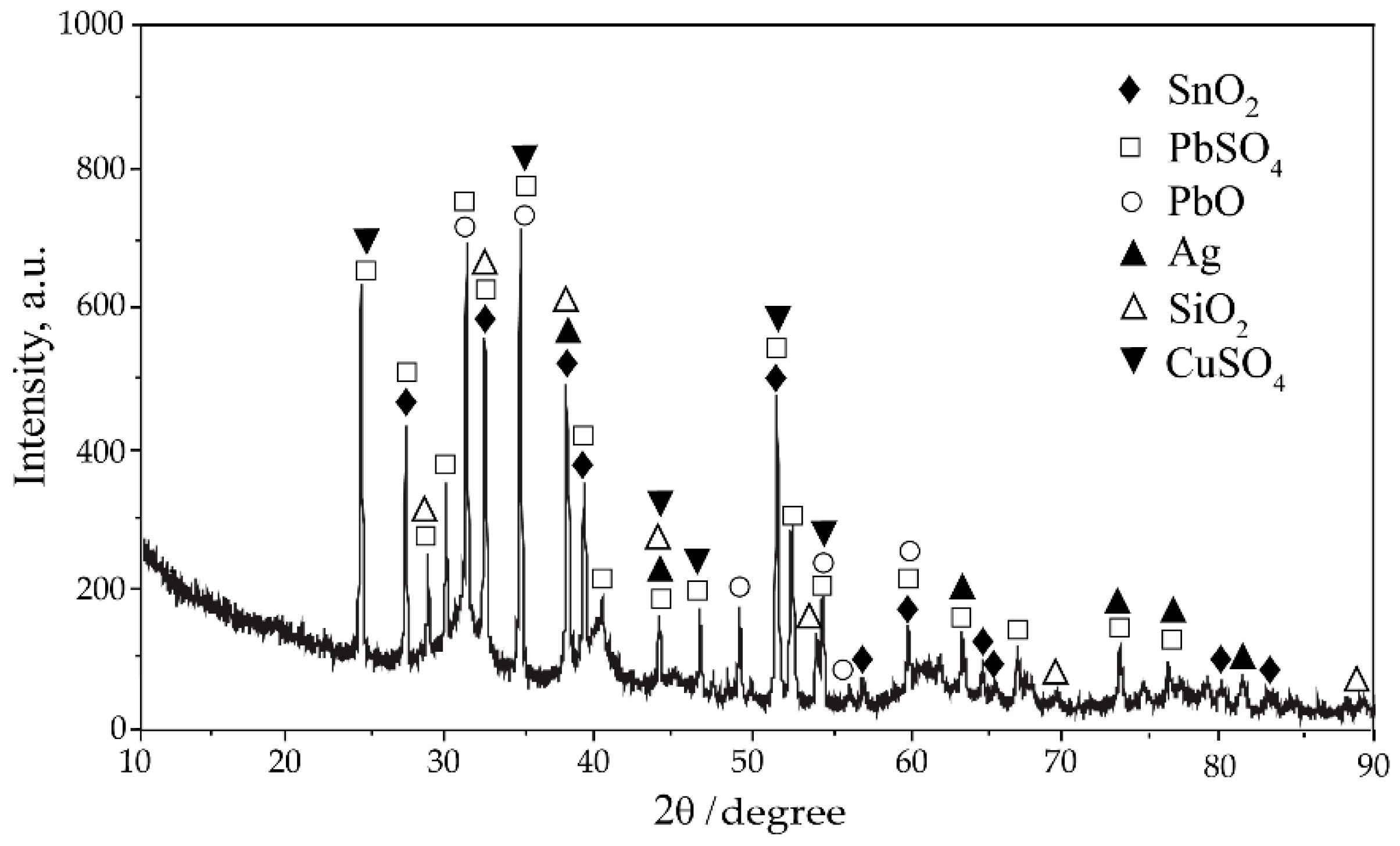
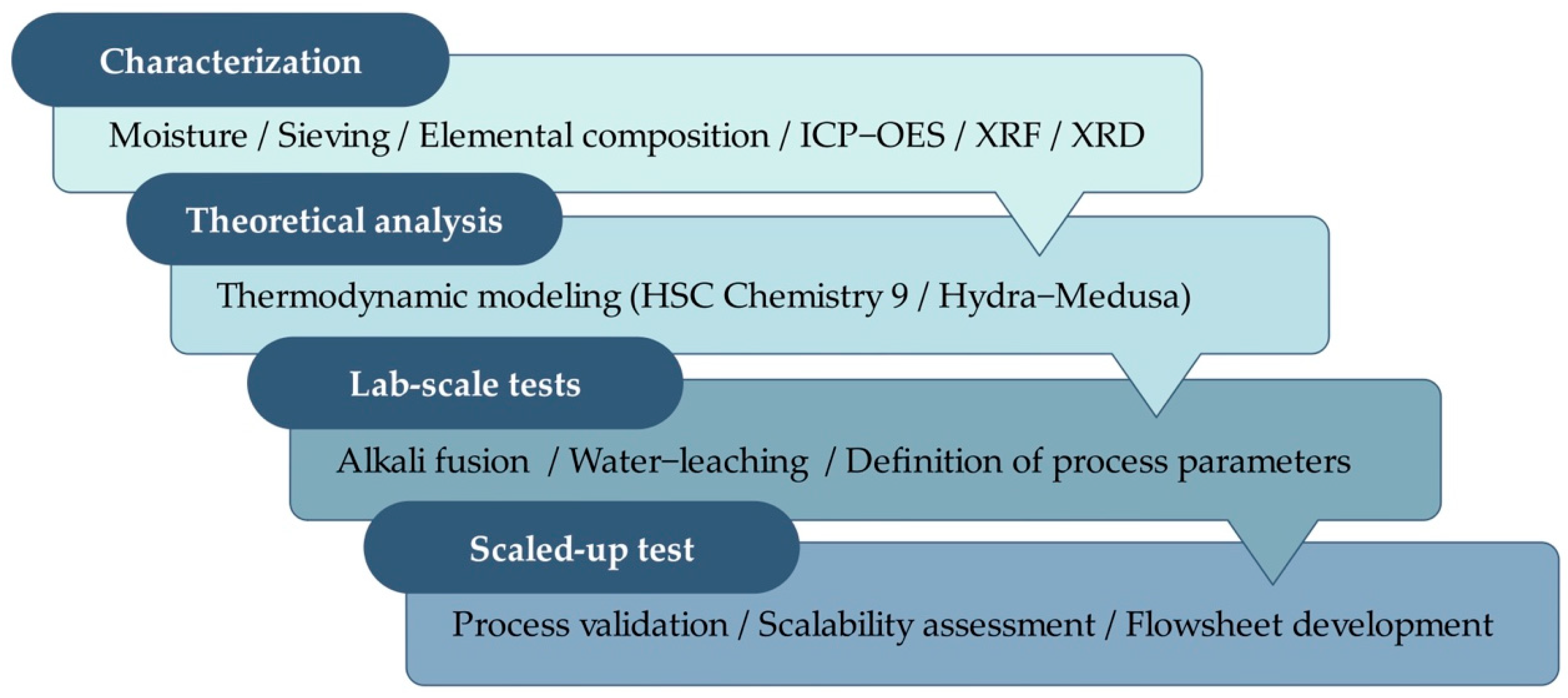
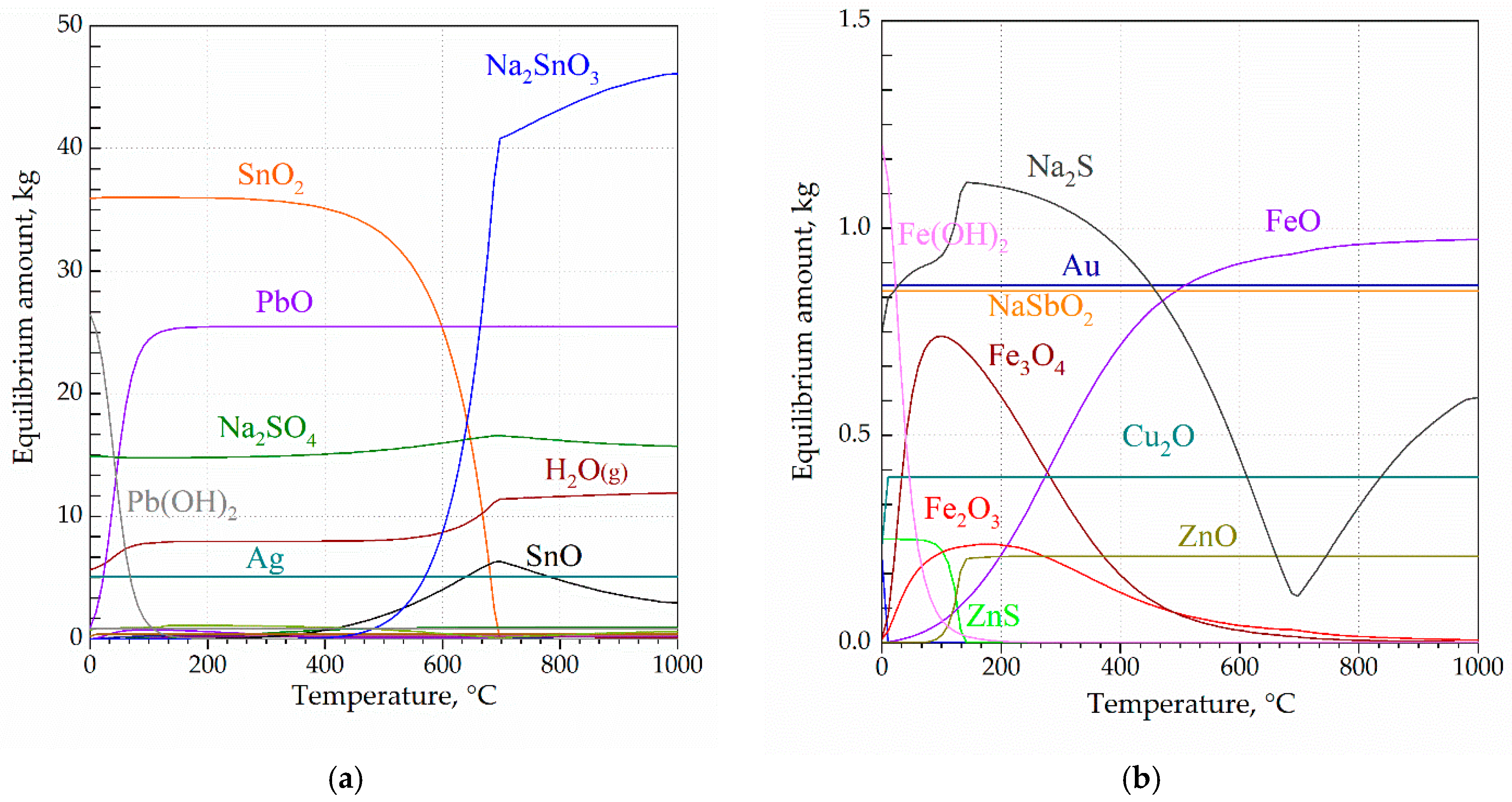
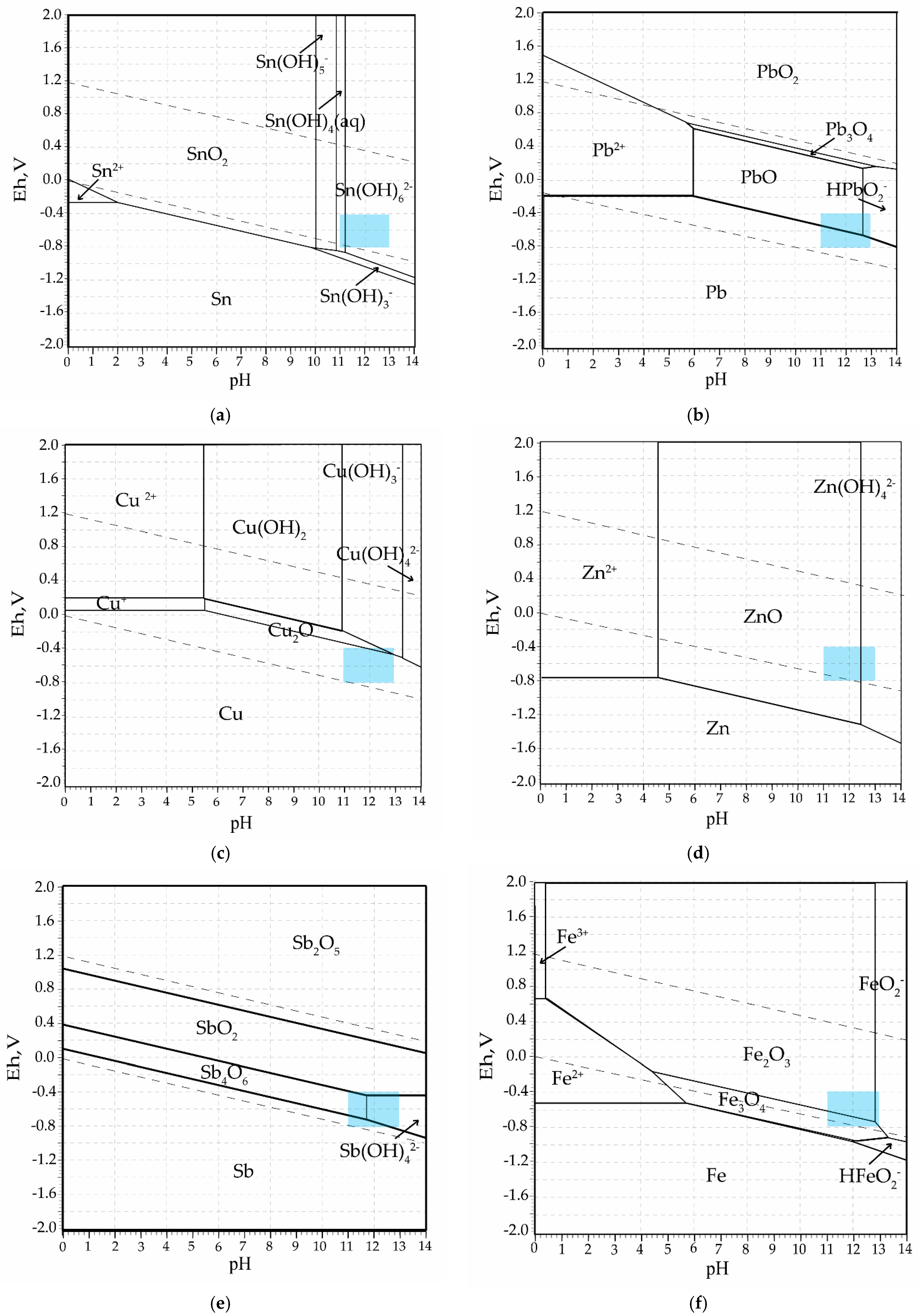
1. Introduction
The growing demand for base, precious, and technological metals necessitates the exploration of alternative sources in addition to conventional primary exploitation. These alternative sources, although commonly considered as waste, possess compositions rich in metals that make them valuable secondary raw materials. In addition to serving as a source of essential and economically significant metals, this approach is consistent with the principles of cleaner production, environmental protection, and the objectives of a circular economy [
1,
2].
In the field of primary metallurgy, numerous studies have been devoted to the optimization of leaching and extraction processes to enhance metal recovery efficiencies and reduce environmental impact [
3,
4]. Careful control of process parameters is essential to achieve high metal yields, and the same principle applies to the valorization of secondary materials.
One of the valuable secondary sources is anode slime, a by-product generated during the electro-hydrometallurgical refining stage of copper production (copper anode slime—CAS). Given that global copper production in 2024 reached approximately 23 million tons, of which nearly 80% was produced via pyrometallurgical routes, the associated generation of CAS is estimated at approximately 2–20 kg per ton of refined Cu [
5,
6]. More than 70% of its total composition consists of base metals such as Cu, Ni, Sb, Sn, and Pb [
7,
8,
9]. Although it also contains hazardous elements such as As and Cd, which classify CAS as hazardous waste, its significant content of precious and platinum group metals makes it an attractive material for further processing and valorization [
10,
11,
12]. Considering that global copper demand continues to outpace supply and that only about 20% of total copper production originates from secondary sources, the need to enhance recycling and recovery efforts is becoming increasingly urgent [
13].
