Abstract
Organophosphorus compounds (OPC) are a large class of organic compounds that provide a wide range of applications, and their importance has grown steadily in recent years. In each category and family, these compounds have similarities and differences. Due to their immense variety, these chemicals have various properties and, therefore, various applications. In fact, various works have been published recently that present the main applications of OPC, especially in metal extraction. Despite their extemsive range of use, optimizing their performance as extractant agents remains a challenge due to their structural variability and sensitivity to process parameters. This review provides a critical analysis of pentavalent OPCs, focusing on how their chemical nature influences heavy metal extraction efficiency. For the first time, we present a novel classification system for OPCs based on phosphorus valency and heteroatom coordination, offering a framework to guide future research. Our findings reveal that the direct coordination of the phosphorus to heteroatoms such as oxygen, sulfur, and nitrogen has a great influence on the physicochemical characteristics of the extractant and the metal extraction efficiency. This observation is in line with Pearson’s Hard and Soft Acids and Bases (HSAB) theory in the sense that it demonstrates that altering the heteroatom alters the metal affinity of the ligand. As a result, these structural modifications can improve the extraction performance by up to 40% for some heavy metals, highlighting the potential for optimized molecular designs to maximize industrial applications. In the future, this work offers a solid foundation for future studies on the rational design of organophosphorus-based extractants. Using HSAB theory and our novel classification system, researchers can rationally design OPCs for their target metal with unparalleled precision. These results have transformative impacts on metal recovery efficiency-intensive sectors like mining, waste recycling, and clean energy technologies.
1. Introduction
The synthetic organophosphorus compounds (OPCs) have many uses and applications, such as nerve agents [1,2,3] and chemical warfare weapons [4,5], but their major application concerns the manufacture of pesticides and insecticides [6,7,8]. OPCs are also the main components of flame retardant [9,10]; other applications of organophosphorus compounds have been developed in different fields, particularly the extraction and complexation of metals such as copper and gold [11,12].
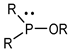
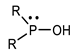
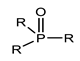
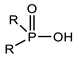
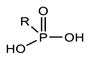
Organophosphorus compounds are often phosphoric acid esters (organophosphates derivatives). These chemicals have a general structure comprising a P = O and/or P = S group, a branching group containing R3, which is sensitive to hydrolysis and exchangeable with nucleophilic reagents, and two substituents, R1 and R2, that have increased stability vis-à-vis hydrolysis (Figure 1) [13,14].
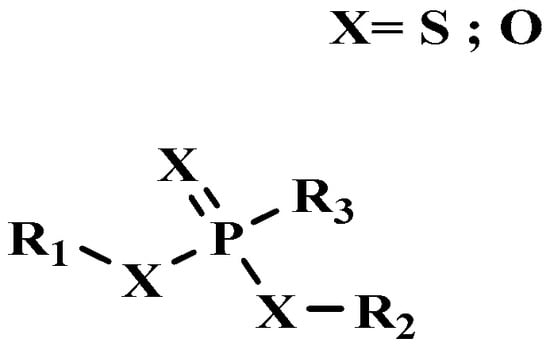
Figure 1.
General structure of organophosphorus compounds.
The two terms “organophosphorus compound-OPC” and “organophosphate-OP” refer to the group of synthetic organic compounds comprising one or more phosphorus atoms (while organophosphates (OPs) are a specific subclass of OPCs) [15]. In this review, we are particularly interested in organophosphates derivatives based on trivalent and/or pentavalent phosphorus (the most common organophosphorus chemicals) [16].
2. Main Families of OPCs and Their Applications
Historically, the first known application of organophosphorus compounds concerns the manufacture of pesticides and insecticides. The data in the literature agree that the chemistry of organophosphate began in the early 1820s, when Jean Louis Lassaigne synthesized the first organophosphate compounds, including alkylated phosphates, by reacting phosphoric acid with alcohols in 1854 [17]. The French chemist Philippe de Clermont described the synthesis of tetraethyl pyrophosphate (TEPP) at a meeting of the French Academy of Sciences [18]. In 1932, the German chemist Willy Lange, alongside Gerda von Krueger, one of his students, first described the effects of organophosphates on the cholinergic nervous system [19,20,21], being related to nerve agents [22,23]. Many molecules have been synthesized, but their real interest did not appear until 1936, when the German chemist Gerhard Schrader, employed at the Bayer AG division of the company IG Farben, highlighted the neurotoxic properties by experimenting with these compounds as insecticides [1,17,24,25].
The synthesis of these products saw a significant surge. During his research into insecticides that kill insects by cardio-circulatory, Dr. Gerhard Schrader synthesized a new class of organophosphorus compounds known as organophosphates [25,26]. This work led to his discovery of the following nerve agents: Tabun in 1936, Sarin in 1939, and Soman in 1944 [17,27]. Shortly thereafter, Dr. Schrader also discovered parathion, which became the first commercialized insecticide based on organophosphorus (although it is now prohibited) [2,20,28]. The high toxicity of some of these molecules aroused the interest of the German military, which subsequently thought of using them as chemical warfare agents [4,5,27,29]. In 1952, three teams—Ghost (England), Schrader (Germany), and Tamlin (Sweden)—developed a new OPC, the VX [30,31], whose toxicity makes it one of the most toxic xenobiotics [32,33]. The chemistry of OPCs has grown very strongly, resulting in many other applications of OPCs. OPCs are, for instance, considered to be excellent extraction reagents of metal ions, and can form stable complexes due to their favorable physicochemical properties in hydrometallurgical processes [12,34,35,36,37]. The chemical structure, chemical properties, and physical properties of organophosphorus compounds allow us to better classify the OPC group that we present below.
2.1. Classification of Organophosphorus Compounds
The electronic structure of phosphorus [Ne]3s23p3 allows it to be formally trivalent or pentavalent by either using only the three unpassed electrons or all of the electrons in its valence layer. The vast majority of organophosphorus compounds form three (σ3), four (σ4), or five bonds (σ5) with other atoms [38,39]. OPCs are optionally ester, amide, or thiol derivatives of phosphoric acids, and their derivatives, such as phosphonic, phosphorothioic, or phosphonothionic acid, which makes their classification complex.
The complexity of the classification of OPC is due to the variety of atoms or substituents bound directly to the phosphorus atom, the type of aliphatic and/or aromatic chains, and the position to which its substituents are attached [40,41]. Until today, no system has been universally accepted for the nomenclature and the classification of OPC, although the Anglo-American system reached an agreement to adopt an “international nomenclature” instead of individual systems of the four countries (Germany, Sweden, United Kingdom, and the United States of America) [42,43]. This is why a new classification is developed in this manuscript. It will allow for a more precise and simpler description of these compounds, thus facilitating readability via classification of OPC, both according to the degree of valence and according to their family group (OP, Thio-OP, amido-OP, etc.), with illustration and examples.
This review covers only tri- and pentavalent phosphorus families, given that these chemicals are the first most provided extractants used in the hydrometallurgical industry in general, and solvent extraction plants specifically. According to the valency of phosphorus, we subdivided OPs into two groups that are shown in Table 1.

Table 1.
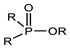
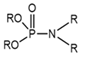
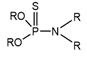
(i) A group of trivalent phosphorus derivatives (PIII), including phosphines, phosphites, phosphinites, phosphonites, phosphoreous acid, phosphoneous acids, and phosphineous acids, and (ii) the pentavalent group (PV), including phosphine oxides, phosphinic acids, phosphonic acids, phosphoric acids ester (Dialkyl hydrogen phosphate), organophosphates, thio-organophosphates, etc. Structural classification of the main tri and pentavalent organophosphorus compounds.
Obviously, OPC does not constitute a homogeneous class. The large structural variability of organophosphorus derivatives makes it very diverse in terms of characteristics and corresponding physical and chemical properties, allowing for the wide use of these products. Therefore, according to its molecular structure, OPC has multiple applications in various fields. OP is part of hundreds of product formulations, including insecticides [25], pesticides [22], nerve agents [44], flame retardants [9], lubricant-additive oils [45]), plasticizers, corrosion inhibitors (antioxidants) [46,47], flotation agents [13,48], and industrial solvents and extracting agents [20,44]. Moreover, OPs are also the main component used in the pharmaceutical industry; they are also the active ingredient of drugs, used for various treatments [49], including cyclophosphamide for the treatment of cancer [50] and isoflurophate for the treatment of esotropia and bisphosphonates for treatment of osteoporosis [51,52,53].
2.2. Properties of the Main Organophosphorus Compounds
The family of organophosphorus compounds consists of a great diversity of products, whether for their aliphatic or aromatic structures, or their physicochemical properties, and, consequently, their reactivity. OPCs are divided into various classes according to the oxidation degree of phosphorus (pentavalent or trivalent) and the substituents’ nature, including the presence of one and/or several oxygen atoms, or another atom, including chalcogens (the most common is sulfur) and halogens (Chlorine, Fluorine, etc.). The physicochemical properties of OPC, such as solubility, volatility, stability, toxicity, and reactivity, are highly variable and depend on the nature of substituents bound to the phosphorus atom, such as oxygen, sulfur, nitrogen, side chains, or halogens. At the same time, these properties depend on the type of bonds binding substituents to the phosphorus atom, which can be a covalent bond or a double bond.
