Transformation of TiN to TiNO Films via In-Situ Temperature-Dependent Oxygen Diffusion Process and Their Electrochemical Behavior
Abstract
1. Introduction
2. Materials and Methods
2.1. Thin Film Deposition
2.2. Characterization Techniques
2.3. Electrochemical Measurements
3. Results and Discussion
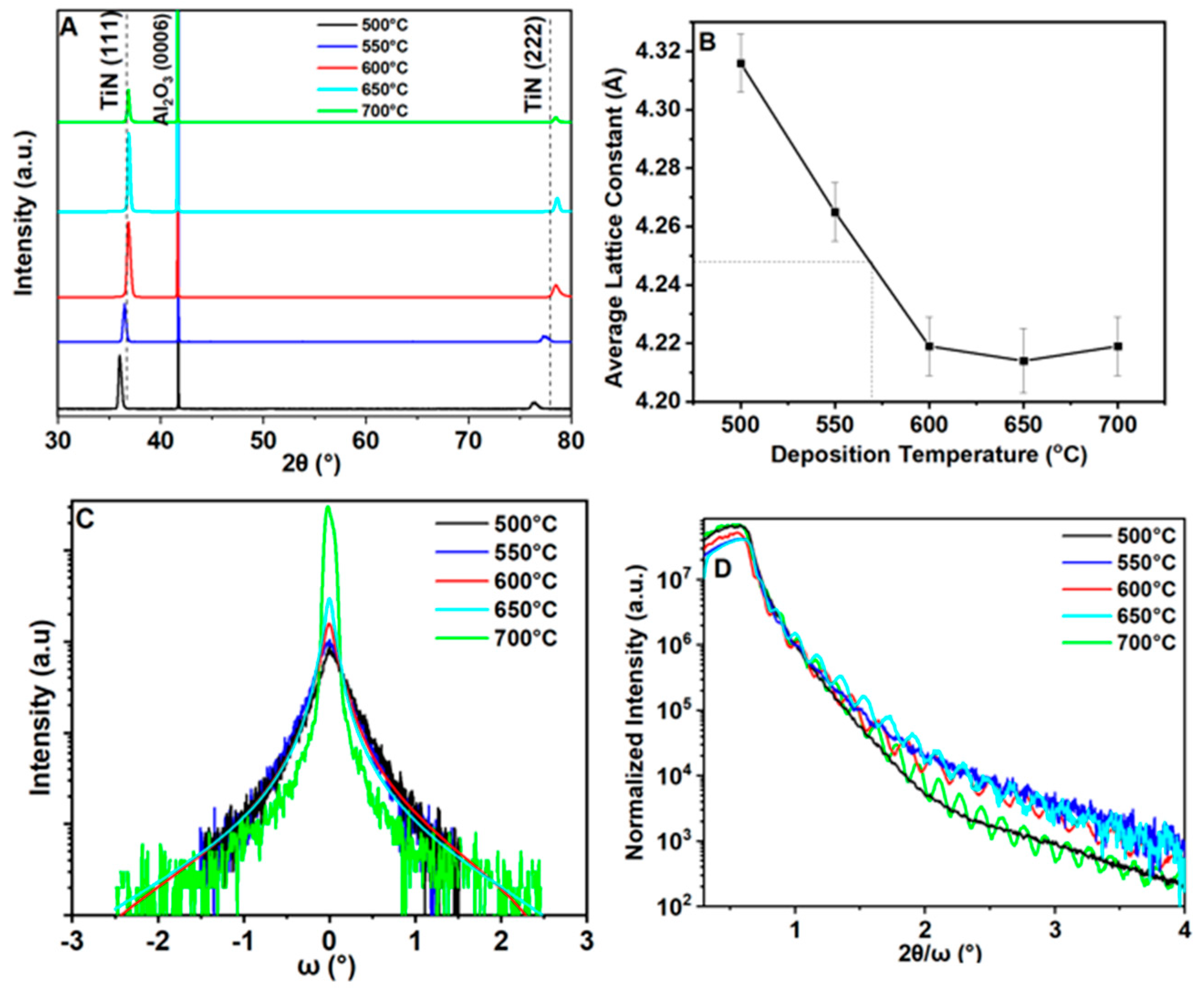
3.1. Texture and Phase Composition
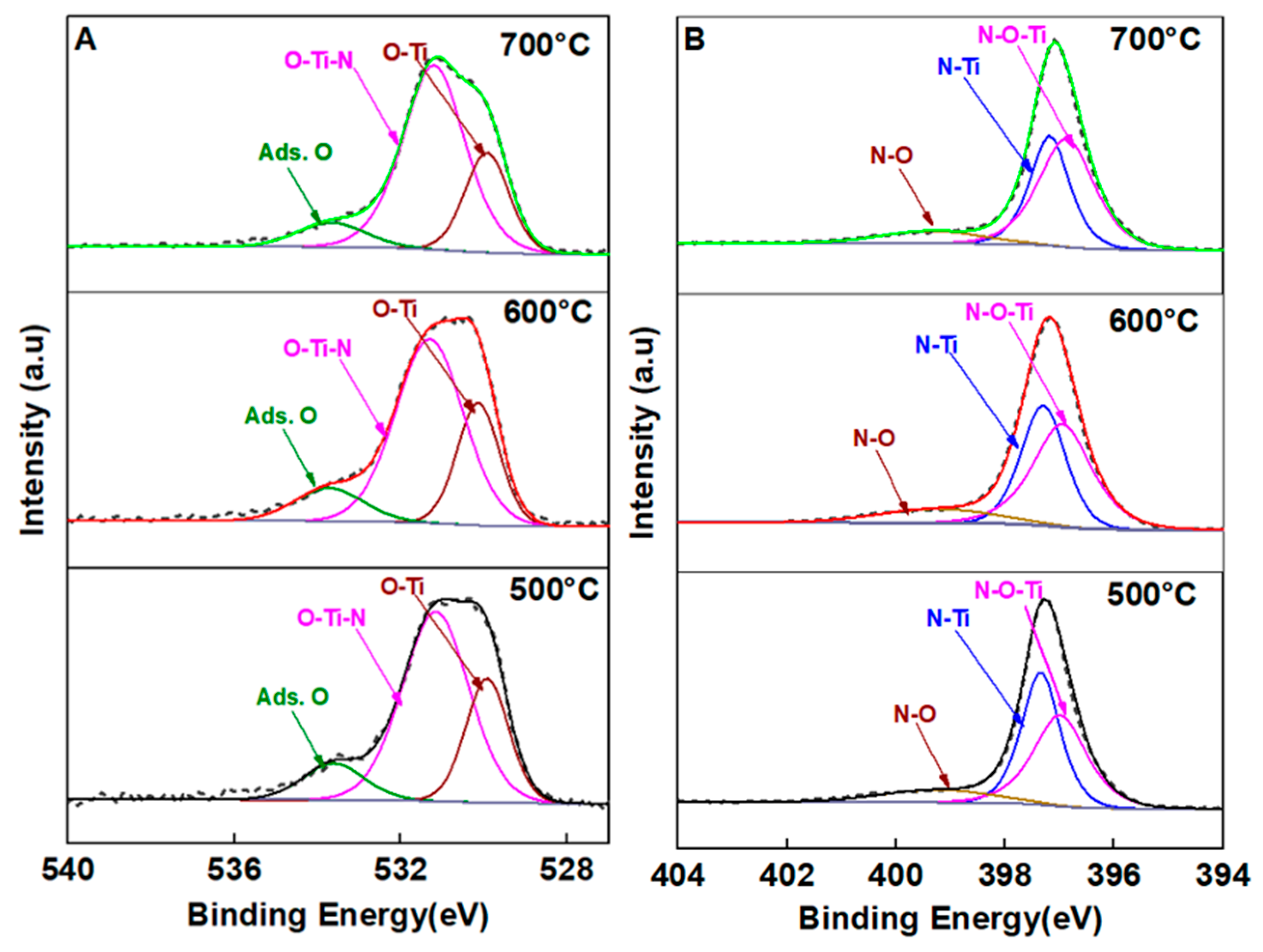
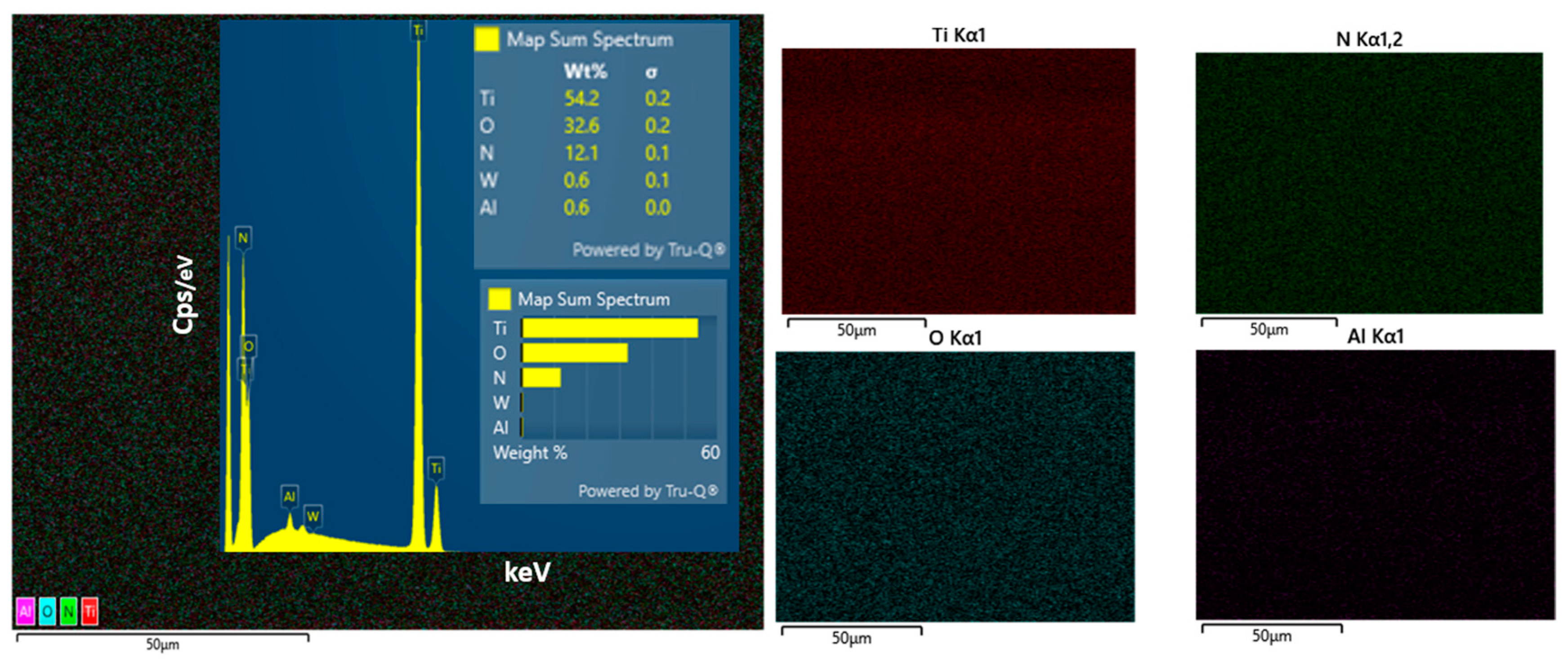
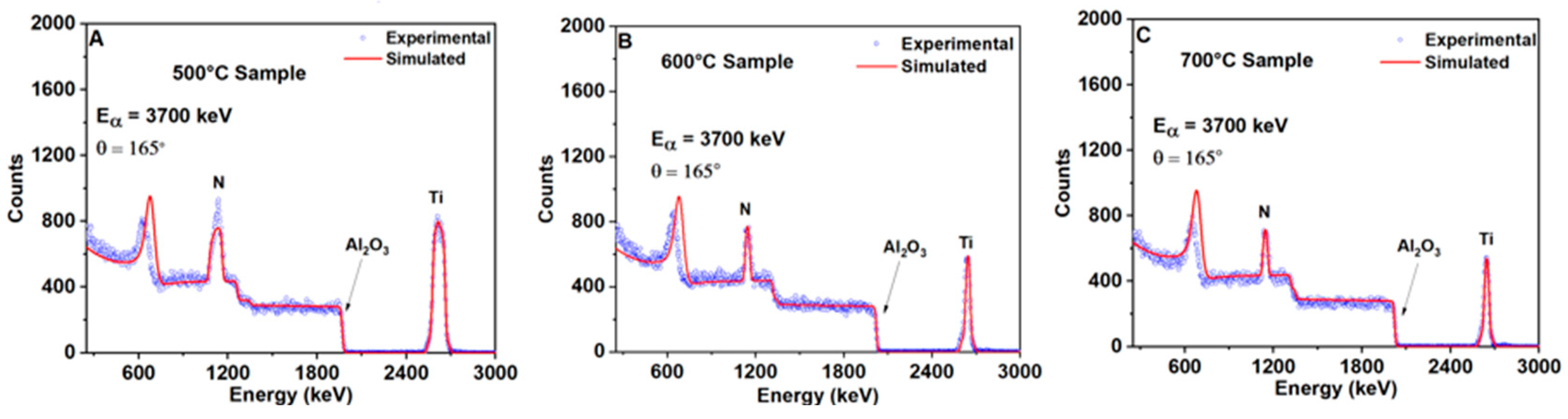
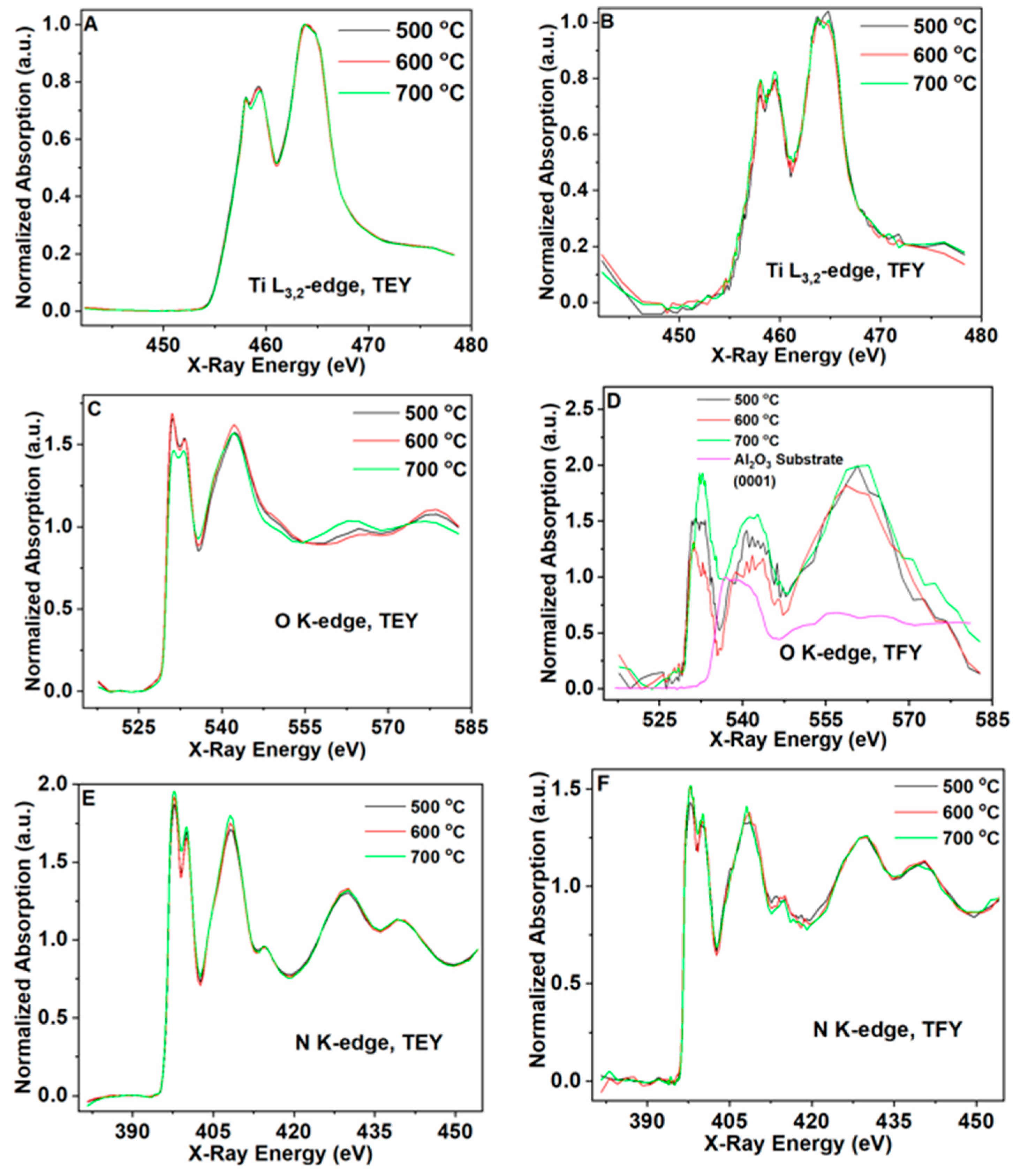
3.2. Chemical Composition
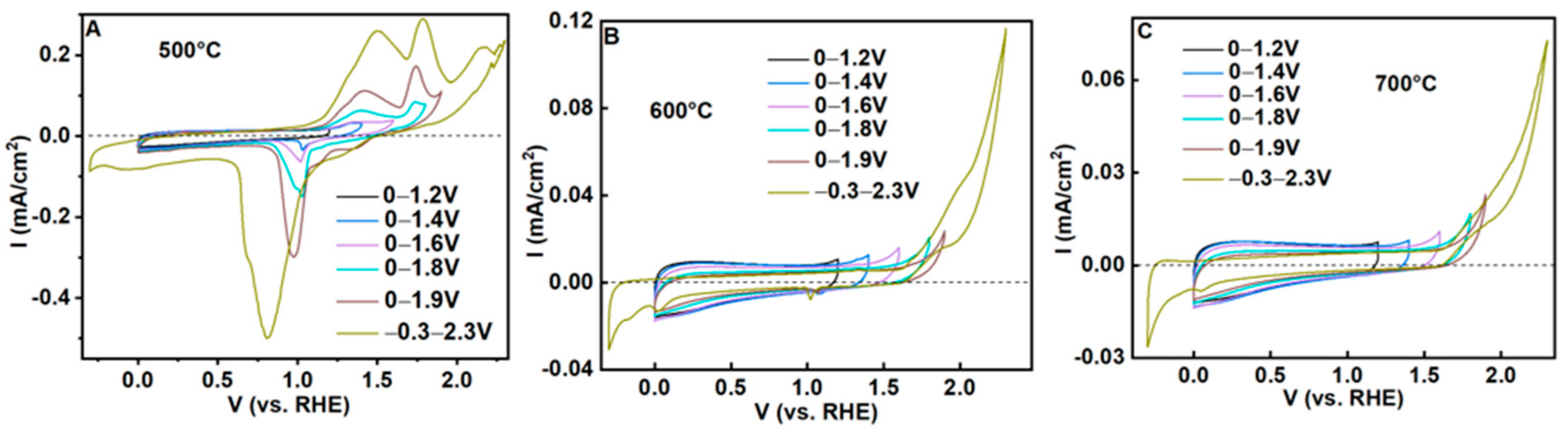
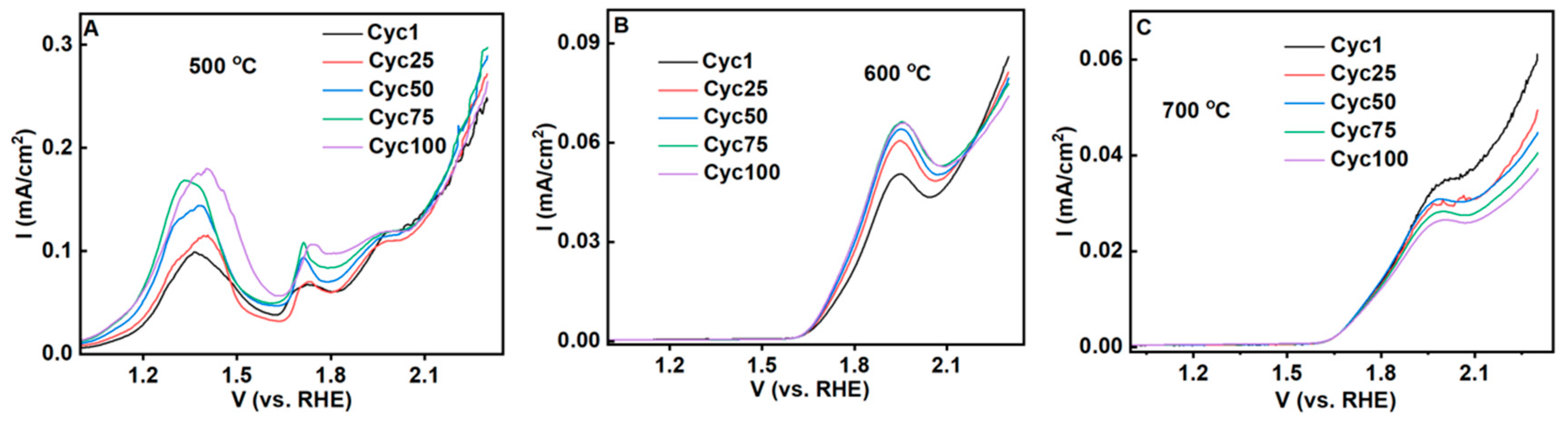
3.3. Electrochemical Properties
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| TiNO | Titanium oxynitride |
| PLD | Pulsed laser deposition |
| XRD | X-ray diffraction |
| XRR | X-ray reflectometry |
| XPS | X-ray photo electron spectroscopy |
| XAS | X-ray absorption spectroscopy |
| Ti | Titanium nitride |
| TiO2 | Titanium dioxide |
| CVD | Chemical vapor deposition |
| ALD | Atomic layer deposition |
| TEY | Total electron yield |
| TFY | Total fluorescent yield |
| CV | Cyclic voltammetry |
| LSV | Linear sweep voltammetry |
| EIS | Electrochemical impedance spectroscopy |
| OER | Oxygen evolution reaction |
| ORC | Omega rocking curve |
| OCP | Open circuit potential |
References
- Gebauer, C.; Fischer, P.; Wassner, M.; Diemant, T.; Jusys, Z.; Hüsing, N.; Behm, R.J. Performance of titanium oxynitrides in the electrocatalytic oxygen evolution reaction. Nano Energy 2016, 29, 136–148. [Google Scholar] [CrossRef]
- Mucha, N.R.; Som, J.; Choi, J.; Shaji, S.; Gupta, R.K.; Meyer, H.M.; Cramer, C.L.; Elliott, A.M.; Kumar, D. High-Performance Titanium Oxynitride Thin Films for Electrocatalytic Water Oxidation. ACS Appl. Energy Mater. 2020, 3, 8366. [Google Scholar] [CrossRef]
- Mohamed, S.H.; Zhao, H.; Romanus, H.; El-Hossary, F.M.; El-Kassem, M.A.; Awad, M.A.; Rabia, M.; Lei, Y. Optical, water splitting and wettability of titanium nitride/titanium oxynitride bilayer films for hydrogen generation and solar cells applications. Mater. Sci. Semicond. Process. 2020, 105, 104704. [Google Scholar] [CrossRef]
