Efficient and Green Flotation Separation of Molybdenite from Chalcopyrite Using 1-Thioglycerol as Depressant
Abstract
1. Introduction
2. Experimental Materials and Methods
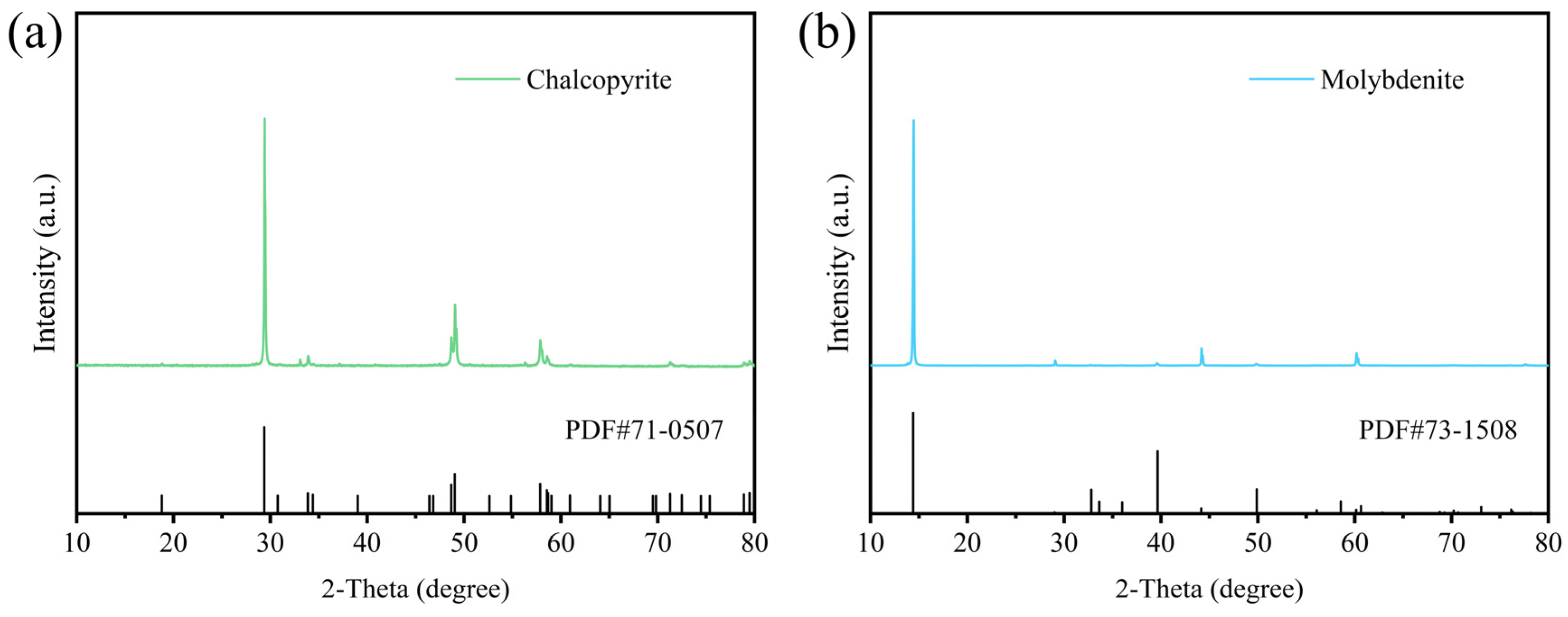
2.1. Materials and Reagents
2.2. Micro-Flotation Experiments
2.3. Zeta Potential Measurements
2.4. FT-IR Measurements
2.5. Contact Angle Measurements
2.6. XPS Analysis
3. Results and Discussion
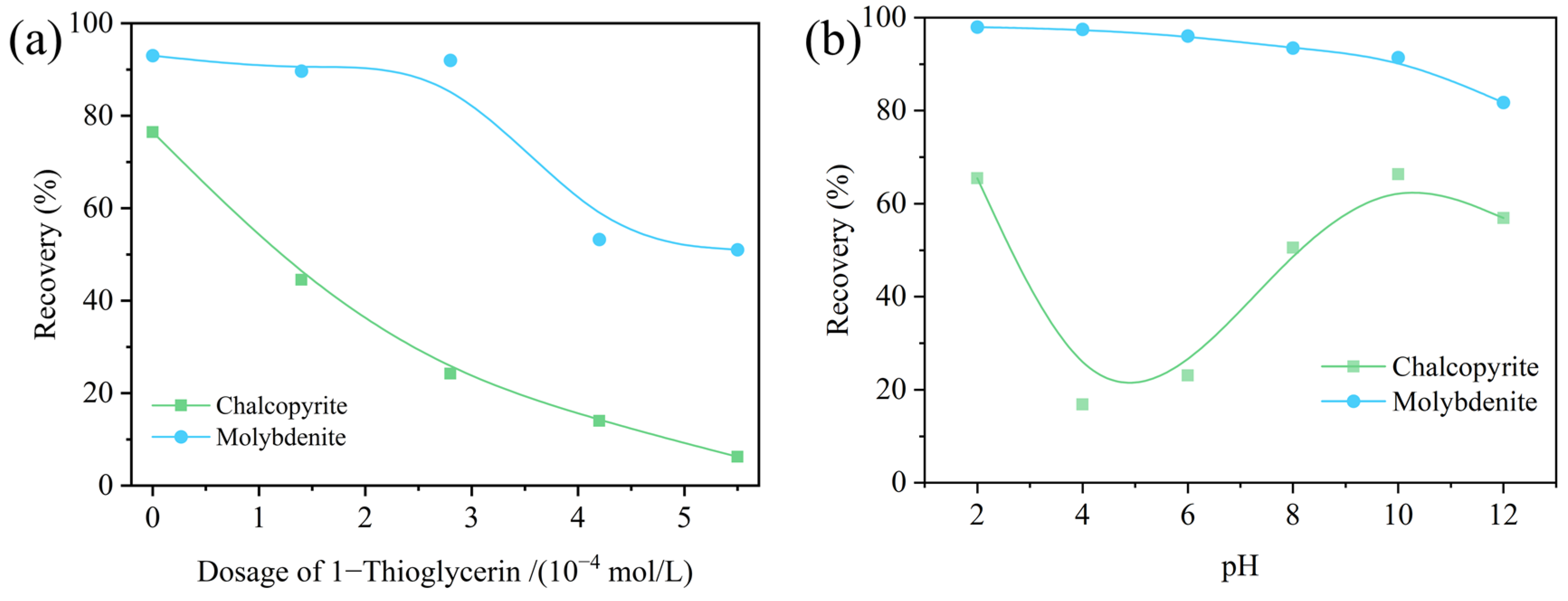
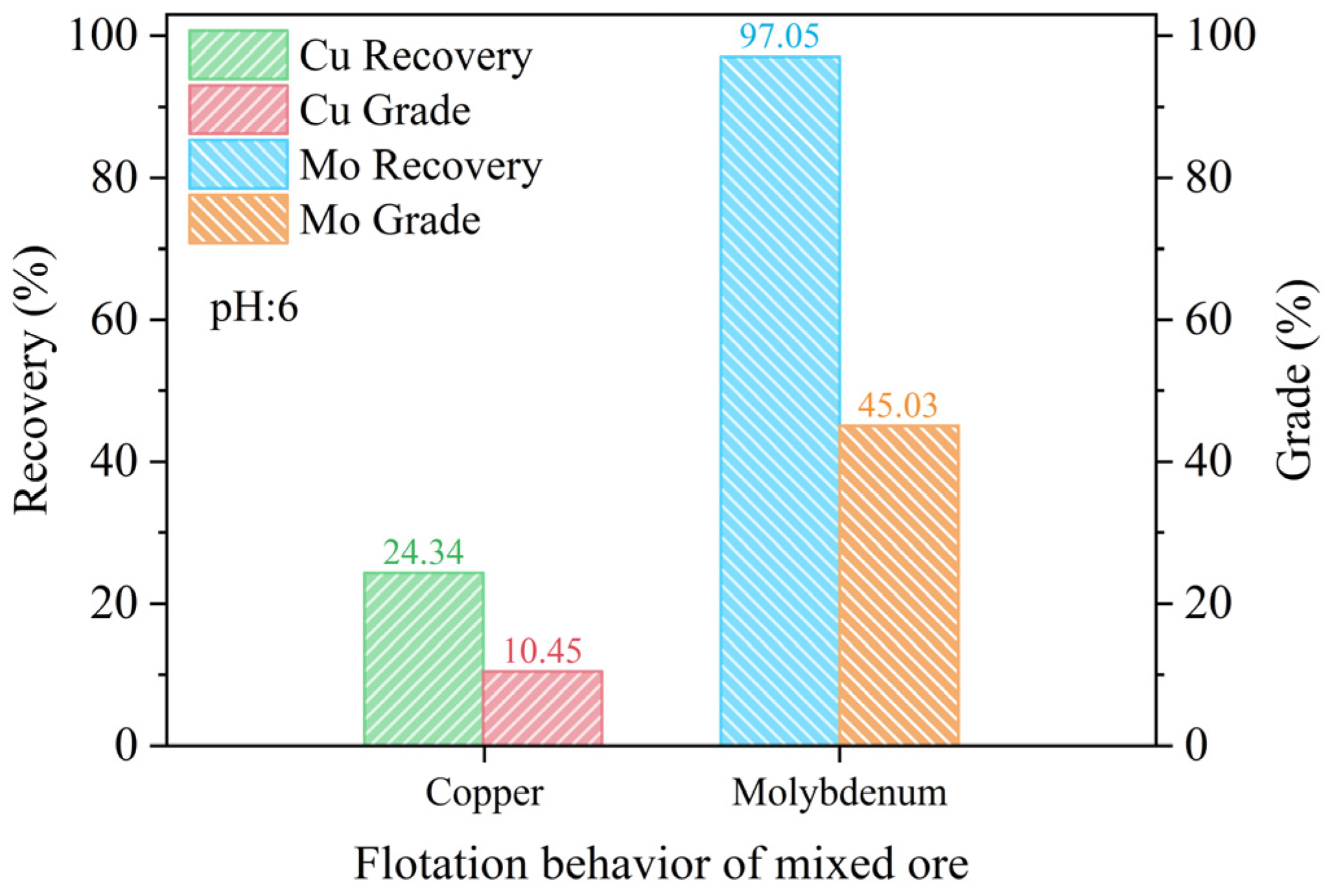
3.1. Flotation Experiments
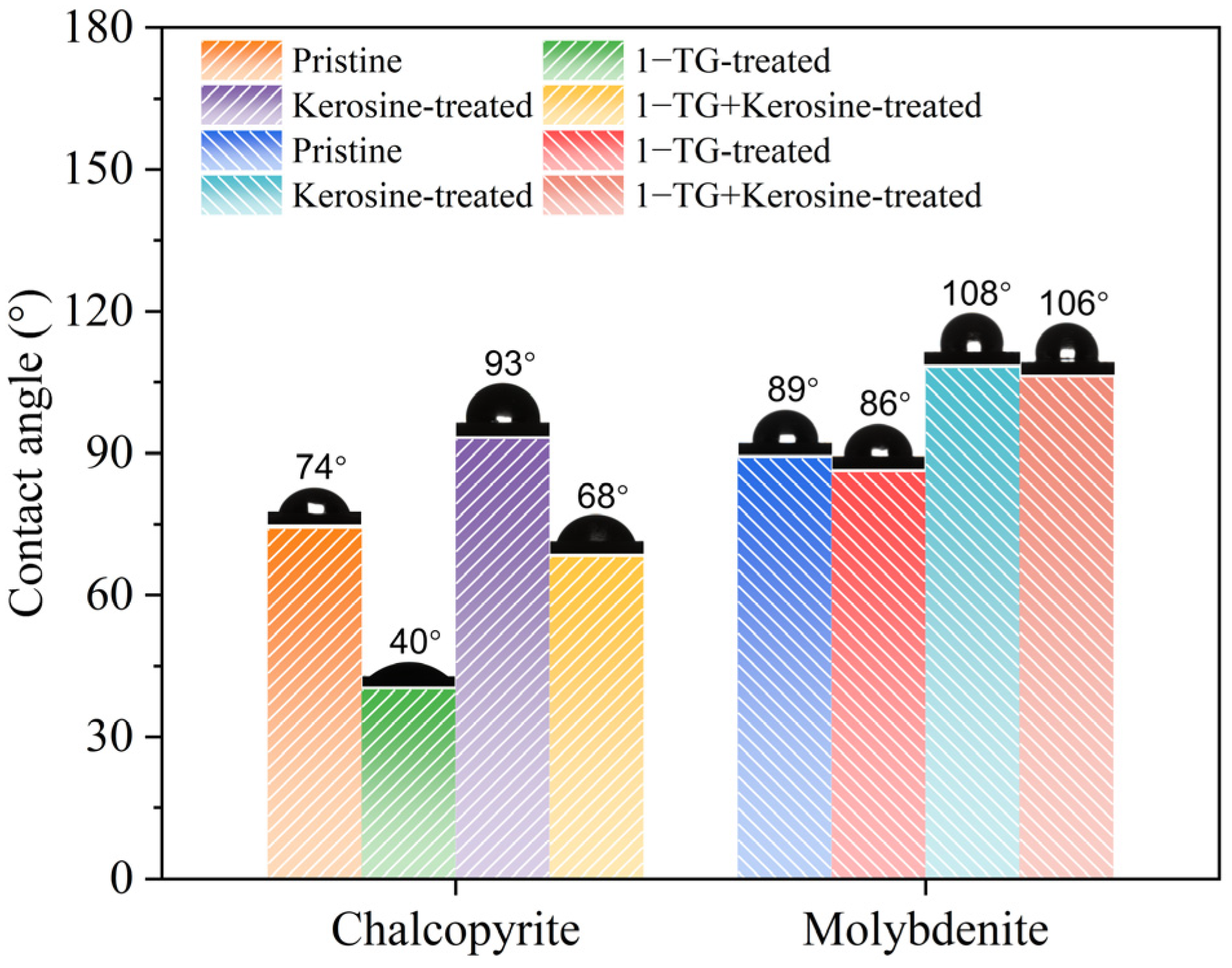
3.2. Contact Angle Test
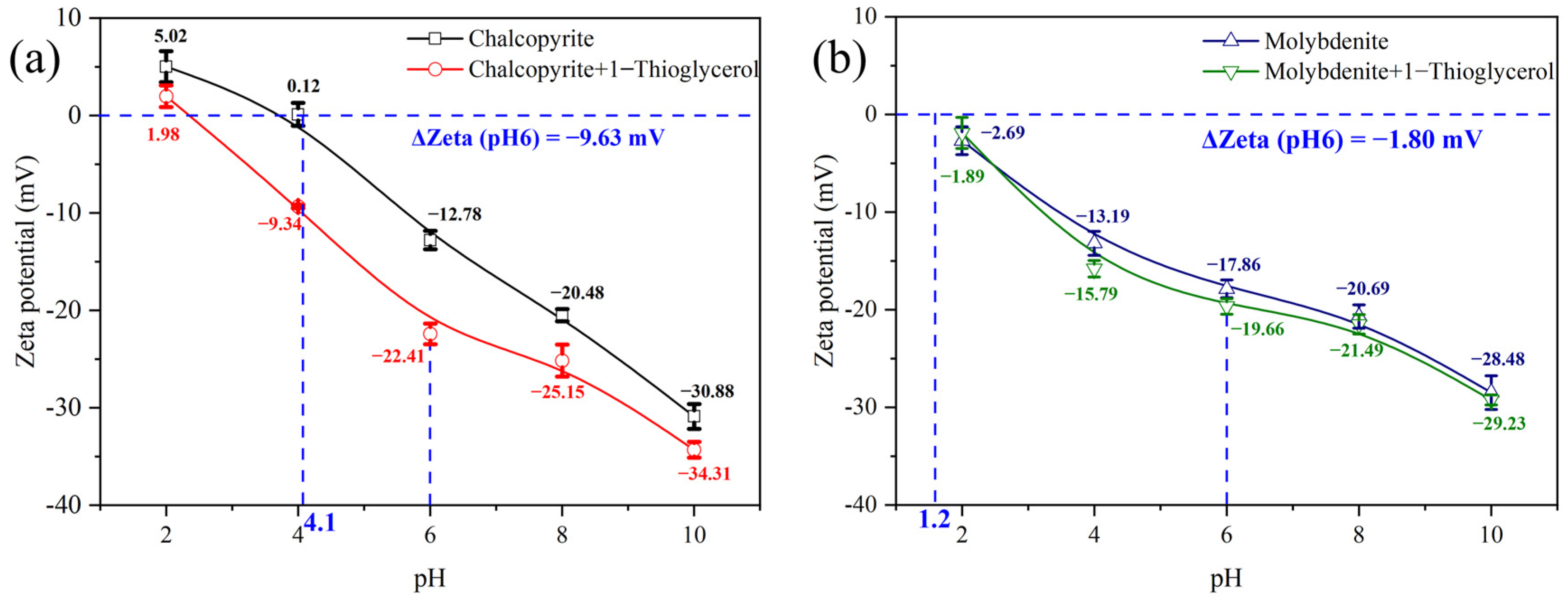
3.3. Zeta Potential Characterizations
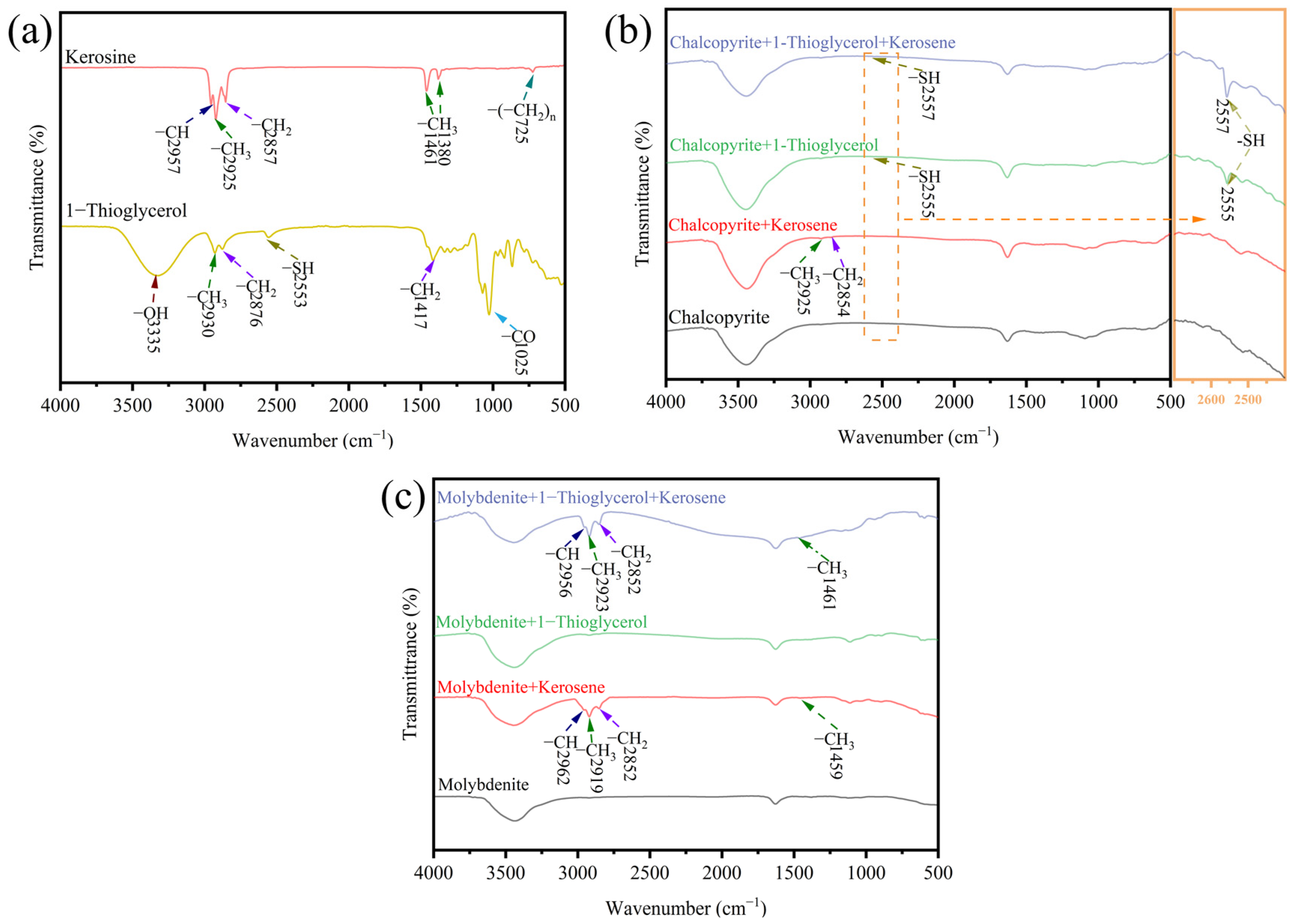
3.4. FT-IR Analysis
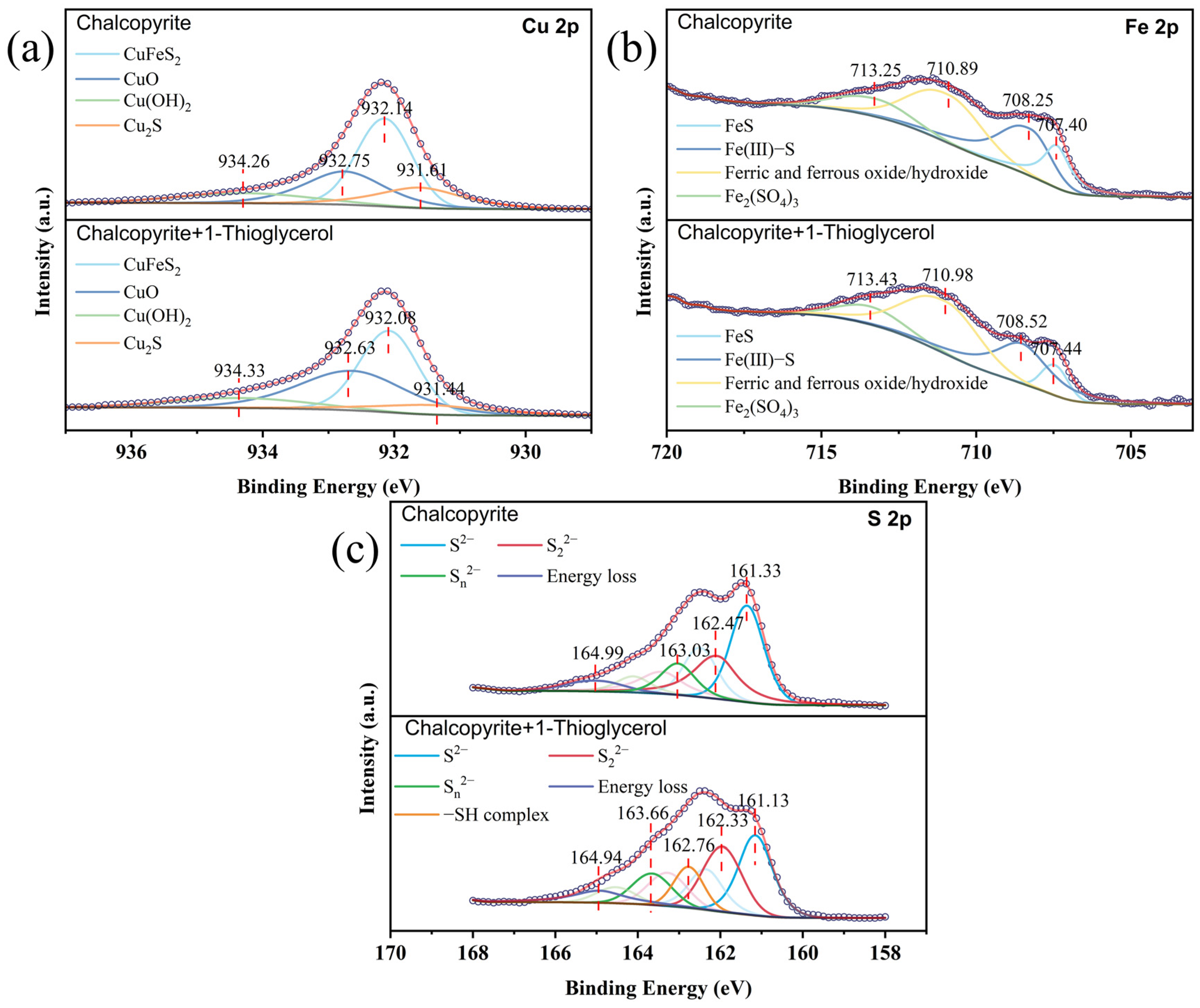
3.5. XPS Analysis
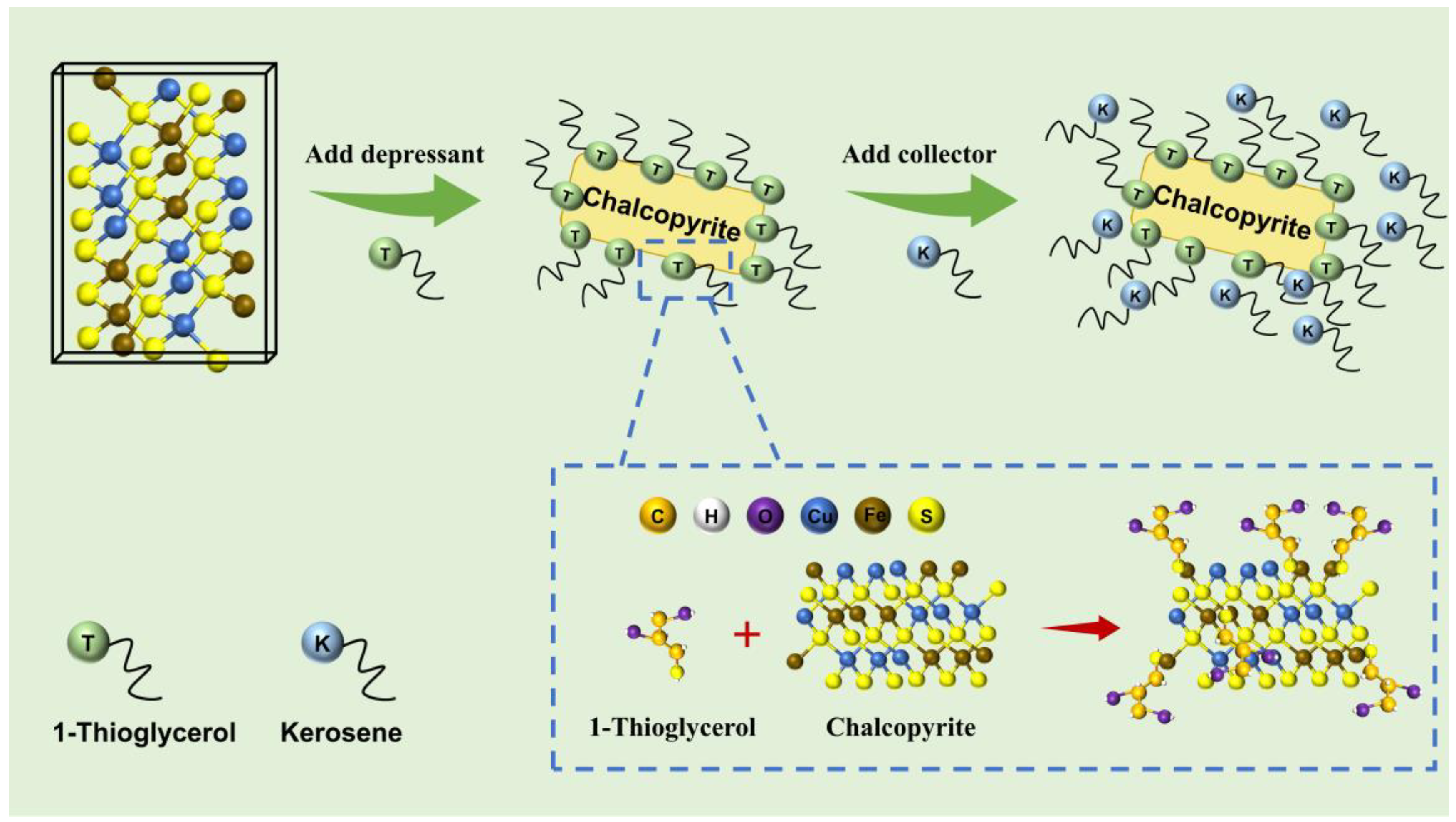
3.6. Possible Adsorption Mechanism
4. Conclusions
- (1)
- 1-TG demonstrated a strong depression effect on chalcopyrite flotation, with minimal impact on molybdenite when kerosene was used as the collector at pH 6.
- (2)
- Robust adsorption was realized between 1-TG and the chalcopyrite surface, which realized the selectively enhanced hydrophilicity of the chalcopyrite surface and the inhibition of subsequent collector adsorption.
- (3)
- The interaction between 1-TG and chalcopyrite was driven by the chemosorption of the S-H group with active iron sites on the chalcopyrite surface.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Qin, X.; Liu, J.; Yu, Y.; Hao, J.; Gao, H.; Li, D.; Dai, L. Novel application of depressant sodium mercaptoacetate in flotation separation of chalcopyrite and pyrite. Adv. Powder Technol. 2023, 34, 104141. [Google Scholar] [CrossRef]
