Material Characterization and Technological Properties of Biocompatible Ti-12Al-42Nb Spherical Powder Alloy for Additive Manufacturing of Personal Medical Implants
Abstract
1. Introduction
| Alloy | Concentration, wt.% | References | |||||
|---|---|---|---|---|---|---|---|
| Ti | Al | V | Nb | O | N | ||
| Titanium Grade 4 | Balance | - | - | - | ≤0.4 | ≤0.05 | [15,21] |
| Ti-6Al-4V | Balance | 6.70 | 3.71 | - | 0.145 | 0.022 | [15,21] |
| Ti-6Al-2Nb | Balance | 6.11 | - | 2.42 | 0.141 | 0.041 | [15,21] |
| Ti-6Al-4Nb | Balance | 5.91 | - | 4.84 | 0.095 | 0.012 | [15,21] |
| Ti-6Al-6Nb | Balance | 5.85 | - | 7.16 | 0.145 | 0.031 | [15,21] |
| Ti-6Al-7Nb | Balance | 5.93 | - | 8.36 | 0.149 | 0.033 | [15,21] |
| Ti-6Al-10Nb | Balance | 5.90 | - | 10.45 | 0.083 | 0.014 | [15,21] |
| Ti-10Al-42 Nb | Balance | 9.52 | - | 42.24 | - | - | [22] |
| Ti-11Al-44Nb | Balance | 11.24 | - | 43.80 | 0.110 | 0.003 | [23] |
| Ti-22Al-25Nb | Balance | 22.78 | 0.05 | 41.37 | 0.008 | 0.002 | [24,25,26,27] |
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
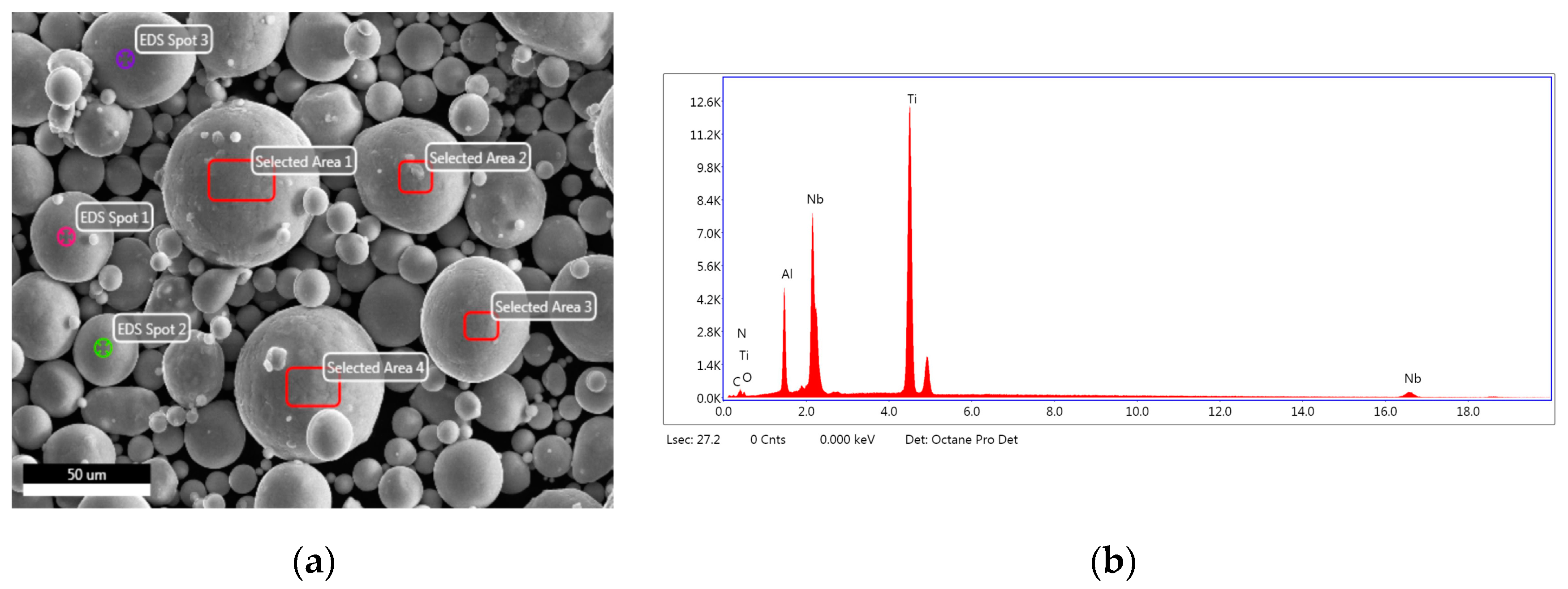
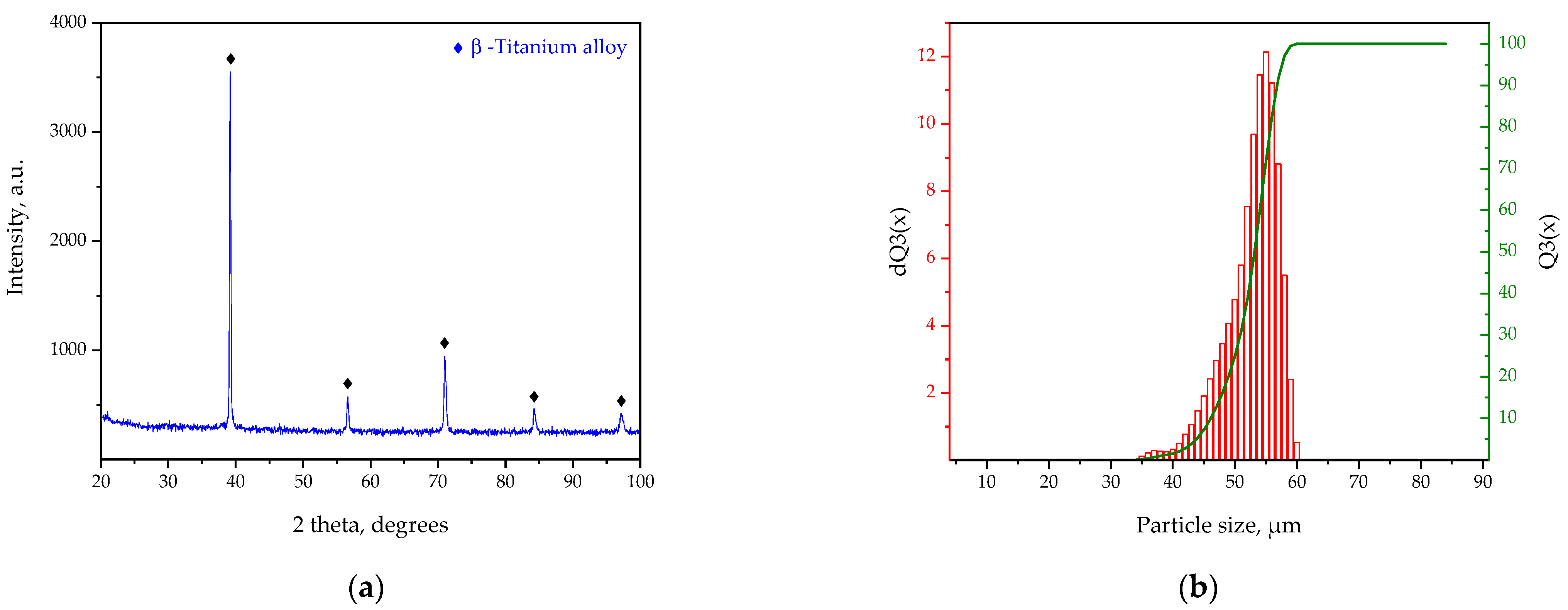
- XRD analysis showed that the Ti-12Al-42Nb powder alloy had hexagonal titanium beta phase-stabilized niobium, as verified by COD 9008554 reference pattern. X-ray elemental analysis indicated that the Ti-12Al-42Nb powder alloy contained 45.7 wt.% of titanium, 12 wt.% of aluminum, 41.3 wt.% niobium, 0.6 wt.% of silicon, and 0.4 wt.% of phosphorous as impurities;
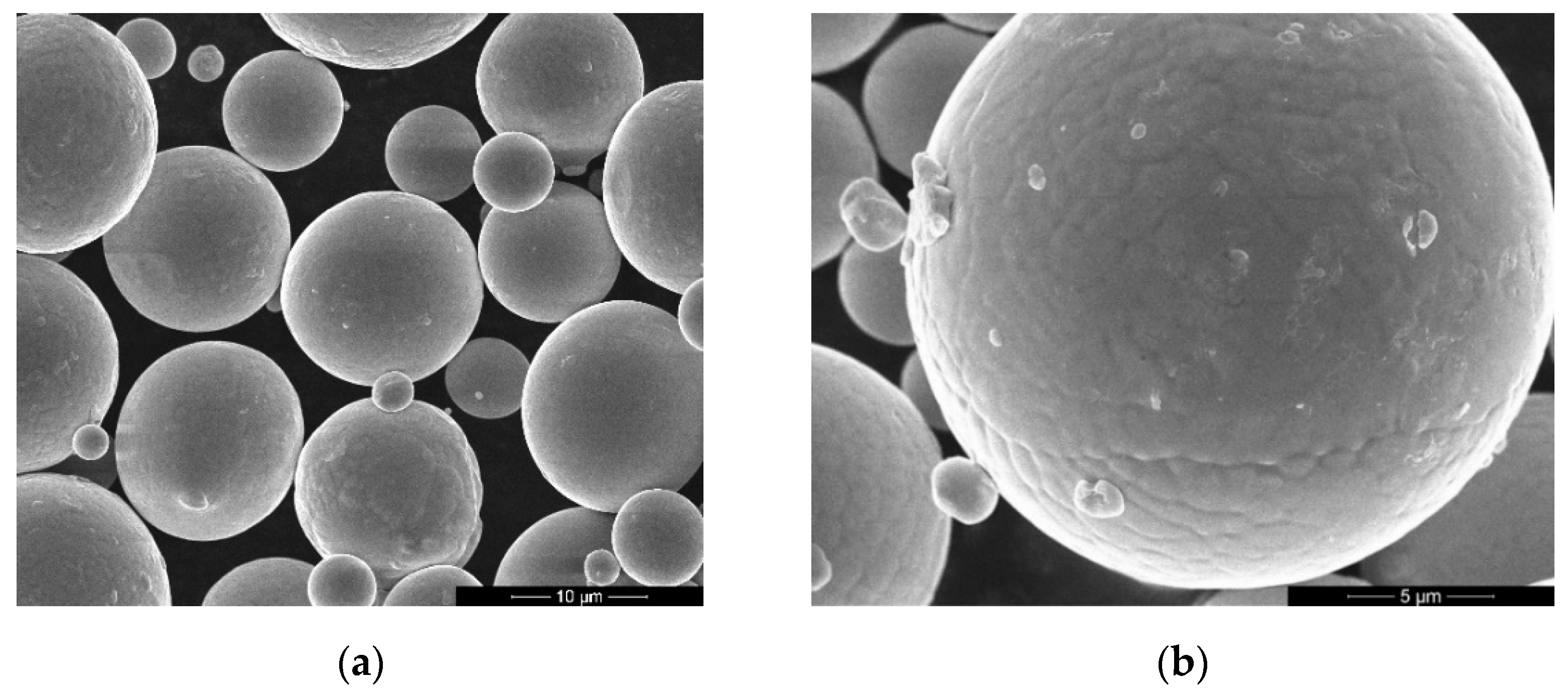
- Thermal-induced porosity (TIP) with a pore size of 2 µm in diameter was indicated by means of scanning electron microscopy of particle cross-sections in the Ti-12Al-42Nb powder alloy produced by electrode induction melting inert gas atomization (EIGA). SEM indicated ultra- and microcrystalline structures with grain sizes from 0.61 to 2.36 µm and an isometrical shape with clearly visible grain boundaries;
