Construction of CuCo2O4@NiFe-LDH Core–Shell Heterostructure for High-Performance Hybrid Supercapacitors
Abstract
1. Introduction
2. Experimental Process
2.1. Synthesis of CuCo2O4 Nanowire Arrays
2.2. Synthesis of CuCo2O4@NiFe-LDH
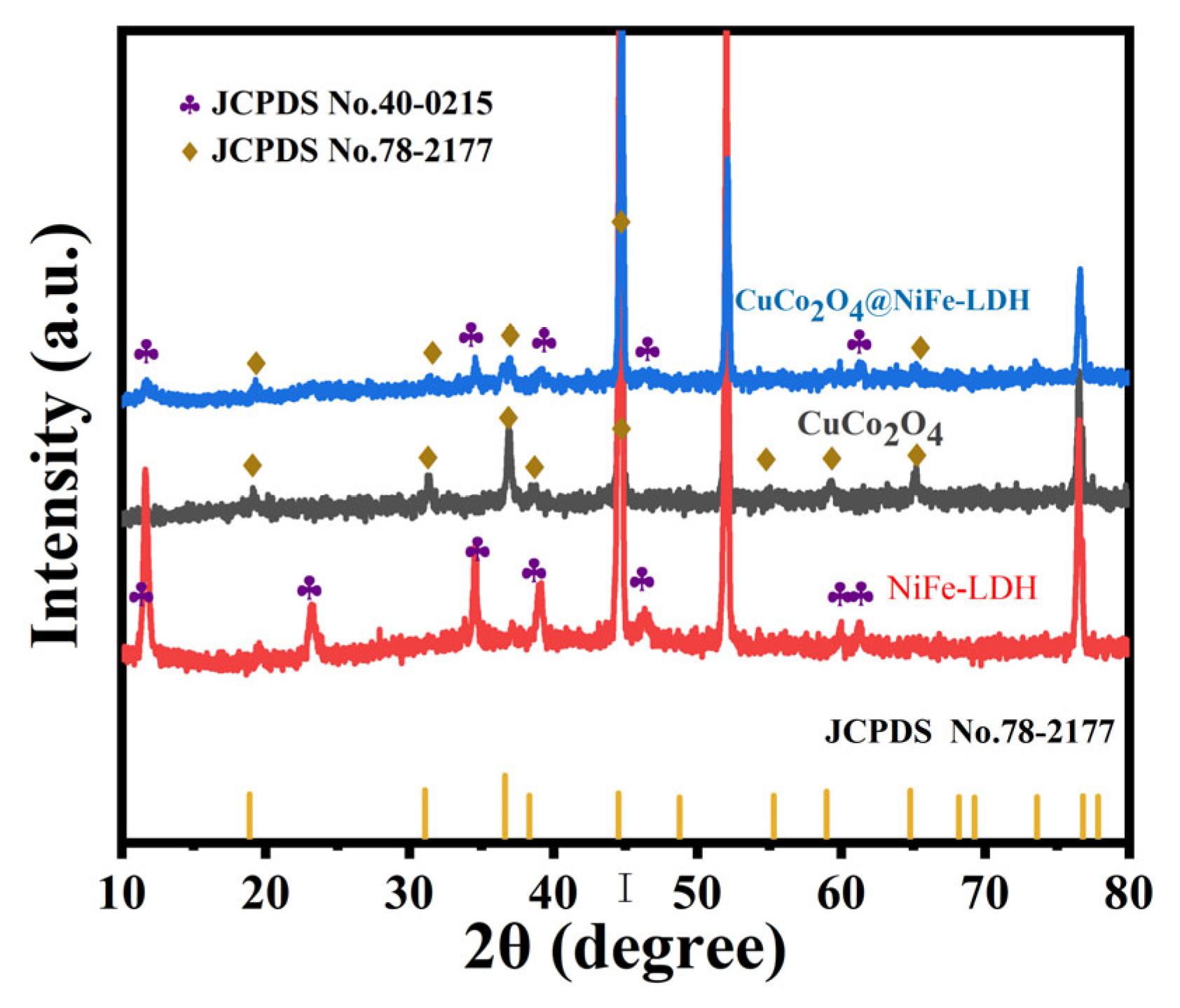
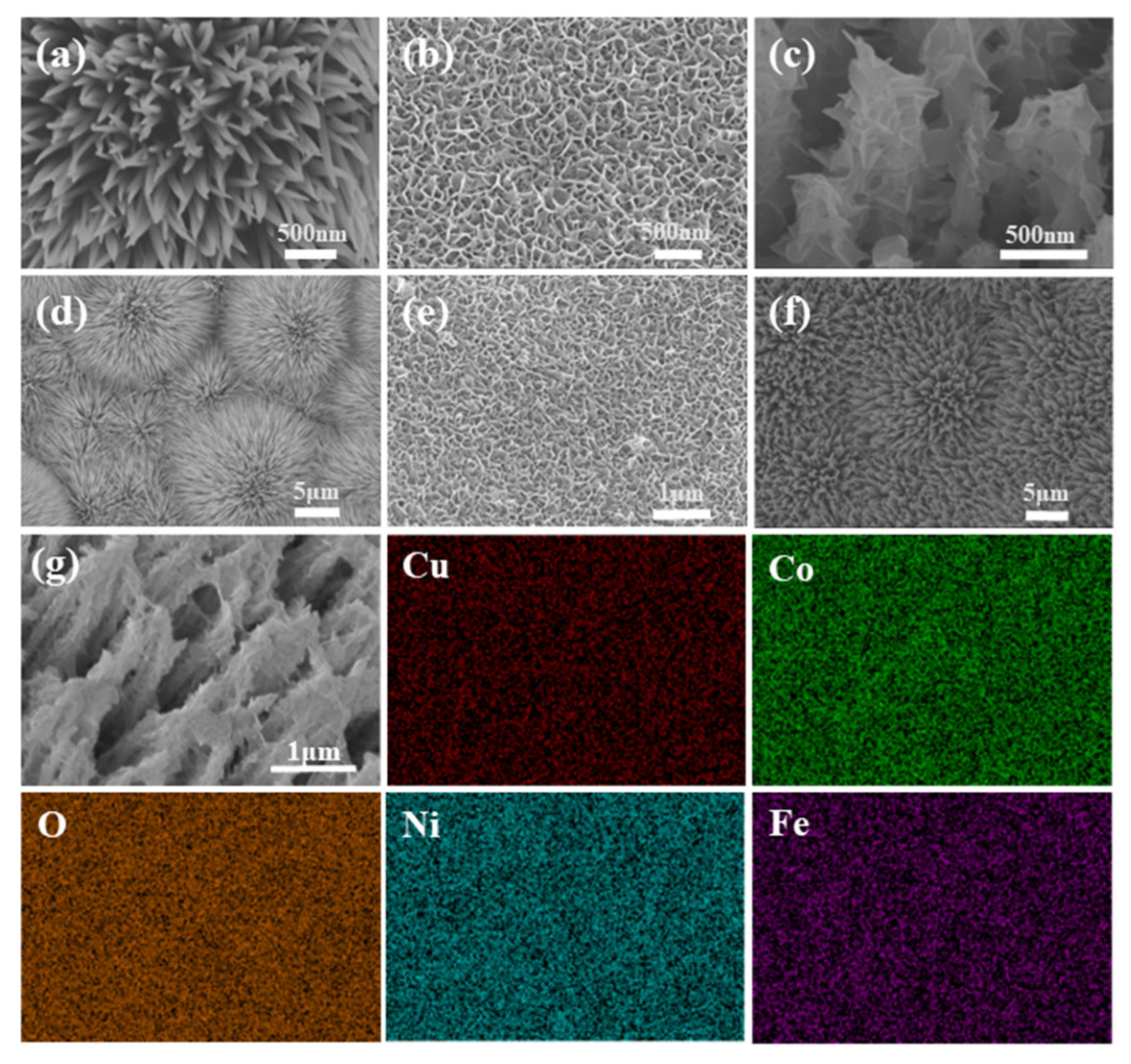
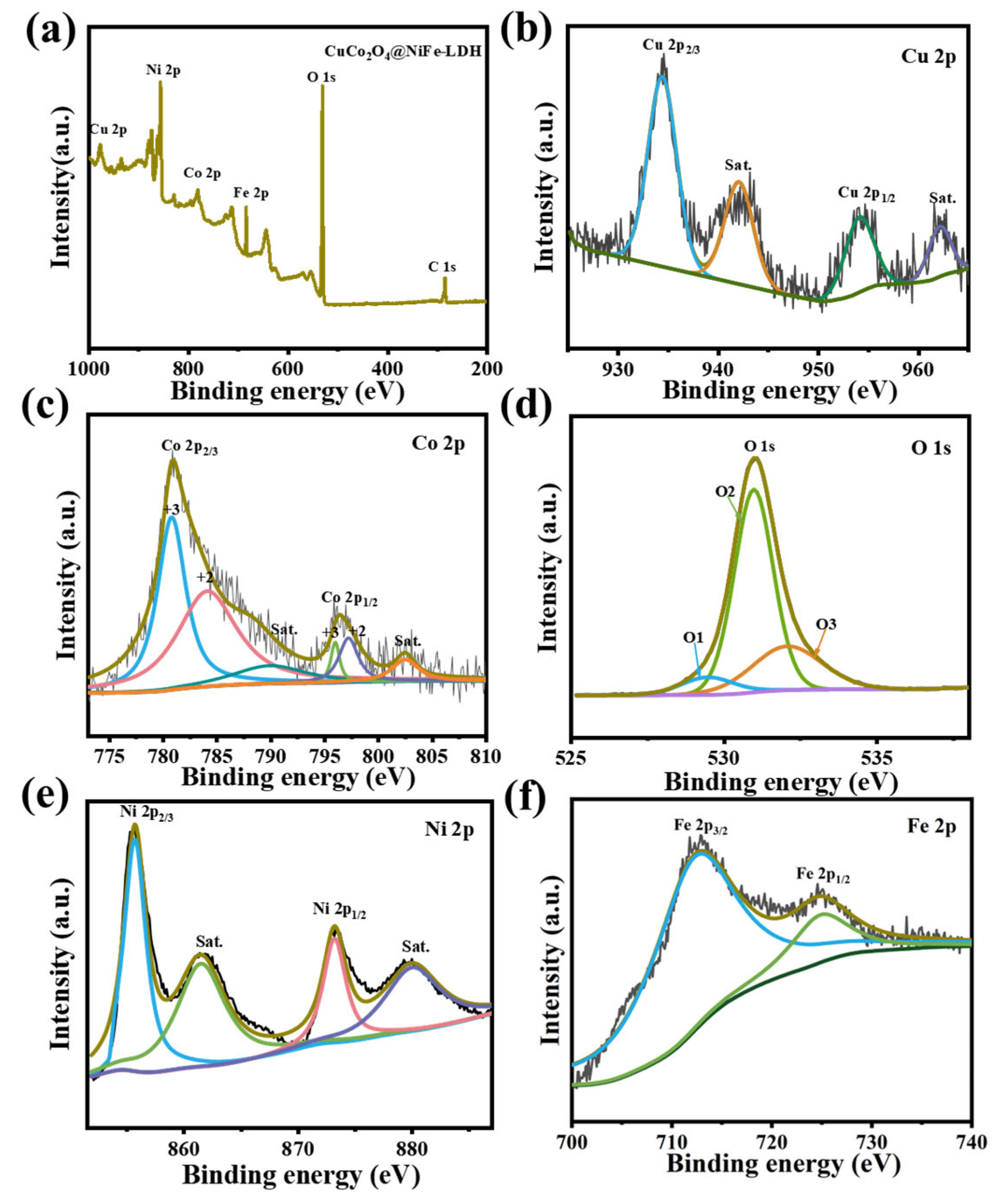
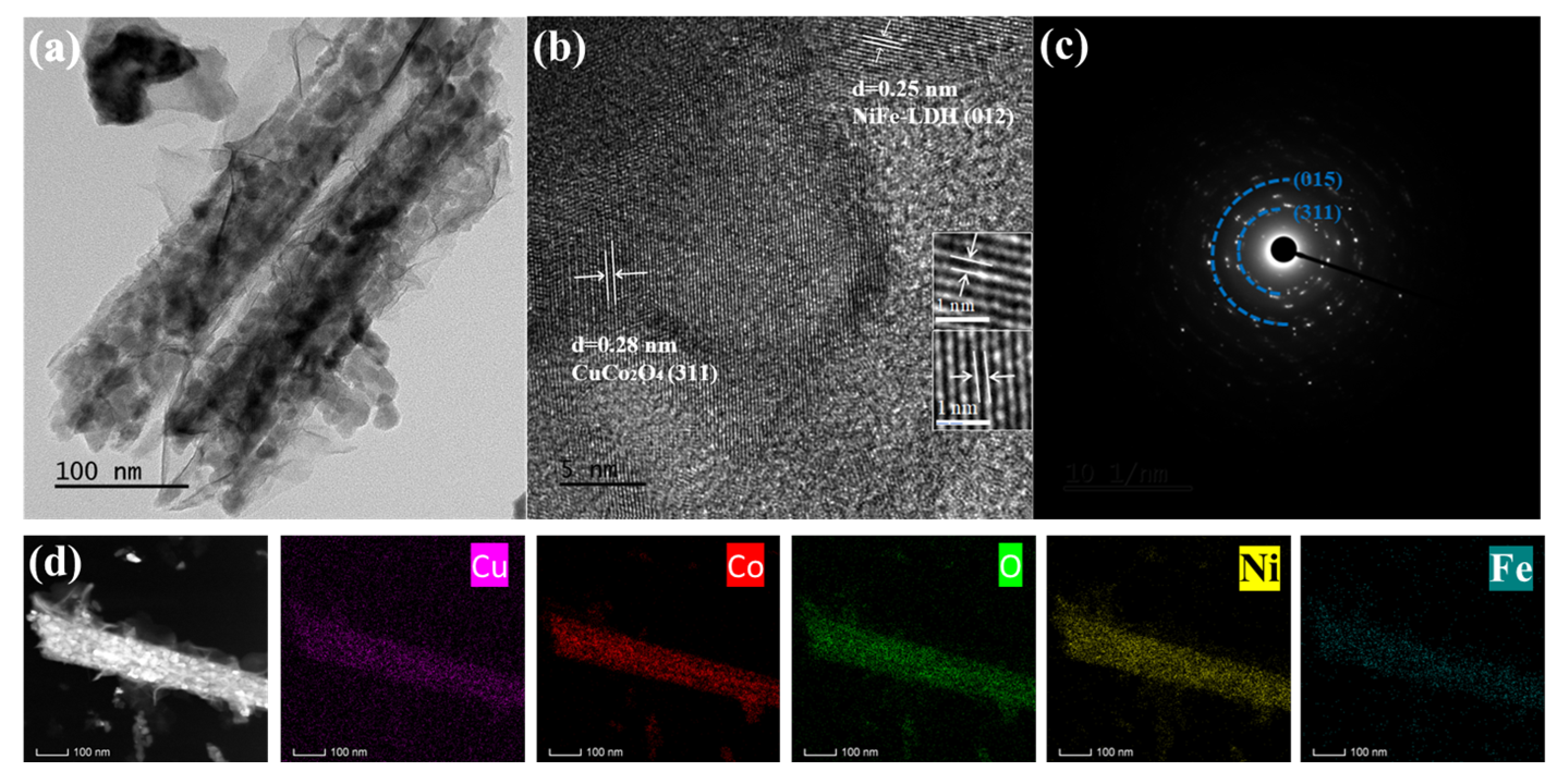
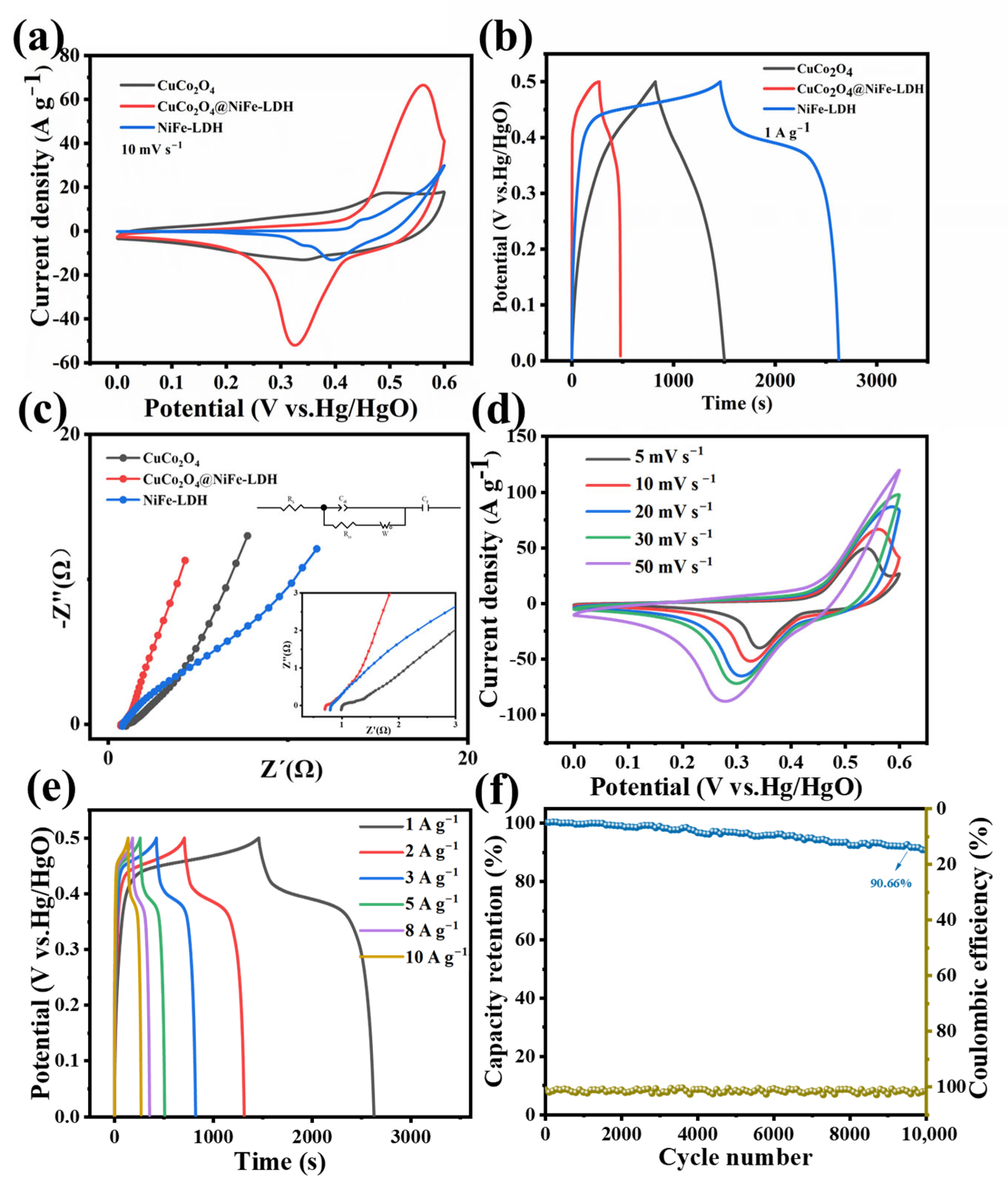
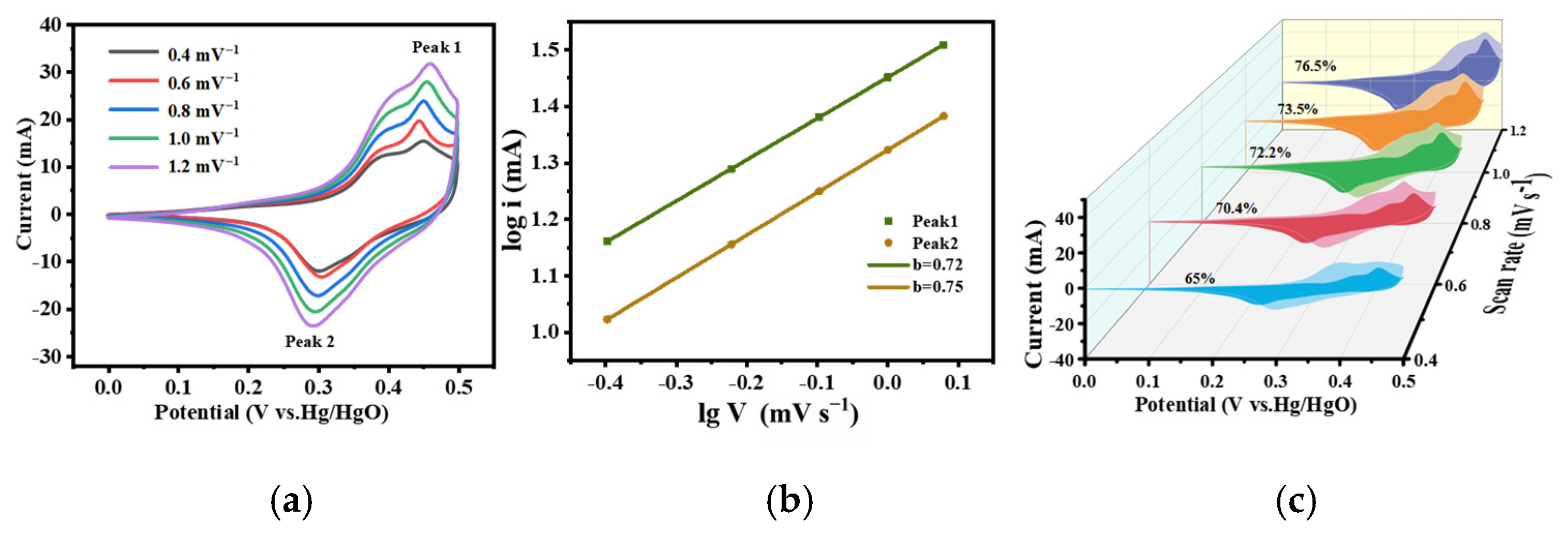
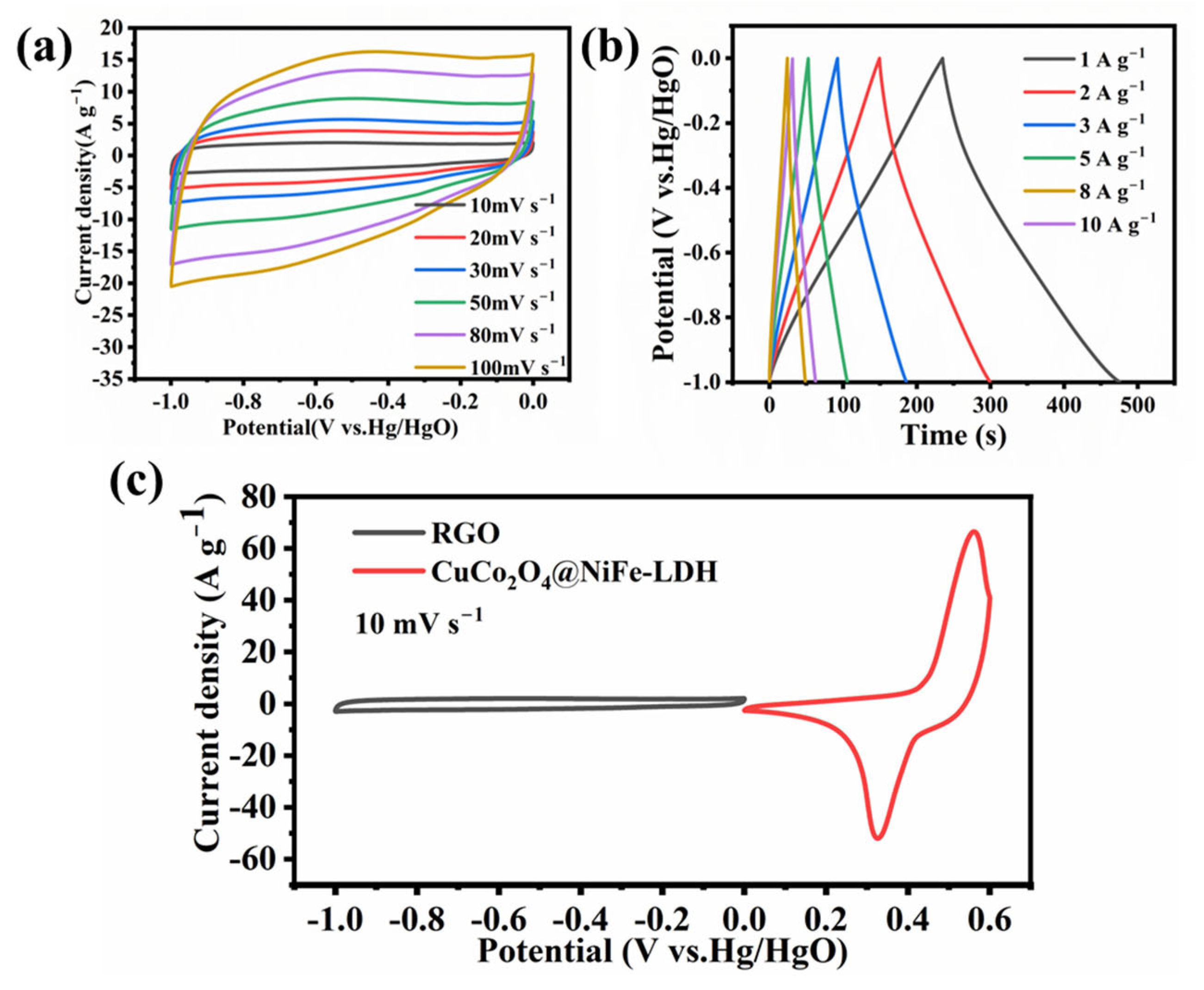
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yougbare, S.; Chou, H.; Yang, C.; Krisnawati, D.I.; Jazidie, A.; Nuh, M.; Kuo, T. Facet-dependent gold nanocrystals for effective photothermal killing of bacteria. J. Hazard. Mater. 2021, 407, 124617. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Wang, J.; Wang, J. Morphology-controllable synthesis of CuCo2O4 arrays on Ni foam as advanced electrodes for supercapacitors. J. Alloys Compd. 2019, 789, 193–200. [Google Scholar] [CrossRef]
- Okoro, G.; Husain, S.; Saukani, M.; Mutalik, C.; Yougbare, S.; Hsiao, Y.; Kuo, T. Emerging trends in nanomaterials for photosynthetic biohybrid systems. ACS Mater. Lett. 2023, 5, 95–115. [Google Scholar] [CrossRef]
- Mutalik, C.; Okoro, G.; Krisnawati, D.I.; Jazidie, A.; Rahmawati, E.Q.; Rahayu, D.; Hsu, W.; Kuo, T. Copper sulfide with morphology-dependent photodynamic and photothermal antibacterial activities. J. Colloid. Interface Sci. 2022, 607, 1825–1835. [Google Scholar] [CrossRef]
- Mutalik, C.; Okoro, G.; Chou, H.; Lin, I.; Yougbare, S.; Chang, C.; Kuo, T. Phase-dependent 1t/2h-MoS2 nanosheets for effective photothermal killing of bacteria. ACS Sustain. Chem. Eng. 2022, 10, 8949–8957. [Google Scholar] [CrossRef]
