Abstract
The inhibitory effect of the alkaloid extract from Chimarrhis cymosa on C38 steel corrosion in 1M HCl was examined through electrochemical investigations. An inhibition efficiency of 90% was achieved with 200 mg/L of the alkaloid extract from Chimarrhis cymosa at 25 °C. Potentiodynamic polarization revealed that the extract acts as a mixed-type inhibitor. Nyquist plots showed that an increase in the concentration of the alkaloid extract from Chimarrhis cymosa led to an increase in charge-transfer resistance and a decrease in double-layer capacitance, resulting in enhanced inhibition efficiency. The adsorption of inhibitor molecules followed the Langmuir adsorption isotherm. XPS analysis confirmed the formation of an inhibitor layer on the steel surface containing the Chimarrhis cymosa alkaloidic extract.
1. Introduction
Many inhibitors currently in use are synthesized from low-cost raw materials or derived from organic compounds that incorporate heteroatoms, such as nitrogen, sulfur, phosphorus, or oxygen, within their aromatic rings or carbon chains. However, the majority of these anticorrosive substances present toxicity risks to both human health and the environment. These compounds can cause damage to the nervous system and disrupt essential biochemical and enzymatic processes, potentially resulting in both temporary and permanent damage. This toxicity can manifest during either the synthesis or application of these compounds. Furthermore, their non-biodegradability contributes to pollution concerns. Plants have long been acknowledged as sources of natural compounds, some of which possess complex molecular structures and diverse physical, biological, and chemical properties. While most of these extracted compounds find utility primarily in pharmaceuticals and biofuels, their use is particularly appealing due to their biodegradability, environmental friendliness, low cost, and widespread availability. Consequently, numerous research endeavors have been devoted to exploring plant-derived substances as corrosion inhibitors for metals and alloys across various corrosive environments [1,2,3,4,5].
Alkaloids represent a broader category of secondary substances that have garnered significant interest from pharmacists and pharmaceutical industries due to their toxic or medicinal properties. Some examples include the opium salt discovery by Derosne in 1803, quinine by Pelletier and Caventou in 1821, the semi-synthesis of vincamine by LeMen and Levy in 1975, and the semi-synthesis of taxol by Potier in 1992. Presently, there are over 5500 known alkaloids, with this list continuously expanding. Given that all alkaloids contain nitrogen (N), often within a heterocyclic structure, they emerge as promising candidates for effectively inhibiting metal corrosion.
Alkaloids extracted from various plant sources have been extensively researched for their effectiveness as corrosion inhibitors in acidic environments. These alkaloids, including papaverine, strychnine, quinine, piperine, liriodenine, oxoanalobine, nicotine, brucine, berberine, harmane, and others, have shown significant inhibitory effects on steel corrosion in media such as sulfuric acid and hydrochloric acid [6,7,8,9,10,11,12,13]. Overall, previous studies highlight the potential of alkaloids as corrosion inhibitors, offering insights for industrial corrosion control applications.
The aim of this study is to investigate the inhibitory effect of alkaloid extracts from Chimarrhis cymose (AECC), a naturally occurring substance, on the corrosion behavior of C38 steel in a 1M HCl solution. Polarization curves and electrochemical impedance spectroscopy were conducted to explore the mechanism of corrosion inhibition. X-ray photoelectron spectroscopy was also performed to investigate the corrosion inhibition mechanism of AECC on steel in acidic solution. The adsorption mode and the corrosion inhibition mechanism on the steel surface were analyzed and discussed.
2. Materials and Methods
2.1. Specimen Preparation and Solution for Corrosion Tests
The corrosion tests involved electrodes fabricated from sheets of C38 steel. These steel strips contained 0.36 wt% carbon (C), 0.66 wt% manganese (Mn), 0.27 wt% silicon (Si), 0.02 wt% sulfur (S), 0.015 wt% phosphorus (P), 0.21 wt% chromium (Cr), 0.02 wt% molybdenum (Mo), 0.22 wt% copper (Cu), and 0.06 wt% aluminum (Al), with the remaining composition being iron (Fe). The specimens were embedded in epoxy resin, leaving a functional area of 0.78 cm2. The working surface was then polished using 180 and 1200 grit grinding papers, followed by cleaning with distilled water and ethanol. The corrosive solutions (1M HCl) were prepared by diluting 37% HCl (analytical reagent grade) with doubly distilled water.
