Research on Mechanism of Methanol–Hydrogen Co-Transport Inhibiting Hydrogen Embrittlement in Pipeline Steel
Abstract
1. Introduction
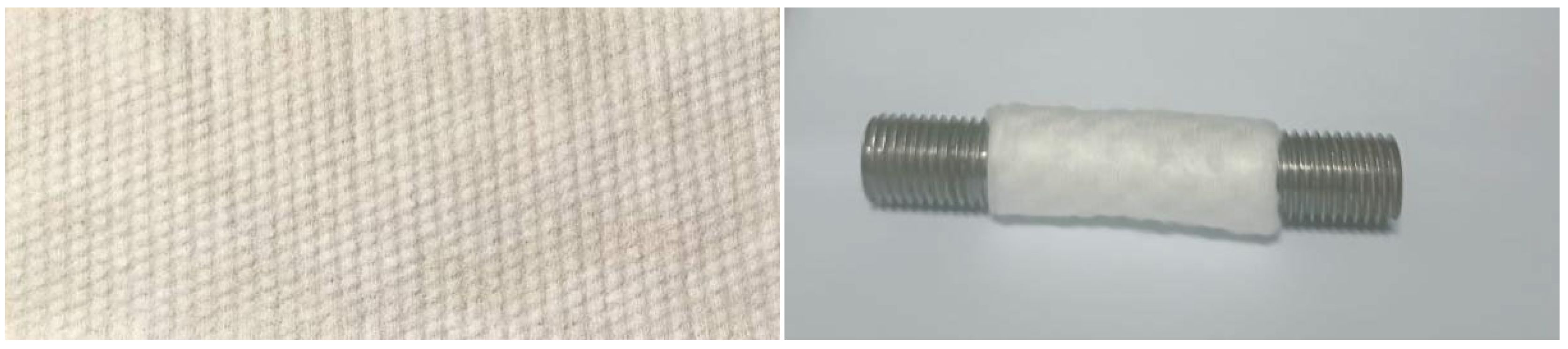
2. Experimental Materials and Methods
2.1. Metal Round Rod Material
