Corrosion Behavior Analysis of Novel Sn-2.5Ag-1.0Bi-0.8Cu-0.05Ni and Sn-1.8Bi-0.75Cu-0.065Ni Pb-Free Solder Alloys via Potentiodynamic Polarization Test
Abstract
1. Introduction
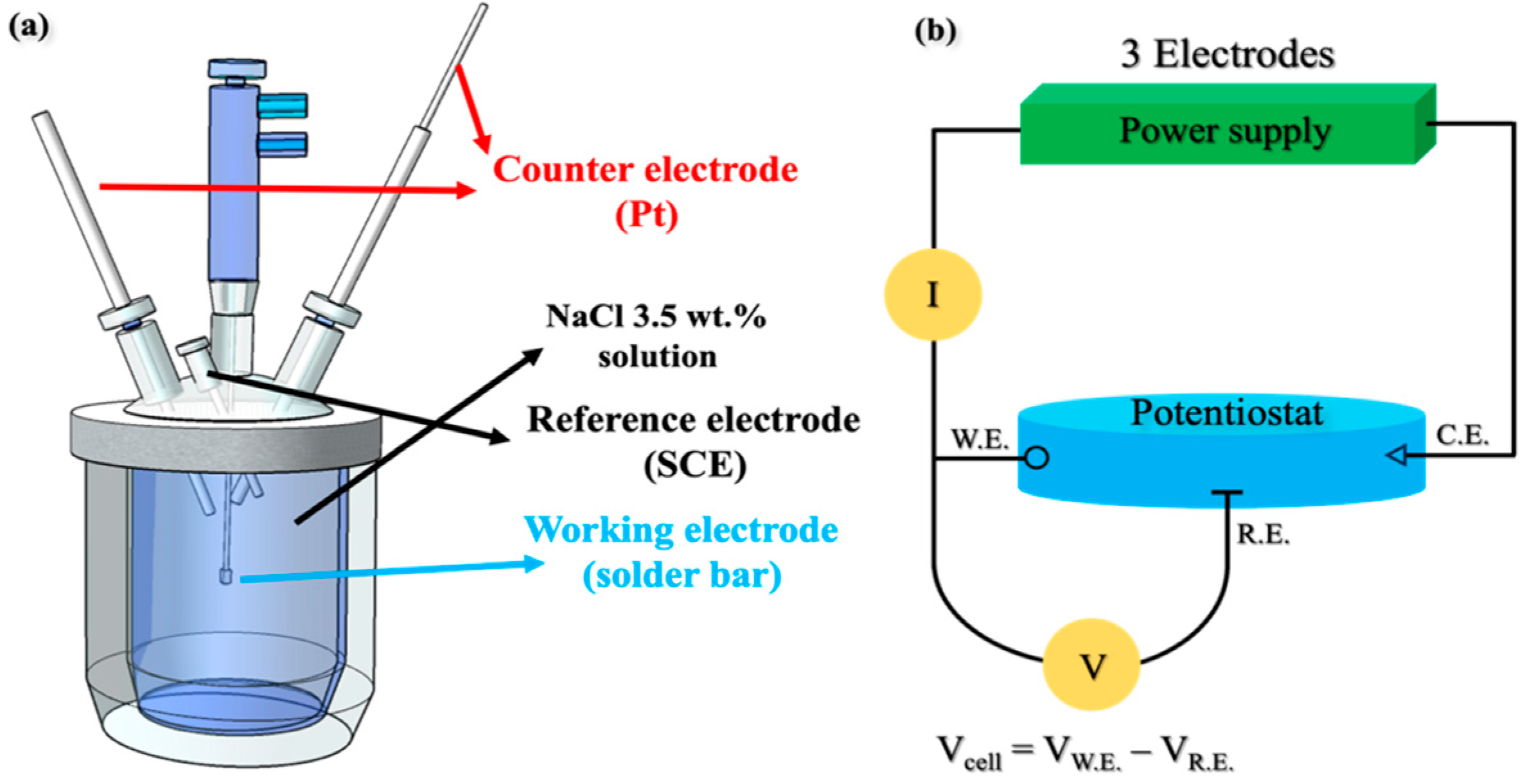
2. Materials and Methods
3. Results and Discussion
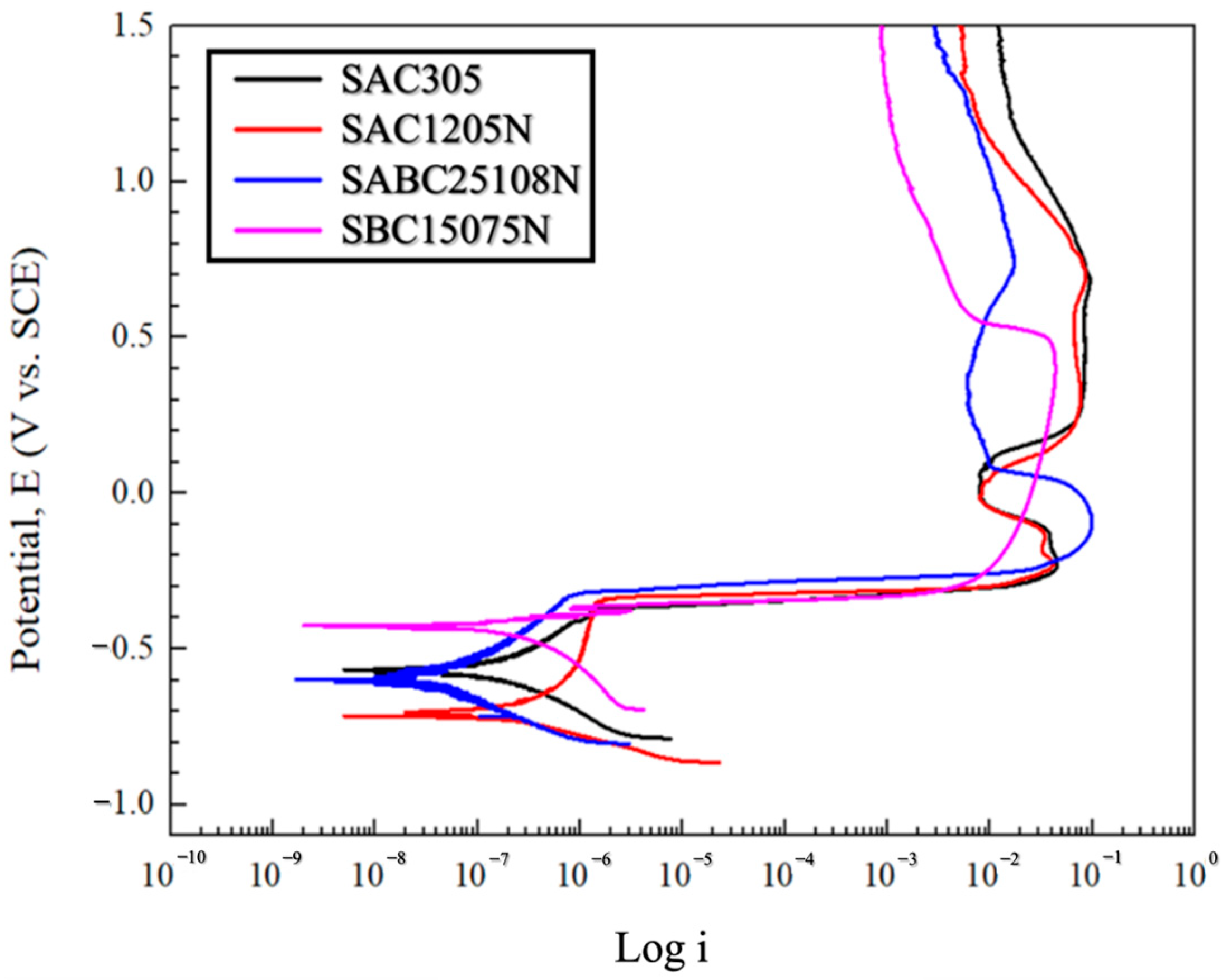
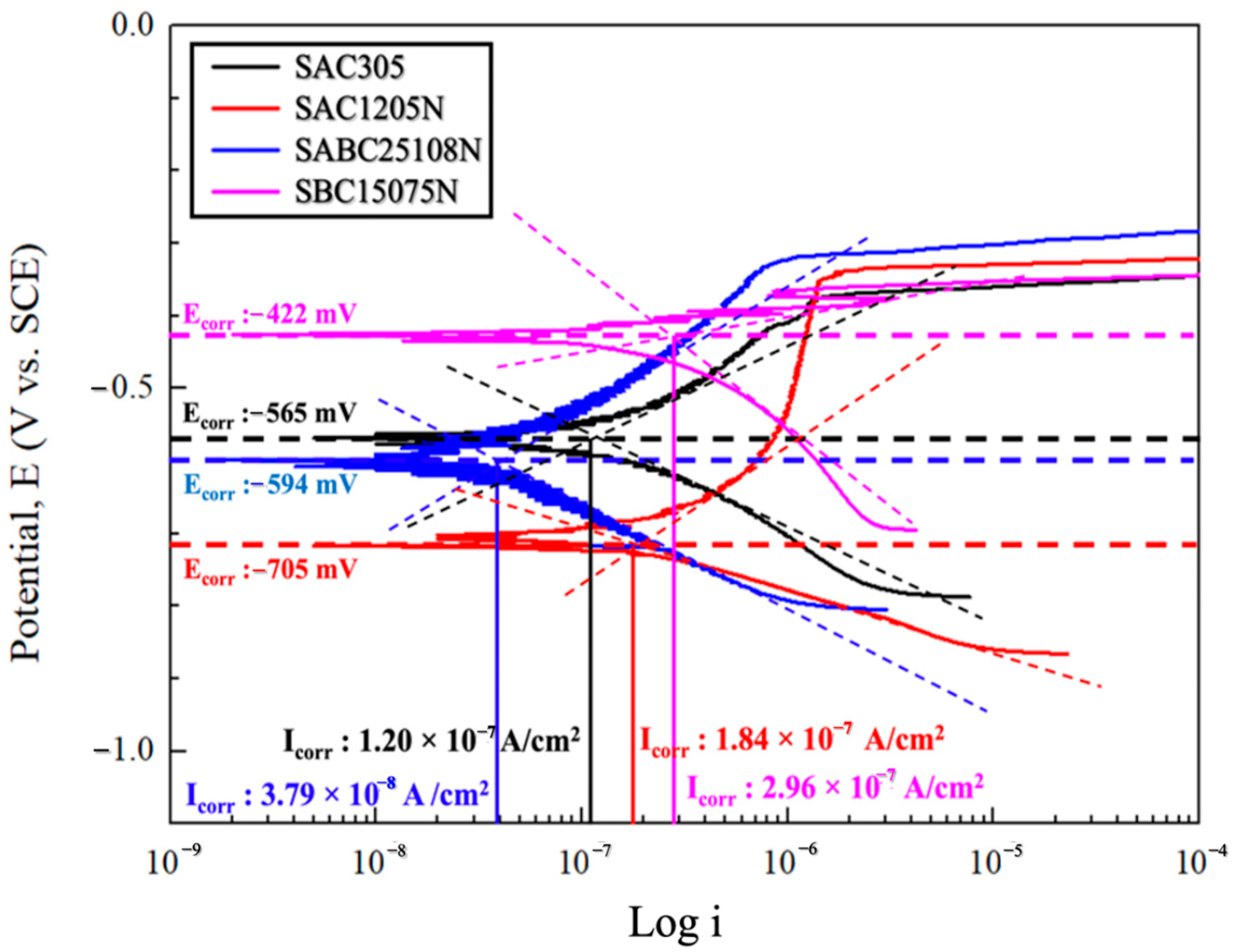
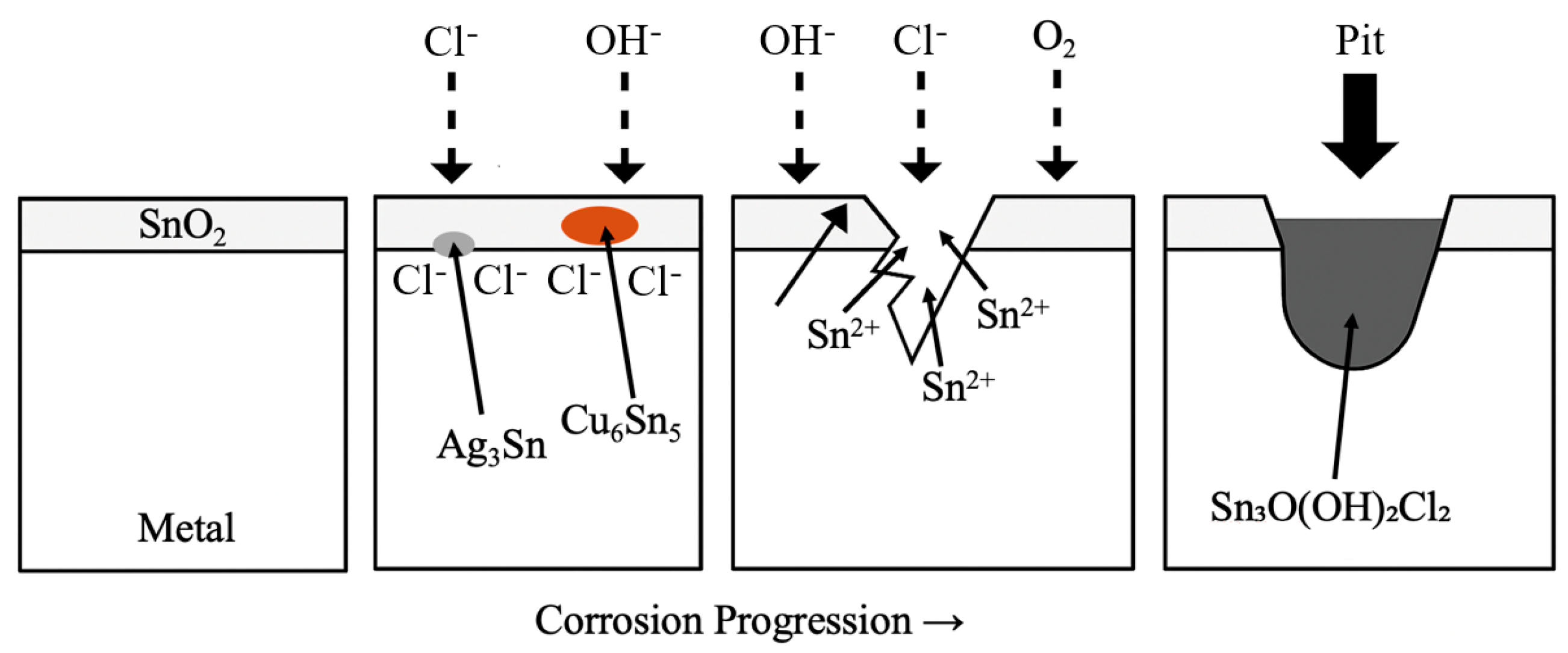
3.1. Potentiodynamic Polarization Test
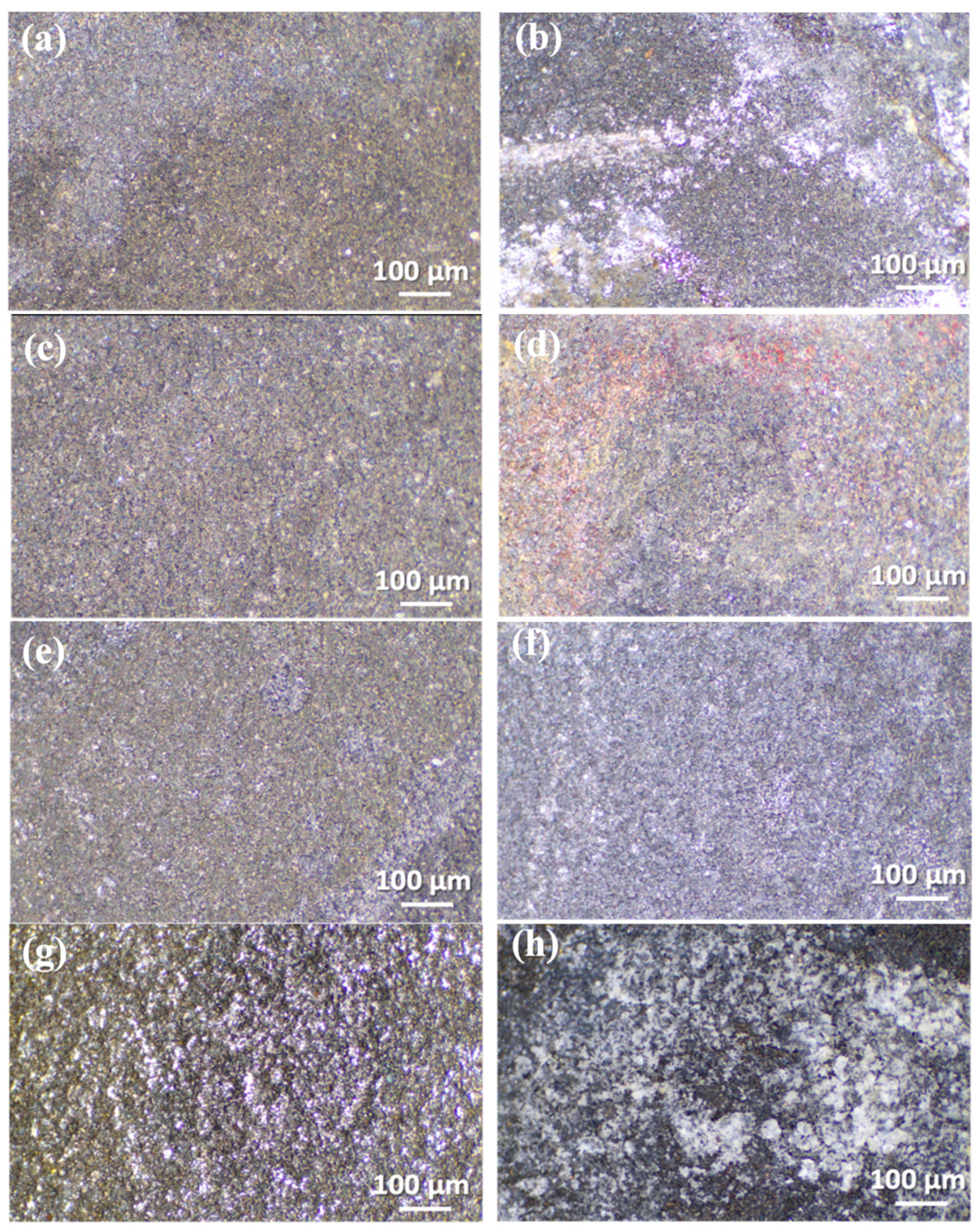
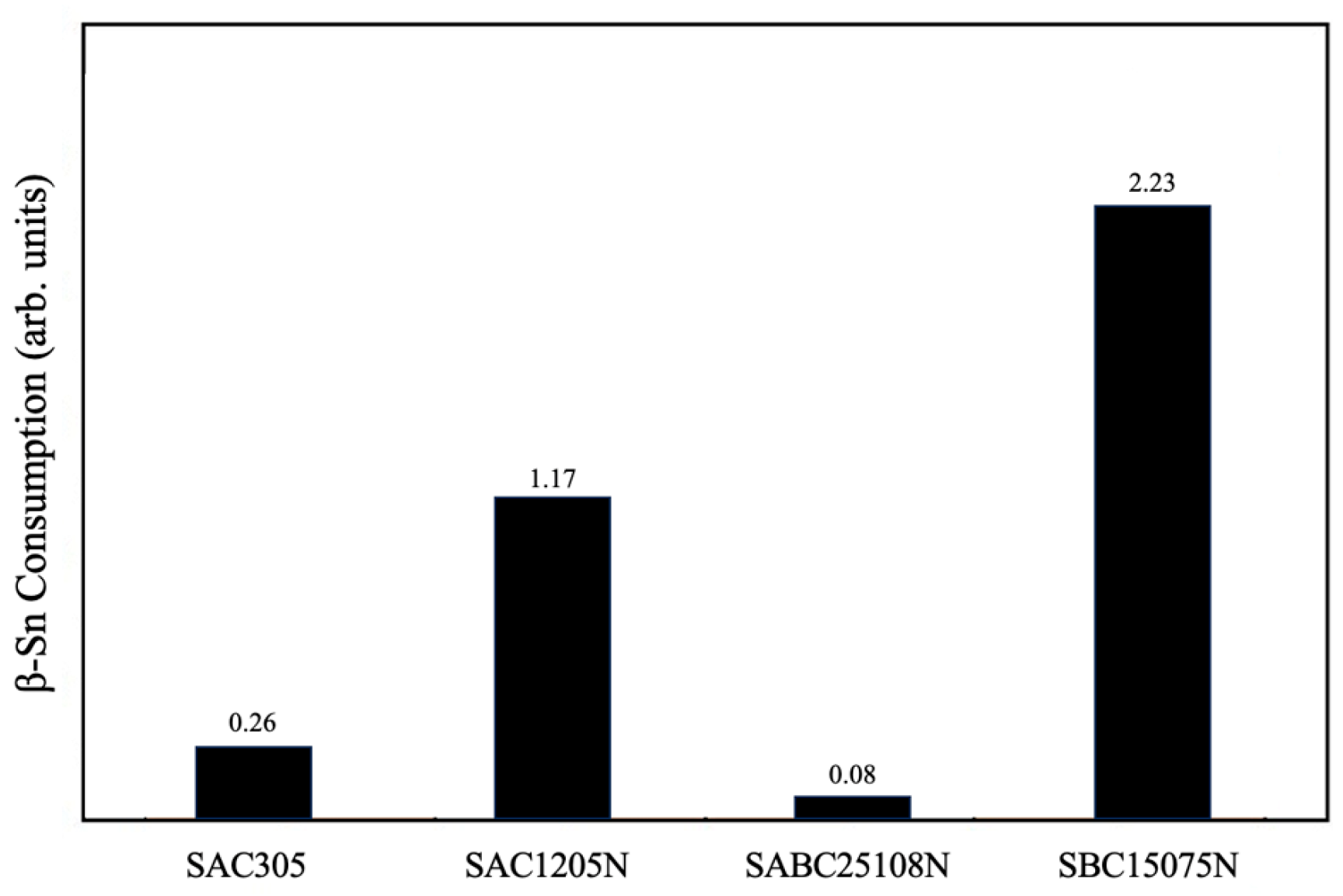
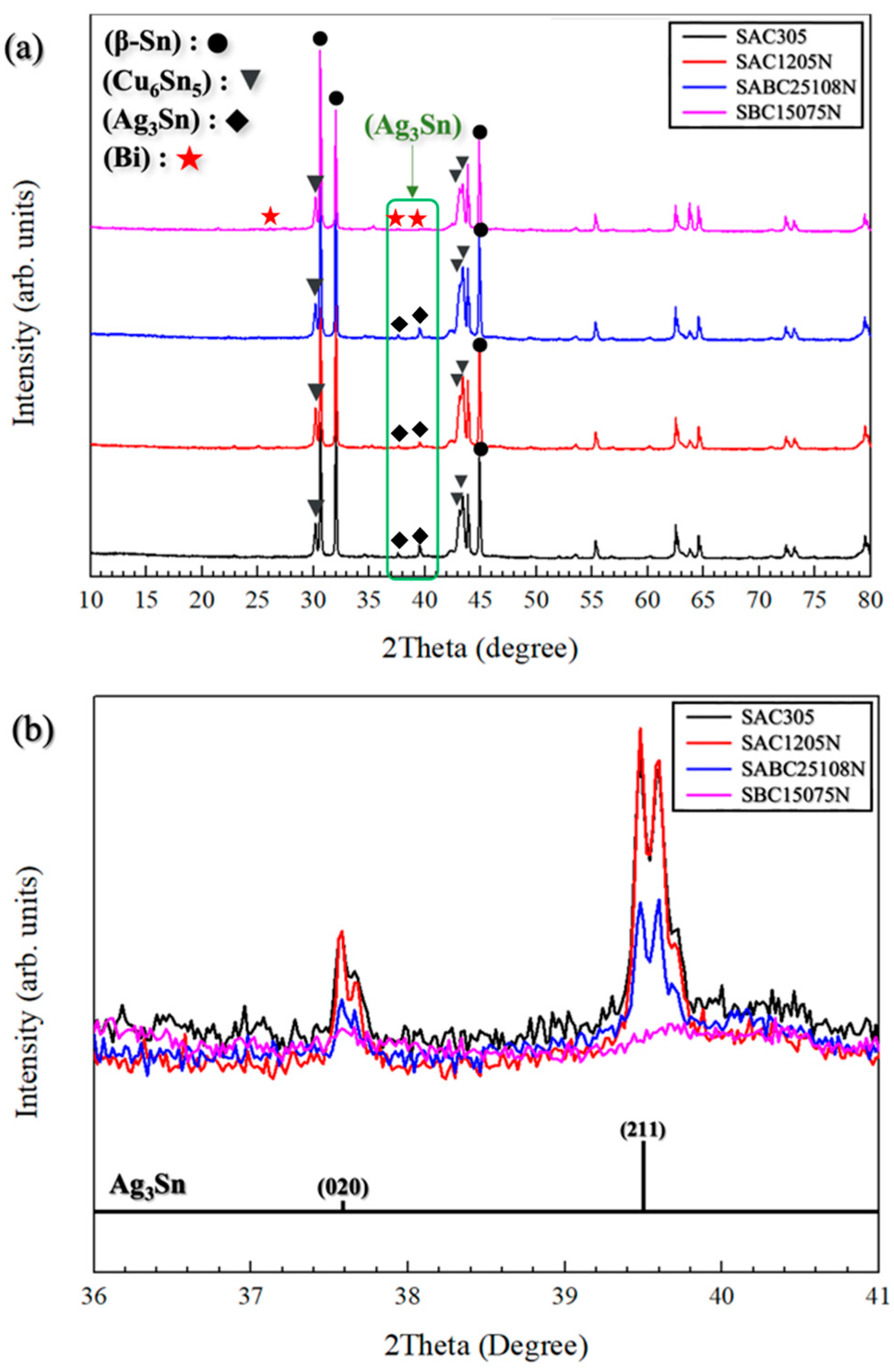
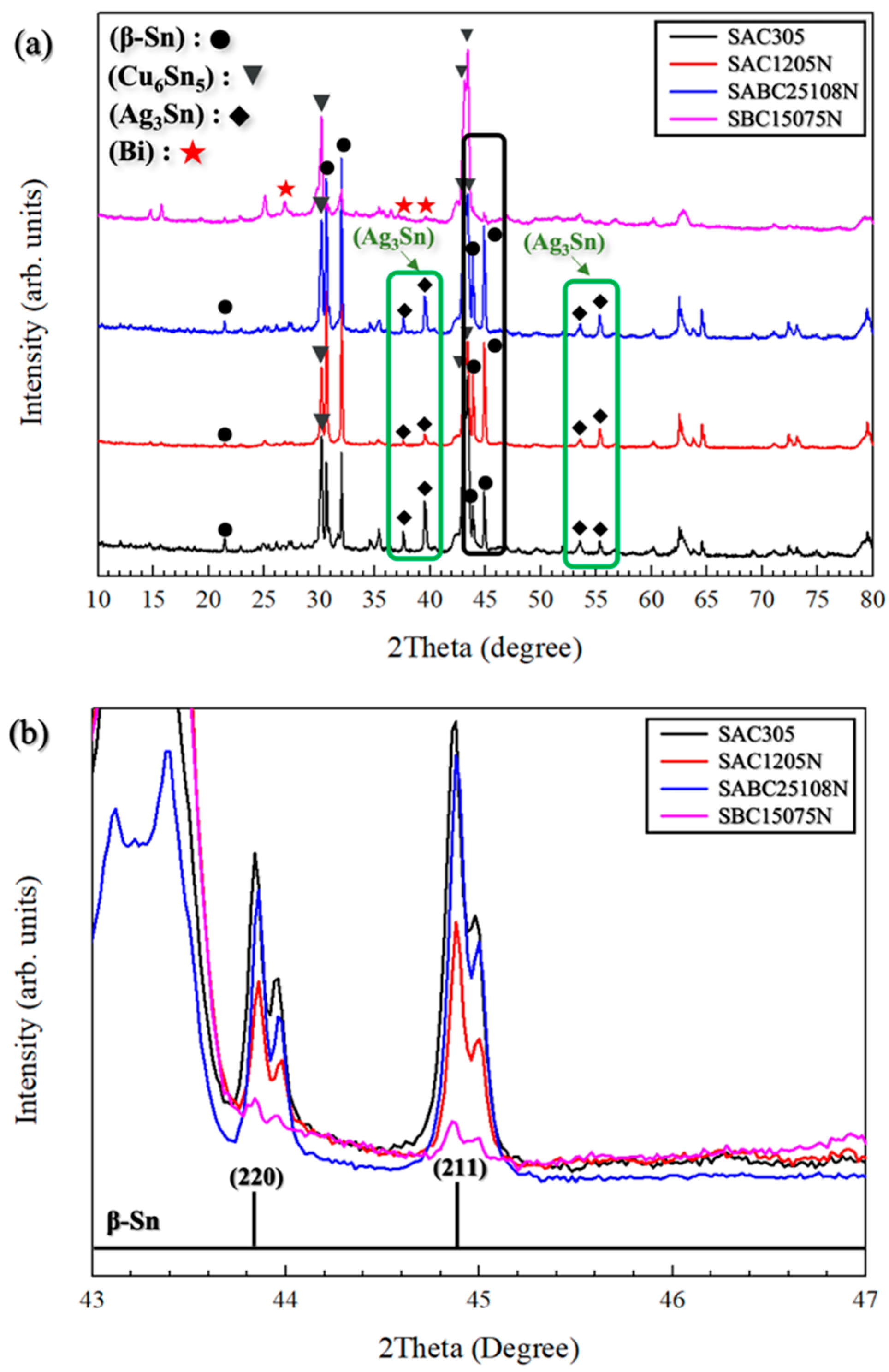
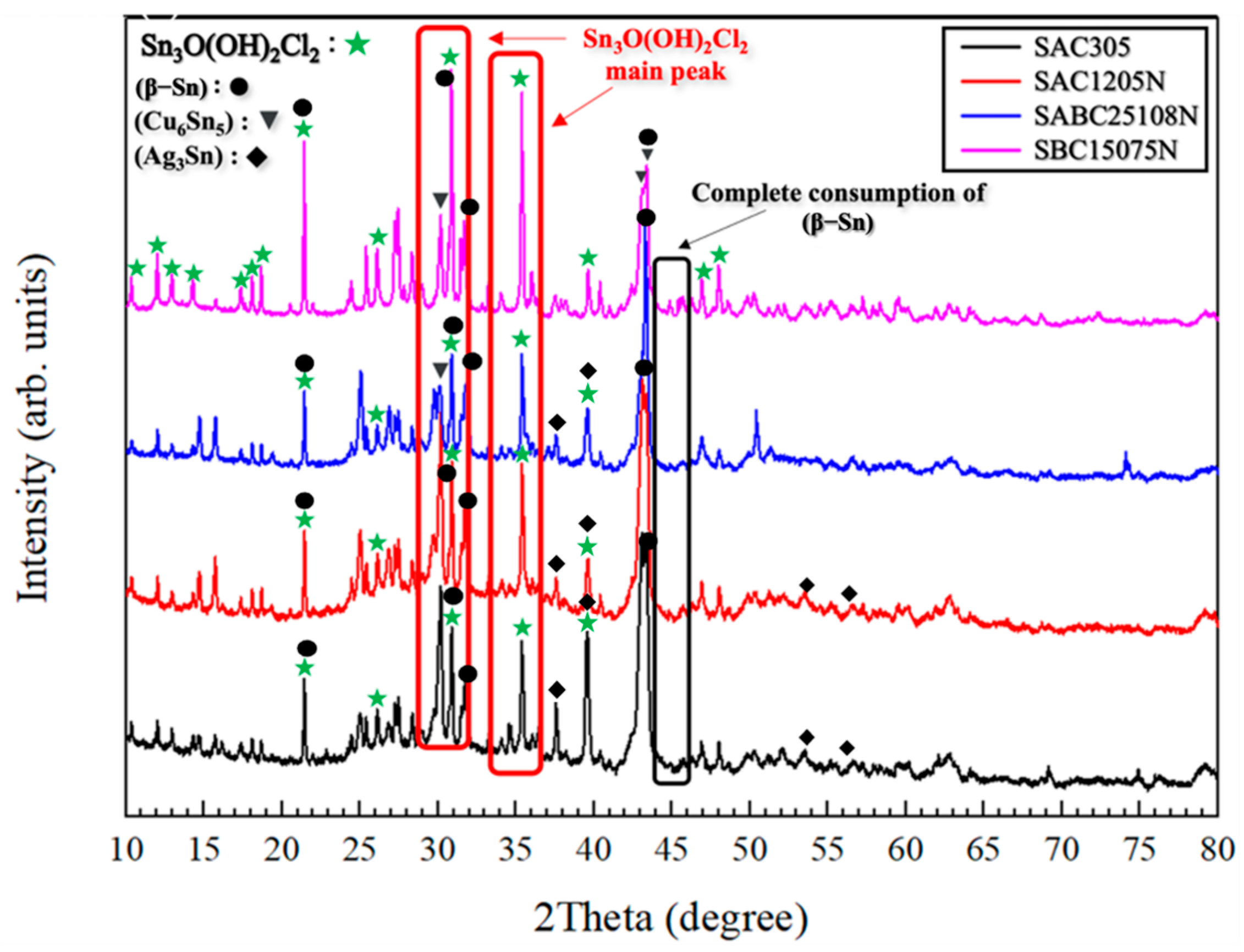
3.2. Microstructural Characterization
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Herat, S. Green electronics through legislation and lead free soldering. Clean 2008, 36, 145–151. [Google Scholar] [CrossRef]
- Suganuma, K. Advances in lead-free electronics soldering. Curr. Opin. Solid State Mater. Sci. 2001, 5, 55–64. [Google Scholar] [CrossRef]
- Terachima, S.; Kariya, Y.; Hosoi, T.; Tanaka, M. Effect of silver content on thermal fatigue life of Sn-xAg-0.5 Cu flip-chip interconnects. J. Electron. Mater. 2003, 32, 1527–1533. [Google Scholar] [CrossRef]
- Syed, A.; Scanlan, J.; Cha, S.W.; Kang, W.J.; Sohn, E.S.; Kim, T.S.; Ryu, C.G. Impact of package design and materials on reliability for temperature cycling, bend, and drop loading conditions. In Proceedings of the 58th Electronic Components and Technology Conference, Lake Buena Vista, FL, USA, 27–30 May 2008. [Google Scholar]
- Zhao, X.J.; Caers, J.F.J.M.; De Vries, J.W.C.; Kloosterman, J.; Wong, E.H.; Rajoo, R. Improvement of mechanical impact resistance of BGA packages with Pb-free solder bumps. In Proceedings of the 8th Electronics Packaging Technology Conference, Singapore, 6–8 December 2006. [Google Scholar]
- Birzer, C.; Rakow, B.; Steiner, R.; Walter, J. Drop test reliability improvement of lead-free fine pitch BGA using different solder ball composition. In Proceedings of the 7th Electronic Packaging Technology Conference, Singapore, 7–9 December 2005. [Google Scholar]
