Effect on the Electrochemical Properties of PEO Films Produced on Commercially Pure Titanium Using Multicomponent Oxide Coatings
Abstract
1. Introduction
2. Materials and Methods
2.1. Surface Preparation
2.2. Surface Modification
2.3. Surface Characterization
2.4. Electrochemical Measurements
3. Results and Discussion
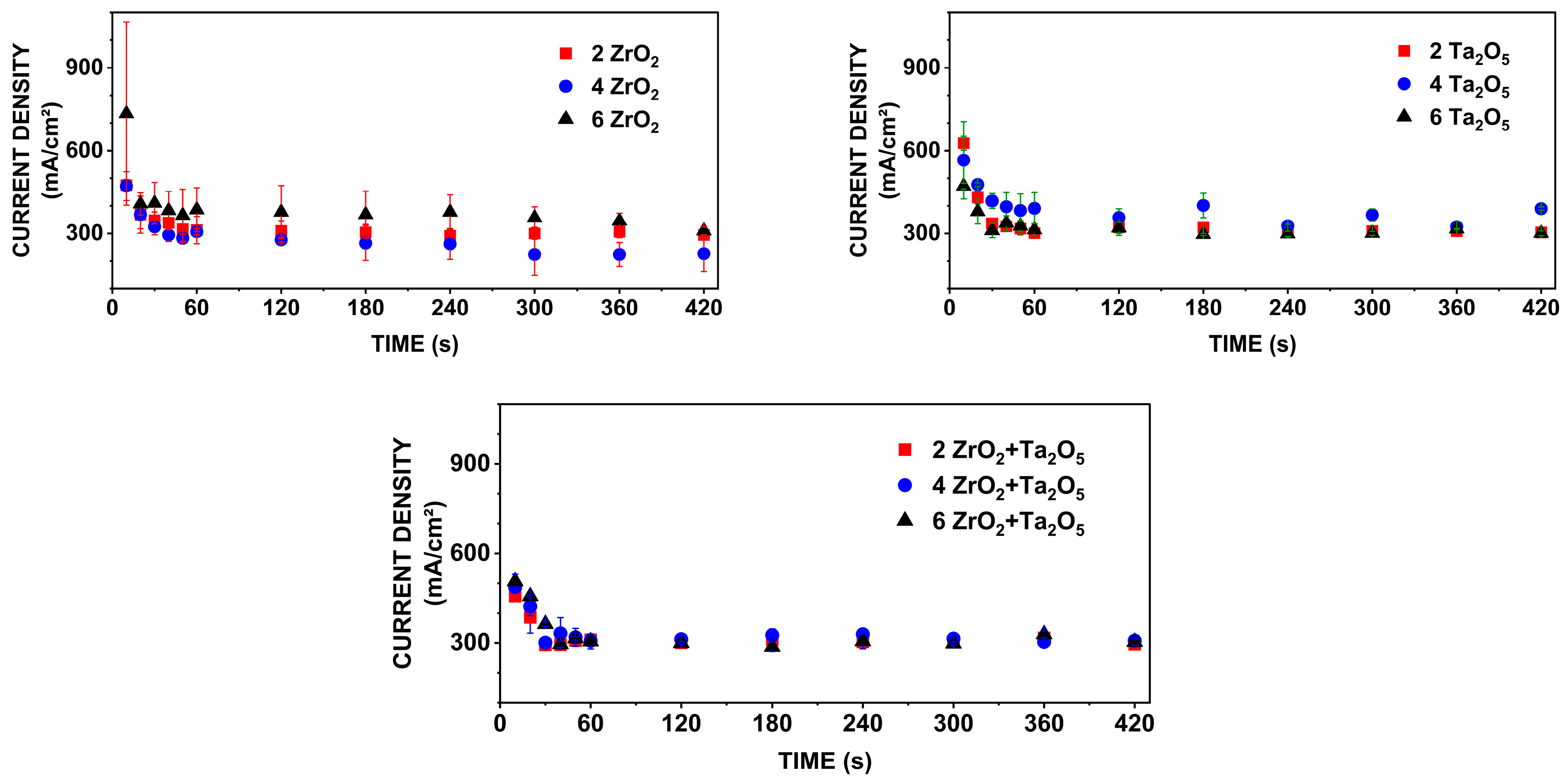
3.1. Current-Time Curves
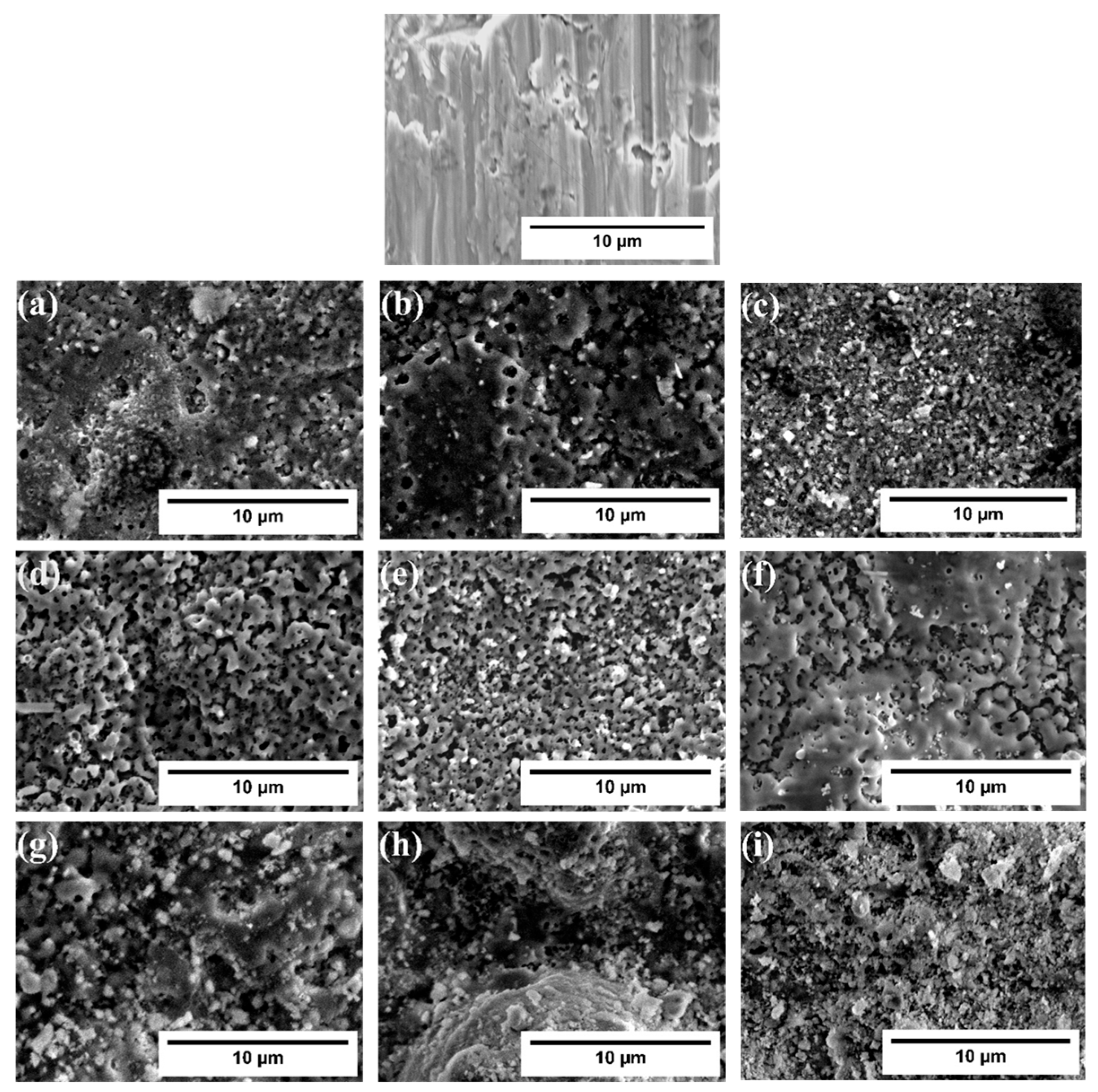
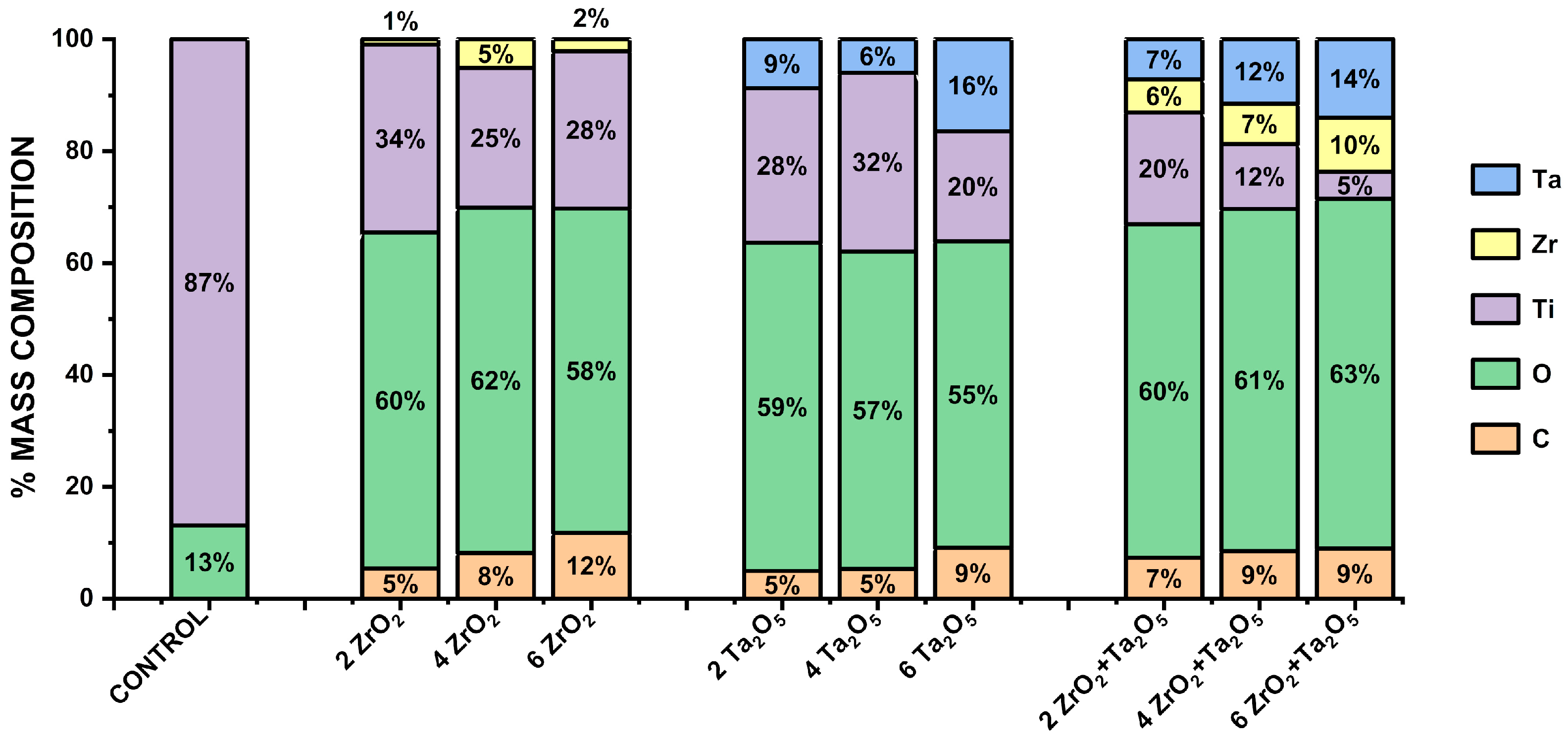
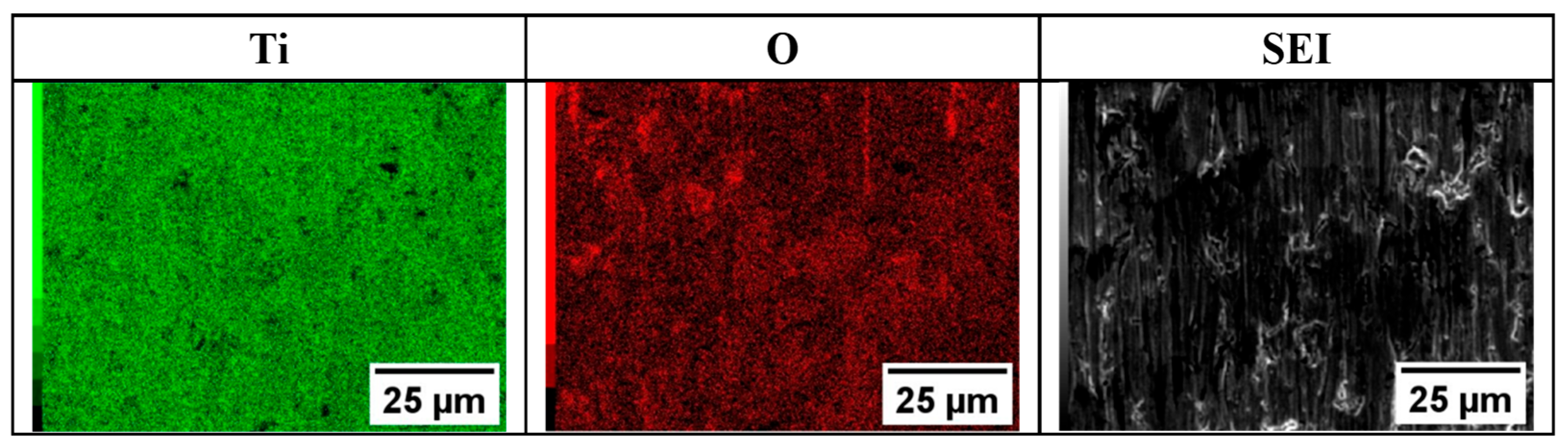
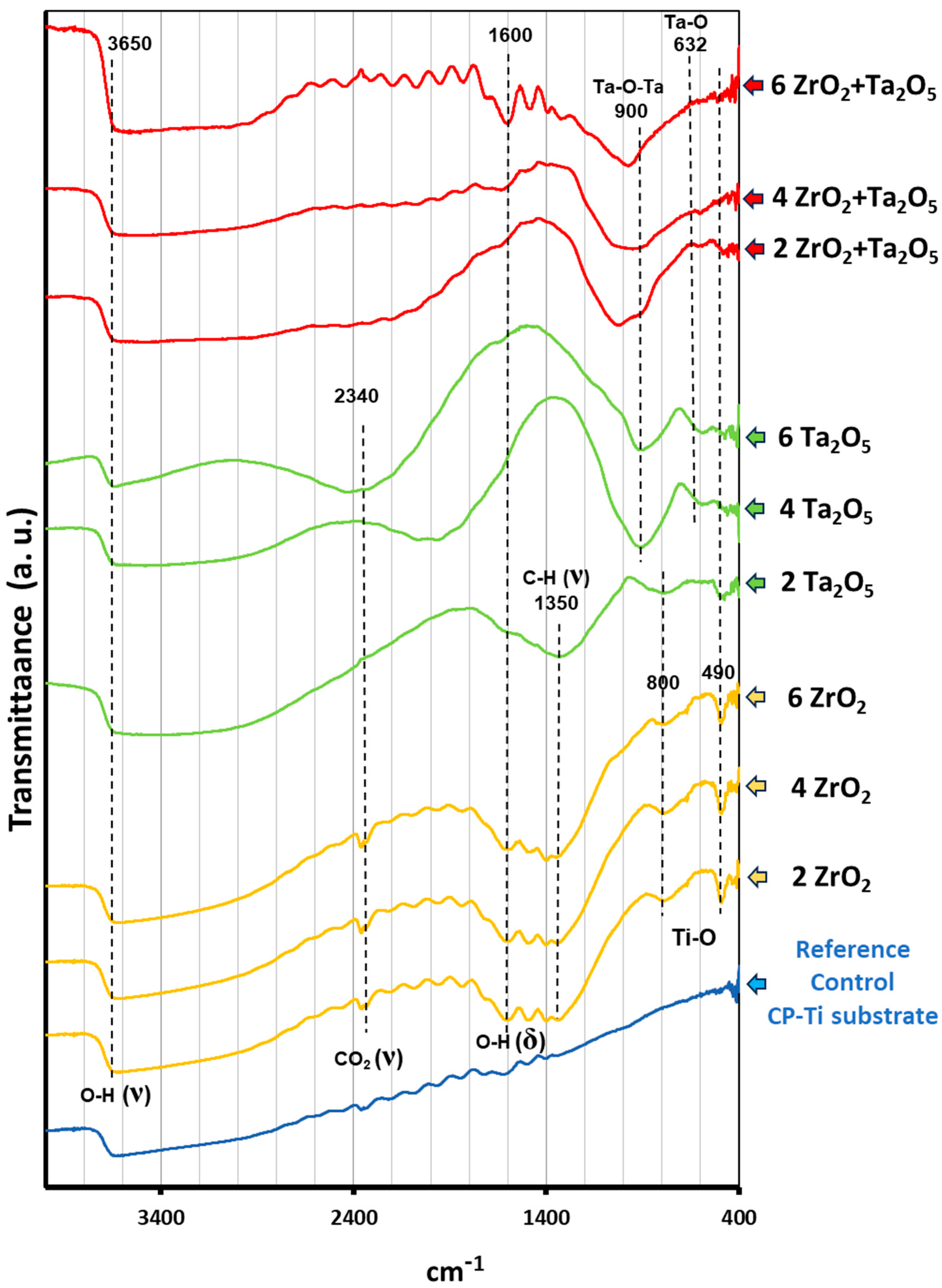
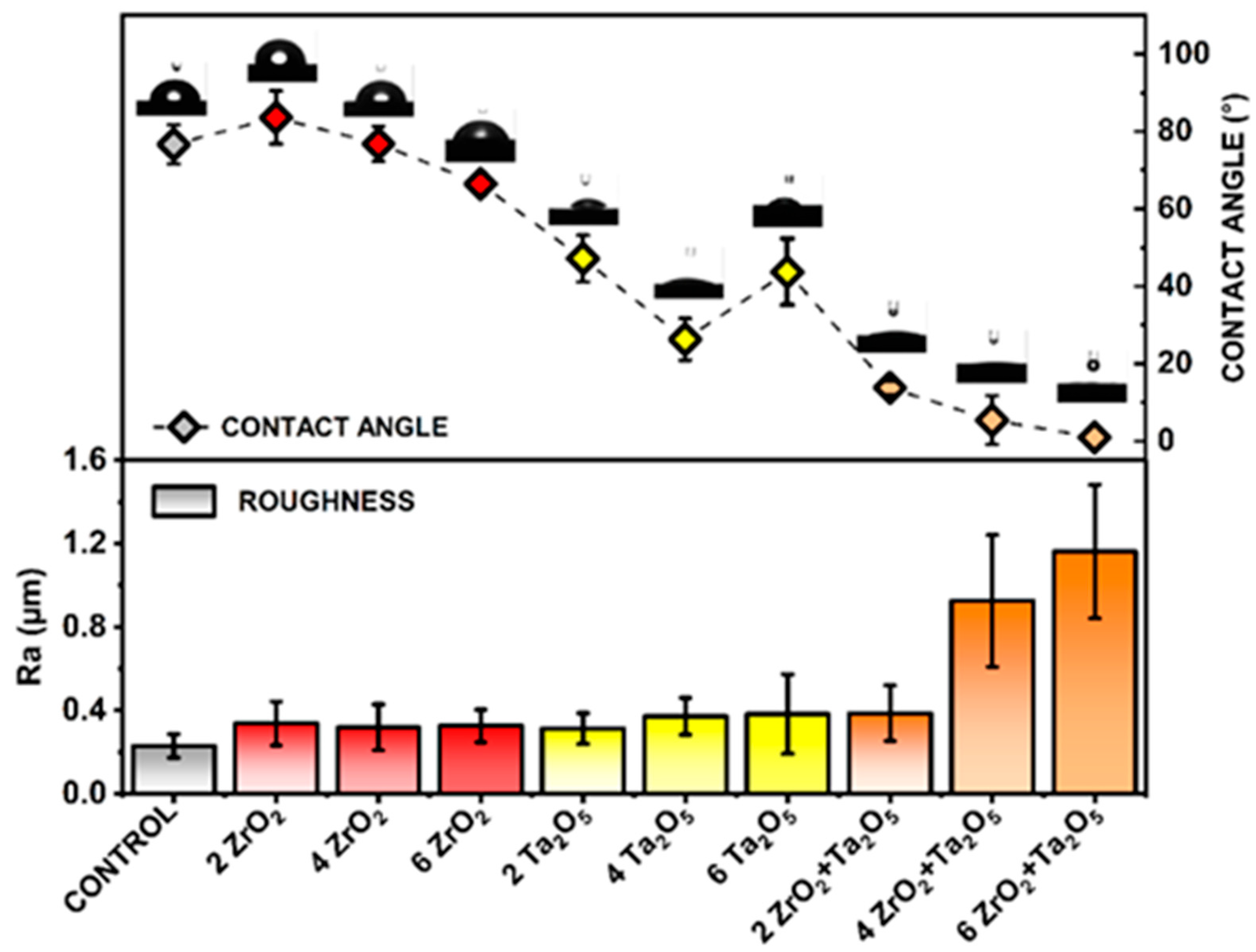
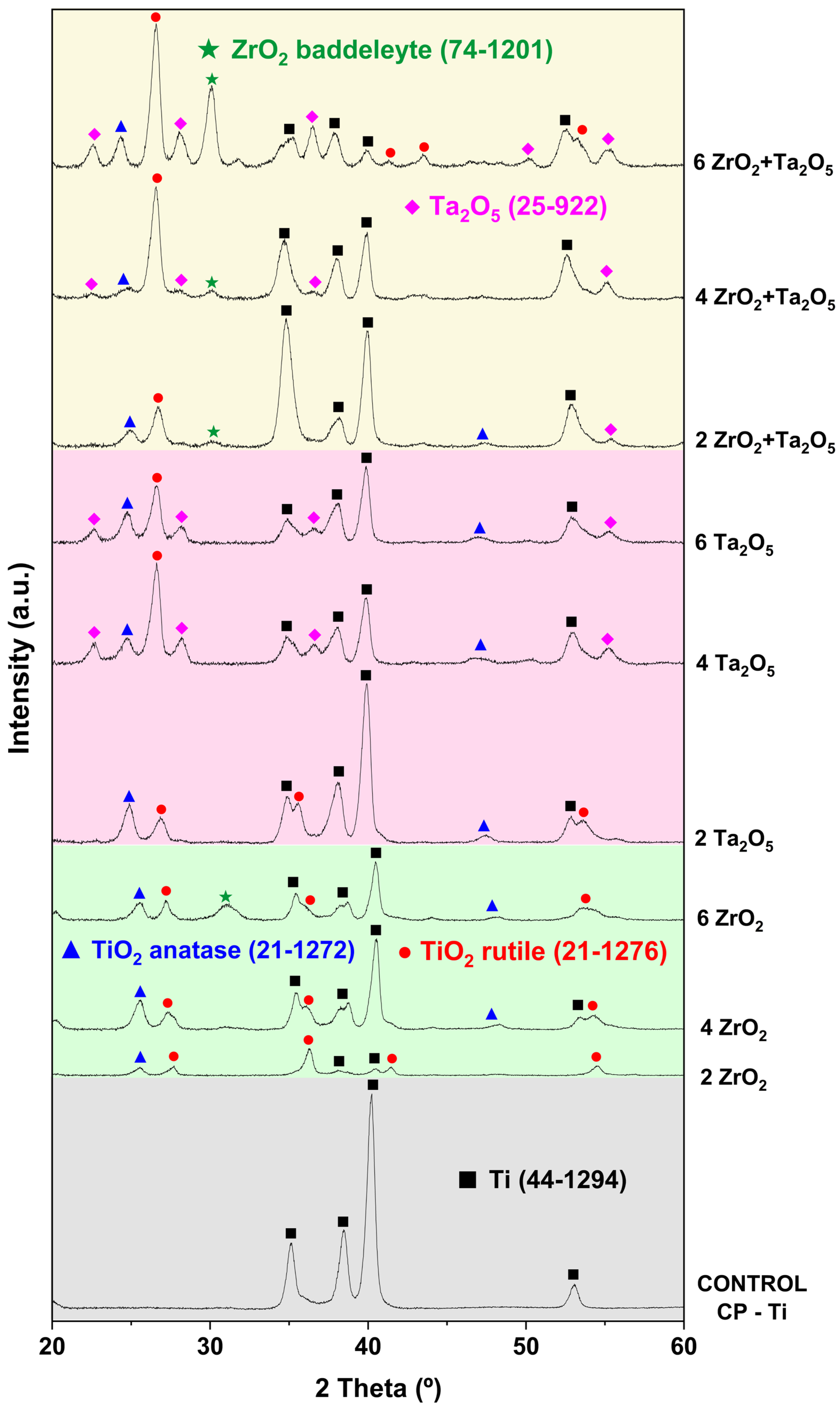
3.2. Morphological and Compositional Analysis
3.3. Electrochemical Analysis
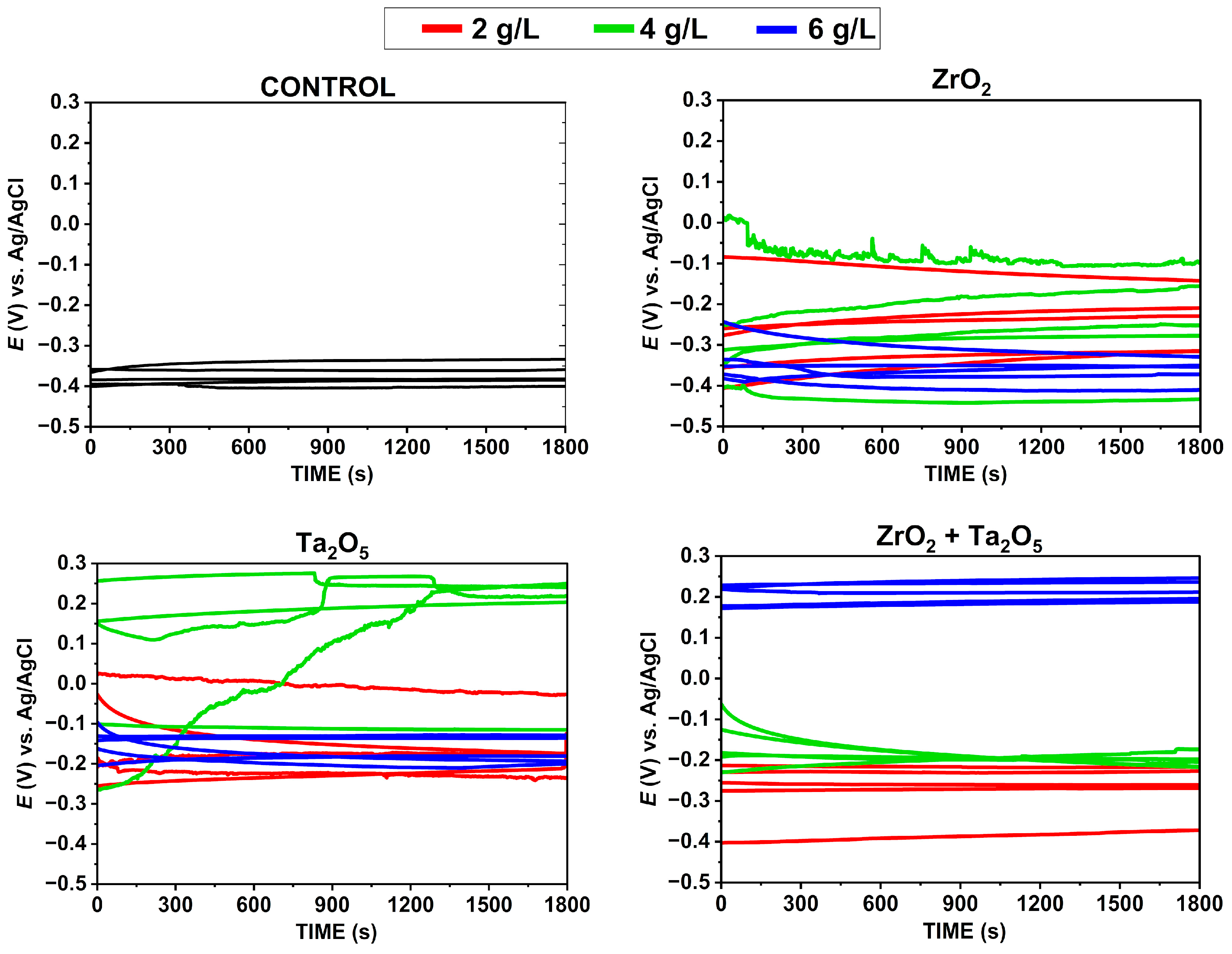
3.3.1. Open Circuit Potential — OCP Measurements
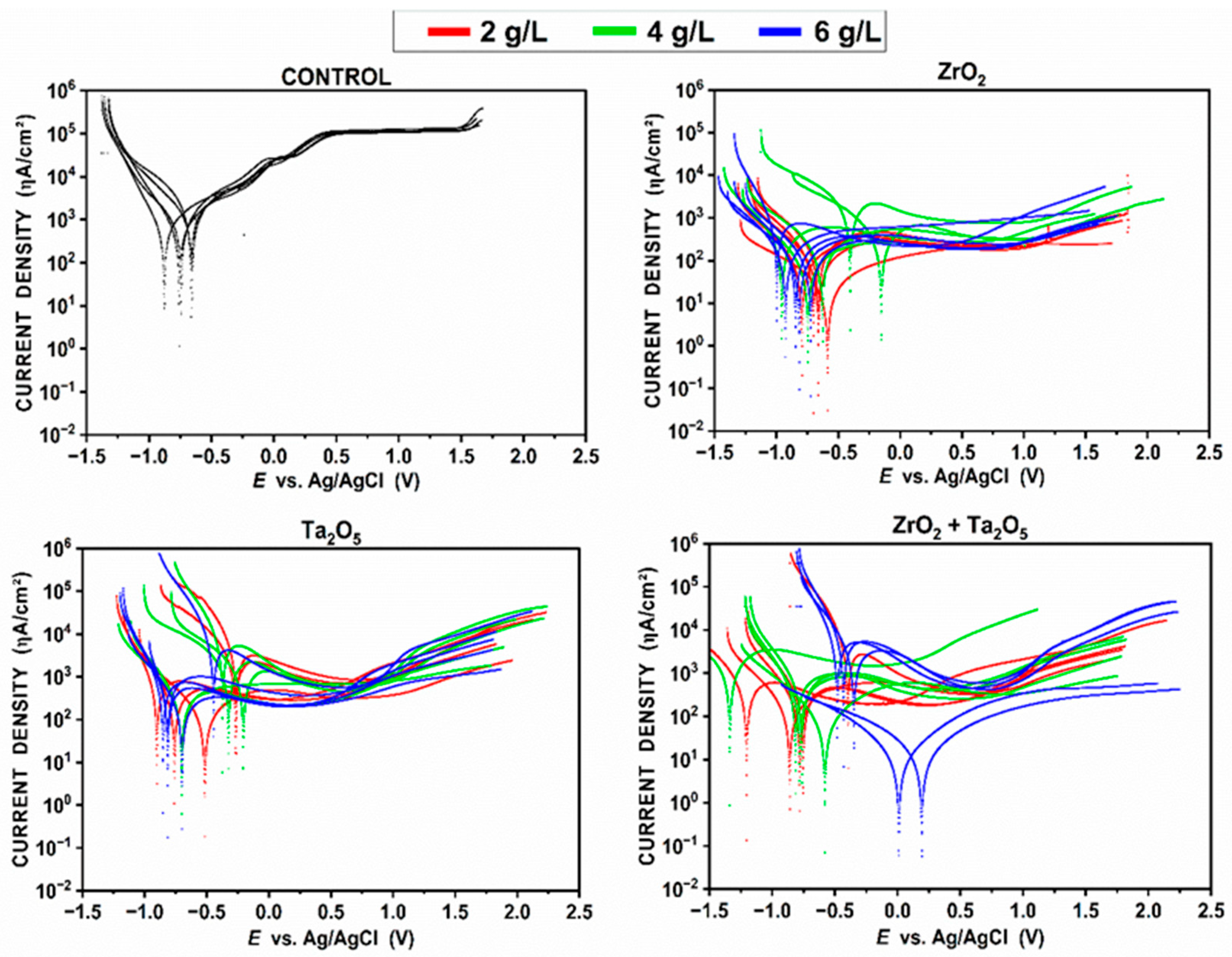
3.3.2. Polarization Direct Scanning—PDS Measurements
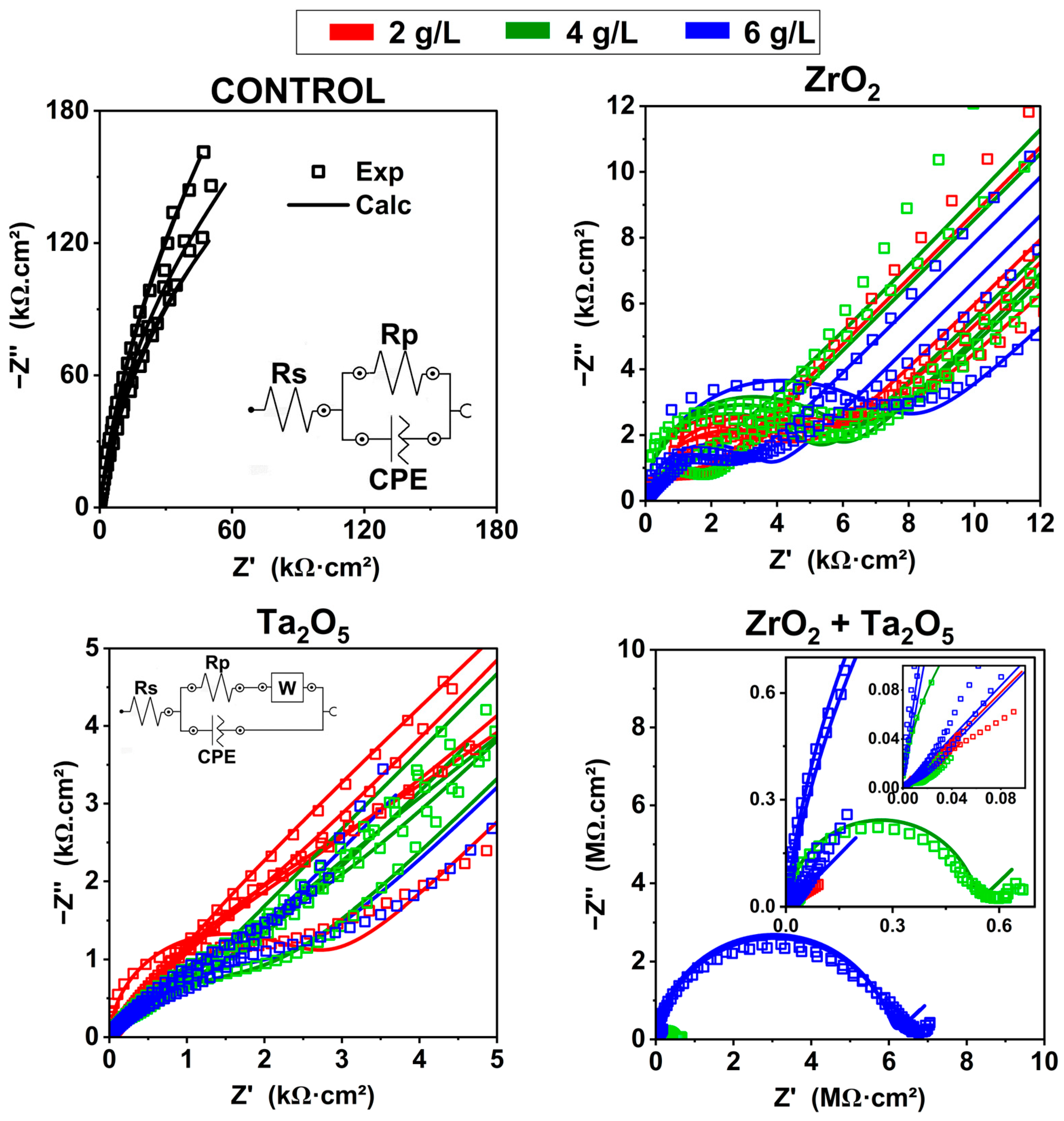
3.3.3. Electrochemical Impedance Spectroscopy—EIS Measurements
3.4. General Considerations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MDPI | Multidisciplinary Digital Publishing Institute |
| CP-Ti | Commercially pure titanium |
| PEO | Plasma electrolytic oxidation |
| SEM | Scanning electron microscope |
| EDX | Energy dispersive X-ray spectroscopy |
| XRD | X-ray diffraction |
| FTIR | Fourier transform infrared spectroscopy |
| MAO | Micro-arc oxidation |
| SP | São Paulo |
| MG | Minas Gerais |
| AZ | Arizona |
| NJ | New Jersey |
| USA | United States of America |
| PGSTAT | Potentiostat/galvanostat |
| AG | Corporation (Aktiengesellschaft) |
| OCP | Open circuit potential |
| EIS | Electrochemical impedance spectroscopy |
| PDS | Polarization direct scanning |
| ICDD | International center for diffraction data |
| Ecorr | Corrosion potential |
| Rs | Solution resistance |
| Rp | Polarization resistance |
| CPE | Constant phase element |
| W | Warburg element |
| AMG | Advanced Metallurgical Group |
References
- Chen, Y.; Zhang, H.; Wang, B.; Huang, J.; Zhou, M.; Wang, L.; Xi, Y.; Jia, H.; Xu, S.; Liu, H.; et al. A Review of Research on Improving Wear Resistance of Titanium Alloys. Coatings 2024, 14, 786. [Google Scholar] [CrossRef]
- Lin, Z.; Song, K.; Yu, X. A Review on Wire and Arc Additive Manufacturing of Titanium Alloy. J. Manuf. Process. 2021, 70, 24–45. [Google Scholar] [CrossRef]
- Zuo, D.; Zhang, Z.; Lai, Y.; Zhang, G. Research on Failure Mechanism of Condenser Titanium Tube in Nuclear Power Plant. J. Phys. Conf. Ser. 2021, 2044, 012122. [Google Scholar] [CrossRef]
- Destefani, J.D. Properties and Selection: Nonferrous Alloys and Special-Purpose Materials; ASM International: Materials Park, OH, USA, 2023; Volume 2, ISBN 978-1-62708-026-2. [Google Scholar]