2.2. Experimental Environment
2.3. Experimental Facilities
2.4. Experimental Methods
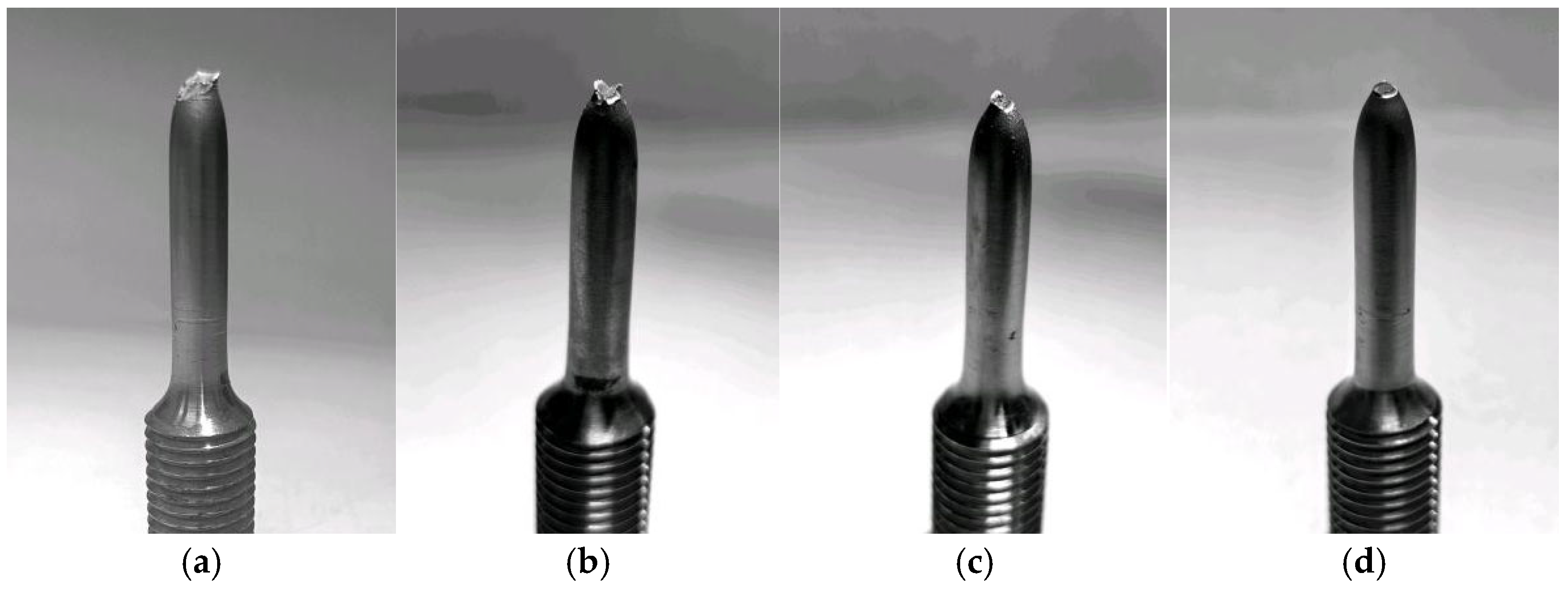
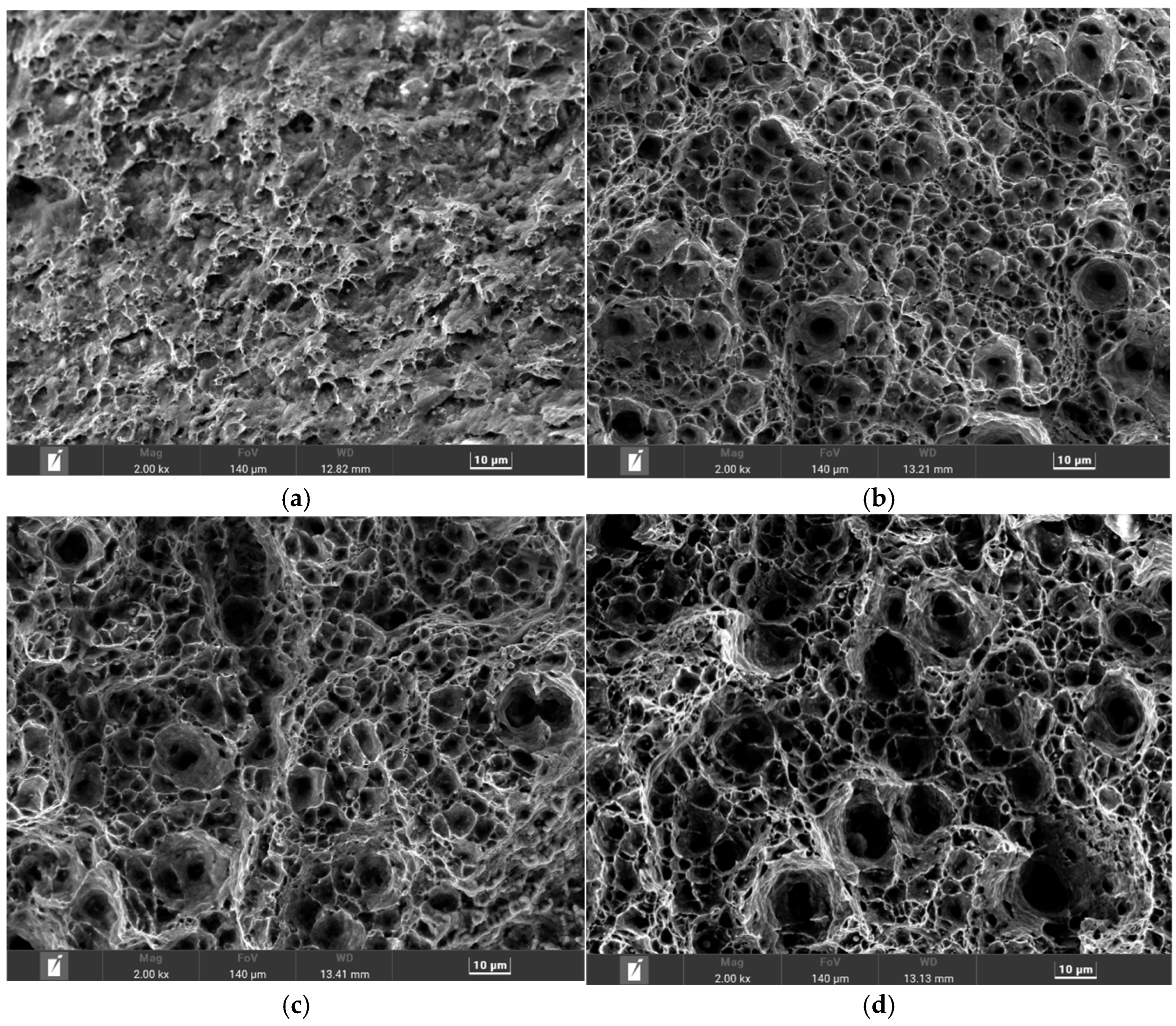
3. Experiment Results and Analyses
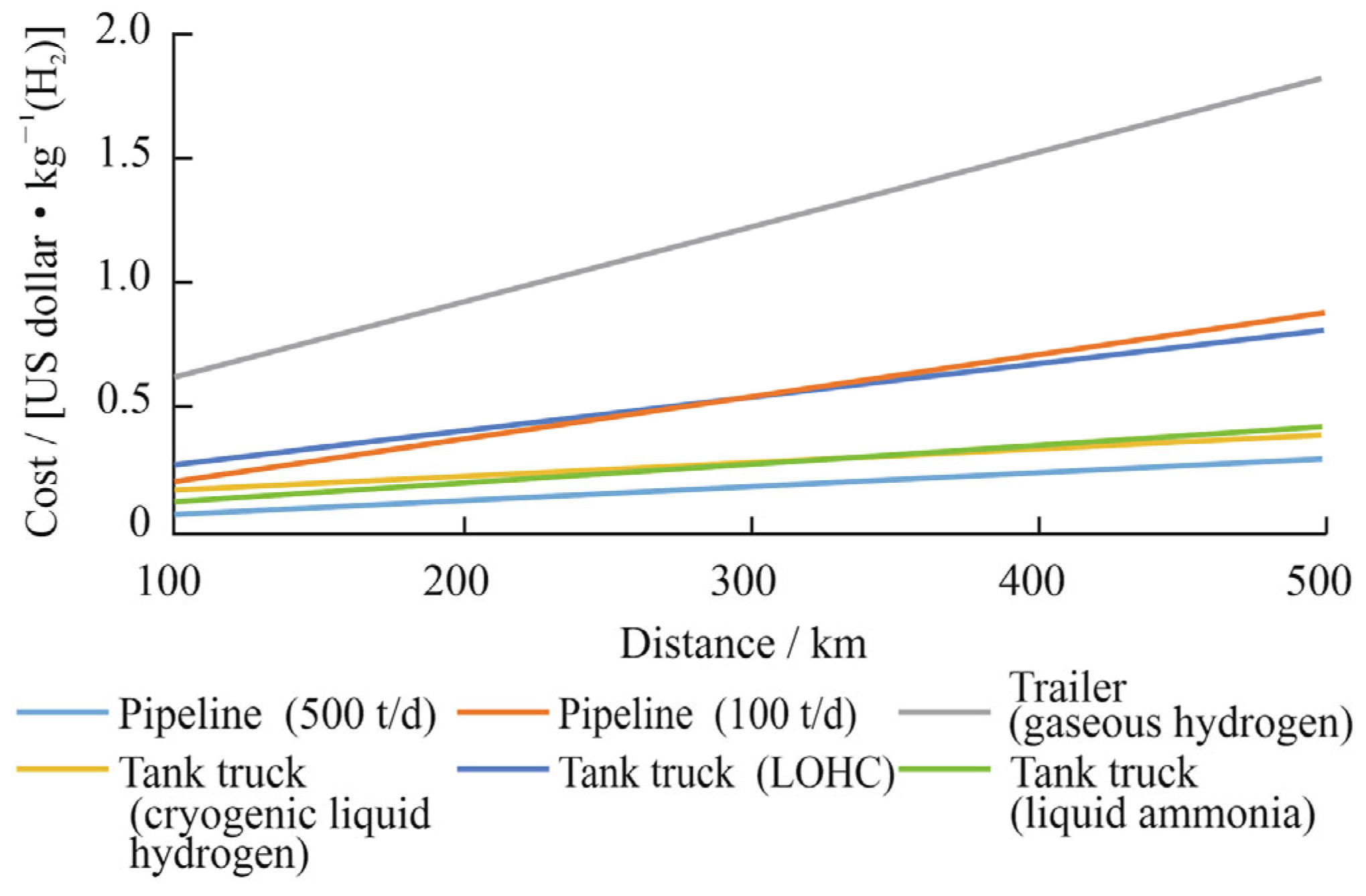
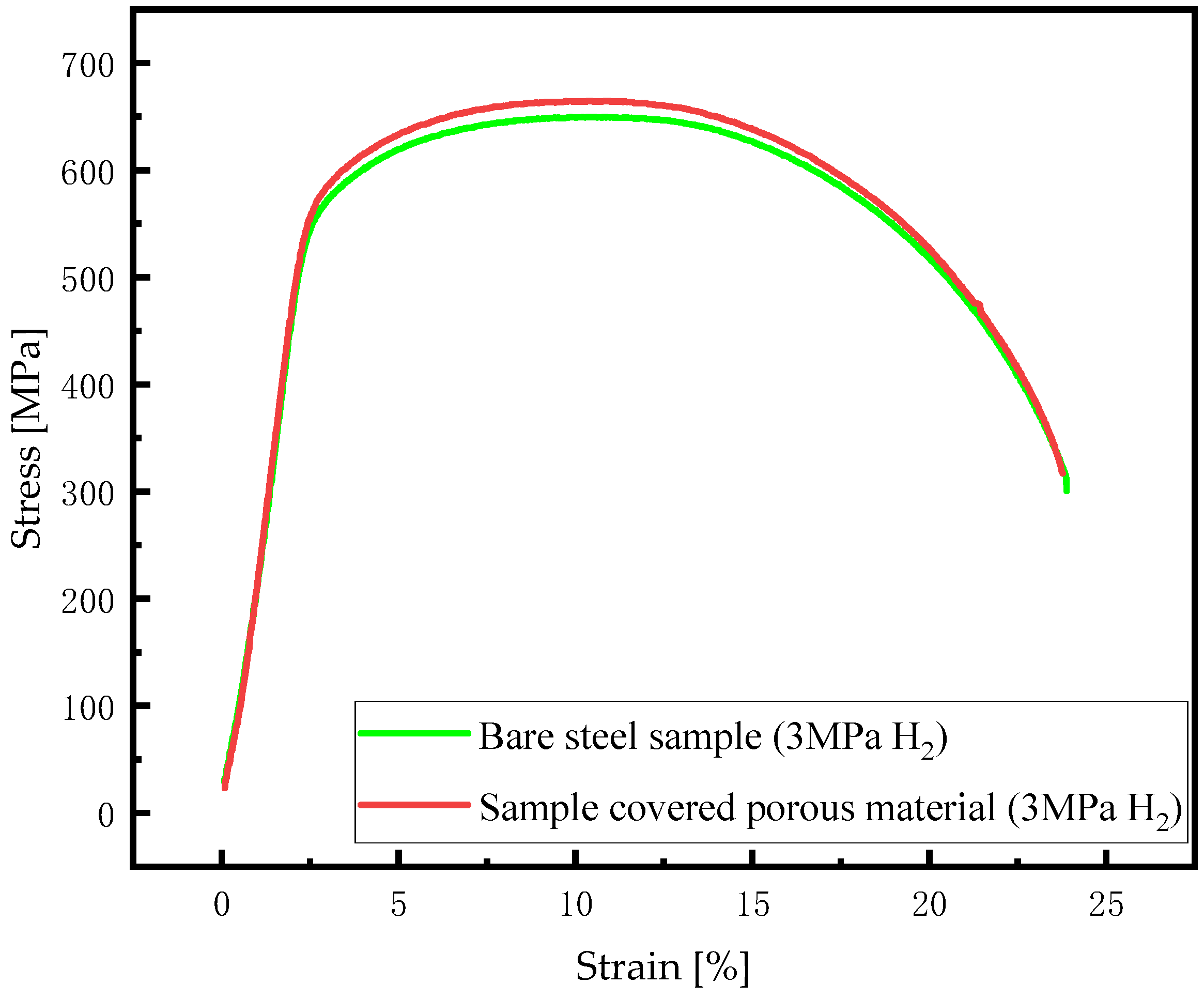
