Abstract
Titanium (Ti) alloys are widely used in biomedical applications but face challenges like poor biological activity and corrosion at modular interfaces. Strontium (Sr)-doped micro-arc oxidation (MAO) surfaces are proposed to improve biocompatibility and tribocorrosion resistance. This study examines the electrochemical behaviour of Ti surfaces treated with 0.0013 M and 0.13 M Sr-doped MAO via open circuit potential, potentiodynamic polarisation, and electrochemical impedance spectroscopy in a basic physiological solution at 37 °C. The results indicate that higher Sr concentrations led to lower passivation current densities (more than two times lower than at the lowest Sr concentration) and reduced barrier layer capacitance (more than one and a half times lower than at the lowest Sr concentration), suggesting improved corrosion resistance for Sr-enriched MAO treatments on Ti implants.
1. Introduction
Ti and its alloys, known for their low density, high specific strength, and excellent biocompatibility, have found extensive use in medical applications, particularly in orthopaedics and dentistry [1]. Nevertheless, titanium and its alloys possess relatively poor biological activity, as well as susceptibility to corrosion at modular interfaces, particularly in areas where mechanical abrasion of the oxide film, or fretting, occurs [2,3].
To address these limitations, surface modification treatments have garnered considerable attention. Techniques such as MAO, physical or chemical vapour deposition, plasma spraying, and sol–gel processes have been widely explored to enhance the surface properties of titanium alloys. MAO, also referred to as plasma electrolytic oxidation (PEO), is a well-established process used to improve the surface characteristics of titanium, magnesium, and aluminium alloys. By incorporating different elements and compounds during the oxidation process, MAO layers can achieve enhanced wear resistance, corrosion resistance, and improved biological activity [3].
One element that has shown promise in improving the biological performance of titanium surfaces is Sr. Naturally present in bone tissue, particularly in areas of high metabolic turnover, Sr enhances osteoblast activity and differentiation while inhibiting osteoclast proliferation. The incorporation of Sr into surface coatings has demonstrated a clear positive impact on bone cell activity, which can be directly attributed to its presence in the coating material [4]. Hence, several in vitro studies [5,6,7,8,9], as well as both in vitro and in vivo studies [10,11,12,13,14,15] conducted on Sr-doped MAO-treated Ti [5,6,7,10,11,12,13,14], Ti6Al4V [8,9], and Ti35Nb2Ta3Zr [15] surfaces, have reported favourable biological properties.
On the other hand, in order to better understand both the release of Sr ions and the degradation behaviour of the surfaces, the electrochemical response of Sr-doped MAO-treated surfaces also needs to be well understood. However, the number of studies investigating the electrochemical behaviour of these surfaces is quite limited compared to those examining their biological properties. Within those, some researchers investigated the electrochemical response of MAO-treated Ti-based surfaces with and without the incorporation of Sr. Yu et al. [16] explored the effect of Sr and Si incorporation on MAO layers formed on Ti6Al4V surfaces in a Ca- and P-containing electrolyte, employing potentiodynamic polarisation and EIS measurements in 9 g/L NaCl solution. Sr incorporation was achieved by adding 0.0075–0.03 M Sr(CH3COO)2·0.5H2O to the MAO electrolyte. The authors reported that the corrosion potential of Sr-doped MAO surfaces was lower than that of MAO surfaces without Sr addition, while the passive region extended to higher potentials. Moreover, based on EIS data, the polarisation resistance of the Sr-doped MAO surfaces was higher than that of the undoped MAO surfaces. Liu et al. [6] produced Sr-doped MAO surfaces on Ti by adding 15 g/L Sr(CH3COO)2 to the electrolyte. The electrochemical behaviour was assessed using potentiodynamic polarisation and EIS measurements in simulated body fluid (SBF). The authors concluded that Sr-doped MAO surfaces exhibited better corrosion resistance than those produced without Sr. Finally, Kung et al. [17] examined the MAO-treated surfaces produced with varying amounts of Sr by adding 0.0013–0.013 M Sr(OH)2·8H2O to the electrolyte. The electrochemical response was evaluated through OCP monitoring and potentiodynamic polarisation measurements in SBF. The authors reported that all MAO surfaces, both with and without Sr additions, exhibited similar polarisation curves.
Some of the present authors had previously produced Sr-doped MAO surfaces on commercially pure grade 2 Ti surfaces in a Ca- and P-containing electrolyte with additions of 0.0013, 0.013, and 0.13 M Sr of Sr(OH)2·8H2O to the electrolyte. As stated before, along with the favourable biological response [5], surfaces having a higher amount of Sr exhibited less mechanical damage and lower COF during tribocorrosion tests executed in 9 g/L of a NaCl solution against a 10 mm diameter alumina ball under 0.5 N normal load and 1 Hz of frequency during 10 min due to the increase in the rutile phase within the MAO surfaces [18]. However, the electrochemical response of these surfaces compared to those produced without Sr addition across a wide range of Sr content, remains unknown. Therefore, this study aims to explore the electrochemical behaviour of 0.0013 M and 0.13 M Sr-doped MAO surfaces formed on Ti through OCP monitoring, potentiodynamic polarisation tests, and EIS measurements in a simple physiological solution at 37 °C.
2. Materials and Methods
MAO treatment was performed on ground (#320 SiC paper) and etched (Kroll’s reagent; HF:HNO3:H2O) commercial pure Ti (CP Ti grade 2, Goodfellow) plates measuring 20 × 20 × 2 mm. Prior to MAO treatment, the samples were cleaned ultrasonically in propanol (10 min), distilled water (5 min), and then dried in warm air.
