Research on the Influence of Mo on the Corrosion Mechanism of 1%Ni Weathering Steel in Simulated Marine Atmospheric Environments
Abstract
1. Introduction
2. Experiments
2.1. Samples
2.2. Corrosion Test
2.3. Analysis of the Corrosion Products
2.4. Electrochemical Measurements
3. Results
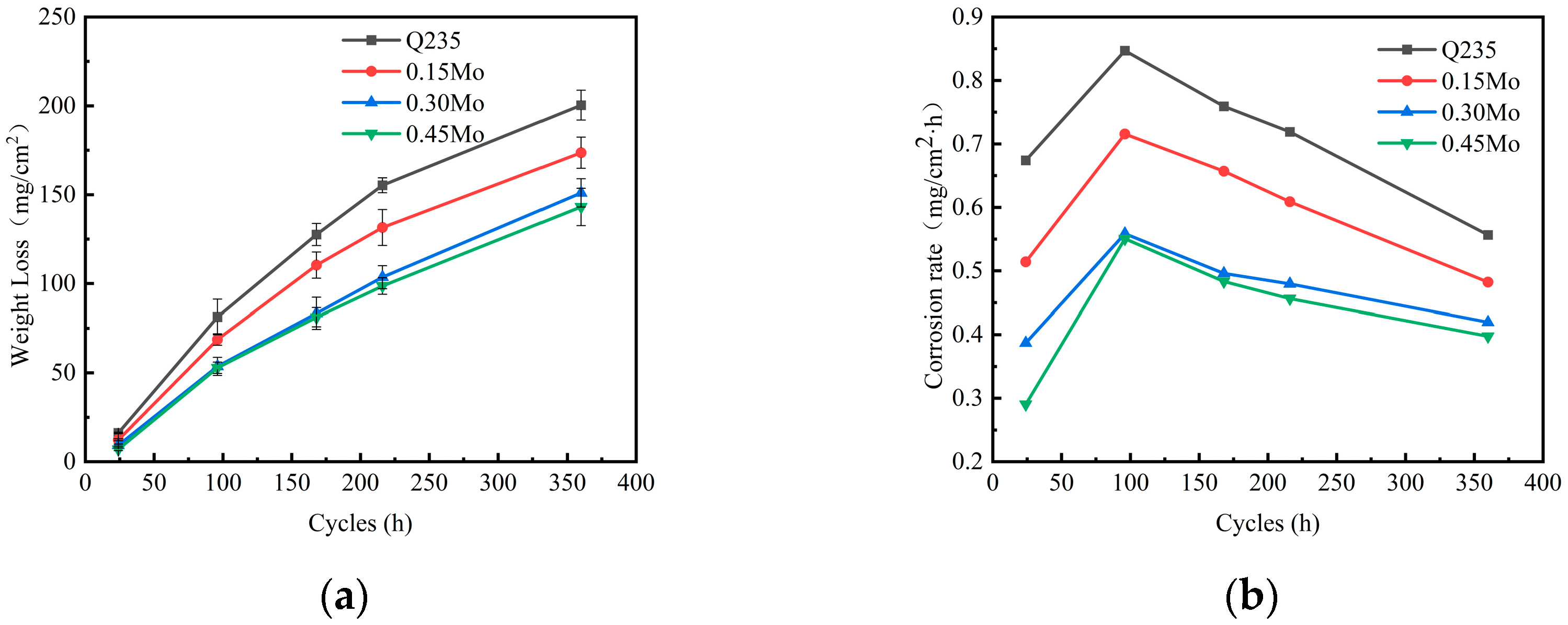
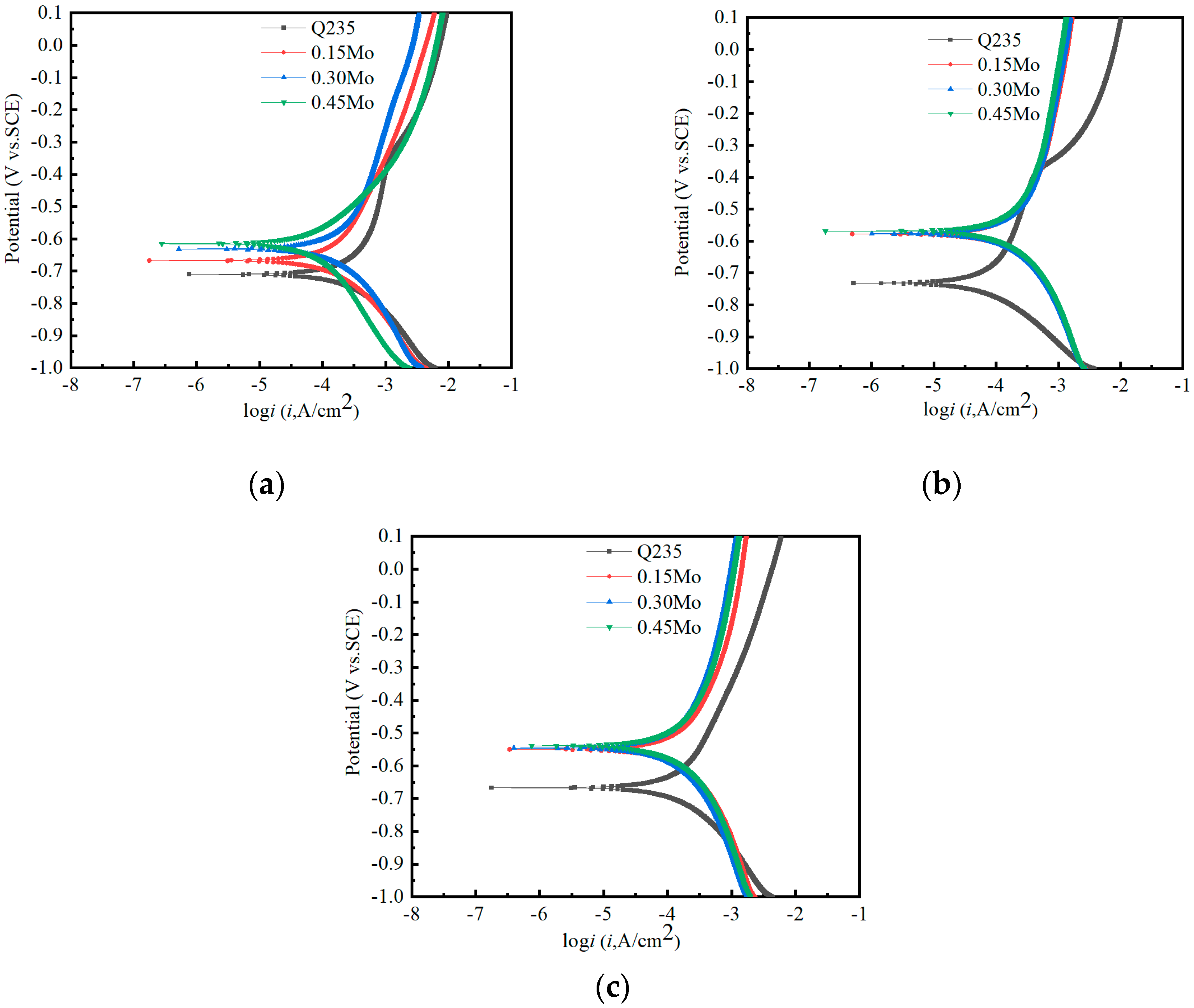
3.1. Corrosion Kinetics
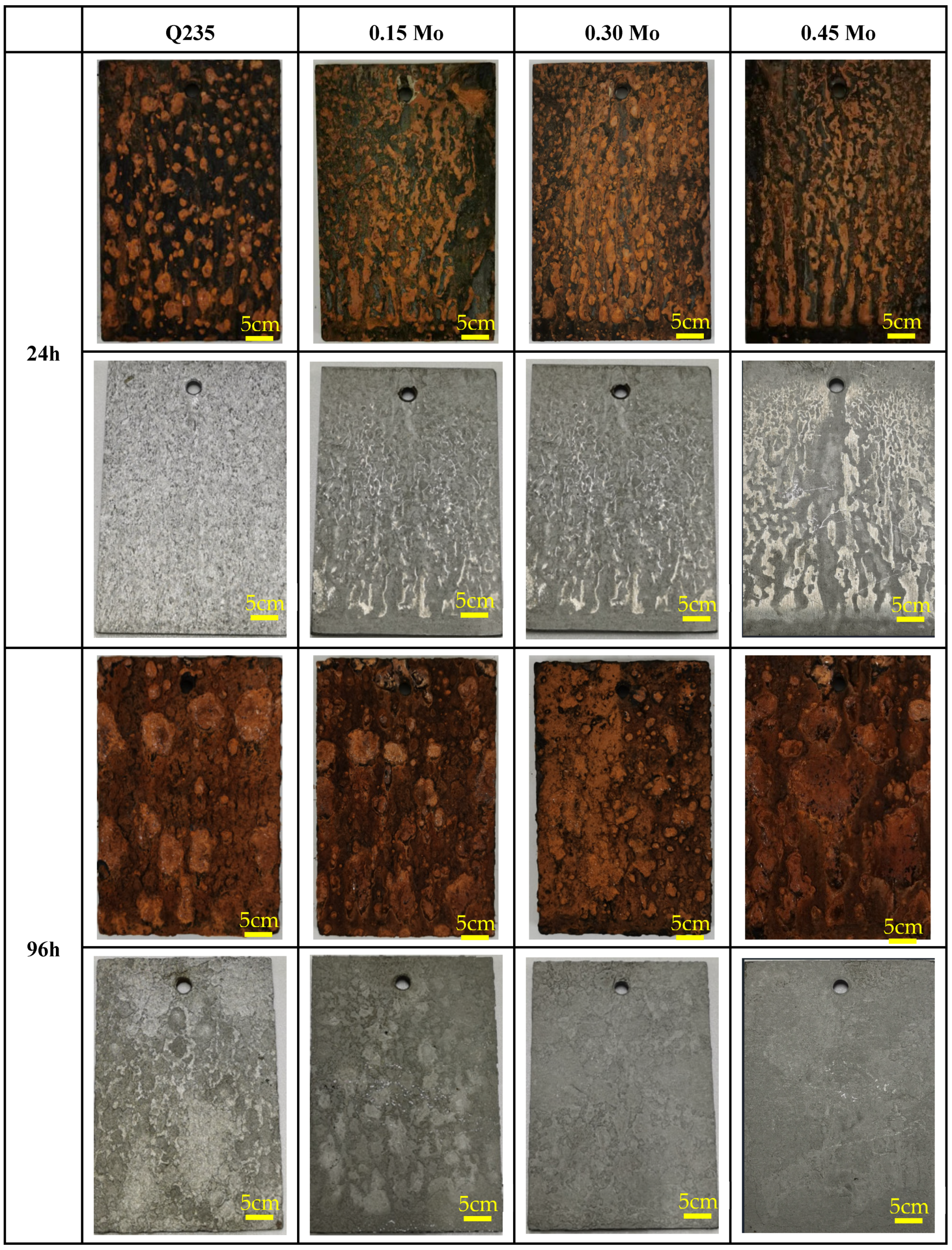