2.2. Preparation of Plant Extract
The leaves from Chimarrhis cymosa were collected in the north of Martinique near rivers and then air-dried at room temperature (30–35 °C). The total alkaloids were obtained through the alkaloid extraction protocol, which involved a two-step process. The first step was a solid–liquid extraction of the plant powder using a 5% ammonia solution and dichloromethane (CH2Cl2). In the second step, a liquid–liquid extraction of the organic solution was carried out using a 3% hydrochloric acid solution. The acidic aqueous phases obtained sequentially were alkalinized with a 25% ammonia (NH4OH) solution and then re-extracted with dichloromethane. The organic phases were then dried with anhydrous sodium sulfate (Na2SO4) and evaporated to dryness, yielding a mixture of alkaloids called total alkaloids for each extract [11,12,13]. The alkaloid extract from Chimarrhis cymosa (AECC) was utilized in a concentration range of 25–200 mg/L in acidic media.
2.3. Electrochemical Measurements
Electrochemical assessments, encompassing potentiodynamic polarization curves and electrochemical impedance spectroscopy (EIS), were conducted in a three-electrode cell configuration. The working electrode comprised a C38 steel specimen, while a platinum wire served as the counter electrode, and a saturated calomel electrode (SCE) functioned as the reference electrode. The surface area of the counter electrode was four times larger than that of the working electrode. It was a platinum plate of 2 × 3 cm. Prior to each set of polarization measurements covering a wider potential range and EIS experiments, the electrode underwent a corrosion process to establish its open-circuit potential (OCP) over a period of 3 h. This duration was necessary to achieve a quasi-steady state for the OCP, which corresponds to the corrosion potential (Ecorr) of the working electrode. Anodic and cathodic polarization curves were generated using a constant sweep rate of 20 mV/min, ranging from −300 mV to 300 mV relative to the open-circuit potential. EIS measurements were conducted with AC signals of 5 mV peak-to-peak amplitude under varying conditions within a frequency range of 100 kHz to 10 mHz. These electrochemical measurements were executed using a VMP3 potentiostat (Bio-Logic, Claix, France). After three hours of immersion, the electrochemical measurements were conducted, starting with EIS, followed by linear polarization (LP) measurements. Each experimental measurement was repeated three times to ensure the reliability and reproducibility of the results.
2.4. XPS
The steel disks were immersed in a 1M HCl solution containing the AECC as an inhibitor. After removal from the solution, the disks were rinsed with ethanol and dried in air at room temperature. X-ray photoelectron spectroscopy (XPS) was used to analyze the surface composition of the disks using a VG ESCALAB 220 XL spectrometer (VG Scientific, West Sussex, UK). The binding energy scale was calibrated using Cu 2p3/2, Ag 3d5/2, and Au 4f7/2 peak positions and internally referenced to the C 1s energy at 285 eV for aliphatic-like species. The outer layer atomic composition was quantified, and spectral simulation of the experimental peaks was performed using the software provided by VG Scientific (XPS Peak 4.1).
3. Results and Discussion
3.1. Polarization Curves
Figure 1 presents the cathodic and anodic polarization curves obtained for C38 steel in 1M hydrochloric acid solutions without and with various concentrations of AECC. As can be observed in Figure 1, the presence of AECC in the solution significantly affects both the anodic and cathodic reactions, indicating that AECC acts as a mixed-type inhibitor. The addition of AECC to the hydrochloric acid solution reduces the anodic dissolution of iron and also retards the cathodic hydrogen evolution reaction, as described by Equations (1) and (2). These results suggest that AECC can effectively inhibit the corrosion of C38 steel in acidic solutions.
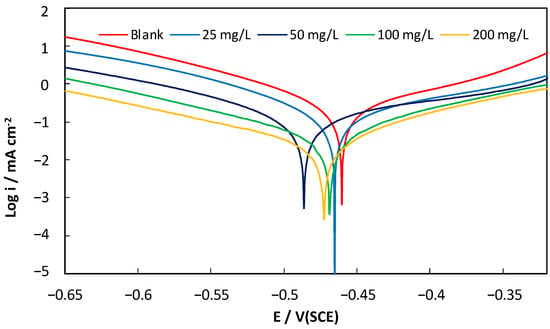
Figure 1.
Polarization curves for C38 steel in 1M HCl containing different concentrations of AECC.
The polarization curves for the 1M HCl solutions containing the inhibitor, in all cases, exhibit similar shapes to those of the uninhibited HCl solutions. The addition of AECC decreases the corrosion rate, but it does not alter other aspects of the corrosion behavior, suggesting that the electrochemical reactions responsible for corrosion are not modified by the inhibitor. Furthermore, the absence of significant changes in the cathodic and anodic Tafel slopes in the presence of AECC indicates that both the hydrogen evolution and anodic metal dissolution reactions are slowed down by the surface blocking effect of the inhibitor. This suggests that the inhibitive action of AECC may be attributed to the adsorption of extract molecules on the electrode surface.