- Almeraya-Calderón, F.; Jáquez-Muñoz, J.M.; Lara-Banda, M.; Zambrano-Robledo, P.; Cabral-Miramontes, J.A.; Lira-Martínez, A.; Estupinán-López, F.; Gaona Tiburcio, C. Corrosion Behavior of Titanium and Titanium Alloys in Ringer’s Solution. Int. J. Electrochem. Sci. 2022, 17, 220751. [Google Scholar] [CrossRef]
- Aliofkhazraei, M.; Macdonald, D.D.; Matykina, E.; Parfenov, E.V.; Egorkin, V.S.; Curran, J.A.; Troughton, S.C.; Sinebryukhov, S.L.; Gnedenkov, S.V.; Lampke, T.; et al. Review of Plasma Electrolytic Oxidation of Titanium Substrates: Mechanism, Properties, Applications and Limitations. Appl. Surf. Sci. Adv. 2021, 5, 100121. [Google Scholar] [CrossRef]
- Yerokhin, A.L.; Nie, X.; Leyland, A.; Matthews, A. Characterisation of Oxide Films Produced by Plasma Electrolytic Oxidation of a Ti–6Al–4V Alloy. Surf. Coat. Technol. 2000, 130, 195–206. [Google Scholar] [CrossRef]
- Liu, R.; Xie, Y.; Jin, Y.; Cui, Y.; Liu, L.; Wang, F. Stress Corrosion Cracking of the Titanium Alloys under Hydrostatic Pressure Resulting from the Degradation of Passive Films. Acta Mater. 2023, 252, 118946. [Google Scholar] [CrossRef]
- Kyrylenko, S.; Warchoł, F.; Oleshko, O.; Husak, Y.; Kazek-Kęsik, A.; Korniienko, V.; Deineka, V.; Sowa, M.; Maciej, A.; Michalska, J.; et al. Effects of the Sources of Calcium and Phosphorus on the Structural and Functional Properties of Ceramic Coatings on Titanium Dental Implants Produced by Plasma Electrolytic Oxidation. Mater. Sci. Eng. C 2021, 119, 111607. [Google Scholar] [CrossRef]
- Sathish, M.; Radhika, N.; Saleh, B. A Critical Review on Functionally Graded Coatings: Methods, Properties, and Challenges. Compos. Part B Eng. 2021, 225, 109278. [Google Scholar] [CrossRef]
- Costa, R.C.; Nagay, B.E.; Dini, C.; Borges, M.H.R.; Miranda, L.F.B.; Cordeiro, J.M.; Souza, J.G.S.; Sukotjo, C.; Cruz, N.C.; Barão, V.A.R. The Race for the Optimal Antimicrobial Surface: Perspectives and Challenges Related to Plasma Electrolytic Oxidation Coating for Titanium-Based Implants. Adv. Colloid. Interface Sci. 2023, 311, 102805. [Google Scholar] [CrossRef]
- Pesode, P.; Barve, S. Surface Modification of Titanium and Titanium Alloy by Plasma Electrolytic Oxidation Process for Biomedical Applications: A Review. Mater. Today Proc. 2021, 46, 594–602. [Google Scholar] [CrossRef]
- Kaseem, M.; Fatimah, S.; Nashrah, N.; Ko, Y.G. Recent Progress in Surface Modification of Metals Coated by Plasma Electrolytic Oxidation: Principle, Structure, and Performance. Prog. Progress. Mater. Sci. 2021, 117, 100735. [Google Scholar] [CrossRef]
- Molaei, M.; Fattah-alhosseini, A.; Nouri, M.; Nourian, A. Systematic Optimization of Corrosion, Bioactivity, and Biocompatibility Behaviors of Calcium-Phosphate Plasma Electrolytic Oxidation (PEO) Coatings on Titanium Substrates. Ceram. Int. 2022, 48, 6322–6337. [Google Scholar] [CrossRef]
