Enhancement of Corrosion Resistance of MAO/Polydopamine/Polylactic Acid-Coated AZ31 Magnesium Alloy for Biomedical Applications
Abstract
1. Introduction
2. Materials and Methods
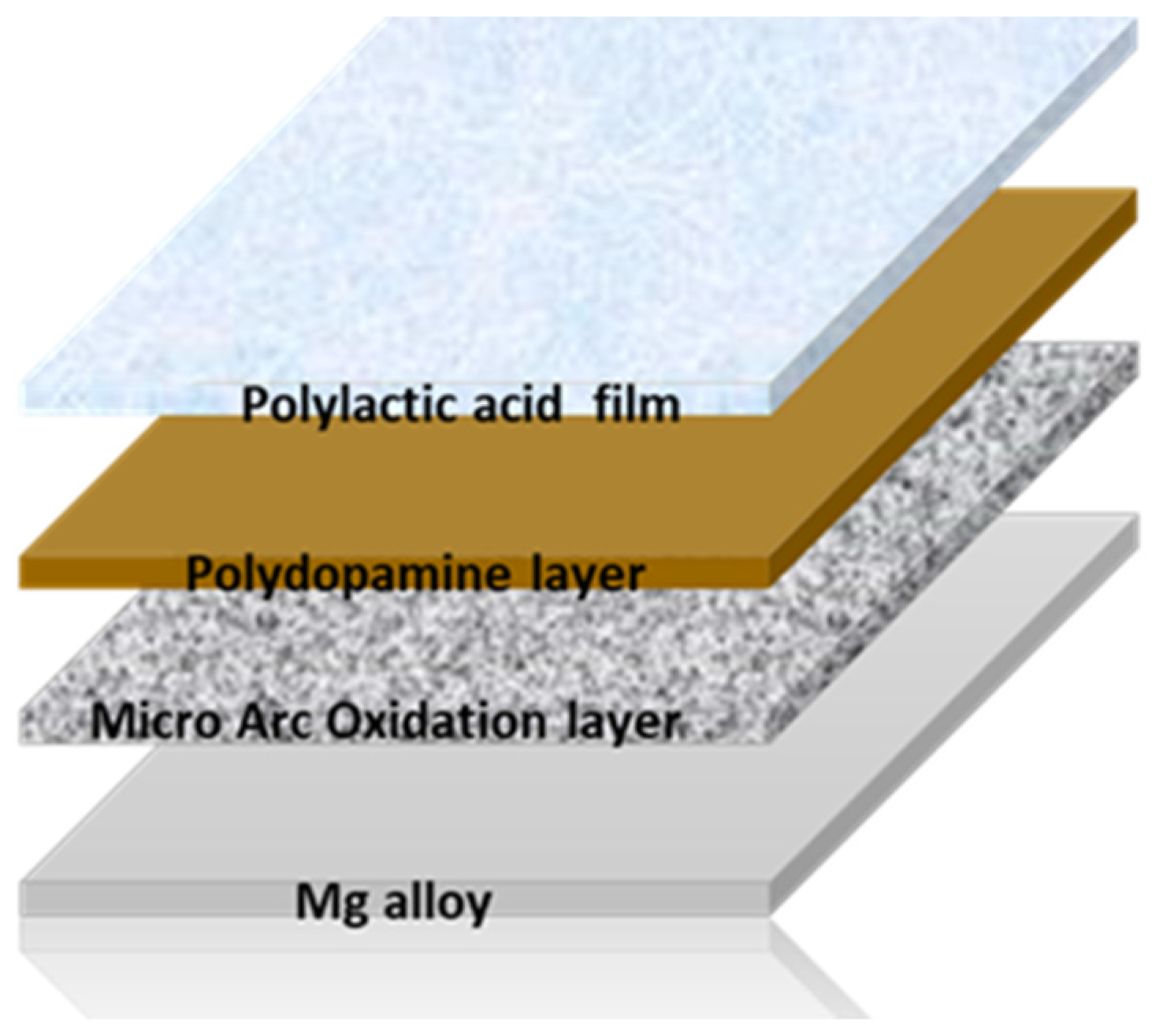
2.1. Sample Preparation
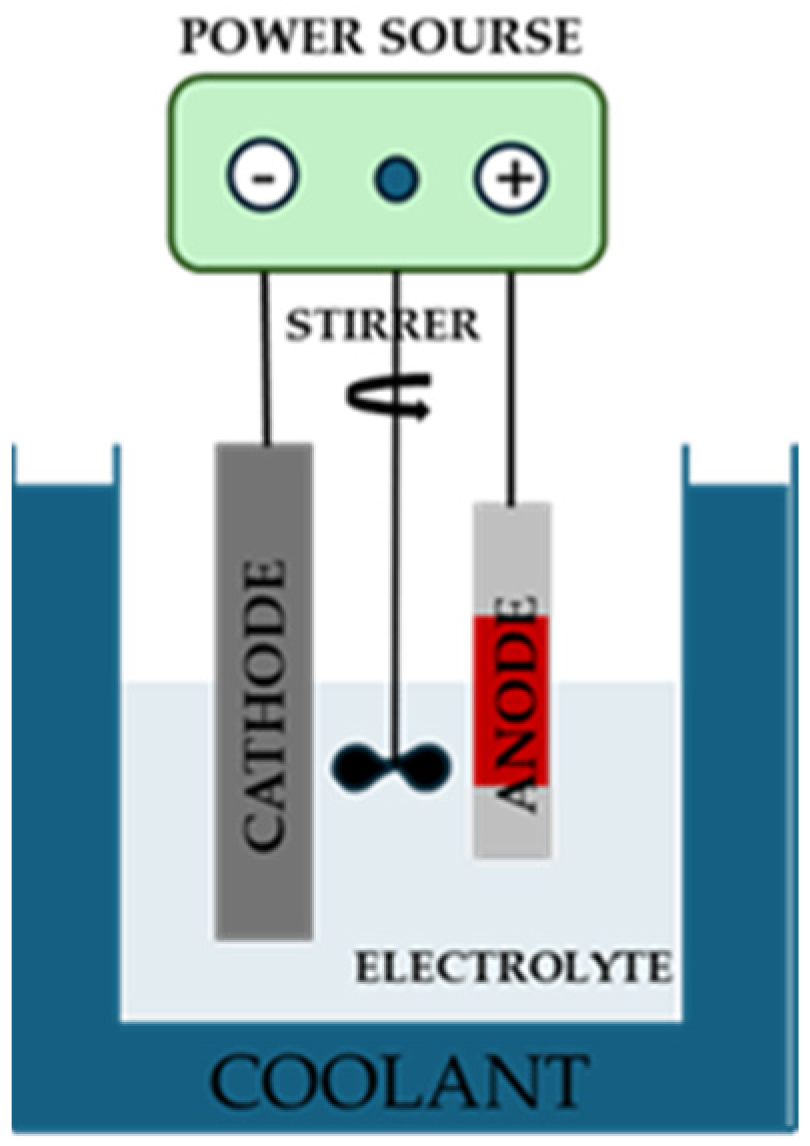
2.1.1. Treatment: Micro-Arc Oxidation
2.1.2. Bio-Inspired Coating
2.1.3. Polylactic Acid Film
2.2. Surface Characterizations
3. Results
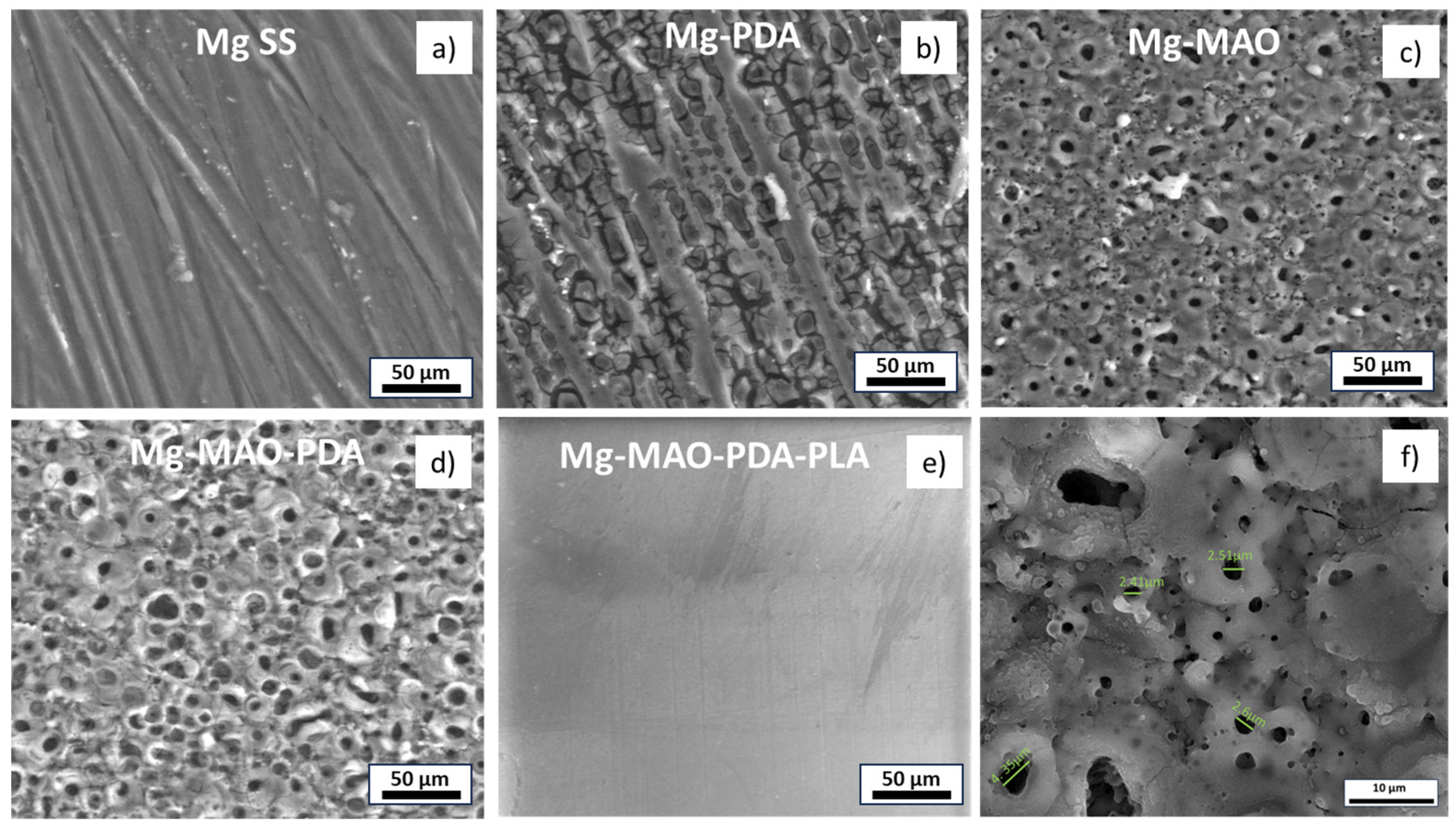
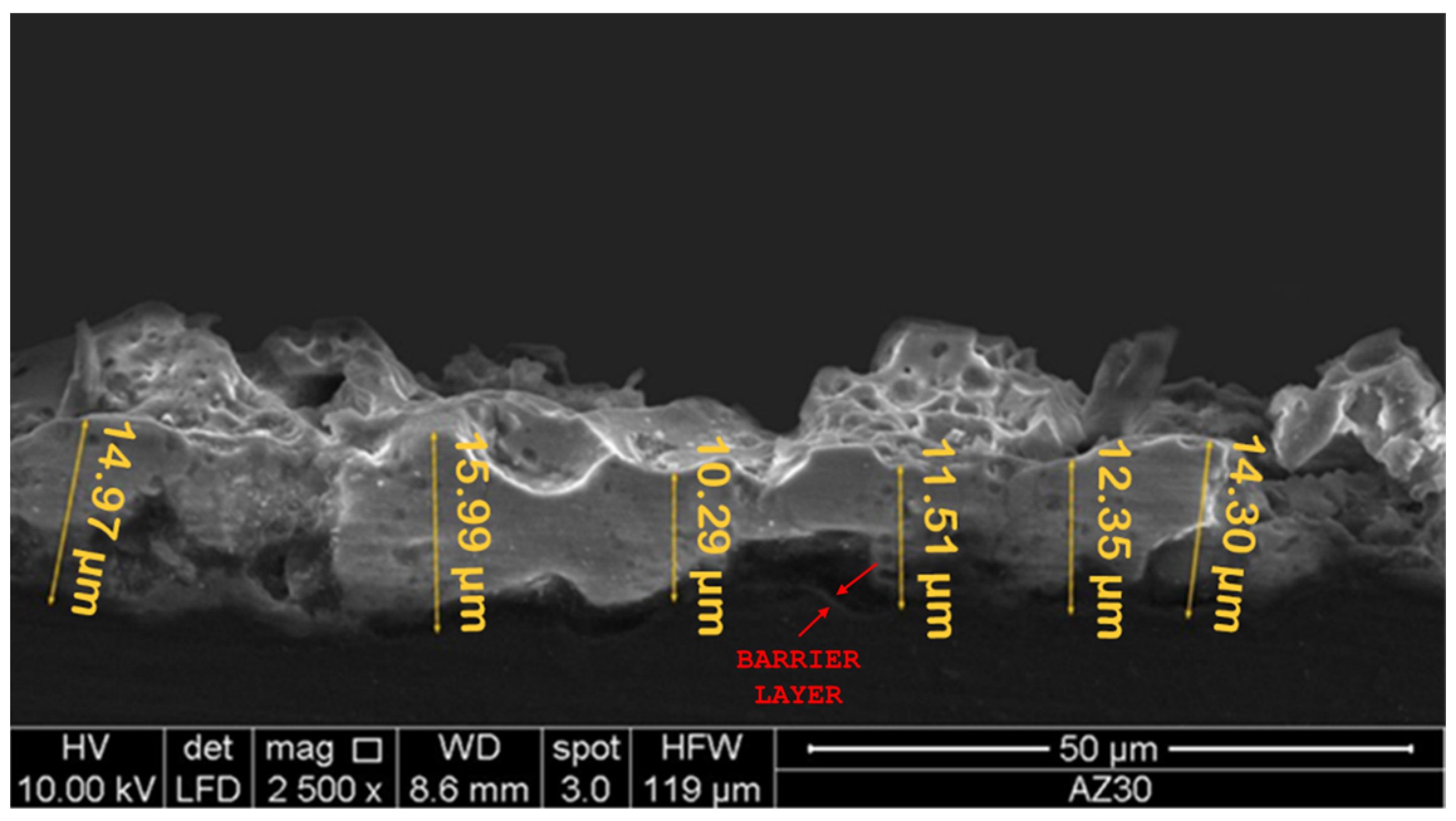