- Zhou, G.; Gao, X.; Wen, S.; Wu, X.; Zhang, L.; Wang, T.; Zhao, P.; Yin, J.; Zhu, W. Magnesium-regulated oxygen vacancies of cobalt-nickel layered double hydroxide nanosheets for ultrahigh performance asymmetric supercapacitors. J. Colloid. Interface Sci. 2022, 612, 772–781. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, Q.; Ma, T.; Li, Z.; Tan, X.; Bateer, B. Design of thin-layer porous nickel cobalt sulfide for high-performance asymmetric supercapacitors. J. Alloys Compd. 2023, 945, 168902. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, X.; Yu, X.; Chen, X.; Jiang, J.; Han, S. Sponge-like 3d flower-like core-shell heterostructure CuCo2O4@CuCo2S4 as advanced electrodes for high-performance supercapacitor. J. Power Sources 2022, 551, 232186. [Google Scholar] [CrossRef]
- Wu, X.; Meng, L.; Wang, Q.; Zhang, W.; Wang, Y. High flexibility and large energy density asymmetric fibered-supercapacitor based on unique NiCo2O4@MnO2 core-shell nanobrush arrays electrode. Electrochim. Acta 2019, 295, 532–539. [Google Scholar] [CrossRef]
- Shuja, A.; Khan, H.R.; Murtaza, I.; Ashraf, S.; Abid, Y.; Farid, F.; Sajid, F. Supercapacitors for energy storage applications: Materials, devices and future directions: A comprehensive review. J. Alloys Compd. 2024, 1009, 176924. [Google Scholar] [CrossRef]
- Saleki, F.; Mohammadi, A.; Moosavifard, S.E.; Hafizi, A.; Rahimpour, M.R. MOF assistance synthesis of nanoporous double-shelled CuCo2O4 hollow spheres for hybrid supercapacitors. J. Colloid. Interface Sci. 2019, 556, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Yang, J.; Jia, K.; Liu, S.; Hu, C.; Qiu, J. Boosting supercapacitor performance of graphene by coupling with nitrogen-doped hollow carbon frameworks. Chem.-Eur. J. 2020, 26, 2897–2903. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Wang, H.; Zhang, Q.; Li, Y. High-performance flexible asymmetric supercapacitors based on 3d porous graphene /MnO2 nanorod and graphene/ag hybrid thin-film electrodes. J. Mater. Chem. C 2013, 1, 1245–1251. [Google Scholar] [CrossRef]
