1. Introduction
This work aims to generate valuable information for industries that require the separation of boehmite from sources, whether from mineral sources for aluminum extraction or from synthetic sources for water-treatment purposes. In the aluminum-extraction industry, the findings of this paper could help enhance the operating conditions of the microbubble flotation process using SDS and PO collectors. Moreover, insights from this work may have a positive impact on the boehmite water-treatment sector, as the flotation process reduces floc separation time and replaces large volumes of settling equipment with a flotation column. For any of the applications mentioned above, there is currently limited literature on the flotation of mineral or synthetic boehmite, underscoring the significance of this work in addressing this information gap.
Froth flotation is undoubtedly one of the most important and versatile techniques used for separation processes (e.g., selective separation of minerals). Billions of tons of minerals are processed by froth flotation worldwide each year. Currently, froth flotation also finds applications outside the mining industry. For example, it is used to treat water effluents, separate inked paper fiber, and treat effluents generated by petroleum refineries [
1]. Also, froth flotation is extensively applied in several industries such as the textile, medical, food, and medical industries, as well in remediation of environmental contamination [
2].
Bauxite consists mainly of aluminum minerals such as gibbsite (Al(OH)
3), boehmite (γ-AlO(OH)), and diaspore (α-AlO(OH)), which is the most important ore for the production of metallic aluminum by the Bayern process [
3,
4]. Bauxite is often naturally accompanied by quartz and iron minerals, and froth flotation is as a viable alternative approach to separation from these impurities [
5,
6,
7]. Boehmite contains more than 40% aluminum, so its recovery and concentration are topics of great interest in aluminum processing [
8,
9,
10]. A discussion of some previous works on boehmite flotation under different conditions is presented below. The literature deals with the recovery of natural and synthetic boehmite.
Birinci and Gök [
11] studied the recovery of boehmitic bauxite by reverse flotation, using dodecylamine to float the silicates and starch to depress bauxites. In that study, the results showed that the recovery of aluminum-containing minerals is highly dependent on particle size and the degree of mineral liberation (obtaining a maximum recovery of 50% at a size close to 50 µm). Longhua et al. [
12] studied the adsorption mechanism of sodium oleate (NaOL) on the surface of boehmite and measured its zeta potential with and without this reagent. They also performed flotation tests with different particle sizes and studied the effect of pH on recovery. The results show that the adsorption of anionic surfactant (NaOL) shifts the isoelectric point (IEP) to a more acidic value (from pH 7.5 to 5.5). Additionally, NaOL preferentially adsorbs on the surface of boehmite, which exhibits a positive surface charge at pH < IEP. However, the adsorption of anionic carboxyl functional groups of sodium oleate still occurs on the negative surface charges of boehmite at pH > IEP. The authors propose a chemisorption mechanism because, at pH > IEP, electrostatic attraction between the anionic surfactant and the negatively charged solids is not possible, but adsorption by hydrogen bonds may occur. On the other hand, Longhua et al. [
12] concluded that maximum recovery of boehmite is obtained at a pH close to 9.5 for particle sizes in relatively coarse fractions (range 38 µm to 75 µm). The shift of the IEP to more acidic values allow us to identify a pH zone in which boehmite particles appear to be more readily floatable, at the point where the zeta potential becomes less negative. These results seem to align with those of Alonso [
13], who conducted a study on this topic and analyzed the adsorption of dodecyl sulfate species on synthetic boehmite, characterizing its zeta potential in acid and alkaline conditions. He concluded that if surfactant has a charge opposite that of one of the determinant ions adsorbed on the boehmite surface, adsorption is spontaneous (at pH = 4.7). The positive charge of the solids is decreased by neutralization of their surface charges. However, when the solid’s electric charge is the same as that of the surfactant, adsorption occurs, but only at high concentrations of surfactant.
Sonawane et al. [
14] studied the use of various surfactants to float aluminum hydroxides and suggested the use of a mixture of NaOL and SDS, both anionic surfactants, at a 1:3 ratio, to improve the recovery of aluminum hydroxide. They explained that the formation of a stable froth zone was promoted under these operation conditions and that this enhanced recovery by true flotation. Wang et al. [
15] used molecular dynamics simulations to study the effect of temperature on the flotation efficiency of diaspore using sodium oleate. Their results revealed that an increase in temperature enhances reagent dispersion and mineral–reagent interactions. However, an excessive increase in temperature may negatively affect collector adsorption. Sun et al. [
16] investigated the use of benzohydroxamic acid (BHA) and oleate sodium (NaOL) to float AlO(OH)ores. Their results showed that with this collector mixture of NaOL/BHA, it is possible to selectively float the AlO(OH) from bauxite with kaolinite, obtaining recoveries ranging from 86.42% to 90.85%.
