-order kinetics, indicating a chemically controlled process. The activation energies (76.54 kJ/mol for Li and 97.22 kJ/mol for Co) confirm the reaction-controlled nature of the leaching.
- Optimal leaching conditions were established at 6 M NH3, 1.5 M (NH4)2CO3, L:S ratio of 10:1, and 70 °C for 5 h, achieving 82.5% lithium and 96.1% cobalt recovery.
- The ammonia stripping process was optimized for energy efficiency, with operations at 95–98 °C providing the best balance between rapid NH3 removal and energy consumption. At 98 °C, energy demand was reduced to ~282 kJ/mol, a sevenfold improvement over lower temperature operations.
- A stepwise separation strategy was developed, involving selective lithium precipitation at pH 10.7–10.8, followed by controlled ammonia stripping to precipitate cobalt at pH 8.8–9.0.
Author Contributions
Conceptualization, R.N. and K.K.; methodology, A.B., B.M. and L.M.; investigation, L.M., A.B. and B.M.; resources, R.N.; writing—original draft preparation, K.K., L.M. and A.B.; writing—review and editing, R.N. and L.M.; project administration, R.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Science Committee of the Ministry of Science and Higher Education of the Republic of Kazakhstan (Grant no. AP23489870).
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Fan, E.; Li, L.; Wang, Z.; Lin, J.; Huang, Y.; Yao, Y.; Chen, R.; Wu, F. Sustainable recycling technology for Li-ion batteries and beyond: Challenges and future prospects. Chem. Rev. 2020, 120, 7020–7063. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.; Mu, D.; Lu, Z.; Li, R.; Liu, Z.; Wang, Y.; Tian, S.; Dai, C. A comprehensive review on the recycling of spent lithium-ion batteries: Urgent status and technology advances. J. Clean. Prod. 2022, 340, 130535. [Google Scholar] [CrossRef]
- Natarajan, S.; Aravindan, V. An urgent call to spent LIB recycling: Whys and wherefores for graphite recovery. Adv. Energy Mater. 2020, 10, 2002238. [Google Scholar] [CrossRef]
- Hh Du, M.; Du, K.D.; Guo, J.Z.; Liu, Y.; Aravindan, V.; Yang, J.L.; Zhang, K.-Y.; Gu, Z.-Y.; Wang, X.-T.; Wu, X.L. Direct reuse of oxide scrap from retired lithium-ion batteries: Advanced cathode materials for sodium-ion batteries. Rare Met. 2023, 42, 1603–1613. [Google Scholar] [CrossRef]
- Zheng, S.-H.; Wang, X.-T.; Gu, Z.-Y.; Lü, H.-Y.; Zhang, X.-Y.; Cao, J.-M.; Guo, J.-Z.; Deng, X.-T.; Wu, Z.-T.; Zeng, R.-H.; et al. Direct and rapid regeneration of spent LiFePO4 cathodes via a high-temperature shock strategy. J. Power Sources 2023, 587, 233697. [Google Scholar] [CrossRef]
- Du, M.; Lü, H.; Du, K.; Zheng, S.; Wang, X.; Deng, X.; Zeng, R.; Wu, X. Upcycling the spent graphite/LiCoO2 batteries for high-voltage graphite/LiCoPO4-co-workable dual-ion batteries. Int. J. Miner. Metall. Mater. 2024, 31, 1745–1751. [Google Scholar] [CrossRef]
- Zhou, M.; Li, B.; Li, J.; Xu, Z. Pyrometallurgical technology in the recycling of a spent lithium ion battery: Evolution and the challenge. ACS EST Eng. 2021, 1, 1369–1382. [Google Scholar] [CrossRef]