- Sahoo, B.B.; Pandey, V.S.; Dogonchi, A.S.; Mohapatra, P.K.; Thatoi, D.N.; Nayak, N.; Nayak, M.K. A state-of-art review on 2d material-boosted metal oxide nanoparticle electrodes: Supercapacitor applications. J. Energy Storage 2023, 65, 107335. [Google Scholar] [CrossRef]
- Kumar, S.A.; Sahoo, S.; Laxminarayana, G.K.; Rout, C.S. Electrochemical deposition for cultivating nano- and microstructured electroactive materials for supercapacitors: Recent developments and future perspectives. Small 2024, 20, 2402087. [Google Scholar] [CrossRef]
- Sun, J.; Tian, X.; Xu, C.; Chen, H. Porous CuCo2O4 microtubes as a promising battery-type electrode material for high-performance hybrid supercapacitors. J. Materiomics 2021, 7, 1358–1368. [Google Scholar] [CrossRef]
- Che, X.; Jin, J.; Zhang, Y.; Liu, S.; Wang, M.; Yang, J. Fabrication of coal-based oxygen-rich porous carbon nanosheets for high-performance supercapacitors. New Carbon Mater. 2023, 38, 1050–1058. [Google Scholar] [CrossRef]
- Meng, Y.; Liang, C.; Jiang, D.; Zhang, Y.; Su, J.; Xu, X.; Lu, M. Ion modified cobalt-based layered double hydroxides and its derivatives as electrode materials for supercapacitors: A review and perspective. J. Energy Storage 2023, 74, 109105. [Google Scholar] [CrossRef]
- Wan, L.; Wang, P. Recent progress on self-supported two-dimensional transition metal hydroxides nanosheets for electrochemical energy storage and conversion. Int. J. Hydrogen Energy 2021, 46, 8356–8376. [Google Scholar] [CrossRef]
- Li, Y.; He, G.; Huang, Z.; Huangfu, H.; Li, Z.; Qiao, Y.; Shi, Z. High-performance NF-loaded f-NiFe-LDH/rGO nanoflower/nanosheet composite electrode material achieving enhanced specific capacitance and energy density for supercapacitor. J. Energy Storage 2024, 101, 113954. [Google Scholar] [CrossRef]