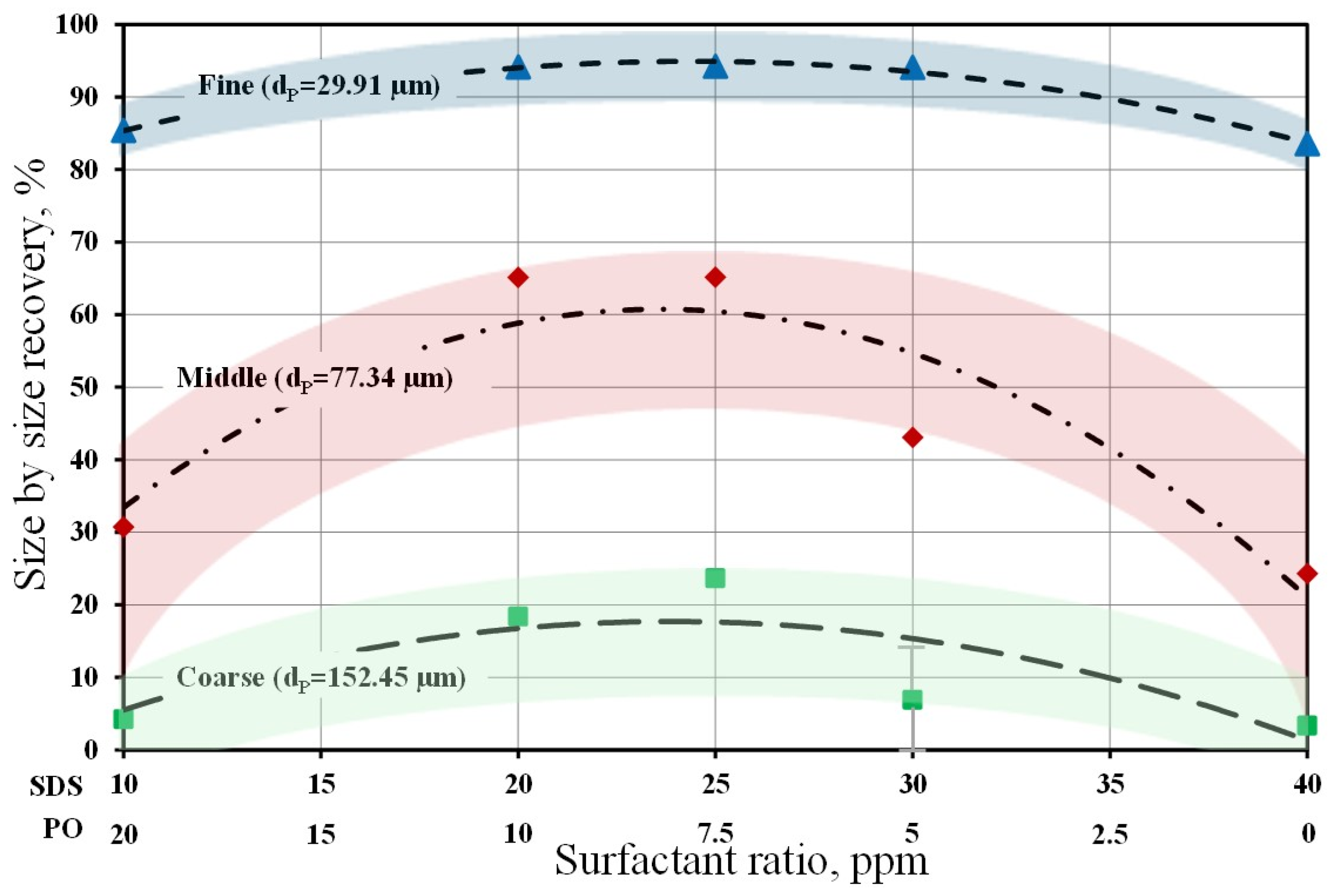
This literature review shows that two of the most important factors for improving boehmite recovery are the selection of the boehmite particle size to be floated and the reagent scheme. Although there is limited information on boehmite recovery by flotation, particle size, reagent scheme, and pH conditions are important variables in this process. However, it is worth noting that there is no information on how the reagent ratio can modify the characteristics of the bubbles produced and, consequently, boehmite flotation.
Boehmite is also found in large quantities as a waste material from many water-treatment plants (synthetic boehmite) that utilize the electrocoagulation process with aluminum anodes [
17,
18,
19]. This paper may interest some in the oil and food industries, as boehmite is a foam stabilizer that can easily adsorb on the gas−liquid interfaces generated in these industries [
20]. As mentioned earlier, one potential application for boehmite flotation is to speed up the separation of its flocs from the slurry (water−flocs) generated after the electrocoagulation process. Therefore, to clarify this section, the electrocoagulation process is briefly outlined as follows. Electrocoagulation involves using electrolytic cells with cathodes and anodes. During this process, the metal anode is electrooxidized and dissolved to produce the coagulants, often oxyhydroxides, needed to remove contaminants. Electrocoagulation uses chemical, electrochemical, and physicochemical phenomena for water treatment, destabilizing colloidal pollutants. The cathode is typically made of stainless steel. In summary, electrocoagulation consists of three sequential steps for generating coagulated ions: (i) the electrolytic oxidation of the anode produces the coagulants; (ii) the contaminants are adsorbed onto the suspended flocs (in this case, boehmite); and (iii) subsequently, the suspended flocs destabilize, forming clots that initiate a gradual sedimentation process [
21]. As proposed in this work, the flotation process with microbubbles can replace this gradual-sedimentation step (separation from the water).
Other complex and expensive chemical methods, like those described in the following lines, have proven effective in producing boehmite. Zhonglin et al. [
22] synthesized porous boehmite microspheres using a hydrothermal method involving high pressures and temperatures (150 °C) and the use of autoclaves. These microspheres were used to remove Congo red pigment and yielded high removal rates. Anvari et al. [
23] synthesized boehmite nanoparticles using the sol−gel method, and these nanoparticles subsequently were bonded to chitosan particles by coprecipitation with sodium tripolyphosphate. The product was used as an efficient adsorbent to remove the anionic pigment Congo red and the nonionic pigment bromothymol blue. Boehmite nanoparticles can also be synthesized by the ultrasound-assisted hydrothermal method (550 ◦C) from clove extract for water treatment; in this work, the adsorption efficiency was evaluated by removing copper ions [
24]. Movasati et al. [
25] studied the synthesis of boehmite using a hydrothermal method to produce small crystals of this oxyhydroxide, which is highly efficient for pollutant removal. This process uses aluminum nitrate, urea, calcium chloride, and xanthan gum, which are subjected to a reaction for 12 h in the autoclave.