- Terashima, S.; Kohno, T.; Mizusawa, A.; Arai, K.; Okaga, O.; Wakabayashi, T.; Tanaka, M.; Tatsumi, K. Improvement of thermal fatigue properties of Sn-Ag-Cu lead-free solder interconnects on Casio’s wafer-level packages based on morphology and grain boundary character. J. Electron. Mater. 2009, 38, 33–38. [Google Scholar] [CrossRef]
- Chen, Z.; Shi, Y.; Xia, Z.; Yan, Y. Properties of lead-free solder SnAgCu containing minute amounts of rare earth. J. Electron. Mater. 2003, 32, 235–243. [Google Scholar] [CrossRef]
- Xiao, W.; Shi, Y.; Lei, Y.; Xia, Z.; Guo, F. Comparative study of microstructures and properties of three valuable SnAgCuRE lead-free solder alloys. J. Electron. Mater. 2006, 35, 1095–1103. [Google Scholar] [CrossRef]
- Tsao, L.C. An investigation of microstructure and mechanical properties of novel Sn3.5Ag0.5Cu–XTiO2 composite solders as functions of alloy composition and cooling rate. Mater. Sci. Eng. A 2011, 529, 41–48. [Google Scholar] [CrossRef]
- Gain, A.K.; Fouzder, T.; Chan, Y.C.; Sharif, A.; Wong, N.B.; Yung, W.K.C. The influence of addition of Al nano-particles on the microstructure and shear strength of eutectic Sn–Ag–Cu solder on Au/Ni metallized Cu pads. J. Alloys Compd. 2010, 506, 216–223. [Google Scholar] [CrossRef]
- Shen, J.; Chan, Y.C. Effects of ZrO2 nanoparticles on the mechanical properties of Sn–Zn solder joints on Au/Ni/Cu pads. J. Alloys Compd. 2009, 477, 552–559. [Google Scholar] [CrossRef]
- Lee, I.; Lee, Y.-W.; Valliappan, A.; Lee, T.-K. Crack propagation mechanism study on bismuth contained Sn base lead free solder under thermo-mechanical stress. In Proceedings of the SMTA International Conference, Rosemont, IL, USA, 14–18 October 2018. [Google Scholar]
- Son, J.Y.; Lee, S.G.; Park, J.W.; Lee, Y.W.; Jung, S.B. Modifying of tin based solder composition with optimized Bi, Ni and Cu contents for high TC and drop shock reliability. In Proceedings of the 24th Electronics Packaging Technology Conference, Singapore, 7–9 December 2022. [Google Scholar]