Most organophosphate molecules are poorly soluble in water; moreover, they are very soluble in the majority of organic solvents (chloroform, carbon tetrachloride, ether, alcohol, toluene, carbon sulfide, and various hydrocarbons) and have low volatility, but are very fat-soluble, hydrolyzed with the formation of non-toxic, water-soluble derivatives; they are very stable at temperatures less than or equal to 60° and in extraction processes; and they can be used in several cycles and for several months without risk of decomposition. These molecules often bind together by hydrogen bonds and form polymers whose degree of polymerization depends on the polarity of the solvent [54].
Organophosphorus compounds (OPCs) as extractants have exhibited superiority in heavy metal recovery across various extraction methods. These compounds, characterized by phosphorus–carbon bonds, include a range of functional groups, such as phosphoric acid esters (e.g., D2EHPA), phosphonic acids (e.g., PC88A), phosphine oxides (e.g., TOPO), and thiophosphorus derivatives (e.g., Cyanex®301). They owe their extractive properties to the fact that they are able to form stable complexes with metal ions through different mechanisms like cation exchange, solvation, and chelation. The oxidation state of the phosphorus center (III or V) and the character of heteroatoms directly bonded to it (O, S, N) exert a strong influence on their metal-binding selectivity and affinity, following Pearson’s Hard–Soft Acid–Base (HSAB) principle. This structural flexibility allows OPCs to be tailored to target specific heavy metals for specific industrial operations, from hydrometallurgical processing to environmental remediation [55,56].
OP-based extracting reagents have high thermal stability. These extractants act by cation exchange (case of acid extractants), by solvation (case of neutral extractants), or by chelation (case of mixtures of acid and neutral extractants) of metals contained in solutions according to the following rules [57]:
- ∗
- Acid Dissociation Constant (pKa) and Metal Extraction Efficiency: The acid dissociation constant (pKa) of the organophosphorus acids plays a significant role in metal extraction procedures. The more negative the pKa value of the organophosphorus acid, the more acidic it will be, leading to better proton release and assisting metal complexation. This consequently leads to higher metal distribution coefficient (D) and lower pH1/2 value (the pH value for 50% extraction of the metal). Further, the hydrophobic character of the produced metal complex greatly controls extraction effectiveness; the more hydrophobic the complexes, the more favorably they will distribute into the organic phase, hence the increased metal distribution coefficient.
- ∗
- Extractant Hydrophobicity and Partitioning Behavior: Hydrophobicity of the extractant itself is also a critical consideration in determining metal extraction. The greater the partition coefficient (Kp) of the extractant between organic and aqueous phases, the more hydrophobic it becomes, which allows for greater transfer of the metal complex to the organic phase. Such enhanced hydrophobicity is typically related to a lower pH1/2 value because the extractant is able to work well even at lower acidic pH. Additionally, the metal–ligand complex stability constant (β) gives an idea about how much the ligand favors the metal ion; the higher the β value, the tighter the bonding, thereby increasing the metal distribution coefficient and lowering the pH1/2 value.
Commercially, the most common properties required for organophosphorus(OP)-based extractants include [57,58,59]: high selectivity, high load capacity, rapid phase separation without formation of stable emulsions, fast extraction kinetics, easy pickling and recovery of solvents, low solubility in the aqueous phase, and good solubility in a cheap organic diluent, as well as thermal and hydrolytic stability
3. Influence of the Chemical Nature of Organophosphorus Additives on Metal Extraction Performance
Organophosphorus products are the most efficient extraction reagents [59,60] used in hydrometallurgy processes [19,61]. Their chemical nature differs mainly in the types of atoms and substituents, their structural arrangement in the molecule, the length of the chain, the type and strength of chemical bonds, reactivity, and chemical stability. The importance of these chemical factors in achieving the performance targets of metal mining has been extensively studied. The table below shows that the chemical structure of organophosphorus extractants influences their performance, including the extraction, precipitation, or flotation of metals [25,62,63,64,65].
Table 2 provides a comprehensive overview and highlights the main key parameters that determine the relationship between extractant structure, process conditions, and extraction efficiency, ultimately leading towards optimizing heavy metal extraction by OPCS in acidic media. These parameters include the chemical characteristics of the extractants, such as their functional groups, molecular weight, and hydrophobicity, which are significant factors in determining their selectivity and affinity towards target heavy metals. Furthermore, the table confirms the importance of extraction process conditions and shows its impact, including pH, temperature, contact time, extraction medium, and extractant and metal ion concentrations. Through an objective analysis of these variables, the data show how optimizing the structural properties of extractants and operating conditions can lead to an optimal extraction performance. This systematic approach not only improves the recovery of heavy metals, but also renders the process economically viable and environmentally sustainable, particularly when operating in acidic environments where complications such as competing ion interactions and extractant stability are prevalent.

Table 2.
Metals extraction by organophosphorus extractant on different medium (solution)/solvents.
- -
- Extraction Efficiency Across Metals:
The Table 2 demonstrates that OPCs exhibit varying degrees of extraction efficiency for different metals, depending on their chemical properties and the following extraction conditions:
- ○
- Scandium (III) exists mostly as a hydroxide species under pH 5.5, i.e., (Sc(OH)3) or Sc(OH)4−, depending on solution conditions. In our case (media rich in chloride), Sc(OCl)3 can also form, but this is not as common unless excessive chloride concentration is encountered.
- ○
- Based on HSAB theory, scandium (III) is a “hard acid” due to its small ionic radius, high charge density, and preference for hard donor ligands such as oxygen. This classification agrees with its strong bonding with oxygen donor ligands, such as those found in TBP (−(P(=O)(OR)3). Given the above explanation, we now provide a comparative analysis of TBP and TOPO extraction behavior:
- •
- TBP (Tributyl Phosphate) is an oxygen donor ligand, and it reacts strongly with hard acids like scandium (III). The capacity to form stable Sc(III)-TBP complexes justifies the description of scandium (III) as a hard acid. This is consistent with HSAB theory.
- •
- TOPO (Tri-n-Octylphosphine Oxide) contains both a soft phosphorus donor and a hard oxygen donor. While TOPO can coordinate with borderline as well as hard acids, its coordination with scandium (III) is primarily through the oxygen donor, again confirming scandium (III)’s hard acid status. However, the softer phosphorus donor in TOPO introduces some ambiguity, which should be demonstrated experimentally.
- ○
- Cyanex® 272 and Cyanex® 301 achieve near-complete extraction (>98%) for several metals, including Fe, Ga, Cu, Co, and Zn, under optimized conditions. This underscores their versatility and effectiveness as cationic exchangers.
- ○
- Sodium diethyl dithiophosphate achieves a Cd extraction yield of 93.1%, demonstrating its suitability for soft Lewis acids like cadmium.
For certain metals (e.g., Fe with Cyanex® 302), the extraction efficiency drops significantly (40%), indicating selective interactions based on metal ion characteristics and extractant donor groups.
The molecular structure of organophosphorus-based extractants (OPC) is the major determinant of their performance in extraction. Functional groups play a strong role in determining effectiveness; phosphinic and dithiophosphinic acids (e.g., Cyanex® 272 and Cyanex® 301) exhibit good chelating ability and high compatibility with borderline hard acids, while sulfur-containing extractants (e.g., sodium diethyl dithiophosphate) exhibit high selectivity for soft acids like Cd(II) and Cu(I), as predicted by HSAB theory principles.
The hydrophobicity of the organophosphorus-based extractant agent is also affected by alkyl chain length; longer chains encourage organic phase partitioning, but excessively long chains might decrease selectivity for smaller metal ions, as in the case of tetradecylphosphonic acid (C14). Last but not least, extractants with a mixture of hard and soft donor atoms (such as TOPO) show intermediate behavior. They bind both hard and borderline acids, offering versatility but requiring careful process condition optimization.
The extraction efficiency is impacted directly by the process parameters, such as pH, contact duration, temperature, and diluent type, which have a direct impact on the following:
- -
- pH: OPCs function best at acidic pH ranges (pH 2–4, for instance), where metal ions are present as free cations or weakly solvated ions. For example, Cyanex® 301 functions optimally in terms of extraction yields for Cu, Zn, and Co at pH 2, while Cyanex® 272 optimally functions for Fe and Ga at the same pH.
- -
- Contact Time and Temperature: Shorter contact times (e.g., 10 min) and moderate temperatures (e.g., 25 °C) can provide high extraction efficiencies, as seen with Cyanex® 301 and 302.
- -
- Diluent Effects: Kerosene and toluene are low-polarity, general-purpose diluents with high efficacy for stabilizing organic phase complexes. The diluent choice could influence extraction kinetics and phase separation efficiency.