The electrochemical corrosion kinetic parameters obtained from the linear I-E plots within the potential range of ±25 mV from the corrosion potential are given in Table 1. The inhibitory efficiencies (EI (%)) were obtained with Equation (3).
where Rp and Rpinh correspond, respectively, to the polarization resistance without and with the inhibitor.

Table 1.
Polarization parameters and the corresponding inhibition efficiency for the corrosion of C38 steel in 1M HCl containing different concentrations of AECC at 25 °C.
The polarization results presented in Table 1 indicate that there is no significant shift in the corrosion potential (Ecorr) with the addition of the AECC inhibitor, and there is no clear correlation between Ecorr and the inhibitor concentration. However, the polarization resistance (Rp) values increase with increasing AECC concentration, suggesting that the inhibitor is effective in reducing the corrosion rate. The inhibition efficiency (IE%) also increases with increasing inhibitor concentration, reaching a maximum value of 89% at 200 mg/L, indicating that the inhibitor is highly effective at this concentration. These results suggest that AECC acts as a mixed-type inhibitor, affecting both the anodic and cathodic reactions, and its inhibitive action may be related to the adsorption of extract molecules on the electrode surface.
3.2. Electrochemical Impedance Spectroscopy
Electrochemical impedance spectroscopy is a widely used and effective technique for studying corrosion processes. In this study, EIS was used to investigate the effect of concentration on surface coverage. Figure 2 shows the Nyquist plot obtained at the open-circuit potential after 3 h of immersion. The shape of the Nyquist plots indicates that the curves consist of a large capacitive loop with one capacitive time constant in Bode-phase plots, as shown in Figure 3. The capacitive loops suggest that the corrosion process is primarily controlled by charge transfer. The general shape of the curves is similar for all samples, indicating that the corrosion mechanism remains unchanged with the addition of the inhibitor. The diameter of the Nyquist plots increases with increasing alkaloid extract concentration, indicating that the inhibition efficiency is proportional to the inhibitor concentration. These results suggest that the AECC exhibits inhibition behavior towards the corrosion of C38 steel in a 1M HCl solution. The addition of AECC resulted in an increase in the impedance modulus at 0.01 Hz (|Z|0.01 Hz) from 2.11 Ω cm2 in the uninhibited solution to 2.23, 2.41, 2.55, and 2.67 Ω cm2 with increasing concentrations of 25, 50, 100, and 200 mg/L, respectively. This improvement in corrosion resistance was accompanied by an increase in the maximum phase angles (αmax) from −49.5° to −52.8°, −56.6°, −60.5°, and −63.9°, indicating enhanced shielding properties.
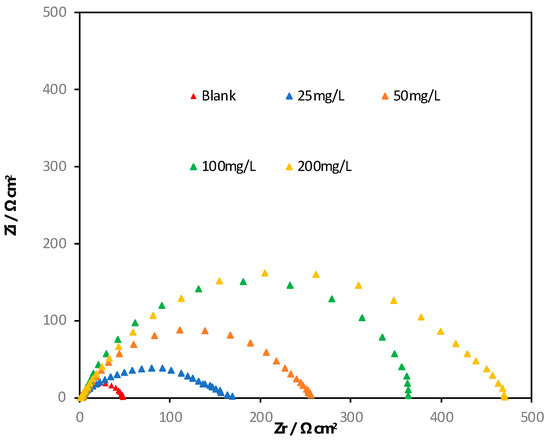
Figure 2.
Nyquist plots for C38 steel in 1M HCl in the absence and presence of different concentrations of AECC.
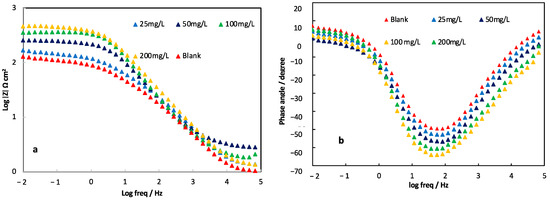
Figure 3.
Bode plots, Log |Z| vs. freq (a) and phase angle vs. freq (b), for C38 steel in 1M HCl in the absence and presence of different concentrations of AECC.
The Nyquist plots obtained for the inhibitor were analyzed using an equivalent circuit that includes a constant phase element (CPE) to improve the accuracy of the fit, as shown in Figure 4. Figure 4 also demonstrates a good fit of the experimental data obtained in this case. In fact, an excellent parametric fit of the various experimental impedance spectra was achieved using this model. Moreover, the experimental and simulated spectra are well correlated, with a χ2 coefficient on the order of 10−3, thereby validating the model.
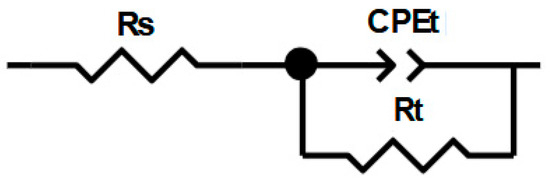
Figure 4.