- Holzer, A.; Windisch-Kern, S.; Ponak, C.; Raupenstrauch, H. A novel pyrometallurgical recycling process for lithium-ion batteries and its application to the recycling of LCO and LFP. Metals 2021, 11, 149. [Google Scholar] [CrossRef]
- Kwon, O.S.; Sohn, I.L. Fundamental thermokinetic study of a sustainable lithium-ion battery pyrometallurgical recycling process. Resour. Conserv. Recycl. 2020, 158, 104809. [Google Scholar] [CrossRef]
- Makuza, B.; Tian, Q.; Guo, X.; Chattopadhyay, K.; Yu, D. Pyrometallurgical options for recycling spent lithium-ion batteries: A comprehensive review. J. Power Sources 2021, 491, 229622. [Google Scholar] [CrossRef]
- Larouche, F.; Tedjar, F.; Amouzegar, K.; Houlachi, G.; Bouchard, P.; Demopoulos, G.P.; Zaghib, K. Progress and status of hydrometallurgical and direct recycling of Li-ion batteries and beyond. Materials 2020, 13, 801. [Google Scholar] [CrossRef] [PubMed]
- Asadi Dalini, E.; Karimi, G.; Zandevakili, S.; Goodarzi, M. A review on environmental, economic and hydrometallurgical processes of recycling spent lithium-ion batteries. Miner. Process. Extr. Metall. Rev. 2021, 42, 451–472. [Google Scholar] [CrossRef]
- Cerrillo-Gonzalez, M.D.M.; Villen-Guzman, M.; Vereda-Alonso, C.; Rodriguez-Maroto, J.M.; Paz-Garcia, J.M. Towards sustainable lithium-ion battery recycling: Advancements in circular hydrometallurgy. Processes 2024, 12, 1485. [Google Scholar] [CrossRef]
- Thompson, D.; Hyde, C.; Hartley, J.M.; Abbott, A.P.; Anderson, P.A.; Harper, G.D. To shred or not to shred: A comparative techno-economic assessment of lithium ion battery hydrometallurgical recycling retaining value and improving circularity in LIB supply chains. Resour. Conserv. Recycl. 2021, 175, 105741. [Google Scholar] [CrossRef]
- Swain, B. Recovery and recycling of lithium: A review. Sep. Purif. Technol. 2017, 172, 388–403. [Google Scholar] [CrossRef]
- Yao, Y.; Zhu, M.; Zhao, Z.; Tong, B.; Fan, Y.; Hua, Z. Hydrometallurgical processes for recycling spent lithium-ion batteries: A critical review. ACS Sustain. Chem. Eng. 2018, 6, 13611–13627. [Google Scholar] [CrossRef]
- Baum, Z.J.; Bird, R.E.; Yu, X.; Ma, J. Lithium-ion battery recycling—Overview of techniques and trends. ACS Energy Lett. 2022, 7, 712–719. [Google Scholar] [CrossRef]
- Saim, A.K. Ammoniacal leaching for the extraction of valuable metals from secondary resources: A review. Miner. Process. Extr. Metall. Rev. 2025, 46, 284–305. [Google Scholar] [CrossRef]
- Zhang, X.M.; Senanayake, G. A review of ammoniacal thiosulfate leaching of gold: An update useful for further research in non-cyanide gold lixiviants. Miner. Process. Extr. Metall. Rev. 2016, 37, 385–411. [Google Scholar] [CrossRef]
- Li, M.; Liu, B.; Zheng, S.; Wang, S.; Du, H.; Dreisinger, D.B.; Zhang, Y. A cleaner vanadium extraction method featuring non-salt roasting and ammonium bicarbonate leaching. J. Clean. Prod. 2017, 149, 206–217. [Google Scholar] [CrossRef]
- Nie, W.; Zhang, R.; He, Z.; Zhou, J.; Wu, M.; Xu, Z.; Chi, R.; Yang, H. Research progress on leaching technology and theory of weathered crust elution-deposited rare earth ore. Hydrometallurgy 2020, 193, 105295. [Google Scholar] [CrossRef]