The selectivity of OPCs for specific metals is influenced by interfering ions and the physicochemical environment, as follows:
- -
- Selective Extraction: Cyanex® 301 is highly selective for Cu compared to other metals in sulfate solutions, which renders it useful for hydrometallurgical applications in mixed-metal streams. Sodium diethyl dithiophosphate selectively extracts Cd in acidic medium, even with trivalent iron present.
- -
- Competing Ions: Competitive ions (e.g., protons, sulfate, chloride) may reduce the efficiency of extraction by competing for coordination sites or metal-extractant compound instability.
While the OPCs are found to be outstanding for the purpose of heavy metal extraction, the efficiency of extraction by OPCs is also affected by the following weaknesses.
- -
- Strengths:
- Highly selective and tunable owing to structural change.
- Ability to blend with a broad range of extractive media from acidic to close to neutral solutions.
- Developed scalability for industrial application.
- -
- Limitation:
- Reduced extraction efficiency for soft acids when oxygen donor ligands are employed.
- Sensitivity of process parameters such as pH, temperature, and diluent polarity.
- Environmental concerns over the disposal of spent extractants and organic solvents.
3.1. Thermodynamic Fundamentals of Metal-OPC Extraction
Organophosphorus compounds (OPCs) constitute a versatile group of ligands with excellent heavy metal selectivity, and they play a key role in a number of extraction processes, including solvent extraction, adsorption, membrane separation, and precipitation. Their versatility in functional groups—phosphoric acid esters (e.g., D2EHPA), phosphonates (e.g., Ionquest 801), thiophosphinates (e.g., Cyanex 301), and phosphine oxides (e.g., Cyanex 923)—underpin selective metal recovery by mechanisms ranging from ion exchange and chelation to solvation and interfacial complexation [34,79].
The efficiency of OPC-mediated extraction depends on thermodynamic driving forces, which control the spontaneity (ΔG°), energetics (ΔH°), and structural reorganization (ΔS°) of metal–ligand interactions. Sulfur-containing OPCs, for instance, are extremely selective for soft heavy metals (e.g., Cd2+, Hg2+) on the basis of favorable ΔG° values, while phosphoryl oxygen groups are the most effective in extracting borderline acids (e.g., Cu2+, Zn2+). In addition to solvent extraction, such principles also govern solid-phase systems (e.g., functional resins) and hybrid processes (e.g., liquid membranes), in which pH, temperature, and ligand design are adjusted to optimize the recovery yields and the energy efficiency [80,81]. Here, the thermodynamic foundations of OPC-heavy metal interactions are explained, highlighting their generalizability to extraction science. By correlating the molecular structure with thermodynamic characteristics, we describe ways of designing OPCs to a specific metal or industrial process, from wastewater treatment to hydrometallurgy [82,83].
The solvent extraction process can usually be described by the following general equation:
where
Mn+(aq) + n HL(org)⇌ MLn (org) + nH+(aq)
Mn+(aq) is the metal ion (with charge n+) in the aqueous phase.
HL(org) is the cationic exchanger (acidic extractant, e.g., D2EHPA, LIX reagents, or Versatic acid) in the organic phase.
MLn(org) is the metal-extractant complex formed in the organic phase.
H+(aq) is the proton released into the aqueous phase.
The equilibrium constant (Kex) for this reaction is given by Equation (2), as follows:
The Gibbs free energy change (ΔG) associated with this process is related to the equilibrium constant by
where T is the absolute temperature and R is the gas constant. The enthalpy (ΔH) and entropy (ΔS) changes can be determined using the van’t Hoff Equation (4), as follows:
ΔG = −RTlnKex
LnKex = −∆H°/RT + ∆S°/R
By determining Kex at different temperatures, the thermodynamic parameters ΔH° and ΔS° can be calculated, which can provide information on the nature of the extraction process.
Factors Influencing Thermodynamics
Molecular Structure of Extractants
Organophosphorus molecules’ structure significantly influences their thermodynamics. For example, Cyanex 272 (bis(2,4,4-trimethylpentyl) phosphinic acid) forms stable complexes with divalent metal ions due to its chelating ability [84].
Cyanex 923 (a neutral phosphine oxide) is less interactive but highly selective for certain metals [63,70].
Temperature Dependence
Temperature plays a crucial role in terms of efficiency in extraction. Lower temperatures are favorable for exothermic reactions (ΔH < 0), while higher temperatures are favorable for endothermic reactions (ΔH > 0). The entropy change (ΔS) is a measure of disorder of the system and positive values indicate greater randomness.
Solvent Effects
The choice of diluent (e.g., kerosene, toluene) affects the thermodynamics of the extraction by changing the stability and solubility of the metal-extractant complex. Polar diluents can enhance extraction efficiency by stabilizing ionic species, while nonpolar solvents enhance the extraction of neutral complexes.
Nature of the Metal Ion
The size, charge, and hydration energy of the metal ion influence its affinity for the extractant.
Hard metal ions (e.g., Fe3+, Al3+) prefer hard donor atoms (e.g., oxygen), while soft metal ions (e.g., Cu+, Ag+) prefer soft donor atoms (e.g., sulfur).
Kinetic Factors
The rate of complex formation depends on factors such as diffusion, mixing, and the activation energy of the reaction.
Slow kinetics can limit the efficiency of extraction, even if the thermodynamics are favorable.
3.2. Case Study: Copper Extraction with Cyanex 301
Cyanex 301 (bis(2,4,4-trimethylpentyl)dithiophosphinic acid) is widely used for copper extraction. The thermodynamic process analysis shows the following:
- Enthalpy Change (ΔH): The reaction is exothermic (ΔH < 0), which indicates intense bonding between copper ions and the extractant.
- Entropy Change (ΔS): The negative change in entropy indicates decreased randomness with the formation of a complex.
- Gibbs Free Energy (ΔG): The negative value confirms spontaneity in the extraction process.
These findings highlight the optimization of temperature and pH to maintain maximum recovery of copper
Organophosphorus solvent extraction of metals is a thermodynamically driven process that works based on equilibrium constants, enthalpy–entropy trade-offs, and a few key operational parameters, such as pH, temperature, and extractant concentration. Management and optimization of these variables greatly enhance the efficiency and selectivity of metal recovery, rendering them usable in hydrometallurgy, nuclear reprocessing, and environmental remediation.
Future advances in process integration and extractant design will further improve the sustainability and cost-effectiveness of metal extraction technology, making them more applicable to industrial separations and resource recovery.
3.3. Influence of the Atom Directly Bound to Phosphorus
The direct binding of phosphorus to heteroatom, including oxygen, sulfur, and nitrogen, influences the metal extraction properties and efficiency; this is because, by modifying the main function of organophosphorus (changing heteroatom linked to phosphorus), the ligand’s affinity for the metal will change. According to Pearson’s Hard and Soft Acid–Base (HSAB) concept, this affects both the efficiency and the extraction yield [85,86].
Organophosphorus-based extractants have been widely used in a variety of applications since the 1940s. Furthermore, the theory of R. Pearson [87] is mainly used to qualitatively interpret the selectivity of chemical reactions, and also to explain the stability of certain complexes [88]. According to the Hard–Soft concept, the complexation of a soft Lewis acid, such as Pt2+, Pd2+, Ag+, Au+, Hg2+, and Cd2+ with a soft Lewis base, should occur with high selectivity [89].
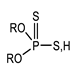
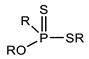
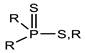
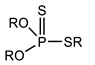
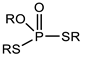
Through the comparison of the reactivity of Thioorganophosphate extractants, Brian K. Tait has shown that the use of Bis(2,4,4-trimethylpentyl) dithiophosphinic acid (Cyanex ®301) for the extraction of copper, zinc, iron, and cobalt under hard conditions (very high acidities) is more efficient than bis(2,4,4-trimethylpentyl) monothiophosphinic acid (Cyanex®302). The structural difference between Cyanex® 301 and 302 (Figure 2) explains their distinct reactivity. The metal extraction can reach up to 100% for the Bis(2,4,4-trimethylpentyl) dithiophosphinic acid, due to the replacement of oxygen atoms of phosphinic acid (of Cyanex ®302) by sulfur atoms, leading to a change in their physicochemical properties and chemical characteristics. The Bis(2,4,4-trimethylpentyl) dithiophosphinic acid’s acidity is stronger than bis(2,4,4-trimethylpentyl) monothiophosphinic acid (Cyanex ®302); however, the degradation of Bis(2,4,4-trimethylpentyl) dithiophosphinic acid in the presence of strong oxidizing agents limits the extraction range in industrial applications. Moreover, the stripping of metal ions from the loaded phase of Bis(2,4,4-trimethylpentyl) dithiophosphinic acid becomes more difficult [90,91].