- Wei, X.; Su, S.; Zhang, Q. Construction of hierarchical NiCoP@NiFe-LDH nanoflakes heterostructure for high energy asymmetric supercapacitor. ChemElectroChem 2023, 10, e202201166. [Google Scholar] [CrossRef]
- Chen, C.; Li, S.; Wang, F.; Shen, L.; Sun, S.; Xu, Y.; Li, H. Facile synthesis of NiFe-LDH/CB/S nanocomposite electrode for sustainable hybrid supercapacitors. Energy Fuels 2023, 37, 12416–12426. [Google Scholar] [CrossRef]
- Wang, P.; Lin, Y.; Xu, Q.; Xu, Z.; Wan, L.; Xia, Y.; Wang, B. Acid-corrosion-induced hollow-structured NiFe-layered double hydroxide electrocatalysts for efficient water oxidation. ACS Appl. Energ. Mater. 2021, 4, 9022–9031. [Google Scholar] [CrossRef]
- Yu, Y.; Li, M.; Zhou, J.; Sun, X.; Jiang, Z.; Li, Y.; Wang, C. Structural designs of advanced wood-based thick electrodes for high-performance eco-supercapacitors. Nano Today 2024, 55, 102154. [Google Scholar] [CrossRef]
- Li, Q.; Liu, M.; Huang, F.; Zuo, X.; Wei, X.; Li, S.; Zhang, H. Co9S8@MnO2 core-shell defective heterostructure for high-voltage flexible supercapacitor and zn-ion hybrid supercapacitor. Chem. Eng. J. 2022, 437, 135494. [Google Scholar] [CrossRef]
- Zhao, W.; Xu, X.; Wu, N.; Zhao, X.; Gong, J. Dandelion-like CuCo2O4@NiMn-LDH core / shell nanoflowers for excellent battery-type supercapacitor. Nanomaterials 2023, 13, 730. [Google Scholar] [CrossRef]
- Zhang, C.; Sui, Q.; Lu, L.; Zou, Y.; Xu, F.; Sun, L.; Cai, D.; Xiang, C. Hollow core-shell CuCo2O4@MoNi-layered double hydroxides as an electrode material for supercapacitors. J. Energy Storage 2023, 61, 106691. [Google Scholar] [CrossRef]
