Abstract
In the present study, fatigue performance of 6061-T6 and 6082-T6 commercially available extruded aluminum alloys in dry air and 3.5 wt% NaCl-saturated environment was investigated and compared. It was found that the aggressive chloride environment accelerated fatigue failure by up to an order of magnitude compared to laboratory air. Furthermore, alloy 6061-T6 shows more predictable fatigue life, having less scatter in its time to failure in a corrosive environment. The presence of localized pitting corrosion, particularly in Fe-rich intermetallic phases, provides initiation sites for fatigue cracks, leading to premature failure in both alloys. The corrosion fatigue cracks dominantly propagate through the grain interiors rather than along grain boundaries, indicating a tendency to transgranular crack propagation mechanisms. The effect of different loading frequencies (10 Hz and 0.2 Hz) on the corrosion fatigue life of 6061-T6 alloy showed a slightly enhanced fatigue life at the higher frequency. It was also found that alloy 6061-T6 was susceptible to pitting corrosion in NaCl-saturated environments with concentrations ranging between 0.5 wt% and 3.5 wt% without exhibiting significant changes in fatigue life.
1. Introduction
Due to modern engineering and sustainability requirements, lightweight structures are becoming more and more necessary in service. Weight reduction in the transportation sector can be achieved either by using less material or by substituting lighter materials, providing higher functionality of vehicles. Aluminum alloys exhibit good performance and remain lightweight alternatives to steel in the automotive industry. Replacing steel with aluminum reduces the weight of a vehicle by 60–65% [1]. Also, it improves fuel efficiency. In particular, fuel consumption of an aluminum vehicle can be decreased by 20% when compared to steel. Even the increasing use of fiber-reinforced polymer composites in the automotive sector has not altered aluminum’s relevance. For economic and sustainability reasons, polymer composites production remains small-scale [1,2].
In addition to their light weight, aluminum alloys are attractive due to their good formability, weldability, and corrosion resistance under atmospheric conditions. Nevertheless, the exposure of aluminum components to severe environmental conditions, in particular salty water containing chlorides, causes their degradation. The combination of cyclic loading and corrosive environments during operation can lead to earlier initiation of fatigue cracks and structural failures. This is why corrosion fatigue is an issue for the application of aluminum in transportation in regions close to the sea or where road salts are used in winter. The environmentally exacerbated cracking in aluminum alloys is usually initiated by passive film breakdown and localized pitting or intergranular corrosion [3,4]. Subjected to aggressive environmental conditions, pit growth occurs until it transitions into cracking governed by anodic dissolution and hydrogen embrittlement mechanisms. Moreover, as well as surface imperfections, secondary phases such as precipitates at grain boundaries and intermetallic phases can be a root cause of localized corrosion in aluminum. The predominant type of corrosion (pitting or intergranular) in aluminum can be affected, among other factors, by the composition and the microstructure of the alloy.
The susceptibility to pitting and intergranular corrosion of Al-Mg-Si (6xxx series) alloys has also been widely documented in the literature. This susceptibility is primarily associated with the presence of secondary phases in the aluminum matrix. The extruded alloys usually contain a relatively high concentration of Fe, which forms several kinds of Fe-rich intermetallic phases by consuming Al, Cu, Mn or Si. Fe-rich intermetallic phases can be the root cause of localized pitting corrosion in Al-Mg-Si [5,6]. With the addition of Cu, not only Cu-containing precipitates, but also thin Cu-rich films along grain boundaries act as cathodic areas relative to the surrounding aluminum matrix, accelerating anodic dissolution in grains and causing intergranular corrosion of Al-Mg-Si alloys [3,7,8]. Although the presence of Cu is of primary importance for intergranular corrosion of 6xxx series alloys, their susceptibility to it can also be affected by the presence of Mg and Si. For example, alloys containing 0.14 wt% of Cu and a Mg/Si ratio of 2.13 showed predominantly pitting corrosion, whereas with a Mg/Si ratio of 0.55, significant intergranular corrosion was observed [9]. In the case of corrosion fatigue, crack initiation is an important stage connected with the pit–crack transition time which depends on the susceptibility of the alloy to localized corrosion. Therefore, the objective of this study was to investigate the fatigue and corrosion performance of the widely used 6061-T6 and 6082-T6 aluminum alloys with a focus on their different composition. Also, in contrast to previous similar studies [10,11], this research focuses on corrosion fatigue at a low frequency of 0.2 Hz, since crack tip conditions can be changed at frequencies below 1 Hz and crack propagation mechanisms during corrosion fatigue can be affected, as was reported in [12]. In addition, characterization of the microstructure and initial surface defects was carried out to better understand the corrosion, fatigue and corrosion fatigue mechanisms affecting material degradation. Furthermore, an in-depth understanding of the underlying corrosion fatigue mechanisms can provide a better prediction of the material’s service life.
2. Materials and Methods
In the present study, tests were performed on the commercial aluminum alloys 6061-T6 and 6082-T6 (Constellium ES, Děčín, Czech Republic) supplied as extruded round bars of 15 mm in diameter. According to the X-ray fluorescent analysis, the content of the main alloying elements of the alloys is given in Table 1. The manufacturer’s specification and laboratory control of tensile properties are also given in Table 2.

Table 1.
Chemical composition of the 6061-T6 and 6082-T6 aluminum alloys.

Table 2.
Mechanical properties of the 6061-T6 and 6082-T6 alloys.