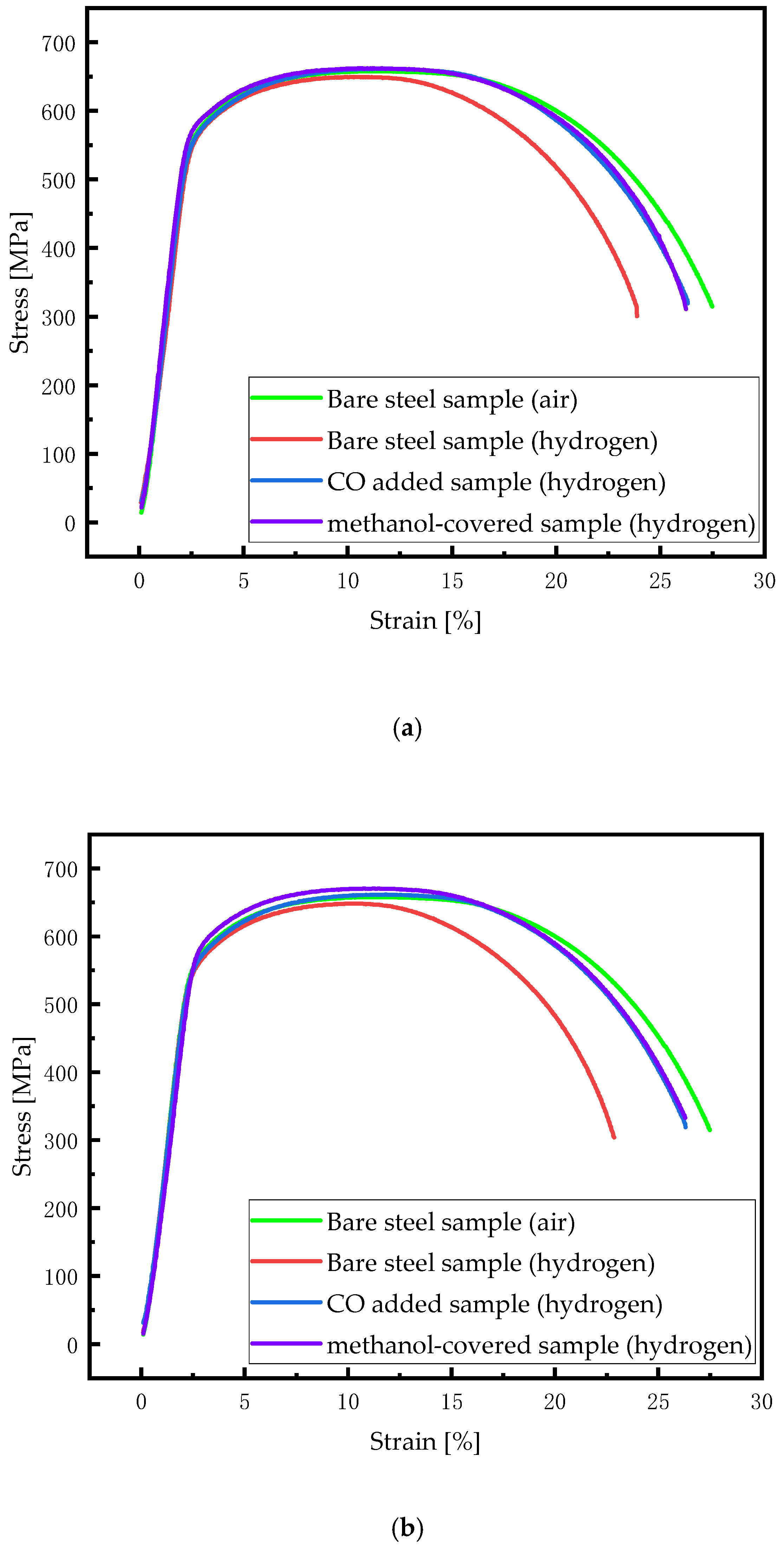
3.1. Effect of Porous Material on Hydrogen Embrittlement Behavior of X80 Steel
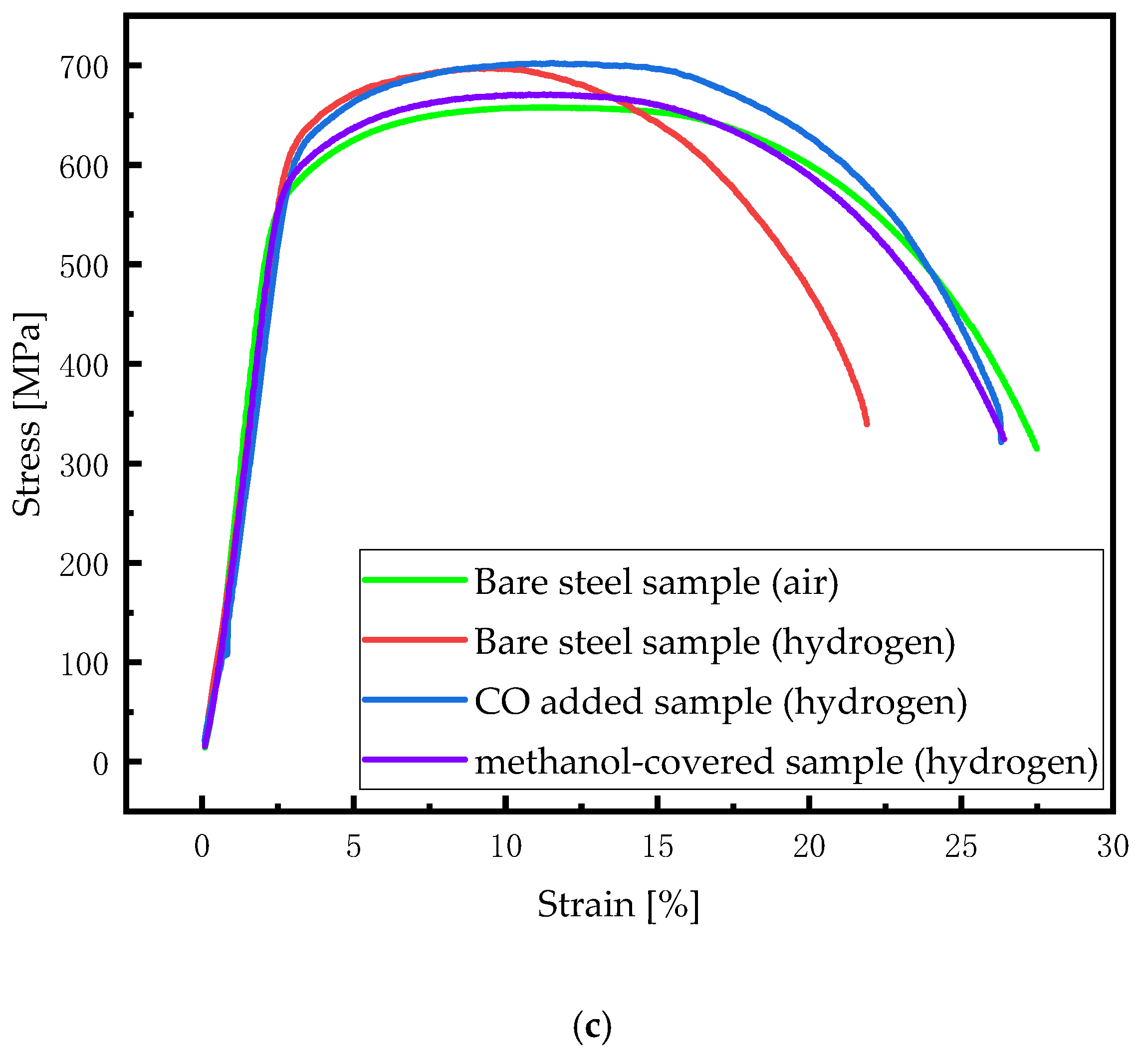
3.2. Effect of Methanol on Mechanical Properties of Pipeline Steel in Hydrogen Environment