The equivalent circuit used to fit the impedance data recorded for a C38 electrode in 1M HCl in the presence of different concentrations of AECC; adapted from [12].
The CPE was used as a substitute for the capacitive element to account for the frequency dispersion observed in the plots, which is characteristic of solid electrodes and attributed to surface heterogeneity [14,15,16].
The impedance of the CPE is defined by two parameters, Q and n, and is described by the equation [17,18]:
where Q is the CPE constant, ω is the angular frequency (in rad.s−1), i, such as i2 = −1, is an imaginary number, and n is a CPE exponent that can be used as a gauge of the heterogeneity in or roughness of the surface. The corresponding impedance data are given in Table 2. The Rt values were used to calculate the inhibition efficiency (IE) according to the following equation:
where Rt0 and Rt are, respectively, the charge transfer resistance in the absence and the presence of alkaloid extracts. The results obtained are shown in Table 2.

Table 2.
The values of the elements of the equivalent circuit required for fitting the EIS data for C38 steel in 1M HCl, both in the absence and presence of different concentrations of AECC, along with the corresponding inhibition efficiency.
The results of this study indicate that the alkaloid extract inhibits the corrosion of C38 steel through adsorption onto the metal surface. The impedance spectra reveal a single capacitive loop, suggesting that the extract adsorbs via straightforward surface coverage, and it functions as a primary interface inhibitor [19]. The proportional factor n of the constant phase element (CPE) varies regularly with the inhibitor concentration, and the changes in the values of the charge transfer resistance and n can be attributed to the gradual substitute of water molecules by the extract on the surface, leading to a decrease in the number of active sites for corrosion [19]. The increase in the coefficient n with increasing extract concentration indicates a decrease in the heterogeneity in the steel surface due to adsorption of the extract. The inhibition efficiencies derived from the EIS measurements align with those obtained from polarization measurements, further confirming the effectiveness of this plant extract as a corrosion inhibitor.
3.3. Surface Analysis Results
The presence of the alkaloid extract on the steel surface was investigated using X-ray photoelectron spectroscopy (XPS), with a focus on the characteristic N1s signals, as alkaloids are nitrogen-containing organic compounds. The XPS spectra were obtained for the steel surface exposed to AECC in the 1M HCl solutions to assess the presence and chemical state of nitrogen on the surface, as shown in Figure 5. The results revealed key information regarding the interaction of the alkaloids with the steel surface, particularly in relation to the binding state of nitrogen, which is crucial for understanding the corrosion inhibition mechanism of the extract. All XPS spectra exhibited complex features that were resolved into individual components using a deconvolution fitting procedure. This procedure employed a non-linear least squares algorithm with a Shirley baseline and a Gaussian–Lorentzian combination to assign the complex forms to their corresponding species. XPS Peak-Fit 4.1 software was utilized for this analysis.
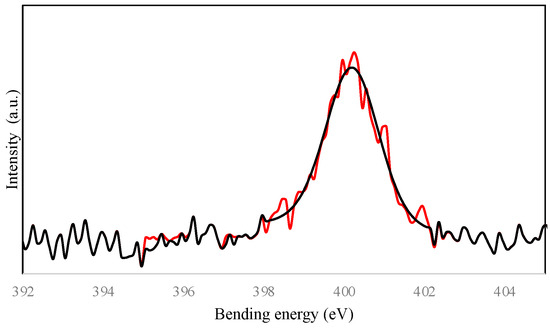
Figure 5.
N1s spectra for the steel surface exposed to AECC in 1M HCl solutions; (red) Experimental results and (black) fitted results.
The N1s spectrum of the steel surface exposed to the alkaloid extract in the 1M HCl solutions exhibited a single peak at approximately 400.7 eV, which can be assigned to adsorbed organic nitrogen groups [20]. This peak was shifted to a slightly higher binding energy compared to the pure extract, indicating the formation of a complex between the nitrogen atoms of the extract and the vacant d orbitals of iron on the steel surface through donor–acceptor interactions, resulting in the formation of an organometallic complex such as Fe–plant extract. This shift in the N1s spectrum provides clear evidence for the direct interaction between the alkaloid extract and the steel surface.