Specimens for fatigue tests with a gauge diameter of 6 mm and a gauge length of 18 mm were machined in the longitudinal direction of extrusion. The geometry and the size of specimens were according to ISO Standard (EN ISO 11782-1) [13]. In order to obtain conditions similar to those in service, all tests were performed without additional treatment of the specimen surface (grinding or polishing). The specimens were fabricated using CNC turning, and the surface roughness (Ra) of the gauge section was lower than 1 μm. After machining, all specimens were wiped with petrol and ethanol and further cleaned in an ultrasonic acetone bath to remove any contamination. Measuring of the surface roughness before testing was performed with a profilometer (HOMMEL-ETAMIC W10, Jenoptik Industrial Metrology Germany GmbH, Villingen-Schwenningen, Germany). For all specimens, the value of Ra ranged from 200 μm to 700 μm.
Fully reversed cantilever type fatigue tests with sinusoidal wave form were performed in ambient air and a corrosive environment at frequencies of 4 Hz and 0.2 Hz, respectively. Maximum stress (σmax) levels ranged from 65% to 40% of the tensile yield strength of the two alloys. Two test rigs were employed for testing in different environments to reduce testing time. Corrosion fatigue tests were conducted using Empa’s SwRK (Schwingungsrisskorrosion Prüfstand, Empa, Dübendorf, Switzerland) testing machine (Figure 1a,b), while the tests in air were performed in a servo-hydraulic machine. In the SwRK machine, eight specimens were simultaneously tested in a displacement-controlled mode. Each specimen was placed in the environmental chamber, where the transparent part was connected to the bottom part with a rubber sleeve (Figure 1b).
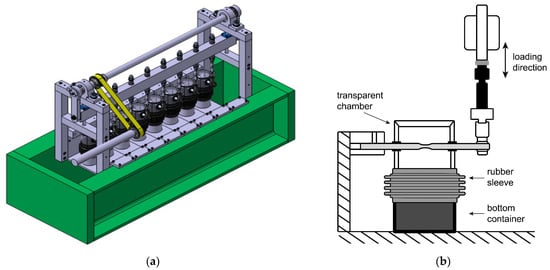
Figure 1.
Experimental SwRK setup for corrosion fatigue test (a), and corrosion chamber with test specimen (b).
Before testing, the bottom part was filled with NaCl solution to maintain a saturated environment inside the chamber (later named “NaCl-saturated environment”). The neutral 3.5 wt% NaCl solution was prepared from laboratory-grade sodium chloride (provided by EMSURE®, Merck, Darmstadt, Germany) and deionized water following Standard Practice B117-19 [14]. During testing, the gauge area of each specimen was periodically (every other day) sprayed with 3.5 wt% NaCl solution. The wetting of the surface was checked every day, and if necessary, the spraying was repeated. Also, additional tests were performed at frequencies of 0.2 Hz and 10 Hz under a load of 175 MPa in different NaCl-saturated environments (0.5 wt%, 2.0 wt%, 3.5 wt%) to determine the effects of frequency and chloride concentrations on the corrosion fatigue of the 6061-T6 alloy. The temperature and relative humidity during the testing were in the range of 20–24 °C and 30–60%, respectively (later named as “air”).
The microstructure of the samples was characterized using optical microscopy (OM, AxioImager m2m, Carl Zeiss) and scanning electron microscopy (SEM, ZEISS GeminiSEM 460, Carl Zeiss AG, Oberkochen, Germany). Energy-dispersive X-ray spectroscopy (EDS) was performed using an Oxford Instruments® X-Max detector and analyzed via AZtec® 6.0 software. For microscopic observations, the central parts of the specimens parallel to the extrusion direction were selected. The cross-sections were prepared by standard grinding procedure using SiC papers (up to #2500), followed by polishing with 6 μm and 3 μm diamond suspensions and finally using 0.05 μm silica. After each polishing step, the samples were cleaned with deionized water and ethanol and left to dry. To reveal the grain structure, a two-step etching procedure developed by Papageorge et al. [15] including modified versions of Keller’s and Weck’s reagent was used. The fraction and the size of the grains were statistically evaluated using ImageJ 1.54g software. To ensure adequate statistics, at least four images were used for the mapping of one specimen.
3. Results
3.1. Microstructure
The microstructures of the 6061-T6 and 6082-T6 aluminum alloys obtained by SEM are shown in Figure 2. The microstructure of both alloys includes two main types of coarse intermetallic phases. The EDS analysis revealed that Si, Mn, Fe and Cu were enriched in the bright phase contrasts, while only Al, Mg and Si were present in the dark-contrast particles. These results imply that the bright and dark contrasts correspond to the Fe-rich intermetallic and the MgSi equilibrium phase in Al-Mg-Si alloys, respectively. The dominant constituent Fe-rich phases in the 6061-T6 and 6082-T6 alloys were identified as the compounds of AlFeSiCu and AlFeSiMn, respectively (Figure 2a,b). Also, these intermetallics were regularly distributed in the matrix and aligned in strips in the direction of extrusion.
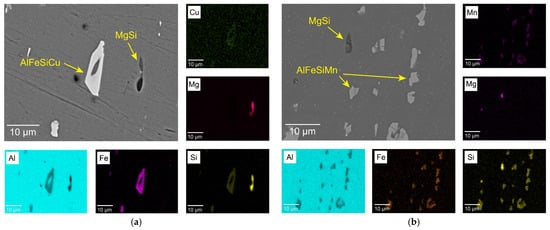
Figure 2.
SEM images of microstructure with corresponding EDS elemental maps of the 6061-T6 (a) and 6082-T6 (b) alloys.
Since grain size affects mechanical properties in polycrystalline materials, the determination and comparison of grain size, shape, and their distribution were performed for both alloys. To reveal the grain structure, chemical etchants such as Kroll’s, Keller’s, Barker’s and Weck’s reagents are commonly used in metallography for aluminum alloys. Nevertheless, some alloys, especially aluminum 6061-T6, are regarded as difficult to etch for purposes of high grain contrast. This was confirmed to be problematic with the mentioned etchants for the two alloys investigated in this study. Improved results were achieved with a two-step etching technique developed by Papageorge [15]. This two-step etching created a good contrast without significant pitting of the polished surface, as can be seen on the microstructures obtained using SEM in Figure 3a,c.