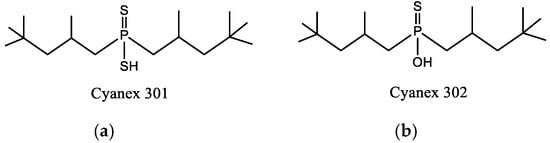
Figure 2.
(a) Molecular Structure of Bis(2,4,4-trimethylpentyl) dithiophosphinic acid (Cyanex ®301); (b) Molecular structure of Bis(2,4,4-trimethylpentyl) monothiophosphinic acid (Cyanex ®302) [91].
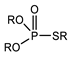
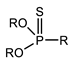
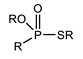
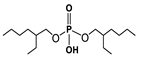
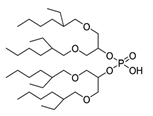
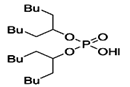
K. Omelchuk et al. have shown that, in organophosphate acids, the presence of oxygen atoms—directly bound to phosphorus—promotes the extraction of the metal from acid media. Contrariwise, the oxygen atoms within hydrophobic alkyl chains (e.g., in Figure 3) have a negative effect on the metal extraction yield due to the increase in acidity constant, caused by the inductive and attractive effects of oxygen in the presence of proton H+ [89,92]. This distinction is exemplified by comparing Di(2-ethylhexyl) phosphoric acid (D2EHPA) (Figure 3a) and Bis(1,3-di-2-ethylhexyloxypropan-2-yl) phosphoric acid (EHPA) (Figure 3b). While D2EHPA’s oxygen atoms are directly linked to phosphorus (enhancing metal coordination), EHPA’s oxygen atoms reside in ether linkages within its hydrophobic chains, reducing extraction efficiency under acidic conditions.
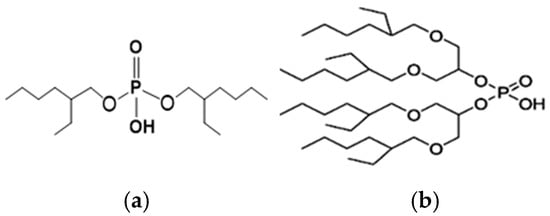
Figure 3.
(a) Molecular structure of Di-(2-ethylhexyl) phosphoric acid (D2EHPA) and (b) Molecular structure of Bis(1,3-di-2-ethylhexyloxypropan-2-yl) phosphoric acid EHPA [89].
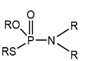
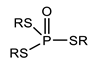
According to Tercero, N et al., dithiophosphinates and dithiophosphates share a similar structure (as illustrated in Figure 4), except for the fact that the alkyl substituents of dithiophosphinates are directly attached to the phosphorus atom, while for dithiophosphates the substituents are bonded to oxygen atoms attached to the central phosphorus atom. This difference has a profound effect on the OP chemical properties in their interactions with metals and minerals. In the case of dithiophosphates, their interactions with metals can be partially understood in terms of acid–base/hard–soft concepts, where there is a soft donor appearance that will tend to bind to the metal acceptor soft ions or metal sites on the minerals [90].
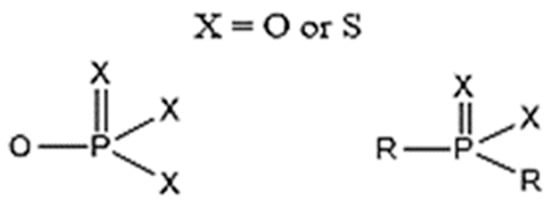
Figure 4.
Molecular structure of phosphonates, phosphinates, and their derivatives [90].
On the other hand, thio-organophosphate decomposes under strong oxidation and hydrolytic hard conditions. However, dithiophosphinic acids exhibit great hydrolytic stability that allows them to be used in solvent extraction of metal in an acidic solution under a wide range of conditions [90,93].
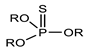
The patent filed by Hoechst Aktiengesellschaft shows that the use of dithiophosphate and/or dithiophosphonate (molecular structures shown in Figure 5) associated with various adsorption agents allows for extraction of up to 98% of heavy metal compounds such as cadmium, mercury, and lead contained in phosphoric acid and its derivatives [92].
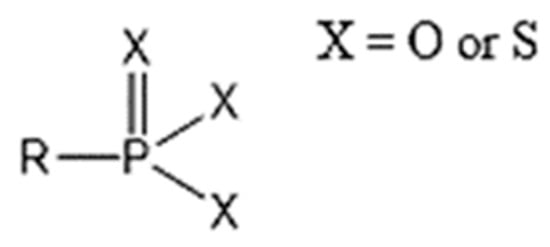
Figure 5.
Molecular structures of phosphonate and thiophosphonate derivatives [92].
This is in agreement with the Hard and Soft Acid–Base (HSAB) concept, whose donor atoms of the most common Lewis bases have increasing electronegativity of the order of S < Br < N < CI < O < F. Ops’ ligands substitution by sulfur should be beneficial for metal ions extraction [88], because sulfur is an easily oxidizable element [94] that has a high affinity for metals’ transition, particularly heavy metals [88], and many organophosphate complexes containing sulfur bound to a transition metal have been studied. The wide variety of these extractants results from the diversity of Ops’ structures and the different heteroatoms bond to the phosphorus of these substances.
3.4. Influence of the Nature of the Attached Group (Substituents R)
The efficiency of organophosphorus compounds (OPCs) as heavy metal recovery extractants depends mainly on the nature of the atom attached to the phosphorus center directly. This atom, whether oxygen, sulfur, or an organic carbon-containing group, determines the physicochemical properties of the extractant, e.g., hydrophobicity, acidity, and metal ion binding preference. Altered structures around the phosphorus atom play a decisive role in determining the extraction process, from complexation tochelation and selectivity.
In this section, we systematically explore the effect of the atom directly bonded to phosphorus on the extraction efficiency of OPCs. For a complete analysis, we examine significant parameters, such as alkyl chain length, functional group type, and extraction medium (e.g., acidic, neutral, or basic conditions). These parameters are crucial in the context of optimizing the structure of OPC-based extractants for specific heavy metals and real-world applications.
The data in Table 3 summarize the influence of carbon chain length and functional groups on extraction yields of various heavy metals. The table also includes information about the medium of extraction, which further describes the interplay between molecular structure and process conditions. By analyzing these trends, we try to highlight the relationship between structural properties of OPCs and the ability to selectively and efficiently extract target metals. Not only do we highlight here the necessity for tailoring extractant structures, but we also provide meaningful insights for next-generation extraction system design.

Table 3.
Influence of carbon numbers of OPC-Based extractants on heavy metal extraction yield.
Table 4 illustrates the comparison between the extraction of cadmium by different extractants, particularly between the commercial molecules of the dithiophosphate family and the dithiophosphinate family. It is expected, for example, that the branching of the alkyl chains of the organophosphate in the vicinity of the acid group is responsible for a decrease in the extraction yield, dinylphenyl dithiophosphate, and dicresyl dithiophosphate [107], the congested structure of the extractant which would not promote hydrogen bond interactions [89].

Table 4.
Influence of carbon numbers on cadmium extraction yield.
After showing the influence of the carbon number and position of the alkylated chain on the performance of metal extraction, a complete description of the presence of oxygen in the alkylated chain, and on the physicochemical behaviors of organophosphates as an extractant, is necessary [84]. The results compiled in the table above are derived from a study by Omelchuk et al. [89], which investigates the effects of structural changes in new organophosphorus cationic exchangers on the solvent extraction of cobalt, nickel, and manganese from acidic chloride media [79,91,108].
The results presented in Table 5 present valuable data on the influence of structural differences in organophosphorus extractants and their impact on the cobalt (Co(II)) extraction yield from acidic chloride solutions. Specifically, our results establish that the presence of oxygen atoms close to the branched carbonylated chains significantly reduces the hydrophobic character of the extractant. This hydrophobicity reduction negatively affects the extractant’s ability to carry cobalt complexes into the organic phase and lower extraction efficiency.

Table 5.
Oxygen number effect of OP-based extractants on cobalt (II) extraction efficiency.
Furthermore, the findings prove there is a clear-cut relationship between the number of oxygen atoms in the alkyl chains of the extractants and the organophosphorus compound’s acidity constant (pKa). As the number of oxygen atoms increases, so too does the acidity constant of the extractant, leading to a corresponding decrease in the yield of Co(II) extraction. This is caused by the rising polarity of the extractant molecules, which reduces their interaction with hydrophobic metal complexes and their efficiency in solvent extraction processes.
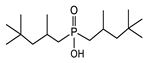
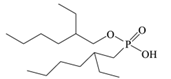
A good example of this effect can be observed in comparison between two of the most widely used extractants, EHPA (2-ethylhexylphosphonic acid) and D2EHPA (di-(2-ethylhexyl) phosphoric acid). Our results indicate that D2EHPA extracts cobalt from hydrochloric acid solutions more effectively than EHPA. This difference in performance can be attributed to the structural characteristics of D2EHPA, which possesses a more balanced pair of hydrophobicity and acidity, enabling it to stabilize and efficiently extract cobalt complexes. Under the same conditions, the higher polarity and stronger acidity of EHPA appear to hinder its extraction efficiency. These findings corroborate with the observations of Omelchuk et al. [89], which highlighted the pivotal role played by structural variation in organophosphorus cationic exchangers toward their extraction efficiency.