After a review of the current literature on methods of boehmite generation, it can be deduced that its electrolytic generation using aluminum anodes (by electrocoagulation) is easier and possibly more economical, which adds interest to this research work.
The knowledge generated in the present work should be useful in devising applications for these industrial subproducts. It is worth mentioning that boehmite is used in wastewater treatment because when the flocs are formed during the electrocoagulation process, the boehmite flocs adsorb contaminants due to their surface properties. Dura [
26] studied the use of boehmite generated with aluminum−zinc alloys as anodes in an electrocoagulation cell to successfully remove phosphates, Zn
2+, and toxic colorants. Mamelkina et al. [
27] have shown that the same boehmite flocs can be used for the removal of nitrate, cyanide, and toxic metals from mining waters. Hur et al. [
28] proved that undesirable tungstate species in soil and aquatic systems can be adsorbed on the surface of boehmite. Similar conclusions were reached by Hashima et al. [
29] in their study on phosphate removal from contaminated water, which they conducted using an electrochemical cell that produces boehmite flocs. More recently, Sadri et al. [
30] studied the use of boehmite to adsorb orthophosphate and pyrophosphate at temperatures ranging from 10 to 50 °C and pH values between 3.5 and 11.5. They found that the boehmite can adsorb higher amounts of these reagents at lower pH because the zeta potential of boehmite is more positive and suitable for the adsorption of the studied fertilizers.
Once boehmite flocs have formed, their separation from water is very difficult. The gravimetric separation of the boehmite flocs from water is a slow process because the flocs have very low sedimentation velocities and require high additions of flocculants to accelerate the separation. In spite of the