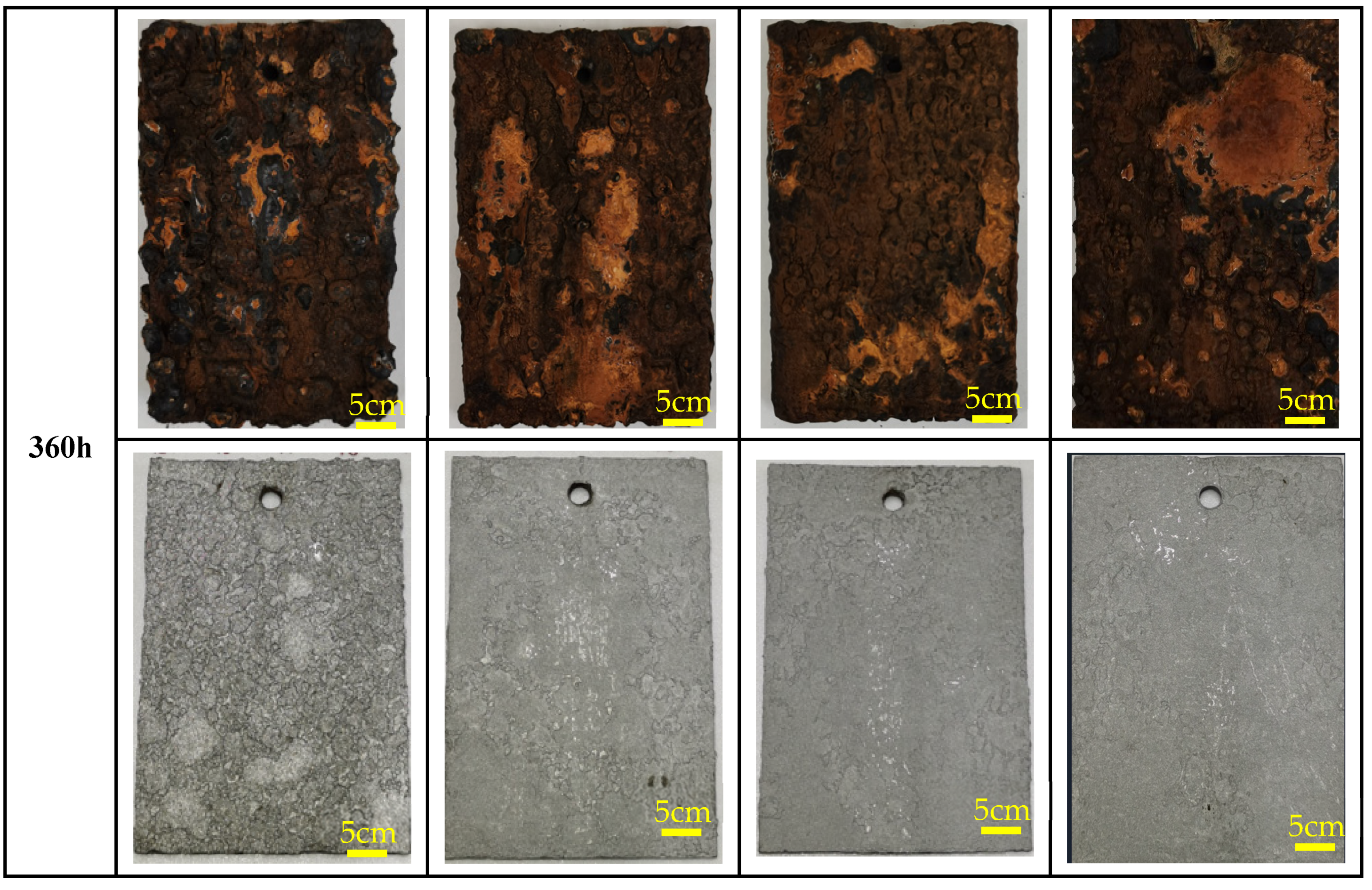
3.2. Macroscopic Studies of Corrosion Products
3.3. Composition of the Rust Layer
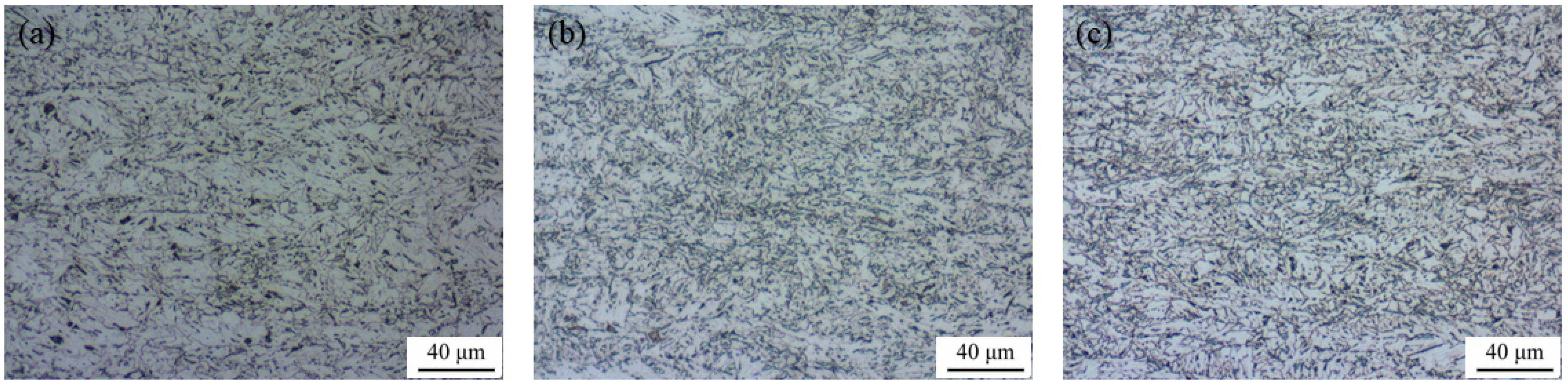
3.4. Microstructure Studies of Corrosion Products
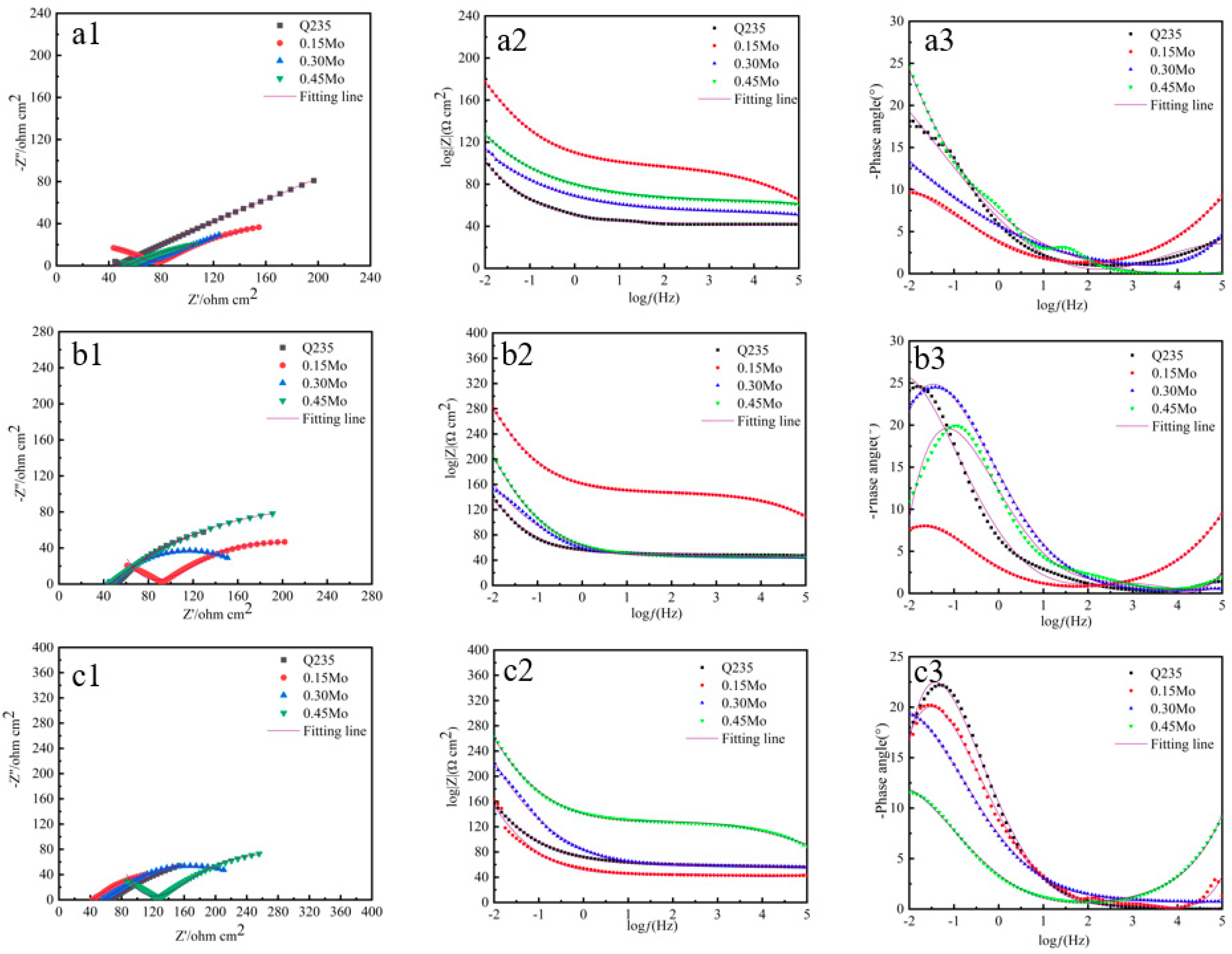
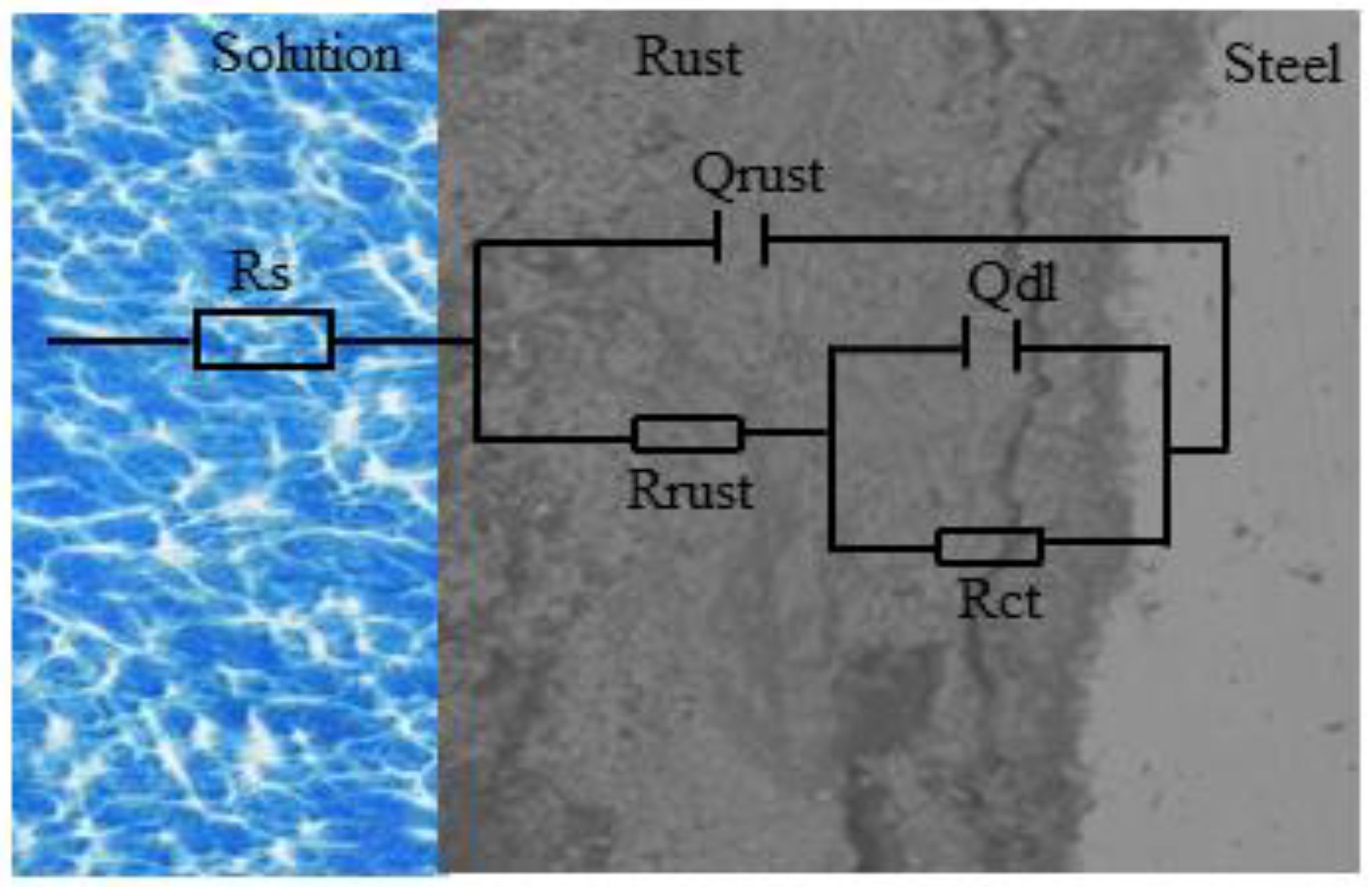
3.5. Electrochemical Properties of the Rust Layer
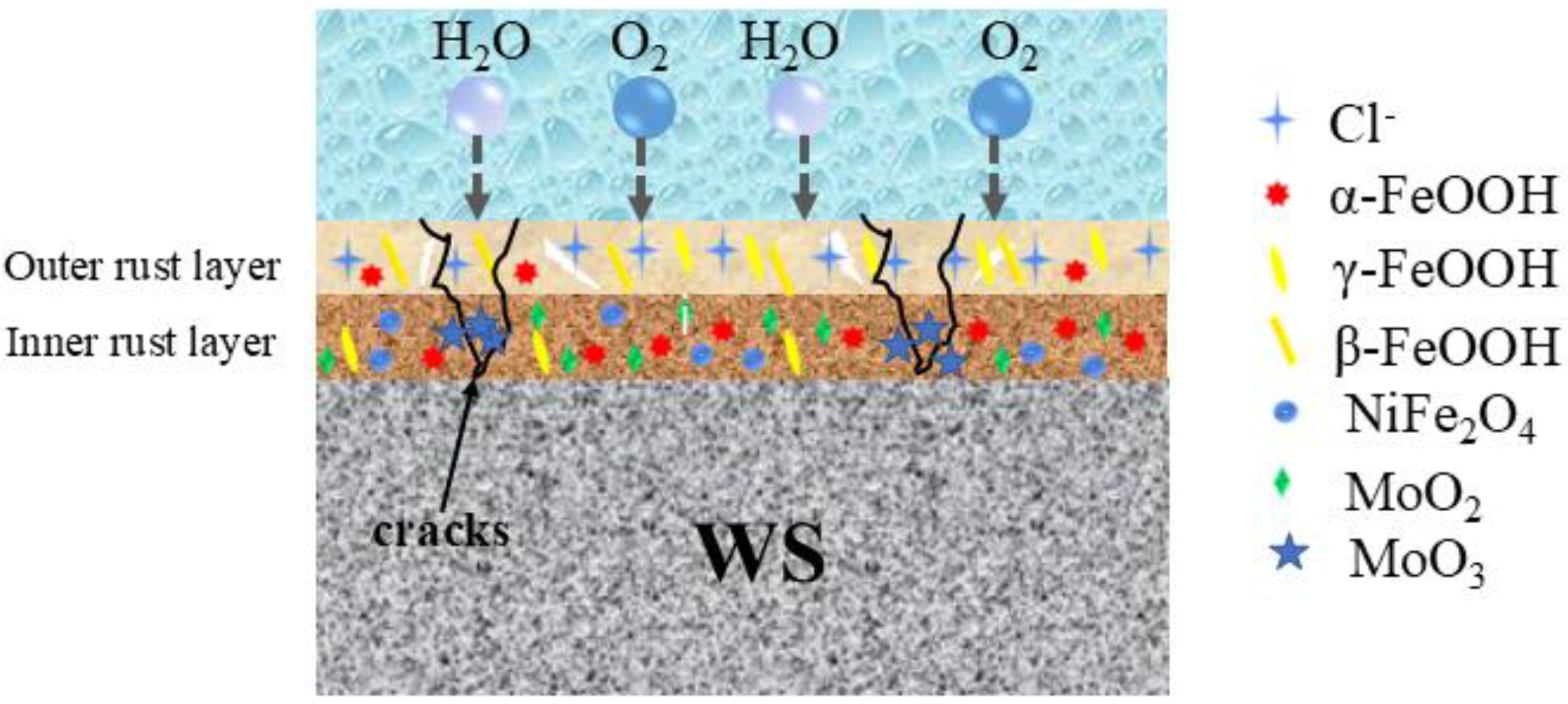
4. Discussion
5. Conclusions
- (1)
- Adding a specific amount of Mo to 1%Ni weathering steel (WS) significantly enhances its corrosion resistance. The increasing Mo content promotes the formation of a stable and compact rust layer, which markedly increases the proportion of α-FeOOH. This transformation endows the rust layer with effective physical barrier properties, thereby providing superior protection against corrosion.
- (2)
- The presence of Mo leads to the formation of MoO2 and MoO3 deposits within the holes and cracks of the rust layer. These deposits serve multiple functions. Compaction and Uniformity: They make the rust layer more compact and uniform. Trapping Corrosive Particles: They trap corrosive particles, preventing further penetration. Promotion of α-FeOOH Formation: They facilitate the formation of α-FeOOH, which contributes to the rust layer’s protective ability by forming a physical barrier.