- Yang, J.; Chen, L.; Xue, Z.; Yang, K.; Shao, Y.; Zeng, J.; Gao, Y. Performance evaluation of PHGMS technology for superfine chalcopyrite-molybdenite separation. Sep. Purif. Technol. 2024, 336, 126136. [Google Scholar] [CrossRef]
- Lunk, H.-J.; Hartl, H. Discovery, properties and applications of molybdenum and its compounds. ChemTexts 2017, 3, 13. [Google Scholar] [CrossRef]
- Hao, J.; Liu, J.; Yu, Y.; Gao, H.; Qin, X.; Bai, X. Depressants for separation of chalcopyrite and molybdenite: Review and prospects. Miner. Eng. 2023, 201, 108209. [Google Scholar] [CrossRef]
- Castro, S.; Lopez-Valdivieso, A.; Laskowski, J.S. Review of the flotation of molybdenite. Part I: Surface properties and floatability. Int. J. Miner. Process. 2016, 148, 48–58. [Google Scholar] [CrossRef]
- Yuan, D.; Cadien, K.; Liu, Q.; Zeng, H. Separation of talc and molybdenite: Challenges and opportunities. Miner. Eng. 2019, 143, 105923. [Google Scholar] [CrossRef]
- Liu, G.-y.; Lu, Y.-p.; Zhong, H.; Cao, Z.-f.; Xu, Z.-h. A novel approach for preferential flotation recovery of molybdenite from a porphyry copper–molybdenum ore. Miner. Eng. 2012, 36–38, 37–44. [Google Scholar] [CrossRef]