- Janders, M.; Egert, U.; Stelzle, M.; Nisch, W. Novel thin film titanium nitride micro-electrodes with excellent charge transfer capability for cell stimulation and sensing applications. In Proceedings of the 18th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Amsterdam, The Netherlands, 31 October–3 November 1996. [Google Scholar] [CrossRef]
- Chen, T.-T.; Liu, H.-P.; Wei, Y.-J.; Chang, I.-C.; Yang, M.-H.; Lin, Y.-S.; Chan, K.-L.; Chiu, H.-T.; Lee, C. Porous titanium oxynitride sheets as electrochemical electrodes for energy storage. Nanoscale 2014, 6, 5106–5112. [Google Scholar] [CrossRef] [PubMed]
- Chris-Okoro, I.; Som, J.; Cherono, S.; Liu, M.; Nalawade, S.S.; Lu, X.; Wise, F.; Aravamudhan, S.; Kumar, D. Effect of Substrate Temperature on the Electrochemical and Supercapacitance Properties of Pulsed Laser-Deposited Titanium Oxynitride Thin Films. J. Electrochem. Energy Convers. Storage 2025, 22, 011010. [Google Scholar] [CrossRef]
- Chatenet, M.; Pollet, B.G.; Dekel, D.R.; Dionigi, F.; Deseure, J.; Millet, P.; Braatz, R.D.; Bazant, M.Z.; Eikerling, M.; Staffell, I.; et al. Water electrolysis: From textbook knowledge to the latest scientific strategies and industrial developments. Chem. Soc. Rev. 2022, 51, 4583–4762. [Google Scholar]
- Wang, S.; Lu, A.; Zhong, C. Hydrogen production from water electrolysis: Role of catalysts. Nano Converg. 2021, 8, 4. [Google Scholar] [CrossRef]
- Guo, H.; Chen, W.; Shan, Y.; Wang, W.; Zhang, Z.; Jia, J. Microstructures and properties of titanium nitride films prepared by pulsed laser deposition at different substrate temperatures. Appl. Surf. Sci. 2015, 357, 473. [Google Scholar] [CrossRef]
- Serro, A.P.; Completo, C.; Colaço, R.; Dos Santos, F.; Da Silva, C.L.; Cabral, J.M.S.; Araújo, H.; Pires, E.; Saramago, B.A. Comparative study of titanium nitrides, TiN, TiNbN and TiCN, as coatings for biomedical applications. Surf. Coat. Technol. 2009, 203, 3701–3707. [Google Scholar] [CrossRef]