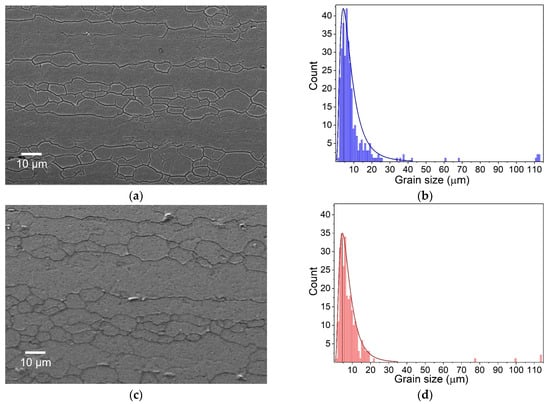
Figure 3.
SEM images of grain structures and histograms of grain size distributions of 6061-T6 (a,b) and 6082-T6 (c,d) alloys.
The geometry of the grains in the 6061-T6 and 6082-T6 alloys varied from relatively equiaxed for the smallest grains to elongated for the largest grains. These elongated grains were especially prominent along the extrusion direction. The smallest grains tended to be finer and more uniform in size, whereas the largest grains exhibited an elongated morphology, indicating significant deformation along the extrusion axis. The histogram of the grain size distribution, obtained from SEM images using ImageJ software, revealed that the smallest grains ranged from 2 µm to 10 µm in size, while the length of the larger, elongated grains exceeded 100 microns for both alloys. The fraction of finer grains in the alloy’s microstructure may influence the alloy’s mechanical properties, such as its hardness.
3.2. Corrosion Fatigue (S-N) Behavior
The S-N fatigue curves of the aluminum alloys 6061-T6 and 6082-T6 in air and in 3.5 wt% NaCl-saturated environment are shown in Figure 4, as a function of the stress amplitude and the total number of cycles to failure.
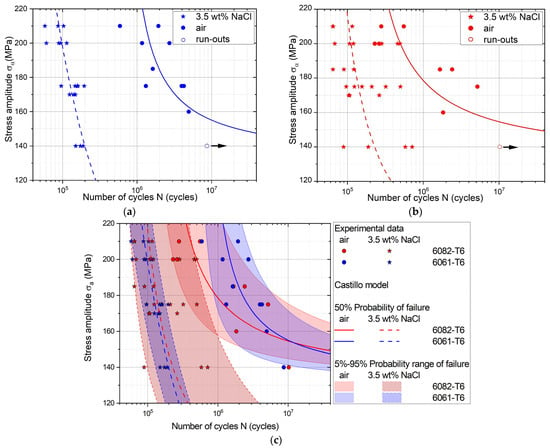
Figure 4.
Fatigue data for aluminum alloys 6061-T6 (a) and 6082-T6 (b) obtained in air and 3.5 wt% NaCl-saturated environment. The comparison of the S-N curves evaluated by the proposed Castillo and Fernández-Canteli probabilistic model (c).
Both alloys, when subjected to a corrosive environment, showed a significant reduction in fatigue life. For example, the fatigue life of alloy 6061-T6 at a stress amplitude of 210 MPa decreased by a factor of 15, from 1.2 × 106 cycles in air to 8.3 × 104 cycles in NaCl-saturated environment. When the stress level reached 140 MPa, material failure of samples started after 1.5 × 105 cycles in the corrosive media, while in air the material had high fatigue resistance and withstood more than 8 × 106 fatigue cycles. The reduction of fatigue life in 3.5 wt% NaCl-saturated environment indicates that the corrosive environment significantly deteriorated the fatigue properties of the alloy, especially in the low stress region.
As shown in Figure 4b,c, alloy 6082-T6 exhibits greater scatter in fatigue data obtained in a corrosive environment. At upper stress levels (approximately 60% of the yield strength), the fatigue data in both the corrosive environment and air partially overlapped. When the maximum cyclic stress was reduced to 140 MPa, the average fatigue life in the 3.5 wt% NaCl-saturated environment was 5.95 × 105 cycles, while in air, the fatigue life exceeded 10⁷ cycles. The data scatter persisted even at lower stress levels. This increased scatter in 6082-T6 indicates greater variability in fatigue life across specimens, with a broader distribution of cycles to failure, causing higher uncertainty in fatigue life predictions of this alloy.
The Basquin model is widely used to characterize the fatigue behavior of materials. However, when fatigue data exhibit significant scatter, the deterministic Basquin model may not provide sufficient accuracy. In the present study, to improve fatigue life predictions the software program ProFatigue 1.1 was used for derivation of the probabilistic S–N field from experimental fatigue data. In accordance with the probabilistic fatigue model developed by Castillo and Fernández-Canteli [16], the obtained data were analyzed and fitted to a three-parameter Weibull distribution by the following equation:
where B is a threshold value of the lifetime, C is the endurance limit, and λ, δ and β are the location, scale and shape Weibull model parameters, respectively.
The fitted median S–N curves (failure probability of 50%) for the 6061-T6 and 6082-T6 alloys are shown in Figure 4c. We found a slight difference in the slope of the fatigue curves within the tested stress range in the corrosive environment, suggesting that the fatigue behavior of the materials in NaCl-saturated environment is similar. However, the 6061-T6 alloy had no estimated endurance limit (estimated fatigue threshold) whereas the 6082-T6 alloy exhibited an estimated fatigue threshold based on the Castillo model of approximately 50 MPa. The threshold for both alloys tested in air was approximately 130 MPa.