- Bulatovic, S.M.; Wyslouzil, D.M.; Kant, C. Operating practices in the beneficiation of major porphyry copper/molybdenum plants from Chile: Innovated technology and opportunities, a review. Miner. Eng. 1998, 11, 313–331. [Google Scholar] [CrossRef]
- Yi, G.; Macha, E.; Van Dyke, J.; Ed Macha, R.; McKay, T.; Free, M.L. Recent progress on research of molybdenite flotation: A review. Adv. Colloid Interface Sci. 2021, 295, 102466. [Google Scholar] [CrossRef]
- Yang, B.; Zeng, M.; Zhu, H.; Huang, P.; Li, Z.; Song, S. Selective depression of molybdenite using a novel eco-friendly depressant in Cu-Mo sulfides flotation system. Colloids Surf. A Physicochem. Eng. Asp. 2021, 622, 126683. [Google Scholar] [CrossRef]
- Pan, C.-L.; Wei, X.-X.; Zhang, X.-G.; Xu, Y.-W.; Xu, P.-F.; Luo, Y.-C. 2-((5-Mercapto-1,3,4-thiadiazol-2-yl)thio)acetic acid as a novel chalcopyrite depressant for selective flotation separation of molybdenite from chalcopyrite. Miner. Eng. 2022, 183, 107625. [Google Scholar] [CrossRef]
- Petrus, H.T.B.M.; Hirajima, T.; Sasaki, K.; Okamoto, H. Effects of sodium thiosulphate on chalcopyrite and tennantite: An insight for alternative separation technique. Int. J. Miner. Process. 2012, 102–103, 116–123. [Google Scholar] [CrossRef]
- Li, M.; Wei, D.; Liu, Q.; Liu, W.; Zheng, J.; Sun, H. Flotation separation of copper–molybdenum sulfides using chitosan as a selective depressant. Miner. Eng. 2015, 83, 217–222. [Google Scholar] [CrossRef]
- Liao, R.; Feng, Q.; Wen, S.; Liu, J. Flotation separation of molybdenite from chalcopyrite using ferrate(VI) as selective depressant in the absence of a collector. Miner. Eng. 2020, 152, 106369. [Google Scholar] [CrossRef]