- Faruque, M.K.; Kwadwo, M.; Xu, Z.; Kumar, D. Fabrication, characterization, and mechanism of vertically aligned titanium nitride nanowires. Appl. Surf. Sci. 2012, 260, 36–41. [Google Scholar] [CrossRef]
- Mucha, N.R.; Som, J.; Shaji, S.; Fialkova, S.; Apte, P.R.; Balasubramanian, B.; Shield, J.E.; Anderson, M.; Kumar, D. Electrical and optical properties of titanium oxynitride thin films. J. Mater. Sci. 2020, 55, 5123–5134. [Google Scholar] [CrossRef]
- LeClair, P.; Berera, G.P.; Moodera, J.S. Titanium nitride thin films obtained by a modified physical vapor deposition process. Thin Solid Films 2000, 376, 9–15. [Google Scholar] [CrossRef]
- Ali, S.; Magnusson, R.; Pshyk, O.; Birch, J.; Eklund, P.; Le Febvrier, A. Effect of O/N content on the phase, morphology, and optical properties of titanium oxynitride thin films. J. Mater. Sci. 2023, 58, 10975. [Google Scholar] [CrossRef]
- El-Hossary, F.M.; Negm, N.Z.; Abd El-Rahman, A.M.; Raaif, M.; Abd Elmula, A.A. Properties of titanium oxynitride prepared by RF plasma. Adv. Chem. Eng. Sci. 2014, 5, 1. [Google Scholar] [CrossRef]
- Acosta, E. Thin Films/Properties and Applications. In Thin Films; Intech Open: London, UK, 2021. [Google Scholar] [CrossRef]
- Geremew, T. Thin Film Deposition and Characterization Techniques. J3DPA 2022, 1, 1. [Google Scholar] [CrossRef]
- Chrisey, D.B.; Hubler, G.K. Pulsed Laser Deposition of Thin Films; John Wiley & Sons: Hoboken, NJ, USA, 1994. [Google Scholar]
- Roy, M.; Sarkar, K.; Som, J.; Pfeifer, M.A.; Craciun, V.; Schall, J.D.; Aravamudhan, S.; Wise, F.W.; Kumar, D. Modulation of Structural, Electronic, and Optical Properties of Titanium Nitride Thin Films by Regulated In Situ Oxidation. ACS Appl. Mater. Interfaces 2023, 15, 4733–4742. [Google Scholar] [CrossRef]
- Shepelin, N.A.; Tehrani, Z.P.; Ohannessian, N.; Schneider, C.W.; Pergolesi, D.; Lippert, T. A practical guide to pulsed laser deposition. Chem. Soc. Rev. 2023, 52, 2294. [Google Scholar] [CrossRef]