- (3)
- Only some of the Mo ions migrate to the vicinity of the Ni element for the hydrolysis reaction, which led to the difficultly in improving the corrosion rate of the steel with a high Mo content. Hence, the content of the Mo element can be controlled at 0.3–0.5% for the 1Ni WS.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jia, J.; Cheng, X.; Yang, X.; Li, X.; Li, W. A study for corrosion behavior of a new-type weathering steel used in harsh marine environment. Constr. Build. Mater. 2020, 259, 119760. [Google Scholar] [CrossRef]
- Morcillo, M.; Chico, B.; Diaz, I.; Cano, H.; Fuente, D.D.L. Atmospheric corrosion data of weathering steels. A review. Corros. Sci. 2013, 77, 6–24. [Google Scholar] [CrossRef]
- Wu, W.; Cheng, X.; Zhao, J.; Li, X. Benefit of the corrosion product film formed on a new weathering steel containing 3% nickel under marine atmosphere in Maldives. Corros. Sci. 2019, 165, 108416. [Google Scholar] [CrossRef]
- Zhang, B.; Liu, W.; Sun, Y.; Yang, W.; Chen, L.; Xie, J.; Li, W. Corrosion behavior of the 3 wt% Ni weathering steel with replacing 1 wt% Cr in the simulated tropical marine atmospheric environment. J. Phys. Chem. Solids 2023, 175, 111221. [Google Scholar] [CrossRef]
- Diaz, I.; Cano, H.; Fuente, D.D.L.; Chico, B.; Vega, J.M.; Morcillo, M. Atmospheric corrosion of Ni-advanced weathering steels in marine atmospheres of moderate salinity. Corros. Sci. 2013, 76, 348–360. [Google Scholar] [CrossRef]
- Usami, A.; Kihira, H.; Kusunoki, T. 3%Ni Weathering Steel Plate for Uncoated Bridges at High Airborne Salt Environment. Shinnittetsu Giho 2003, 19–21. Available online: https://www.nipponsteel.com/en/tech/report/nsc/pdf/n8706.pdf (accessed on 1 April 2025).
- Yu, Q.; Yang, X.; Dong, W.; Wang, Q.; Zhang, F.; Gu, X. Layer-by-layer investigation of the multilayer corrosion products for different Ni content weathering steel via a novel Pull-off testing. Corros. Sci. 2022, 195, 109988. [Google Scholar] [CrossRef]
- Jia, J.; Liu, Z.; Cheng, X.; Du, C.; Li, X. Development and optimization of Ni-advanced weathering steel: A review. Corros. Commun. 2021, 2, 82–90. [Google Scholar] [CrossRef]
- Cheng, X.; Jin, Z.; Liu, M.; Li, X. Optimizing the nickel content in weathering steels to enhance their corrosion resistance in acidic atmospheres. Corros. Sci. 2017, 115, 135–142. [Google Scholar] [CrossRef]
- Zhu, C.; Gao, X.; Zhu, C.; Li, W.; Liu, T.; Du, L.; Wu, H. A study on corrosion behavior of advanced weathering steel relying on Ni–Mo synergy under marine atmosphere. J. Mater. Res. Technol. 2024, 30, 8469–8481. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, T.; Wu, W.; Jiang, S.; Yang, J.; Liu, Z. Optimizing the resistance of Ni-advanced weathering steel to marine atmospheric corrosion with the addition of Al or Mo. Constr. Build. Mater. 2021, 279, 122341. [Google Scholar] [CrossRef]