The composition of CAS, particularly the ratios of metals it contains, is largely determined by the nature of the input material for copper refining, specifically whether and to what extent secondary raw materials such as electronic waste (e-waste) are introduced alongside primary copper ores. With the continuous increase in global e-waste generation, recognized as an important carrier of valuable metals, its integration into copper smelting has become significant. Although the proportion of e-waste varies considerably among refineries, major operators are gradually adopting mixed-feed smelting (co-processing), combining 60–40% copper concentrate with 40–60% e-waste, instead of the typical <20% of e-waste addition [
14]. Thus, CAS generated from co-processing routes, referred to as non-standard anode slime, exhibits a far more heterogeneous, inherited, chemical composition compared to conventional CAS generated by refining of anodes made solely from copper concentrate. These non-standard CAS materials generally contain elevated levels of lead and tin (predominantly as metastannic acid, H
2SnO
5 × 9H
2O), along with increased fractions of precious and technological metals [
15,
16]. The comprehensive review of Moosavi-Khoonsari [
5] provides an extensive summary of CAS compositions collected from over 100 smelters worldwide, presenting the characteristic features and processing approaches for this material; however, it does not clearly distinguish between standard and non-standard anode slimes. Although comprehensive studies addressing the composition and behavior of non-standard CAS remain scarce, their pronounced heterogeneity—both in elemental diversity and concentration—is evident, reflecting the inherited composition of input materials and the specific pyrometallurgical and electrorefining processes from which the CAS originates.
CAS valorization has been the subject of numerous studies, yet the optimal processing strategy is defined by its physicochemical characteristics [
11,
17]. Processing methods are generally categorized into traditional hybrid routes, integrating hydro-, pyro-, and electrometallurgical refining stages and purely hydrometallurgical approaches [
10,
18,
19,
20]. However, recovery of its metallic constituents remains a considerable challenge, primarily due to the strong chemical interactions among base metals (e.g., adhesion, encapsulation), which hinder efficient metal recovery [
21,
22,
23]. A particular challenge arises from the complex phase composition of the CAS, characterized by stable and insoluble compounds such as metastannic acid [
24]. This insoluble hydrated tin oxide forms under the anode refining process conditions and is especially prominent in non-standard copper anode slimes due to the increased tin input resulting from e-waste co-processing. These inert phases further complicate the already demanding treatment process [
25,
26].
To overcome these limitations, the alkali fusion–leaching approach was selected in this study as it enables the transformation of metastannic acid into soluble species, thus improving selectivity and overall recovery efficiency.
Alkali fusion has emerged as an effective method for the treatment of various metallurgical wastes and by-products, particularly in cases where traditional approaches are insufficient to meet the requirements of sustainable and environmentally responsible processing. This method is frequently applied to CAS to alter its phase composition, thereby facilitating subsequent processing, i.e., leaching [
27,
28]. Its primary applications include the removal of base metals, enrichment of CAS with precious metals by concentrating them, and the recovery of Pb and Sn. The core of the process involves the reaction of PbSO
4 and SnO
2 in alkaline medium, possibly with additives such as nitrates, at elevated temperatures, leading to the formation of soluble salts—plumbates and stannates [
29,
30]. Leaching procedures involve water, acid, or sulfide media, depending on the target metals [
31]. A combination of NaOH fusion (1:1 CAS mass ratio; 700 °C, 2 h), water leaching, and synergistic acid leaching with H
2SO
4, H
2O
2, and tartaric acid has been shown to remove Pb, Sn, S, As, Cu, Ni, and Sb (76.39, 79.29, 87.72, 95.20, 99.68, 81.99, and 99.94%, respectively) while enriching the residue with precious metals [
32]. A fusion system consisting of NaOH and Na
2CO
3 as the alkaline medium and NaNO
3 as the oxidizing agent, in a mass ratio of 1:3:3 relative to the sample, was employed at 600 °C for 40 min to induce phase transformation of Sn, Pb, Al, and Zn with efficiencies of 84.5, 73.97, 95.22, and 78.87%, respectively. After leaching, Cu and precious metals remained concentrated in the residue and were further separated by selective acid leaching [
33]. However, most studies have focused on standard CAS, but the efficiency of suggested processes on non-standard ones remain unknown.
Accordingly, the objective of this study is to optimize the alkali fusion–leaching process for non-standard CAS, characterized by elevated contents of lead, tin (present as metastannic acid), and precious metals. The research integrates thermodynamic modeling, experimental investigation, and scaled-up validation to establish the correlation between fusion parameters, type and dosage of additives, phase transformations, dissolution behavior, and leaching efficiency to achieve selective tin separation into leachate and enrichment of precious metals in the lead phase (solid residue), suitable for further valorization. By optimizing the process, this study can provide a framework for enhancing metal recovery efficiency from non-standard CAS, addressing industrial needs driven by increased e-waste co-processing, which aligns with the principles of cleaner production, environmental protection, and the objectives of a circular economy.