- The Ti-12Al-42Nb powder alloy has the following technological properties: particle size distributions of d10—15.72 µm, d50—38.28 µm, and d100—64.48 µm; powder flow density meter—196 sec, powder bulk density—2.79 g/cm3; true density—5.34 g/cm3; powder sphericity coefficient—1.02; and calculated specific surface area—2209.79 cm2/cm3;
- Reducing melting method showed that the Ti-12Al-42Nb powder alloy had a very small amount of impurities, such as oxygen 0.0087 ± 0.0018 wt.%, nitrogen 0.0360 ± 0.004 wt.%, and hydrogen impurities 0.0012 wt. ± 0.0002%. Infrared absorption of CO2 and SO2 gases from the Ti-12Al-42Nb powder alloy during oxidative melting indicated small impurities of sulfur 0.0016 ± 0.001 wt.% and carbon 0.022 ± 0.0003 wt.%;
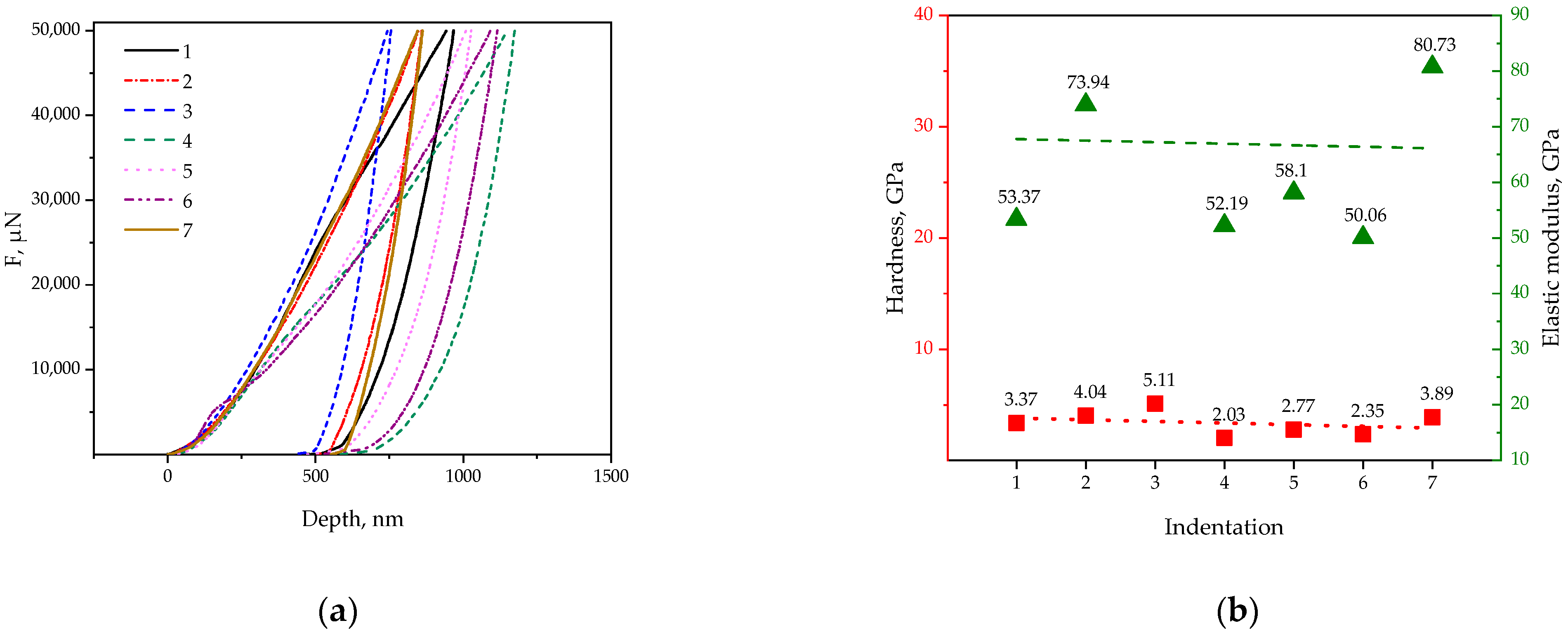
- Nanoindentation showed that the Ti-12Al-42Nb powder alloy had a microhardness (H) of 3.4 ± 1.1 GPa, elastic modulus (E) of 67 ± 19 GPa, indentation of maximum depth of 970 ± 150 nm, and ratio of elastic work to the total work of indentation (ηit) of 54 ± 18%;
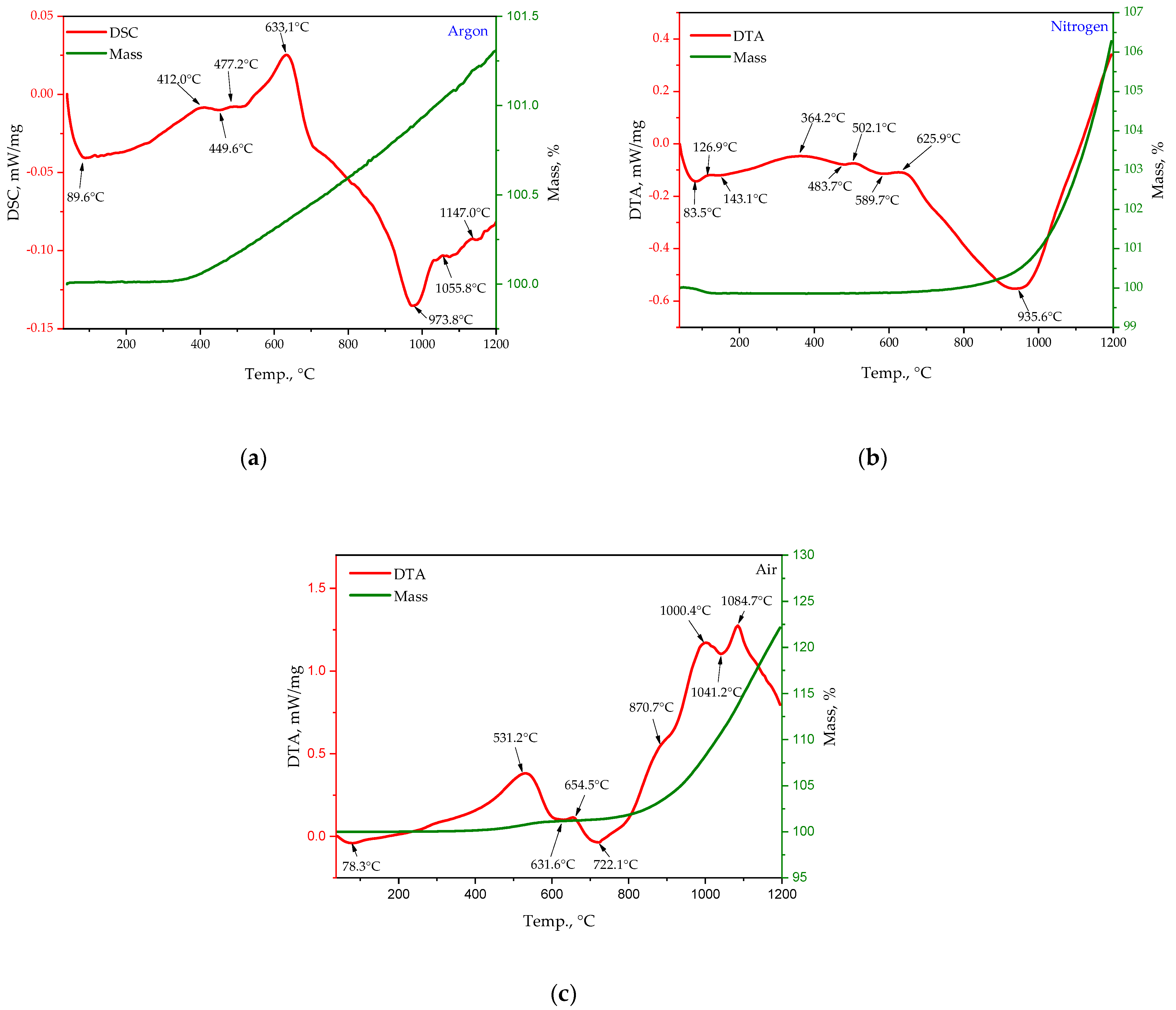
- STA in argon showed that the Ti-12Al-42Nb powder alloy’s weight was stable until 380 °C, and then, it gradually increased from 100 mass. % to 101.26 mass. % at 1200 °C (Figure 3a). Different peaks on the DSC curve were indicated in the temperature range from 400 °C to 700 °C and were related to the particle surface’s slight oxidation of the Ti-12Al-42Nb powder alloy, with the formation of TiO2 and Al2O3. A γAl2O3 θAl2O3 phase transformation occurred in temperature range of 900–1100 °C as well as a θAl2O3 αAl2O3 phase transformation in the temperature range of 1100–1200 °C;