The electrolyte utilised for the MAO process was composed of a blend of 0.02 M β-glycerophosphate disodium salt pentahydrate (β-GP, C3H7Na2O6P·5H2O from Alfa Aesar,, Ward Hill, MA, US), 0.35 M calcium acetate monohydrate (CA, Ca(CH3CO2)2·H2O from Alfa Aesar) and varying concentrations of strontium hydroxide octahydrate (Sr, Sr(OH)2·8H2O from Sigma Aldrich, St. Louis, MO, USA) as detailed in Table 1. Electrical conductivity was measured (Consort C3230 2CH pH/mV/EC/DO/ISE) for the three conditions of electrolyte at room temperature, with at least 5 measurements per condition. The MAO process used a potentiostatic regime at a voltage of 300 V for 1 min at room temperature. The anode (CP Ti samples, 9.6 cm2) and cathode (Pt foil, 13 cm2) were positioned 8 cm apart and connected to a DC power supply (GPR-30H10D). A magnetic stirrer operating at 200 rpm was employed to generate a turbulent flow regime (Figure 1a). Following the MAO process, the samples were rinsed with distilled water.

Table 1.
Group nomenclature, electrolyte composition, and electrolyte conductivity.
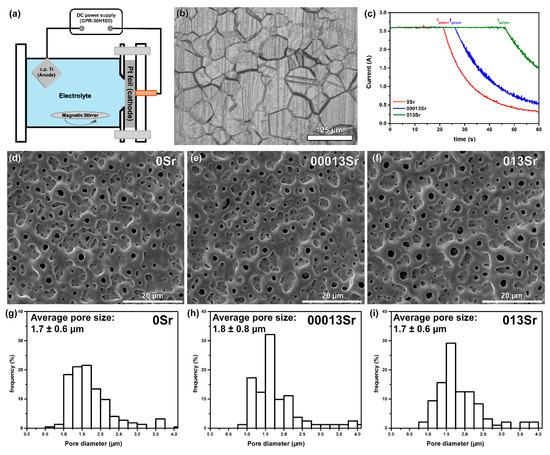
Figure 1.
MAO treatment: (a) schematic representation of the MAO treatment set-up; (b) OM image of untreated Ti after etching; (c) current evolution during MAO treatment; secondary electron (SE) SEM images of MAO-treated (d) 0Sr, (e) 00013Sr, and (f) 013Sr surfaces; pore size distribution on MAO-treated (g) 0Sr, (h) 00013Sr, and (i) 013Sr surfaces.
The morphology and chemistry of the MAO surfaces were analysed using a field emission gun scanning electron microscope (FEG-SEM, FEI Nova 200, Hillsboro, OR, USA) equipped with EDAX - Pegasus X4M energy-dispersive X-Ray spectroscopy (EDS), Mahwah, NJ, USA. The microstructure of untreated surfaces was examined using a Leica DM2500 optical microscope (OM).
Electrochemical tests included OCP, EIS, and potentiodynamic polarisation tests. All tests were conducted in a basic physiological solution containing 9 g/L of NaCl at body temperature (37 °C). To maintain a constant testing temperature, the electrochemical cell was placed in a climate chamber (Nahita incubator 636 Plus), which also functioned as a Faraday cage. The sample surfaces served as the working electrode (exposed area of 0.38 cm2), a platinum mesh was used as the counter electrode, and a saturated Ag/AgCl electrode was used as the reference electrode, connected to a Gamry Instruments Reference 600+ potentiostat, Warminster, PA, USA. All potentials are reported with respect to Ag/AgCl.
Measurements of OCP, EIS, and potentiodynamic polarisation were conducted in a sequential manner. The OCP was continuously monitored for a minimum of one hour and considered stable when the voltage fluctuation (ΔE) was less than 60 mV h⁻1. EIS was performed at the final OCP value, covering a frequency range from 105 to 10⁻2 Hz, with seven data points collected per frequency decade. A sinusoidal signal with an amplitude of 10 mV was employed to ensure the electrode’s response remained linear. Following the EIS measurements, the OCP was recorded for an additional 10 min, after which potentiodynamic polarisation tests commenced at −0.250 V relative to the OCP, increasing to 1.5 VAg/AgCl at a scanning rate of 0.5 mV s⁻1. To verify the consistency of the results, each experiment was repeated at least three times, and the outcomes are reported as the average ± standard deviation.
3. Results and Discussion
A representative OM image of the etched untreated Ti surface is shown Figure 1b. The grinding marks remained visible after etching; moreover, the typical granular structure of Ti can also be observed. In addition, to remove surface contaminants, the chemical etching may also tailor the topography and roughness of Ti surfaces. The microstructure consists of equiaxed grains with a variation in grain size, where finer and coarser grains are present.
The variation in current during the MAO treatment is shown in Figure 1c. From the current evolution plot, two stages can be observed. The first stage is characterised by a constant current, while the second stage shows a decrease in the current. The constant current is due to the limiting current (2.5 A) of the power supply and was observed until the chosen voltage of 300 V was reached. Once 300 V was achieved, as indicated by tgal/pot, the MAO treatment transitioned from galvanostatic to potentiostatic mode (the second stage), during which a decrease in current was observed. The tgal/pot values are 26 ± 4, 30 ± 4, and 44 ± 3 s for 0Sr, 00013Sr, and 013Sr treatment conditions, respectively. As the Sr concentration in the MAO electrolyte increased, the tgal/pot also increased. The conductivity of the MAO electrolyte rose from approximately 24 up to 31 ms/cm as the concentration of strontium hydroxide octahydrate increased from 0 to 0.13 M.
In the first stage, the voltage increases rapidly, leading to the formation of a barrier layer whose thickness continues to increase until the breakdown voltage is reached. Once the breakdown voltage is attained, the potential rise slows, and the spark discharges begin to occur. The spark discharges promote the formation of numerous sparks that spread across the substrate/electrolyte interface, resulting in a porous layer [19,20].
As per Ikonopisov’s theory of electrical breakdown during the formation of barrier anodic films [21], the relationship between the conductivity (k) of a given electrolyte and the breakdown voltage (Vb) is described by Equation (1):
where aB and bB are constant values for a given metal and electrolyte composition. Thus, according to this equation, the breakdown voltage increases when the electrolyte conductivity decreases. In the present study, it can be assumed that the breakdown voltage decreases as the Sr addition increased, which may explain the differences in tgal/pot for the different concentrations of Sr. The initial rise in voltage during the MAO process, leading up to the breakdown voltage, is primarily influenced by the electrolyte concentration/conductivity, while the subsequent voltage increase is driven by the growth of the layer thickness and the formation of the MAO layers on the bulk metal surface [19,22].