commercial importance of boehmite, there are very few reports available on natural and synthetic boehmite or on boehmite present in flocs recovered by froth flotation. In this research, froth flotation is proposed as an alternative method to concentrate fine particles of synthetic boehmite. In addition, operating conditions (namely, collector ratio and particle-size distribution) were evaluated to optimize their effects on recovery, with particular attention to how bubble size can influence boehmite recovery. This information could be used to design more efficient water-treatment processes.
2. Materials and Methods
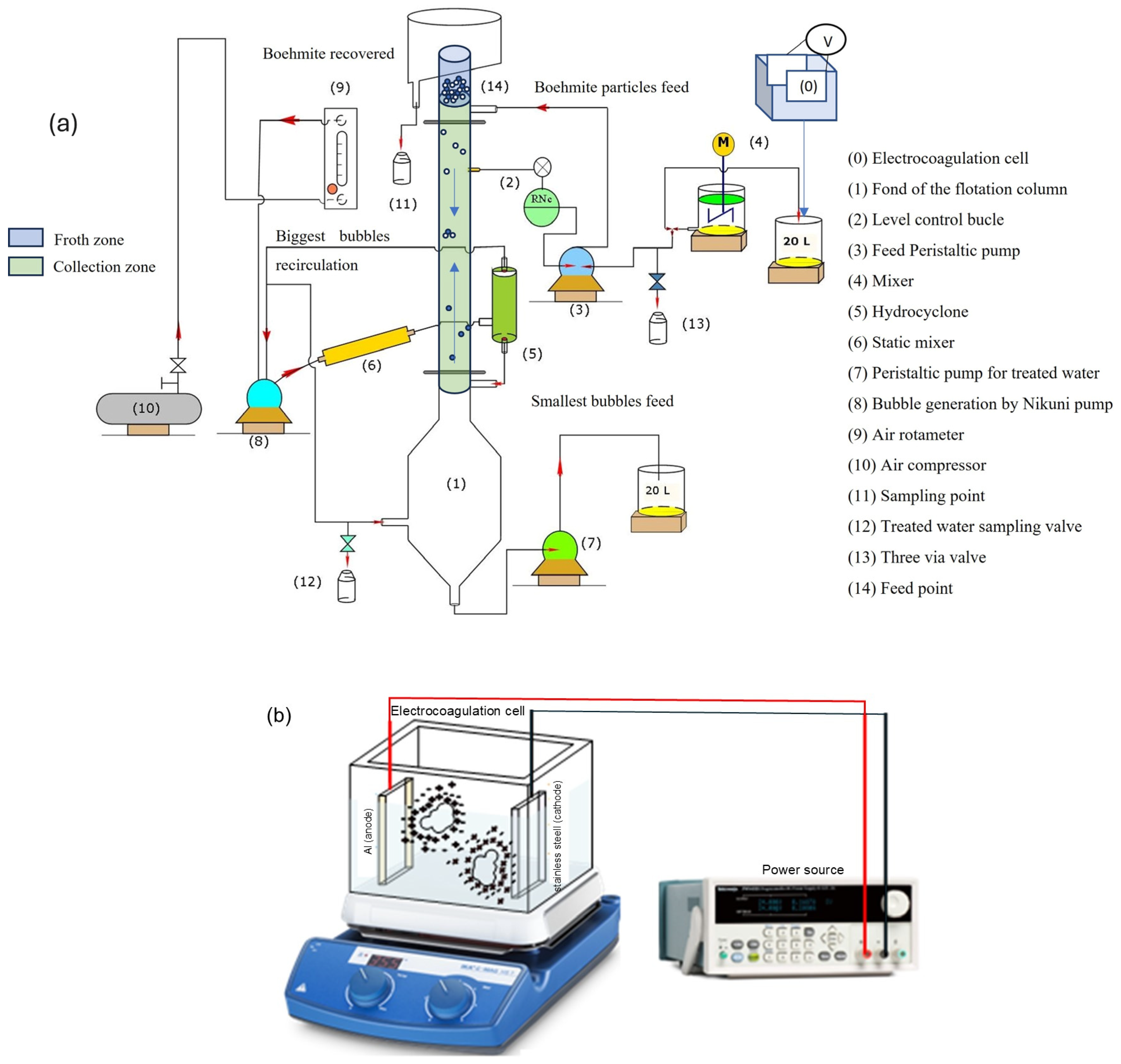
The experimental system is shown schematically in
Figure 1. Boehmite particles were obtained by drying the flocs produced during electrocoagulation using aluminum anodes (point 0). The synthetic boehmite was produced in an electrochemical cell (5 L) operating in batch mode at 25 °C, initial pH = 8.3, and equipped with a magnetic mixer device (IKA
®, C–MAG, Wilmington, NC, USA) at 120 rpm (
Figure 1b). The electrodes included an aluminum anode (6105 alloy) and a 304 stainless steel plate as a cathode. Both electrodes had a rectangular geometry (length = 10 cm, height = 15.9 cm) and were connected in parallel and separated by 10 cm. The effective electricity-transfer area was 159 cm
2, and only the front walls were in electric contact with an aqueous solution (electrolyte). The production of boehmite was accomplished with deionized water (0.05 μS/cm) and 0.01 M of NaCl (Sigma-Aldrich
®, St., Louis, MO, USA, 99%), and the initial electrical conductivity of the aqueous solution was 20 mS/cm. Once the electrolyte was in the electrochemical cell, the cathode and anode were positioned in the cell and electrically connected to the source of power (Tektronix
® pw4305, Beaverton, OR, USA) to give them a current density of 8.92 mA/ cm