- STA in nitrogen illustrated that the Ti-12Al-42Nb powder alloy weight’s was stable until 900 °C in nitrogen, and it gradually increased from 100 mass. % to 106.3 mass. % at 1200 °C, and the DTA curve increased at a steady rate as well (Figure 3b). The nitrogen adsorbed at the surface diffused into the Ti-12Al-42Nb powder alloy, with the formation of an interstitial solution of nitrogen on the surface of particles until 500 °C. In the temperature range of 500–900 °C, the concentration of nitrogen on the gas/particle interface became greater, and a new Ti2N phase formed. The phase transformation of Ti2N into TiN started at 935.6 °C and continued until 1200 °C;
- STA in air indicated the Ti-12Al-42Nb powder alloy’s oxidation started at 300 °C, and the sample’s weight increased sharply to 122.14 mass. % at 1200 °C (Figure 6c). The DTA curve indicated a peak at 531.32 °C related to the Ti-12Al-42Nb powder alloy’s oxidation and the formation of a titanium oxide (TiO2) layer on the particle surface, mainly composed of anatase TiO2. The next peak at 654.5 °C indicated the formation of aluminum oxide γAl2O3 as an additional intermediate layer on the Ti-12Al-42Nb particles. There was also formation of Nb-based suboxides NbOx and NbOy between the temperatures 270 °C and 500 °C and 330 °C and 500 °C, respectively, as well as Nb-based oxides such as NbO in the temperature range from 500 to 700 °C, NbO2 in the temperature range of 650–810 °C, and Nb2O5 at 870 °C. Also, the DTA curve indicated a phase transformation of Al2O3 from γAl2O3 to θAl2O3 in the temperature range from 900 °C to 1041.4 °C, with the extremum at 1000.4 °C, as well as a phase transformation of TiO2 from anatase to rutile in the temperature range from 1000 °C to 1200 °C. Also, a phase transformation from θAl2O3 to αAl2O3 was indicated at the same temperatures, with the extremum at 1084.7 °C.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Niinomi, M.; Nakai, M.; Hieda, J. Development of New Metallic Alloys for Biomedical Applications. Acta Biomater. 2012, 8, 3888–3903. [Google Scholar] [CrossRef] [PubMed]
- Lapin, J.; Gabalcová, Z.; Bajana, O.; Daloz, D. Effect of Heat Treatments on the Microstructure and Mechanical Properties of a Cast Intermetallic Ti-44Al-4Nb-4Zr-0.2Si-0.3B Alloy. Kovove Mater. 2006, 44, 297. [Google Scholar]