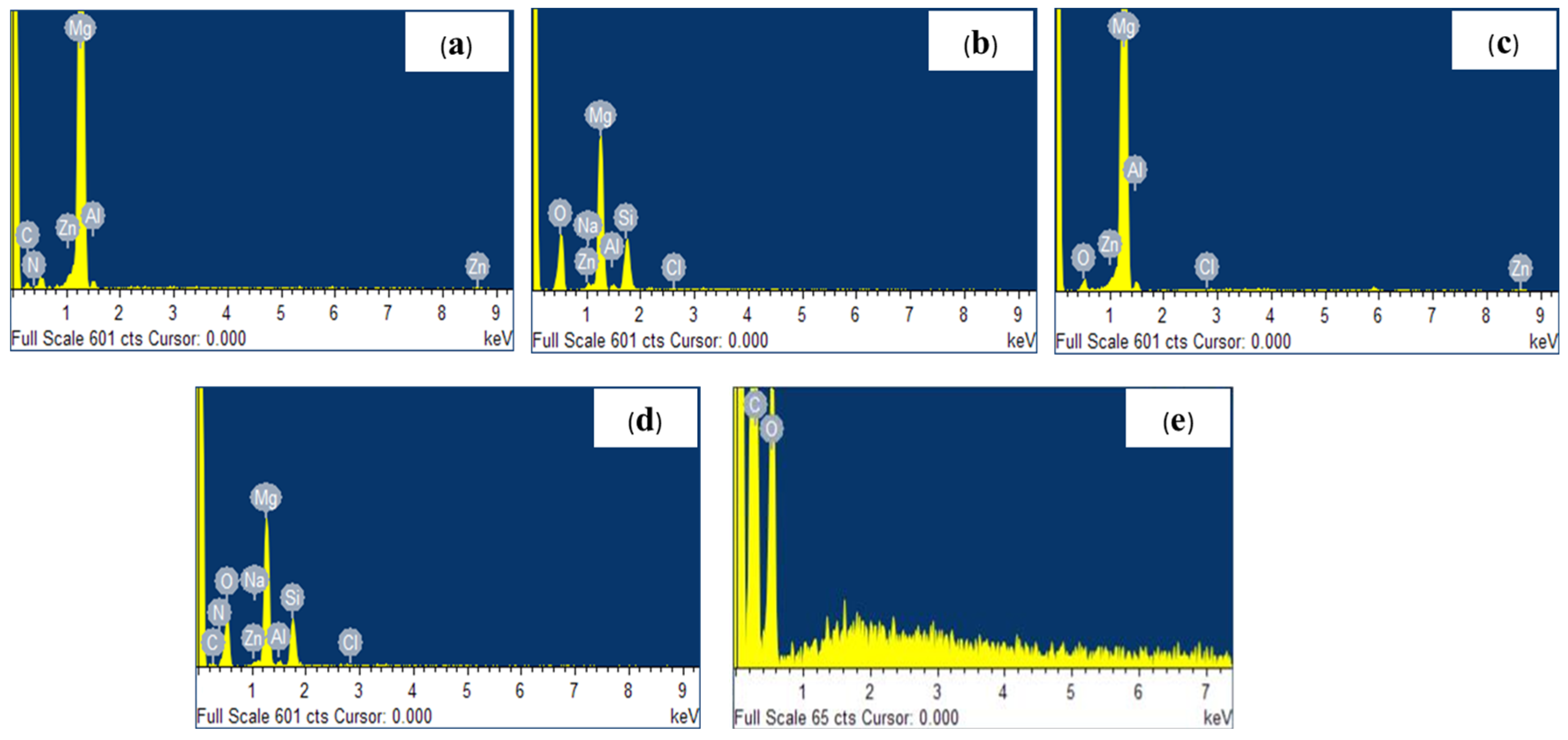
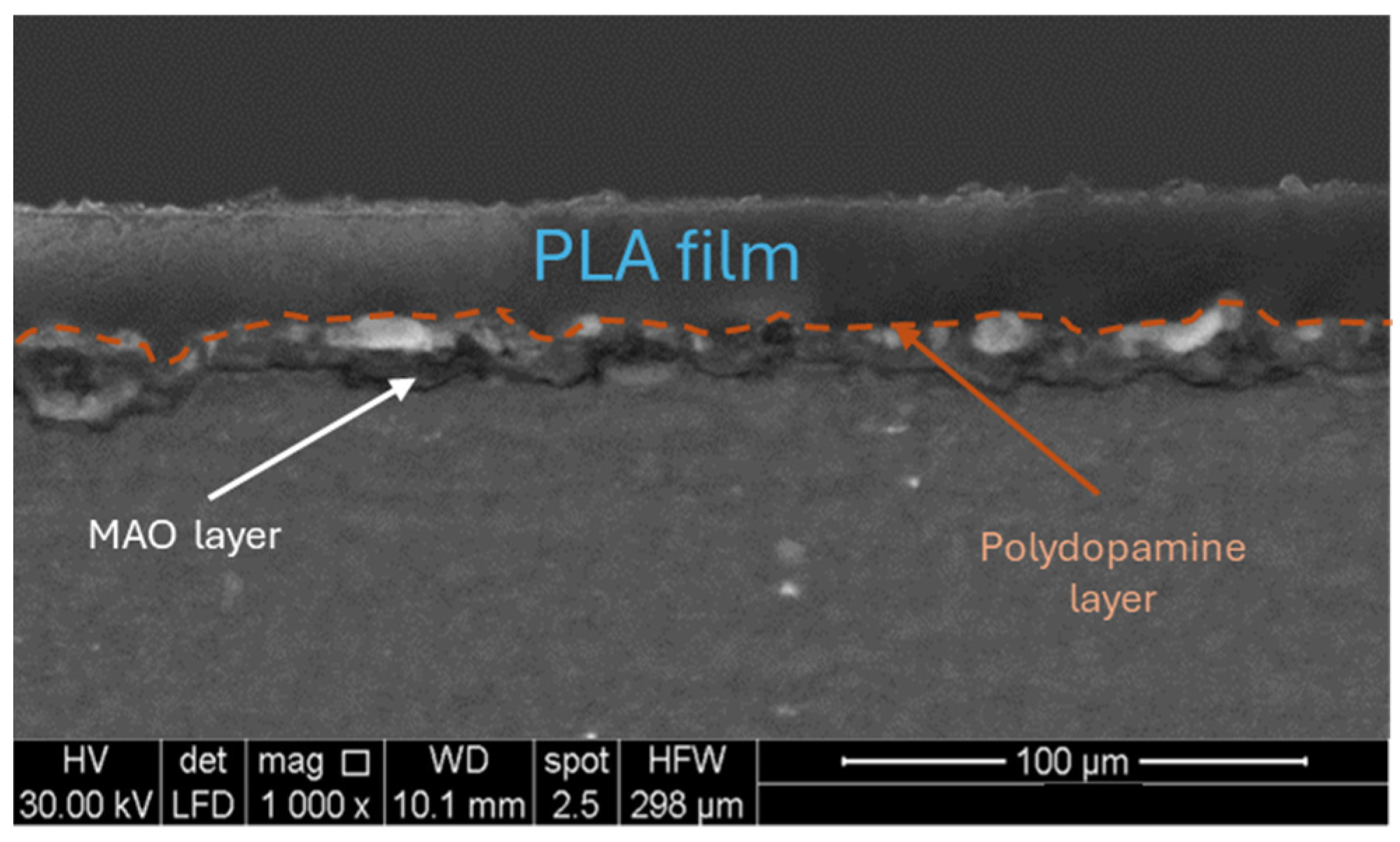
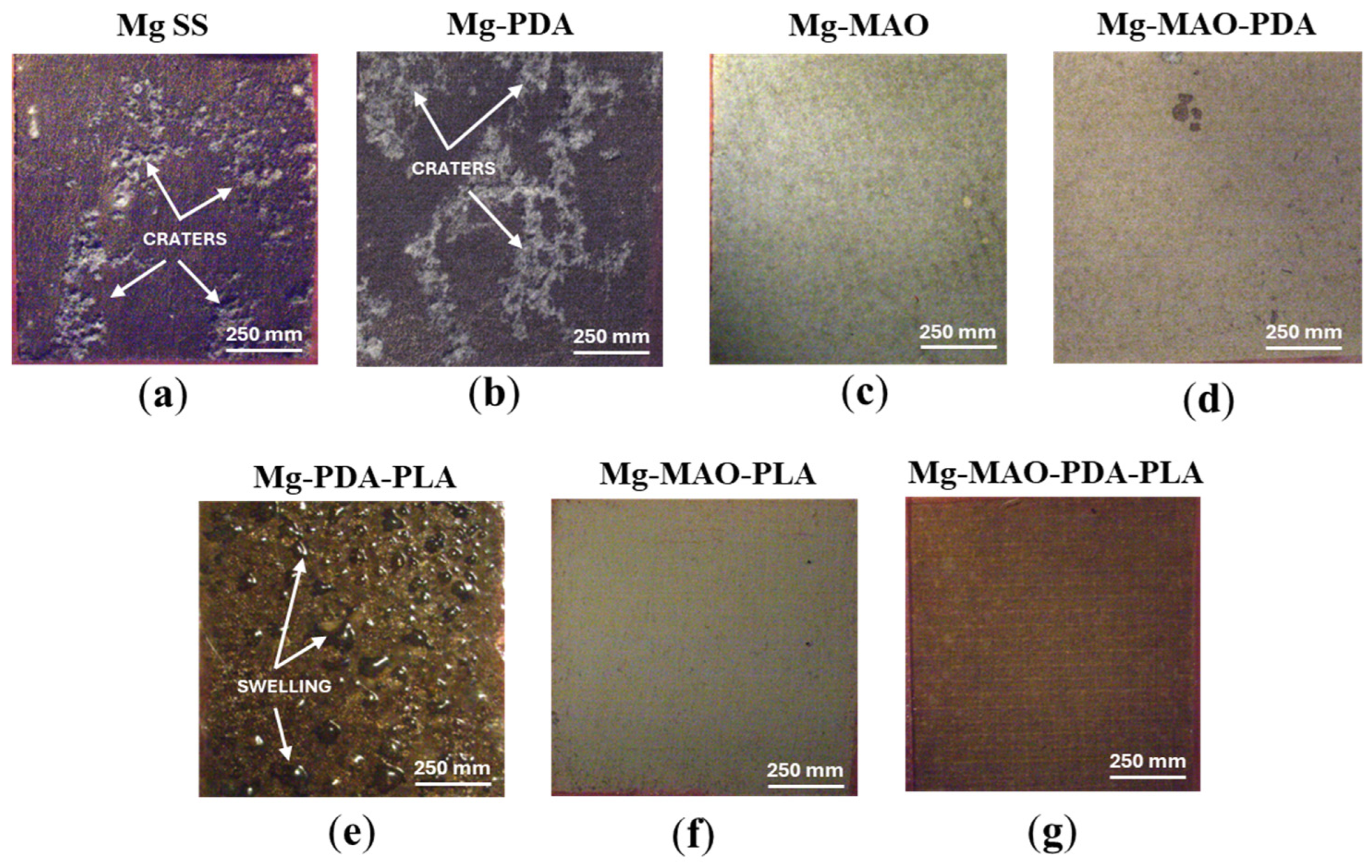
3.1. Morphological and Chemical Analysis
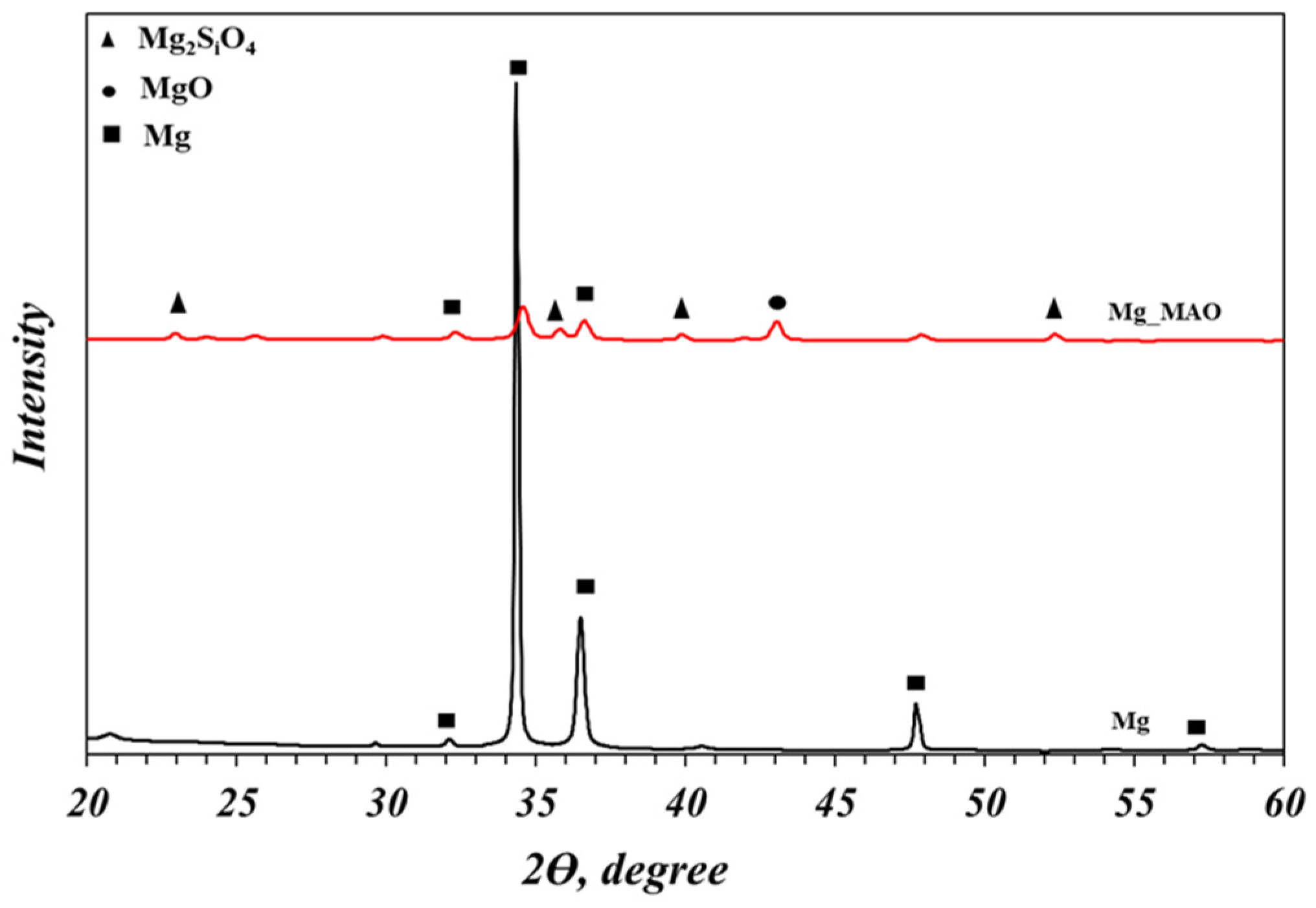
3.2. Crystallographic Analysis
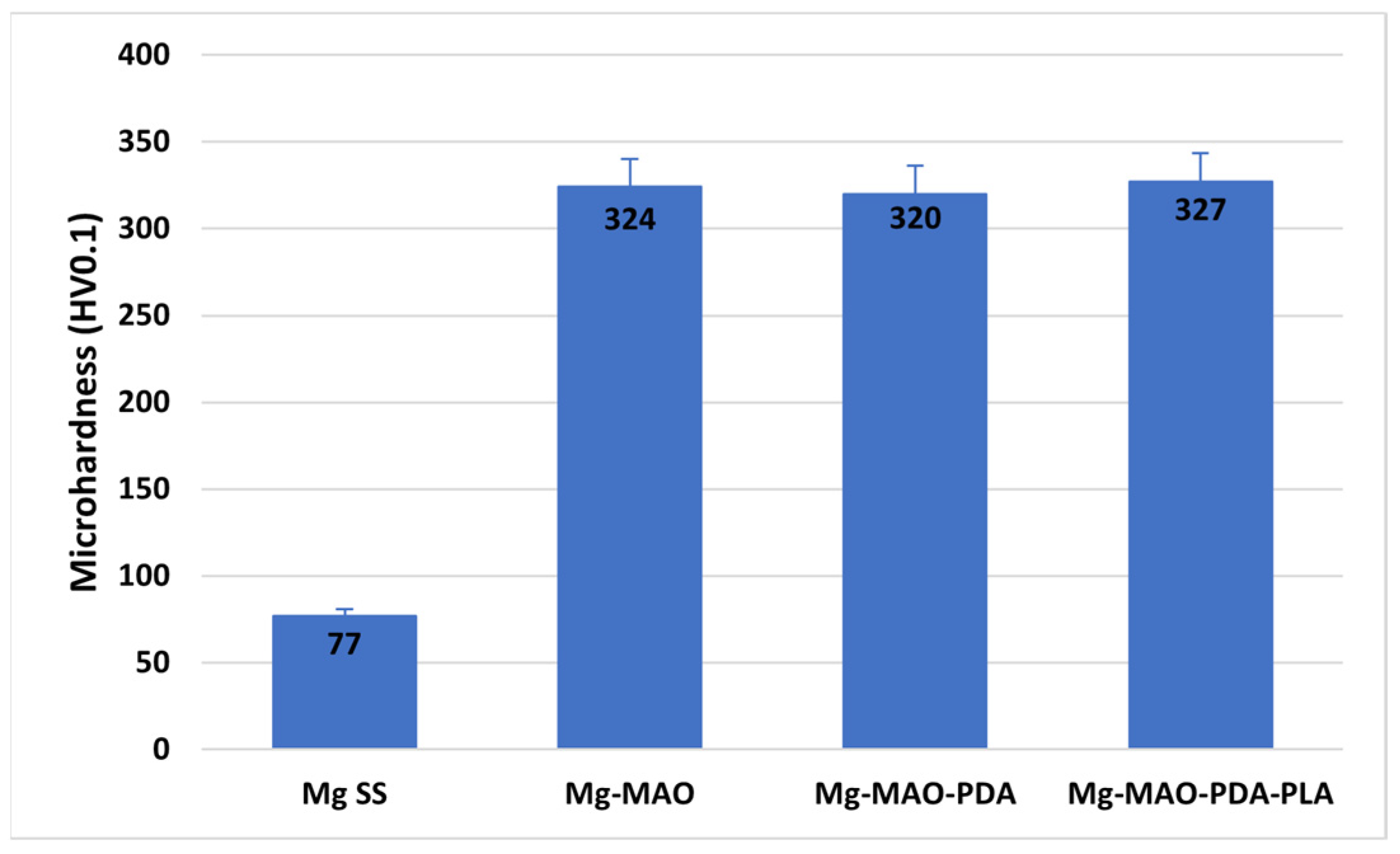
3.3. Micro-Hardness Test
3.4. Roughness Measurements
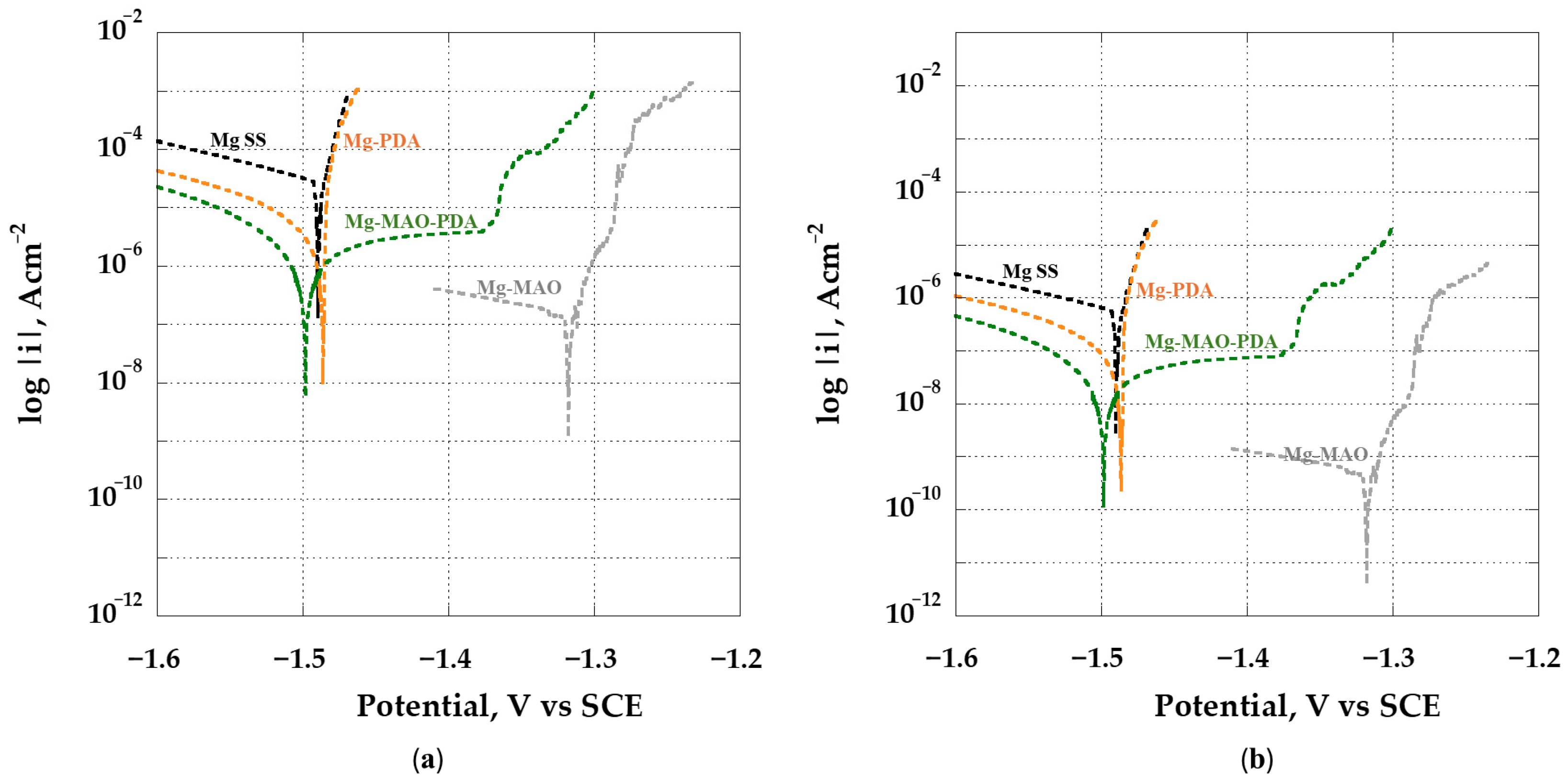
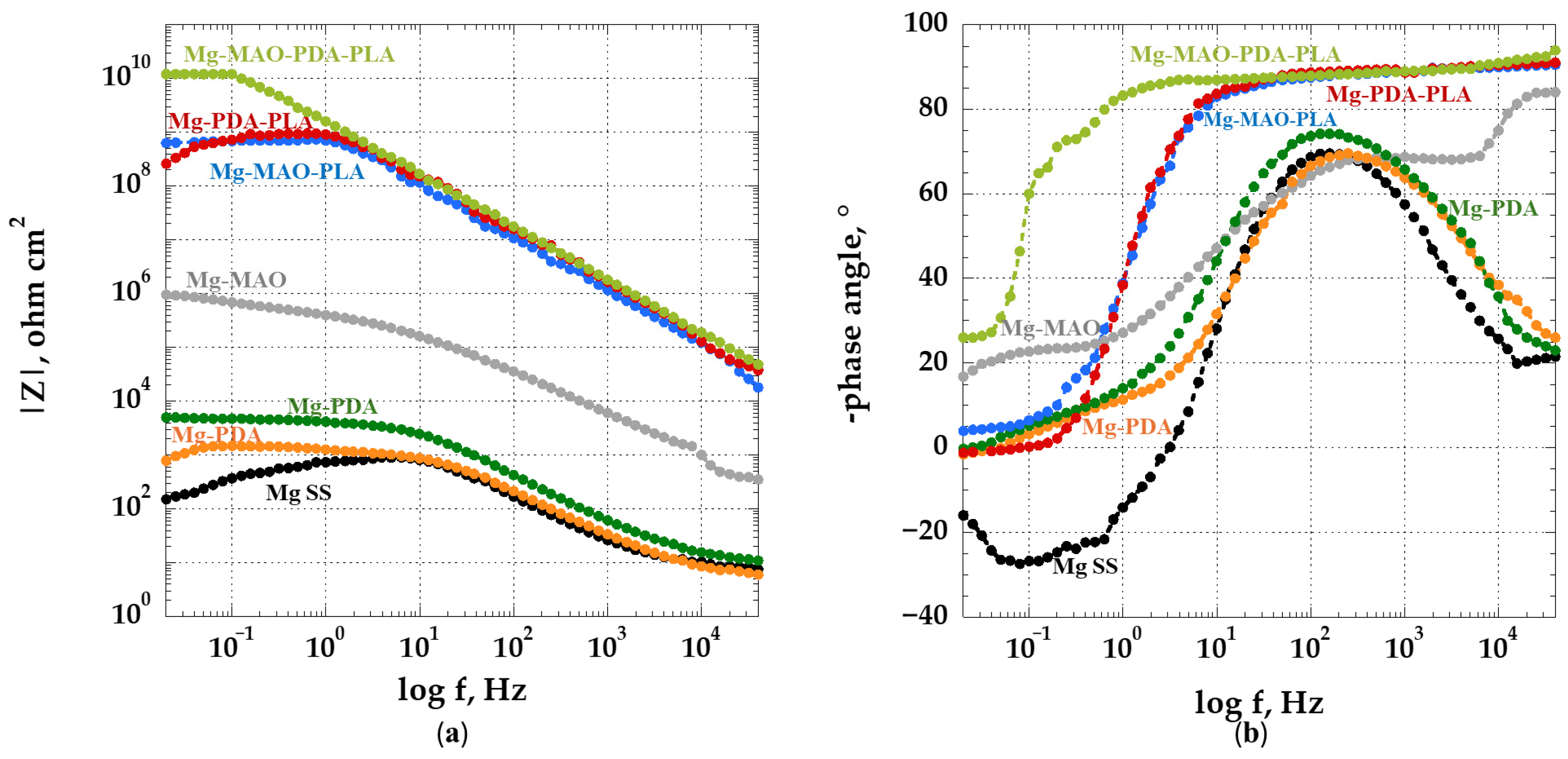
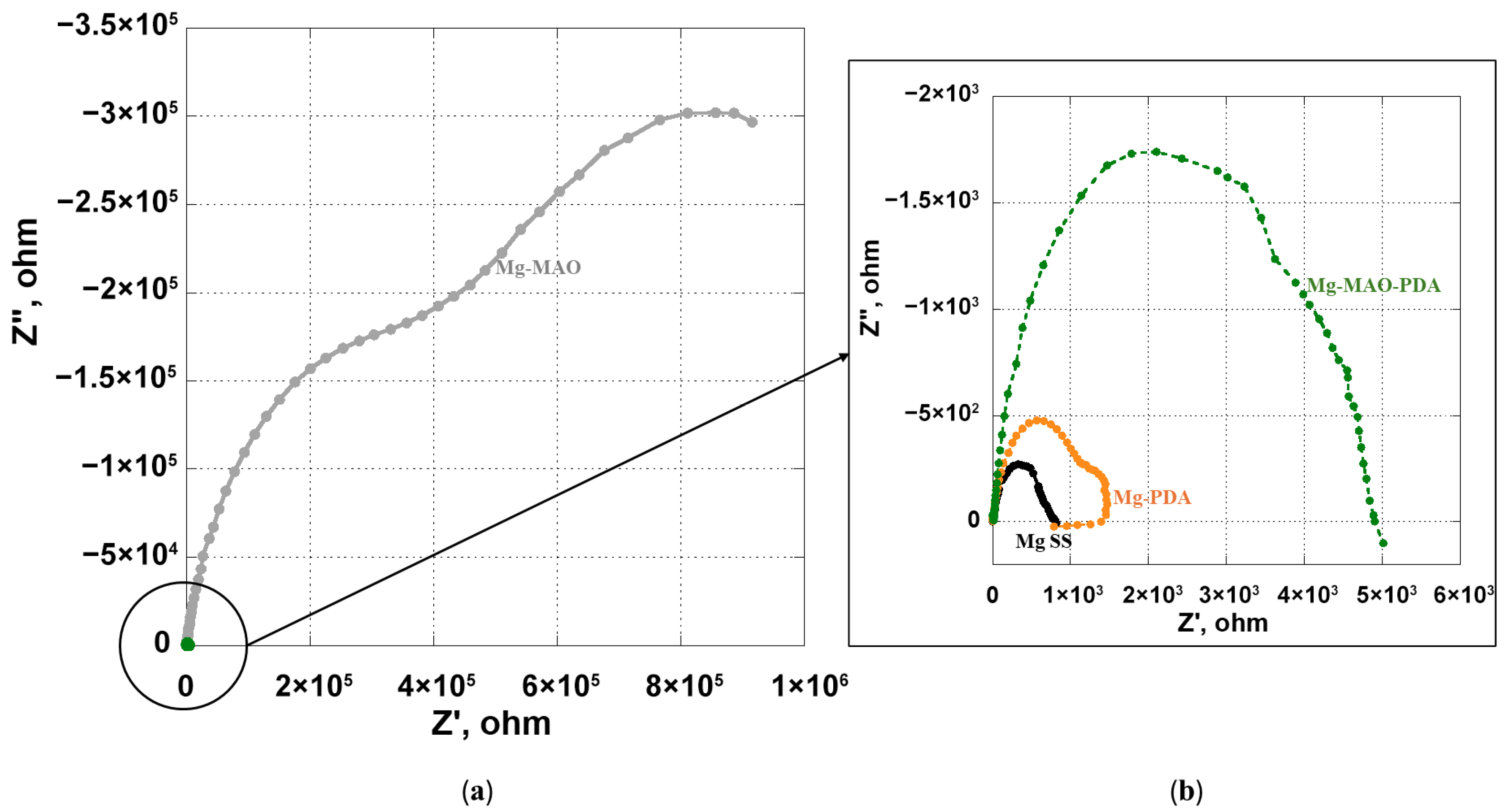
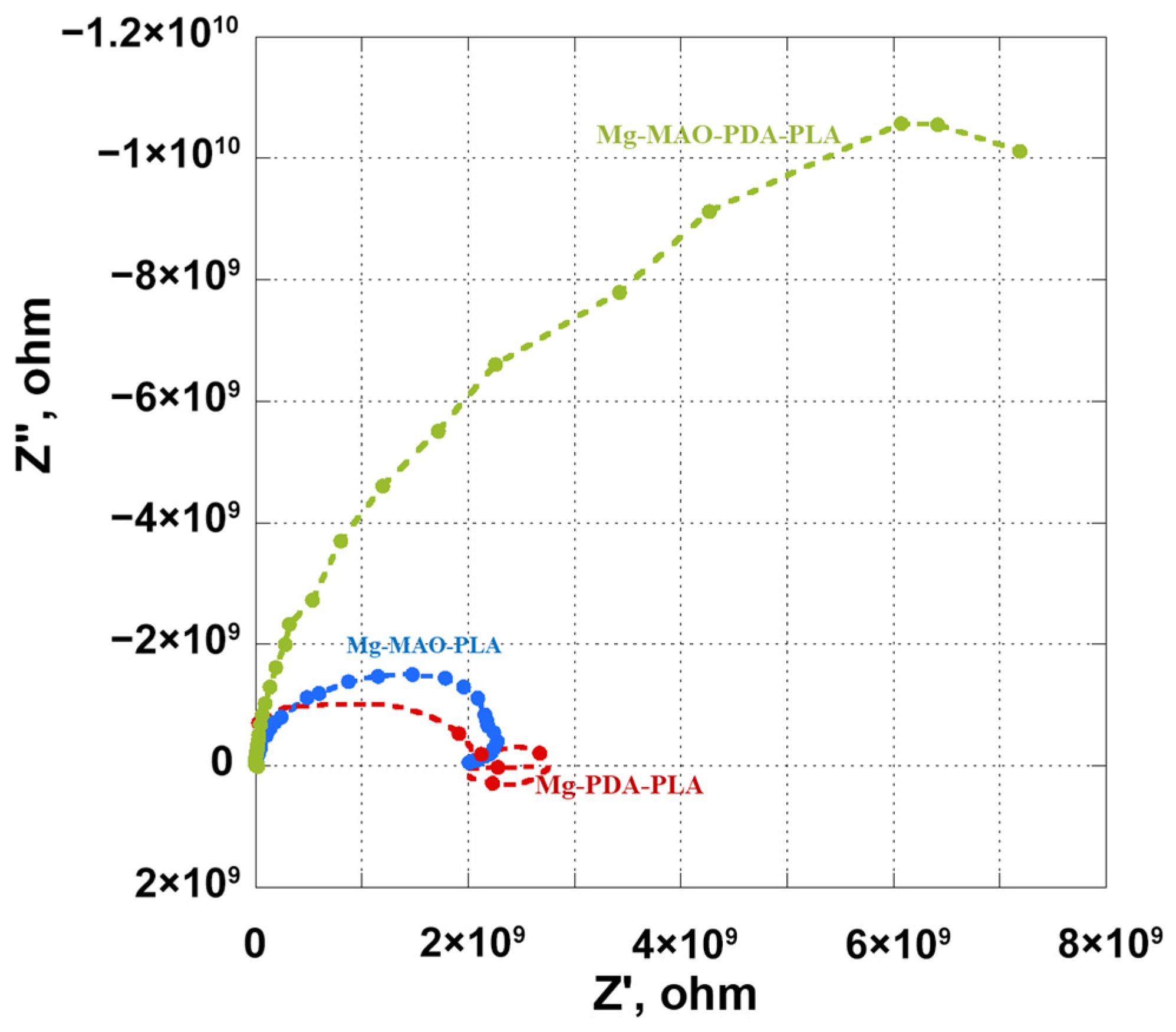
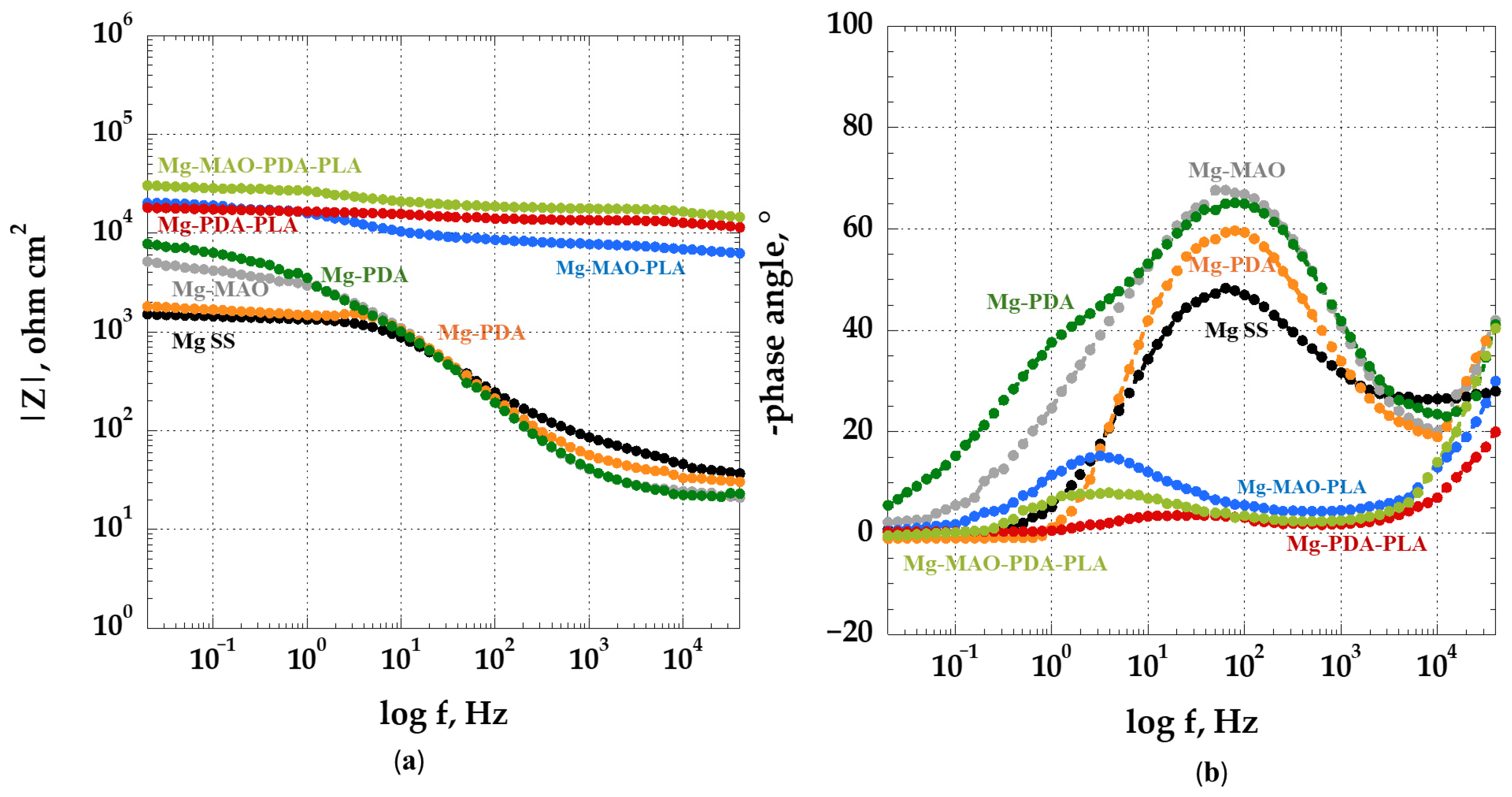