- Jadhav, P.; Bongale, A.; Kumar, S. A Review of Process Characteristics of Plasma Electrolytic Oxidation of Aluminium Alloy. J. Phys. Conf. Ser. 2021, 1854, 012030. [Google Scholar] [CrossRef]
- Simchen, F.; Sieber, M.; Kopp, A.; Lampke, T. Introduction to Plasma Electrolytic Oxidation—An Overview of the Process and Applications. Coatings 2020, 10, 628. [Google Scholar] [CrossRef]
- da Silva, A.C.N.; Ribeiro, R.P.; Rangel, E.C.; da Cruz, N.C.; Correa, D.R.N. Production of Porous ZrO2–TiO2 Ceramic Coatings on the Biomedical Ti-6Al-4V Alloy via AC PEO Treatment and Their Effects on the Corrosion Behavior in 0.9% NaCl. Coatings 2024, 14, 866. [Google Scholar] [CrossRef]
- Sikdar, S.; Menezes, P.V.; Maccione, R.; Jacob, T.; Menezes, P.L. Plasma Electrolytic Oxidation (PEO) Process—Processing, Properties, and Applications. Nanomaterials 2021, 11, 1375. [Google Scholar] [CrossRef]
- Tsai, D.-S.; Chou, C.-C. Influences of Growth Species and Inclusions on the Current–Voltage Behavior of Plasma Electrolytic Oxidation: A Review. Coatings 2021, 11, 270. [Google Scholar] [CrossRef]
- Magić, M.; Čolović, B.; Vasilijić, S.; Tadić, N.; Stojadinović, S.; Jokanović, V. Nanodesigned Coatings Obtained by Plasma Electrolytic Oxidation of Titanium Implant and Their Cytotoxicity. J. Appl. Biomater. Funct. Mater. 2021, 19, 2280800018822252. [Google Scholar] [CrossRef]
- Hou, W.; Lou, N.; Wang, H.; Cai, Z.; Xing, Z.; Piao, Z. Research on the Microstructure and Corrosion-Wear Resistance Mechanism of Ti–6Al–4V Alloy under Cryogenic Burnishing. J. Mater. Res. Technol. 2025, 35, 5854–5871. [Google Scholar] [CrossRef]
- Li, J.; Zhu, C.; Cao, F.; Sun, Q. Nanoscale Pitting Corrosion of Commercially Pure Ti in Solution Containing Fluoride Ion. Mater. Chem. Phys. 2024, 323, 129650. [Google Scholar] [CrossRef]
- Molaei, M.; Nouri, M.; Babaei, K.; Fattah-Alhosseini, A. Improving Surface Features of PEO Coatings on Titanium and Titanium Alloys with Zirconia Particles: A Review. Surf. Interfaces 2021, 22, 100888. [Google Scholar] [CrossRef]
- de Proença, J.P.; Ribeiro, R.P.; Rangel, E.C.; da Cruz, N.C.; Pinto, B.O.; Grandini, C.R.; Correa, D.R.N. Alloying Element Depletion and Phase Transition in Stainless Steel 304 Induced by PEO Treatment in KOH- and TaOH-Rich Electrolyte. Crystals 2023, 13, 1480. [Google Scholar] [CrossRef]
- Marcuz, N.; Ribeiro, R.P.; Rangel, E.C.; da Cruz, N.C.; Correa, D.R.N. The Effect of PEO Treatment in a Ta-Rich Electrolyte on the Surface and Corrosion Properties of Low-Carbon Steel for Potential Use as a Biomedical Material. Metals 2023, 13, 520. [Google Scholar] [CrossRef]