- Zhang, D.; Guan, L.; Li, Z.; Pan, G.; Tan, X.; Li, L. Simulation of island aggregation influenced by substrate temperature, incidence kinetic energy and intensity in pulsed laser deposition. Appl. Surf. Sci. 2006, 253, 874–880. [Google Scholar] [CrossRef]
- Bele, M.; Jovanovič, P.; Marinko, Z.; Drev, S.; Šelih, V.S.; Kovač, J.; Gaberšček, M.; Koderman Podboršek, G.; Draẑič, G.; Hodnik, N.; et al. Increasing the oxygen-evolution reaction performance of nanotubular titanium oxynitride-supported Ir nanoparticles by a strong metal–support interaction. ACS Catal. 2020, 10, 13688–13700. [Google Scholar] [CrossRef]
- Tian, X.; Luo, J.; Nan, H.; Zou, H.; Chen, R.; Shu, T.; Li, X.; Li, Y.; Song, H.; Liao, S.; et al. Transition Metal Nitride Coated with Atomic Layers of Pt as a Low-Cost, Highly Stable Electrocatalyst for the Oxygen Reduction Reaction. J. Am. Chem. Soc. 2016, 138, 1575. [Google Scholar] [CrossRef]
- Harrington, G.F.; Santiso, J. Back-to-Basics tutorial: X-ray diffraction of thin films. J. Electroceram. 2021, 47, 141. [Google Scholar] [CrossRef]
- Callister, W.D.; Rethwisch, D.G. Fundamentals of Materials Science and Engineering, 10th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2020. [Google Scholar]
- Rasic, D.; Sachan, R.; Chisholm, M.F.; Prater, J.; Narayan, J. Room Temperature Growth of Epitaxial Titanium Nitride Films by Pulsed Laser Deposition. Cryst. Growth Des. 2017, 17, 6634. [Google Scholar] [CrossRef]
- Rumble, J. CRC Handbook of Chemistry and Physics, 102nd ed.; CRC Press: Boca Raton, FL, USA, 2017; Chapter: Ionic Conductivity and Diffusion at Infinite Dilution. [Google Scholar]
- Goswami, A. Thin Film Fundamentals; New Age International: New Delhi, India, 1996. [Google Scholar]
- Sambles, J.R. The resistivity of thin metal films—Some critical remarks. Thin Solid Films 1983, 106, 321–331. [Google Scholar] [CrossRef]
- Saha, N.C.; Tompkins, H.G. Titanium nitride oxidation chemistry: An X-ray photoelectron spectroscopy study. J. Appl. Phys. 1992, 72, 3072–3079. [Google Scholar] [CrossRef]