According to several authors [9,23,24,25,26,27,28,29], the growth of MAO layers on Ti-based bulk materials in Ca-P-rich electrolytes occurs in four steps. As soon as the MAO treatment starts (Stage I), the Ti-based surface is instantly covered by a continuous layer of amorphous titanium oxide film, also known as the barrier layer. As the voltage increases (Stage II), the barrier film becomes thicker, and gas evolution may occur during this stage, leading to the formation of fine porosities in the layer. However, due to the discharge channels, such porosities may vary in size and morphology. The following stage (Stage III) is characterised by a further increase in the applied voltage, which provokes spark initiation on the most outer layer of TiO2, with sparks spreading across the surface and becoming stronger until the end of this step. The intense sparks cause localised melting zones on bulk metal and also result in the ionisation of electrolyte components such as H2O, calcium acetate, and phosphate. The anions, such as phosphate and oxygen, may be transferred to the bulk metal through two mechanisms, namely electromigration and diffusion. In contrast, the movement of cations, such as calcium, to the anode is primarily controlled by diffusion. Thus, the TiO2 doped with Ca-P spreads across the layer surface through the discharge channels. Finally, in the last stage (Stage IV), a volcano-like structure forms due to localised plasma, the power and type of the plasma arc, and the existing ions located in the discharge channels. The MAO process continues even when the applied voltage becomes constant; however, this results in stronger arcs, albeit in lower quantities.
The surface morphology following the MAO treatments is shown in Figure 1d–f. It is evident that a multiscale porous structure was formed, with cracks apparent on the surfaces of all sample groups. Furthermore, the addition of Sr did not significantly affect the morphology of the MAO layers; there were no marked changes in either the pore size distribution or the average pore size (Figure 1g–i). These results corroborate previous studies, which found no significant effect of Sr concentration on the pore size and morphology of MAO layers [5,9,30].
Moreover, the addition of Sr into the electrolyte led to a decrease in the number of cracks present in the MAO layer, with crack densities measured as 70 ± 13, 40 ± 15, and 42 ± 13 cracks/cm2 for 0Sr, 00013Sr, and 013Sr, respectively. The presence of cracks may be explained by the rapid cooling of the melt in contact with the electrolyte, which leads to a high thermal gradient [24,31,32]. On the other hand, as the concentration of Sr rises, the intensity of sparks also increases, leading to a greater amount of molten oxide. However, the higher discharge intensity results in more ablation of the molten oxide, which decreases its accumulation on the surface. This situation is not beneficial for the formation of oxide films and may be associated with a decrease in the number of cracks [9,33].
The EDS analysis of the MAO layer surfaces (see Figure 2 and Table 2) reveals that, in addition to Ti, the MAO layers also contain O, Ca, P, and Sr elements. By adding Sr to the MAO electrolyte, the amount of Ca incorporated into the MAO layers increased. On the other hand, for the lowest concentration of Sr, the incorporation of P also increased; however, for the highest concentration, there was a slight decrease in P content. The incorporation of Ca and Sr is easier compared to P because the electric field created during the MAO attracts the cations (Ca2+ and Sr2+) present in the electrolyte to the cathode. In this way, the Ca2+ and Sr2+ are easily combined with OH− and then incorporated into the MAO layer as the voltage increases [30]. According to Zhang et al. [9], there is no significant difference in P content between the outer and inner MAO layers. However, there is a compositional gradient regarding the Ca and Sr content in the MAO layers, with the content of these elements in the outermost MAO layer being higher than in the inner layer. The authors explained this behaviour by noting that the positive charges of these elements and the electric field prevent their movement to the anode. Regarding the Ca/P ratio, as the concentration of Sr increased, the ratio also increased. These findings were also reported by other authors [34,35]. Nan et al. [35] reported that the Sr/(Ca+Sr) ratio in the MAO layers was lower than the ratio in the MAO electrolyte, indicating that the Ca element can penetrate the MAO layers more easily than Sr under the same conditions.
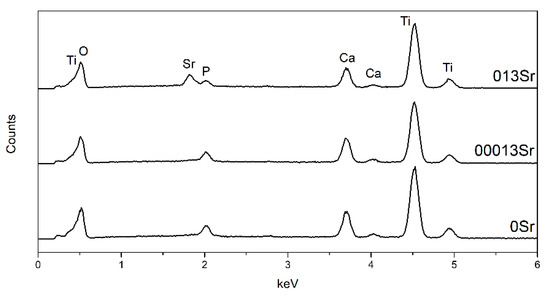
Figure 2.
EDS spectra of MAO-treated surfaces.

Table 2.
Elemental concentrations (at.%) from EDS analysis of MAO-treated surfaces, along with Ca/P ratios.
The less negative the OCP value, the lower the tendency of the metal to corrode. It can be assumed that OCP values can be used to evaluate the probability of corrosion occurrence in a material [36]. Thus, the OCP provides qualitative information regarding the thermodynamics of the system, indicating the susceptibility of a material to corrosion. The evolution of OCP during the last 10 min of immersion in NaCl is shown in Figure 3a. Therefore, by observing the OCP evolution presented in Figure 3a and the EOCP values in Table 3, it can be stated that all MAO-treated samples exhibited a considerably lower tendency to corrosion compared to untreated Ti. On the other hand, the addition of Sr resulted in more positive OCP values; however, no significant difference was observed between the lower and higher Sr concentrations when considering the values along with the standard deviation.
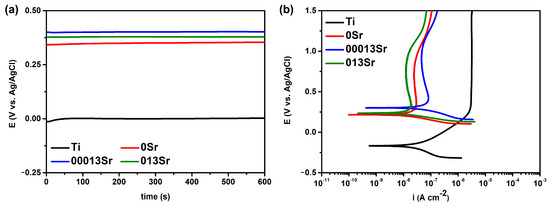
Figure 3.