- Li, D.; Conway, P.P.; Liu, C. Corrosion characterization of tin–lead and lead free solders in 3.5 wt% NaCl solution. Corros. Sci. 2008, 50, 995–1004. [Google Scholar] [CrossRef]
- Nguyen, C.K.; Clark, B.N.; Stone, K.R.; Edwards, M.A. Acceleration of galvanic lead solder corrosion due to phosphate. Corros. Sci. 2011, 53, 1515–1521. [Google Scholar] [CrossRef]
- Cheng, C.Q.; Yang, F.; Zhao, J.; Wang, L.H.; Li, X.G. Leaching of heavy metal elements in solder alloys. Corros. Sci. 2011, 53, 1738–1747. [Google Scholar] [CrossRef]
- Hu, J.; Luo, T.; Hu, A.; Li, M.; Mao, D. Electrochemical corrosion behaviors of Sn-9Zn-3Bi-xCr solder in 3.5% NaCl solution. J. Electron. Mater. 2011, 40, 1556–1562. [Google Scholar] [CrossRef]
- Yan, X.; Liu, S.; Long, W.; Huang, J.; Zhang, L.; Chen, Y. The effect of homogenization treatment on microstructure and properties of ZnAl15 solder. Mater. Des. 2013, 45, 440–445. [Google Scholar] [CrossRef]
- Jumali, N.; Mohamad, A.A.; Mohd Nazeri, M.F. Corrosion properties of Sn-9Zn solder in acidic solution. Mater. Sci. Forum 2017, 888, 365–372. [Google Scholar] [CrossRef]
- Pu, C.; Li, C.; Dong, T.; Miao, Y.; Gao, P.; Zhang, X.; Peng, J.; Yi, J. Effect of Ag addition on the microstructure and corrosion properties of Sn–9Zn lead-free solder. J. Mater. Res. Technol. 2023, 27, 6400–6411. [Google Scholar] [CrossRef]
- Lopez, K.G.; Xiao, J.; Crokett, C.; Lytle, C.; Grubbs, H.; Edwards, M. Zinc orthophosphate can reduce nitrate-induced corrosion of lead solder. ACS EST Water 2024, 4, 3153–3162. [Google Scholar]
- Pu, C.; Li, C.; Miao, Y.; Lu, Q.; Peng, J.; Xu, Z.; Zhang, X.; Yi, J. Electrochemical corrosion behavior and corrosion mechanism of Sn-9Zn-xGe solder alloys in NaCl solution. Corros. Sci. 2024, 228, 111809. [Google Scholar] [CrossRef]
- Osório, W.R.; Spinelli, J.E.; Afonso, C.R.M.; Peixoto, L.C.; Garcia, A. Microstructure, corrosion behaviour and microhardness of a directionally solidified Sn–Cu solder alloy. Electrochim. Acta 2011, 56, 8891–8899. [Google Scholar] [CrossRef]
- Osório, W.R.; Freitas, E.S.; Spinelli, J.E.; Garcia, A. Electrochemical behavior of a lead-free Sn-Cu solder alloy in NaCl solution. Corros. Sci. 2014, 80, 71–81. [Google Scholar] [CrossRef]