- Mishra, R.K.; Choi, G.J.; Choi, H.J.; Singh, J.; Mirsafi, F.S.; Rubahn, H.; Mishra, Y.K.; Lee, S.H.; Gwag, J.S. Voltage holding and self-discharge phenomenon in ZnO-Co3O4 core-shell heterostructure for binder-free symmetric supercapacitors. Chem. Eng. J. 2022, 427, 131895. [Google Scholar] [CrossRef]
- Tamtam, M.R.; Wang, R.; Koutavarapu, R.; Choi, G.S.; Shim, J. Core-shell Mn@Al-MOF: A multidimensional hierarchical advanced electrode material for high-performance supercapacitors. J. Alloys Compd. 2025, 1013, 178517. [Google Scholar] [CrossRef]
- Chavan, G.T.; Dubal, D.P.; Morankar, P.J.; Jeon, C.; An, J.; Song, K. Hierarchical CoMn-LDH and heterostructured composites for advanced supercapacitors and electrocatalysis applications. Materials 2025, 18, 604. [Google Scholar] [CrossRef]
- Joshi, B.; Samuel, E.; Huh, J.; Kim, S.; Ali, A.; Rahaman, M.; Lee, H.; Yoon, S.S. Hierarchically decorated ZnO/CoOx nanoparticles on carbon fabric for high energy-density and flexible supercapacitors. Ceram. Int. 2024, 50, 43324–43333. [Google Scholar] [CrossRef]
- Zhou, Y.; Si, J.; Wang, H.; Li, X.; Zhang, S.; Deng, C. Co9S8@NiFe-LDH bifunctional electrocatalysts as high-efficiency cathodes for Zn-air batteries. Energy Fuels 2023, 37, 9619–9625. [Google Scholar] [CrossRef]
- Li, X.; Ren, J.; Sridhar, D.; Xu, B.B.; Algadi, H.; El-Bahy, Z.M.; Ma, Y.; Li, T.; Guo, Z. Progress of layered double hydroxide-based materials for supercapacitors. Mater. Chem. Front. 2023, 7, 1520–1561. [Google Scholar] [CrossRef]
- Sun, P.; Zhang, J.; Huang, J.; Wang, L.; Wang, P.; Cai, C.; Lu, M.; Yao, Z.; Yang, Y. Bimetallic MOF-derived (CuCo) Se nanoparticles embedded in nitrogen-doped carbon framework with boosted electrochemical performance for hybrid supercapacitor. Mater. Res. Bull. 2021, 137, 111196. [Google Scholar] [CrossRef]
- Shi, D.; Ji, Y.; Lu, F.; Yao, J. Facile and rapid construction of NiFe-LDH foam with abundant oxygen vacancies as high-performance supercapacitor negative electrode. Mater. Lett. 2023, 350, 134884. [Google Scholar] [CrossRef]