(a) The OCP evolution during the last 10 min of stabilisation; (b) potentiodynamic polarisation curves.

Table 3.
Open circuit potential (EOCP), corrosion potential (E(i=0)), and passivation current density (ipass) for all tested groups.
Figure 3b shows the representative polarisation curves for all sample groups. The average corrosion potential (E(i=0)) and passive current density (ipass) are presented in Table 3. The results clearly show a reduction in ipass for the MAO-treated samples, indicating that the corrosion resistance of Ti was increased by the MAO treatment. Among the MAO treatments, the lowest passivation current density was found for the 013Sr condition, followed by the 00013Sr and 0Sr conditions. The corrosion potential exhibited a notable rise from untreated titanium to all groups subjected to MAO treatment, with Sr incorporation seeming to have a positive impact on the corrosion potential.
The E(i=0) values were lower than those recorded for EOCP for both the untreated and MAO-treated groups. This behaviour may be explained by the applied cathodic potentials during the potentiodynamic scan, which alter the surface state of the samples, namely by rupturing the passive film, leading to a lower E(i=0) than EOCP [37,38,39,40]. Moreover, it is assumed that hydrogen evolution from water reduction occurs during a cathodic polarisation, while under anodic polarisation, dissolution or passivation mechanisms may occur in the anodic domain [41]. In the present study, the cathodic currents of MAO-treated groups appeared to be lower than those of untreated Ti; thus, it can be assumed that hydrogen evolution is inhibited by the MAO layers.
Based on the potentiodynamic polarisation curves, untreated Ti displayed the characteristic behaviour of a passive metal, with a clearly defined passivation plateau beginning at around 0.450 VAg/AgCl. The passivation plateau remained stable up to the highest potential values. A passivation plateau was also observed for all MAO-treated samples; however, the extension of the plateau was shorter. The passivation plateau of the MAO-treated groups is followed by a slight increase in the current densities, which may be attributed to the degradation of the protective film, likely due to the dissolution of incorporated elements such as Ca, P, and Sr. The increase in current density after the passive region on MAO layers has also been reported by other authors [37,42,43,44,45,46]. While the passive range decreases from untreated Ti to all MAO-treated Ti, the current density values remained significantly lower than those of untreated Ti, even beyond the initial passive range and reaching the maximum applied potential. This demonstrates an apparent enhancement in corrosion behaviour following MAO treatment. The ipass values showed a significant drop (approximately two orders of magnitude) from untreated Ti to MAO-treated groups, with 013Sr presenting the lowest ipass value, followed by 0Sr and 00013Sr, respectively. Some of the present authors [5] produced MAO layers under identical conditions to this study and reported that while there were no differences in the percentage of rutile phase between the MAO layers produced with no Sr (0Sr) and the lower Sr content (00013Sr), the highest Sr content (013Sr) led to an increase in rutile content. It has been reported that MAO layers with higher rutile content may improve the corrosion behaviour compared to layers that are less rutile [47]. Additionally, the improved corrosion behaviour of MAO-treated Ti is attributed by several authors to the presence of a thick oxide layer formed during the treatment [42,43,44,45,46,48].
Yu et al. [16] observed that the corrosion potential on MAO-treated Ti6Al4V surfaces doped with Sr was lower than that of untreated Ti6Al4V and MAO-treated samples without the addition of Sr, while the passive region was shifted to higher potentials. The authors attributed these findings to the influence of surface pores on electrochemical reactions. As a result, surfaces with pores exhibit a lower corrosion potential compared to those without, since the external potential keeps the activation potential within the pores, even when a high potential is applied to the area where the passivation film forms. However, this trend regarding the corrosion potential (E(i=0)) was not observed in the current study, as there were no significant differences in pore size among the surfaces treated with MAO.
Figure 4a,b display the EIS plots for all sample groups, represented as Nyquist and Bode diagrams, respectively. The Nyquist diagram (Figure 4a) reveals that the MAO-treated samples exhibit a larger semicircle diameter, indicating greater corrosion resistance compared to untreated Ti. In the Bode diagram for untreated Ti, at high frequencies (102 Hz to 105 Hz), |Z| remains constant, and the phase angle is close to 0°, reflecting the electrolyte resistance response. At low and mid-range frequencies, the phase angle approaches 90°, which is indicative of the typical capacitive behaviour associated with a compact oxide film.
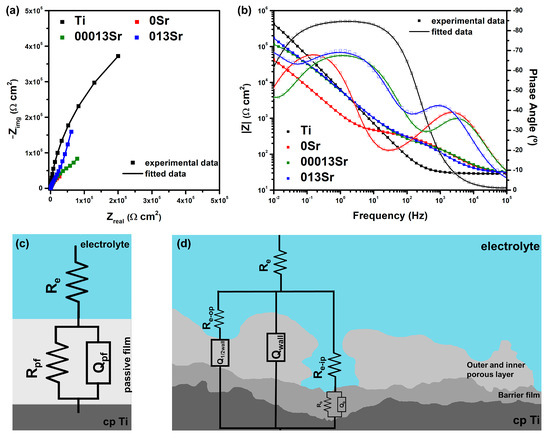
Figure 4.
EIS spectra presented as (a) Nyquist and (b) Bode diagrams, along with the EECs used to fit EIS data for (c) untreated Ti and (d) MAO-treated groups.
Figure 4c,d illustrates the equivalent electrical circuit (EEC) used to model EIS results. The quality of the proposed electrical equivalent circuit (EEC) was assessed based on its goodness of fit (χ2). The EEC presented in Figure 4c represents the passive film formed on the surface of untreated Ti samples for fitting the experimental data. The circuit includes Re—electrolyte resistance; Rpf—passive film resistance; and Qpf—constant phase element (CPE), which account for the non-ideal capacitance of the passive film.