- Lapin, J. Creep of a Cast Intermetallic TiAl-Based Alloy. Kovove Mater. 2005, 43, 81–92. [Google Scholar]
- Lapin, J. Comparative Study of Creep of Cast Ti-46Al-2W-0.5Si and Ti-45Al-2W-0.6Si-0.7B Alloys. Kovove Mater. 2006, 44, 57. [Google Scholar]
- Leyens, C.; Peters, M. (Eds.) Titanium and Titanium Alloys: Fundamentals and Applications. John Wiley & Sons: Hoboken, NJ, USA, 2006. [Google Scholar]
- Kudrman, J.; Fousek, J.; Březina, V.; Míková, R.; Veselý, J. Titanium Alloys for Implants in Medicine. Kovove Mater. 2007, 45, 199. [Google Scholar]
- Niinomi, M.; Nakai, M. Titanium-Based Biomaterials for Preventing Stress Shielding between Implant Devices and Bone. Int. J. Biomater. 2011, 2011, 836587. [Google Scholar] [CrossRef]
- Abdel-Hady, M.; Hinoshita, K.; Morinaga, M. General Approach to Phase Stability and Elastic Properties of β-Type Ti-Alloys Using Electronic Parameters. Scr. Mater. 2006, 55, 477–480. [Google Scholar] [CrossRef]
- Abdel-Hady, M.; Fuwa, H.; Hinoshita, K.; Kimura, H.; Shinzato, Y.; Morinaga, M. Phase Stability Change with Zr Content in β-Type Ti-Nb Alloys. Scr. Mater. 2007, 57, 1000–1003. [Google Scholar] [CrossRef]
- Abdel-Hady, M.; Hinoshita, K.; Fuwa, H.; Murata, Y.; Morinaga, M. Change in Anisotropy of Mechanical Properties with β-Phase Stability in High Zr-Containing Ti-Based Alloys. Mater. Sci. Eng. A 2008, 480, 167–174. [Google Scholar] [CrossRef]
- Abdel-Hady, M.; Morinaga, M. Modification of Phase Stability and Mechanical Properties by the Addition of O and Fe into Β−Ti Alloys. Int. J. Mod. Phys. B 2009, 23, 1559–1565. [Google Scholar] [CrossRef]
- Aldea, E.; Dicu, M.M.; Gleizes, A.; Demetrescu, I. The modification of titanium dioxide MOCVD coating in TiAlNb after immersion in artificial saliva. In Proceedings of the 14th Nordic-Baltic Conference on Biomedical Engineering and Medical Physics, Riga, Latvia, 16–20 June 2008. [Google Scholar]
