Quantitative and Qualitative Analysis of Atmospheric Effects on Carbon Steel Corrosion Using an ANN Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Collection and Variable Selection
2.2. Design of Model Architecture
2.3. Model Validation
2.4. Feature Visualization
3. Results and Discussions
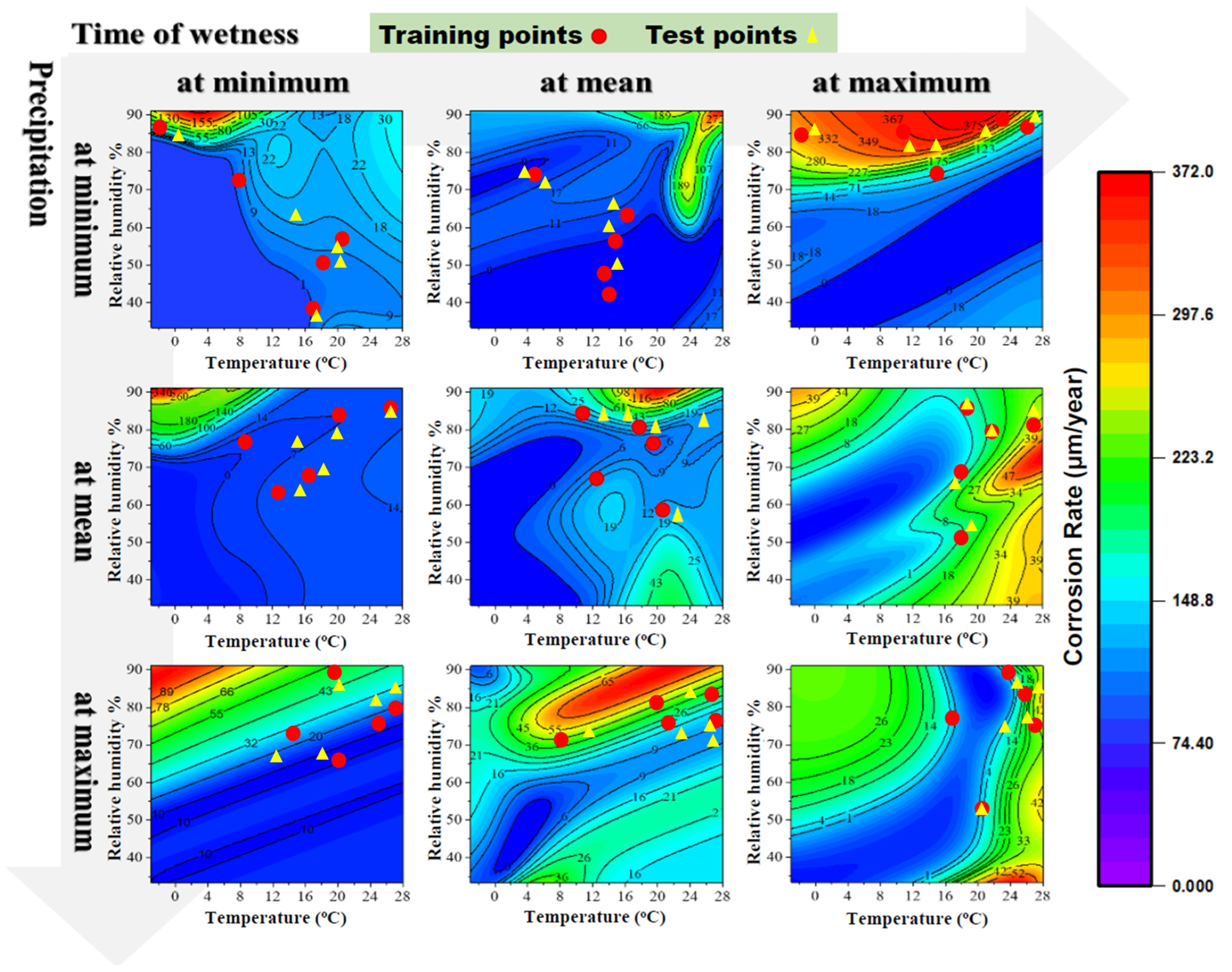
3.1. Heat Map
3.2. Predicted Corrosion Rate of Carbon Steel at Various Atmospheric Conditions
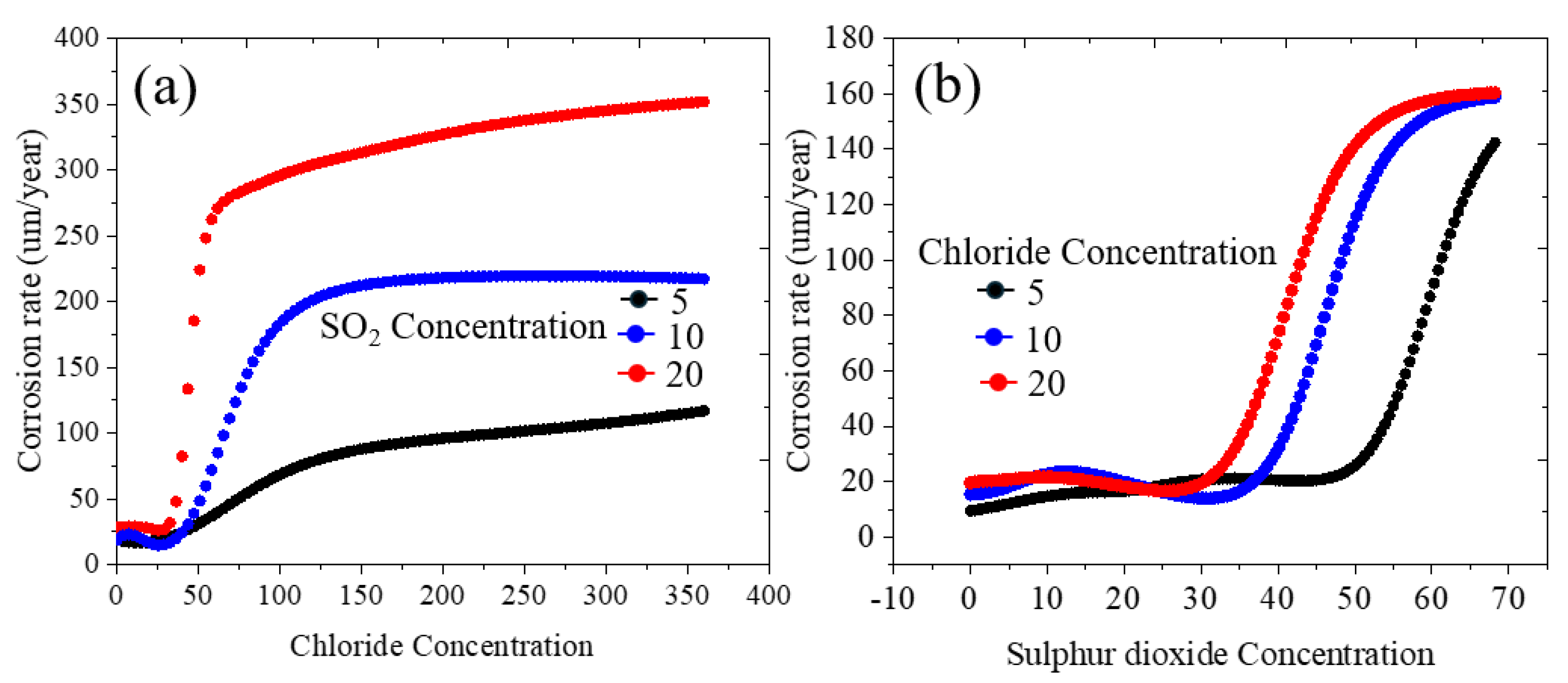
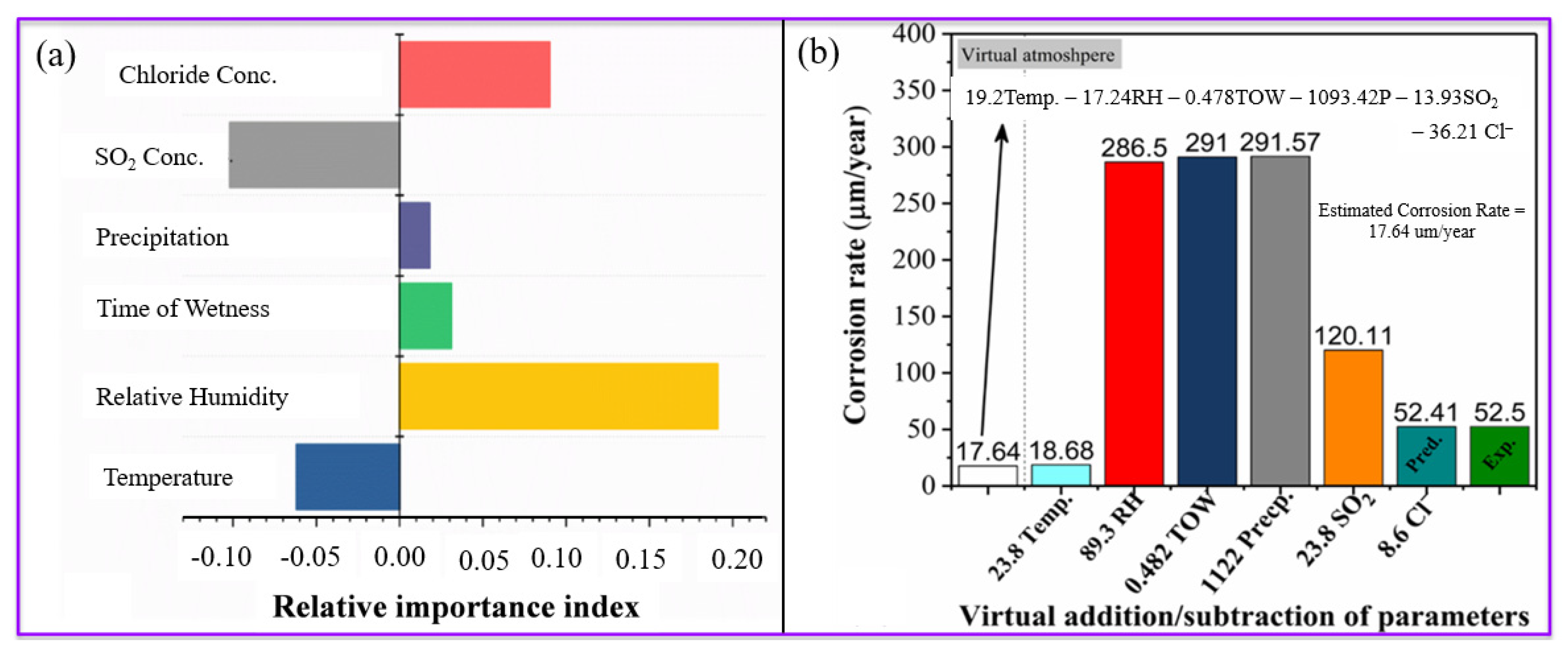
3.3. Qualitative and Quantitative Estimation of Corrosion Rate
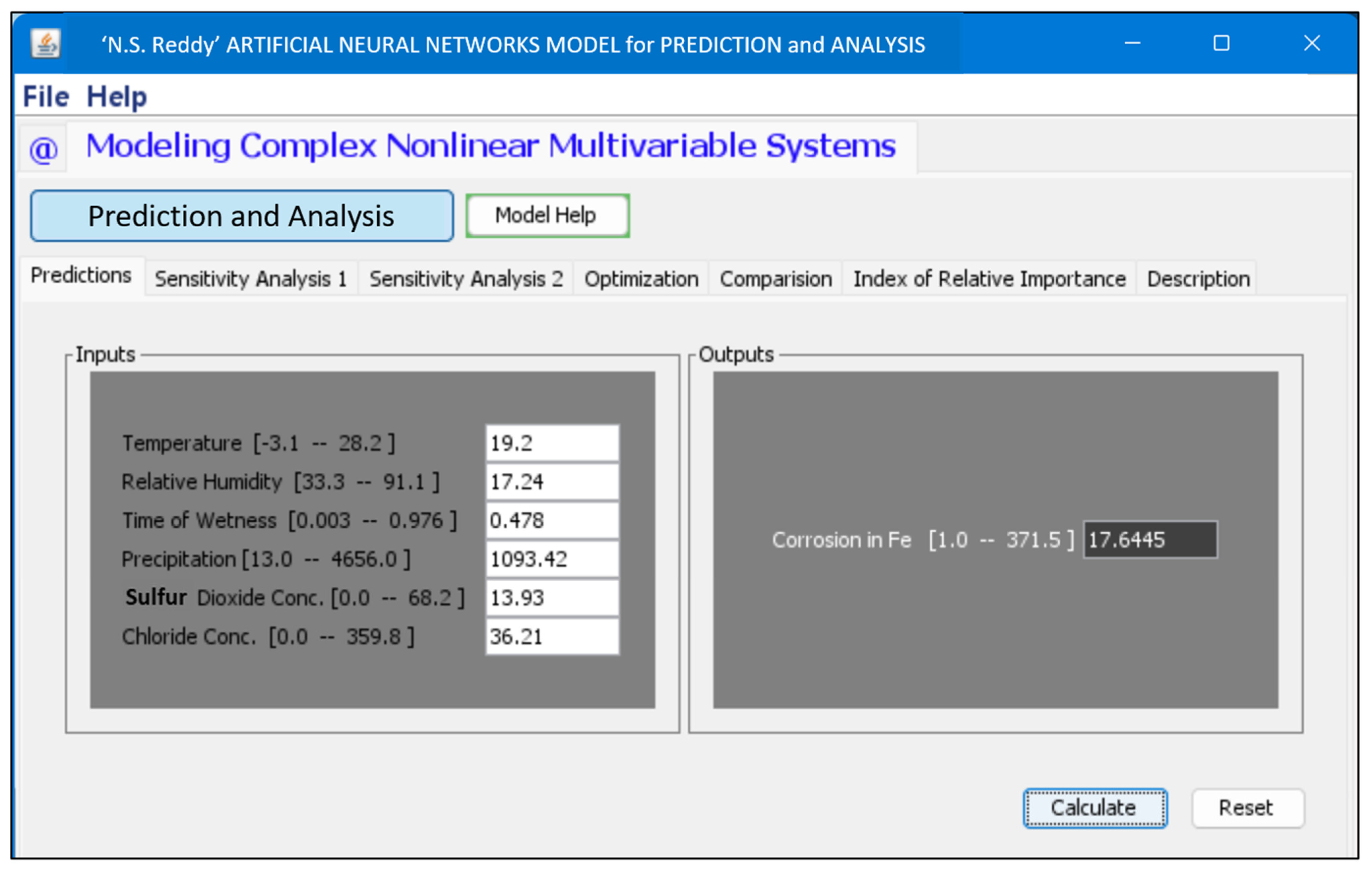
3.4. GUI for Corrosion Rate Prediction Using ANN
3.5. Limitations and Future Work
4. Conclusions
- The developed ANN model showed excellent accuracy in predicting the corrosion rate of carbon steels with an R2 of 97.2% for the training dataset and 95.6% for the testing dataset, demonstrating its effectiveness in capturing the complex relationships between atmospheric variables and corrosion rates.
- Among the atmospheric variables evaluated, relative humidity (RH) was found to have the most significant impact on corrosion rates, surpassing the influence of temperature and sulfur dioxide (SO2) concentration.