- Huang, H.; Shuai, G.; Wei, X.; Yin, C. Effects of sulfur addition on the wettability and corrosion resistance of Sn-0.7Cu lead-free solder. Microelectron. Reliab. 2017, 74, 15–21. [Google Scholar] [CrossRef]
- Yang, W.; Du, Z.; Yu, S.; Li, Y.; Feng, J.; Wei, X.; Li, Q.; Zhan, Y. The effect of rare earths additions on the microstructure and the corrosion behavior of Sn-0.7Cu 0.075Al solder alloy. Materials 2019, 12, 3731. [Google Scholar] [CrossRef]
- Huang, H.; Lu, D.; Shuai, G.; Wei, X. Effects of phosphorus addition on the corrosion resistance of Sn-0.7Cu lead-free solder alloy. T. Indian. I. Metals 2016, 69, 1537–1543. [Google Scholar]
- Yan, Z.; Xian, A.P. Pitting corrosion behavior of Sn-0.7Cu lead free alloy in simulated marine atmospheric environment and the effect of trace Ga. Acta Metal. Sin. 2011, 47, 1327–1334. [Google Scholar]
- Yan, Z.; Xian, A.P. Corrosion of Ga-doped Sn-0.7Cu solder in simulated marine atmosphere. Metall. Mater. Trans. A 2012, 44, 1462–1474. [Google Scholar] [CrossRef]
- Freitas, E.S.; Osório, W.R.; Spinelli, J.E.; Garcia, A. Mechanical and corrosion resistances of a Sn-0.7wt%Cu lead-free solder alloy. Microelectron. Reliab. 2014, 54, 1392–1400. [Google Scholar] [CrossRef]
- Bui, Q.V.; Nam, N.D.; Noh, B.I.; Kar, A.; Kim, J.G.; Jung, S.B. Effect of Ag addition on the corrosion properties of Sn-based solder alloys. Mater. Corros. 2010, 61, 30–33. [Google Scholar] [CrossRef]
- Ameer, M.A.; Ghoneim, A.A.; Fekry, A.M. The electrochemical behavior of Sn-Ag binary alloys in sulfate solutions. Mater. Corros. 2010, 61, 580–589. [Google Scholar] [CrossRef]
- Ameer, M.A.; Fekry, A.M.; Ghoneim, A.A. Electrochemical behavior of Sn-Ag alloys in alkaline solutions. Corrosion 2009, 65, 589–594. [Google Scholar] [CrossRef]
- Wang, H.; Gao, Z.; Liu, Y.; Li, C.; Ma, Z.; Yu, L. Evaluation of cooling rate on electrochemical behavior of Sn-0.3Ag-0.9Zn solder alloy in 3.5wt% NaCl solution. J. Mater. Sci. Mater. Electron. 2014, 26, 11–22. [Google Scholar] [CrossRef]