- Kesler, M.S.; Goyel, S.; Rios, O.; Cupid, D.M.; Seifert, H.J.; Ebrahimi, F. A Study of Phase Transformation in a TiAlNb Alloy and the Effect of Cr Addition. Mater. Sci. Eng. A 2010, 527, 2857–2863. [Google Scholar] [CrossRef]
- Bean, G.E.; Kesler, M.S.; Manuel, M.V. Effect of Nb on Phase Transformations and Microstructure in High Nb Titanium Aluminides. J. Alloys Compd. 2014, 613, 351–356. [Google Scholar] [CrossRef]
- Bartáková, S.; Prachár, P.; Kudrman, J.; Brezina, V.; Podhorná, B.; Cernochova, P.; Vanek, J.; Strecha, J. New Titanium β-Alloys for Dental Implantology and Their Laboratory-Based Assays of Biocomatibility. Scr. Medica 2009, 82, 76–82. [Google Scholar]
- Albretsen, J. The Toxicity of Iron, an Essential Element. Vet. Med. 2006, 201, 82–90. [Google Scholar]
- Brewer, G.J. Risks of Copper and Iron Toxicity during Aging in Humans. Chem. Res. Toxicol. 2010, 23, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Park, S.J.; Yong, T.S. Effect of Iron on Adherence and Cytotoxicity of Entamoeba Histolytica to CHO Cell Monolayers. Korean J. Parasitol. 2008, 46, 37–40. [Google Scholar] [CrossRef] [PubMed]
- Konushkin, S.V.; Kirsankin, A.A.; Mikhailova, A.V.; Rumyantsev, B.A.; Luk’yanov, A.S.; Kaplan, M.A.; Gorbenko, A.D.; Sergienko, K.V.; Nasakina, E.O.; Kolmakov, A.G.; et al. Technology for Production of a Ti–26Nb Alloy. Russ. Metall. (Met.) 2023, 2023, 1773–1777. [Google Scholar] [CrossRef]
- Sovar, M.M.; Aldea, E.; Mitran, V.; Miculescu, F.; Demetrescu, I. Cell Growth on TiAlNb Alloy as a Function of Bioactivation Method. In Key Engineering Materials; Trans Tech Publications Ltd.: Baech, Switzerland, 2008; Volume 361–363 II, pp. 1131–1134. [Google Scholar]
- ASTM F67-13; Specification for Unalloyed Titanium, for Surgical Implant Applications (UNS R50250, UNS R50400, UNS R50550, UNS R50700). ASTM International: West Conshohocken, PA, USA, 2013.