The 0Sr group exhibited two time constants, with one corresponding to the high frequencies, which shifted to higher-frequency values as time elapses. The other MAO-treated groups with the addition of Sr displayed similar behaviour to the 0Sr group, but the resistance corresponding to the low-frequency time constant was higher than that of the 0Sr group. All MAO-treated groups presented a phase angle near −70° for the lowest-frequency range (10−2–10−1 Hz).
Some of the present authors [5,37,49] described the MAO layer as a triplex structure, starting with a barrier film at the metal/oxide interface, which is formed in the initial moments of the MAO treatment. Two porous layers, an inner and an outer layer, grow after the barrier film; the inner layer is characterised by small pores, while the outer porous layer presents larger pores. The interface between the inner and outer porous layers in Ti MAO coatings may influence their formation, structural characteristics, and overall performance. This transition zone is where critical physical, chemical, and electrochemical processes occur, affecting the development and functionality of both layers. Additionally, during the MAO process, the inner porous layer forms first as the anodic treatment progresses, resulting in a compact yet porous structure. The outer porous layer then develops due to continued plasma discharge, leading to increased porosity and roughness. The interface between these layers governs the material transition and significantly impacts layer thickness and uniformity [50,51].
Additionally, the MAO layer is composed of anatase, which forms near the inner porous layer, while a higher amount of rutile is observed on the surface of the outer porous layer. Furthermore, no significant differences were observed in either the structure or thickness of the MAO layers with varying amounts of Sr added [18]. Based on this triplex structure, Alves et al. [37] proposed the EEC presented in Figure 4d. The EEC features a pair, Rbf/Qbf, linked to the barrier film, while the thicker MAO layers are represented by Qwall and Q1/2wall, reflecting the intact porous wall and the porous structure beneath the outer pores, respectively. Parallel resistors with the constant phase elements (CPEs) are not included, as the considerable thickness of the oxide films would result in excessively high values. Additionally, three resistors are incorporated to represent the overall electrolyte resistance (Re) and the resistances of the solution contained within the inner pores (Re-ip) and outer pores (Re-op). Details of all EEC parameters are listed in Table 4.

Table 4.
Parameters obtained from the EIS data using the proposed EECs.
The equation describes the impedance of a CPE, where is the admittance of the CPE, is the angular frequency, is the exponential factor, and represents the imaginary unit. When the exponential factor is equal to one, the CPE response corresponds to that of a capacitor. If is 0, it corresponds to a resistor, and if is −1, it corresponds to an inductor. However, when , a non-ideal capacitor can be described by a CPE. The surface roughness and heterogeneities influence the value of .
To estimate the thickness of each anodic layer, it is necessary to convert the Q values into capacitance (C) [52]. Thus, by following Brug’s equation [53], it was possible to derive Equations (2)–(4) for the passive film of untreated Ti (Cpf), Cwall, and C1/2wall for the MAO-treated porous layers, respectively. Additionally, the capacitance of the barrier film in MAO-treated samples was calculated according to Equation (5), which considers a resistor in parallel with a CPE [53]:
The barrier film of the MAO samples (Cbf) was compared with the passive film (Cpf) naturally formed on the surface of untreated Ti. It was observed that the Cbf values were consistently lower compared to the capacitance of the passive film formed on untreated Ti. This difference can be attributed to the distinct formation mechanisms of these films. The passive film is formed spontaneously and naturally on the Ti surface, whereas the barrier film is formed under an applied potential, which promotes and accelerates its formation. Regarding the Cbf of MAO-treated samples, it was noted that the barrier film formed during the MAO treatment with the highest addition of Sr exhibited the lowest capacitance, while no significant differences were observed between 0Sr and 00013Sr conditions. This behaviour may be attributed to the increase in the conductivity of the electrolyte when Sr was added, which led to a decrease in the breakdown voltage and resulted in differences in the barrier film thickness.
Taking into account the standard deviation for Cwall and C1/2wall, it was observed that both capacitances did not change significantly with the MAO treatment conditions. According to Equations (6) and (7), the capacitance values depend on the thickness:
where ε is the dielectric constant of the oxide film, ε0 is the vacuum permittivity, A is the area, and d is the thickness. Considering ε0 = 8.854 × 10−14 Fcm−1 and ε0 = 100 (typical dielectric constant for TiO2 [54,55]), it may be possible to estimate the thickness of each layer. Nonetheless, a significant concern with this estimation is that the EIS results might be heavily influenced by the capacitance of the space-charge layer, which is likely thinner than the actual oxide film [56,57]. Additionally, the MAO layers formed under different treatment conditions led to oxide films with varying characteristics. Although in all cases, the MAO layers are characterised as a mixture of anatase and rutile, the amount of each phase may differ under each condition, thus leading to different responses.
4. Conclusions
This study investigated the electrochemical behaviour of Ti surfaces treated with Sr-doped MAO using strontium hydroxide octahydrate (Sr(OH)2·8H2O) concentrations of 0.0013 M and 0.13 M. The addition of Sr increased the conductivity of the electrolyte and decreased the breakdown potential, with the effect being more pronounced at higher Sr concentrations. Potentiodynamic polarisation measurements demonstrated that samples treated with higher Sr concentrations exhibited the lowest ipass, suggesting improved overall corrosion resistance.
EIS analysis suggested a similar electrochemical mechanism for all MAO-treated samples, regardless of Sr addition. However, slightly lower average capacitance values for the barrier layer were observed in samples with higher Sr concentrations, which may point to improved barrier properties. Although clear differences were not discernible due to the variability in the data, the observed trends highlight the potential influence of Sr on the electrochemical performance of MAO layers.
The findings highlight the importance of Sr content in governing the corrosion behaviour of MAO-treated Ti surfaces. Future work should focus on exploring a broader range of Sr concentrations and conducting atomic-level characterisations of the MAO layers, especially the barrier layer, to gain deeper insights into the influence of Sr incorporation on the electrochemical mechanisms in these surfaces.