- Osório, W.R.; Garcia, L.R.; Peixoto, L.C.; Garcia, A. Electrochemical behavior of a lead-free SnAg solder alloy affected by the microstructure array. Mater. Des. 2011, 32, 4763–4772. [Google Scholar] [CrossRef]
- Rosalbino, F.; Angelini, E.; Zanicchi, G.; Marazza, R. Corrosion behaviour assessment of lead-free Sn-Ag-M (M=In, Bi, Cu) solder alloys. Mater. Chem. Phys. 2008, 109, 386–391. [Google Scholar] [CrossRef]
- Vuong, B.X.; Vu, N.S.H.; Manh, T.D.; Vaka, M.; Du, D.X.; Nam, N.D. Role of cerium in microstructure and corrosion properties of Sn-1.0Ag solder alloys. Mater. Lett. 2018, 228, 309–313. [Google Scholar] [CrossRef]
- Wierzbicka-Miernik, A.; Guspiel, J.; Zabdyr, L. Corrosion behavior of lead-free SAC-type solder alloys in liquid media. Arch. Civ. Mech. Eng. 2015, 15, 206–213. [Google Scholar] [CrossRef]
- Qiao, C.; Wang, M.; Hao, L.; Liu, X.; Jiang, X.; An, X.; Li, D. Temperature and NaCl deposition dependent corrosion of SAC305 solder alloy in simulated marine atmosphere. J. Mater. Sci. Technol. 2021, 75, 252–264. [Google Scholar] [CrossRef]
- Curioni, M. The behaviour of magnesium during free corrosion and potentiodynamic polarization investigated by real-time hydrogen measurement and optical imaging. Electrochim. Acta 2014, 120, 284–292. [Google Scholar] [CrossRef]
- Brooks, E.; Tobias, M.; Krautsak, K.; Ehrensberger, M. The influence of cathodic polarization and simulated inflammation on titanium electrochemistry. J. Biomed. Mater. Res. B Appl. Biomater. 2014, 102, 1445–1453. [Google Scholar] [CrossRef]
- Bou-Saleh, Z.; Shahryari, A.; Omanovic, S. Enhancement of corrosion resistance of a biomedical grade 316LVM stainless steel by potentiodynamic cyclic polarization. Thin Solid Film. 2007, 515, 4727–4737. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Z.; Xu, A.; Liu, X. Understanding pitting corrosion behavior of AZ91 alloy and its MAO coating in 3.5% NaCl solution by cyclic potentiodynamic polarization. J. Magnes. Alloys 2022, 10, 1368–1380. [Google Scholar] [CrossRef]
- Zhao, T.; Liu, Z.; Du, C.; Sun, M.; Li, X. Effects of cathodic polarization on corrosion fatigue life of E690 steel in simulated seawater. Int. J. Fatigue 2018, 110, 105–114. [Google Scholar] [CrossRef]