- A graphical user interface (GUI) is provided, which offers an interactive and user-friendly platform for visualizing the effects of infinite combinations of various atmospheric parameters. With a GUI, one can easily predict the effect of a single variable, or the combined effect of two variables, can see the trend of increasing or decreasing the corrosion rate with a particular variable, and can also see the sensitivity analysis without prior knowledge of the programming.
- The model provides practical benefits in several key scenarios: (a) enabling condition-based maintenance for coastal infrastructure subject to variable corrosion risks, (b) optimizing inspection schedules for industrial facilities under changing climate conditions, (c) improving protective coating application timing for transportation infrastructure such as bridges and railways, and (d) helping utility companies prioritize maintenance resources across geographically distributed assets based on location-specific corrosion risk profiles.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhi, Y.; Jin, Z.; Lu, L.; Yang, T.; Zhou, D.; Pei, Z.; Wu, D.; Fu, D.; Zhang, D.; Li, X. Improving atmospheric corrosion prediction through key environmental factor identification by random forest-based model. Corros. Sci. 2021, 178, 109084. [Google Scholar] [CrossRef]
- Pei, Z.; Zhang, D.; Zhi, Y.; Yang, T.; Jin, L.; Fu, D.; Cheng, X.; Terryn, H.A.; Mol, J.M.C.; Li, X. Towards understanding and prediction of atmospheric corrosion of an Fe/Cu corrosion sensor via machine learning. Corros. Sci. 2020, 170, 108697. [Google Scholar] [CrossRef]
- Schweitzer, P.A. Atmospheric Degradation and Corrosion Control; CRC Press: Boca Raton, FL, USA, 1999. [Google Scholar]
- Chico, B.; De la Fuente, D.; Díaz, I.; Simancas, J.; Morcillo, M. Annual atmospheric corrosion of carbon steel worldwide. An integration of ISOCORRAG, ICP/UNECE and MICAT databases. Materials 2017, 10, 601. [Google Scholar] [CrossRef] [PubMed]
- Ishtiaq, M.; Inam, A.; Tiwari, S.; Seol, J.B. Microstructural, mechanical, and electrochemical analysis of carbon doped AISI carbon steels. Appl. Microsc. 2022, 52, 10. [Google Scholar] [CrossRef]
- LeBozec, N.; Jonsson, M.; Thierry, D. Atmospheric corrosion of magnesium alloys: Influence of temperature, relative humidity, and chloride deposition. Corrosion 2004, 60, 356–361. [Google Scholar] [CrossRef]
- Wang, X.; Li, X.; Tian, X. Influence of temperature and relative humidity on the atmospheric corrosion of zinc in field exposures and laboratory environments by atmospheric corrosion monitor. Int. J. Electrochem. Sci. 2015, 10, 8361–8373. [Google Scholar] [CrossRef]
- Cai, Y.; Zhao, Y.; Ma, X.; Zhou, K.; Chen, Y. Influence of environmental factors on atmospheric corrosion in dynamic environment. Corros. Sci. 2018, 137, 163–175. [Google Scholar] [CrossRef]
- Mansfeld, F. Atmospheric corrosion rates, time-of-wetness and relative humidity. Mater. Corros. 1979, 30, 38–42. [Google Scholar] [CrossRef]
- Jain, S.; Jain, R.; Wagri, N.K.; Sikarwar, A.S.; Khaire, S.J.; Dewangan, S.K.; Jeon, Y.; Ahn, B. Reducing experimental dependency: Machine-learning-based prediction of Co effects on the mechanical properties of AlCrFeNiCox high-entropy alloys. Mater. Today Commun. 2025, 44, 112055. [Google Scholar] [CrossRef]