2 for two h. This procedure was repeated several times to produce the mass of boehmite required for all the flotation tests. The flocs of boehmite were filtered and dried for two h at 105 °C and subsequently crushed and powdered in a laboratory ceramic ball mill.
Although, in the electrocoagulation process, the contaminants are generally adsorbed simultaneously during the formation of boehmite flocs, in this work, no contaminant-removal stage was performed. The aim was to simplify the experimental work because the focus of this study was the flotation of said synthetic boehmite flocs, since it is well known that these particles are excellent contaminant adsorbents. The slurry with synthetic boehmite and reagents was fed to the mixer for conditioning (point 4) and then fed to the flotation column (point 14) through the peristaltic pump (point 3). There was a collection zone where particles and fine bubbles could collide and join. Boehmite-particle enrichment occurred in the froth zone, and the boehmite was then recovered in the final concentrate (point 11). Particles not attached to bubbles in the collection zone fell to the bottom of the column flotation cell (1). The sampling point for tailings was at point 12, and clear water was obtained at point 7. Tests were performed in a continuous circuit, avoiding recycling and distortion of bubble-particle aggregates to obtain reproducible results. A convenient device for supplying small bubbles was developed by using a bubble hydrocyclone (point 5). This device sorted the bubbles: the smallest bubbles were fed to the column, and the biggest bubbles were redirected to a Nikuni
® centrifugal pump (Nikuni Co., Ltd., Kawasaki, Kanagawa, Japan), which can generate microbubbles [
31,
32].
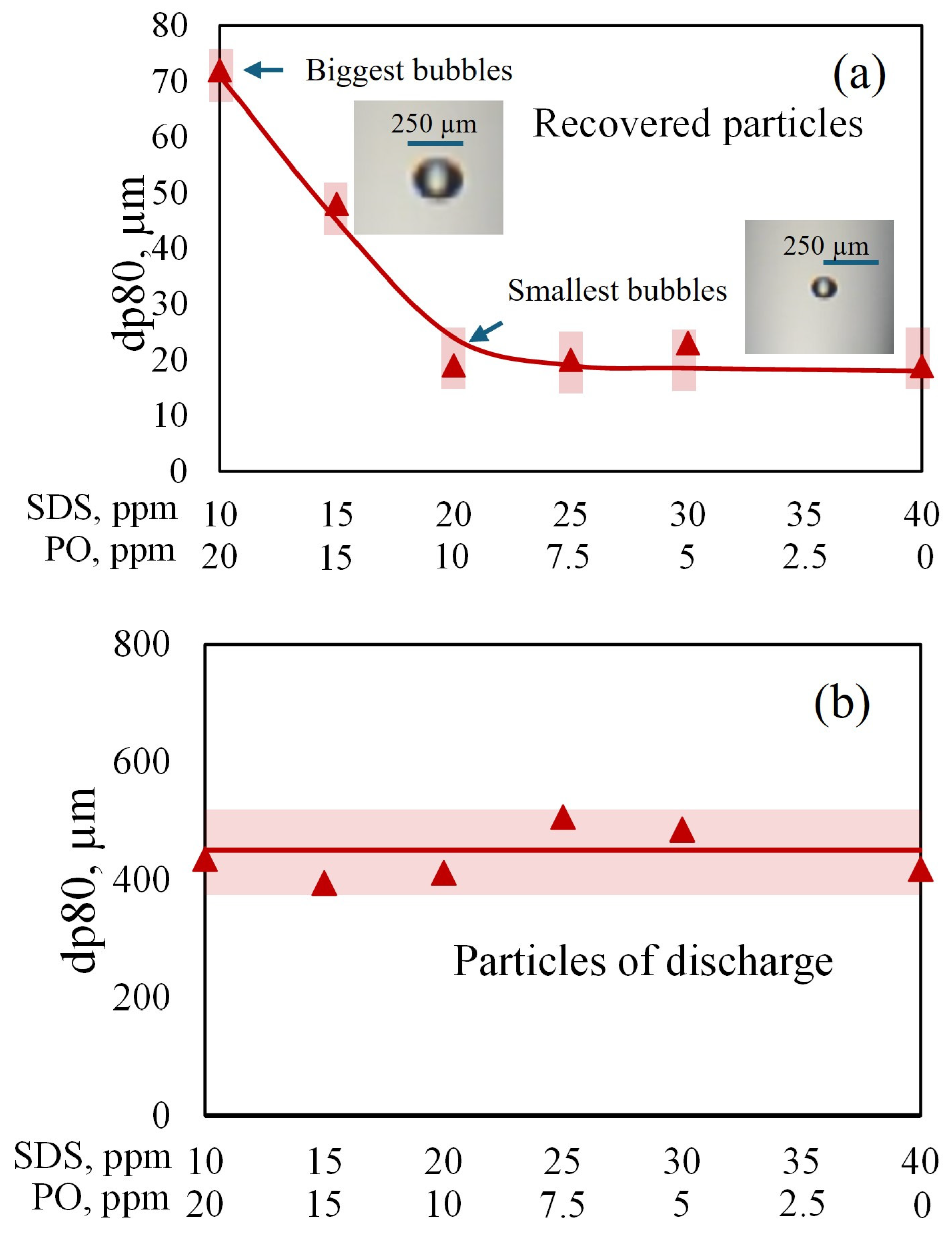