- Wagner, C.D.; Naumkin, A.V.; Kraut-Vass, A.; Allison, J.W.; Powell, C.J.; Rumble, J.R., Jr. NIST Standard Reference Database 20; Version 3.4 (Web Version); National Institute of Standards and Technology: Gaithersburg, MD, USA, 2003; Volume 20899. [Google Scholar]
- Greczynski, G.; Hultman, L. A step-by-step guide to perform x-ray photoelectron spectroscopy. J. Appl. Phys. 2022, 132, 011101. [Google Scholar] [CrossRef]
- Stevie, F.A.; Donley, C.L. Introduction to X-ray photoelectron spectroscopy. J. Vac. Sci. Technol. A 2020, 38, 063204. [Google Scholar] [CrossRef]
- Jaeger, D.; Patscheider, J. A complete and self-consistent evaluation of XPS spectra of TiN. J. Electron Spectrosc. Relat. Phenom. 2012, 185, 523–534. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Lau, L.W.; Gerson, A.R.; Smart, R.S.C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Sc, Ti, V, Cu and Zn. Appl. Surf. Sci. 2010, 257, 887–898. [Google Scholar] [CrossRef]
- Chan, M.; Lu, F. X-ray photoelectron spectroscopy analyses of titanium oxynitride films prepared by magnetron sputtering using air/Ar mixtures. Thin Solid Films 2009, 517, 5006–5009. [Google Scholar] [CrossRef]
- Odusanya, A.A.; Schall, J.D.; Pfeifer, M.A.; Wright, J.; Martin, C.; Craciun, V.; Kumar, D. Optical properties of unoxidized and oxidized titanium nitride thin films. J. Mater. Sci. Mater. Eng. 2025, 20, 2. [Google Scholar] [CrossRef]
- Chris-Okoro, I.; Cherono, S.; Akande, W.; Nalawade, S.; Liu, M.; Martin, C.; Craciun, V.; Kim, R.S.; Mahl, J.; Cuk, T.; et al. Optical and Plasmonic Properties of High-Electron-Density Epitaxial and Oxidative Controlled Titanium Nitride Thin Films. The J. Phys. Chem. C 2025, 129, 3762–3774. [Google Scholar] [CrossRef]
- Zukalova, M.; Prochazka, J.; Bastl, Z.; Duchoslav, J.; Rubacek, L.; Havlicek, D.; Kavan, L. Facile conversion of electrospun TiO2 into titanium nitride/oxynitride fibers. Chem. Mater. 2010, 22, 4045–4055. [Google Scholar] [CrossRef]