- Suyantara, G.P.W.; Miki, H.; Ochi, D.; Aoki, Y.; Ura, K.; Berdakh, D.; Ulmaszoda, A.; Dwitama, E.P.; Sasaki, K.; Hirajima, T. Sodium metabisulfite as a copper depressant in the selective flotation of copper-molybdenum concentrate using seawater. Adv. Powder Technol. 2023, 34, 104258. [Google Scholar] [CrossRef]
- Sarquís, P.E.; Menéndez-Aguado, J.M.; Mahamud, M.M.; Dzioba, R. Tannins: The organic depressants alternative in selective flotation of sulfides. J. Clean. Prod. 2014, 84, 723–726. [Google Scholar] [CrossRef]
- Yao, W.; Wu, Y.; Li, M.; Cui, R.; Li, J.; Yang, Z.; Fu, Y.; Pan, Z.; Wang, D.; Zhang, M. Exploring Dextrin as an Eco-Friendly Depressant for Selective Flotation Separation of Scheelite and Calcite Minerals. Miner. Process. Extr. Metall. Rev. 2023, 46, 143–152. [Google Scholar] [CrossRef]
- Huang, W.; Liu, R.; Jiang, F.; Tang, H.; Wang, L.; Sun, W. Adsorption mechanism of 3-mercaptopropionic acid as a chalcopyrite depressant in chalcopyrite and galena separation flotation. Colloids Surf. A Physicochem. Eng. Asp. 2022, 641, 128063. [Google Scholar] [CrossRef]
- Yang, B.; Huang, P.; An, Q. An efficient chalcopyrite depressant for Cu-Mo separation and its interaction mechanism: Adsorption configuration and DFT calculations. J. Mol. Liq. 2022, 345, 118171. [Google Scholar] [CrossRef]