Author Contributions
Conceptualization, A.C.A. and F.T.; methodology, A.C.A., C.D. and F.T.; validation, A.C.A. and C.D.; formal analysis, A.C.A., C.D. and F.T.; investigation, C.D.; writing—original draft, A.C.A.; writing—review and editing, A.C.A. and F.T.; supervision, F.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Scientific and Technological Research Council of Turkey (TUBITAK) with the project grant no. 222M088.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cui, S.; Shi, A.; Xie, Y.; Yu, H.; Wei, Y.; Yang, L.; Qin, G.; Zhang, E. Feasibility study on Ti-15Mo-7Cu with low elastic modulus and high antibacterial property. BioMetals 2022, 35, 1225. [Google Scholar]
- Rodrigues, D.C.; Robert, U.M.; Jacobs, J.J.; Gilbert, J.L.; Urban, R.M. In vivo severe corrosion and hydrogen embrittlement of retrieved modular body titanium alloy hip-implants. J. Biomed. Mater. Res. Part B Appl. Biomater. 2010, 88, 206. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Ma, F.; Liu, P.; Qi, S.; Li, W.; Zhang, K.; Chen, X. Review of micro-arc oxidation of titanium alloys: Mechanism, properties and applications. J. Alloys Compd. 2023, 948, 169773. [Google Scholar]
- Capuccini, C.; Torricelli, P.; Sima, F.; Boanini, E.; Ristoscu, C.; Bracci, B.; Socol, G.; Fini, M.; Mihailescu, I.N.; Bigi, A. Strontium-substituted hydroxyapatite coatings synthesized by pulsed-laser deposition: In vitro osteoblast and osteoclast response. Acta Biomater. 2008, 4, 1885. [Google Scholar] [PubMed]
- Costa, A.I.; Gemini-Piperni, S.; Alves, A.C.; Costa, N.A.; Checca, N.R.; Leite, P.E.; Rocha, L.A.; Pinto, A.M.P.; Toptan, F.; Rossi, A.L.; et al. TiO2 bioactive implant surfaces doped with specific amount of Sr modulate mineralization. Mater. Sci. Eng. C 2021, 120, 111735. [Google Scholar]
- Liu, L.; Ma, F.; Kang, B.; Liu, P.; Qi, S.; Li, W.; Zhang, K.; Chen, X. Preparation and mechanical and biological performance of the Sr-containing microarc oxidation layer on titanium implants. Surf. Coatings Technol. 2023, 463, 129530. [Google Scholar]
- Liu, L.; Ma, F.; Liu, P.; Qi, S.; Li, W.; Zhang, K.; Chen, X. Preparation and antibacterial properties of ZnSr-doped micro-arc oxidation coatings on titanium. Surf. Coatings Technol. 2023, 462, 129469. [Google Scholar]
- Nguyen, A.-N.N.; Kung, K.-C.C.; Chen, K.-C.C.; Hsu, C.-W.W.; Huang, C.-L.L.; Lee, T.-M.M. Characteristics and biological responses of selective laser melted Ti6Al4V modified by micro-arc oxidation. J. Dent. Sci. 2024, 19, 1426. [Google Scholar]
- Zhang, Z.-Y.; Huang, T.-Y.; Zhai, D.-J.; Wang, H.-B.; Feng, K.-Q.; Xiang, L. Study on strontium doped bioactive coatings on titanium alloys surfaces by micro-arc oxidation. Surf. Coatings Technol. 2022, 451, 129045. [Google Scholar] [CrossRef]
- Lu, W.; Zhou, C.; Ma, Y.; Li, J.; Chen, Y.; Jiang, J.; Dong, L.; He, F. Improved osseointegration of strontium-modified titanium implant by regulating angiogenesis and macrophage polarization. Biomater. Sci. 2022, 10, 2198–2214. [Google Scholar] [CrossRef]
- Liu, W.; Wang, D.; He, G.; Li, T.; Zhang, X. A novel porous titanium with engineered surface for bone defect repair in load-bearing position. J. Biomed. Mater. Res. Part A 2024, 112, 1083. [Google Scholar]
- Shen, X.; Fang, K.; Yie, K.H.R.; Zhou, Z.; Shen, Y.; Wu, S.; Zhu, Y.; Deng, Z.; Ma, P.; Ma, J.; et al. High proportion strontium-doped micro-arc oxidation coatings enhance early osseointegration of titanium in osteoporosis by anti-oxidative stress pathway. Bioact. Mater. 2022, 10, 405. [Google Scholar]
- Wang, D.; Chen, M.W.; Wei, Y.J.; Geng, W.B.; Hu, Y.; Luo, Z.; Cai, K.Y. Construction of Wogonin Nanoparticle-Containing Strontium-Doped Nanoporous Structure on Titanium Surface to Promote Osteoporosis Fracture Repair. Adv. Healthc. Mater. 2022, 11, 2201405. [Google Scholar]
- Zhang, W.; Cao, H.; Zhang, X.; Li, G.; Chang, Q.; Zhao, J.; Qiao, Y.; Ding, X.; Yang, G.; Liu, X.; et al. A strontium-incorporated nanoporous titanium implant surface for rapid osseointegration. Nanoscale 2016, 8, 5291. [Google Scholar]
- Liu, W.; Cheng, M.; Wahafu, T.; Zhao, Y.; Qin, H.; Wang, J.; Zhang, X.; Wang, L. The in vitro and in vivo performance of a strontium-containing coating on the low-modulus Ti35Nb2Ta3Zr alloy formed by micro-arc oxidation. J. Mater. Sci. Mater. Med. 2015, 26, 1. [Google Scholar]
- Yu, J.-M.; Cho, H.-R.; Choe, H.-C. Electrochemical characteristics of Sr/Si-doped hydroxyapatite coating on the Ti alloy surface via plasma electrolytic oxidation. Thin Solid Films 2022, 746, 139124. [Google Scholar]
- Kung, K.C.; Lee, T.M.; Lui, T.S. Bioactivity and corrosion properties of novel coatings containing strontium by micro-arc oxidation. J. Alloys Compd. 2010, 508, 384. [Google Scholar]