- Yang, M.; Lin, X.; Xu, X.; Chen, J.; Huang, W. Microstructure and Phase Evolution in Ti60-Ti2AlNb Gradient Material Prepared by Laser Solid Forming. Acta Metall. Sin. 2009, 45, 729–736. [Google Scholar]
- Che, Q.; He, W.; Li, H.; Cheng, K.; Wang, Y. Microstructure and Property of Ti2AlNb Alloy by Selective Electron Beam Melting. J. Mater. Eng. 2022, 50, 156–164. [Google Scholar] [CrossRef]
- Zhou, Y.H.; Li, W.P.; Zhang, L.; Zhou, S.Y.; Jia, X.; Wang, D.W.; Yan, M. Selective Laser Melting of Ti–22Al–25Nb Intermetallic: Significant Effects of Hatch Distance on Microstructural Features and Mechanical Properties. J. Mater. Process Technol. 2020, 276, 116398. [Google Scholar] [CrossRef]
- Zhou, Y.H.; Li, W.P.; Wang, D.W.; Zhang, L.; Ohara, K.; Shen, J.; Ebel, T.; Yan, M. Selective Laser Melting Enabled Additive Manufacturing of Ti–22Al–25Nb Intermetallic: Excellent Combination of Strength and Ductility, and Unique Microstructural Features Associated. Acta Mater. 2019, 173, 117–129. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, B.; Bai, Q.; Xie, G. Correlation of Microstructure and Mechanical Properties of Ti2AlNb Manufactured by SLM and Heat Treatment. Intermetallics 2021, 139, 107367. [Google Scholar] [CrossRef]
- Zhou, Y.H.; Wang, D.W.; Song, L.J.; Mukhtar, A.; Huang, D.N.; Yang, C.; Yan, M. Effect of Heat Treatments on the Microstructure and Mechanical Properties of Ti2AlNb Intermetallic Fabricated by Selective Laser Melting. Mater. Sci. Eng. A 2021, 817, 141352. [Google Scholar] [CrossRef]
- Wang, Y.H.; Lin, J.P.; Xu, X.J.; He, Y.H.; Wang, Y.L.; Chen, G.L. Effect of Fabrication Process on Microstructure of High Nb Containing TiAl Alloy. J. Alloys Compd. 2008, 458, 313–317. [Google Scholar] [CrossRef]
- Soni, N.; Renna, G.; Leo, P. Advancements in Metal Processing Additive Technologies: Selective Laser Melting (SLM). Metals 2024, 14, 1081. [Google Scholar] [CrossRef]
- Strumza, E.; Hayun, S.; Barzilai, S.; Finkelstein, Y.; Ben David, R.; Yeheskel, O. In Situ Detection of Thermally Induced Porosity in Additively Manufactured and Sintered Objects. J. Mater. Sci. 2019, 54, 8665–8674. [Google Scholar] [CrossRef]
- Pesode, P.; Barve, S. A Review—Metastable β Titanium Alloy for Biomedical Applications. J. Eng. Appl. Sci. 2023, 70, 25. [Google Scholar] [CrossRef]
- Illarionov, A.G.; Stepanov, S.I.; Naschetnikova, I.A.; Popov, A.A.; Soundappan, P.; Thulasi Raman, K.H.; Suwas, S. A Review—Additive Manufacturing of Intermetallic Alloys Based on Orthorhombic Titanium Aluminide Ti2AlNb. Materials 2023, 16, 991. [Google Scholar] [CrossRef]
- Tan, J.H.; Wong, W.L.E.; Dalgarno, K.W. An Overview of Powder Granulometry on Feedstock and Part Performance in the Selective Laser Melting Process. Addit. Manuf. 2017, 18, 228–255. [Google Scholar] [CrossRef]
- Mergulhão, M.V.; Das Neves, M.D.M. Characteristics of Biometallic Alloy to Additive Manufacturing Using Selective Laser Melting Technology. J. Biomater. Nanobiotechnol. 2018, 9, 89–99. [Google Scholar] [CrossRef]
- Olakanmi, E.O.; Cochrane, R.F.; Dalgarno, K.W. A Review on Selective Laser Sintering/Melting (SLS/SLM) of Aluminium Alloy Powders: Processing, Microstructure, and Properties. Prog. Mater. Sci. 2015, 74, 401–477. [Google Scholar] [CrossRef]
- Maamoun, A.H.; Elbestawi, M.; Dosbaeva, G.K.; Veldhuis, S.C. Thermal Post-Processing of AlSi10Mg Parts Produced by Selective Laser Melting Using Recycled Powder. Addit. Manuf. 2018, 21, 234–247. [Google Scholar] [CrossRef]
- Maamoun, A.H.; Elbestawi, M.A.; Veldhuis, S.C. Influence of Shot Peening on Alsi10mg Parts Fabricated by Additive Manufacturing. J. Manuf. Mater. Process. 2018, 2, 40. [Google Scholar] [CrossRef]
- Liu, G.; Wang, Z.; Fu, T.; Li, Y.; Liu, H.; Li, T.; Gong, M.; Wang, G. Study on the Microstructure, Phase Transition and Hardness for the TiAl-Nb Alloy Design during Directional Solidification. J. Alloys Compd. 2015, 650, 45–52. [Google Scholar] [CrossRef]
- Illarionov, A.; Mukanov, G.; Stepanov, S.; Kuznetsov, V.; Karelin, R.; Andreev, V.; Yusupov, V.; Korelin, A. Microstructure and Physico-Mechanical Properties of Biocompatible Titanium Alloy Ti-39Nb-7Zr after Rotary Forging. Metals 2024, 14, 497. [Google Scholar] [CrossRef]
- Niinomi, M. Mechanical Biocompatibilities of Titanium Alloys for Biomedical Applications. J. Mech. Behav. Biomed. Mater. 2008, 1, 30–42. [Google Scholar] [CrossRef]
- Shastov, A.L.; Ermakov, A.M.; Popkov, A.V.; Kononovich, N.A.; Gorbach, E.N.; Stogov, M.V. Assessment of the Need for Bioactive Implants with Antimicrobial Properties in the Treatment of Patients with Orthopedic Pathology Complicated by Infection. Biomed. Eng. 2024, 58, 55–58. [Google Scholar] [CrossRef]