Our study draws on such observations by highlighting the dual impact of hydrophobicity and acidity in affecting the efficacy of organophosphorus extractants for cobalt recovery. Such observations highlight the necessity for the molecular structure of extractants to be altered to meet particular metal extraction requirements to optimize their physicochemical properties.
In summary, the present work ascertains that the reduction of alkyl chain oxygen atoms of organophosphorus extractants, with the correct balance between hydrophobicity and acidity, is of crucial importance to enhance cobalt extraction efficiency. Synthesis of new extractants with appropriately optimized structural features in future research would target enhancing cobalt recovery efficiency and selectivity from acidic chloride solutions.
4. Conclusions
Nowadays, organophosphorus chemistry offers a wide range of applications; it has become a field of science in its own right. Organophosphorus chemical applications are numerous, varied, and booming in recent years.
The most common category of organophosphorus derivatives is organophosphate compounds, which are currently used in a wide range of applications. Among the multiple applications, in this review we are interested in the extraction of heavy metals using the most widespread chemical method in hydrometallurgical separation and purification. OP-based extractants are now experiencing an accentuated development for the extraction of metals on an industrial scale. They act by cation exchange or complexation, whose extraction efficiency can more easily reach 100% by varying the pH of the solution and other extraction parameters that would affect their chemical stability and their selectivity.
As the mean outcome of this review, the organophosphate family is classified for the first time according to the valence number of phosphorus, and according to the nature and number of heteroatoms bound to phosphorus. To better understand the influence of the structure of OPC-based extraction agents on metal extraction performance, we have studied the following key parameters:
- ∗
- Influence of the atom directly linked to phosphorus on the extraction,
- ∗
- Influence of the alkyl chain of the OPC on the extraction of metals,
- ∗
- Influence of the presence of oxygen in the alkyl chain on the extraction of metals.
Regarding the extraction of heavy metals using organophosphorus extractants, our critical review, as well as our in-depth analysis of numerous pieces of research, establishes that organophosphorus extractants demonstrate very selective extraction of heavy metals.
Nonetheless, an increase in the number of oxygen atoms branched to alkyl substituents results in the reduced extraction yield, particularly for soft and borderline acid (such as Cd, Cu, Pt, Pb, Co, Ni, etc.). In the same way, the increase in the degree of branching of the alkyl, substituted at the organophosphorus core, indorses an unfavorable effect on the extraction yield of heavy metals.
5. Future Perspectives
The prospect for organophosphorus-based metal separations is very bright, with the impetus of improvements in molecular engineering and environmentally friendly process design. Central to this will be efforts towards bespoke extractant architectures, where structural characteristics can be optimized—e.g., by tuning branching and chain length—so that selectivity and extraction kinetics for the desired metals, such as rare earths (REEs) and platinum-group metals, are significantly improved. Green chemistry thinking will be the key driver, with focus directed toward creating low-toxicity, biodegradable variants minimizing the environmental footprint at no cost to performance. Coupling hybrid systems—organophosphorus ligands with nanomaterials or polymeric matrices—can potentially unlock heretofore unseen extraction yields and multi-metal recovery potential. At the same time, process optimization through artificial intelligence and online monitoring will create precision control of variables such as pH and temperature, optimizing at scale. Critically, such innovations will address immediate industrial needs, ranging from urban e-waste mining to clean REE refining, and in doing so will overcome pragmatic hurdles like solvent viscosity and phase stability. In the marriage of molecular science and scalable engineering, organophosphorus chemistry can lead the next generation of metal recovery technologies into alignment with both economic feasibility and planetary responsibility.
While this review focuses on the extraction of heavy metals, it is important to note that organophosphorus compounds also play a critical role in the separation and purification of rare earth elements (REEs). Future reviews could explore the application of HSAB theory and other principles to optimize OPC-based extractants for REE recovery.
Author Contributions
Conceptualization, M.E. and N.H.; methodology, R.B. and A.B.; validation, R.B. and A.B.; formal analysis, H.M.; investigation, H.M.; resources, H.M.; data curation, M.E.; writing—review and editing, N.H. and H.M.; visualization, A.B. and R.B.; supervision, H.M., N.H. and F.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Guignet, M.; Lein, P.J. Neuroinflammation in organophosphate-induced neurotoxicity. Adv. Neurotoxicol. 2019, 3, 35–79. Available online: https://linkinghub.elsevier.com/retrieve/pii/S2468748018300225 (accessed on 6 February 2025).
- Richardson, J.R.; Fitsanakis, V.; Westerink, R.H.S.; Kanthasamy, A.G. Neurotoxicity of pesticides. Acta Neuropathol. 2019, 138, 343–362. [Google Scholar] [CrossRef]
- Merwin, S.J.; Obis, T.; Nunez, Y.; Re, D.B. Organophosphate neurotoxicity to the voluntary motor system on the trail of environment-caused amyotrophic lateral sclerosis: The known, the misknown, and the unknown. Arch. Toxicol. 2017, 91, 2939–2952. [Google Scholar] [CrossRef]
- Kloske, M.; Witkiewicz, Z. Novichoks—The A group of organophosphorus chemical warfare agents. Chemosphere 2019, 221, 672–682. [Google Scholar] [CrossRef]
- MSD Manual Professional Edition. Nerve Chemical-Warfare Agents—Injuries; Poisoning. Available online: https://www.msdmanuals.com/professional/injuries-poisoning/mass-casualty-weapons/nerve-chemical-warfare-agents (accessed on 22 January 2025).
- Fu, H.; Tan, P.; Wang, R.; Li, S.; Liu, H.; Yang, Y.; Wu, Z. Advances in organophosphorus pesticides pollution: Current status and challenges in ecotoxicological, sustainable agriculture, and degradation strategies. J. Hazard. Mater. 2022, 424, 127494. [Google Scholar] [CrossRef]
- Farkhondeh, T.; Mehrpour, O.; Forouzanfar, F.; Roshanravan, B.; Samarghandian, S. Oxidative stress and mitochondrial dysfunction in organophosphate pesticide-induced neurotoxicity and its amelioration: A review. Environ. Sci. Pollut. Res. 2020, 1, 27. [Google Scholar] [CrossRef]
- Jaga, K.; Dharmani, C. Sources of exposure to and public health implications of organophosphate pesticides. Rev. Panam. Salud. Pública. 2003, 14, 171–185. [Google Scholar] [CrossRef] [PubMed]
- Pantelaki, I.; Voutsa, D. Organophosphate flame retardants (OPFRs): A review on analytical methods and occurrence in wastewater and aquatic environment. Sci. Total Environ. 2019, 649, 247–263. [Google Scholar] [CrossRef] [PubMed]
- Yao, C.; Yang, H.; Li, Y. A review on organophosphate flame retardants in the environment: Occurrence, accumulation, metabolism and toxicity. Sci. Total Environ. 2021, 795, 148837. [Google Scholar] [CrossRef]
- Solvent Extraction in the Hydrometallurgical Processing and Purification of Metals: Process Design and Selected Applications. Available online: https://www.researchgate.net/publication/281136739_Solvent_extraction_in_the_hydrometallurgical_processing_and_purification_of_metals_process_design_and_selected_applications (accessed on 22 January 2025).
- Merroune, A.; Ait Brahim, J.; Berrada, M.; Essakhraoui, M.; Achiou, B.; Mazouz, H.; Beniazza, R. A comprehensive review on solvent extraction technologies of rare earth elements from different acidic media: Current challenges and future perspectives. J. Ind. Eng. Chem. 2024, 139, 1–17. [Google Scholar] [CrossRef]
- Keglevich, G. Organophosphorus Chemistry 2018; MDPI: Basel, Switzerland, 2020; p. 602. [Google Scholar]
- Bradford, T.E. Organophosphates in aircraft cabin air. Ph.D. Thesis, University of Central Lancashire, Preston, UK, 2023. Available online: https://www.uclan.ac.uk/research/index.php (accessed on 17 February 2025).
- Morodo, R.; Bianchi, P.; Monbaliu, J.C.M. Continuous Flow Organophosphorus Chemistry. Eur. J. Org. Chem. 2020, 2020, 5236–5277. [Google Scholar] [CrossRef]
- Eto, M. Organophosphorus Pesticides; CRC Press: Boca Raton, FL, USA, 2018; Available online: https://www.taylorfrancis.com/books/mono/10.1201/9781351075305/organophosphorus-pesticides-morifusa-eto (accessed on 17 February 2025).