4. Analysis of Methanol’s Mechanism
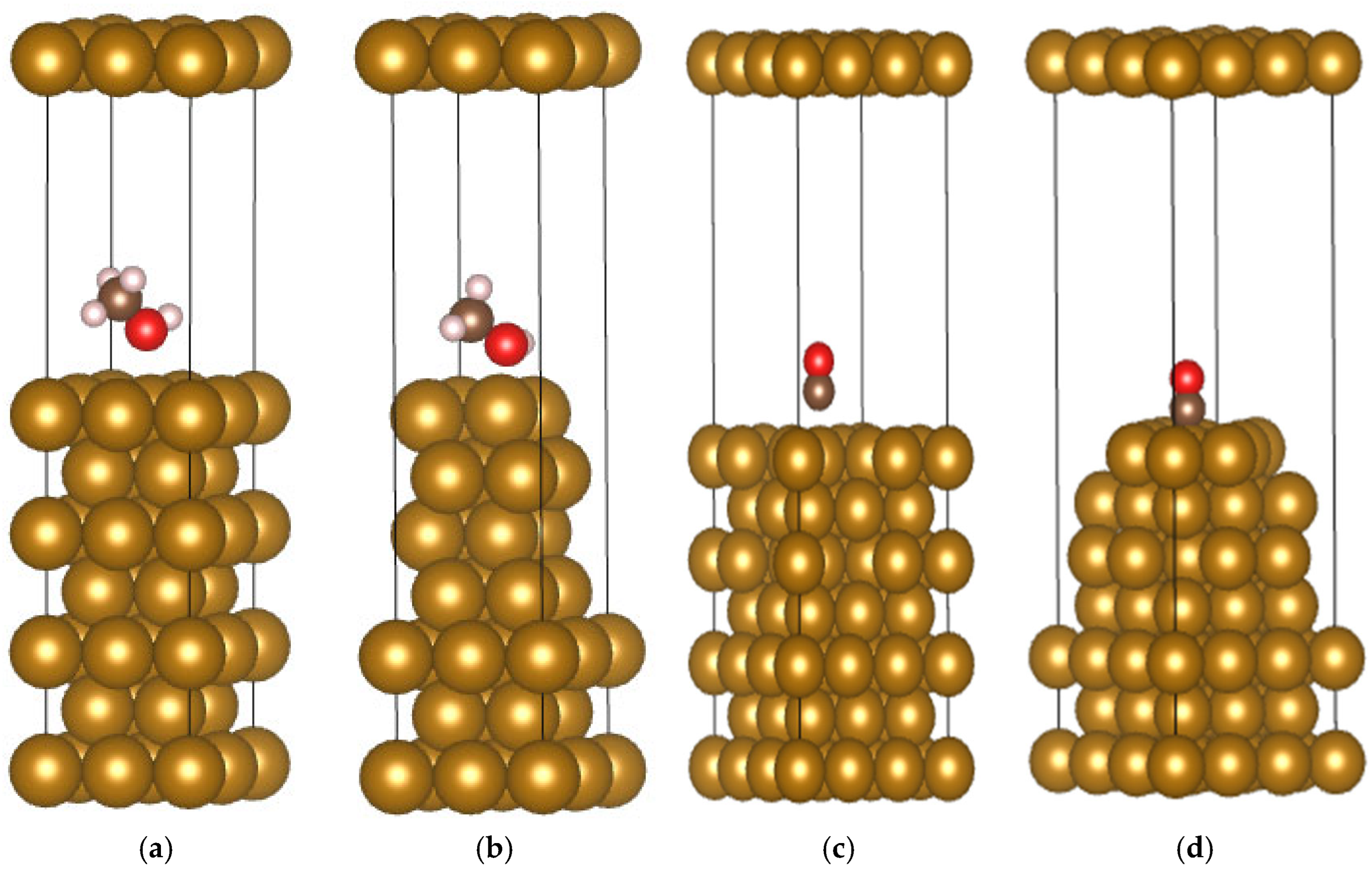
4.1. Adsorption Behavior of Methanol on Pipeline Steel Surfaces
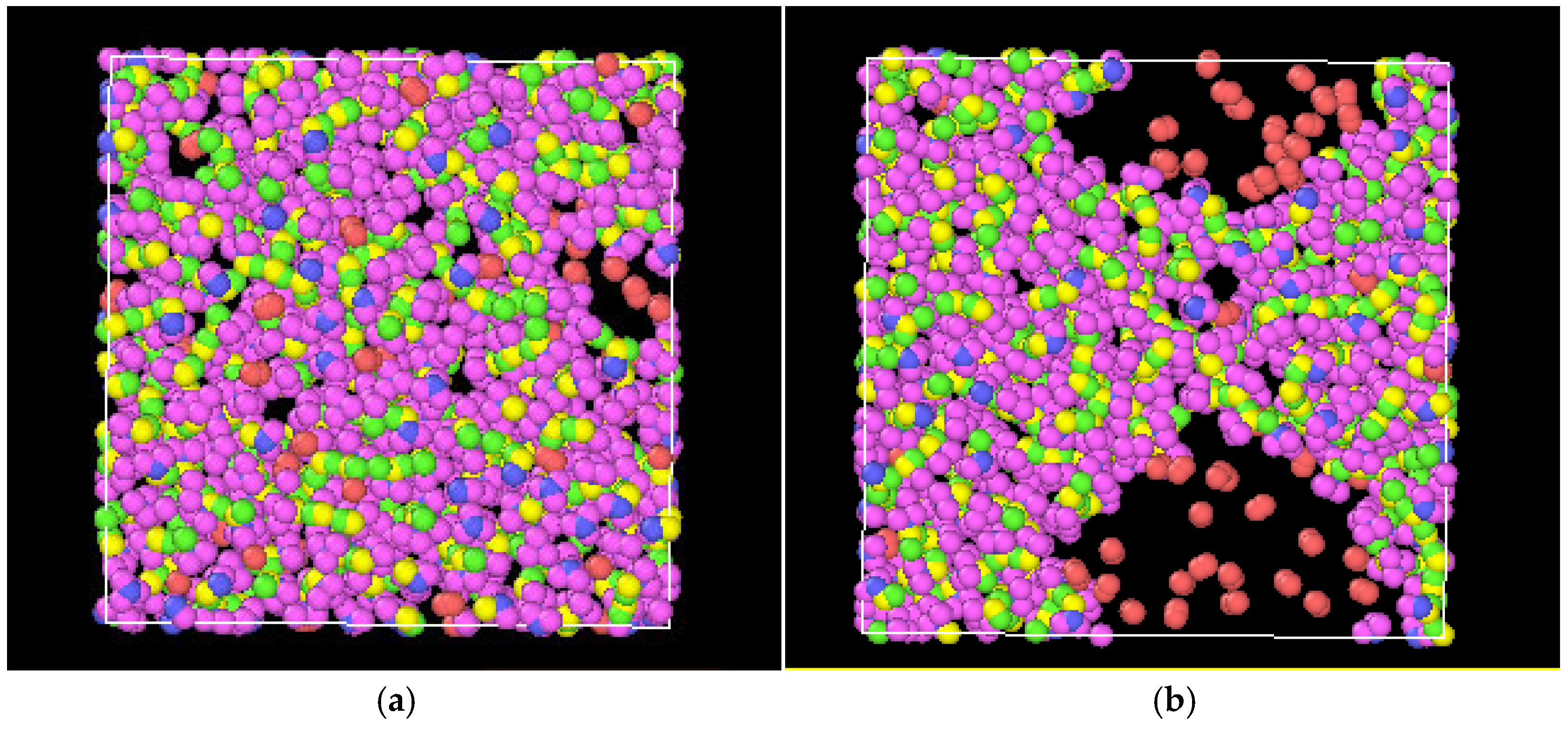
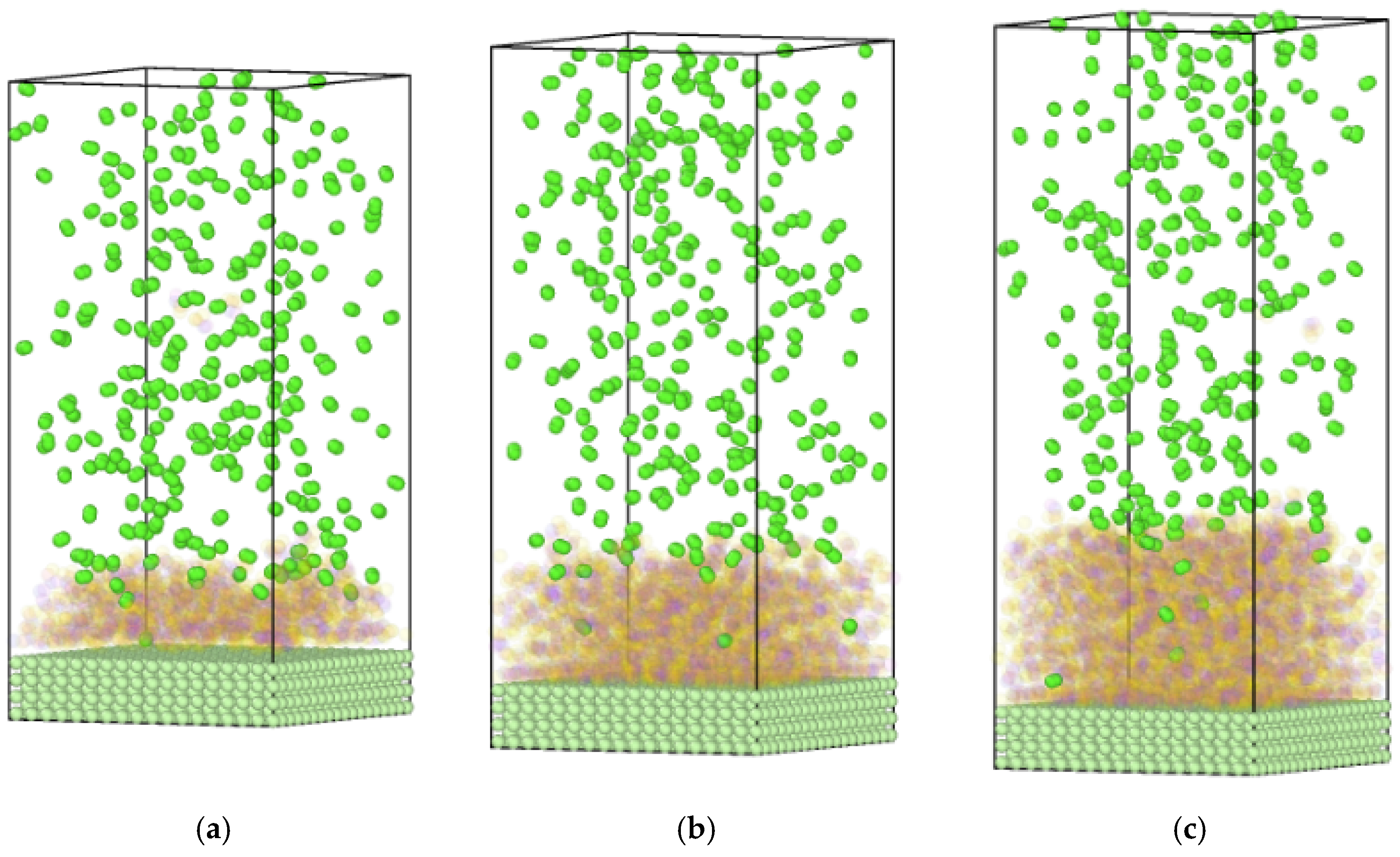