The microbubble-generating system (8) was composed of a centrifugal pump, a static mixing tube (6), an air rotameter (9), an air compressor (10), and a hydrocyclone (5), which was used to separate the smallest bubbles from the largest ones. Note that a fraction of the descending treated water was recycled to the bubble generator, since this centrifugal pump requires a mixture of water and air for its correct operation. The lower area of the column had a large cross-section (1) to prevent microbubbles from being entrained towards the treated water discharge. It is worth noting that the microbubble generator (8) and the classification system (5; hydrocyclone) were very efficient for sorting the bubbles by size and introducing only smaller bubbles into the column. The superficial velocity of the gas, Jg, was kept constant (Jg = air flow rate/cross-sectional area of the column; cm/s).
Figure 1.
(a) Schematic representation of experimental rig for boehmite microflotation. (b) Electrochemical cell for boehmite generator (point 0).
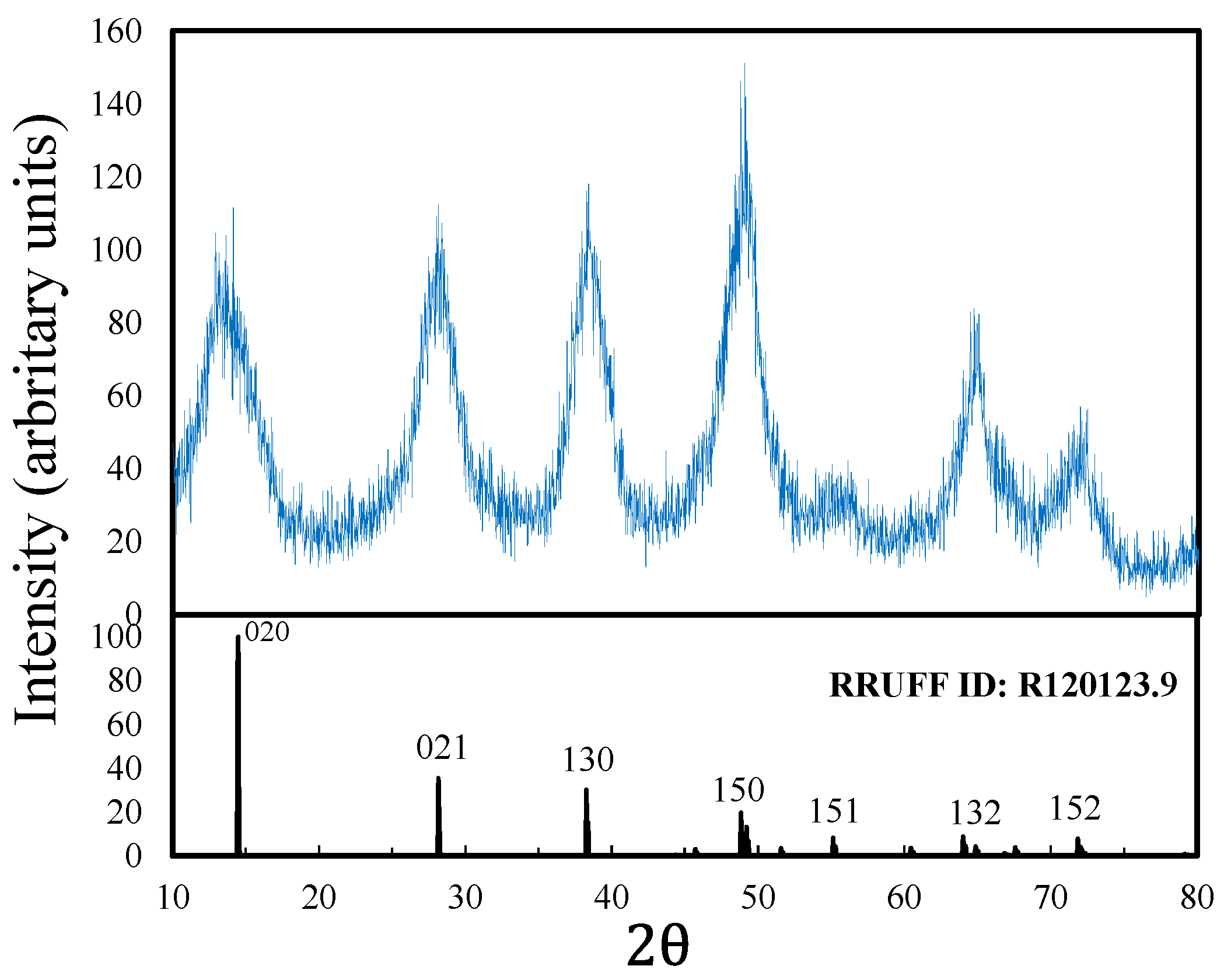
For the flotation tests, the boehmite slurry was chemically conditioned with SDS (Aldrich, 98%) and potassium oleate (PO; Aldrich, 40%), and the pH of the slurry was modified using 0.5 M HCl and NaOH solutions. The synthetic boehmite was characterized by X-ray diffraction (XRD; Bruker D8 Advance diffractometer CuKa = 1.5406 Å, Billerica, MA, USA) and scanning electron microscopy (SEM; JEOL 6300, Tokyo, Japan), coupled with energy-dispersive X-ray spectroscopy (EDS). Particle-size distribution was analyzed by laser ray diffraction (Beckman Coulter LS13320, Barcelona, Spain). The XRD spectrum of fine-ground boehmite is shown in
Figure 2. The XRD spectrum suggests the presence of orthorhombic boehmite due to the co-occurrence of the peaks at distance 2
with the peaks on the reference card (reference RRUFF ID: R120123.9).
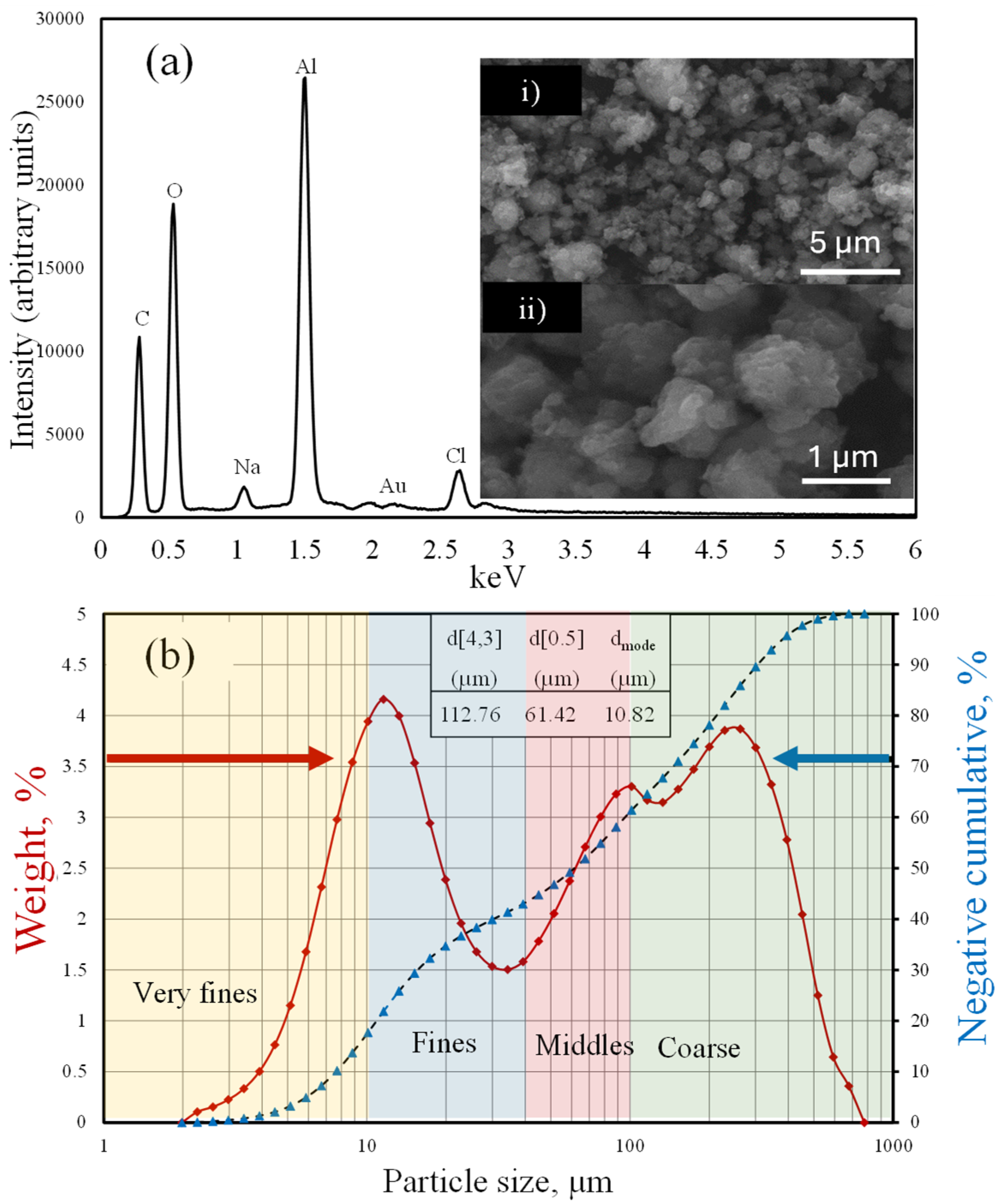
The elemental compositions and morphologies of these particles were analyzed by EDS and SEM, respectively, and the results are presented in
Figure 3a,b.