- Vale, A.; Lotti, M. Organophosphorus and carbamate insecticide poisoning. Handb. Clin. Neurol. 2015, 31, 149–168. Available online: https://linkinghub.elsevier.com/retrieve/pii/B978044462627100010X (accessed on 17 February 2025).
- Petroianu, G.A. Synthesis of tetraethyl pyrophosphate (TEPP): From physician Abbot and pharmacist Riegel to chemist Nylen. Pharmazie 2015, 70, 427–434. [Google Scholar] [PubMed]
- Flett, D. Solvent extraction in hydrometallurgy: The role of organophosphorus extractants. J. Organomet. Chem. J. Organomet. Chem. 2005, 690, 2426–2438. [Google Scholar] [CrossRef]
- Costa, L.G. Organophosphorus Compounds at 80: Some Old and New Issues. Toxicol. Sci. 2018, 162, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Jacquet, P.; Poirier, L.; Daudé, D.; Chabrière, E. Intoxication aux organophosphorés: Vers des traitements enzymatiques. Ann. Pharm. Fr. 2019, 77, 349–362. [Google Scholar] [CrossRef]
- Camacho-Pérez, M.R.; Covantes-Rosales, C.E.; Toledo-Ibarra, G.A.; Mercado-Salgado, U.; Ponce-Regalado, M.D.; Díaz-Resendiz, K.J.G.; Girón-Pérez, M.I. Organophosphorus Pesticides as Modulating Substances of Inflammation through the Cholinergic Pathway. Int. J. Mol. Sci. 2022, 23, 4523. [Google Scholar] [CrossRef]
- Pereira, E.F.R.; Aracava, Y.; DeTolla, L.J.; Beecham, E.J.; Basinger, G.W.; Wakayama, E.J.; Albuquerque, E.X. Animal Models That Best Reproduce the Clinical Manifestations of Human Intoxication with Organophosphorus Compounds. J. Pharmacol. Exp. Ther. 2014, 350, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Chemical; Chemical & Engineering News. The Nazi Origins of Deadly Nerve Gases. Available online: https://cen.acs.org/articles/94/i41/Nazi-origins-deadly-nerve-gases.html (accessed on 22 February 2025).
- Burke, R.D.; Todd, S.W.; Lumsden, E.; Mullins, R.J.; Mamczarz, J.; Fawcett, W.P.; Albuquerque, E.X. Developmental neurotoxicity of the organophosphorus insecticide chlorpyrifos: From clinical findings to preclinical models and potential mechanisms. J. Neurochem. 2017, 142, 162–177. [Google Scholar] [CrossRef]
- Aghabiklooei, A.; Mostafazadeh, B.; Farzaneh, E.; Morteza, A. Review: Does organophosphate poisoning cause cardiac injury? Pak. J. Pharm. Sci. 2013, 26, 1247–1250. [Google Scholar]
- Black, R.M.; Harrison, J.M. PATAI’S Chemistry of Functional Groups. In The Chemistry of Organophosphorus Chemical Warfare Agents, 1st ed.; Hartley, F.R., Ed.; Wiley: Hoboken, NJ, USA, 1996; pp. 781–840. Available online: https://onlinelibrary.wiley.com/doi/10.1002/0470034351.ch10 (accessed on 17 February 2025).
- Yang, D. Quinone Methide Precursors as Resurrectors of Aged Acetylcholinesterase: A Synthetic Study of Quinone Methide Precursors. Ph.D. Thesis, The Ohio State University, Columbus, OH, USA, 2019. Available online: https://kb.osu.edu/items/56278dfb-5fa5-4107-87a8-22790fb16ecd (accessed on 17 February 2025).
- Kumar, V.; Kim, H.; Pandey, B.; James, T.D.; Yoon, J.; Anslyn, E.V. Recent advances in fluorescent and colorimetric chemosensors for the detection of chemical warfare agents: A legacy of the 21st century. Chem. Soc. Rev. 2023, 52, 663–704. [Google Scholar] [CrossRef]
- Chai, P.R.; Boyer, E.W.; Al-Nahhas, H.; Erickson, T.B. Toxic chemical weapons of assassination and warfare: Nerve agents VX and sarin. Toxicol. Commun. 2017, 1, 21–23. [Google Scholar] [CrossRef] [PubMed]
- Doyle, G. What Is VX Nerve Agent? A Deadly Weapon, Rarely Seen. The New York Times, 24 February 2017. Available online: https://www.nytimes.com/2017/02/24/world/asia/vx-nerve-agent-kim-jong-nam.html (accessed on 17 February 2025).
- Casida, J.E. Organophosphorus Xenobiotic Toxicology. Annu. Rev. Pharmacol. Toxicol. 2017, 57, 309–327. [Google Scholar] [CrossRef] [PubMed]
- Olivier, T.; Marine, B. Organophosphorous Compound—An Overview|ScienceDirect Topics. 2022. Available online: https://www.sciencedirect.com/topics/earth-and-planetary-sciences/organophosphorous-compound (accessed on 18 February 2025).
- Yudaev, P.A.; Kolpinskaya, N.A.; Chistyakov, E.M. Organophosphorous extractants for metals. Hydrometallurgy 2021, 201, 105558. [Google Scholar] [CrossRef]
- Ibrahim, T.H. An Overview of the Physiochemical Nature of Metal-Extractant Species in Organic Solvent/Acidic Organophosphorus Extraction Systems. Sep. Sci. Technol. 2011, 46, 2157–2166. [Google Scholar] [CrossRef]
- Batchu, N.K.; Li, Z.; Verbelen, B.; Binnemans, K. Structural effects of neutral organophosphorus extractants on solvent extraction of rare-earth elements from aqueous and non-aqueous nitrate solutions. Sep. Purif. Technol. 2021, 255, 117711. [Google Scholar] [CrossRef]
- Baes, C.F. The Synergistic Effects in Organophosphate Extraction Systems. Nucl. Sci. Eng. 1963, 16, 405–412. [Google Scholar] [CrossRef]
- Baumgartner, T.; Réau, R. Organophosphorus π-Conjugated Materials. Chem. Rev. 2006, 106, 4681–4727. [Google Scholar] [CrossRef]
- Ung, S.P.M.; Li, C.J. From rocks to bioactive compounds: A journey through the global P (V) organophosphorus industry and its sustainability. RSC Sustain. 2023, 1, 11–37. [Google Scholar] [CrossRef]
- Favre, H.A.; Powell, W.H.; Nomenclature of Organic Chemistry. The Royal Society of Chemistry. 2013. Available online: https://books.rsc.org/books/book/180/Nomenclature-of-Organic-Chemistry (accessed on 25 February 2025).
- Chemistry LibreTexts. 2015. 3.1: Functional Groups. Available online: https://chem.libretexts.org/Bookshelves/Organic_Chemistry/Organic_Chemistry_(Morsch_et_al.)/03%3A_Organic_Compounds-_Alkanes_and_Their_Stereochemistry/3.01%3A_Functional_Groups (accessed on 25 February 2025).
- Biochemical Nomenclature Committees, International Union of Biochemistry and Molecular Biology. Available online: https://iubmb.org/about/committees/biochemical-nomenclature-committees/ (accessed on 25 February 2025).
- IUBMB Nomenclature Home Page. Available online: https://iubmb.qmul.ac.uk/ (accessed on 25 February 2025).
- Steindl, D.; Boehmerle, W.; Körner, R.; Praeger, D.; Haug, M.; Nee, J.; Eckardt, K.U. Novichok nerve agent poisoning. Lancet 2021, 397, 249–252. [Google Scholar] [CrossRef]
- Ahmed, N.; Nassar, A. Lubricating Oil Additives. In Tribology—Lubricants and Lubrication; 2011; Available online: https://www.researchgate.net/publication/221918100_Lubricating_Oil_Additives (accessed on 13 June 2024). [CrossRef]
- Ouksel, L.; Bourzami, R.; Chafaa, S.; Chafai, N. Solvent and catalyst-free synthesis, corrosion protection, thermodynamic, MDS and DFT calculation of two environmentally friendly inhibitors: Bis-phosphonic acids. J. Mol. Struct. 2020, 1222, 128813. [Google Scholar] [CrossRef]
- Zhu, Y.; Liu, X.; Tang, Y.; Xu, K.; Tang, X.; Zhu, L.; Xiong, B. Recent Advances in the Synthesis of Commercially Available Phosphite Antioxidants. ChemistryOpen 2025, 14, e202400135. [Google Scholar] [CrossRef]
- Bahri, Z.; Razaghi Zonoz, T.; Zakeri Khatir, M. Removal of organic reagent from synthetic wastewater using ion flotation. Iran. J. Chem. Chem. Eng. Res. Artic. Vol. 2023, 42, 810–820. Available online: https://www.ijcce.ac.ir/index.php/article_252607_f89dfee27082d428cbdf42dc23d686ed.pdf (accessed on 23 April 2025).
- A Global Analysis of Research Outputs on Neurotoxicants from 2011–2020: Adverse Effects on Humans and the Environment. Available online: https://www.mdpi.com/2076-3417/12/16/8275 (accessed on 26 February 2025).