- Zhang, S.; Feng, Q.; Wen, S.; Xian, Y.; Liu, J.; Liang, G. Flotation separation of chalcopyrite from molybdenite with sodium thioglycolate: Mechanistic insights from experiments and MD simulations. Sep. Purif. Technol. 2024, 342, 126958. [Google Scholar] [CrossRef]
- Zhang, X.; Jiang, X.; Luo, A.; Chen, J.; Meng, Y. A modified co-polyacrylamide to depress molybdenite in Cu/Mo separation and its competitive adsorption mechanism between reagents. Sep. Purif. Technol. 2024, 337, 126239. [Google Scholar] [CrossRef]
- Hao, J.; Liu, J.; Lai, H.; Liao, R.; Gao, H.; bai, X. A new application of Cu2+ on differential modification to promote copper-molybdenum separation with a novel chalcopyrite depressant amidinothiourea. Sep. Purif. Technol. 2025, 353, 128282. [Google Scholar] [CrossRef]
- Huang, P.; Wang, L.; Liu, Q. Depressant function of high molecular weight polyacrylamide in the xanthate flotation of chalcopyrite and galena. Int. J. Miner. Process. 2014, 128, 6–15. [Google Scholar] [CrossRef]
- Lei, Y.; Zhou, Y.; Li, Y.; Liu, Y.; Gao, H.; Lin, L.; Xu, H. Intelligent controlled release of sodium thioglycolate in poloxamer-cationic surfactant temperature-sensitive hydrogels. Colloids Surf. A Physicochem. Eng. Asp. 2023, 677, 132222. [Google Scholar] [CrossRef]
- Aboulaich, A.; Balan, L.; Ghanbaja, J.; Medjahdi, G.; Merlin, C.; Schneider, R. Aqueous Route to Biocompatible ZnSe:Mn/ZnO Core/Shell Quantum Dots Using 1-Thioglycerol as Stabilizer. Chem. Mater. 2011, 23, 3706–3713. [Google Scholar] [CrossRef]