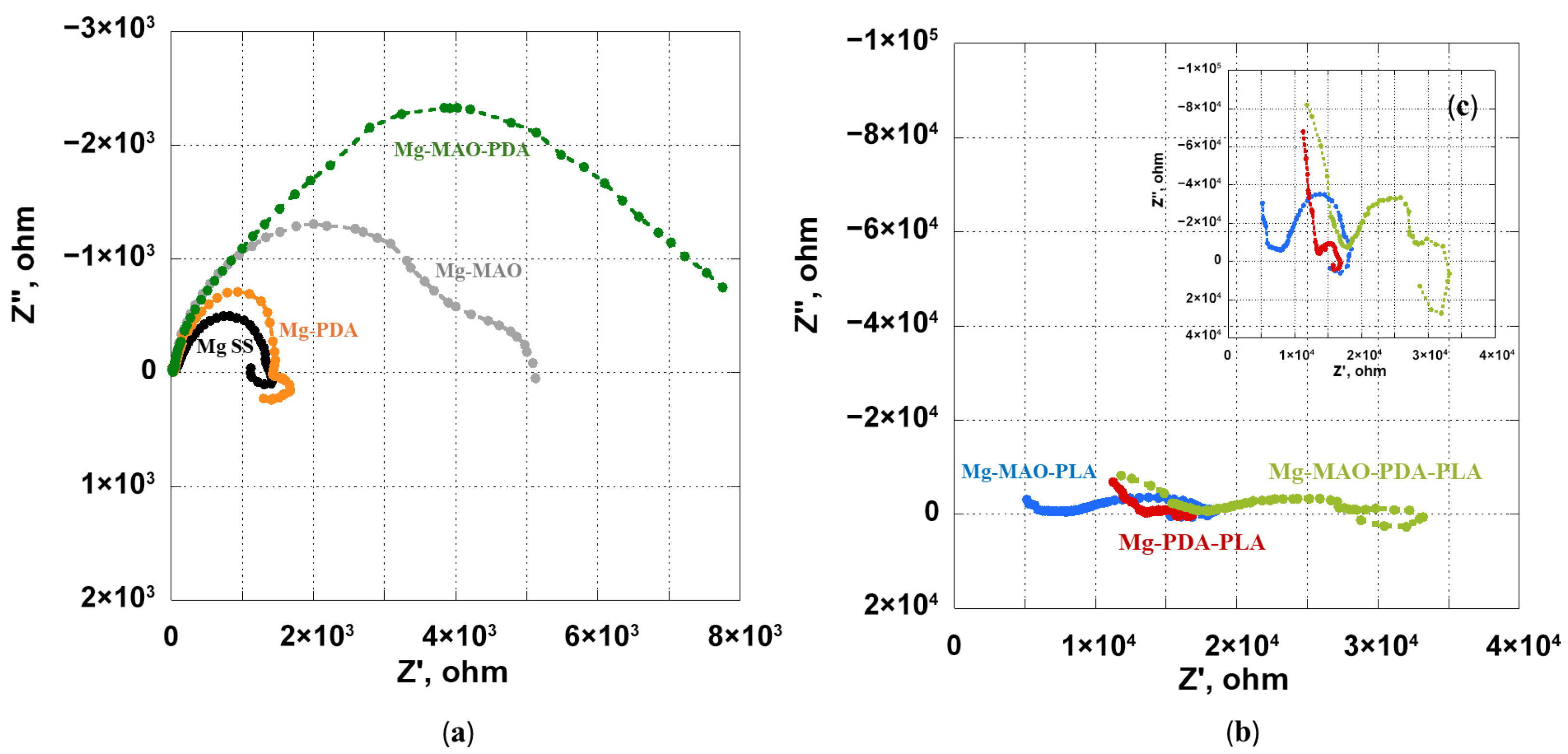
3.5. Electrochemical In Vitro Characterization
4. Conclusions
- The electrochemical treatment involved the classical pancake-like morphology, characterized by many small pores, which were enlarged and filled with the polydopamine layer, whose thickness was nanometric. The thickness of the anodic oxide was of about 13 µm, while the PLA layer showed a thickness of about 25 µm.
- The EDX analysis, in addition to the elements composing the substrate, revealed the presence of oxygen, silicon, and sodium after the anodization treatment, as well as the presence of nitrogen and carbon when the polydopamine layer was applied and carbon and oxygen after the deposition of the PLA film.
- The XRD analysis showed the crystallographic structure of the oxide coating, revealing the magnesium oxide and the magnesium silicate as principal compounds.
- The micro-hardness measurements revealed a higher hardness of the oxide coating compared to the untreated sample. The presence of the polydopamine and the PLA film did not affect the value.
- The roughness measurement highlighted the great extension of the area after the MAO treatment due to high porosity, as revealed through the Sdr parameters (very high for the Mg-MAO specimen), and the smoothing effect of the polydopamine, as attested to in the Sp and Sv values’ parameters, suggesting the sealing of oxide pores. In addition, the presence of the PLA film gave the surface neat, square patterning, which could improve cellular adhesion.