- Ahlmann, M.; Hempel, G. The effect of cyclophosphamide on the immune system: Implications for clinical cancer therapy. Cancer Chemother. Pharmacol. 2016, 78, 661–671. [Google Scholar] [CrossRef] [PubMed]
- CoLab DFP in the Handling of Esotropia. Available online: https://colab.ws/articles/10.1080%2F0065955x.1965.11981462 (accessed on 26 February 2025).
- CoLab Clinical Experience with Isoflurophate as an AID in Orthoptic Treatment. Available online: https://colab.ws/articles/10.1080%2F0065955x.1960.11981318 (accessed on 26 February 2025).
- Mechanisms of Action of Bisphosphonates|Annual Reviews. Available online: https://www.annualreviews.org/content/journals/10.1146/annurev.pharmtox.38.1.375 (accessed on 26 February 2025).
- El-Ebiary, A.A.; Elsharkawy, R.E.; Soliman, N.A.; Soliman, M.A.; Hashem, A.A. N-acetylcysteine in Acute Organophosphorus Pesticide Poisoning: A Randomized, Clinical Trial. Basic. Clin. Pharmacol. Toxicol. 2016, 119, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Qiu, X. RAITANIR Processes for Removing Heavy Metals from Phosphoric Acid Solutions. EP3691992B1. 2022. Available online: https://patents.google.com/patent/EP3691992B1/en (accessed on 31 August 2024).
- Samrane, K.; Latifi, M.; Khajouei, M.; Bouhaouss, A. Comprehensive analysis and relevant developments of cadmium removal technologies in fertilizers industry. Miner. Eng. 2023, 201, 108189. [Google Scholar] [CrossRef]
- Modeling of the Extraction of Uranium (VI) from Concentrated Phosphoric Acid by Synergistic Mixtures of Bis-(2-Ethylhexyl)-Phosphoric Acid and Tri-N-Octylphosphine Oxide. Available online: https://www.researchgate.net/publication/257131173_Modeling_of_the_extraction_of_uranium_VI_from_concentrated_phosphoric_acid_by_synergistic_mixtures_of_bis-2-ethylhexyl-phosphoric_acid_and_tri-n-octylphosphine_oxide (accessed on 26 February 2025).
- Ritcey, G.M.; Ashbrook, A.W. Solvent Extraction: Principles and Applications to Process Metallurgy; Elsevier Scientific Publishing Company: Amsterdam, The Netherlands, 1984; pp. 386–737. Available online: https://books.google.co.ma/books/about/Solvent_Extraction_Principles_and_Applic.html?id=IP7mxAEACAAJ&redir_esc=y (accessed on 26 February 2025).
- CoLab, A.N. Frumkin Institute of Physical Chemistry and Electrochemistry of the Russian Academy of Sciences. Available online: https://colab.ws/organizations/004dzkd51 (accessed on 26 February 2025).
- Kathryn, H.S.; Jilska, M.P.; Geoff, S. Chapter 6: Equipment in Solvent Extraction Processing. In Solvent Extraction, Principles and Applications to Process Metallurgy, 2nd ed.; ResearchGate; 2006; pp. 253–419. Available online: https://www.researchgate.net/publication/259742607_’Solvent_Extraction_Principles_and_Applications_to_Process_Metallurgy’ (accessed on 26 March 2025).
- Tiei Liquid/Liquid Mixing and Separation. Types of Equipment for Liquid Liquid Extraction and Liquid Liquid Extraction Method. Available online: https://www.tyextractor.com/product/ (accessed on 27 February 2025).
- Basualto, C.; Valenzuela, F.; Molina, L.; Munoz, J.P.; Fuentes, E.; Sapag, J. Study of the solvent extraction of the lighter lanthanide metal ions by means of organophosphorus extractants. J. Chil. Chem. Soc. 2012, 58, 1785–1789. [Google Scholar] [CrossRef]
- Kedari, C.; Coll, T.; Fortuny, A.; Sastre, A. Third Phase Formation in the Solvent Extraction System Ir(IV)—Cyanex 923. Solvent. Extr. Ion. Exch. 2005, 23, 545–559. [Google Scholar] [CrossRef]
- Suresh, A.; Rao, C.B.; Srinivasulu, B.; Sreenivasan, N.L.; Subramaniam, S.; Sabharwal, K.N.; Sivaraman, N.; Srinivasan, T.G.; Natarajan, R.; Rao, P.V. Development of Alternate Extractants for Separation of Actinides. Energy Procedia 2013, 39, 120–126. [Google Scholar] [CrossRef]
- Draa, M.; Belaid, T.; Benamor, M. Extraction of Pb(II) by XAD7 impregnated resins with organophosphorus extractants (DEHPA, Ionquest 801, Cyanex 272). Sep. Purif. Technol. 2004, 40, 77–86. [Google Scholar] [CrossRef]
- Mellah, A.; Benachour, D. The solvent extraction of zinc, cadmium and chromium from phosphoric acid solutions by tri-n butyl phosphate in kerosene diluent. Sep. Purif. Technol. 2007, 56, 220–224. [Google Scholar] [CrossRef]
- Zhang, P.; You, S.; Zhang, L.; Feng, S.; Hou, S. A solvent extraction process for the preparation of ultrahigh purity scandium oxide. Hydrometallurgy 1997, 47, 47–56. [Google Scholar] [CrossRef]
- Thakur, N.V. Extraction studies of base metals (Mn, Cu, Co and Ni) using the extractant 2-ethylhexyl 2-ethylhexyl phosphonic acid, PC 88A. Hydrometallurgy 1998, 48, 125–131. [Google Scholar] [CrossRef]
- Yacouba, A.R.C.; Ibrahim, S.L.; Mamane, O.S.; Mohamed, M.; Natatou, I. Extraction liquide-liquide de l’uranium et de quelques métaux restrictifs issus des jus uranifères de la République du Niger par l’acide di-2-éthylhexylphosphorique et l’oxyde de trioctylphosphine. Int. J. Biol. Chem. Sci. 2018, 12, 2424–2437. [Google Scholar] [CrossRef]
- Li, D.; Wang, C. Solvent extraction of Scandium(III) by Cyanex 923 and Cyanex 925. Hydrometallurgy 1998, 48, 301–312. [Google Scholar] [CrossRef]
- Zhao, Z.; Cui, L.; Guo, Y.; Li, H.; Cheng, F. Recovery of gallium from sulfuric acid leach liquor of coal fly ash by stepwise separation using P507 and Cyanex 272. Chem. Eng. J. 2020, 381, 122699. [Google Scholar] [CrossRef]
- Tait, B.K. The extraction of some base metal ions by Cyanex 301, Cyanex 302 and their binary extractant mixtures with Aliquat 336. Solvent Extr. Ion. Exch. 1992, 10, 799–809. [Google Scholar] [CrossRef]
- Bessiere, J.; Bruant, M.; Jdid, E.A.; Blazy, P. Flottation ionique du cadmium et de l’arsenic par un dithiophosphate dans les solutions concentrées en acide phosphorique. Int. J. Miner. Process 1986, 16, 63–74. [Google Scholar] [CrossRef]
- Jdid, E.A.; Blazy, P.; Bessiere, J.; Floreancig, A. Process for the Purification of Wet-Process Phosphoric Acid by Removal of Cadmium. US4634580A. 1987. Available online: https://patents.google.com/patent/US4634580A/en (accessed on 31 March 2025).
- Kouzbour, S.; Gourich, B.; Gros, F.; Vial, C.; Allam, F.; Stiriba, Y. Comparative analysis of industrial processes for cadmium removal from phosphoric acid: A review. Hydrometallurgy 2019, 188, 222–247. [Google Scholar] [CrossRef]
- Merred, D.; Nagaraj, D.R. Procede Pour Eliminer Les Impuretes Metalliques Contenues Dans De L’acide Phosphorique De Procedes Par Voie Humide Et Compositions Correspondantes. MA27719A1. 2006. pp. 1–19. Available online: http://patent.ompic.ma/publication-server/html-document?PN=MA27719%20MA%2027719&iDocId=4549 (accessed on 15 December 2024).
- Rickelton, W.A. Phosphine and Its Derivatives. In Kirk-Othmer Encyclopedia of Chemical Technology; American Cancer Society: Atlanta, GA, USA, 2000; Available online: https://onlinelibrary.wiley.com/doi/abs/10.1002/0471238961.1608151918090311.a01 (accessed on 31 March 2025).
- Bierman, L.W.; Polinsky, S.M.; Hempel, D.A.; Humberger, R.B. Process for the Recovery of Cadmium and Other Metals from Solution. US4511541A. 1985. Available online: https://patents.google.com/patent/US4511541A/en (accessed on 31 March 2025).