- Nishimura, T.; Kodama, T. Clarification of chemical state for alloying elements in iron rust using a binary-phase potential–pH diagram and physical analyses. Corros. Sci. 2003, 45, 1073–1084. [Google Scholar] [CrossRef]
- Hao, L.; Zhang, S.; Dong, J.; Ke, W. A study of the evolution of rust on Mo–Cu-bearing fire-resistant steel submitted to simulated atmospheric corrosion. Corros. Sci. 2012, 54, 244–250. [Google Scholar] [CrossRef]
- Teramoto, K.H.A. An X-ray photo-electron spectroscopic study on the role of molybdenum in increasing the corrosion resistance of ferritic stainless steels in HC1. Corros. Sci. 1979, 19, 3–14. [Google Scholar]
- Sun, Y.; Liu, W.; Dong, B.; Zhang, T.; Chen, L.; Yang, W.; Li, H.; Zhang, B.; Xie, J.; Cui, J. Designing Weathering Steel with Optimized Mo/Ni Ratios for Better Corrosion Resistance in Simulated Tropical Marine Atmosphere. Met. Mater. Int. 2024, 30, 909–927. [Google Scholar] [CrossRef]
- TB/T 2375-1993 (1993); Test Method for Periodic Immersion Corrosion of Weathering Steels for Railway. Ministry of Railways: Beijing, China, 1993.
- Wang, B.; Liu, L.; Cheng, X.; Wu, W.; Liu, C.; Zhang, D.; Li, X. Advanced multi-image segmentation-based machine learning modeling strategy for corrosion prediction and rust layer performance evaluation of weathering steel. Corros. Sci. 2024, 237, 112334. [Google Scholar] [CrossRef]
- Kamimura, T.; Hara, S.; Miyuki, H.; Yamashita, M.; Uchida, H. Composition and protective ability of rust layer formed on weathering steel exposed to various environments. Corros. Sci. 2006, 48, 2799–2812. [Google Scholar] [CrossRef]
- Cano, H.; Neff, D.; Maricllo, M.; Dillmann, P.; Diaz, I.; de la Fuente, D. Characterization of corrosion products formed on Ni 2.4 wt%-Cu 0.5 wt%-Cr 0.5 wt% weathering steel exposed in marine atmospheres. Corros. Sci. 2014, 87, 438–451. [Google Scholar] [CrossRef]
- Zhang, T.; Liu, W.; Chen, L.; Dong, B.; Yang, W.; Fan, Y.; Zhao, Y. On how the corrosion behavior and the functions of Cu, Ni and Mo of the weathering steel in environments with different NaCl concentrations. Corros. Sci. 2021, 192, 109851. [Google Scholar] [CrossRef]
- Antunes, R.A.; Costa, I.; Faria, D.L.A.D. Characterization of corrosion products formed on steels in the first months of atmospheric exposure. Mater. Res. 2003, 6, 403–408. [Google Scholar] [CrossRef]
- Yu, Q.; Dong, W.; Yang, X.; Wang, Q.; Zhang, F. Insights into the corrosion mechanism and electrochemical properties of the rust layer evolution for weathering steel with various Cl deposition in the simulated atmosphere. Mater. Res. Express 2021, 8, 36515–36518. [Google Scholar] [CrossRef]
- Liu, H.; Huang, F.; Yuan, W.; Hu, Q.; Liu, J.; Cheng, Y.F. Essential role of element Si in corrosion resistance of a bridge steel in chloride atmosphere. Corros. Sci. 2020, 173, 108758. [Google Scholar] [CrossRef]
- Mansfeld, F. Tafel slopes and corrosion rates obtained in the pre-Tafel region of polarization curves. Corros. Sci. 2005, 47, 3178–3186. [Google Scholar] [CrossRef]