3.3. Pitting Corrosion and Crack Propagation Path
Corrosion fatigue cracking is closely associated with pit formation, which acts as a stress concentrator and facilitates crack initiation. Pits typically develop due to localized corrosion originating at cavities or intermetallic phases, where microstructural inhomogeneities promote anodic dissolution of material. In order to investigate the initiation of corrosion fatigue cracks, the surface morphologies of specimens subjected to a corrosive environment were examined using SEM. Almost circular pits were observed on the surface of both alloys tested at 175 MPa (Figure 5a,b). Also, some relatively close corrosion pits were observed to be coalescing or merging, and this may be associated with the distribution of intermetallic phases and microstructural heterogeneity. The surface morphology of specimens tested at the higher stress amplitude of 210 MPa was less affected by pitting corrosion, and only rarely were pits observed in both alloys.
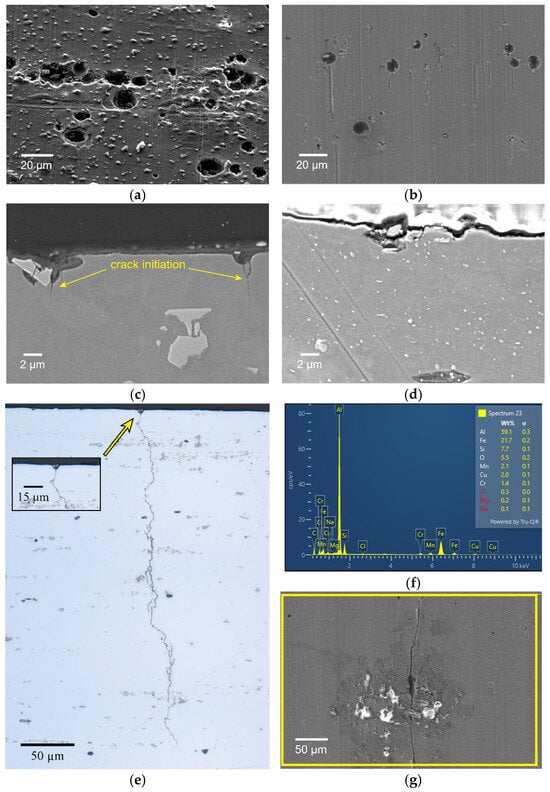
Figure 5.
SEM images of corrosion pits on the surface and on the cross-sections of 6061-T6 (a,c) and 6082-T6 (b,d) alloy tested at 175 MPa. OM image of the cross-section of the 6061-T6 alloy with fatigue crack passing through the corrosion pit (e). EDX spectrum of Fe-rich intermetallic phase in the pit in 6061-T6 alloy (f). Crack initiation from the pits in 6082-T6 alloy (g).
The cross-sections of the 6061-T6 and 6082-T6 samples were also examined by SEM to identify surface degradation due to corrosion. Figure 5c,d illustrates the cross-sectional SEM images of the materials with microscopic pitting corrosion sites. It was found that the depth of the observed pits was about 2 and 7 µm. Furthermore, some pits had intermetallic debris at the bottom (Figure 5c). EDX analysis identified the presence of Fe-rich intermetallics along with corrosion products in these pits (Figure 5f). Also, small micron-sized cracks originating from the pits were detected, confirming that these pits serve as stress concentration sites for crack initiation. As shown in Figure 5e,g, the surface pits were starting points (or links) for corrosion fatigue crack growth in both alloys.
The fracture surfaces of both alloys in 3.5 wt% NaCl-saturated environment show typical fracture patterns corresponding to the main stages of fatigue crack propagation (Figure 6a,c–f): regions of fatigue crack initiation; regions corresponding to the stable growth stage with different growth rates and formation of striations; and regions with dimples corresponding to final rupture. As can be seen from the high-magnification image of the initiation site of 6061-T6 alloy tested at 175 MPa (Figure 6b), the corrosion fatigue crack initiated at the localized corroded area on the surface. Corrosion products were observed at the crack initiation sites to some extent, indicating that the corrosion in NaCl-saturated environment assisted fatigue crack nucleation. Also, multi-site cracks were observed to be originating on the fracture surface, and the presence of ratchet marks indicated that the cracks were initiated at different planes.
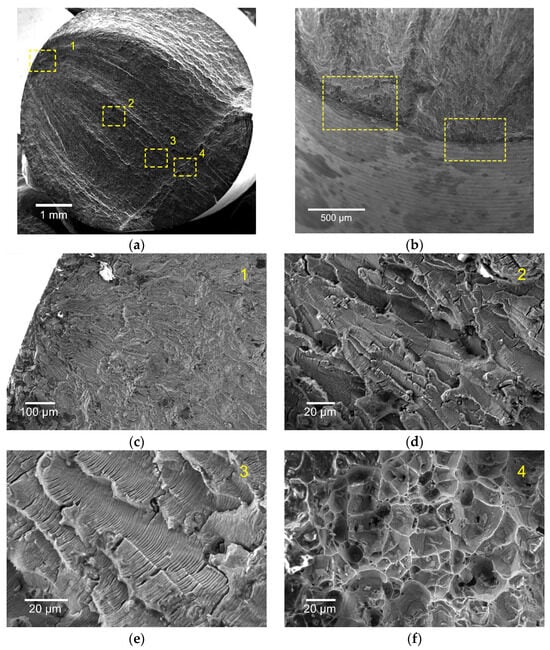
Figure 6.
Fracture surface (a) and typical fracture patterns (c–f) of the 6061-T6 alloy tested at 210 MPa in 3.5 wt% NaCl-saturated environment. Fatigue crack initiation sites on the fracture surface of the 6061-T6 alloy tested at 175 MPa in corrosive environment (b).
In a corrosive environment, crack propagation becomes a critical stage for fatigue failure, as corrosion accelerates crack growth by weakening the material at the crack tip through anodic dissolution and hydrogen embrittlement [17]. Therefore, the influence of the corrosive environment on the crack propagation path of both alloys was compared. To observe the evolution of crack paths through the material structure, samples containing fatigue cracks were characterized using SEM. To reveal the grain structure, polished cross sections from the corrosion-fatigued samples were etched by the two-step Papageorge technique [15].