- Ahmed, S.L.; Thamida, S.K.; Narasaiah, N. Novel optimization technique to determine polarization characteristics of a corroding metal. Electrochem. Energy Technol. 2017, 3, 1–8. [Google Scholar]
- Bellezze, T.; Giuliani, G.; Roventi, G. Study of stainless steels corrosion in a strong acid mixture. Part 1: Cyclic potentiodynamic polarization curves examined by means of an analytical method. Corros. Sci. 2018, 130, 113–125. [Google Scholar] [CrossRef]
- Saidman, S.B.; Bessone, J.B. Cathodic polarization characteristics and activation of aluminium in chloride solutions containing indium and zinc ions. J. Appl. Electrochem. 1997, 27, 731–737. [Google Scholar] [CrossRef]
- Li, M.; Luo, S.; Zeng, C.; Shen, J.; Lin, H.; Cao, C. Corrosion behavior of TiN coated type 316 stainless steel in simulated PEMFC environments. Corros. Sci. 2004, 46, 1369–1380. [Google Scholar] [CrossRef]
- Liu, X.; MacDonald, D.D.; Wang, M.; Xu, Y. Effect of dissolved oxygen, temperature, and pH on polarization behavior of carbon steel in simulated concrete pore solution. Electrochim. Acta 2021, 366, 137437. [Google Scholar] [CrossRef]
- Ramón, J.E.; Martínez, I.; Gandía-Romero, J.M.; Soto, J. Improved Tafel-based potentiostatic approach for corrosion rate monitoring of reinforcing steel. J. Nondestruct. Eval. 2022, 41, 70. [Google Scholar] [CrossRef]
- Cui, S.; Zhai, H.; Tong, W.; Li, W.; Li, X.; Fan, X.; Xiong, D. Passivation behavior and surface chemistry of Fe-based amorphous coating in NaCl solution: Influence of applied potential and immersion time. Intermetallics 2024, 172, 108391. [Google Scholar] [CrossRef]
- Siddiqui, M.A.; Ren, L.; Macdonald, D.D.; Yang, K. Effect of Cu on the passivity of Ti–xCu (x = 0, 3 and 5 wt%) alloy in phosphate-buffered saline solution within the framework of PDM-II. Electrochim. Acta 2021, 386, 138466. [Google Scholar] [CrossRef]
- Pandey, A.K.; Gautam, R.K.; Behera, C.K. Corrosion and wear behavior of Ti–5Cu-xNb biomedical alloy in simulated body fluid for dental implant applications. J. Mech. Behav. Biomed. Mater. 2023, 137, 105533. [Google Scholar] [CrossRef]