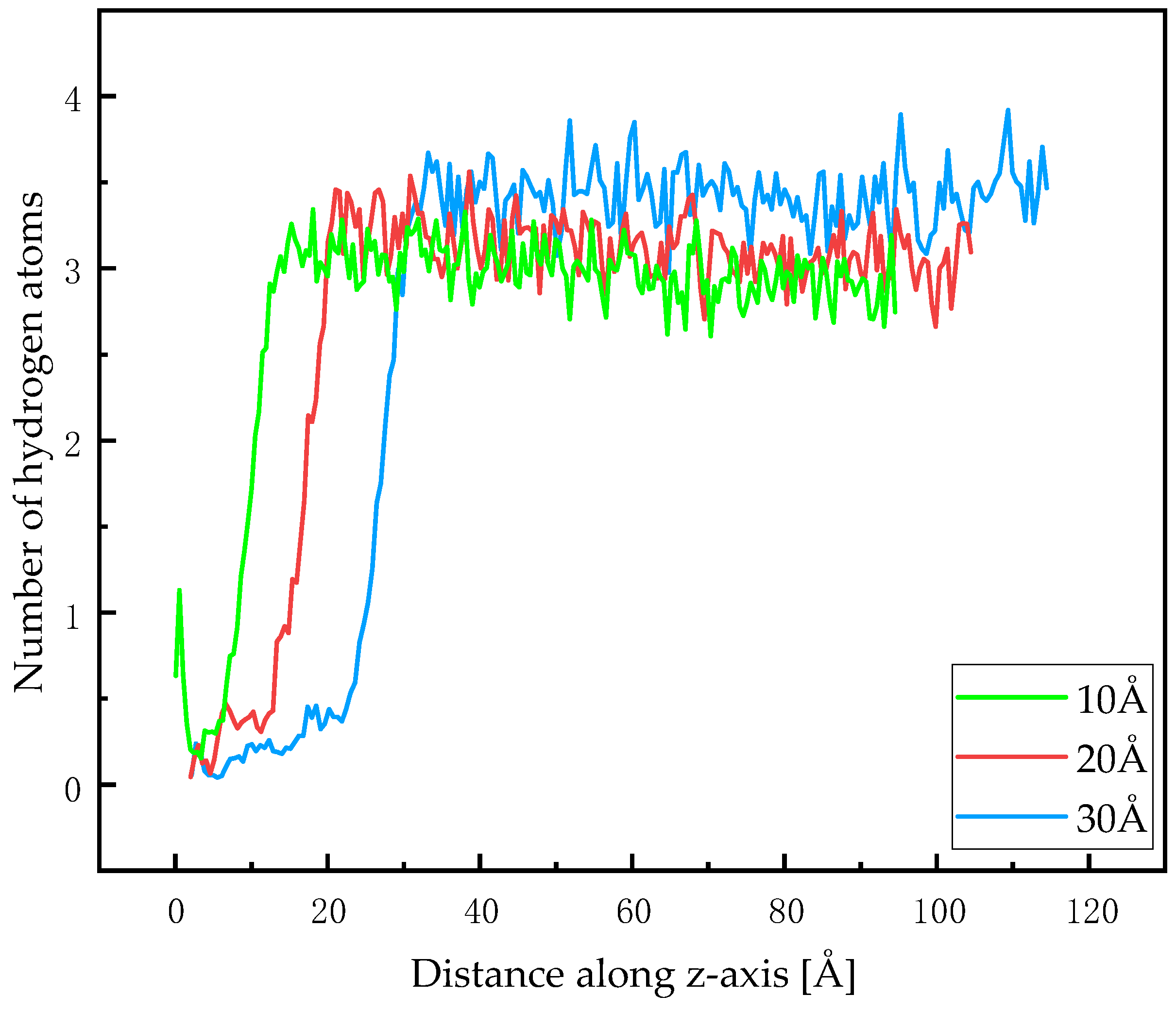
4.2. Diffusion Behavior of Hydrogen in Methanol Liquid Films
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| HIC | Hydrogen-induced cracking |
| HEI | Hydrogen embrittlement index |
| FS | Fracture strain |
| DFT | Density functional theory |
| PBE | Perdew Burke Ernzerhof |
| GCA | Generalized gradient approximation |
References
- U.S. Department of Energy. Hydrogen Strategy Enabling a Low-Carbon Economy; U.S. Department of Energy: Washington, DC, USA, 2020; pp. 51–52.