- Qi, Y.; Meng, F.; Yi, X.; Shu, J.; Chen, M.; Sun, Z.; Sun, S.; Xiu, F.R. A novel and efficient ammonia leaching method for recycling waste lithium ion batteries. J. Clean. Prod. 2020, 251, 119665. [Google Scholar] [CrossRef]
- Ku, H.; Jung, Y.; Jo, M.; Park, S.; Kim, S.; Yang, D.; Rhee, K.; An, E.-M.; Sohn, J.; Kwon, K. Recycling of spent lithium-ion battery cathode materials by ammoniacal leaching. J. Hazard. Mater. 2016, 313, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Li, B.; Yuan, C.; Ni, S.; Li, L. Recycling valuable metals from spent lithium-ion batteries by ammonium sulfite-reduction ammonia leaching. Waste Manag. 2019, 93, 153–161. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, N.; Hu, F.; Ye, L.; Xi, Y.; Yang, S. Thermal treatment and ammoniacal leaching for the recovery of valuable metals from spent lithium-ion batteries. Waste Manag. 2018, 75, 469–476. [Google Scholar] [CrossRef]
- Li, D.; Zhang, B.; Ou, X.; Zhang, J.; Meng, K.; Ji, G.; Li, P.; Xu, J. Ammonia leaching mechanism and kinetics of LiCoO2 material from spent lithium-ion batteries. Chin. Chem. Lett. 2021, 32, 2333–2337. [Google Scholar] [CrossRef]
- Wang, S.; Wang, C.; Lai, F.; Yan, F.; Zhang, Z. Reduction-ammoniacal leaching to recycle lithium, cobalt, and nickel from spent lithium-ion batteries with a hydrothermal method: Effect of reductants and ammonium salts. Waste Manag. 2020, 102, 122–130. [Google Scholar] [CrossRef]
- Wang, C.; Wang, S.; Yan, F.; Zhang, Z.; Shen, X.; Zhang, Z. Recycling of spent lithium-ion batteries: Selective ammonia leaching of valuable metals and simultaneous synthesis of high-purity manganese carbonate. Waste Manag. 2020, 114, 253–262. [Google Scholar] [CrossRef]
- Ou, H.; Zhang, J.; Shen, A.; Chen, Y.; Wang, C. A simplified method for the recycling of spent lithium-ion batteries via manganese selective recovery by anoxic ammonia leaching and spontaneous precipitation. J. Power Sources 2024, 590, 233799. [Google Scholar] [CrossRef]
- Daminescu, D.; Duteanu, N.; Ciopec, M.; Negrea, A.; Negrea, P.; Nemeş, N.S.; Pascu, B.; Lazău, R.; Berbecea, A. Kinetic Modelling the Solid–Liquid Extraction Process of Scandium from Red Mud: Influence of Acid Composition, Contact Time and Temperature. Materials 2023, 16, 6998. [Google Scholar] [CrossRef]
- Lie, J.; Tanda, S.; Liu, J.C. Subcritical water extraction of valuable metals from spent lithium-ion batteries. Molecules 2020, 25, 2166. [Google Scholar] [CrossRef]
- Lie, J.; Liu, J.C. Closed-vessel microwave leaching of valuable metals from spent lithium-ion batteries (LIBs) using dual-function leaching agent: Ascorbic acid. Sep. Purif. Technol. 2021, 266, 118458. [Google Scholar] [CrossRef]
- Yin, K.; Chan, W.P.; Dou, X.; Lisak, G.; Chang, V.W.C. Kinetics and modeling of trace metal leaching from bottom ashes dominated by diffusion or advection. Sci. Total Environ. 2020, 719, 137203. [Google Scholar] [CrossRef]
- Liu, C.; Liu, P.; Xu, H.; Zeng, G.; Luo, X.; Wang, Z.; Yang, L.; Deng, C.; He, J. Oriented conversion of spent LiCoO2-lithium battery cathode materials to high-value products via thermochemical reduction with common ammonium oxalate. Resour. Conserv. Recycl. 2023, 190, 106782. [Google Scholar] [CrossRef]