- The beneficial effect of the applied coatings was highlighted in the electrochemical tests, which showed a better response for surface-modified specimens in terms of corrosion kinetics. In particular, from the potentiodynamic polarization was observed an improvement in the corrosion current density and the corrosion potential for the anodized specimen. Different behavior was apparent after the application of the polydopamine on the anodic oxide, which reduced the corrosion potential, but it involved a passivity range by ennobling the corrosion behavior. The EIS analysis confirmed the results of the potentiodynamic polarization, and during the immersion time of 168 h, it revealed the best corrosion resistance for the specimen covered with all coatings.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Amukarimi, S.; Mozafari, M. Biodegradable Magnesium-based Biomaterials: An Overview of Challenges and Opportunities. MedComm 2021, 2, 123–144. [Google Scholar] [CrossRef]
- De Baaij, J.H.; Hoenderop, J.G.; Bindels, R.J. Magnesium in Man: Implications for Health and Disease. Physiol. Rev. 2015, 95, 1–46. [Google Scholar] [CrossRef] [PubMed]
- Zindani, D.; Kumar, K.; Paulo Davim, J. 4—Metallic Biomaterials—A Review. In Mechanical Behaviour of Biomaterials; Davim, J.P., Ed.; Woodhead Publishing: Sawston, UK, 2019; pp. 83–99. ISBN 978-0-08-102174-3. [Google Scholar]
- Kirkland, N.T. Magnesium Biomaterials: Past, Present and Future. Corros. Eng. Sci. Technol. 2012, 47, 322–328. [Google Scholar] [CrossRef]
- Witte, F. The History of Biodegradable Magnesium Implants: A Review: The THERMEC’2009 Biodegradable Metals. Acta Biomater. 2010, 6, 1680–1692. [Google Scholar] [CrossRef]
- Witte, F.; Fischer, J.; Nellesen, J.; Crostack, H.-A.; Kaese, V.; Pisch, A.; Beckmann, F.; Windhagen, H. In Vitro and in Vivo Corrosion Measurements of Magnesium Alloys. Biomaterials 2006, 27, 1013–1018. [Google Scholar] [CrossRef]
- Wu, C.S.; Zhang, Z.; Cao, F.H.; Zhang, L.J.; Zhang, J.Q.; Cao, C.N. Study on the Anodizing of AZ31 Magnesium Alloys in Alkaline Borate Solutions. Appl. Surf. Sci. 2007, 253, 3893–3898. [Google Scholar] [CrossRef]
- Dai, D.; Wang, H.; Li, J.-Z.; Wu, X.-D. Environmentally Friendly Anodization on AZ31 Magnesium Alloy. Trans. Nonferrous Met. Soc. China Engl. Ed. 2008, 18, s380–s384. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, G.; Wei, M. Controlling the Biodegradation Rate of Magnesium Using Biomimetic Apatite Coating. J. Biomed. Mater. Res. B Appl. Biomater. 2009, 89B, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.-L.; Wu, H.-I.; Chen, Z.-H.; Wang, H.-M.; Zhang, Z.; Wu, Y.-W. Corrosion Properties of AZ31 Magnesium Alloy and Protective Effects of Chemical Conversion Layers and Anodized Coatings. Trans. Nonferrous Met. Soc. China Engl. Ed. 2007, 17, 502–508. [Google Scholar] [CrossRef]
- Dabalà, M.; Brunelli, K.; Napolitani, E.; Magrini, M. Cerium-Based Chemical Conversion Coating on AZ63 Magnesium Alloy. Surf. Coat. Technol. 2003, 172, 227–232. [Google Scholar] [CrossRef]
- Scharnagl, N.; Blawert, C.; Dietzel, W. Corrosion Protection of Magnesium Alloy AZ31 by Coating with Poly(Ether Imides) (PEI). Surf. Coat. Technol. 2009, 203, 1423–1428. [Google Scholar] [CrossRef]
- Raja, V.S.; Venugopal, A.; Saji, V.S.; Sreekumar, K.; Nair, R.S.; Mittal, M.C. Electrochemical Impedance Behavior of Graphite-Dispersed Electrically Conducting Acrylic Coating on AZ31 Magnesium Alloy in 3.5 Wt.% NaCl Solution. Prog. Org. Coat. 2010, 67, 12–19. [Google Scholar] [CrossRef]
- Kim, Y.-K.; Park, I.-S.; Lee, K.-B.; Bae, T.-S.; Jang, Y.-S.; Oh, Y.-M.; Lee, M.-H. Effect upon Biocompatibility and Biocorrosion Properties of Plasma Electrolytic Oxidation in Trisodium Phosphate Electrolytes. Biointerphases 2016, 11, 011006. [Google Scholar] [CrossRef] [PubMed]
- Pezzato, L.; Gennari, C.; Franceschi, M.; Brunelli, K. Influence of Silicon Morphology on Direct Current Plasma Electrolytic Oxidation Process in AlSi10Mg Alloy Produced with Laser Powder Bed Fusion. Sci. Rep. 2022, 12, 14329. [Google Scholar] [CrossRef]
- Yeshmanova, G.; Blawert, C.; Serdechnova, M.; Wieland, D.C.F.; Starykevich, M.; Gazenbiller, E.; Höche, D.; Smagulov, D.; Zheludkevich, M.L. Effect of Electrolyte Composition on the Formation of PEO Coatings on AA2024 Aluminium Alloy. Surf. Interfaces 2024, 44, 103797. [Google Scholar] [CrossRef]
- Sarian, M.N.; Iqbal, N.; Sotoudehbagha, P.; Razavi, M.; Ahmed, Q.U.; Sukotjo, C.; Hermawan, H. Potential Bioactive Coating System for High-Performance Absorbable Magnesium Bone Implants. Bioact. Mater. 2022, 12, 42–63. [Google Scholar] [CrossRef]
- Peng, F.; Wang, D.; Tian, Y.; Cao, H.; Qiao, Y.; Liu, X. Sealing the Pores of PEO Coating with Mg-Al Layered Double Hydroxide: Enhanced Corrosion Resistance, Cytocompatibility and Drug Delivery Ability. Sci. Rep. 2017, 7, 8167. [Google Scholar] [CrossRef]
- Sreekanth, D.; Rameshbabu, N. Development and Characterization of MgO/Hydroxyapatite Composite Coating on AZ31 Magnesium Alloy by Plasma Electrolytic Oxidation Coupled with Electrophoretic Deposition. Mater. Lett. 2012, 68, 439–442. [Google Scholar] [CrossRef]
- Barberi, J.; Saqib, M.; Dmitruk, A.; Opitz, J.; Naplocha, K.; Beshchasna, N.; Spriano, S.; Ferraris, S. Characterization of Tannic Acid-Coated AZ31 Mg Alloy for Biomedical Application and Comparison with AZ91. Materials 2024, 17, 343. [Google Scholar] [CrossRef]
- Li, X.; Cong, Y.; Sui, J.; Li, X. An Investigation on the Degradation Behaviors of Mg Wires/PLA Composite for Bone Fixation Implants: Influence of Wire Content and Load Mode. Sci. Eng. Compos. Mater. 2021, 28, 39–47. [Google Scholar] [CrossRef]
- Butt, M.S.; Bai, J.; Wan, X.; Chu, C.; Xue, F.; Ding, H.; Zhou, G. Mg Alloy Rod Reinforced Biodegradable Poly-Lactic Acid Composite for Load Bearing Bone Replacement. Surf. Coat. Technol. 2017, 309, 471–479. [Google Scholar] [CrossRef]
- Negrescu, A.-M.; Necula, M.-G.; Gebaur, A.; Golgovici, F.; Nica, C.; Curti, F.; Iovu, H.; Costache, M.; Cimpean, A. In Vitro Macrophage Immunomodulation by Poly(ε-Caprolactone) Based-Coated AZ31 Mg Alloy. Int. J. Mol. Sci. 2021, 22, 909. [Google Scholar] [CrossRef]
- Heise, S.; Virtanen, S.; Boccaccini, A.R. Tackling Mg Alloy Corrosion by Natural Polymer Coatings—A Review. J. Biomed. Mater. Res. A 2016, 104, 2628–2641. [Google Scholar] [CrossRef] [PubMed]
- Keerthiga, G.; Prasad, M.J.N.V.; Vijayshankar, D.; Singh Raman, R.K. Polymeric Coatings for Magnesium Alloys for Biodegradable Implant Application: A Review. Materials 2023, 16, 4700. [Google Scholar] [CrossRef]
- Zeng, R.-C.; Cui, L.; Jiang, K.; Liu, R.; Zhao, B.-D.; Zheng, Y.-F. In Vitro Corrosion and Cytocompatibility of a Microarc Oxidation Coating and Poly (l-Lactic Acid) Composite Coating on Mg–1Li–1Ca Alloy for Orthopedic Implants. ACS Appl. Mater. Interfaces 2016, 8, 10014–10028. [Google Scholar] [CrossRef]
- Khalili, V.; Ghaleh, H.; Asl, H.N.; Ege, D.; Dikici, B.; Kaseem, M.; Breisch, M.; Frenzel, J.; Eggeler, G. Assessing the Properties of Biodegradable Magnesium Alloy AZ31 Protected by a Polymer Layer on a Plasma Electrolytic Oxidized (PEO) Surface. Surf. Coat. Technol. 2024, 487, 131002. [Google Scholar] [CrossRef]
- Muñoz, M.; Torres, B.; Mohedano, M.; Matykina, E.; Arrabal, R.; López, A.J.; Rams, J. PLA Deposition on Surface Treated Magnesium Alloy: Adhesion, Toughness and Corrosion Behaviour. Surf. Coat. Technol. 2020, 388, 125593. [Google Scholar] [CrossRef]
- Daavari, M.; Atapour, M.; Mohedano, M.; Matykina, E.; Arrabal, R.; Nesic, D. Biological Performance of Duplex PEO + CNT/PCL Coating on AZ31B Mg Alloy for Orthopedic and Dental Applications. J. Funct. Biomater. 2023, 14, 475. [Google Scholar] [CrossRef] [PubMed]
- Usmaniya, N.; Radhakrishna Pillai, S.; Palanivel, M.; Edalacheruvu, L.; Chennampalli, P.; Vaithiyanathan, P.; Parfenov, E.; Lingamaneni, R.K.; Nagumothu, R. Effect of Polycaprolactone Coating on the Corrosion and Biological Characteristics of Plasma Electrolytic Oxidised ZM21 Magnesium Alloy. Surf. Coat. Technol. 2023, 471, 129915. [Google Scholar] [CrossRef]
- Chafiq, M.; Chaouiki, A.; Salghi, R.; Ko, Y.G. Fabrication of Branch-like Aph@LDH-MgO Material through Organic-Inorganic Hybrid Conjugation for Excellent Anti-Corrosion Performance. J. Magnes. Alloys 2023, 11, 2469–2485. [Google Scholar] [CrossRef]
- Wei, Z.; Tian, P.; Liu, X.; Zhou, B. Hemocompatibility and Selective Cell Fate of Polydopamine-Assisted Heparinized PEO/PLLA Composite Coating on Biodegradable AZ31 Alloy. Colloids Surf. B Biointerfaces 2014, 121, 451–460. [Google Scholar] [CrossRef] [PubMed]
- Silverman, H.G.; Roberto, F.F. Understanding Marine Mussel Adhesion. Mar. Biotechnol. 2007, 9, 661–681. [Google Scholar] [CrossRef]
- Tian, P.; Xu, D.; Liu, X. Mussel-Inspired Functionalization of PEO/PCL Composite Coating on a Biodegradable AZ31 Magnesium Alloy. Colloids Surf. B Biointerfaces 2016, 141, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Acquesta, A.; Russo, P.; Monetta, T. Plasma Electrolytic Oxidation Treatment of AZ31 Magnesium Alloy for Biomedical Applications: The Influence of Applied Current on Corrosion Resistance and Surface Characteristics. Crystals 2023, 13, 510. [Google Scholar] [CrossRef]