- Feng, X.; Jiao, Q.; Chen, W.; Dang, Y.; Dai, Z.; Suib, S.L.; Zhang, J.; Zhao, Y.; Li, H.; Feng, C. Cactus-like NiCo2S4@NiFe-LDH hollow spheres as an effective oxygen bifunctional electrocatalyst in alkaline solution. Appl. Catal. B—Environ. 2021, 286, 119869. [Google Scholar] [CrossRef]
- Huang, K.; Dong, R.; Wang, C.; Li, W.; Sun, H.; Geng, B. Fe-Ni layered double hydroxide arrays with homogeneous heterostructure as efficient electrocatalysts for overall water splitting. ACS Sustain. Chem. Eng. 2019, 7, 15073–15079. [Google Scholar] [CrossRef]
- Luo, D.; Yong, Y.; Hou, J.; Guo, W.; Liu, H.; Zhao, X.; Xiang, J.; Zongzong, N.; Han, Y.; Yan, M. Construction of hierarchical NiCo2O4@NiFe-LDH core-shell heterostructure for high-performance positive electrode for supercapacitor. ChemNanoMat 2022, 8, e202200137. [Google Scholar] [CrossRef]
- Chu, X.; Meng, F.; Yang, H.; Zhang, W.; Qin, T.; Wang, Z.; Molin, S.; Jasinski, P.; Zheng, W. Cu-doped layered double hydroxide constructs the performance-enhanced supercapacitor via band gap reduction and defect triggering. ACS Appl. Energ. Mater. 2022, 5, 2192–2201. [Google Scholar] [CrossRef]
- Yuan, J.; Li, Y.; Lu, G.; Gao, Z.; Wei, F.; Qi, J.; Sui, Y.; Yan, Q.; Wang, S. Controlled synthesis of flower-like hierarchical NiCo-layered double hydroxide integrated with metal-organic framework-derived Co@C for supercapacitors. ACS Appl. Mater. Interfaces 2023, 15, 36143–36153. [Google Scholar] [CrossRef]
- Du, X.; Su, H.; Zhang, X. Metal-organic framework-derived cu-doped Co9S8 nanorod array with less low-valence co sites as highly efficient bifunctional electrodes for overall water splitting. ACS Sustain. Chem. Eng. 2019, 7, 16917–16926. [Google Scholar] [CrossRef]