- Molaei, M.; Fattah-alhosseini, A.; Nouri, M.; Mahmoodi, P.; Navard, S.H.; Nourian, A. Enhancing Cytocompatibility, Antibacterial Activity and Corrosion Resistance of PEO Coatings on Titanium Using Incorporated ZrO2 Nanoparticles. Surf. Interfaces 2022, 30, 101967. [Google Scholar] [CrossRef]
- Webber, L.P.; Chan, H.-L.; Wang, H.-L. Will Zirconia Implants Replace Titanium Implants? Appl. Sci. 2021, 11, 6776. [Google Scholar] [CrossRef]
- Li, Y.-J.; Hsieh, Y.-H.; Lin, W.-T.; Tran, H.-C.; Huang, J.-W.; Kuo, T.-Y.; Chien, C.-S. Comparison of Laser Cladding Properties of Tantalum and Tantalum Pentoxide Powders on Titanium Substrates. Int. J. Adv. Manuf. Technol. 2024, 132, 5457–5471. [Google Scholar] [CrossRef]
- Marcuz, N.; Ribeiro, R.P.; Rangel, E.C.; Cruz, N.C.; Possato, L.G.; Coan, K.S.; Grandini, C.R.; Correa, D.R.N. Exploiting the Effect of PEO Parameters on the Surface of AISI 1020 Low-Carbon Steel Treated in a TaOH-Rich Electrolyte. Surf. Coat. Technol. 2024, 477, 130374. [Google Scholar] [CrossRef]
- Mashtalyar, D.V.; Imshinetskiy, I.M.; Kashepa, V.V.; Nadaraia, K.V.; Piatkova, M.A.; Pleshkova, A.I.; Fomenko, K.A.; Ustinov, A.Y.; Sinebryukhov, S.L.; Gnedenkov, S.V. Effect of Ta2O5 Nanoparticles on Bioactivity, Composition, Structure, in Vitro and in Vivo Behavior of PEO Coatings on Mg-Alloy. J. Magnes. Alloys 2024, 12, 2360–2379. [Google Scholar] [CrossRef]
- Li, H.; Ding, Y.; Hu, X.; Li, W.; Liao, C.; Ding, Z. Microstructure and Performance of Compositionally Graded Ta2O5/Ti Thin Films on Ti6Al4V Alloy Deposited by Magnetron Sputtering. Surf. Coat. Technol. 2024, 494, 131443. [Google Scholar] [CrossRef]
- Li, H.; Ding, Y.; Hu, X.; Li, W.; Ding, Z. A Comparative Study of TiO2, Ta2O5 and Nb2O5 Coated Ti6Al4V Titanium Alloy for Biomedical Applications. Ceram. Int. 2024, 50, 50444–50453. [Google Scholar] [CrossRef]
- Wu, S.; He, M.; Yang, M.; Zhang, B.; Wang, F.; Li, Q. Near-Infrared Spectroscopy Study of Serpentine Minerals and Assignment of the OH Group. Crystals 2021, 11, 1130. [Google Scholar] [CrossRef]
- Amaral, M.M.; Yukuhiro, V.Y.; Vicentini, R.; Peterlevitz, A.C.; Da Silva, L.M.; Fernandez, P.; Zanin, H. Direct Observation of the CO2 Formation and C–H Consumption of Carbon Electrode in an Aqueous Neutral Electrolyte Supercapacitor by in-Situ FTIR and Raman. J. Energy Chem. 2022, 71, 488–496. [Google Scholar] [CrossRef]
- Wang, H.; Abruña, H.D. Identifying Adsorbed OH Species on Pt and Ru Electrodes with Surface-Enhanced Infrared Absorption Spectroscopy through CO Displacement. J. Am. Chem. Soc. 2023, 145, 18439–18446. [Google Scholar] [CrossRef]