It can be observed that these particles contained Al, O, Cl, and Na and that the percentages of Al and O corresponded to the stoichiometric contents in boehmite. Au traces were attributed to a layer sprayed on the material during sample preparation to increase the sample conductivity. The micrographs in
Figure 3(a-i,a-ii) reveal that the samples were composed of semi-spherical agglomerates of different particle sizes and irregular surfaces. An important aspect of how synthetic boehmite promotes the removal of various contaminants can be observed. The semi-spherical particles, approximately 1 µm in size, had large surface areas that allowed for greater interaction with the contaminants, promoting their adsorption. However, it should be noted that, for the development of this research, boehmite macro-crystals were generated by using the electrocoagulation method and grinding to obtain the size distribution shown in
Figure 3b.
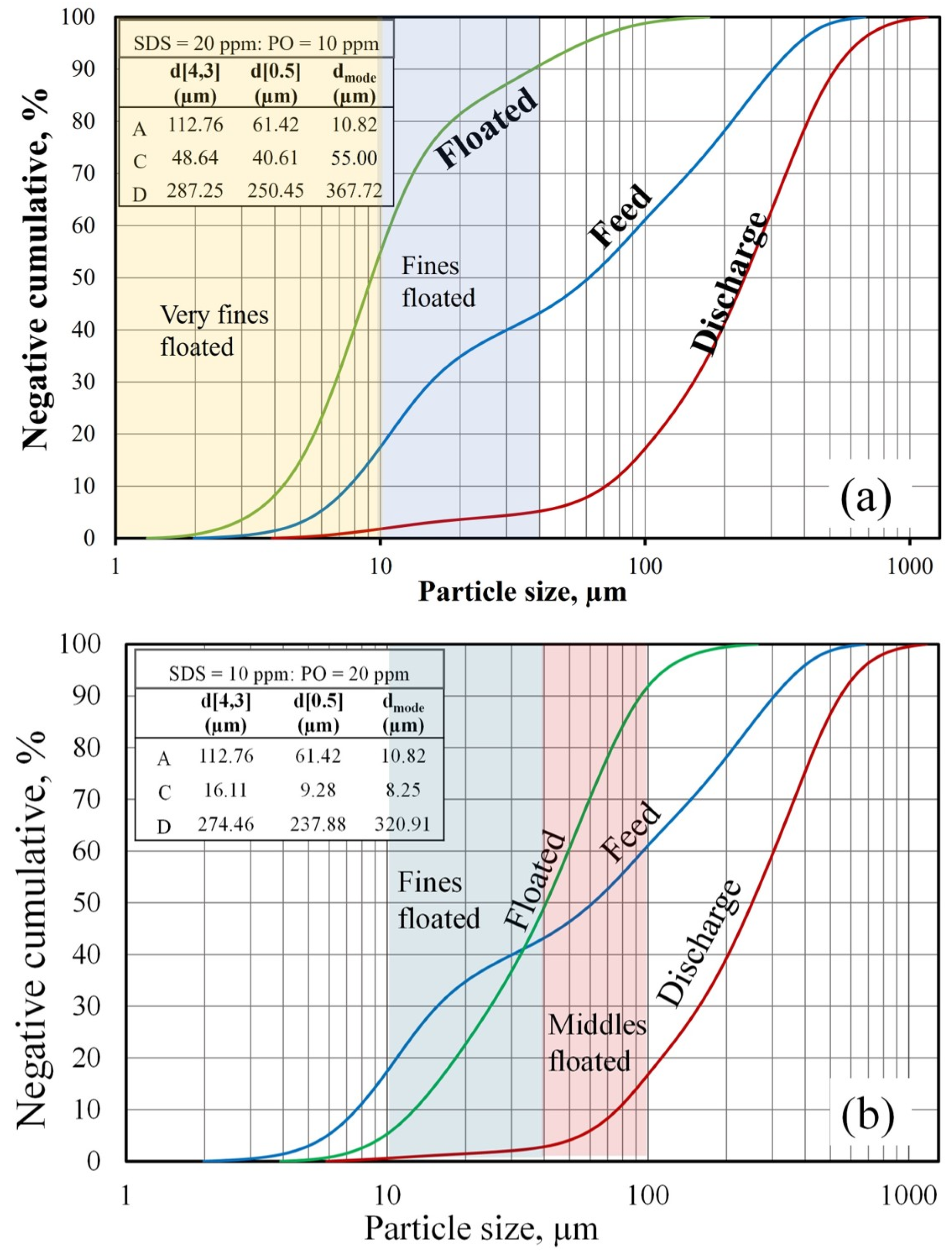
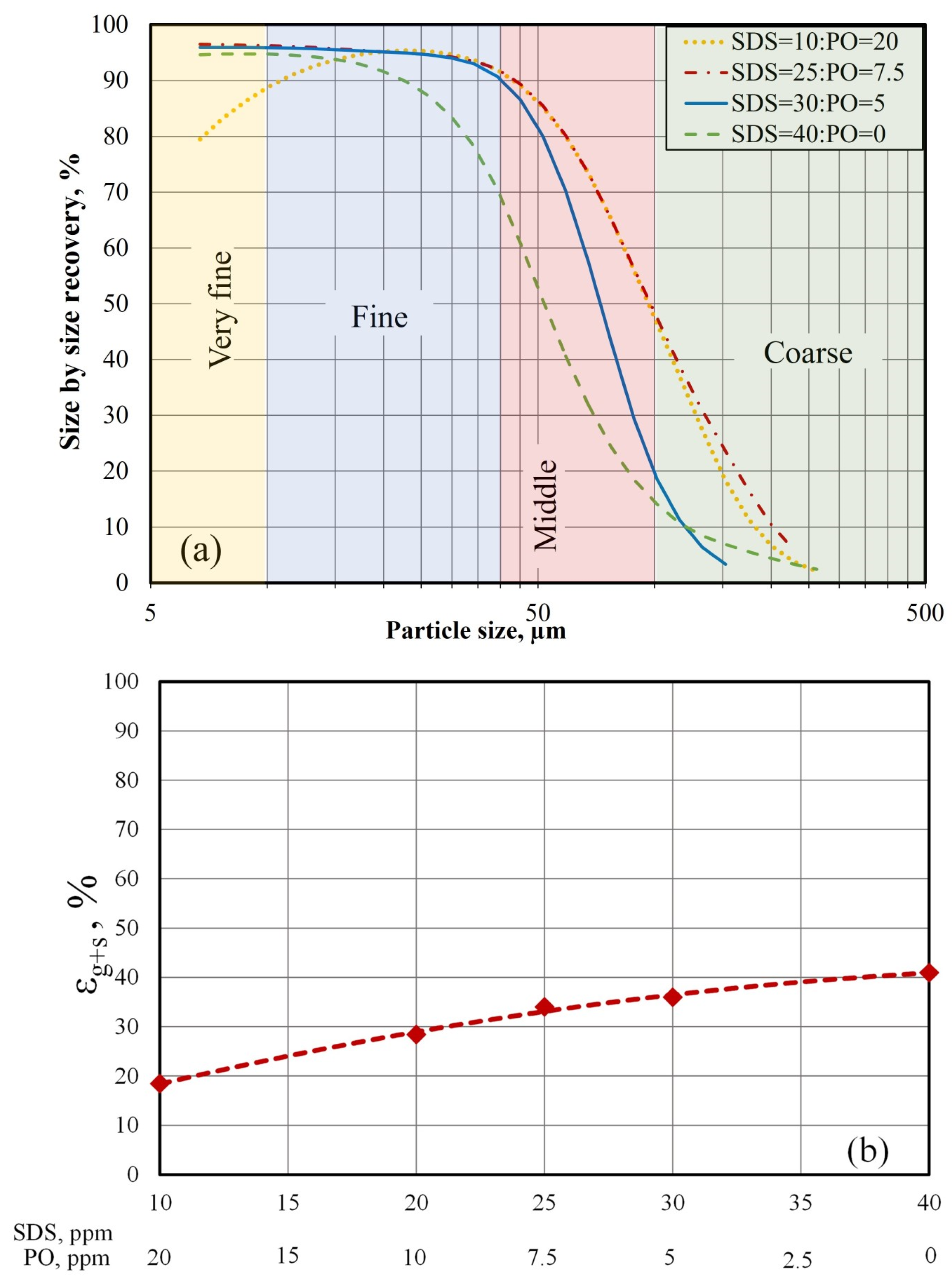
Figure 3b illustrates the bimodal particle-size distribution of the synthetic boehmite, which had a mean particle size (d [4.3] of 112.76 µm, median d [0.5] of 61.42 µm, and d[mode] of 10.82 µm). This bimodal distribution can be associated with different hard characteristics. To further simplify analysis, it was assumed that the boehmite was composed of four types of particles: very fine (<10 µm), fine (10–40 µm), midsized (40–100 µm), and coarse (>100 µm). According to the cumulative distribution curve in