- Radecka, M.; Pamula, E.; Trenczek-Zajac, A.; Zakrzewska, K.; Brudnik, A.; Kusior, E.; Kim-Ngan, N.T.; Balogh, A.G. Chemical composition, crystallographic structure and impedance spectroscopy of titanium oxynitride TiNxOy thin films. Solid State Ion. 2011, 192, 693–698. [Google Scholar] [CrossRef]
- Chen, H.; Lu, F. Oxidation behavior of titanium nitride films. J. Vac. Sci. Technol. A 2005, 23, 1006–1009. [Google Scholar] [CrossRef]
- Moulder, J.F.; Stickle, W.F.; Sobol, P.E.; Bomben, K.D. Handbook of X-Ray Photoelectron Spectroscopy; Physical Electronics Inc.: Eden Prairie, MN, USA, 1995. [Google Scholar]
- McCrory, C.C.; Jung, S.; Peters, J.C.; Jaramillo, T.F. Benchmarking heterogeneous electrocatalysts for the oxygen evolution reaction. J. Am. Chem. Soc. 2013, 135, 16977–16987. [Google Scholar] [CrossRef]
- Avasarala, B.; Haldar, P. Electrochemical oxidation behavior of titanium nitride based electrocatalysts under PEM fuel cell conditions. Electrochim. Acta 2010, 55, 9024–9034. [Google Scholar] [CrossRef]
- Esaka, F.; Furuya, K.; Shimada, H.; Imamura, M.; Matsubayashi, N.; Sato, H.; Nishijima, A.; Kawana, A.; Ichimura, H.; Kikuchi, T. Comparison of surface oxidation of titanium nitride and chromium nitride films studied by x-ray absorption and photoelectron spectroscopy. J. Vac. Sci. Technol. A Vac. Surf. Film. 1997, 15, 2521–2528. [Google Scholar] [CrossRef]
- Logothetidis, S.; Meletis, E.I.; Stergioudis, G.; Adjaottor, A.A. Room temperature oxidation behavior of TiN thin films. Thin Solid Films 1999, 338, 304–313. [Google Scholar] [CrossRef]
- Zhang, Y.; Marschilok, A.C.; Takeuchi, K.J.; Kercher, A.K.; Takeuchi, E.S.; Dudney, N.J. Understanding how structure and crystallinity affect performance in solid-state batteries using a glass ceramic LiV3O8 cathode. Chem. Mater. 2019, 31, 6135–6144. [Google Scholar] [CrossRef]
- Wang, H.; Fan, W.; Yang, S.; Gong, G.; Chen, S.; Jiao, L.; You, F.; Qi, J. Deeply understanding electrocatalytic oxygen evolution reaction from the perspective of defect structures. Chem. Eng. J. 2024, 499, 156124. [Google Scholar] [CrossRef]