- Hosseini, M.; Khalil-Allafi, J.; Safavi, M.S.; Ghalandarzadeh, A. Fascinating Osteoblast Compatibility and Antibacterial Activity of HA-Ta2O5 Composite Coating Deposited by Plasma Electrolytic Oxidation. J. Alloys Compd. 2025, 1023, 180193. [Google Scholar] [CrossRef]
- García-Contreras, L.A.; Flores-Flores, J.O.; Arenas-Alatorre, J.Á.; Chávez-Carvayar, J.Á. Synthesis, Characterization and Study of the Structural Change of Nanobelts of TiO2 (H2Ti3O7) to Nanobelts with Anatase, Brookite and Rutile Phases. J. Alloys Compd. 2022, 923, 166236. [Google Scholar] [CrossRef]
- Bright, T.J.; Watjen, J.I.; Zhang, Z.M.; Muratore, C.; Voevodin, A.A.; Koukis, D.I.; Tanner, D.B.; Arenas, D.J. Infrared Optical Properties of Amorphous and Nanocrystalline Ta2O5 Thin Films. J. Appl. Phys. 2013, 114, 083515. [Google Scholar] [CrossRef]
- Brandsrud, M.A.; Blümel, R.; Solheim, J.H.; Kohler, A. The Effect of Deformation of Absorbing Scatterers on Mie-Type Signatures in Infrared Microspectroscopy. Sci. Rep. 2021, 11, 4675. [Google Scholar] [CrossRef]
- Bharti, B.; Kumar, S.; Kumar, R. Superhydrophilic TiO2 Thin Film by Nanometer Scale Surface Roughness and Dangling Bonds. Appl. Surf. Sci. 2016, 364, 51–60. [Google Scholar] [CrossRef]
- Yu, S.; Lu, X.; Lu, H. Marine Microbial Biofilms on Diverse Abiotic Surfaces. Front. Mar. Sci. 2025, 12, 1482946. [Google Scholar] [CrossRef]
- Knisz, J.; Eckert, R.; Gieg, L.M.; Koerdt, A.; Lee, J.S.; Silva, E.R.; Skovhus, T.L.; An Stepec, B.A.; Wade, S.A. Microbiologically Influenced Corrosion—More than Just Microorganisms. FEMS Microbiol. Rev. 2023, 47, fuad041. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.-H.; Lu, Y.-J.; Lan, W.-C.; Ruslin, M.; Lin, H.-Y.; Ou, K.-L.; Saito, T.; Tsai, H.-Y.; Lee, C.-H.; Cho, Y.-C.; et al. Surface Properties and Biocompatibility of Anodized Titanium with a Potential Pretreatment for Biomedical Applications. Metals 2021, 11, 1090. [Google Scholar] [CrossRef]
- Babaei, M.; Dehghanian, C.; Babaei, M. Electrochemical Assessment of Characteristics and Corrosion Behavior of Zr-Containing Coatings Formed on Titanium by Plasma Electrolytic Oxidation. Surf. Coat. Technol. 2015, 279, 79–91. [Google Scholar] [CrossRef]
- Kant, R.; Goel, H. In Situ Electrochemical Impedance Spectroscopic Method for Determination of Surface Roughness and Morphological Convexity. J. Phys. Chem. Lett. 2021, 12, 10025–10033. [Google Scholar] [CrossRef]
- Ahounbar, E.; Mousavi Khoei, S.M.; Urgen, M.; Shokouhimehr, M. Characteristics of the Hierarchical Porous TiO2 Layer Synthesized on Ti via Plasma Electrolytic Oxidation: Role of the Applied Voltage. Ceram. Int. 2021, 47, 8279–8289. [Google Scholar] [CrossRef]