As shown in Figure 7a,b, cracks propagate through large, elongated grains and finer grains in both alloys. Also, dominant cracks partly display path deflection on the grain boundaries and the appearance of small crack branches. Deflection of cracks was observed on the intermetallic phases, and in some sub-grain zones of the large grains the crack path followed a zig-zag pattern. In summary, the observations of crack paths confirmed that the transgranular propagation mode was dominant in both alloys.
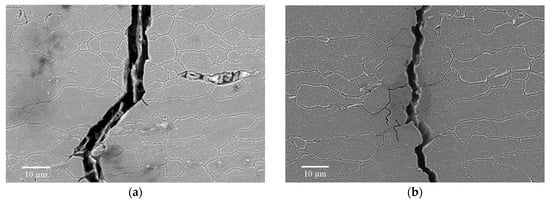
Figure 7.
Corrosion fatigue crack propagation path in the 6061-T6 (a) and 6082-T6 alloy (b) tested at 175 MPa.
3.4. Effect of Other Variables on Corrosion Fatigue
The effect of frequency and environment on fatigue damage evolution was investigated only for the 6061-T6 alloy. The corrosion fatigue results were obtained at a constant amplitude of 175 MPa at 0.2 Hz and 10 Hz in 0.5 wt%, 2.0 wt% and 3.5 wt% NaCl-saturated environment, as shown in Figure 8.
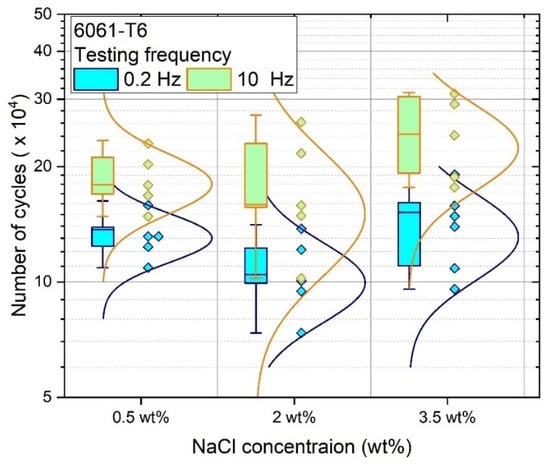
Figure 8.
Fatigue life of aluminum alloy 6061-T6 tested in 0.5, 2.0, and 3.5 wt% NaCl solutions at loading frequencies of 0.2 Hz and 10 Hz.
As shown in Figure 8, a more pronounced influence of corrosion was observed at lower fatigue frequencies. Specimens tested at a frequency of 0.2 Hz displayed reduced fatigue life in comparison with specimens cycled at 10 Hz. For example, the average fatigue life of the alloy in 3.5 wt% NaCl-saturated environment was 2.45 × 105 cycles at 10 Hz, and 1.44 × 105 cycles at 0.2 Hz. The difference in fatigue life of samples tested at different frequencies in 2.0 wt% NaCl-saturated environment was around 0.75 × 105 cycles. A similar tendency was observed in the 0.5 wt% NaCl environment. Meanwhile, when the concentration of the solution was changed from 0.5 wt% to 3.5 wt%, no obvious changes in fatigue life were found. The data obtained overlapped, indicating similar responses of the alloy for the selected testing conditions. Microscopic examination of the surfaces showed the formation of pits after testing in each environment.
4. Discussion
The corrosion fatigue data presented in Section 3.2 indicate a distinct reduction in the fatigue life of the 6061-T6 and 6082-T6 alloys in 3.5 wt% NaCl-saturated environment compared to the results obtained in air. The decrease in fatigue life can be attributed to the premature material degradation caused by the detrimental effect of the environment. It is well known that Cl− ions can easily penetrate aluminum oxide film, weakening its strength and leading to metal dissolution. This process results in corrosion-induced pitting in localized areas of the surface. Additionally, changes in fatigue stresses during testing can accelerate the rupture of the oxide film, causing constant contact between the metal and the corrosive environment. This contact facilitates electrochemical reactions and leads to faster metal degradation. Thus, anodic dissolution can have a greater impact on fatigue life at higher stress levels, while pitting corrosion is dominant at lower stress. At higher stress amplitudes, materials can often yield locally, especially near stress concentrators like pit tips. This plastic deformation can blunt sharp features, reducing the effective stress intensity at those points. At the same time, cyclic loading provides sufficient mechanical driving force to form slip steps that act as stress concentrators and oxide film rupture sites. In particular, such results were obtained for alloys of the 7xxx series by Lin and Yang [18]. The fractographic data from their study has also shown that at high stress levels, the crack initiation sites were related to the anodic slip dissolution process. A similar explanation could be applied to the results obtained for the 6061-T6 and 6082-T6 alloys in the present study. However, such conclusions would require a more detailed study of the intensity of pitting on the alloy surfaces at different stress levels and their involvement in crack initiation. In addition, the steep slope of both S-N curves in corrosive environments for both alloys could indicate similarities in the crack initiation mechanism within the selected stress levels. Also, the different scatter in the fatigue life data of the 6082-T6 and 6061-T6 alloys can be attributed to microstructural characteristics affecting the alloys’ response to corrosion fatigue. Alloy 6082-T6 experienced greater scatter, which may be explained by its microstructural heterogeneity that makes it susceptible to localized corrosion and leads to variability in fatigue life. In contrast, the more uniform microstructure of the 6061-T6 alloy may lead to a more consistent initiation and propagation of cracks, reducing variability in fatigue life.