- Wang, L.; Peng, C.; Lin, H.; Zhao, B. Unraveling the role of defects in electrocatalysts for water splitting: Recent advances and perspectives. Energy Fuels 2022, 36, 11660–11690. [Google Scholar] [CrossRef]
- Lazanas, A.C.; Prodromidis, M.I. Electrochemical impedance spectroscopy—A tutorial. ACS Meas. Sci. Au 2023, 3, 162–193. [Google Scholar] [CrossRef] [PubMed]
- Bredar, A.R.; Chown, A.L.; Burton, A.R.; Farnum, B.H. Electrochemical Impedance Spectroscopy of Metal Oxide Electrodes for Energy Applications. ACS Appl. Energy Mater. 2020, 3, 66. [Google Scholar] [CrossRef]
- Balaguera, E.H.; Allagui, A. Limit capacitance of the constant phase element. J. Energy Storage 2024, 90, 111801. [Google Scholar] [CrossRef]
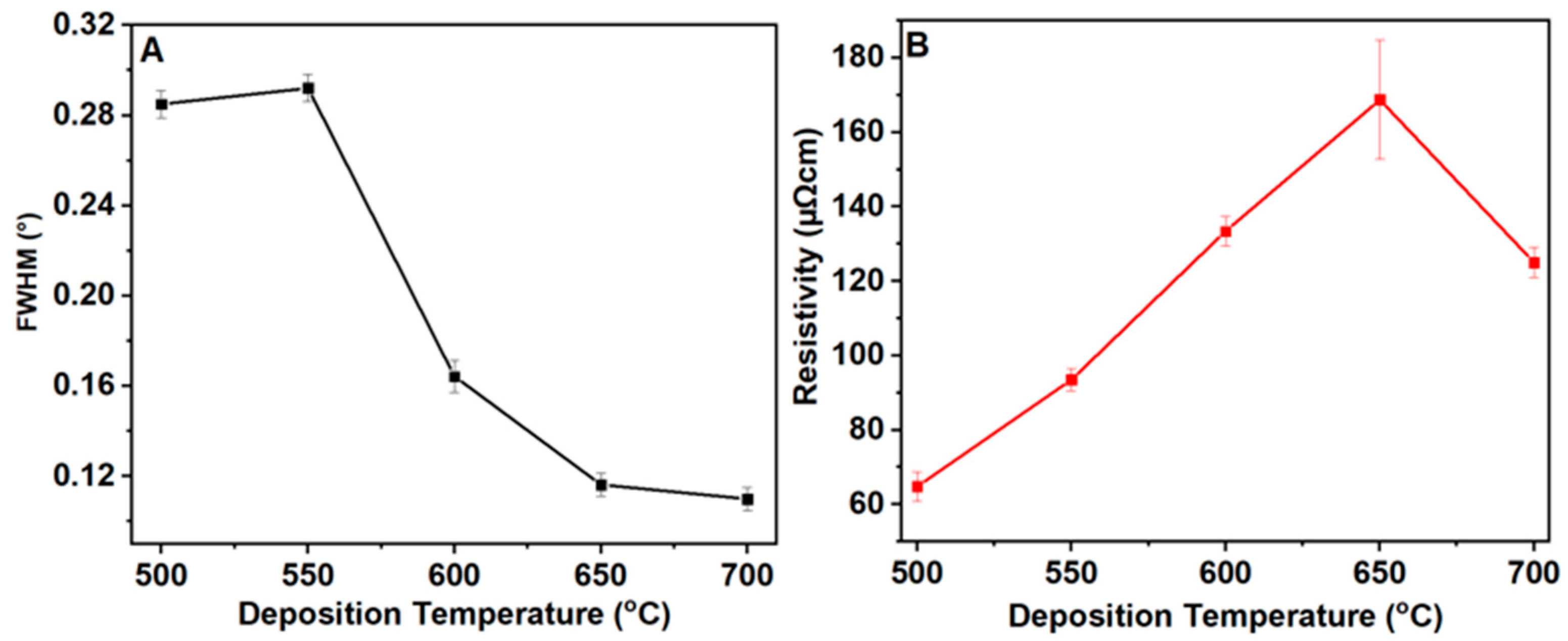


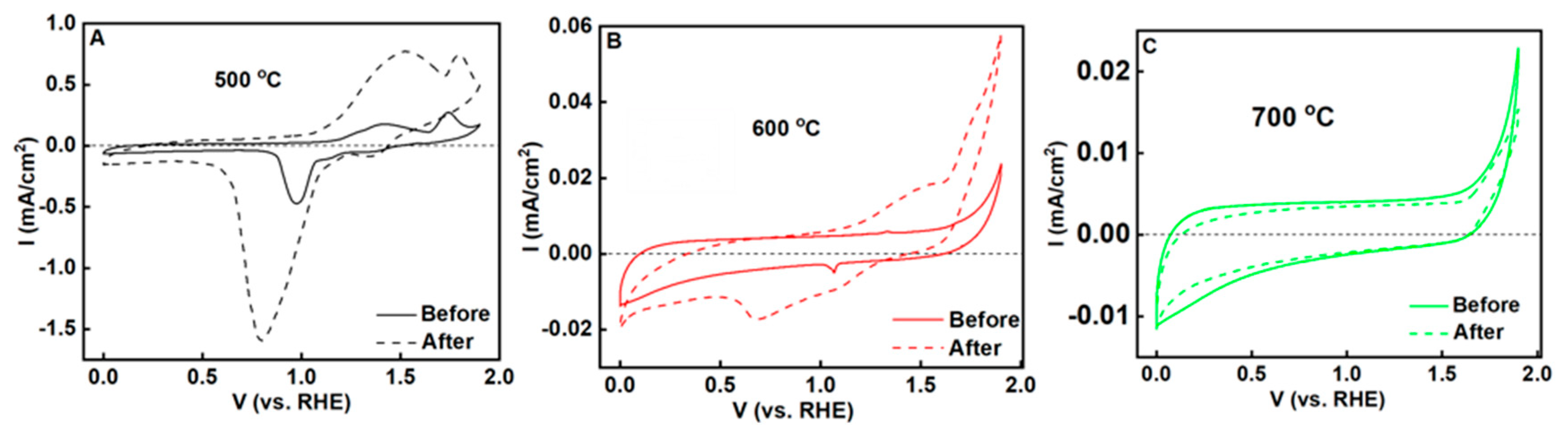
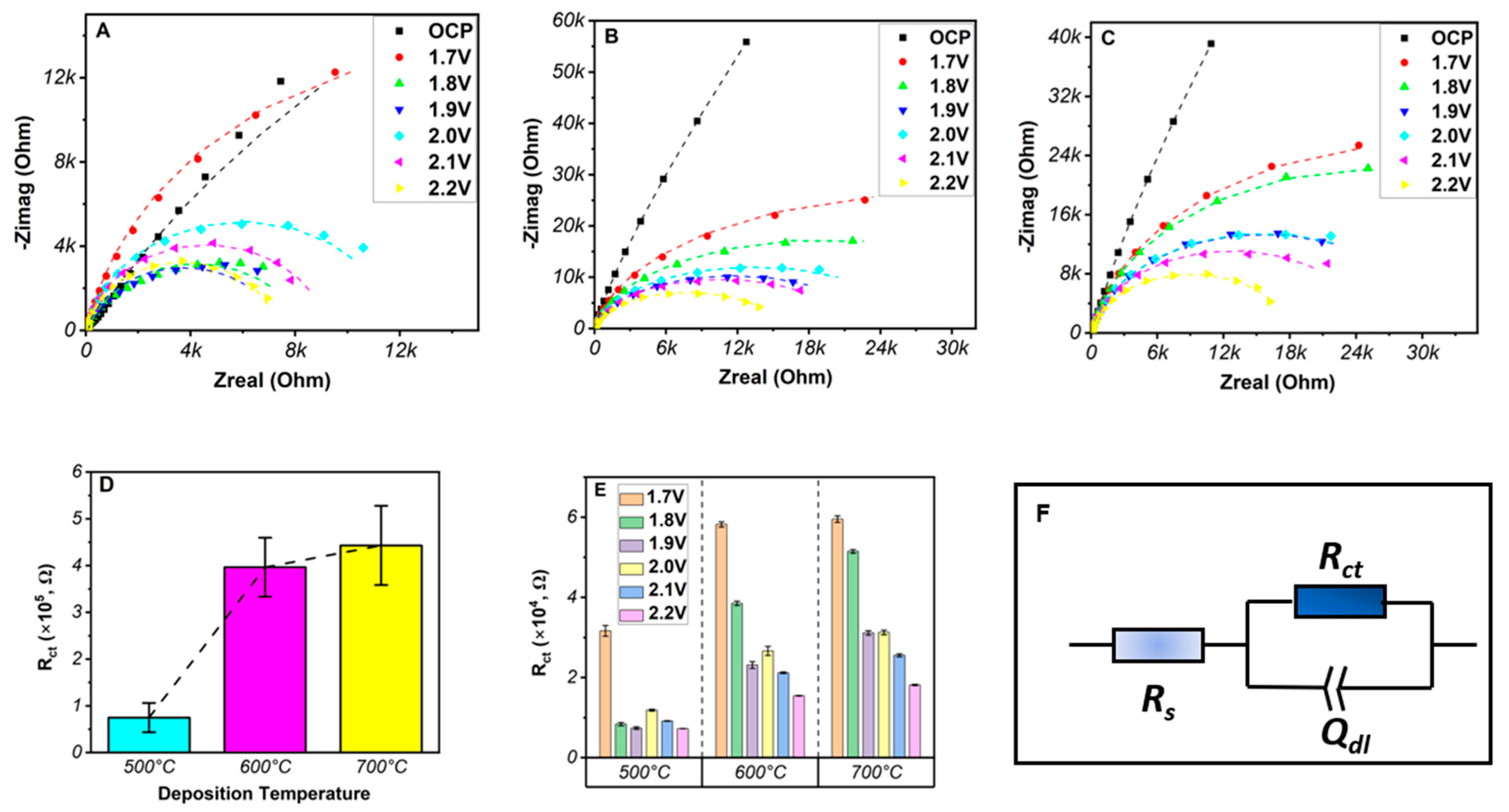
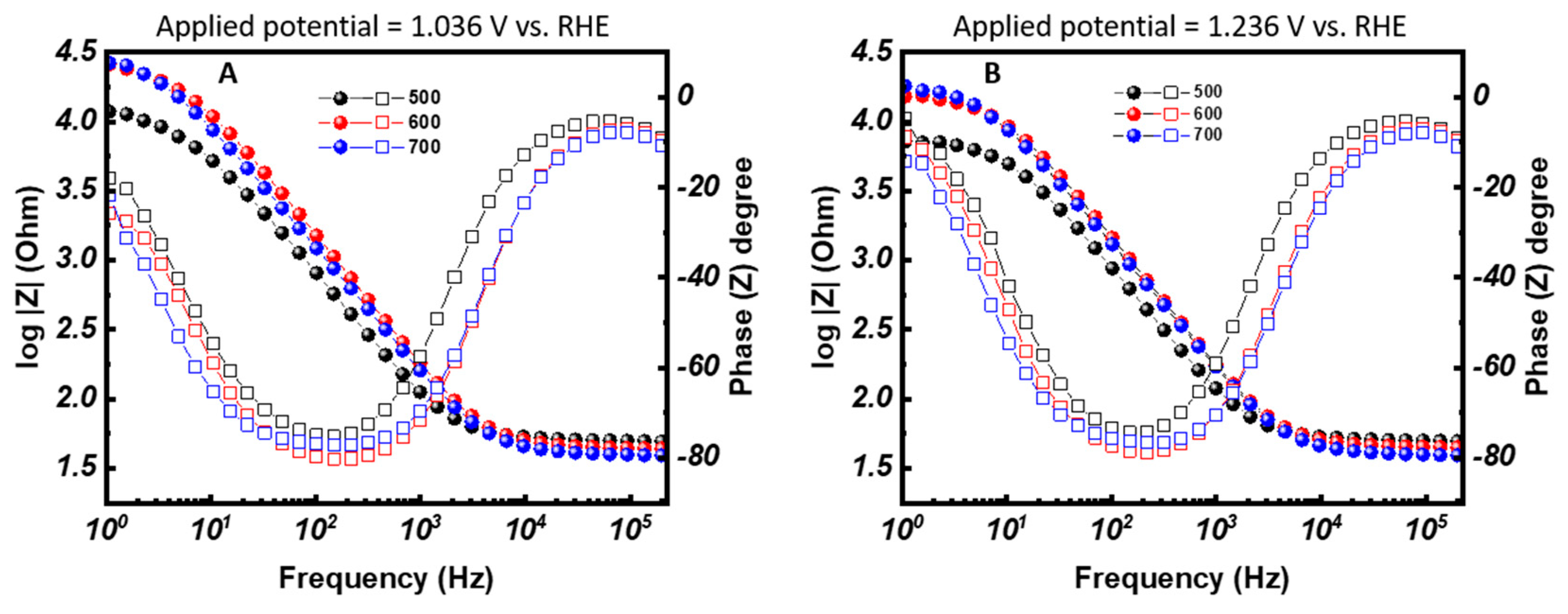
| Sample | Temperature (°C) | Sheet Resistance (Ω) | Film Thickness (nm) | Resistivity (µΩcm) |
|---|---|---|---|---|
| 1 | 500 | 6.04 | 107.3 ± 2.0 | 64.78 ± 4 |
| 2 | 550 | 19.09 | 49.0 ± 1.7 | 93.54 ± 3 |
| 3 | 600 | 27.29 | 48.9 ± 2.0 | 133.45 ± 4 |
| 4 | 650 | 41.98 | 40.2 ± 7.5 | 168.76 ± 16 |
| 5 | 700 | 25.60 | 48.8 ± 2.0 | 125.05 ± 4 |
| Temperature (°C) | N1s (%) | Ti2p (%) | O1s (%) |
|---|---|---|---|
| 500 | 43.27 | 43.74 | 12.99 |
| 600 | 41.69 | 44.51 | 13.80 |
| 700 | 35.04 | 44.84 | 20.12 |
| Assignment | Binding Energy (eV) | FWHM (eV) |
|---|---|---|
| Ti-N 2p 3/2 | 455.05 | 1.20 |
| Ti-N 2p ½ | 460.95 | 1.44 |
| Ti-N-O 2p 3/2 | 456.12 | 2.3 ± 0.02 |
| Ti-N-O 2p 1/2 | 462.10 | 2.40 |
| Ti-O2 2p 3/2 | 458.51 | 2.4 |
| Ti-O2 2p ½ | 464.21 | 2.4 |
| Plasmon Ti-N 2p 3/2 | 457.95 | 1.36 |
| Plasmon Ti-N 2p 1/2 | 463.75 | 1.36 |
| Plasmon Ti-N-O 2p 3/2 | 459.03 ± 0.06 | 2.4 |
| Plasmon Ti-N-O 2p 1/2 | 464.48 ± 0.06 | 2.4 |
| N 1s (N-Ti) | 397.31 ± 0.1 | 1.1 |
| N 1s (N-O-Ti) | 396.86 | 1.0 |
| N 1s (N-O) | 399.10 | 2.3 |
| O1s (O-Ti) | 529.97 | 1.27 ± 0.03 |
| O1s(O-Ti-N) | 531.2 | 1.9 ± 0.07 |
| O1s (Ads O) | 533.65 | 1.9 ± 0.01 |