- Gutiérrez-Menchaca, J.; Cabral-Miramontes, J.A.; Garay-Tapia, A.M.; Torres-Torres, D.; GaonaTiburcio, C.; Jaquez-Muñoz, J.M.; Almeraya-Calderón, F. Influence of Microstructure on Corrosion Behavior of Zn-Al-Sr Alloys in Sodium Chloride Solution. Int. J. Electrochem. Sci. 2022, 17, 221038. [Google Scholar] [CrossRef]
- Gowtham, S.; Hariprasad, S.; Arunnellaiappan, T.; Rameshbabu, N. An Investigation on ZrO2 Nano-Particle Incorporation, Surface Properties and Electrochemical Corrosion Behaviour of PEO Coating Formed on Cp-Ti. Surf. Coat. Technol. 2017, 313, 263–273. [Google Scholar] [CrossRef]
- Sowa, M.; Simka, W. Electrochemical Impedance and Polarization Corrosion Studies of Tantalum Surface Modified by DC Plasma Electrolytic Oxidation. Materials 2018, 11, 545. [Google Scholar] [CrossRef]
- Zehra, T.; Kaseem, M.; Hossain, S.; Ko, Y.-G. Fabrication of a Protective Hybrid Coating Composed of TiO2, MoO2, and SiO2 by Plasma Electrolytic Oxidation of Titanium. Metals 2021, 11, 1182. [Google Scholar] [CrossRef]


| Nomenclature | Electrolyte (g/L) | ||
|---|---|---|---|
| KOH | ZrO2 | Ta2O5 | |
| 2 ZrO2 | 2 | 2 | - |
| 4 ZrO2 | 4 | ||
| 6 ZrO2 | 6 | ||
| 2 Ta2O5 | 2 | - | 2 |
| 4 Ta2O5 | 4 | ||
| 6 Ta2O5 | 6 | ||
| 2 ZrO2 + Ta2O5 | 2 | 1 | 1 |
| 4 ZrO2 + Ta2O5 | 2 | 2 | |
| 6 ZrO2 + Ta2O5 | 3 | 3 | |
| Control | EIS Measurement Replicates | ||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| Rs | 10.96 (0.6) | 8.99 (0.6) | 10.21 (0.6) | 12.61 (0.9) | 11.58 (0.7) |
| (Ω·cm²) | |||||
| Rp | 4.79 × 105 (4.4) | 6.33 × 105 (4.5) | 5.22 × 105 (4.7) | 1.06 × 106 (12.9) | 1.03 × 106 (8.2) |
| (Ω·cm²) | |||||
| CPE-Y0 | 9.29 × 105 (0.4) | 7.69 × 105 (0.4) | 9.61 × 105 (0.3) | 8.29 × 105 (0.6) | 7.52 × 105 (0.4) |
| (S·sn·cm−2) | |||||
| CPE-n | 0.93 (0.1) | 0.92 (0.1) | 0.91 (0.1) | 0.92 (0.2) | 0.92 (0.1) |
| ZrO2 | EIS Measurement Replicates | |||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||
| Rs (Ω·cm2) | 2 ZrO2 | 10.87 (fixed) | 10.87 (fixed) | 21.40 (23.5) | 10.87 (fixed) | 10.87 (fixed) |
| 4 ZrO2 | 10.87 (fixed) | 10.87 (fixed) | 22.50 (14.5) | 10.87 (0.1) | 10.87 (0.1) | |
| 6 ZrO2 | 10.87 (fixed) | 10.87 (fixed) | 10.87 (fixed) | 10.87 (fixed) | 22.24 (7.0) | |
| Rp (Ω·cm2) | 2 ZrO2 | 6.64 × 103 (4.5) | 2.12 × 105 (5.6) | 1.29 × 103 (2.9) | 5.12 × 103 (2.0) | 4.27 × 103 (1.4) |
| 4 ZrO2 | 5.54 × 103 (1.9) | 4.56 × 103 (1.6) | 3.34 × 103 (14.6) | 1.48 × 103 (2.0) | 5.20 × 103 (1.9) | |
| 6 ZrO2 | 7.13 × 103 (2.3) | 2.24 × 103 (2.5) | 1.27 × 104 (3.1) | 3.39 × 103 (3.8) | 3.96 × 103 (22.5) | |
| CPE-Y0 (S·sn·cm−2) | 2 ZrO2 | 7.6 × 10−8 (28.5) | 6.0 × 10−9 (23.8) | 9.8 × 10−10 (20.7) | 2.0 × 10−9 (21.1) | 1.8 × 10−9 (16.3) |
| 4 ZrO2 | 4.2 × 10−10 (17.8) | 3.0 × 10−10 (15.9) | 1.4 × 10−5 (13.3) | 6.4 × 10−10 (27.0) | 5.6 × 10−10 (20.7) | |
| 6 ZrO2 | 8.8 × 10−10 (26.0) | 1.0 × 10−9 (29.9) | 2.3 × 10−9 (25.9) | 1.5 × 10−9 (27.6) | 2.2 × 10−5 (9.0) | |