As described in Section 3.3, pits can serve as precursors for crack initiation in both 6061-T6 and 6082-T6 aluminum alloys. Surface irregularities and microstructural heterogeneity of materials commonly contribute to localized corrosion, promoting pit nucleation and growth. 6xxx series alloys are generally known to be susceptible to localized corrosion, which is closely related to the presence of grain boundary precipitates and coarse intermetallic compounds. According to the EDS analysis, two main micrometric intermetallic compounds are present in both alloys: Fe-rich compounds, and Mg2Si particles. The galvanic interaction of intermetallic particles with aluminum plays an important role in understanding localized corrosion and environmentally assisted cracking of aluminum alloys.
Iron is one of the main impurity elements in commercial aluminum alloys and due to its very low solid solubility in the matrix, it appears predominantly in the form of intermetallic particles. Fe-rich phases act as catalytic sites for cathodic reactions and pit nucleation. In aggressive environments, the aluminum matrix near Fe-rich intermetallic phases preferentially undergoes anodic dissolution, as follows:
Al → Al3+ + 3e−.
This reaction leads to the dissolution of aluminum into Al3+ ions, releasing electrons into the surrounding environment. Additionally, the cathodic reaction, occurring at the Fe-rich intermetallic phases, involves the reduction of oxygen in neutral environments:
O2 + 2H2O + 4e− → 4OH−.
These reactions drive localized pitting, and weakening the aluminum matrix. Also, previous investigators have already reported the formation of cavities around Fe-rich intermetallics in aluminum alloys [5]. Moreover, the change in the cathodic reactivity of the intermetallic particles depends on their composition and plays a critical role in trenching. For example, research by Kakinuma et al. [19] has shown that deep trenches, which become the initiation site for pitting, were formed around Al–Fe–Si particles but not around Al–Fe particles in aluminum AA1050. At the same time, iron remains mostly stable and partially dissolves under aggressive conditions, forming corrosion products such as Fe (OH)3. As shown in Figure 5c,d, a secondary phase containing Fe was also detected within the concave pits of both alloys, confirming that Fe-rich intermetallic phases act as initiation sites for localized corrosion and pit formation. Differences in the composition of these intermetallics may result in variations in their electrochemical behavior, leading to trenching and influencing the depth and size of the pits.
In the case of the micrometric anodic Mg2Si particles, water releases Mg ions and leads to formation of the oxide SiO2 and hydrogen [20]:
Mg2 Si + 6H2O → SiO2 + 2Mg2+ + 4H2 + 4OH−.
A significant role in localized corrosion is played by the size, distribution, and composition of the intermetallic phases, as these were noted to also have a pronounced impact on the electrochemical characteristics of aluminum alloys [21]. In order to precisely evaluate and compare the corrosion behavior of aluminum with intermetallics of varying compositions and distributions, further targeted investigation is required.
After crack initiation, the cracking of the material results from the combined effects of mechanical stress and electrochemical reactions occurring in a localized region at the crack tip. Therefore, accurately characterizing the chemical and electrochemical conditions at the crack tip is essential for determining the mechanism responsible for crack growth. Bland and Loke [12] have shown that under cyclic loading, advection can mitigate the occluded nature of the crack tip. However, its influence diminishes as the loading frequency decreases (lower than 1 Hz) and the stress ratio increases (from 0.1 to 0.8). Under these conditions, corrosion fatigue can shift towards being governed by stress corrosion cracking mechanisms. Moreover, the transition from cyclic to environmentally assisted static cracking conditions can change the degradation behavior of the material’s structure. The difference in crack propagation between stress corrosion and corrosion fatigue cracking in the A7N01P-T4 aluminum alloy has been shown by Shen et al. [22]. The crack-tip propagation path through grains was analyzed by the EBSD technique, and the authors showed that the corrosion fatigue crack path was mainly transgranular and partly intergranular, while the stress corrosion cracking fracture mode was primarily intergranular, accompanied with minor transgranular cracking. Also, segregated precipitates at the grain boundaries and high-angle grain boundaries were susceptible to cracking. Another study by Ringdalen et al. [23] examined grain boundary precipitates in 6xxx series aluminum alloys using transmission electron microscopy and density functional theory simulations. The findings indicated that these precipitates weaken grain boundaries by reducing decohesion energies, thereby acting as initiation sites for intergranular fracture. It was also reported that the addition of Cu in 6xxx series alloys can lead to susceptibility to intergranular cracking due to the formation of Cu-rich phases at grain boundaries. These precipitates often act as cathodic sites, accelerating selective anodic dissolution of the adjacent aluminum matrix and propagating intergranular attack.
In the results we obtained for the aluminum alloys 6061-T6 and 6082-T6, predominantly transgranular propagation of cracks in corrosive environments was observed. When the crack crossed the grain boundaries in some regions it was deflected, since the grain boundary can provide higher resistance to crack propagation. Also, regardless of the number of times the crack deflects, it will ultimately propagate perpendicularly to the extrusion direction of the alloy. In certain regions along the main crack path in the 6082-T6 alloy, micron-sized crack branches were observed propagating along grain boundaries and in a transgranular manner, which can be attributed to the localized effects of the corrosive environment. Observed similarities in the dominant transgranular crack paths for both alloys suggest that their corrosion fatigue mechanisms are primarily governed by the interaction of the mechanical stresses with localized electrochemical reactions at the crack tip within grains, rather than weakening of grain boundaries. However, an investigation of the distribution of precipitates at the grain boundaries and a comparison of the fatigue crack growth rate could provide additional information on the effect of the alloy composition on the crack propagation mechanism.