- Liu, H.; Azimi, G. Process analysis and study of factors affecting the lithium carbonate crystallization from sulfate media during lithium extraction. Hydrometallurgy 2021, 199, 105532. [Google Scholar] [CrossRef]
- Xu, Z.G.; Sun, S.Y. Preparation of battery-grade lithium carbonate with lithium-containing desorption solution. Metals 2021, 11, 1490. [Google Scholar] [CrossRef]
- Marcinov, V.; Klimko, J.; Takáčová, Z.; Pirošková, J.; Miškufová, A.; Sommerfeld, M.; Dertmann, C.; Friedrich, B.; Oráč, D. Lithium production and recovery methods: Overview of lithium losses. Metals 2023, 13, 1213. [Google Scholar] [CrossRef]
- Stallmeister, C.; Friedrich, B. Holistic investigation of the inert thermal treatment of industrially shredded NMC 622 lithium-ion batteries and its influence on selective lithium recovery by water leaching. Metals 2023, 13, 2000. [Google Scholar] [CrossRef]
- Becci, A.; Amato, A.; Rodríguez Maroto, J.M.; Beolchini, F. Prediction model for Cu chemical leaching from printed circuit boards. Ind. Eng. Chem. Res. 2019, 58, 20585–20591. [Google Scholar] [CrossRef]
- Mashifana, T.; Ntuli, F.; Okonta, F. Leaching kinetics on the removal of phosphorus from waste phosphogypsum by application of shrinking core model. South Afr. J. Chem. Eng. 2019, 27, 1–6. [Google Scholar] [CrossRef]
- Laidler, K.J. The development of the Arrhenius equation. J. Chem. Educ. 1984, 61, 494. [Google Scholar] [CrossRef]
- Li, H.; Zhao, L.; Wang, L.; Liang, J.; Yan, H.; Liu, J. Leaching kinetics of secondary zinc oxide in a NH3–NH4HCO3–H2O System. Crystals 2021, 11, 496. [Google Scholar] [CrossRef]
- Chindo, S.Y.; Omoniyi, K.I.; Raji, M.A. Chalcopyrite leaching in ammonia-ammonium chloride solutions: Insight into the dissolution kinetic studies. J. Sustain. Mater. Process. Manag. 2022, 2, 90–97. [Google Scholar] [CrossRef]
- Gueye, R.S.; Gaye, N.; Baldé, M.; Diedhiou, A.; Diouf, N.; Awa, S.G.; Ndoye, I.; Tine, Y.; Seck, M.; Fall, D.; et al. Review of Research on Li-Ion Batteries Waste Management. Open J. Inorg. Chem. 2022, 12, 19–38. [Google Scholar] [CrossRef]
- Duan, X.; Zhu, W.; Ruan, Z.; Xie, M.; Chen, J.; Ren, X. Recycling of lithium batteries—A review. Energies 2022, 15, 1611. [Google Scholar] [CrossRef]
- Liu, X.; Huang, K.; Xiong, H.; Dong, H. Ammoniacal leaching process for the selective recovery of value metals from waste lithium-ion batteries. Environ. Technol. 2023, 44, 211–225. [Google Scholar] [CrossRef] [PubMed]
- Asselin, E. Thermochemistry of the Fe, Ni and Co-NH 3-H 2 O systems as they relate to the Caron process: A review. Min. Metall. Explor. 2011, 28, 169–175. [Google Scholar]
- Formica, M.; Fusi, V.; Micheloni, M.; Pontellini, R.; Romani, P. Cryptand ligands for selective lithium coordination. Coord. Chem. Rev. 1999, 184, 347–363. [Google Scholar] [CrossRef]
- Zhang, J.; Mani, R.; Louhi-Kultanen, M. Process monitoring of cobalt carbonate precipitation by reactions between cobalt sulfate and sodium carbonate solutions to control product morphology and purity. Hydrometallurgy 2024, 224, 106232. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).