- Erdemir, E.; Suna, G.; Liv, L.; Eğlence-Bakır, S.; Şahin, M.; Karakuş, E. Smartphone-assisted dual-channel discriminative detection of Hg(II) and Cu(II) ions with a simple, unique, readily available probe. Sens. Actuators B Chem. 2023, 382, 133487. [Google Scholar] [CrossRef]
- Wang, L.; Lyu, W.; Zhou, W.; Zhang, H. The role of sodium phytate in the flotation separation of smithsonite from calcite. Miner. Eng. 2022, 187, 107775. [Google Scholar] [CrossRef]
- You, K.; Kim, K.; Han, S.; Kwon, S. Direct measurement of interaction force between solid surface and air bubble: Relationship between interaction force and contact angle. Miner. Eng. 2020, 152, 106358. [Google Scholar] [CrossRef]
- Zhao, L.; Zhuang, L.; Zhang, Z. The direct observation and distribution regularity of contact angle for spherical particle attached to air bubble in static three-phase system. Miner. Eng. 2023, 201, 108191. [Google Scholar] [CrossRef]
- Kruszelnicki, M.; Polowczyk, I.; Kowalczuk, P.B. Insight into the influence of surface wettability on flotation properties of solid particles—Critical contact angle in flotation. Powder Technol. 2024, 431, 119056. [Google Scholar] [CrossRef]
- Zhao, Q.; Yang, H.; Tong, L.; Jin, R.; Ma, P. Understanding the effect of grinding media on the adsorption mechanism of cyanide to chalcopyrite surface by ToF–SIMS, XPS, contact angle, zeta potential and flotation. Colloids Surf. A Physicochem. Eng. Asp. 2022, 644, 128799. [Google Scholar] [CrossRef]