- Cho, M.; Gim, J.; Kim, J.H.; Kang, S. Development of an Artificial Neural Network Model to Predict the Tensile Strength of Friction Stir Welding of Dissimilar Materials Using Cryogenic Processes. Appl. Sci. 2024, 14, 9309. [Google Scholar] [CrossRef]
- Kamrunnahar, M.; Urquidi-Macdonald, M. Prediction of corrosion behavior using neural network as a data mining tool. Corros. Sci. 2010, 52, 669–677. [Google Scholar] [CrossRef]
- Zadeh Shirazi, A.; Mohammadi, Z. A hybrid intelligent model combining ANN and imperialist competitive algorithm for prediction of corrosion rate in 3C steel under seawater environment. Neural Comput. Appl. 2017, 28, 3455–3464. [Google Scholar] [CrossRef]
- Birbilis, N.; Cavanaugh, M.K.; Sudholz, A.D.; Zhu, S.M.; Easton, M.A.; Gibson, M.A. A combined neural network and mechanistic approach for the prediction of corrosion rate and yield strength of magnesium-rare earth alloys. Corros. Sci. 2011, 53, 168–176. [Google Scholar] [CrossRef]
- Wen, Y.F.; Cai, C.Z.; Liu, X.H.; Pei, J.F.; Zhu, X.J.; Xiao, T.T. Corrosion rate prediction of 3C steel under different seawater environment by using support vector regression. Corros. Sci. 2009, 51, 349–355. [Google Scholar] [CrossRef]
- Shi, J.; Wang, J.; Macdonald, D.D. Prediction of primary water stress corrosion crack growth rates in Alloy 600 using artificial neural networks. Corros. Sci. 2015, 92, 217–227. [Google Scholar] [CrossRef]
- Pintos, S.; Queipo, N.V.; Troconis de Rincón, O.; Rincón, A.; Morcillo, M. Artificial neural network modeling of atmospheric corrosion in the MICAT project. Corros. Sci. 2000, 42, 35–52. [Google Scholar] [CrossRef]
- Lee, S.; Narayana, P.L.; Seok, B.W.; Panigrahi, B.B.; Lim, S.-G.; Reddy, N.S. Quantitative estimation of corrosion rate in 3C steels under seawater environment. J. Mater. Res. Technol. 2021, 11, 681–686. [Google Scholar] [CrossRef]
- Chen, B.; Ge, B.; Zhang, X.; Yang, D.; Yang, P.; Lu, W.; Min, J.; Ming, P.; Zhang, C. Role of oxide layer on corrosion resistance and surface conductivity of titanium bipolar plates for proton exchange membrane fuel cell. J. Power Sources 2024, 624, 235637. [Google Scholar] [CrossRef]
- Kordas, G. Corrosion Barrier Coatings: Progress and Perspectives of the Chemical Route. Corros. Mater. Degrad. 2022, 3, 376–413. [Google Scholar] [CrossRef]
- Pilch, O.; Faltejsek, P.; Hurby, V.; Krbata, M. The Corrosion Resistance of Turbocharger Stator after Plasma Nitriding Process. Manuf. Technol. 2017, 17, 360–364. [Google Scholar] [CrossRef]
- Yadav, A.; Panjikar, S.; Raman, R.K.S. Graphene-Based Impregnation into Polymeric Coating for Corrosion Resistance. Nanomaterials 2025, 15, 486. [Google Scholar] [CrossRef]
- Song, Z.; Song, H.; Liu, J.; Liu, H. Tuning heterogeneous microstructure and improving mechanical properties of twin-roll strip cast low carbon steels by on-line heat treatments. J. Mater. Res. Technol. 2024, 30, 5894–5904. [Google Scholar] [CrossRef]
- ISO 9223; Corrosion of Metals and Alloys—Corrosivity of Atmospheres—Classification, Determination and Estimation. International Organization for Standardization: Geneva, Switzerland, 2012.
- ASTM G92; Standard Practice for Characterization of Atmospheric Test Sites. ASTM International: West Conshohocken, PA, USA, 2020.