- Lee, H.; Dellatore, S.M.; Miller, W.M.; Messersmith, P.B. Mussel-Inspired Surface Chemistry for Multifunctional Coatings. Science 2007, 318, 426–430. [Google Scholar] [CrossRef]
- Monetta, T.; Acquesta, A.; Bellucci, F. Evaluation of Roughness and Electrochemical Behavior of Titanium in Biological Environment. Metall. Ital. 2014, 106, 13–21. [Google Scholar]
- Acquesta, A.; Carangelo, A.; Monetta, T. TiO2 Nanotubes on Ti Dental Implant. Part 3: Electrochemical Behavior in Hank’s Solution of Titania Nanotubes Formed in Ethylene Glycol. Metals 2018, 8, 489. [Google Scholar] [CrossRef]
- Pourbaix, M. Atlas of Electrochemical Equilibria in Aqueous Solutions; National Association of Corrosion Engineers: Houston, TX, USA, 1974; ISBN 978-0-915567-98-0. [Google Scholar]
- Zhou, Z.; Zheng, B.; Lang, H.; Qin, A.; Ou, J. Corrosion Resistance and Biocompatibility of Polydopamine/Hyaluronic Acid Composite Coating on AZ31 Magnesium Alloy. Surf. Interfaces 2020, 20, 100560. [Google Scholar] [CrossRef]
- Carangelo, A.; Acquesta, A.; Monetta, T. Durability of AZ31 Magnesium Biodegradable Alloys Polydopamine Aided. Part 2: Ageing in Hank’s Solution. J. Magnes. Alloys 2019, 7, 218–226. [Google Scholar] [CrossRef]
- Chu, C.L.; Han, X.; Xue, F.; Bai, J.; Chu, P.K. Effects of Sealing Treatment on Corrosion Resistance and Degradation Behavior of Micro-Arc Oxidized Magnesium Alloy Wires. Appl. Surf. Sci. 2013, 271, 271–275. [Google Scholar] [CrossRef]
- Ghanbari, A.; Bordbar-Khiabani, A.; Warchomicka, F.; Sommitsch, C.; Yarmand, B.; Zamanian, A. PEO/Polymer Hybrid Coatings on Magnesium Alloy to Improve Biodegradation and Biocompatibility Properties. Surf. Interfaces 2023, 36, 102495. [Google Scholar] [CrossRef]
- Wu, T.; Blawert, C.; Serdechnova, M.; Karlova, P.; Dovzhenko, G.; Florian Wieland, D.C.; Stojadinovic, S.; Vasilic, R.; Wang, L.; Wang, C.; et al. Role of Phosphate, Silicate and Aluminate in the Electrolytes on PEO Coating Formation and Properties of Coated Ti6Al4V Alloy. Appl. Surf. Sci. 2022, 595, 153523. [Google Scholar] [CrossRef]
- Schlüter, K.; Zamponi, C.; Piorra, A.; Quandt, E. Comparison of the Corrosion Behaviour of Bulk and Thin Film Magnesium Alloys. Corros. Sci. 2010, 52, 3973–3977. [Google Scholar] [CrossRef]
- Farshid, S.; Kharaziha, M.; Atapour, M.; Di Franco, F.; Santamaria, M. Duplex Plasma Electrolytic Oxidation/Hydroxyapatite- Polydopamine Coating on WE43 Alloy for Bone Implants: Long-Term Corrosion Resistance and Biological Properties. Surf. Coat. Technol. 2024, 493, 131262. [Google Scholar] [CrossRef]
- Barati Darband, G.; Aliofkhazraei, M.; Hamghalam, P.; Valizade, N. Plasma Electrolytic Oxidation of Magnesium and Its Alloys: Mechanism, Properties and Applications. J. Magnes. Alloys 2017, 5, 74–132. [Google Scholar] [CrossRef]
- Li, N.; Ling, N.; Fan, H.; Liang, K.; Zhang, J.; Wang, L. Smart Functionalization of PEO Coating on AZ31B Magnesium Alloy by a Novel Facile One-Step Sealing Method. Surf. Coat. Technol. 2023, 467, 129674. [Google Scholar] [CrossRef]
- Cai, S.; Wu, C.; Yang, W.; Liang, W.; Yu, H.; Liu, L. Recent Advance in Surface Modification for Regulating Cell Adhesion and Behaviors. Nanotechnol. Rev. 2020, 9, 971–989. [Google Scholar] [CrossRef]
- Walboomers, X.; Croes, H.; Ginsel, L.; Jansen, J. Contact Guidance of Rat Fibroblasts on Various Implant Materials. J. Biomed. Mater. Res. 1999, 47, 204–212. [Google Scholar] [CrossRef]
- Rusen, L.; Cazan, M.; Mustaciosu, C.; Filipescu, M.; Sandel, S.; Zamfirescu, M.; Dinca, V.; Dinescu, M. Tailored Topography Control of Biopolymer Surfaces by Ultrafast Lasers for Cell–Substrate Studies. Appl. Surf. Sci. 2014, 302, 256–261. [Google Scholar] [CrossRef]
- Parandakh, A.; Anbarlou, A.; Tafazzoli-Shadpour, M.; Ardeshirylajimi, A.; Khani, M. Substrate Topography Interacts with Substrate Stiffness and Culture Time to Regulate Mechanical Properties and Smooth Muscle Differentiation of Mesenchymal Stem Cells. Colloids Surf. B Biointerfaces 2018, 173, 194–201. [Google Scholar] [CrossRef]
- Shukla, A.; Slater, J.H.; Culver, J.C.; Dickinson, M.E.; West, J.L. Biomimetic Surface Patterning Promotes Mesenchymal Stem Cell Differentiation. ACS Appl. Mater. Interfaces 2016, 8, 21883–21892. [Google Scholar] [CrossRef] [PubMed]
- Ber, S.; Torun Köse, G.; Hasırcı, V. Bone Tissue Engineering on Patterned Collagen Films: An in Vitro Study. Biomaterials 2005, 26, 1977–1986. [Google Scholar] [CrossRef] [PubMed]
- Nikkhah, M.; Edalat, F.; Manoucheri, S.; Khademhosseini, A. Engineering Microscale Topographies to Control the Cell–Substrate Interface. Biomaterials 2012, 33, 5230–5246. [Google Scholar] [CrossRef] [PubMed]
- Nikkhah, M.; Strobl, J.S.; De Vita, R.; Agah, M. The Cytoskeletal Organization of Breast Carcinoma and Fibroblast Cells inside Three Dimensional (3-D) Isotropic Silicon Microstructures. Biomaterials 2010, 31, 4552–4561. [Google Scholar] [CrossRef] [PubMed]
- Altomare, L.; Gadegaard, N.; Visai, L.; Tanzi, M.C.; Farè, S. Biodegradable Microgrooved Polymeric Surfaces Obtained by Photolithography for Skeletal Muscle Cell Orientation and Myotube Development. Acta Biomater. 2010, 6, 1948–1957. [Google Scholar] [CrossRef]
- Song, G.L.; Atrens, A. Corrosion Mechanisms of Magnesium Alloys. Adv. Eng. Mater. 1999, 1, 11–33. [Google Scholar] [CrossRef]
- Nachtsheim, J.; Ma, S.; Burja, J.; Batič, B.Š.; Markert, B. Tuning the Long-Term Corrosion Behaviour of Biodegradable WE43 Magnesium Alloy by PEO Coating. Surf. Coat. Technol. 2023, 474, 130115. [Google Scholar] [CrossRef]
- Liu, L.J.; Schlesinger, M. Corrosion of Magnesium and Its Alloys. Corros. Sci. 2009, 51, 1733–1737. [Google Scholar] [CrossRef]
- Standing Committee on the Scientific Evaluation of Dietary Reference Intakes. Dietary Reference Intakes for Calcium, Phosphorus, Magnesium, Vitamin D, and Fluoride; National Academies Press: Washington, DC, USA, 1999. [Google Scholar]
- CHENG, Y.; QIN, T.; WANG, H.; ZHANG, Z. Comparison of Corrosion Behaviors of AZ31, AZ91, AM60 and ZK60 Magnesium Alloys. Trans. Nonferrous Met. Soc. China 2009, 19, 517–524. [Google Scholar] [CrossRef]
- Zhang, F.; Ma, A.; Song, D.; Jiang, J.; Lu, F.; Zhang, L.; Yang, D.; Chen, J. Improving In-Vitro Biocorrosion Resistance of Mg-Zn-Mn-Ca Alloy in Hank’s Solution through Addition of Cerium. J. Rare Earths 2015, 33, 93–101. [Google Scholar] [CrossRef]
- Johnston, S.; Shi, Z.; Atrens, A. The Influence of pH on the Corrosion Rate of High-Purity Mg, AZ91 and ZE41 in Bicarbonate Buffered Hanks’ Solution. Corros. Sci. 2015, 101, 182–192. [Google Scholar] [CrossRef]
- Han, G.; Lee, J.-Y.; Kim, Y.-C.; Park, J.H.; Kim, D.-I.; Han, H.-S.; Yang, S.-J.; Seok, H.-K. Preferred Crystallographic Pitting Corrosion of Pure Magnesium in Hanks’ Solution. Corros. Sci. 2012, 63, 316–322. [Google Scholar] [CrossRef]
- Zeng, R.-C.; Sun, L.; Zheng, Y.-F.; Cui, H.-Z.; Han, E.-H. Corrosion and Characterisation of Dual Phase Mg–Li–Ca Alloy in Hank’s Solution: The Influence of Microstructural Features. Corros. Sci. 2014, 79, 69–82. [Google Scholar] [CrossRef]
- Bukovinová, L.; Hadzima, B. Electrochemical Characteristics of Magnesium Alloy AZ31 in Hank’s Solution. Corros. Eng. Sci. Technol. 2012, 47, 352–357. [Google Scholar] [CrossRef]
- Xin, Y.; Liu, C.; Zhang, X.; Tang, G.; Tian, X.; Chu, P.K. Corrosion Behavior of Biomedical AZ91 Magnesium Alloy in Simulated Body Fluids. J. Mater. Res. 2007, 22, 2004–2011. [Google Scholar] [CrossRef]
- Mena Morcillo, E.; Veleva, L. Degradation of AZ31 and AZ91 Magnesium Alloys in Different Physiological Media: Effect of Surface Layer Stability on Electrochemical Behaviour. J. Magnes. Alloys 2020, 8, 667–675. [Google Scholar] [CrossRef]
- Ghanbari, A.; Warchomicka, F.; Sommitsch, C.; Zamanian, A. Investigation of the Oxidation Mechanism of Dopamine Functionalization in an AZ31 Magnesium Alloy for Biomedical Applications. Coatings 2019, 9, 584. [Google Scholar] [CrossRef]
- Xiang, N.; Song, R.; Zhuang, J.; Song, R.; Lu, X.; Su, X. Effects of Current Density on Microstructure and Properties of Plasma Electrolytic Oxidation Ceramic Coatings Formed on 6063 Aluminum Alloy. Trans. Nonferrous Met. Soc. China 2016, 26, 806–813. [Google Scholar] [CrossRef]
- Li, Y.; Li, H.; Xiong, Q.; Wu, X.; Zhou, J.; Wu, J.; Wu, X.; Qin, W. Multipurpose Surface Functionalization on AZ31 Magnesium Alloy by Atomic Layer Deposition: Tailoring the Corrosion Resistant and Electrical Performance. Nanoscale 2017, 9, 8591–8599. [Google Scholar] [CrossRef] [PubMed]
- Jothi, V.; Adesina, A.Y.; Rahman, M.M.; Kumar, A.M.; Ram, J.S.N. Improved Adhesion and Corrosion Resistant Performance of Polyurethane Coatings on Anodized Mg Alloy for Aerospace Applications. J. Mater. Eng. Perform. 2020, 29, 2586–2596. [Google Scholar] [CrossRef]
- ASTM G102-89 (Reapproved 2015); Standard Practice for Calculation of Corrosion Rates and Related Information from Electrochemical Measurements. ASTM International: West Conshohocken, PA, USA, 2015.