- He, T.; Li, H.; Jin, J.; Peng, Y.; Wang, Y.; Wan, H. Improving fine molybdenite flotation using a combination of aliphatic hydrocarbon oil and polycyclic aromatic hydrocarbon. Results Phys. 2019, 12, 1050–1055. [Google Scholar] [CrossRef]
- Chen, X.; Gu, G.-h.; Chen, Z.-x. Seaweed glue as a novel polymer depressant for the selective separation of chalcopyrite and galena. Int. J. Miner. Metall. Mater. 2019, 26, 1495–1503. [Google Scholar] [CrossRef]
- Chung, H.; Ku, M.-S.; Lee, J.-S. Comparison of near-infrared and mid-infrared spectroscopy for the determination of distillation property of kerosene. Vib. Spectrosc. 1999, 20, 155–163. [Google Scholar] [CrossRef]
- Lin, Q.-q.; Gu, G.-h.; Wang, H.; Liu, Y.-c.; Fu, J.-g.; Wang, C.-q. Flotation mechanisms of molybdenite fines by neutral oils. Int. J. Miner. Metall. Mater. 2018, 25, 1–10. [Google Scholar] [CrossRef]
- Zheng, M.; Peng, T.; Wang, Y.; Luo, L.; Shao, W.; Gao, J.; Lu, Q. Flotability of galena and pyrite using 2-mercaptobenzimidazole as a chelating agent: Adsorption characteristics and flotation mechanisms. Powder Technol. 2021, 393, 449–460. [Google Scholar] [CrossRef]
- Shen, Z.; Feng, Q.; Wen, S.; Wang, H.; Cao, J.; Song, Z. Insights into flotation separation mechanism of chalcopyrite from galena using mercaptosuccinic acid as a novel depressant: Experimental and MDS studies. Powder Technol. 2024, 445, 120103. [Google Scholar] [CrossRef]
- Zeng, Y.; Zhang, X.; Dong, Y.; Hua, Z.; Wu, X.; Sun, W.; Wang, L.; Tang, H.; Guan, Q. Flotation behavior and mechanism of tannic acid as a depressant on Cu-Mo flotation separation. J. Ind. Eng. Chem. 2024, 131, 623–634. [Google Scholar] [CrossRef]