| CPE-n | 2 ZrO2 | 0.69 (3.3) | 0.74 (2.8) | 1.04 (1.4) | 0.91 (1.8) | 0.93 (1.4) |
| 4 ZrO2 | 1.02 (1.3) | 1.05 (1.1) | 0.66 (2.4) | 1.05 (2.0) | 1.03 (1.6) | |
| 6 ZrO2 | 0.96 (2.1) | 1.00 (2.3) | 0.89 (2.3) | 0.96 (2.0) | 0.62 (1.6) | |
| W-Y0 (S·s1/2·cm−2) | 2 ZrO2 | 6.1 × 10−6 (3.4) | 5.9 × 10−7 (4.7) | 5.2 × 10−6 (1.3) | 2.7 × 10−6 (1.0) | 3.3 × 10−6 (0.6) |
| 4 ZrO2 | 2.5 × 10−6 (1.2) | 3.1 × 10−6 (1.3) | 5.2 × 10−5 (3.8) | 5.1 × 10−6 (0.8) | 4.1 × 10−6 (2.2) | |
| 6 ZrO2 | 2.7 × 10−6 (1.6) | 3.9 × 10−6 (1.0) | 2.0 × 10−6 (2.2) | 5.4 × 10−6 (2.3) | 4.0 × 10−5 (17.2) | |
| Ta2O5 | EIS Measurement Replicates | |||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||
| Rs (Ω·cm2) | 2 Ta2O5 | 10.87 (fixed) | 26.68 (8.5) | 24.01 (11.8) | 21.01 (12.2) | 25.09 (8.1) |
| 4 Ta2O5 | 10.87 (fixed) | 10.87 (fixed) | 10.87 (fixed) | 10.87 (fixed) | 10.87 (fixed) | |
| 6 Ta2O5 | 10.87 (fixed) | 10.87 (fixed) | 10.87 (fixed) | 10.87 (fixed) | 10.87 (fixed) | |
| Rp (Ω·cm2) | 2 Ta2O5 | 2.40 × 103 (3.9) | 2.17 × 104 (29.0) | 3.92 × 103 (17.6) | 4.65 × 103 (7.6) | 6.47 × 103 (4.9) |
| 4 Ta2O5 | 1.54 × 103 (5.9) | 4.96 × 102 (3.2) | 2.30 × 103 (2.8) | 2.65 × 103 (4.5) | 4.19 × 102 (5.2) | |
| 6 Ta2O5 | 2.28 × 103 (3.5) | 2.30 × 103 (7.6) | 2.63 × 103 (6.0) | 1.06 × 103 (11.1) | 3.65 × 103 (7.2) | |
| CPE-Y0 (S·sn·cm−2) | 2 Ta2O5 | 1.4 × 10−9 (23.5) | 1.5 × 10−5 (5.8) | 1.2 × 10−5 (11.4) | 9.6 × 10−6 (6.7) | 1.0 × 10−5 (4.2) |
| 4 Ta2O5 | 3.8 × 10−6 (10.8) | 5.4 × 10−6 (1.0) | 9.4 × 10−6 (5.9) | 2.6 × 10−6 (7.3) | 2.1 × 10−8 (22.3) | |
| 6 Ta2O5 | 9.4 × 10−10 (29.9) | 3.0 × 10−6 (15.7) | 6.3 × 10−6 (6.0) | 7.7 × 10−6 (17.9) | 9.6 × 10−6 (5.4) | |
| CPE-n | 2 Ta2O5 | 0.99 (1.7) | 0.59 (1.1) | 0.65 (2.0) | 0.68 (1.2) | 0.66 (0.8) |
| 4 Ta2O5 | 0.70 (1.5) | 0.24 (2.4) | 0.61 (1.0) | 0.68 (1.0) | 0.92 (1.7) | |
| 6 Ta2O5 | 1.02 (2.2) | 0.70 (2.1) | 0.65 (0.9) | 0.65 (2.5) | 0.57 (0.9) | |
| W-Y0 (S·s1/2·cm−2) | 2 Ta2O5 | 4.8 × 10−6 (2.6) | 1.3 × 10−5 (20.6) | 2.9 × 10−5 (4.6) | 3.9 × 10−5 (2.2) | 4.0 × 10−5 (1.8) |
| 4 Ta2O5 | 5.1 × 10−5 (2.3) | 2.3 × 10−6 (1.5) | 1.1 × 10−4 (1.9) | 2.6 × 10−5 (1.7) | 1.3 × 10−5 (1.3) | |
| 6 Ta2O5 | 3.1 × 10−6 (1.9) | 7.5 × 10−5 (5.4) | 3.6 × 10−5 (3.9) | 7.5 × 10−5 (4.6) | 2.8 × 10−5 (5.3) | |
| ZrO2 + Ta2O5 | EIS Measurement Replicates | |||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||
| Rs (Ω·cm2) | 2 ZrO2 + Ta2O5 | 8.51 (27.8) | 10.87 (fixed) | 10.87 (fixed) | 10.87 (fixed) | 10.87 (fixed) |
| 4 ZrO2 + Ta2O5 | 10.87 (fixed) | 10.87 (fixed) | 10.87 (fixed) | 13.14 (20.9) | 10.87 (fixed) | |
| 6 ZrO2 + Ta2O5 | 10.87 (fixed) | 10.87 (fixed) | 10.87 (fixed) | 10.87 (fixed) | 10.87 (fixed) | |