The high-resolution XPS spectrum of Fe 2p3/2 was deconvoluted into five distinct peaks, as shown in Figure 6. The peak located at the lowest binding energy of 706.8 eV can be attributed to the presence of metallic iron (Fe0), which is consistent with previous studies [21,22]. The peaks at higher binding energies of 711.0 eV and 715.8 eV are assigned to Fe3+ and can be attributed to the presence of iron oxides and/or hydroxides, such as FeO, Fe2O3, and/or FeOOH [23,24]. The peak at 713.6 eV is indicative of the presence of FeCl3 on the metal surface, which has been reported in previous studies [25,26]. The peak located at approximately 719.7 eV can be ascribed to the satellites of ferric compounds (Fe3+) [24]. Finally, the peak at 725 eV corresponds to Fe 2p1/2 and is accompanied by an associated ghost structure, as previously reported [27]. These results provide insight into the chemical composition and oxidation states of iron on the metal surface.
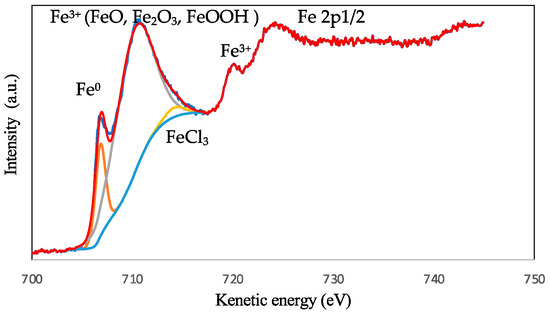
Figure 6.
The XPS deconvoluted profile for the steel surface exposed to AECC in 1M HCl solutions; (red) Experimental results and (other color) fitted results.
3.4. Adsorption Isotherms
Adsorption isotherms play a crucial role in comprehending the mechanism of organo-electrochemical reactions [28]. To assess the adsorption process of AECC on the surface of C38 steel, Langmuir, Temkin, and Frumkin adsorption isotherms were derived using the following equations:
where θ represents the surface coverage of the metal, indicating the fraction of the surface that is covered by the inhibitor molecules. K denotes the adsorption–desorption equilibrium constant, which governs the dynamic equilibrium between the inhibitor molecules adsorbed on the surface and those in the bulk solution. Cinh refers to the concentration of the inhibitor, which influences the extent of surface coverage. The term “a” represents the lateral interaction factor, which characterizes the molecular interactions within the adsorption layer and the surface’s heterogeneity, capturing the influence of these interactions on the adsorption process.
The fractional coverage values of θ, in relation to the inhibitor concentration, can be determined from polarization data as follows:
where and Rt represent the charge transfer resistance in the absence and the presence of AECC, respectively.
Table 3 presents the experimental data used in the adsorption isotherm analysis. These data are crucial for understanding the adsorption behavior and for constructing the corresponding isotherm curves. To determine the most appropriate adsorption isotherm for describing the surface coverage, corresponding plots were generated (Figure 7). These plots exhibit characteristic adsorption isotherms featuring an initial sharp rise followed by a subsequent gradual increase, indicative of the formation of an adsorbed layer on the steel surface. The Langmuir isotherm yielded the best fit by a significant margin (R2 = 0.999). The plots of Cinh/θ versus Cinh produce a linear relationship, confirming that the inhibitor (plant extract) adheres to the Langmuir adsorption isotherm in the 1M HCl medium. This suggests that the adsorbing species from Chimarrhis cymosa occupy typical adsorption sites at the metal/solution interface. However, it is noteworthy that the Langmuir isotherm assumes non-interacting adsorbed molecules, which may not hold true for organic molecules containing polar atoms or groups from heterocyclic compounds. Moreover, the alkaloid extract comprises various heterocyclic compounds alongside the principal active constituent, making it challenging to discuss the adsorption isotherm behavior in terms of the standard free energy of adsorption due to the unknown molecular mass of the extract components.

Table 3.
Experimental data for the adsorption isotherm analysis.
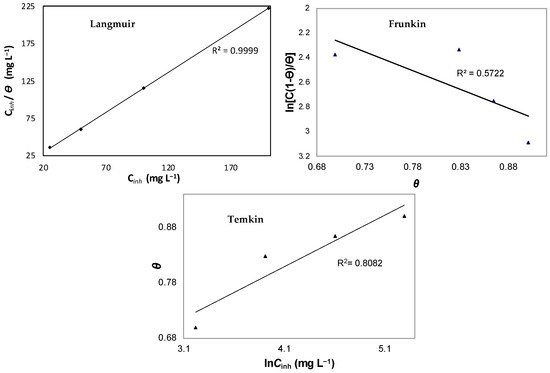
Figure 7.
Langmuir, Frumkin, and Temkin adsorption plots for C38 steel in 1M HCl containing different concentrations of AECC.
3.5. Mechanism of Inhibition
Based on the polarization and impedance measurements, it was observed that the alkaloid extract obtained from Chimarrhis cymosa effectively hampers the corrosion process of C38 steel in a 1M HCl solution. These findings suggest that the inhibition mechanism operates by obstructing the C38 steel surface through the adsorption of inhibitor molecules. This adsorption phenomenon is notably influenced by both the inherent characteristics of the metal and the specific chemical structure of the inhibitor.