- He, H.; Yu, J. Effect of Adsorbed Water on Mechanical and Mechanochemical Properties of Silicate Glasses. J. Non-Cryst. Solids X 2023, 18, 100189. [Google Scholar] [CrossRef]
- Chapman, I.D.; Hair, M.L. The Role of the Surface Hydroxyl Groups in Catalytic Cracking. J. Catal. 1963, 2, 145–148. [Google Scholar] [CrossRef]
- Bernett, M.K.; Zisman, W.A. Effect of Adsorbed Water on Wetting Properties of Borosilicate Glass, Quartz, and Sapphire. J. Colloid. Interface Sci. 1968, 29, 413–423. [Google Scholar] [CrossRef]
- Cuthill, J.R.; Hayes, W.D.; Seebold, R.E. Nitriding Phenomena in Titanium and the 6Al-4V Titanium Alloy. J. Res. Natl. Bur. Standards. Sect. A Phys. Chem. 1960, 64, 119. [Google Scholar] [CrossRef] [PubMed]
- Zhecheva, A.; Sha, W.; Malinov, S.; Long, A. Enhancing the Microstructure and Properties of Titanium Alloys through Nitriding and Other Surface Engineering Methods. Surf. Coat. Technol. 2005, 200, 2192–2207. [Google Scholar] [CrossRef]
- Solntsev, K.A.; Shevtsov, S.V.; Stetsovskii, A.P.; Shashkeev, K.A. The Phenomenon of Bifurcation in the Processes of Oxidative Construction of Thin-Wall Ceramics under Heating of Solid Titanium Preforms. Inorg. Mater. 2010, 46, 177–182. [Google Scholar] [CrossRef]
- Solntsev, K.A.; Chernyavskii, A.S.; Shustorovich, E.M.; Stetsovskii, A.P. Kinetics of Rutile Formation via Oxidation of Titanium in Air at 850 °C. Inorg. Mater. 2004, 40, 829–832. [Google Scholar] [CrossRef]
- Chu, C.L.; Wu, S.K. Ion Nitriding of Titanium Aluminides with 25–53 at.% A1 I: Nitriding Parameters and Microstructure Characterization. Surf. Coat. Technol. 1996, 78, 211–218. [Google Scholar] [CrossRef]
- Zabunova, S.V. A Review on the Possibility for Nitriding of Aluminium Alloys. Annu. J. Tech. Univ. Varna Bulg. 2021, 5, 100–111. [Google Scholar] [CrossRef]
- Kukarenko Vladimir, A.; Konstantinov Valeriy, M. Structure Of Commercial Titanium Subjected to Low-Temperature Ion Nitriding. Mech. Mach. Mech. Mater. 2022, 1, 48–55. [Google Scholar] [CrossRef]
- Mitoraj-Królikowska, M.; Drożdż, E. Some Aspects of Oxidation and Reduction Processes in Ti–Al and Ti–Al–Nb Systems. Materials 2022, 15, 1640. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Aoyagi, K.; Koizumi, Y.; Yang, C.; Bian, H.; Hayasaka, Y.; Fujieda, T.; Chiba, A. Effect of Niobium Addition on Tensile Properties and Oxidation Resistance of a Titanium-Based Alloy. Corros. Sci. 2021, 180, 109198. [Google Scholar] [CrossRef]
- Kovarik, L.; Bowden, M.; Andersen, A.; Jaegers, N.R.; Washton, N.; Szanyi, J. Quantification of High Temperature Transition Al2O3 and Their Phase Transformations. Angew. Chem. Int. Ed. 2020, 59, 21719–21727. [Google Scholar] [CrossRef] [PubMed]
- Hadi, M.; Bayat, O.; Meratian, M.; Shafyei, A.; Ebrahimzadeh, I. Oxidation Properties of a Beta-Stabilized TiAl Alloy Modified by Rare Earth Elements. Oxid. Met. 2018, 90, 421–434. [Google Scholar] [CrossRef]
- Xiong, Y.; Zhu, S.; Wang, F. The Oxidation Behavior of TiAlNb Intermetallics with Coatings at 800 °C. Surf. Coat. Technol. 2005, 197, 322–326. [Google Scholar] [CrossRef]
- Valenza, T.C.; Weber, P.K.; Marquis, E.A. Role of Nitrogen in the High-Temperature Oxidation of Titanium Alloys. Corros. Sci. 2024, 235, 112164. [Google Scholar] [CrossRef]
- Shida, Y.; Anada, H. The Effect of Various Ternary Additives on the Oxidation Behavior of TiA1 in High-Temperature Air. Oxid. Met. 1996, 45, 197–219. [Google Scholar] [CrossRef]
- Gautier, K.; Monceau, D.; Epifano, E.; Connétable, D.; Gheno, T. Study of the Role of Nitrogen in the Oxidation of Titanium-Based Alloys by Changing the Reaction Gas. High Temp. Corros. Mater. 2024, 101, 861–872. [Google Scholar] [CrossRef]
- Gautier, K.; Epifano, E.; Connétable, D.; Monceau, D.; Gheno, T. Effects of Al and Refractory Alloying Elements (W, Ta and Hf) on Oxidation Kinetics, Oxygen Dissolution and Diffusion in Titanium Alloys. Corros. Sci. 2024, 237, 112330. [Google Scholar] [CrossRef]
- Sienkiewicz, J.; Kuroda, S.; Murakami, H.; Araki, H.; Giżyński, M.; Kurzydłowski, K.J. Microstructure and Oxidation Performance of TiAl-(Cr, Nb, Ta) Coatings Fabricated by Warm Spray and High-Velocity Oxy-Fuel Spraying. J. Therm. Spray. Technol. 2019, 28, 563–579. [Google Scholar] [CrossRef]
- Laboureur, D.; Glabeke, G.; Gouriet, J.B. Aluminum Nanoparticles Oxidation by TGA/DSC: Parametric Analysis and Oxide Thickness Determination. J. Therm. Anal. Calorim. 2019, 137, 1199–1210. [Google Scholar] [CrossRef]