- Zhang, W.; Jin, X.; Feng, Z.; Zheng, R.; Cao, J.; Chen, J.; Sun, W.; Xu, S.; Gao, Z. Collectorless flotation separation of molybdenite from complex sulfide minerals employing a bi-carbonyl depressant. Sep. Purif. Technol. 2023, 322, 124207. [Google Scholar] [CrossRef]
- Yang, B.; Yan, H.; Zeng, M.; Huang, P.; Jia, F.; Teng, A. A novel copper depressant for selective flotation of chalcopyrite and molybdenite. Miner. Eng. 2020, 151, 106309. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, F.; He, S.; Sun, W.; Luo, Y.; Tang, H. Efficient and Green Flotation Separation of Molybdenite from Chalcopyrite Using 1-Thioglycerol as Depressant. Metals 2025, 15, 299. https://doi.org/10.3390/met15030299
Jiang F, He S, Sun W, Luo Y, Tang H. Efficient and Green Flotation Separation of Molybdenite from Chalcopyrite Using 1-Thioglycerol as Depressant. Metals. 2025; 15(3):299. https://doi.org/10.3390/met15030299
Chicago/Turabian StyleJiang, Feng, Shuai He, Wei Sun, Yuanjia Luo, and Honghu Tang. 2025. "Efficient and Green Flotation Separation of Molybdenite from Chalcopyrite Using 1-Thioglycerol as Depressant" Metals 15, no. 3: 299. https://doi.org/10.3390/met15030299
APA StyleJiang, F., He, S., Sun, W., Luo, Y., & Tang, H. (2025). Efficient and Green Flotation Separation of Molybdenite from Chalcopyrite Using 1-Thioglycerol as Depressant. Metals, 15(3), 299. https://doi.org/10.3390/met15030299