- Mathew, Z.P.; Shamnamol, G.K.; Greeshma, K.P.; John, S. Insight on the corrosion inhibition of nanocomposite chitosan/boron nitride integrated epoxy coating system against mild steel. Corros. Commun. 2023, 9, 36–43. [Google Scholar] [CrossRef]
- Wan, T.; Chu, K.; Fang, J.; Zhong, C.; Zhang, Y.; Ge, X.; Ding, Y.; Ren, F. A high strength, wear and corrosion-resistant, antibacterial and biocompatible Nb-5 at.% Ag alloy for dental and orthopedic implants. J. Mater. Sci. Technol. 2021, 80, 266–278. [Google Scholar] [CrossRef]
- Hussein, M.A.; Ogunlakin, N.O.; Kumar, A.M.; Sorour, A.A.; Azeem, M.A. Electrochemical and wear properties of biomedical Ti–Nb–Ag alloy. J. Mater. Res. Technol. 2024, 33, 5744–5754. [Google Scholar] [CrossRef]
- Peng, Z.; Ding, D.; Zhang, W.; Gao, Y.; Chen, G.; Xie, Y.; Liao, Y. Tensile property and corrosion performance of Ag microalloying of Al-Cu alloys for positive electrode current collectors of Li-ion batteries. Materials 2022, 15, 5126. [Google Scholar] [CrossRef]
- Lee, H.-M.; Moon, J.T.; Lee, Y.-W.; Kim, H.J.; Lee, S.G.; Lee, J.-H. Solidification behavior and mechanical properties of Sn-2.5Ag-0.8Cu-0.05Ni-1Bi and Sn-0.75Cu-0.065Ni-1.5Bi solder alloys, and microstructures in joints formed using them. Korean J. Met. Mater. 2024, 62, 511–523. [Google Scholar] [CrossRef]
- Chantaramanee, S.; Sungkhaphaitoon, P. Combined effects of Bi and Sb elements on microstructure, thermal and mechanical properties of Sn-0.7 Ag-0.5 Cu solder alloys. Trans. Nonferrous Met. Soc. China 2022, 32, 3301–3311. [Google Scholar] [CrossRef]
- Bhavan, J.S.; Pazhani, A.; Unnikrishnan, T.G. EBSD characterization of Ag3Sn phase transformation in Sn–Ag lead-free solder alloys: A comparative study before and after heat treatment. J. Mater. Sci. Mater. Electron. 2024, 35, 1577. [Google Scholar] [CrossRef]
- Mousa, M.M.; El-Rehim, A.F.A.; Abdelaziz, S.M. Influence of Cr inclusion on the microstructural evolutions and tensile properties of Sn-5 wt% Sb solder alloy. J. Mater. Sci. Mater. Electron. 2024, 35, 1888. [Google Scholar] [CrossRef]