- Costa, A.I.; Viana, F.; Toptan, F. Preliminary tribocorrosion evaluation of bio-functionalized Ti doped with Ca-P-Sr. Mater. Lett. 2021, 283, 128775. [Google Scholar]
- Albella, J.M.; Montero, I.; Martinez-Duart, J.M. A theory of avalanche breakdown during anodic oxidation. Electrochim. Acta 1987, 32, 255. [Google Scholar]
- Cardoso, G.C.; Grandini, C.R.; Rau, J.V. Comprehensive review of PEO coatings on titanium alloys for biomedical implants. J. Mater. Res. Technol. 2024, 31, 311. [Google Scholar]
- Ikonopisov, S. Theory of electrical breakdown during formation of barrier anodic films. Electrochim. Acta 1977, 22, 1077. [Google Scholar]
- Ding, J.; Liang, J.; Hu, L.T.; Hao, J.C.; Xue, Q.J. Effects of sodium tungstate on characteristics of microarc oxidation coatings formed on magnesium alloy in silicate-KOH electrolyte. Trans. Nonferrous Met. Soc. China 2007, 17, 244. [Google Scholar]
- Hussein, R.O.; Nie, X.; Northwood, D.O. An investigation of ceramic coating growth mechanisms in plasma electrolytic oxidation (PEO) processing. Electrochim. Acta 2013, 112, 111. [Google Scholar]
- Ahounbar, E.; Khoei, S.M.M.; Omidvar, H. Characteristics of in-situ synthesized Hydroxyapatite on TiO2 ceramic via plasma electrolytic oxidation. Ceram. Int. 2019, 45, 3118. [Google Scholar]
- Venkateswarlu, K.; Rameshbabu, N.; Sreekanth, D.; Sandhyarani, M.; Bose, A.C.; Muthupandi, V.; Subramanian, S. Role of electrolyte chemistry on electronic and in vitro electrochemical properties of micro-arc oxidized titania films on Cp Ti. Electrochim. Acta 2013, 105, 468. [Google Scholar]
- van Hengel, I.A.J.; Laçin, M.; Minneboo, M.; Fratila-Apachitei, L.E.; Apachitei, I.; Zadpoor, A.A. The effects of plasma electrolytically oxidized layers containing Sr and Ca on the osteogenic behavior of selective laser melted Ti6Al4V porous implants. Mater. Sci. Eng. C 2021, 124, 112074. [Google Scholar]
- Ochiabuto, K.; Pitts, H.; Cao, Y.; Meletis, E. Quantitative modeling of oxide growth in plasma electrolytic oxidation of titanium. Mater. Chem. Phys. 2024, 323, 129633. [Google Scholar]
- Kaseem, M.; Fatimah, S.; Nashrah, N.; Ko, Y.G. Recent progress in surface modification of metals coated by plasma electrolytic oxidation: Principle, structure, and performance. Prog. Mater. Sci. 2021, 117, 100735. [Google Scholar]
- Kuroda, P.A.B.; Rossi, M.C.; Grandini, C.R.; Afonso, C.R.M. Assessment of applied voltage on the structure, pore size, hardness, elastic modulus, and adhesion of anodic coatings in Ca-, P-, and Mg-rich produced by MAO in Ti–25Ta–Zr alloys. J. Mater. Res. Technol. 2023, 26, 4656. [Google Scholar]
- Sato, M.; Chen, P.; Tsutsumi, Y.; Shiota, M.; Hanawa, T.; Kasugai, S. Effect of strontium ions on calcification of preosteoblasts cultured on porous calcium-and phosphate-containing titanium oxide layers formed by micro-arc oxidation. Dent. Mater. J. 2016, 35, 627. [Google Scholar] [CrossRef]
- Baron-Wiecheć, A.; Curioni, M.; Arrabal, R.; Matykina, E.; Skeldon, P.; Thompson, G.E. Plasma electrolytic oxidation of coupled light metals. Trans. IMF 2013, 91, 107. [Google Scholar] [CrossRef]
- Clyne, T.W.; Troughton, S.C. A review of recent work on discharge characteristics during plasma electrolytic oxidation of various metals. Int. Mater. Rev. 2019, 64, 127. [Google Scholar] [CrossRef]
- Zhai, D.-J.; Feng, K.-Q. Preparation of micro/nano-structured ceramic coatings on Ti6Al4V alloy by plasma electrolytic oxidation process. Trans. Nonferrous Met. Soc. China 2019, 29, 2546. [Google Scholar] [CrossRef]
- Yu, J.M.; Choe, H.C. Morphology changes and bone formation on PEO-treated Ti-6Al-4V alloy in electrolyte containing Ca, P, Sr, and Si ions. Appl. Surf. Sci. 2019, 477, 121. [Google Scholar] [CrossRef]
- Nan, K.; Wu, T.; Chen, J.; Jiang, S.; Huang, Y.; Pei, G. Strontium doped hydroxyapatite film formed by micro-arc oxidation. Mater. Sci. Eng. C 2009, 29, 1554. [Google Scholar] [CrossRef]
- Gowtham, S.; Arunnellaiappan, T.; Rameshbabu, N. An investigation on pulsed DC plasma electrolytic oxidation of cp-Ti and its corrosion behaviour in simulated body fluid. Surf. Coatings Technol. 2016, 301, 63. [Google Scholar]
- Alves, A.C.; Wenger, F.; Ponthiaux, P.; Celis, J.P.; Pinto, A.M.; Rocha, L.A.; Fernandes, J.C.S. Corrosion mechanisms in titanium oxide-based films produced by anodic treatment. Electrochim. Acta 2017, 234, 16. [Google Scholar] [CrossRef]
- Mariano, N.A.; Oliveira, R.G.; Braga, E.I.; Rigo, E.C.S. Corrosion characterization of titanium alloys by electrochemical techniques in artificial saliva and SBF solution. Key Eng. Mater. 2009, 396–398, 315. [Google Scholar] [CrossRef]