Two primary modes of adsorption are typically considered: physical adsorption and chemisorption. Physical adsorption requires an electrically charged metal surface and charged species in the solution, while chemisorption involves a transfer of charge between inhibitors and the metal surface, which can occur regardless of whether the metal surface carries positive or negative charges. The presence of a transition metal with vacant orbitals, coupled with an inhibitor containing “π” electrons from heteroatoms with non-bonding electrons, facilitates this adsorption process.
The inhibition of metal corrosion by organic compounds is a complex process that involves adsorption. This adsorption, which can be physical or chemical, depends on several factors, including the metal’s charge and properties, the chemical structure of the organic compound, and the type of electrolyte present. In general, chemical adsorption involves the transfer or sharing of electrons between inhibitor molecules and the “d” vacant orbitals of the metal surface, leading to the formation of coordination bonds. This electron transfer can occur with organic molecules that have non-bonding electrons, as well as with molecules containing multiple bonds or aromatic nuclei with π electrons.
Furthermore, a photochemical analysis of the Chimarrhis cymosa plant extract led to the isolation and identification of cymoside, a monoterpene indole alkaloid, as shown in Figure 8 [29]. In an acidic environment, cymoside can exist in the form of pyrrolidinium cations, with aromatic amines displaying reduced basicity, typically with a pKa value of 5–6. Two protonated forms can be distinguished, with the protonated form 2 being favored due to the conjugation of the nitrogen doublet in case 1 with the π electron system of the aromatic cycle, making it less available for protonation, as shown in Figure 9.
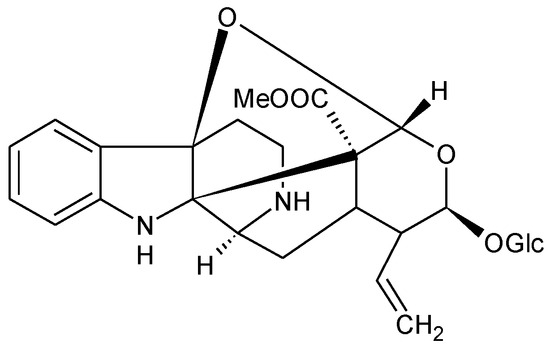
Figure 8.
Chemical formula of cymoside. Reprinted with permission from ref. [29]. 2015, Elsevier.
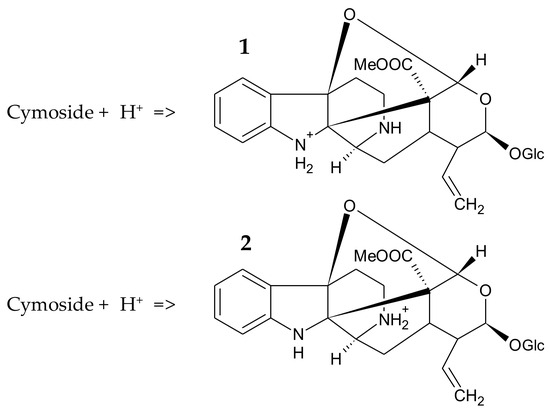
Figure 9.
Protonated forms of cymoside in an acid solution; Form 1 corresponds to protonation of the nitrogen in the five-membered heterocycle and form 2 corresponds to protonation of the nitrogen in the six-membered heterocycle.
The adsorption of cymoside can also occur directly through “donor–acceptor” bonds between the π electrons (heterocyclic compounds and heteroatoms) and the vacant “d” orbitals of iron atoms.
In a hydrochloric acid solution, the interaction between the metal surface and cymoside can occur in two ways:
- These molecules exist in cationic form and can interact with the negatively charged metal surface following the adsorption of chloride ions “Cl−” on the metal surface.
- The adsorption of these molecules can also occur directly through “donor–acceptor” bonds between the π electrons (heterocyclic compounds and heteroatoms) and the vacant “d” orbitals of iron atoms.
- Cymoside is one of several alkaloids present in the extract, and it is not the only active compound. The discussion of the inhibition mechanism focused on cymoside because it is currently the only alkaloid that has been isolated and identified. However, it is important to note that at this pH, any alkaloid present in the extract would be protonated, which could contribute to the observed findings.
4. Conclusions
The corrosion inhibition efficiency of AECC was investigated in 1M HCl using polarization and EIS measurements. The results showed that AECC acted as a mixed-type inhibitor, with inhibition efficiency increasing with inhibitor concentration, reaching 89% efficiency at 200 mg L−1. The EIS measurements data exhibited frequency dispersion behavior, and a constant phase element (CPE) was used for modeling. The inhibition efficiencies calculated from EIS measurements were consistent with those obtained from polarization measurements. The adsorption of AECC on C38 steel in a 1M HCl solution followed the Langmuir adsorption isotherm with a high correlation coefficient. The XPS results provided direct evidence of AECC adsorption onto the steel surface, supporting the model of blocking active sites and preventing corrosion.