- Zeng, R.; Jun, C.; Dietzel, W.; Hort, N.; Kainer, K. Electrochemical Behavior of Magnesium Alloys in Simulated Body Fluids. Trans. Nonferrous Met. Soc. China 2007, 17, s166–s170. [Google Scholar]
- Huang, J.; Li, Z.; Liaw, B.Y.; Zhang, J. Graphical Analysis of Electrochemical Impedance Spectroscopy Data in Bode and Nyquist Representations. J. Power Sources 2016, 309, 82–98. [Google Scholar] [CrossRef]
- Singer, F.; Schlesak, M.; Mebert, C.; Höhn, S.; Virtanen, S. Corrosion Properties of Polydopamine Coatings Formed in One-Step Immersion Process on Magnesium. ACS Appl. Mater. Interfaces 2015, 7, 26758–26766. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, H.; Yuan, D.; Chen, S.; Zhu, C.; Chen, K. The Effect of Polylactic Acid Ordering on the Long-Term Corrosion Protection Capacity of Biodegradable Magnesium Alloys. Int. J. Biol. Macromol. 2024, 282, 135549. [Google Scholar] [CrossRef]
- Alabbasi, A.; Liyanaarachchi, S.; Kannan, M.B. Polylactic Acid Coating on a Biodegradable Magnesium Alloy: An in Vitro Degradation Study by Electrochemical Impedance Spectroscopy. Thin Solid Films 2012, 520, 6841–6844. [Google Scholar] [CrossRef]
- Acquesta, A.; Russo, P.; Monetta, T. Surface Engineering of Magnesium Alloys for the Next Generation of Biodegradable Device; Mechanisms and Machine Science; Springer: Cham, Switzerland, 2024; Volume 162, pp. 376–385. [Google Scholar]
- Monetta, T.; Acquesta, A. Magnesium-Based Biomedical Devices Degradation Control by Means of Multilayer Coatings. In Key Engineering Materials; Trans Tech Publications Ltd.: Bäch, Switzerland, 2023; Volume 967, pp. 137–142. [Google Scholar]
- Xin, Y.; Huo, K.; Tao, H.; Tang, G.; Chu, P.K. Influence of Aggressive Ions on the Degradation Behavior of Biomedical Magnesium Alloy in Physiological Environment. Acta Biomater. 2008, 4, 2008–2015. [Google Scholar] [CrossRef]
- Seyfoori, A.; Mirdamadi, S.; Khavandi, A.; Raufi, Z.S. Biodegradation Behavior of Micro-Arc Oxidized AZ31 Magnesium Alloys Formed in Two Different Electrolytes. Appl. Surf. Sci. 2012, 261, 92–100. [Google Scholar] [CrossRef]
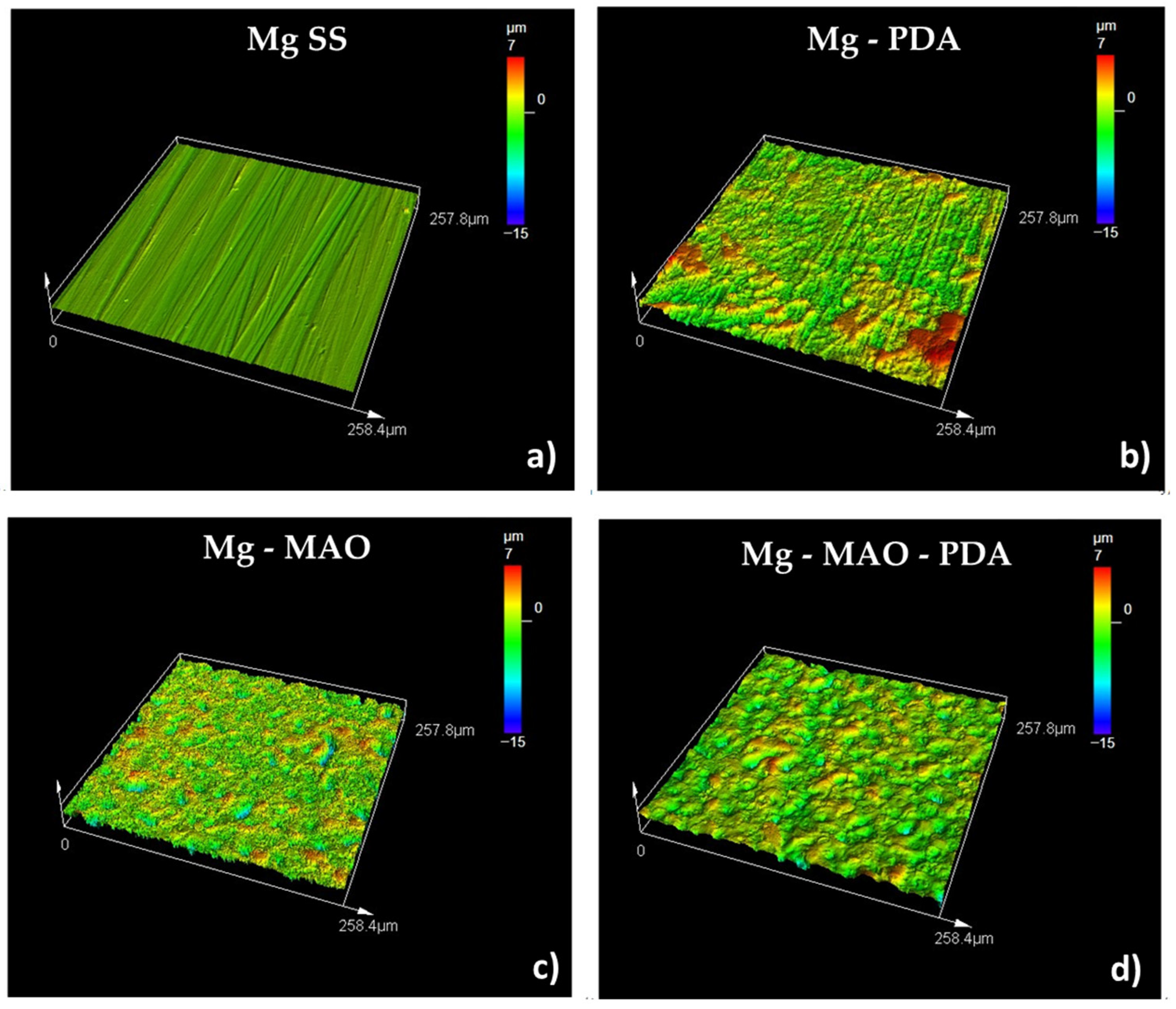
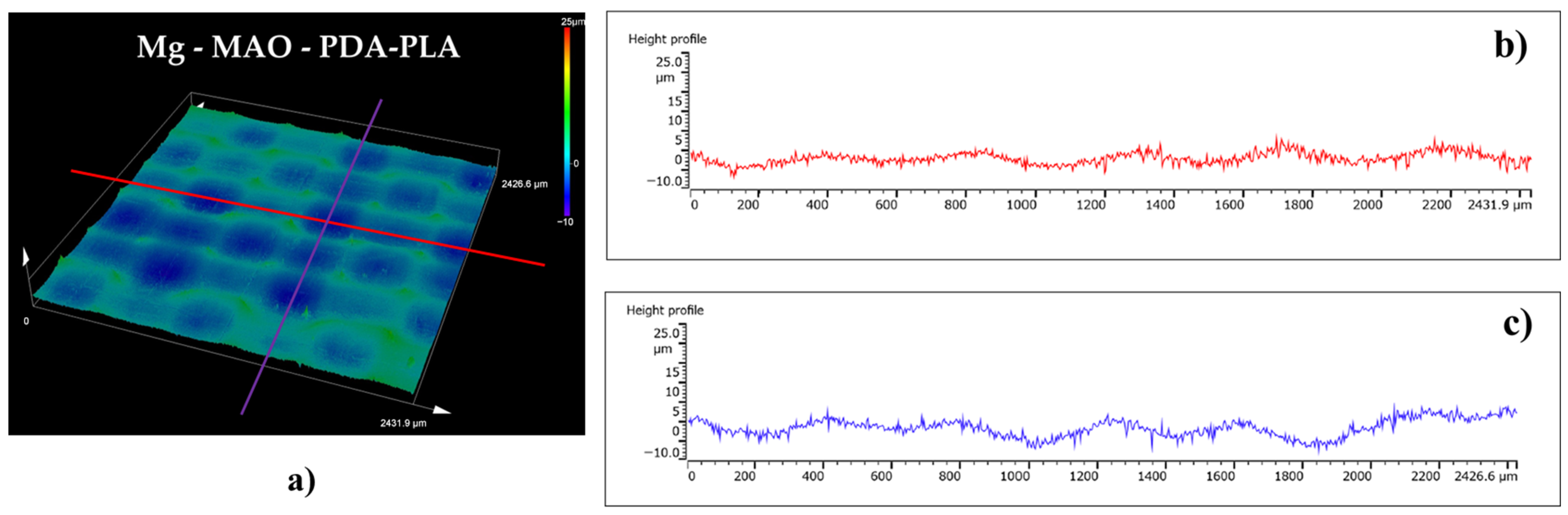
| Sa (µm) | Sq (µm) | Sp (µm) | Sv (µm) | Sku | Ssk | Sdq | Sdr (%) | |
|---|---|---|---|---|---|---|---|---|
| Mg SS | 0.3 ± 0.05 | 0.3 ± 0.02 | 3.1 ± 0.4 | 1.2 ± 0.43 | 4.4 ± 0.2 | 0.1 ± 0.03 | 0.2 ± 0.01 | 2.1 ± 0.05 |
| Mg-MAO | 1.7 ± 0.2 | 2.4 ± 0.1 | 8.0 ± 0.2 | 14.7 ± 1.3 | 6.4 ± 0.4 | −1.4 ± 0.02 | 4.7 ± 0.05 | 289.7 ± 12 |
| Mg-PDA | 1.3 ± 0.3 | 1.7 ± 0.3 | 7.1 ± 0.3 | 5.8 ± 0.2 | 3.7 ± 0.2 | 0.5 ± 0.01 | 1.2 ± 0.02 | 39.7 ± 2.1 |
| Mg-MAO-PDA | 1.2 ± 0.2 | 1.6 ± 0.3 | 5.4 ± 0.2 | 11.4 ± 0.5 | 5.9 ± 0.2 | −0.8 ± 0.02 | 1.0 ± 0.05 | 49.4 ± 1.6 |
| Specimens | Corrosion Potential, V vs. OCP | Corrosion Current Density, A/cm2 | Passivity Current Density, A/cm2 |
|---|---|---|---|
| Mg SS | −1.48 ± 0.2 | (2.65± 0.3) × 10−5 | - |
| Mg-MAO | −1.32 ± 0.4 | (5.99 ± 0.8) × 10−10 | - |
| Mg-PDA | −1.49 ± 0.2 | (1.94 ± 0.3) × 10−7 | - |
| Mg-MAO-PDA | −1.50 ± 0.3 | - | (7.35 ± 0.4) × 10−8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Acquesta, A.; Desiderio, F.; Russo, P.; Stornelli, G.; Di Schino, A.; Monetta, T. Enhancement of Corrosion Resistance of MAO/Polydopamine/Polylactic Acid-Coated AZ31 Magnesium Alloy for Biomedical Applications. Metals 2025, 15, 146. https://doi.org/10.3390/met15020146
Acquesta A, Desiderio F, Russo P, Stornelli G, Di Schino A, Monetta T. Enhancement of Corrosion Resistance of MAO/Polydopamine/Polylactic Acid-Coated AZ31 Magnesium Alloy for Biomedical Applications. Metals. 2025; 15(2):146. https://doi.org/10.3390/met15020146
Chicago/Turabian StyleAcquesta, Annalisa, Fulvia Desiderio, Pietro Russo, Giulia Stornelli, Andrea Di Schino, and Tullio Monetta. 2025. "Enhancement of Corrosion Resistance of MAO/Polydopamine/Polylactic Acid-Coated AZ31 Magnesium Alloy for Biomedical Applications" Metals 15, no. 2: 146. https://doi.org/10.3390/met15020146
APA StyleAcquesta, A., Desiderio, F., Russo, P., Stornelli, G., Di Schino, A., & Monetta, T. (2025). Enhancement of Corrosion Resistance of MAO/Polydopamine/Polylactic Acid-Coated AZ31 Magnesium Alloy for Biomedical Applications. Metals, 15(2), 146. https://doi.org/10.3390/met15020146