Figure 4, 18% of the solids were very fine particles, 20% were fine particles, 15% were midsized particles, and the rest were coarse particles (blue axis).
Each sample of boehmite to be removed was conditioned for 30 min with SDS and PO at pH = 8.3.
Table 1 shows the operating conditions of the tests conducted in the laboratory microflotation column.
Figure 3.
(a) EDS analysis and micrographs of milled boehmite samples (i) size reference 5 µm, and (ii) size reference 1 µm. (b) Particle-size distribution of the boehmite to be anchored.
To estimate the boehmite recovery, the slurry flow of each stream (feed, floated, and tails) was sampled for 2 min during the flotation test. After collection, the slurry samples were filtered using a nylon filter (1 µm pore size). Subsequently, the cake of boehmite particles was dried at 105 °C for 2 h and weighed to evaluate the mass flow rate of solids in each stream of the column.
To evaluate the effect of the SDS:PO ratio on the air holdup (
), the electrical conductivity technique and Maxwell’s model [
33] were used. This model is expressed by the following equation:
where
and
are the electric conductivity of the dispersion mixture (liquid−gas−solid) and the continuous phase (liquid), respectively. The conductivity of the liquid was measured at the end of the experiment, after the solids had been removed and air injection had been stopped. Note that
is equivalent to the sum of the volumetric fractions of air and particles, including the particles attached to the microbubbles.