- Zou, C.; Xiong, B.; Xue, H.; Zheng, D.; Ge, Z.; Wang, Y.; Jiang, L.; Pan, S.; Wu, S. The role of new energy in carbon neutral. Pet. Explor. Dev. 2021, 48, 480–491. [Google Scholar] [CrossRef]
- Demir, M.E.; Dincer, I. Cost assessment and evaluation of various hydrogen delivery scenarios. Int. J. Hydrogen Energy 2017, 43, 10420–10430. [Google Scholar] [CrossRef]
- Cheng, Y.F. Hydrogen transport in aged pipelines II. Technical assessment of the susceptibility to hydrogen embrittlement. In Proceedings of the AMPP/ASM International Calgary Chapter Luncheon, Calgary, AB, Canada, 15 November 2022; p. 8. [Google Scholar]
- Ohaeri, E.; Eduok, U.; Szpunar, J. Hydrogen related degradation in pipeline steel: A review. Int. J. Hydrogen Energy 2018, 43, 14584–14617. [Google Scholar] [CrossRef]
- Cazenave, P.; Jimenez, K.; Gao, M.; Moneta, A.; Hryciuk, P. Hydrogen assisted cracking driven by cathodic protection operated at near−1,200 mV CSE—An onshore natural gas pipeline failure. J. Pipeline Sci. Eng. 2021, 1, 100–121. [Google Scholar] [CrossRef]
- Li, K.; Zeng, Y.; Luo, J. Influence of H2S on the general corrosion and sulfide stress cracking of pipeline steels for supercritical CO2 transportation. Corros. Sci. 2021, 190, 109639. [Google Scholar] [CrossRef]
- Robertson, I.M.; Sofronis, P.; Nagao, A.; Martin, M.L.; Wang, S.; Gross, D.W.; Nygren, K.E. Hydrogen embrittlement understood. Metall. Mater. Trans. B 2015, 46, 1085–1103. [Google Scholar] [CrossRef]
- Wang, J.; Xu, N.; Wu, T.; Xi, X.; Wang, G.; Chen, L. Response of hydrogen diffusion and hydrogen embrittlement to Cu addition in low carbon low alloy steel. Mater. Charact. 2023, 195, 112478. [Google Scholar] [CrossRef]
- Lv, W.; Yu, W.; Wu, Z.; Yan, Y.; Shi, J.; Wang, M. Hydrogen Embrittlement Susceptibility of a Newly Developed Grain-Refined Ultra-High Strength Steel. Materials 2025, 18, 987. [Google Scholar] [CrossRef]
- Chen, Y.S.; Huang, C.; Liu, P.Y.; Yen, H.W.; Niu, R.; Burr, P.; Moore, K.L.; Martínez-Pañeda, E.; Atrens, A.; Cairney, J.M. Hydrogen trapping and embrittlement in metals—A review. Int. J. Hydrogen Energy 2024. [CrossRef]
- Wan, H.; Min, W.; Song, D.; Chen, C. Research progress of hydrogen blocking coatings. Mater. Chem. Phys. 2024, 328, 130028. [Google Scholar] [CrossRef]
- Röthig, M.; Hoschke, J.; Tapia, C.; Venezuela, J.; Atrens, A. A review of gas phase inhibition of gaseous hydrogen embrittlement in pipeline steels. Int. J. Hydrogen Energy 2024, 60, 1239–1265. [Google Scholar] [CrossRef]
- Zou, C.; Li, J.; Zhang, X.; Jin, X.; Xiong, B.; Yu, H.; Liu, X.; Wang, S.; Li, Y.; Zhang, L.; et al. Industrial status, technological progress, challenges, and prospects of hydrogen energy. Nat. Gas Ind. B 2022, 9, 427–447. [Google Scholar] [CrossRef]
- International Energy Agency (IEA). The Future of Hydrogen; IEA: Paris, France, 2019. [Google Scholar]
- Staykov, A.; Yamabe, J.; Somerday, B.P. Effect of hydrogen gas impurities on the hydrogen dissociation on iron surface. Int. J. Quantum Chem. 2014, 114, 626–635. [Google Scholar] [CrossRef]
- Staykov, A.; Komoda, R.; Kubota, M.; Ginet, P.; Barbier, F.; Furtado, J. Coadsorption of CO and H2 on an iron surface and its implication on the hydrogen embrittlement of iron. J. Phys. Chem. C 2019, 123, 30265–30273. [Google Scholar] [CrossRef]
- Liu, C.; Yang, H.; Wang, C.; Zhang, H.; Ding, R.; Ai, L.; Fan, X.; Zhang, R.; Xu, X.; Ning, Y.; et al. Effects of CH4 and CO on hydrogen embrittlement susceptibility of X80 pipeline steel in hydrogen blended natural gas. Int. J. Hydrogen Energy 2023, 48, 27766–27777. [Google Scholar] [CrossRef]
- Zhang, N.; Komoda, R.; Yamada, K.; Kubota, M.; Staykov, A. Ammonia mitigation and induction effects on hydrogen environment embrittlement of SCM440 low-alloy steel. Int. J. Hydrogen Energy 2022, 47, 15084–15093. [Google Scholar] [CrossRef]
- Nagy, H.; Tamany, E.; Ashour, H.; El-Azabawy, O.E.; Zaki, E.G.; Elsaeed, S.M. Polymeric ionic liquids based on benzimidazole derivatives as corrosion inhibitors for X65 carbon steel deterioration in acidic aqueous medium: Hydrogen evolution and adsorption studies. ACS Omega 2020, 5, 30577–30586. [Google Scholar] [CrossRef]
- Bentrah, H.; Rahali, Y.; Chala, A. Gum Arabic as an eco-friendly inhibitor for API 5L X42 pipeline steel in HCl medium. Corros. Sci. 2014, 82, 426–431. [Google Scholar] [CrossRef]
- Campari, A.; Ustolin, F.; Alvaro, A.; Paltrinieri, N. A review on hydrogen embrittlement and risk-based inspection of hydrogen technologies. Int. J. Hydrogen Energy 2023, 48, 35316–35346. [Google Scholar] [CrossRef]