Typically, to reduce the duration of laboratory tests, the load frequency selected for testing is often higher than the load frequency in service. Lower frequencies are considered to promote synergies between corrosion and cyclic loading and to accelerate fatigue crack propagation. It has been shown in previous studies that corrosion fatigue at frequencies above 1 Hz would not be as damaging to the material as corrosion fatigue below this frequency. As corrosion fatigue is a time-dependent process, crack propagation at lower frequencies allows bulk material to be progressively more exposed to an aggressive environment, which stimulates accelerated crack propagation, resulting in a lower fatigue resistance. This effect has been reported for some aluminum alloys [24,25]. In the present study, results obtained for frequencies between 0.2 Hz and 10 Hz for the 6061-T6 and 6082-T6 aluminum alloys showed only a slight difference in fatigue life (factor 1.5–1.7). It can also be hypothesized that the reduction in fatigue frequency allowed larger pits to form and ensured large enough stress concentrations for crack generation. In addition, higher ionic molarities of the solution can cause higher solution conductivities. In this case, ohmic resistances throughout the electrolytic solution will be decreased and larger cathodic areas could be supported. As has been reported previously [26], the susceptibility of aluminum alloy 6061 to pitting corrosion in chloride solution was increased with NaCl concentrations varying within 0.003 wt% to 5.5 wt%. However, in the present study the NaCl solution concentration in the range of 0.5 wt% and 3.5 wt% had no significant effect on the fatigue life of the alloy (Figure 8), indicating that early material failure can occur even in 0.5 wt% solution.
5. Conclusions
In the present study, the corrosion fatigue behavior of heat-treated 6061-T6 and 6082-T6 aluminum alloys was investigated and compared. It was shown that the fatigue life for both alloys in air surpasses 107 cycles at a stress amplitude of 140 MPa. However, in a 3.5 wt% NaCl-saturated environment, both alloys exhibit a fatigue life reduction factor of about 45, indicating a significant effect of corrosion in their premature degradation. With decreasing fatigue strength, no distinct estimated fatigue endurance limit was observed for the two alloys. However, alloy 6061-T6 demonstrated more predictable fatigue life and showed less data scatter in corrosive environments.
Microscopic analysis of 6061-T6 and 6082-T6 alloys revealed that Fe-rich intermetallic phases act as sites for the formation of corrosion pits in the aluminum matrix. Furthermore, it was observed that crack initiation was mostly associated with pitting corrosion on the surface. In both alloys, the crack propagation path predominantly passed through the aluminum matrix in transgranular mode, indicating that the grain boundaries did not weaken due to corrosion under the selected test conditions at a frequency of 0.2 Hz.
The analysis of the frequency effect on the corrosion fatigue behavior of the 6061-T6 alloy, comparing failure data at 10 Hz and 0.2 Hz, showed a slight improvement in fatigue life of factor 1.5–1.7 at the higher frequency. Furthermore, variations in chloride concentration in the NaCl solution, ranging from 0.5 wt% to 3.5 wt%, did not influence the susceptibility of the 6061-T6 alloy to environmentally assisted fatigue cracking, showing that low Cl- concentrations do have a highly detrimental influence on the fatigue resistance of these aluminum alloys.
Author Contributions
Conceptualization, T.A., S.M., J.K., U.H., C.A. and G.P.T.; methodology, T.A., S.M., A.S., J.K. and U.H.; investigation, T.A., A.S. and I.B.; formal analysis, T.A., A.S. and I.B.; visualization, T.A., A.S. and I.B.; writing—original draft preparation, T.A.; writing—review and editing, S.M., J.K., I.B., U.H., C.A. and G.P.T.; supervision, C.A. and G.P.T.; project administration, C.A. and G.P.T.; funding acquisition, G.P.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research was partly funded by the Swiss National Science Foundation (SNSF), grant number IZSEZ0_220283/1.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
The support of ETH student Elia Pezzoni in investigating the frequency effect during a semester project research is gratefully acknowledged. Avramenko T. also acknowledges support by Lau Alexandra from the Joining Technologies and Corrosion Laboratory for providing training in metallography techniques, and trainees Frei Andrin and Moritz Ian for samples preparation.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Kumar, A.; Maithani, R.; Kumar, A.; Kumar, D.; Sharma, S. An All-Aluminium Vehicle’s Design and Feasibility Analysis. Mater. Today Proc. 2022, 64, 1244–1249. [Google Scholar] [CrossRef]
- Pian, W.; Zhou, Y.; Xiao, T. A Review of the Feasibility of Aluminum Alloys, Carbon Fiber Composites and Glass Fiber Composites for Vehicle Weight Reduction in the Automotive Industry. J. Phys. Conf. Ser. 2023, 2608, 012005. [Google Scholar] [CrossRef]