Author Contributions
Conceptualization, M.L.; methodology, M.S.-A. and M.L.; software, M.S.-A.; validation, M.L.; formal analysis, M.S.-A. and M.L.; investigation, M.S.-A. and M.L.; resources, M.L.; data curation, M.S.-A.; writing—original draft preparation, M.S.-A. and M.L.; writing—review and editing, M.S.-A. and M.L. visualization, M.S.-A. and M.L.; supervision, M.L.; project administration, M.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data are openly available in a public repository.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Sesia, R.; Spriano, S.; Sangermano, M.; Ferraris, S. Natural Polyphenols and the Corrosion Protection of Steel: Recent Advances and Future Perspectives for Green and Promising Strategies. Metals 2023, 13, 1070. [Google Scholar] [CrossRef]
- Sutthiruangwong, S.; Wongpaiboon, C.; Sritha, N.; Anukulkich, N. Pitting Potential Improvement of 304 Stainless Steel in Hydrochloric Acid Solution by Terminalia bellirica Fruit Extract. Metals 2023, 13, 262. [Google Scholar] [CrossRef]
- Kwolek, P.; Dychton, K.; Kościelniak, B.; Obłój, A.; Podborska, A.; Wojnicki, M. Gallic Acid as a Potential Green Corrosion Inhibitor for Aluminum in Acidic Solution. Metals 2022, 12, 250. [Google Scholar] [CrossRef]
- Cáceres, L.; Frez, Y.; Galleguillos, F.; Soliz, A.; Gómez-Silva, B.; Borquez, J. Aqueous Dried Extract of Skytanthus acutus Meyen as Corrosion Inhibitor of Carbon Steel in Neutral Chloride Solutions. Metals 2021, 11, 1992. [Google Scholar] [CrossRef]
- Boutoumit, A.; Elhawary, M.; Bellaouchou, A.; Boudalia, M.; Hammani, O.; José Garcia, A.; Amin, H.M.A. Electrochemical, Structural and Thermodynamic Investigations of Methanolic Parsley Extract as a Green Corrosion Inhibitor for C37 Steel in HCl. Coatings 2024, 14, 783. [Google Scholar] [CrossRef]
- Dahmani, M.; Et-Touhami, A.; Al-Deyab, S.S.; Hammouti, B.; Bouyanzer, A. Corrosion Inhibition of C38 Steel in 1 M HCl: A Comparative Study of Black Pepper Extract and Its Isolated Piperine. Int. J. Electrochem. Sci. 2010, 5, 1060–1069. [Google Scholar] [CrossRef]
- Thapa, O.; Magar, J.T.; Oli, H.B.; Rajaure, A.; Nepali, D.; Deval Prasad Bhattarai, D.B.; Mukh, T. Alkaloids of Solanum xanthocarpum stem as green inhibitor for mild steel corrosion in one molar sulphuric acid solution. Electrochem 2022, 3, 820–842. [Google Scholar] [CrossRef]
- Lebrini, M. Alkaloids from Plant Extracts as Corrosion Inhibitors for Metal Alloys. In Corrosion Engineering—Recent Breakthroughs and Innovative Solutions; IntechOpen: London, UK, 2024. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, P.; Liang, Q.; Hou, B. Berberine as a natural source inhibitor for mild steel in 1 M H2SO4. Appl. Surf. Sci. 2005, 252, 1245. [Google Scholar] [CrossRef]
- Chapagain, A.; Acharya, D.; Kumari Das, A.; Chhetri, K.; Bhakta Oli, H.; Prasad Yadav, A. Alkaloid of Rhynchostylis retusa as Green Inhibitor for Mild Steel Corrosion in 1 M H2SO4 Solution. Electrochem 2022, 3, 211–224. [Google Scholar] [CrossRef]
- Ng, J.H.; Almubarak, T.; Nasr-El-Din, H. Natural alkaloid as a non-toxic, environmentally friendly corrosion inhibitor. Can. J. Chem. Eng. 2022, 100, 1214–1225. [Google Scholar] [CrossRef]
- Lebrini, M.; Robert, F.; Roos, C. Inhibition Effect of Alkaloids Extract from Annona Squamosa Plant on the Corrosion of C38 Steel in Normal Hydrochloric Acid Medium. Int. J. Electrochem. Sci. 2010, 5, 1698–1712. [Google Scholar] [CrossRef]
- Faustin, M.; Lebrini, M.; Robert, F.; Roos, C. Corrosion studies of C38 steel by alkaloids extract of a tropical plant type. Int. J. Electrochem. Sci. 2011, 9, 4095–4113. [Google Scholar] [CrossRef]