| Rp (Ω·cm2) | 2 ZrO2 + Ta2O5 | 5.85 × 102 (3.9) | 2.05 × 103 (5.2) | 6.35 × 104 (25.2) | 1.85 × 104 (12.5) | 2.75 × 103 (10.7) |
| 4 ZrO2 + Ta2O5 | 5.32 × 105 (1.2) | 1.09 × 104 (5.0) | 1.32 × 104 (5.8) | 4.04 × 104 (3.9) | 5.06 × 103 (4.7) | |
| 6 ZrO2 + Ta2O5 | 4.55 × 103 (7.8) | 8.74 × 103 (9.9) | 2.79 × 103 (23.8) | 6.07 × 106 (1.3) | 6.05 × 106 (1.3) | |
| CPE-Y0 (S·sn·cm−2) | 2 ZrO2 + Ta2O5 | 2.9 × 10−9 (24.2) | 1.8 × 10−9 (28.4) | 1.5 × 10−5 (5.7) | 4.5 × 10−6 (10.6) | 3.9 × 10−6 (9.9) |
| 4 ZrO2 + Ta2O5 | 2.9 × 10−9 (5.6) | 6.5 × 10−7 (12.6) | 1.1 × 10−6 (10.2) | 1.0 × 10−5 (1.4) | 3.1 × 10−5 (4.6) | |
| 6 ZrO2 + Ta2O5 | 4.6 × 10−6 (7.5) | 7.9 × 10−7 (15.3) | 5.25 × 10−6 (20.6) | 3.3 × 10−9 (3.4) | 2.5 × 10−9 (3.7) | |
| CPE-n | 2 ZrO2 + Ta2O5 | 1.01 (1.7) | 1.00 (2.0) | 0.45 (1.3) | 0.58 (1.8) | 0.77 (1.3) |
| 4 ZrO2 + Ta2O5 | 0.94 (0.7) | 0.74 (1.7) | 0.74 (1.4) | 0.51 (0.3) | 0.51 (1.0) | |
| 6 ZrO2 + Ta2O5 | 0.65 (1.1) | 0.73 (2.0) | 0.67 (2.8) | 0.90 (0.5) | 0.93 (0.5) | |
| W-Y0 (S·s1/2·cm−2) | 2 ZrO2 + Ta2O5 | 8.3 × 10−6 (1.0) | 5.3 × 10−6 (2.4) | 9.1 × 10−6 (13.4) | 2.2 × 10−5 (7.9) | 3.5 × 10−5 (3.9) |
| 4 ZrO2 + Ta2O5 | 8.6 × 10−6 (12.1) | 4.3 × 10−5 (7.6) | 3.3 × 10−5 (6.1) | 1.0 × 10−5 (1.7) | 1.5 × 10−4 (3.8) | |
| 6 ZrO2 + Ta2O5 | 1.9 × 10−5 (1.9) | 1.3 × 10−5 (2.9) | 2.4 × 10−5 (4.6) | 3.3 × 10−6 (17.5) | 3.7 × 10−6 (20.1) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruberti, L.; Acciari, H.A.; Correa, D.R.N.; Pissolitto, Y.B.; Rangel, E.C.; Trivinho-Strixino, F.; da Cruz, N.C. Effect on the Electrochemical Properties of PEO Films Produced on Commercially Pure Titanium Using Multicomponent Oxide Coatings. Metals 2025, 15, 658. https://doi.org/10.3390/met15060658
Ruberti L, Acciari HA, Correa DRN, Pissolitto YB, Rangel EC, Trivinho-Strixino F, da Cruz NC. Effect on the Electrochemical Properties of PEO Films Produced on Commercially Pure Titanium Using Multicomponent Oxide Coatings. Metals. 2025; 15(6):658. https://doi.org/10.3390/met15060658
Chicago/Turabian StyleRuberti, Lauri, Heloisa Andréa Acciari, Diego Rafael Nespeque Correa, Yasmin Bastos Pissolitto, Elidiane Cipriano Rangel, Francisco Trivinho-Strixino, and Nilson Cristino da Cruz. 2025. "Effect on the Electrochemical Properties of PEO Films Produced on Commercially Pure Titanium Using Multicomponent Oxide Coatings" Metals 15, no. 6: 658. https://doi.org/10.3390/met15060658
APA StyleRuberti, L., Acciari, H. A., Correa, D. R. N., Pissolitto, Y. B., Rangel, E. C., Trivinho-Strixino, F., & da Cruz, N. C. (2025). Effect on the Electrochemical Properties of PEO Films Produced on Commercially Pure Titanium Using Multicomponent Oxide Coatings. Metals, 15(6), 658. https://doi.org/10.3390/met15060658