- San Marchi, C.; Somerday, B.P. Effects of high-pressure gaseous hydrogen on structural metals. SAE Trans. 2007, 116, 94–109. [Google Scholar]
- Bai, Z.; Liu, Q.; Lei, J.; Li, H.; Jin, H. A polygeneration system for the methanol production and the power generation with the solar–biomass thermal gasification. Energy Convers. Manag. 2015, 102, 190–201. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, L.; Zhou, Q.; Li, Y.; Liu, L.; Nie, W.; Xu, R.; Zhang, J.; Cheng, Z.; Wang, H.; et al. Carbon dioxide enabled hydrogen storage by methanol: Highly selective and efficient catalysis with well-defined heterogeneous catalysts. Coord. Chem. Rev. 2024, 508, 215775. [Google Scholar] [CrossRef]
- Ishaq, H.; Dincer, I. Evaluation of a wind energy based system for co-generation of hydrogen and methanol production. Int. J. Hydrogen Energy 2020, 45, 15869–15877. [Google Scholar] [CrossRef]
- Yu, C. Study on Microstructure and Properties of Low Temperature X80 Pipeline Steel. Ph.D. Thesis, Xi’an Shiyou University, Xi’an, China, 2020. [Google Scholar]
- Kresse, G.; Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Zeinalipour-Yazdi, C.D.; Cooksy, A.L.; Efstathiou, A.M. CO adsorption on transition metal clusters: Trends from density functional theory. Surf. Sci. 2008, 602, 1858–1862. [Google Scholar] [CrossRef]
- Wang, T.; Tian, X.X.; Li, Y.W.; Wang, J.; Beller, M.; Jiao, H. Coverage-dependent CO adsorption and dissociation mechanisms on iron surfaces from DFT computations. ACS Catal. 2014, 4, 1991–2005. [Google Scholar] [CrossRef]
- Stibor, A.; Kresse, G.; Eichler, A.; Hafner, J. Density functional study of the adsorption of CO on Fe(110). Surf. Sci. 2002, 507, 99–102. [Google Scholar] [CrossRef]
- Sun, X.; Förster, S.; Li, Q.X.; Kurahashi, M.; Suzuki, T.; Zhang, J.W.; Yamauchi, Y.; Baum, G.; Steidl, H. Spin-polarization study of CO molecules adsorbed on Fe (110) using metastable-atom deexcitation spectroscopy and first-principles calculations. Phys. Rev. B 2007, 75, 035419. [Google Scholar] [CrossRef]
- Jorgensen, W.L.; Maxwell, D.S.; Tirado-Rives, J. Development and testing of the OPLS all-atom force field on conformational energetics and properties of organic liquids. J. Am. Chem. Soc. 1996, 118, 11225–11236. [Google Scholar] [CrossRef]
- Liu, G.; Cui, Z.; Wei, J.; Chen, L.; Jiang, B.; Li, D.; Xie, C. Inhibition of hydrogen embrittlement induced by CH4 in pipeline transportation of hydrogen-natural gas mixtures. Oil Gas Storage Transp. 2023, 42, 16–23. [Google Scholar]


| Element | Content | Element | Content | Element | Content | Element | Content |
|---|---|---|---|---|---|---|---|
| Fe | 96.741 | Nb | 0.11 | Cr | 0.24 | Sb | 0.011 |
| Mn | 1.70 | Ti | 0.016 | Mo | 0.24 | Co | 0.008 |
| C | 0.09 | Ni | 0.26 | Si | 0.31 | Se | 0.002 |
| P | 0.004 | V | 0.005 | Cu | 0.19 | As | 0.006 |
| Bi | 0.005 | Pb | 0.005 | Al | 0.036 | Ta | 0.021 |
| Serial Number | Experimental Environment |
|---|---|
| 1 | air |
| 2 | 3 MPa H2 |
| 3 | 3 MPa H2 + CH3OH |
| 4 | 4 MPa H2 |
| 5 | 4 MPa H2 + CH3OH |
| 6 | 5 MPa H2 |
| 7 | 5 MPa H2 + CH3OH |
| Hydrogen Pressure [MPa] | Bare Specimen | Methanol Covered Specimen | CO Added Specimen | |||
|---|---|---|---|---|---|---|
| HEI | FS | HEI | FS | HEI | FS | |
| 3 | 12.66 ± 1.85 | 24.67 ± 0.73 | 4.75 ± 1.11 | 26.10 ± 0.64 | 4.27 ± 1.21 | 26.38 ± 0.38 |
| 4 | 16.93 ± 2.39 | 22.72 ± 0.94 | 6.23 ± 2.98 | 25.58 ± 1.87 | 5.96 ± 1.19 | 25.83 ± 0.59 |
| 5 | 18.49 ± 0.74 | 22.45 ± 0.64 | 6.67 ± 2.85 | 25.50 ± 1.19 | 6.76 ± 1.49 | 25.71 ± 0.61 |
| Adsorbate | h1 [Å] | h2 [Å] | Eads [eV] |
|---|---|---|---|
| methanol | 2.761 | 2.238 | −0.505 |
| CO | 2.576 | 1.768 | −1.912 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, L.; Gu, J.; Yang, H.; Chen, L.; Liu, G. Research on Mechanism of Methanol–Hydrogen Co-Transport Inhibiting Hydrogen Embrittlement in Pipeline Steel. Metals 2025, 15, 429. https://doi.org/10.3390/met15040429
Xiao L, Gu J, Yang H, Chen L, Liu G. Research on Mechanism of Methanol–Hydrogen Co-Transport Inhibiting Hydrogen Embrittlement in Pipeline Steel. Metals. 2025; 15(4):429. https://doi.org/10.3390/met15040429
Chicago/Turabian StyleXiao, Li, Jia Gu, Hongwei Yang, Lei Chen, and Gang Liu. 2025. "Research on Mechanism of Methanol–Hydrogen Co-Transport Inhibiting Hydrogen Embrittlement in Pipeline Steel" Metals 15, no. 4: 429. https://doi.org/10.3390/met15040429
APA StyleXiao, L., Gu, J., Yang, H., Chen, L., & Liu, G. (2025). Research on Mechanism of Methanol–Hydrogen Co-Transport Inhibiting Hydrogen Embrittlement in Pipeline Steel. Metals, 15(4), 429. https://doi.org/10.3390/met15040429