- Wu, X.; Hou, Z.; Xie, X.; Lin, P.; Huo, Y.; Wang, Y.; Zhao, X. Mechanical properties and microstructure evolution of Sn–Bi-based solder joints by microalloying regulation mechanism. J. Mater. Res. Technol. 2024, 31, 3226–3237. [Google Scholar] [CrossRef]

| Composition | Abbreviation |
|---|---|
| Sn-3.0(wt%)Ag-0.5Cu | SAC305 |
| Sn-1.2Ag-0.5Cu-0.05Ni | SAC1205N |
| Sn-2.5Ag-1.0Bi-0.8Cu-0.05Ni | SABC25108N |
| Sn-1.5Bi-0.75Cu-0.065Ni | SBC15075N |
| Reference Electrode | Counter Electrode | Temp. (°C) | Solution | OCP Measurement | Potential Scan Range | Scan Rate (mV/min) |
|---|---|---|---|---|---|---|
| SCE | Pt mesh | 25 (±1) | 3.5 wt% NaCl | 600 s (OCP vs. SCE) | −0.75 V (vs. OCP)~ +1.5 V (vs. R.E.) | 30 |
| Electrolyte | Sample | Ecorr (mV) | Icorr (×10−9 A/cm2) | Corrosion Rate (mm/yr) |
|---|---|---|---|---|
| 3.5 wt% NaCl solution | SAC305 | −565 | 120 | 0.114 |
| SAC1205N | −705 | 184 | 0.175 | |
| SABC25108N | −594 | 37.9 | 0.036 | |
| SBC15075N | −422 | 296 | 0.281 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, S.H.; Lee, J.-H. Corrosion Behavior Analysis of Novel Sn-2.5Ag-1.0Bi-0.8Cu-0.05Ni and Sn-1.8Bi-0.75Cu-0.065Ni Pb-Free Solder Alloys via Potentiodynamic Polarization Test. Metals 2025, 15, 670. https://doi.org/10.3390/met15060670
Jung SH, Lee J-H. Corrosion Behavior Analysis of Novel Sn-2.5Ag-1.0Bi-0.8Cu-0.05Ni and Sn-1.8Bi-0.75Cu-0.065Ni Pb-Free Solder Alloys via Potentiodynamic Polarization Test. Metals. 2025; 15(6):670. https://doi.org/10.3390/met15060670
Chicago/Turabian StyleJung, Sang Hoon, and Jong-Hyun Lee. 2025. "Corrosion Behavior Analysis of Novel Sn-2.5Ag-1.0Bi-0.8Cu-0.05Ni and Sn-1.8Bi-0.75Cu-0.065Ni Pb-Free Solder Alloys via Potentiodynamic Polarization Test" Metals 15, no. 6: 670. https://doi.org/10.3390/met15060670
APA StyleJung, S. H., & Lee, J.-H. (2025). Corrosion Behavior Analysis of Novel Sn-2.5Ag-1.0Bi-0.8Cu-0.05Ni and Sn-1.8Bi-0.75Cu-0.065Ni Pb-Free Solder Alloys via Potentiodynamic Polarization Test. Metals, 15(6), 670. https://doi.org/10.3390/met15060670