- Mccafferty, E. Validation of corrosion rates measured by the Tafel extrapolation method. Corros. Sci. 2005, 47, 3202–3215. [Google Scholar] [CrossRef]
- Hedenstedt, K.; Simic, N.; Wildlock, M.; Ahlberg, E. Kinetic study of the hydrogen evolution reaction in slightly alkaline electrolyte on mild steel, goethite and lepidocrocite. J. Electroanal. Chem. 2016, 783, 1–7. [Google Scholar] [CrossRef]
- Yu, Q.; Dong, W.; Yang, X.; Wang, Q.; Zhang, F. The Role of the Direct Current Electric Field in Enhancing the Protective Rust Layer of Weathering Steel. J. Mater. Eng. Perform. 2021, 30, 6309–6322. [Google Scholar] [CrossRef]
- Chen, Z.; Yu, Q.; Zhao, L.; Zhang, C.; Gu, M.; Wang, Q.; Wang, G. Insight into the role of Si on corrosion resistance of weathering steel in a simulated industrial atmosphere. J. Mater. Res. Technol. 2023, 26, 487–503. [Google Scholar] [CrossRef]
- Hao, L.; Zhang, S.; Dong, J.; Ke, W. Atmospheric corrosion resistance of MnCuP weathering steel in simulated environments. Corros. Sci. 2011, 53, 4187–4192. [Google Scholar] [CrossRef]
- Wu, W.; Cheng, X.; Hou, H.; Liu, B.; Li, X. Insight into the product film formed on Ni-advanced weathering steel in a tropical marine atmosphere. Appl. Surf. Sci. 2018, 436, 80–89. [Google Scholar] [CrossRef]
- Wu, W.; Dai, Z.; Liu, Z.; Liu, C.; Li, X. Synergy of Cu and Sb to enhance the resistance of 3%Ni weathering steel to marine atmospheric corrosion. Corros. Sci. 2021, 183, 109353. [Google Scholar] [CrossRef]
- Niu, G.; Yuan, R.; Wang, E.; Yang, X.; Liu, Z.; Li, Z.; Zhang, Z.; Gong, N.; Li, K.; Su, B. Unraveling the influence of Mo on the corrosion mechanism of Ni-advanced weathering steel in harsh marine atmospheric environments. J. Mater. Sci. Technol. 2024, 195, 41–62. [Google Scholar] [CrossRef]
- Kimura, M.; Kihira, H.; Ohta, N.; Hashimoto, M.; Senuma, T. Control of Fe(O,OH)6 nano-network structures of rust for high atmospheric-corrosion resistance. Corros. Sci. 2005, 47, 2499–2509. [Google Scholar] [CrossRef]
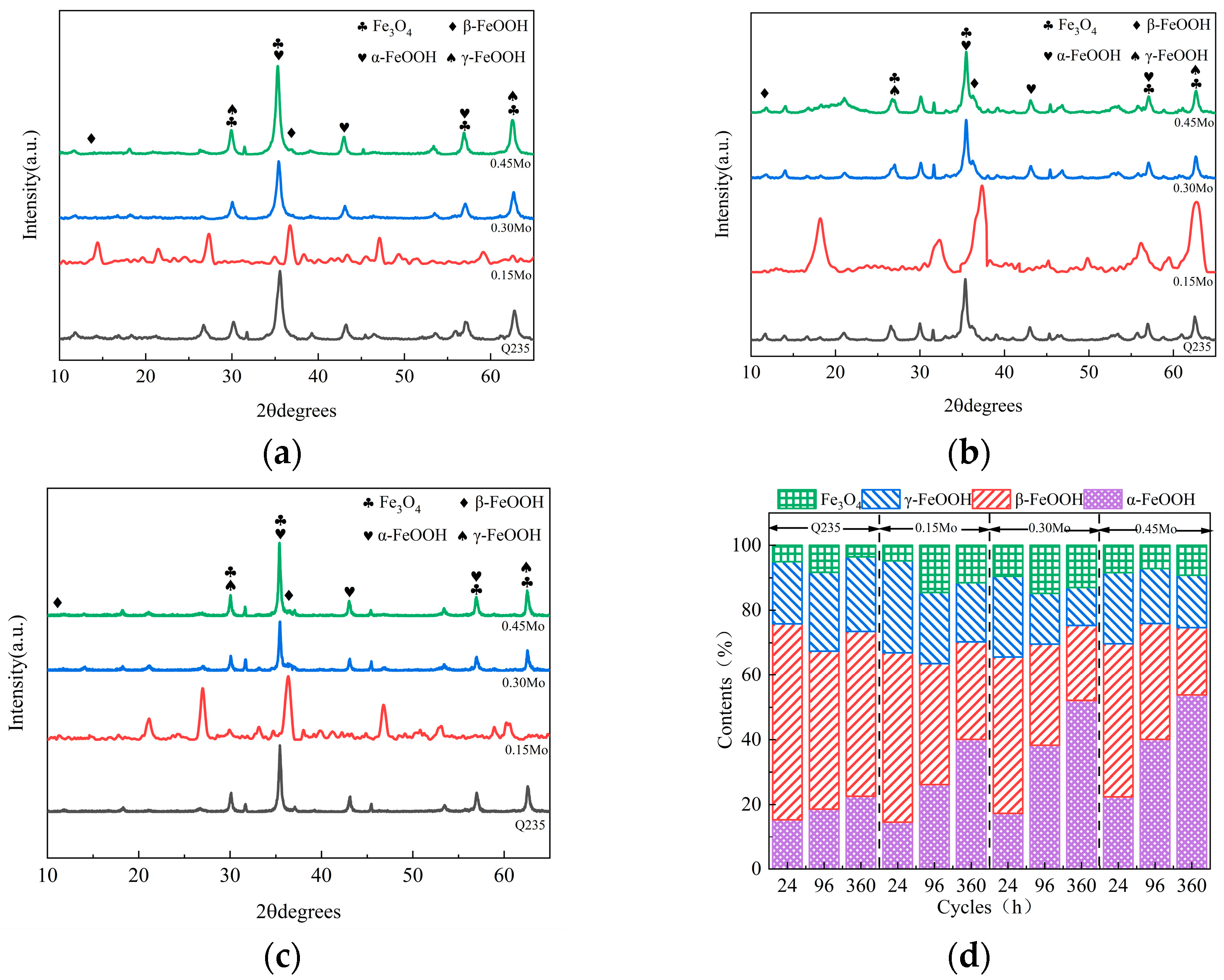
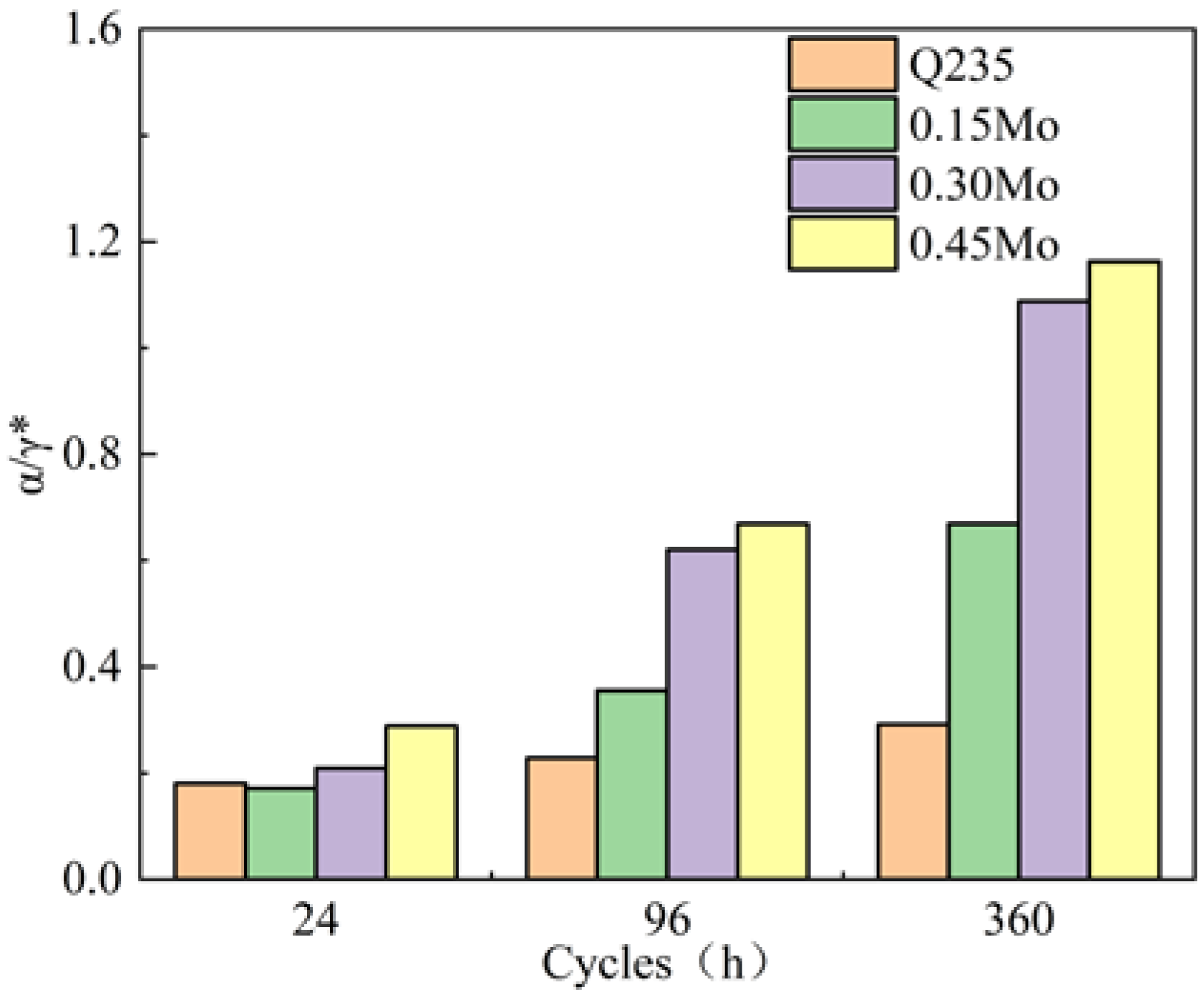
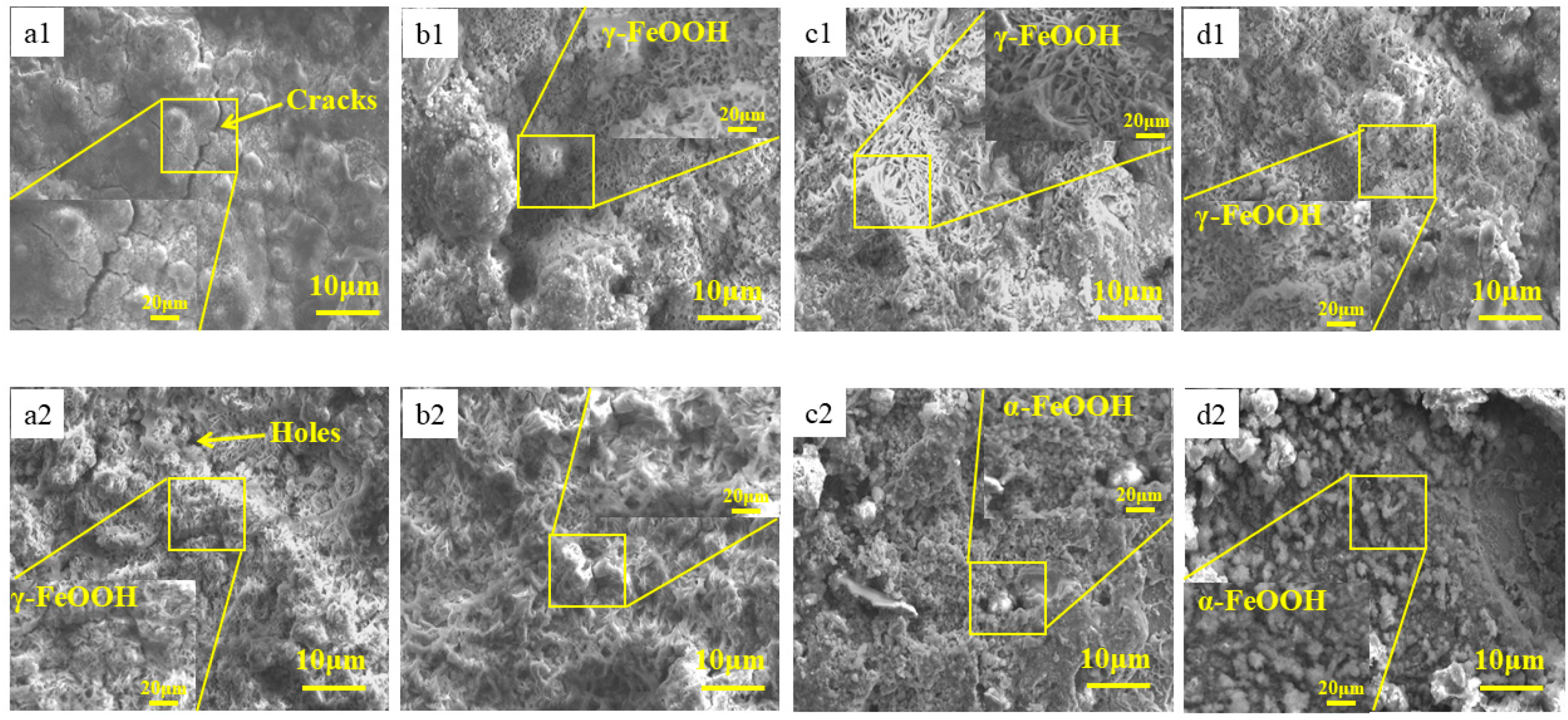
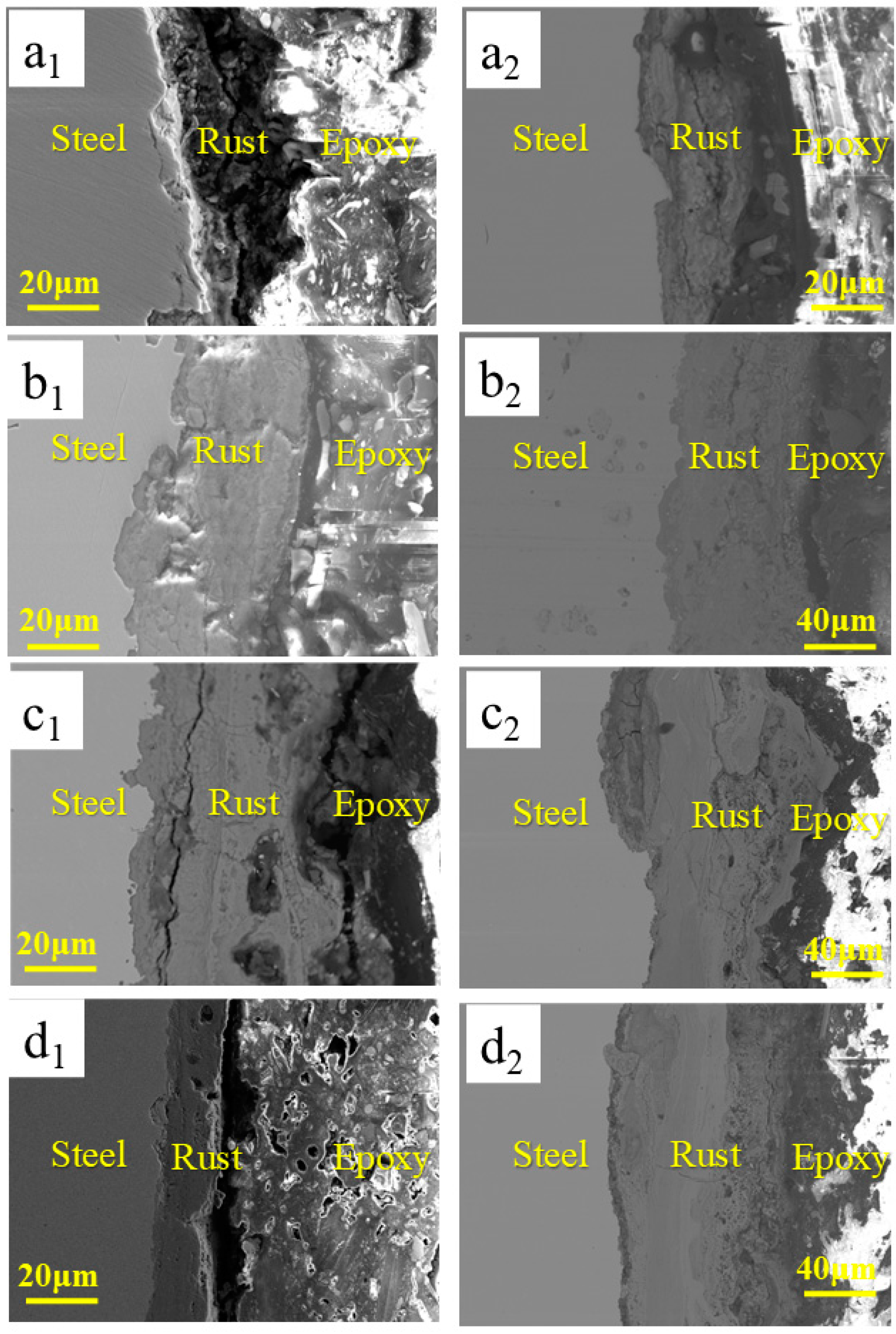
| Steel | C | Si | Mn | P | S | Ni | Mo | Cu | Fe |
|---|---|---|---|---|---|---|---|---|---|
| Q235 | 0.150 | 0.22 | 0.64 | 0.003 | 0.003 | - | - | - | Balance |
| 0.15Mo | 0.053 | 0.20 | 0.92 | 0.003 | 0.002 | 1.30 | 0.16 | 0.59 | Balance |
| 0.30Mo | 0.046 | 0.21 | 0.89 | 0.003 | 0.003 | 1.33 | 0.29 | 0.55 | Balance |
| 0.45Mo | 0.052 | 0.20 | 0.90 | 0.003 | 0.003 | 1.30 | 0.45 | 0.58 | Balance |
| - | Experimental Parameters |
|---|---|
| Solution | 35 g/L ± 1 g/L (3.5%NaCl solution) |
| pH | 7.5–8.2 |
| Temperature | 45 ± 2 °C |
| Relative humidity | 70 ± 5%RH |
| Cycle time | 60 ± 3 min, infiltration time 12 ± 1.5 min |
| Test period | 24 h, 96 h, 168 h, 216 h, 360 h |
| Reference standard | TB/T 2375-1993 |
| Samples | Ecorr/V(vs.SCE) | Icorr/μA·cm−2 | bc/mV·dec−1 | ba/mV·dec−1 |