- Bartawi, E.H.; Mishin, O.V.; Shaban, G.; Grumsen, F.; Nordlien, J.H.; Ambat, R. The Effect of Trace Level Copper Content on Intergranular Corrosion of Extruded AA6082-T6 Alloys. Mater. Chem. Phys. 2023, 309, 128303. [Google Scholar] [CrossRef]
- Szklarska-Smialowska, Z. Pitting Corrosion of Aluminum. Corros. Sci. 1999, 41, 1743–1767. [Google Scholar] [CrossRef]
- Park, J.O.; Paik, C.H.; Huang, Y.H.; Alkire, R.C. Influence of Fe-Rich Intermetallic Inclusions on Pit Initiation on Aluminum Alloys in Aerated NaCl. J. Electrochem. Soc. 1999, 146, 517. [Google Scholar] [CrossRef]
- Birbilis, N.; Cavanaugh, M.K.; Buchheit, R.G. Electrochemical Behavior and Localized Corrosion Associated with Al7Cu2Fe Particles in Aluminum Alloy 7075-T651. Corros. Sci. 2006, 48, 4202–4215. [Google Scholar] [CrossRef]
- Kairy, S.K.; Rometsch, P.A.; Davies, C.H.J.; Birbilis, N. On the Intergranular Corrosion and Hardness Evolution of 6xxx Series Al Alloys as a Function of Si:Mg Ratio, Cu Content, and Aging Condition. Corrosion 2017, 73, 1280–1295. [Google Scholar] [CrossRef]
- Svenningsen, G.; Larsen, M.H.; Walmsley, J.C.; Nordlien, J.H.; Nisancioglu, K. Effect of Artificial Aging on Intergranular Corrosion of Extruded AlMgSi Alloy with Small Cu Content. Corros. Sci. 2006, 48, 1528–1543. [Google Scholar] [CrossRef]
- Zou, Y.; Liu, Q.; Jia, Z.; Xing, Y.; Ding, L.; Wang, X. The Intergranular Corrosion Behavior of 6000-Series Alloys with Different Mg/Si and Cu Content. Appl. Surf. Sci. 2017, 405, 489–496. [Google Scholar] [CrossRef]
- Nguyen, N.; Li, P. Fatigue Behaviour of AA6061-T6 Alloys in the Corrosive Environment. MATEC Web Conf. 2018, 165, 03015. [Google Scholar] [CrossRef]
- Chanyathunyaroj, K.; Phetchcrai, S.; Laungsopapun, G.; Rengsomboon, A. Fatigue Characteristics of 6061 Aluminum Alloy Subject to 3.5% NaCl Environment. Int. J. Fatigue 2020, 133, 105420. [Google Scholar] [CrossRef]
- Bland, L.G.; Locke, J.S. Chemical and Electrochemical Conditions within Stress Corrosion and Corrosion Fatigue Cracks. npj Mater. Degrad. 2017, 1, 12. [Google Scholar] [CrossRef]
- EN ISO 11782-1:1998; Corrosion of Metals and Alloys—Corrosion Fatigue Testing—Part 1: Cycles to Failure Testing. European Committee for Standardization (CEN): Brussels, Belgium, 1998.
- ASTM B117-19; Standard Practice for Operating Salt Spray (Fog) Apparatus. ASTM International: West Conshohocken, PA, USA, 2019.
- Papageorge, W.; Janas, G.; Beach, E. Development of a Versatile Two-Step Etchant to Reveal Grain Boundaries in Multiple Aluminum Alloys. Metallogr. Microstruct. Anal. 2023, 12, 865–871. [Google Scholar] [CrossRef]
- Fernández-Canteli, A.; Przybilla, C.; Nogal, M.; López Aenlle, M.; Castillo, E. ProFatigue: A Software Program for Probabilistic Assessment of Experimental Fatigue Data Sets. Procedia Eng. 2014, 74, 236–241. [Google Scholar] [CrossRef]
- Lynch, S.P. Mechanisms and Kinetics of Environmentally Assisted Cracking: Current Status, Issues, and Suggestions for Further Work. Met. Mater. Trans. A 2013, 44, 1209–1229. [Google Scholar] [CrossRef]
- Lin, C.-K.; Yang, S.-T. Corrosion Fatigue Behavior of 7050 Aluminum Alloys in Different Tempers. Eng. Fract. Mech. 1998, 59, 779–795. [Google Scholar] [CrossRef]
- Kakinuma, H.; Muto, I.; Oya, Y.; Momii, T.; Jin, Y.; Sugawara, Y.; Hara, N. Change in Oxygen Reduction Reactivity of Intermetallics: A Mechanism of the Difference in Trenching around Al–Fe and Al–Fe–Si Particles on AA1050 in NaCl. J. Electrochem. Soc. 2023, 170, 021503. [Google Scholar] [CrossRef]
- L’Haridon-Quaireau, S.; Laot, M.; Colas, K.; Kapusta, B.; Delpech, S.; Gosset, D. Effects of Temperature and pH on Uniform and Pitting Corrosion of Aluminium Alloy 6061-T6 and Characterisation of the Hydroxide Layers. J. Alloys Compd. 2020, 833, 155146. [Google Scholar] [CrossRef]
- Ikeuba, A.I.; Njoku, C.N.; Ekerenam, O.O.; Njoku, D.I.; Udoh, I.I.; Daniel, E.F.; Uzoma, P.C.; Etim, I.N.; Okonkwo, B.O. A Review of the Electrochemical and Galvanic Corrosion Behavior of Important Intermetallic Compounds in the Context of Aluminum Alloys. RSC Adv. 2024, 14, 31921–31953. [Google Scholar] [CrossRef]
- Shen, L.; Chen, H.; Xu, L.-D.; Che, X.-L.; Chen, Y. Stress Corrosion Cracking and Corrosion Fatigue Cracking Behavior of A7N01P-T4 Aluminum Alloy. Mater. Corros. 2018, 69, 207–214. [Google Scholar] [CrossRef]
- Ringdalen, I.G.; Jensen, I.J.T.; Marioara, C.D.; Friis, J. The Role of Grain Boundary Precipitates during Intergranular Fracture in 6xxx Series Aluminium Alloys. Metals 2021, 11, 894. [Google Scholar] [CrossRef]
- Scala, A.; Squillace, A.; Monetta, T.; Mitton, D.B.; Larson, D.; Bellucci, F. Corrosion Fatigue on 2024T3 and 6056T4 Aluminum Alloys. Surf. Interface Anal. 2010, 42, 194–198. [Google Scholar] [CrossRef]
- Menan, F.; Henaff, G. Influence of Frequency and Exposure to a Saline Solution on the Corrosion Fatigue Crack Growth Behavior of the Aluminum Alloy 2024. Int. J. Fatigue 2009, 31, 1684–1695. [Google Scholar] [CrossRef]
- Huang, I.-W.; Hurley, B.L.; Yang, F.; Buchheit, R.G. Dependence on Temperature, pH, and Cl− in the Uniform Corrosion of Aluminum Alloys 2024-T3, 6061-T6, and 7075-T6. Electrochim. Acta 2016, 199, 242–253. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).