- Reddy, N.S.; Krishnaiah, J.; Hong, S.-G.; Lee, J.S. Modeling medium carbon steels by using artificial neural networks. Mater. Sci. Eng. A 2009, 508, 93–105. [Google Scholar] [CrossRef]
- Zhang, X.; He, W.; Odnevall Wallinder, I.; Pan, J.; Leygraf, C. Determination of instantaneous corrosion rates and runoff rates of copper from naturally patinated copper during continuous rain events. Corros. Sci. 2002, 44, 2131–2151. [Google Scholar] [CrossRef]
- Cole, I.S.; Paterson, D.A.; Ganther, W.D. Holistic model for atmospheric corrosion Part 1—Theoretical framework for production, transportation and deposition of marine salts. Corros. Eng. Sci. Technol. 2003, 38, 129–134. [Google Scholar] [CrossRef]
- Xue, F.; Wei, X.; Dong, J.; Wang, C.; Ke, W. Effect of chloride ion on corrosion behavior of low carbon steel in 0.1 M NaHCO3 solution with different dissolved oxygen concentrations. J. Mater. Sci. Technol. 2019, 35, 596–603. [Google Scholar] [CrossRef]
- Xiang, Y.; Wang, Z.; Xu, C.; Zhou, C.; Li, Z.; Ni, W. Impact of SO2 concentration on the corrosion rate of X70 steel and iron in water-saturated supercritical CO2 mixed with SO2. J. Supercrit. Fluids 2011, 58, 286–294. [Google Scholar] [CrossRef]
- Pei, Z.; Cheng, X.; Yang, X.; Li, Q.; Xia, C.; Zhang, D.; Li, X. Understanding environmental impacts on initial atmospheric corrosion based on corrosion monitoring sensors. J. Mater. Sci. Technol. 2021, 64, 214–221. [Google Scholar] [CrossRef]
- Krbata, M.; Ciger, R.; Kohutiar, M.; Sozańska, M.; Eckert, M.; Barenyi, I.; Kianicova, M.; Jus, M.; Beronská, N.; Mendala, B.; et al. Effect of Supercritical Bending on the Mechanical & Tribological Properties of Inconel 625 Welded Using the Cold Metal Transfer Method on a 16Mo3 Steel Pipe. Materials 2023, 16, 5014. [Google Scholar] [CrossRef]



| Fe | C | Mn | Si | S | P | Cr | Ni | Cu | Al |
|---|---|---|---|---|---|---|---|---|---|
| 99.5 | 0.045 | 0.39 | 0.321 | 0.0054 | 0.076 | 0.67 | 0.063 | 0.145 | 0.0058 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Narayana, P.L.; Tiwari, S.; Maurya, A.K.; Ishtiaq, M.; Park, N.; Reddy, N.G.S. Quantitative and Qualitative Analysis of Atmospheric Effects on Carbon Steel Corrosion Using an ANN Model. Metals 2025, 15, 607. https://doi.org/10.3390/met15060607
Narayana PL, Tiwari S, Maurya AK, Ishtiaq M, Park N, Reddy NGS. Quantitative and Qualitative Analysis of Atmospheric Effects on Carbon Steel Corrosion Using an ANN Model. Metals. 2025; 15(6):607. https://doi.org/10.3390/met15060607
Chicago/Turabian StyleNarayana, Pasupuleti L., Saurabh Tiwari, Anoop K. Maurya, Muhammad Ishtiaq, Nokeun Park, and Nagireddy Gari Subba Reddy. 2025. "Quantitative and Qualitative Analysis of Atmospheric Effects on Carbon Steel Corrosion Using an ANN Model" Metals 15, no. 6: 607. https://doi.org/10.3390/met15060607
APA StyleNarayana, P. L., Tiwari, S., Maurya, A. K., Ishtiaq, M., Park, N., & Reddy, N. G. S. (2025). Quantitative and Qualitative Analysis of Atmospheric Effects on Carbon Steel Corrosion Using an ANN Model. Metals, 15(6), 607. https://doi.org/10.3390/met15060607