- Mohanty, R.I.; Mukherjee, A.; Bhanja, P.; Jena, B.K. Novel microporous manganese phosphonate-derived metal oxides as prospective cathode materials for superior flexible asymmetric micro-supercapacitor device. J. Energy Storage 2023, 72, 108730. [Google Scholar] [CrossRef]
- Tian, J.; Zhang, A.; Liu, R.; Huang, W.; Yuan, Z.; Zheng, R.; Wei, D.; Liu, J. Preparation of CoS2 supported flower-like NiFe layered double hydroxides nanospheres for high-performance supercapacitors. J. Colloid. Interface. Sci. 2020, 579, 607–618. [Google Scholar] [CrossRef]
- Yewale, M.A.; Kadam, R.A.; Nakate, U.T.; Teli, A.M.; Kumar, V.; Beknalkar, S.A.; Jadhavar, A.A.; Kadam, S.L.; Shelke, N.T.; Shin, D.K. Sphere-shaped CuCo2O4 nanostructures battery type electrode for supercapacitor via hydrothermal synthesis approach. Colloid Surf. A-Physicochem. Eng. Asp. 2023, 679, 132541. [Google Scholar] [CrossRef]
- Liu, D.; Liu, Y.; Bao, E.; Ren, X.; Liu, X.; Xiang, Y.; Xu, C.; Li, Y.; Chen, H. Porous CuCo2O4/CuO microspheres and nanosheets as cathode materials for advanced hybrid supercapacitors. J. Energy Storage 2023, 68, 107875. [Google Scholar] [CrossRef]
- Li, G.; Song, B.; Cui, X.; Ouyang, H.; Wang, K.; Sun, Y.; Wang, Y. Multidimensional and binary micro CuCo2O4/Nano NiMoO4 for high performance supercapacitors. ACS Sustain. Chem. Eng. 2020, 8, 1687–1694. [Google Scholar] [CrossRef]
- Shrivastav, V.; Dubey, P.; Holdynski, M.; Sundriyal, S.; Tiwari, U.K.; Deep, A. An Insight Into Surface and Diffusion Characteristics of UiO-66@ZIF-8 Core-Shell MOF for Asymmetric Supercapacitors with Unprecedented Energy Density. Adv. Sustain. Syst. 2024, 8, 2400043. [Google Scholar] [CrossRef]
- Liu, Z.; Zhong, Y.; Qiu, Y.; Cui, L.; Yang, W.; Razal, J.M.; Barrow, C.J.; Liu, J. Multilayered and hierarchical structured NiCo double hydroxide nanosheets generated on porous MgCo2O4 nanowire arrays for high performance supercapacitors. Appl. Surf. Sci. 2021, 546, 149133. [Google Scholar] [CrossRef]