- Arunnellaiappan, T.; Babu, N.K.; Krishna, L.R.; Rameshbabu, N. Influence of frequency and duty cycle on microstructure of plasma electrolytic oxidized AA7075 and the correlation to its corrosion behavior. Surf. Coatings Technol. 2015, 280, 136. [Google Scholar] [CrossRef]
- De Assis, S.L.; Wolynec, S.; Costa, I. Corrosion characterization of titanium alloys by electrochemical techniques. Electrochim. Acta 2006, 51, 1815. [Google Scholar] [CrossRef]
- Lu, Y.; Wan, P.; Tan, L.; Zhang, B.; Yang, K.; Lin, J. Preliminary study on a bioactive Sr containing Ca-P coating on pure magnesium by a two-step procedure. Surf. Coatings Technol. 2014, 252, 79. [Google Scholar] [CrossRef]
- Park, I.S.; Woo, T.G.; Jeon, W.Y.; Park, H.H.; Lee, M.H.; Bae, T.S.; Seol, K.W. Surface characteristics of titanium anodized in the four different types of electrolyte. Electrochim. Acta 2007, 53, 863. [Google Scholar]
- Oliveira, F.G.; Ribeiro, A.R.; Perez, G.; Archanjo, B.S.; Gouvea, C.P.; Araújo, J.R.; Campos, A.P.C.; Kuznetsov, A.; Almeida, C.M.; Maru, M.M.; et al. Understanding growth mechanisms and tribocorrosion behaviour of porous TiO2 anodic films containing calcium, phosphorous and magnesium. Appl. Surf. Sci. 2015, 341, 1. [Google Scholar] [CrossRef]
- Park, M.G.; Choe, H.C. Corrosion behaviors of bioactive element coatings on PEO-treated Ti-6Al-4V alloys. Surf. Coatings Technol. 2019, 376, 44. [Google Scholar]
- Mashtalyar, D.V.; Nadaraia, K.V.; Gnedenkov, A.S.; Imshinetskiy, I.M.; Piatkova, M.A.; Pleshkova, A.I.; Belov, E.A.; VFilonina, S.; Suchkov, S.N.; Sinebryukhov, S.L.; et al. Bioactive Coatings Formed on Titanium by Plasma Electrolytic Oxidation: Composition and Properties. Materials 2020, 13, 4121. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Cheng, Y.; Tu, W.; Zhan, T.-Y.; Cheng, Y. The black and white coatings on Ti-6Al-4V alloy or pure titanium by plasma electrolytic oxidation in concentrated silicate electrolyte. Appl. Surf. Sci. 2018, 428, 684. [Google Scholar]
- Shokouhfar, M.; Dehghanian, C.; Montenero, A.; Baradaran, A. Preparation of ceramic coating on Ti substrate by plasma electrolytic oxidation in different electrolytes and evaluation of its corrosion resistance: Part II. Appl. Surf. Sci. 2012, 258, 2416. [Google Scholar]
- Fazel, M.; Salimijazi, H.R.; Golozar, M.A.; Jazi, M.R.G. A comparison of corrosion, tribocorrosion and electrochemical impedance properties of pure Ti and Ti6Al4V alloy treated by micro-arc oxidation process. Appl. Surf. Sci. 2015, 324, 751. [Google Scholar]
- Alves, A.C.; Costa, A.I.; Toptan, F.; Alves, J.L.; Leonor, I.; Ribeiro, E.; Reis, R.L.; Pinto, A.M.P.; Fernandes, J.C.S. Effect of bio-functional MAO layers on the electrochemical behaviour of highly porous Ti. Surf. Coatings Technol. 2020, 386, 125487. [Google Scholar]
- Wang, Y.M.; Guo, L.X.; Ouyang, J.H.; Zhou, Y.; Jia, D.C. Interface adhesion properties of functional coatings on titanium alloy formed by microarc oxidation method. Appl. Surf. Sci. 2009, 255, 6875–6880. [Google Scholar]
- Hu, Y.; Wang, Z.; Ai, J.; Bu, S.; Liu, H. Preparation of Coating on the Titanium Surface by Micro-Arc Oxidation to Improve Corrosion Resistance. Coatings 2021, 11, 230. [Google Scholar] [CrossRef]
- Hsu, C.H.; Mansfeld, F. Technical Note: Concerning the Conversion of the Constant Phase Element Parameter Y 0 into a Capacitance. Corrosion 2001, 57, 747. [Google Scholar]
- Orazem, M.E.; Tribollet, B. Electrochemical Impedance Spectroscopy; John Wiley & Sons: Hoboken, NJ, USA, 2017. [Google Scholar]
- Wypych, A.; Bobowska, I.; Tracz, M.; Opasinska, A.; Kadlubowski, S.; Krzywania-Kaliszewska, A.; Grobelny, J.; Wojciechowski, P. Dielectric properties and characterisation of titanium dioxide obtained by different chemistry methods. J. Nanomater. 2014, 2014, 124814. [Google Scholar]
- Lundstrom, J.; Rinehart, L.; Pate, R.; Smith, T.; Krogh, M.; Huebner, W. Measurement of the dielectric strength of titanium dioxide ceramics. In Proceedings of the 12th IEEE International Pulsed Power Conference, Monterey, CA, USA, 27–30 June 1999; IEEE: Piscataway, NJ, USA, 1999; pp. 10–12. [Google Scholar]
- Çaha, I.; Alves, A.C.; Chirico, C.; Pinto, A.M.; Tsipas, S.; Gordo, E.; Bondarchuk, O.; Deepak, F.L.; Toptan, F. Atomic–scale investigations of passive film formation on Ti-Nb alloys. Appl. Surf. Sci. 2023, 615, 156282. [Google Scholar]
- Blackwood, D.J. Influence of the space-charge region on electrochemical impedance measurements on passive oxide films on titanium. Electrochim. Acta 2000, 46, 563. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).