- Borowski, T.; Zielińska, K.; Spychalski, M.; Adamczyk-Cieślak, B.; Żrodowski, Ł. Effect of Oxidation Temperature on the Properties of Niobium in View of Its Biomedical Applications. Surf. Coat. Technol. 2023, 473, 129911. [Google Scholar] [CrossRef]
- Nico, C.; Monteiro, T.; Graça, M.P.F. Niobium Oxides and Niobates Physical Properties: Review and Prospects. Prog. Mater. Sci. 2016, 80, 1–37. [Google Scholar] [CrossRef]
- Clenny, J.T.; Rosa, C.J. Oxidation Kinetics of Niobium in the Temperature Range of 873 to 1083 K. Metall. Trans. A 1980, 11A, 1385–1389. [Google Scholar] [CrossRef]
- Kovalev, I.A.; Kochanov, G.P.; Zufman, V.Y.; L’vov, L.O.; Shevtsov, S.V.; Demin, K.Y.; Sitnikov, A.I.; Shokodko, A.V.; Strelnikova, S.S.; Chernyavskii, A.S.; et al. Kinetics of High-Temperature Nitridation of Zr–Nb Solid Solutions. Inorg. Mater. 2023, 59, 242–250. [Google Scholar] [CrossRef]
- Banu, A.; Marcu, M.; Petrescu, S.; Ionescu, N.; Paraschiv, A. Effect of Niobium Alloying Level on the Oxidation Behavior of Titanium Aluminides at 850 °C. Int. J. Miner. Metall. Mater. 2016, 23, 1452–1457. [Google Scholar] [CrossRef]

| Element | Dilatation Test | Adherence Test | Screening |
|---|---|---|---|
| Ti | Compatible | Compatible | Compatible |
| Al | Tolerant | Tolerant | Toxic |
| Nb | Compatible | Compatible | Compatible |
| V | Tolerant | Toxic | Tolerant |
| Zr | Compatible | Compatible | Compatible |
| Mo | Tolerant | Tolerant | Tolerant |
| Ta | Compatible | Compatible | Compatible |
| Fe | - | Compatible | Toxic |
| Cu | Toxic | Toxic | Toxic |
| Zn | Toxic | Toxic | Toxic |
| Element | Weight, % | Statistical Error, % |
|---|---|---|
| Ti | 45.70 | 0.75 |
| Al | 12.00 | 3.79 |
| Nb | 41.30 | 0.16 |
| Si | 0.60 | 12.3 |
| P | <0.26 | 10.8 |
| Fe | 0.089 | 11.3 |
| Cu | 0.029 | 18.5 |
| Zn | 0.026 | 17.8 |
| Element | Weight, % | Standard Deviation |
|---|---|---|
| Oxygen | 0.0087 | 0.0018 |
| Nitrogen | 0.0360 | 0.0040 |
| Hydrogen | 0.0012 | 0.0002 |
| Sulfur | 0.0016 | 0.0010 |
| Carbon | 0.0220 | 0.0003 |
| Parameter | Measurement Result | Measurement Units |
|---|---|---|
| Average diameter of particles with percentage fraction distribution | 2–5 (0.28%) | µm |
| 5–10 (2.49%) | ||
| 10–20 (14.56%) | ||
| 20–45 (62.40%) | ||
| 45–75 (20.27%) | ||
| Particle size distribution | d3—10.28 | µm |
| d10—15.72 | ||
| d25—24.08 | ||
| d50—34.12 | ||
| d90—50.59 | ||
| d97—57.67 | ||
| d100—64.48 | ||
| Fluidity of powder | 196 | sec |
| Powder bulk density | 2.79 | [g/c] |
| True density | 5.34 | [g/c] |
| Powder sphericity coefficient | 1.02 | - |
| Specific surface area (BET) | Not indicated | - |
| Specific surface area (calculated) | 2209.79 | [cm2/cm3] |
| Indentation | Hardness, GPa | Elastic Modulus, GPa | MaxDepth, nm | ηit, % |
|---|---|---|---|---|
| 1 | 3.37 | 53.37 | 968.43 | 47.52 |
| 2 | 4.04 | 73.94 | 860.85 | 45.66 |
| 3 | 5.11 | 100.17 | 757.46 | 38.00 |
| 4 | 2.03 | 52.19 | 1175.07 | 83.59 |
| 5 | 2.77 | 58.10 | 1027.66 | 76.49 |
| 6 | 2.35 | 50.06 | 1115.79 | 51.44 |
| 7 | 3.89 | 80.73 | 862.75 | 37.81 |
| Mean value | 3.40 ± 1.10 | 67 ± 19 | 970 ± 150 | 54 ± 18 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anokhin, A.; Kirsankin, A.; Kukueva, E.; Luk’yanov, A.; Chuvikina, M.; Ermakova, E.; Strelnikova, S.; Kupreenko, S. Material Characterization and Technological Properties of Biocompatible Ti-12Al-42Nb Spherical Powder Alloy for Additive Manufacturing of Personal Medical Implants. Metals 2025, 15, 147. https://doi.org/10.3390/met15020147
Anokhin A, Kirsankin A, Kukueva E, Luk’yanov A, Chuvikina M, Ermakova E, Strelnikova S, Kupreenko S. Material Characterization and Technological Properties of Biocompatible Ti-12Al-42Nb Spherical Powder Alloy for Additive Manufacturing of Personal Medical Implants. Metals. 2025; 15(2):147. https://doi.org/10.3390/met15020147
Chicago/Turabian StyleAnokhin, Alexander, Andrey Kirsankin, Elena Kukueva, Alexander Luk’yanov, Maria Chuvikina, Elena Ermakova, Svetlana Strelnikova, and Stepan Kupreenko. 2025. "Material Characterization and Technological Properties of Biocompatible Ti-12Al-42Nb Spherical Powder Alloy for Additive Manufacturing of Personal Medical Implants" Metals 15, no. 2: 147. https://doi.org/10.3390/met15020147
APA StyleAnokhin, A., Kirsankin, A., Kukueva, E., Luk’yanov, A., Chuvikina, M., Ermakova, E., Strelnikova, S., & Kupreenko, S. (2025). Material Characterization and Technological Properties of Biocompatible Ti-12Al-42Nb Spherical Powder Alloy for Additive Manufacturing of Personal Medical Implants. Metals, 15(2), 147. https://doi.org/10.3390/met15020147