|---|---|---|---|---|
| 24 h | ||||
| Q235 | −0.710 ± 0.008 | 154.33 ± 5.79 | −144.3 ± 0.2 | 257.0 ± 1.0 |
| 0.15Mo | −0.667 ± 0.011 | 80.64 ± 6.17 | −165.5 ± 0.4 | 308.6 ± 0.2 |
| 0.30Mo | −0.631 ± 0.003 | 79.62 ± 0.78 | −242.1 ± 0.3 | 207.5 ± 0.2 |
| 0.45Mo | −0.615 ± 0.002 | 80.33 ± 2.47 | −283.2 ± 0.6 | 132.8 ± 0.7 |
| 96 h | ||||
| Q235 | −0.732 ± 0.005 | 103.86 ± 7.34 | −118.3 ± 0.3 | 130.0 ± 0.6 |
| 0.15Mo | −0.578 ± 0.004 | 80.11 ± 1.79 | −125.9 ± 0.4 | 310.7 ± 0.4 |
| 0.30Mo | −0.577 ± 0.003 | 78.14 ± 3.87 | −127.3 ± 0.5 | 328.9 ± 0.6 |
| 0.45Mo | −0.569 ± 0.005 | 79.14 ± 3.57 | −145.3 ± 0.6 | 323.4 ± 0.1 |
| 360 h | ||||
| Q235 | −0.667 ± 0.004 | 80.32 ± 8.77 | −136.7 ± 0.5 | 132.8 ± 0.4 |
| 0.15Mo | −0.550 ± 0.009 | 78.34 ± 5.26 | −110.7 ± 0.9 | 165.0 ± 0.3 |
| 0.30Mo | −0.546 ± 0.005 | 73.63 ± 8.34 | −119.3 ± 0.4 | 207.4 ± 0.6 |
| 0.45Mo | −0.539 ± 0.015 | 53.78 ± 8.49 | −137.2 ± 0.6 | 183.1 ± 0.5 |
| Samples | Cycles/h | Rs/Ω·cm2 | Qrust(Y0) × 10−3 (Ω−1·cm2·sn) | nrust | Rrust/Ω·cm2 | Qdl(Y0) × 10−3 (Ω−1·cm2·sn) | ndl | Rct/Ω·cm2 |
|---|---|---|---|---|---|---|---|---|
| Q235 | 24 | 43.72 | 11.24 | 0.41 | 32.78 | 16.45 | 0.34 | 29.45 |
| 96 | 45.31 | 21.47 | 0.54 | 42.46 | 28.72 | 0.44 | 62.76 | |
| 360 | 51.84 | 36.42 | 0.53 | 57.92 | 41.77 | 0.51 | 69.76 | |
| 0.15Mo | 24 | 73.21 | 13.34 | 0.50 | 37.21 | 11.61 | 0.33 | 68.31 |
| 96 | 92.31 | 27.89 | 0.51 | 51.01 | 22.74 | 0.48 | 72.11 | |
| 360 | 47.31 | 55.41 | 0.62 | 67.33 | 36.41 | 0.60 | 71.46 | |
| 0.30Mo | 24 | 51.27 | 14.31 | 0.55 | 49.62 | 15.48 | 0.40 | 80.19 |
| 96 | 48.78 | 27.75 | 0.59 | 59.67 | 20.79 | 0.40 | 99.48 | |
| 360 | 52.31 | 57.67 | 0.68 | 88.21 | 21.11 | 0.49 | 97.67 | |
| 0.45Mo | 24 | 53.48 | 19.87 | 0.50 | 50.31 | 13.39 | 0.50 | 80.34 |
| 96 | 50.94 | 51.22 | 0.57 | 58.97 | 20.11 | 0.51 | 100.14 | |
| 360 | 92.98 | 58.31 | 0.66 | 86.21 | 22.79 | 0.58 | 109.79 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, C.; Yu, Q.; Wang, S.; Qiao, M.; Wang, Q. Research on the Influence of Mo on the Corrosion Mechanism of 1%Ni Weathering Steel in Simulated Marine Atmospheric Environments. Metals 2025, 15, 430. https://doi.org/10.3390/met15040430
Han C, Yu Q, Wang S, Qiao M, Wang Q. Research on the Influence of Mo on the Corrosion Mechanism of 1%Ni Weathering Steel in Simulated Marine Atmospheric Environments. Metals. 2025; 15(4):430. https://doi.org/10.3390/met15040430
Chicago/Turabian StyleHan, Chengliang, Qiang Yu, Shibiao Wang, Mingliang Qiao, and Qingfeng Wang. 2025. "Research on the Influence of Mo on the Corrosion Mechanism of 1%Ni Weathering Steel in Simulated Marine Atmospheric Environments" Metals 15, no. 4: 430. https://doi.org/10.3390/met15040430
APA StyleHan, C., Yu, Q., Wang, S., Qiao, M., & Wang, Q. (2025). Research on the Influence of Mo on the Corrosion Mechanism of 1%Ni Weathering Steel in Simulated Marine Atmospheric Environments. Metals, 15(4), 430. https://doi.org/10.3390/met15040430