| Sample Name | Characterization | |||||
|---|---|---|---|---|---|---|
| Layers | Thickness (×1015 at./cm2) | Composition | Chemical Formula from Composition | |||
| 700 °C film | 1 | 500 | N | Ti | O | TiN0.64O0.74 |
| 0.27 | 0.42 | 0.31 | ||||
| 2 * | 100,000 | Al | O | Al0.4O0.6 | ||
| 0.4 | 0.6 | |||||
| 600 °C film | 1 | 470 | N | Ti | O | TiN0.71O0.38 |
| 0.34 | 0.48 | 0.18 | ||||
| 2 * | 100,000 | Al | O | Al0.4O0.6 | ||
| 0.4 | 0.6 | |||||
| 500 °C film | 1 | 1170 | N | Ti | O | TiN0.62O0.31 |
| 0.32 | 0.52 | 0.16 | ||||
| 2 * | 100,000 | Al | O | Al0.4O0.6 | ||
| 0.4 | 0.6 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cherono, S.; Chris-Okoro, I.; Liu, M.; Kim, R.S.; Nalawade, S.; Akande, W.; Maria-Diana, M.; Mahl, J.; Hale, C.; Yano, J.; et al. Transformation of TiN to TiNO Films via In-Situ Temperature-Dependent Oxygen Diffusion Process and Their Electrochemical Behavior. Metals 2025, 15, 497. https://doi.org/10.3390/met15050497
Cherono S, Chris-Okoro I, Liu M, Kim RS, Nalawade S, Akande W, Maria-Diana M, Mahl J, Hale C, Yano J, et al. Transformation of TiN to TiNO Films via In-Situ Temperature-Dependent Oxygen Diffusion Process and Their Electrochemical Behavior. Metals. 2025; 15(5):497. https://doi.org/10.3390/met15050497
Chicago/Turabian StyleCherono, Sheilah, Ikenna Chris-Okoro, Mengxin Liu, R. Soyoung Kim, Swapnil Nalawade, Wisdom Akande, Mihai Maria-Diana, Johannes Mahl, Christopher Hale, Junko Yano, and et al. 2025. "Transformation of TiN to TiNO Films via In-Situ Temperature-Dependent Oxygen Diffusion Process and Their Electrochemical Behavior" Metals 15, no. 5: 497. https://doi.org/10.3390/met15050497
APA StyleCherono, S., Chris-Okoro, I., Liu, M., Kim, R. S., Nalawade, S., Akande, W., Maria-Diana, M., Mahl, J., Hale, C., Yano, J., Aravamudhan, S., Crumlin, E., Craciun, V., & Kumar, D. (2025). Transformation of TiN to TiNO Films via In-Situ Temperature-Dependent Oxygen Diffusion Process and Their Electrochemical Behavior. Metals, 15(5), 497. https://doi.org/10.3390/met15050497