- Growcock, F.B.; Jasinski, R.J. Time-resolved impedance spectroscopy of mild steel in concentrated hydrochloric acid. J. Electrochem. Soc. 1989, 136, 2310–2314. [Google Scholar] [CrossRef]
- Li, P.; Lin, J.Y.; Tan, K.L.; Lee, J.Y. Electrochemical impedance and X-ray photoelectron spectroscopic studies of the inhibition of mild steel corrosion in acids by cyclohexylamine. Electrochim. Acta 1997, 42, 605–615. [Google Scholar]
- Lopez, D.A.; Simison, S.N.; de Sanchez, S.R. The influence of steel microstructure on CO2 corrosion. EIS studies on the inhibition efficiency of benzimidazole. Electrochim. Acta 2003, 48, 845–854. [Google Scholar] [CrossRef]
- Stoynov, Z. Impedance modelling and data processing: Structural and parametrical estimation. Electrochim. Acta 1990, 35, 1493–1499. [Google Scholar] [CrossRef]
- Macdonald, J.R. Impedance spectroscopy and its use in analyzing the steady-state AC response of solid and liquid electrolytes. J. Electroanal. Chem. 1987, 223, 25–50. [Google Scholar] [CrossRef]
- McCafferty, E.; Hackerman, N. Double Layer Capacitance of Iron and Corrosion Inhibition with Polymethylene Diamines. J. Electrochem. Soc. 1972, 119, 146–154. [Google Scholar] [CrossRef]
- Riedo, E.; Comin, F.; Chevrier, J.; Schmithusen, F.; Decossas, S.; Sancrotti, M. Role of the Carbon Coating, the Particle Size, and the Agglomeration on the Electronic Conductivity of LiFePO4-Based Electrodes for Lithium-Ion Batteries. Surf. Coat. Technol. 2000, 125, 124. [Google Scholar] [CrossRef]
- Bentiss, F.; Traisnel, M.; Gengembre, L.; Lagrenée, M. Inhibition of acidic corrosion of mild steel by 3,5-diphenyl-4H-1,2,4-triazole. Appl. Surf. Sci. 2000, 161, 194–202. [Google Scholar]
- Castro, V.D.; Ciampi, S. XPS study of the growth and reactivity of FeMnO thin films. Surf. Sci. 1995, 331–333, 294–299. [Google Scholar] [CrossRef]
- Pech-Canul, M.A.; Bartolo-Pérez, P. Inhibition effects of N-phosphono-methyl-glycine/Zn2+ mixtures on corrosion of steel in neutral chloride solutions. Surf. Coat. Technol. 2004, 184, 133–140. [Google Scholar] [CrossRef]
- Tandon, R.K.; Payling, R.; Chenhall, B.E.; Crisp, P.T.; Ellis, J.; Baker, R.S. Application of X-ray photoelectron spectroscopy to the analysis of stainless-steel welding aerosols. Appl. Surf. Sci. 1985, 20, 527–537. [Google Scholar] [CrossRef]
- Sastri, V.S.; Elboujdaini, M.; Brown, J.R.; Perumareddi, J.R. Surface Analysis of Inhibitor Films Formed in Hydrogen Sulfide Medium. Corrosion 1996, 52, 447–452. [Google Scholar] [CrossRef]
- Moulder, F.; Stickle, W.F.; Sobol, P.E.; Bomben, K.D. Handbook of X-Ray Photoelectron Spectroscopy; Perkin-Elmer Corporation: Waltham, MA, USA, 1992. [Google Scholar]
- Lebrini, M.; Lagrenée, M.; Traisnel, M.; Gengembre, L.; Vezin, H.; Bentiss, F. Enhanced corrosion resistance of mild steel in normal sulfuric acid medium by 2,5-bis(n-thienyl)-1,3,4-thiadiazoles: Electrochemical, X-ray photoelectron spectroscopy and theoretical studies. Appl. Surf. Sci. 2007, 253, 9267–9276. [Google Scholar] [CrossRef]
- Khaled, K.F.; Hackerman, N. Investigation of the inhibitive effect of ortho-substituted anilines on corrosion of iron in 1 M HCl solutions. Electrochim. Acta 2003, 48, 2715. [Google Scholar] [CrossRef]
- Lémus, C.; Kritsanida, M.; Canet, A.; Genta-Jouve, G.; Michel, S.; Deguin, B.; Grougnet, R. Cymoside, a monoterpene indole alkaloid with a hexacyclic fused skeleton from Chimarrhis cymosa. Tetrahedron Lett. 2015, 56, 5377–5380. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).