- Rahmati, S.; Adavodi, R.; Hosseini, M.R.; Veglio’, F. Efficient Metal Extraction from Dilute Solutions: A Review of Novel Selective Separation Methods and Their Applications. Metals 2024, 14, 605. [Google Scholar] [CrossRef]
- Wang, L.; Yu, Y.; Huang, X.; Hu, F.; Dong, J.; Yan, L.; Long, Z. Thermodynamics and kinetics of thorium extraction from sulfuric acid medium by HEH (EHP). Hydrometallurgy 2014, 150, 167–172. [Google Scholar] [CrossRef]
- Atanassova, M. Assessment of the equilibrium constants of mixed complexes of rare earth elements with acidic (chelating) and organophosphorus ligands. Separations 2022, 9, 371. [Google Scholar] [CrossRef]
- Soo Kim, J.; Nagaphani Kumar, B.; Lee, J.Y.; Lakshmi Kantam, M.; Ramachandra Reddy, B. Separation and Recovery of Light Rare-Earths from Chloride Solutions using Organophosphorus based Extractants. Sep. Sci. Technol. 2012, 47, 1644–1650. [Google Scholar] [CrossRef]
- Jha, M.K.; Kumar, V.; Jeong, J.; Lee, J.C. Review on solvent extraction of cadmium from various solutions. Hydrometallurgy 2012, 111, 1–9. [Google Scholar] [CrossRef]
- Rodrigues, I.; Deferm, C.; Binnemans, K.; Riaño, S. Separation of cobalt and nickel via solvent extraction with Cyanex-272: Batch experiments and comparison of mixer-settlers and an agitated column as contactors for continuous counter-current extraction. Sep. Purif. Technol. 2022, 296, 121326. [Google Scholar] [CrossRef]
- Hasanzadeh, N.; Nori-Shargh, D. Correlations between hardness, electronegativity, anomeric effect associated with electron delocalizations and electrostatic interactions in 1,4,5,8-tetraoxadecalin and its analogs containing S and Se atoms. Comput. Theor. Chem. 2015, 1051, 1–9. [Google Scholar] [CrossRef]
- Chaquin, P. Absolute electronegativity and hardness: An analogy with classical electrostatics suggests an interpretation of the Parr ‘electrophilicity index’ as a ‘global energy index’ leading to the ‘minimum electrophilicity principle. ’ Chem. Phys. Lett—Chem. Phys. Lett. 2008, 458, 231–234. [Google Scholar] [CrossRef]
- Ritter, S. Hard and Soft Acids and Bases. Chemical & Engineering News. 2003. Available online: https://www.researchgate.net/publication/294637616_Hard_and_soft_acids_and_bases (accessed on 26 February 2025).
- Kathryn Newton. Pearson’s Hard-Soft Acid-Base Concept—Chemistry LibreTexts. In Chemistry of The Elements, Fall. 2023, p. 552. Available online: https://chem.libretexts.org/Courses/Northern_Michigan_University/CH_215%3A_Chemistry_of_the_Elements_Fall_2023/02%3A_Acid-Base_and_Donor-Acceptor_Chemistry/2.07%3A_The_Hard-Soft_Acid-Base_Concept/2.7.02%3A_Pearson%27s_Hard-Soft_Acid-Base_Concept (accessed on 27 February 2025).
- Omelchuk, K.; Szczepański, P.; Shrotre, A.; Haddad, M.; Chagnes, A. Effects of structural changes of new organophosphorus cationic exchangers on a solvent extraction of cobalt, nickel and manganese from acidic chloride media. RSC Adv. 2017, 7, 5660–5668. [Google Scholar] [CrossRef]
- Tercero, N.; Nagaraj, D.; Farinato, R. A Critical Overview of Dithiophosphinate and Dithiophosphate Interactions with Base Metal Sulfides and Precious Metals. Min. Metall. Explor. 2019, 36, 1–12. [Google Scholar] [CrossRef]
- Nguyen, V.N.H.; Nguyen, T.H.; Lee, M.S. Review on the Comparison of the Chemical Reactivity of Cyanex 272, Cyanex 301 and Cyanex 302 for Their Application to Metal Separation from Acid Media. Metals 2020, 10, 1105. [Google Scholar] [CrossRef]
- Hermann, L.; Schneider, G.; Lohrberg, D. Procédé et Installation de Séparation de Métaux Lourds à Partir d’une Matière Première Phosphorique. WO2014177228A1. 2014. Available online: https://patents.google.com/patent/WO2014177228A1/fr (accessed on 27 February 2025).
- Corrosion Behaviour of Copper in Sulphuric Acid in the Presence of N-Methylaniline. Available online: https://www.researchgate.net/publication/299354466_Corrosion_Behaviour_of_Copper_in_Sulphuric_Acid_in_the_Presence_of_N-Methylaniline (accessed on 27 February 2025).
- Alimarin, I.P.; Tat’yana, V.R.; Ivanov, V.M. Extraction with thio and dithio phosphorus acids. Russ. Chem. Rev. 1989, 58, 863. [Google Scholar] [CrossRef]
- Almela, A.; Elizalde, M.P. Solvent extraction of cadmium (II) from acidic media by Cyanex 302. Hydrometallurgy 1995, 37, 47–57. [Google Scholar] [CrossRef]
- Zhou, L.; Zhang, Y.; Zhang, L.; Wu, X.; Jiang, R.; Wang, L. High-value recovery of the iron via solvent extraction from waste nickel-cadmium battery sulfuric acid leachate using saponified D2EHPA. Separations 2023, 10, 251. [Google Scholar] [CrossRef]
- Ayyavoo, S.; Kandasamy, P.; Ramasamy, S. Removal of Mercury in Aqueous Solutions Using Tri n-Butyl Phosphate-Based Polymer Inclusion Membrane. Environ. Eng. Sci. 2022, 39, 650–661. [Google Scholar] [CrossRef]
- Kaur, K.; Singh, G.; Kaur, N.; Singh, N. Cation–π-induced mixed-matrix nanocomposite for the detection and removal of Hg 2+ and azinphos-methyl towards environment remediation. Environ. Sci. Water Res. Technol. 2024, 10, 1595–1609. [Google Scholar] [CrossRef]
- Kuvayskaya, A. Synthesis and Applications of Organophosphonic Acid Compounds as Extractants for Rare Earth Element Separation and Beyond. Ph.D. Thesis, Colorado School of Mines, Golden, CO, USA, 2024. Available online: https://search.proquest.com/openview/c5668b4405f333bc7d3325f46469c325/1?pq-origsite=gscholar&cbl=18750&diss=y (accessed on 27 April 2025).
- Mohammadzadeh, M.; Bagheri, H.; Ghader, S. Solvent Extraction of Nickel and Zinc from Nitric Acid Solution Using D2EHPA: Experimental and Modeling. J. Solut. Chem. 2022, 51, 424–447. [Google Scholar] [CrossRef]
- Zhang, L.; Song, X.; Shao, X.; Wu, Y.; Zhang, X.; Wang, S.; Li, Z. Lead immobilization assisted by fungal decomposition of organophosphate under various pH values. Sci. Rep. 2019, 9, 13353. [Google Scholar] [CrossRef]
- Azzoug, S.; Arous, O.; Kerdjoudj, H. Cadmium (II) and Lead (II) Extraction and Transport in Supported Liquid Membrane Using TOPO and D2EHPA as Mobile Carriers. 2009. Available online: https://www.cabidigitallibrary.org/doi/full/10.5555/20103012981 (accessed on 28 April 2025).
- Juang, R.S.; Chang, Y.T. Kinetics and mechanism for copper(II) extraction from sulfate solutions with bis(2-ethylhexyl)phosphoric acid. Ind. Eng. Chem. Res. 1993, 32, 207–213. [Google Scholar] [CrossRef]
- Purification of Phosphoric Acid by Minimizing Iron, Copper, Cadmium and Fluoride—ScienceDirect. Available online: https://www.sciencedirect.com/science/article/abs/pii/S1383586607005114 (accessed on 30 August 2024).
- Hall, R.E.; Goyden, D.D. Process for Removing Iron, Chromium and Vanadium from Phosphoric acid. US5006319A. 1991. Available online: https://patents.google.com/patent/US5006319A/en (accessed on 30 August 2024).
- Gupta, B.; Begum, Z. Separation and removal of arsenic from metallurgical solutions using bis (2, 4, 4-trimethylpentyl) dithiophosphinic acid as extractant. Sep. Purif. Technol. 2008, 63, 77–85. [Google Scholar] [CrossRef]
- Capanema, R. Mining Chemicals Handbook; Cytec Industries Incorporated: Woodland Park, NJ, USA, 2009; p. 507. [Google Scholar]
- Separation of Cobalt and Nickel from Chloride Leach Solution of Nickel Laterite Ore by Solvent Extraction|Request PDF. Available online: https://www.researchgate.net/publication/299569605_Separation_of_cobalt_and_nickel_from_chloride_leach_solution_of_nickel_laterite_ore_by_solvent_extraction (accessed on 23 April 2025).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).