- Arbi, H.M.; Koyyada, G.; Anil Kumar, Y.; Kumar Kulurumotlakatla, D.; Kim, J.H.; Moniruzzaman, M.; Alzahmi, S.; Obaidat, I.M. Hierarchically Developed Ni(OH)2@MgCo2O4 Nanosheet Composites for Boosting Supercapacitor Performance. Nanomaterials 2023, 13, 1414. [Google Scholar] [CrossRef]
- Han, L.; Liu, X.; Chen, Y.; Cui, Z.; Hua, Y.; Wang, C.; Zhao, X.; Liu, X. High energy density pouch-type supercapacitor achieved by MOFs derived 3D hollow N-doped carbon with Fe2O3 and hierarchical CuCo2S4@NiFe-LDH core-shell nanostructures. Electrochim. Acta 2023, 467, 143131. [Google Scholar] [CrossRef]
- Wang, J.; Li, J.; Wang, F.; Zhang, X.; Wu, X.; Zhang, J.; Niu, S.; Chen, L. Introducing Ce into the interface of NiCo-LDH@PBAs to build multi core–shell electrode with superior stability of supercapacitor. Chem. Eng. J. 2024, 488, 150932. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Li, M.; Xue, C.; Wei, F. Construction of CuCo2O4@NiFe-LDH Core–Shell Heterostructure for High-Performance Hybrid Supercapacitors. Metals 2025, 15, 659. https://doi.org/10.3390/met15060659
Chen Y, Li M, Xue C, Wei F. Construction of CuCo2O4@NiFe-LDH Core–Shell Heterostructure for High-Performance Hybrid Supercapacitors. Metals. 2025; 15(6):659. https://doi.org/10.3390/met15060659
Chicago/Turabian StyleChen, Yang, Man Li, Chengyu Xue, and Fuxiang Wei. 2025. "Construction of CuCo2O4@NiFe-LDH Core–Shell Heterostructure for High-Performance Hybrid Supercapacitors" Metals 15, no. 6: 659. https://doi.org/10.3390/met15060659
APA StyleChen, Y., Li, M., Xue, C., & Wei, F. (2025). Construction of CuCo2O4@NiFe-LDH Core–Shell Heterostructure for High-Performance Hybrid Supercapacitors. Metals, 15(6), 659. https://doi.org/10.3390/met15060659