4. Conclusions
In this study, a clear correlation between alkali fusion parameters, phase transformations, and leaching efficiency for a non-standard CAS with a complex chemical and phase composition that challenges conventional recovery routes has been established. The application of an integrated thermodynamic modeling, laboratory optimization, and scaled-up validation approach to non-standard CAS enabled a distinct separation of metals, where tin was selectively dissolved into the liquid phase, while lead and precious metals were concentrated in the solid product, demonstrating validated scalability.
Thermodynamic modeling provided insight into the transformation behavior of key components and the optimal conditions for both the fusion and leaching steps. The correspondence between the modeling and experimental results confirms the overall process selectivity and supports the experimental observation. Minor deviations between the modeling and experimental results are attributed to the complexity of the system and the use of a real sample in the laboratory tests.
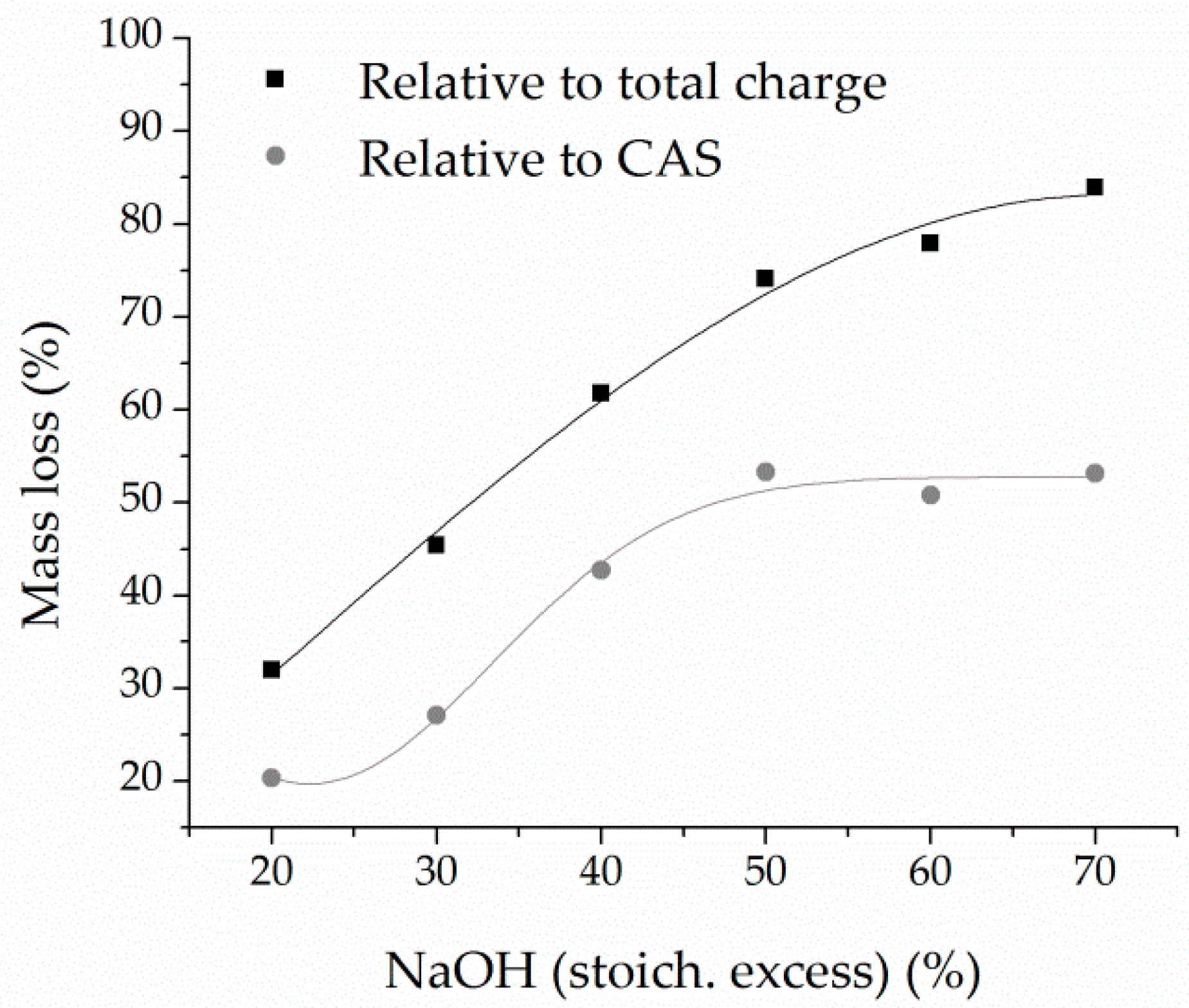
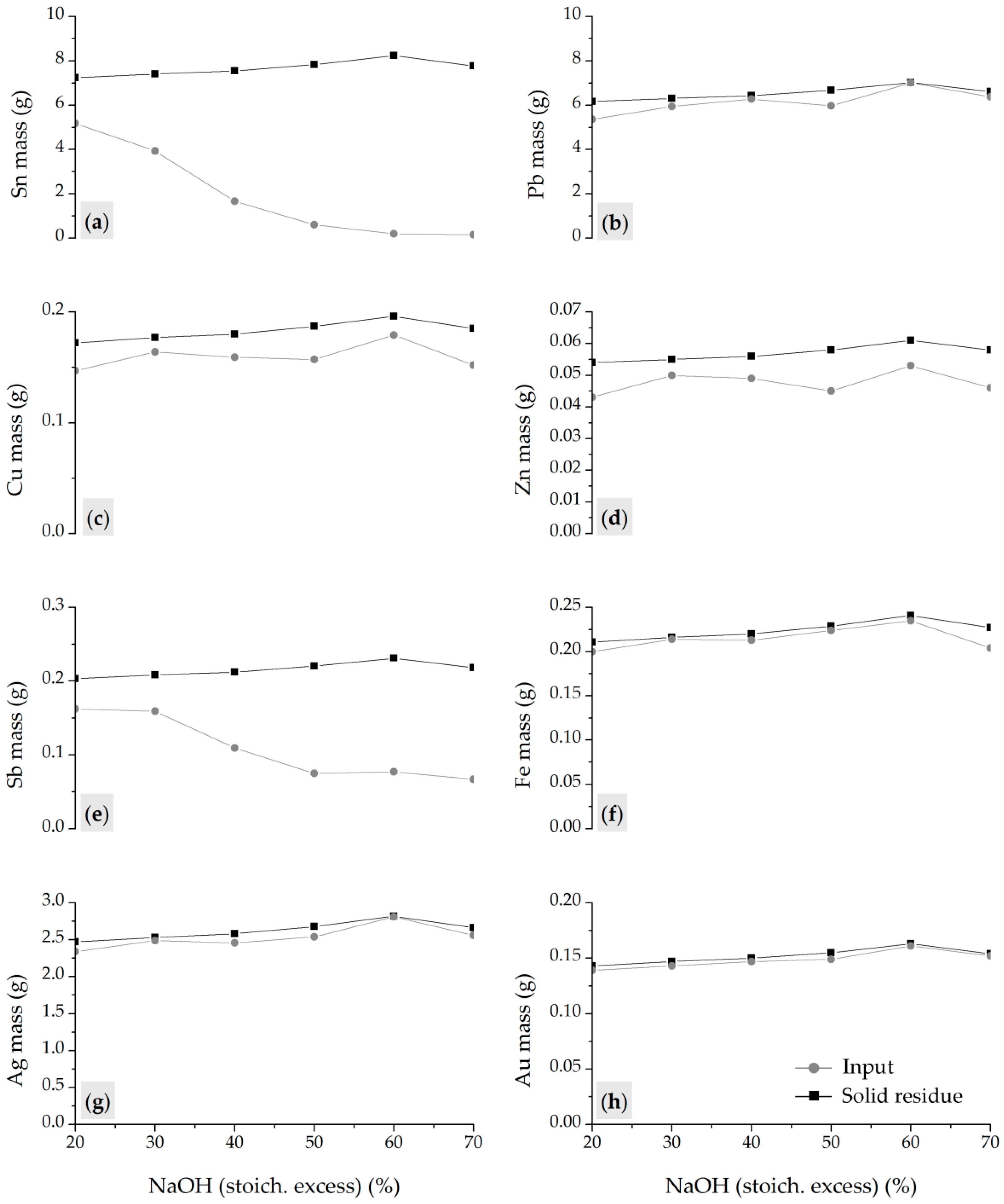
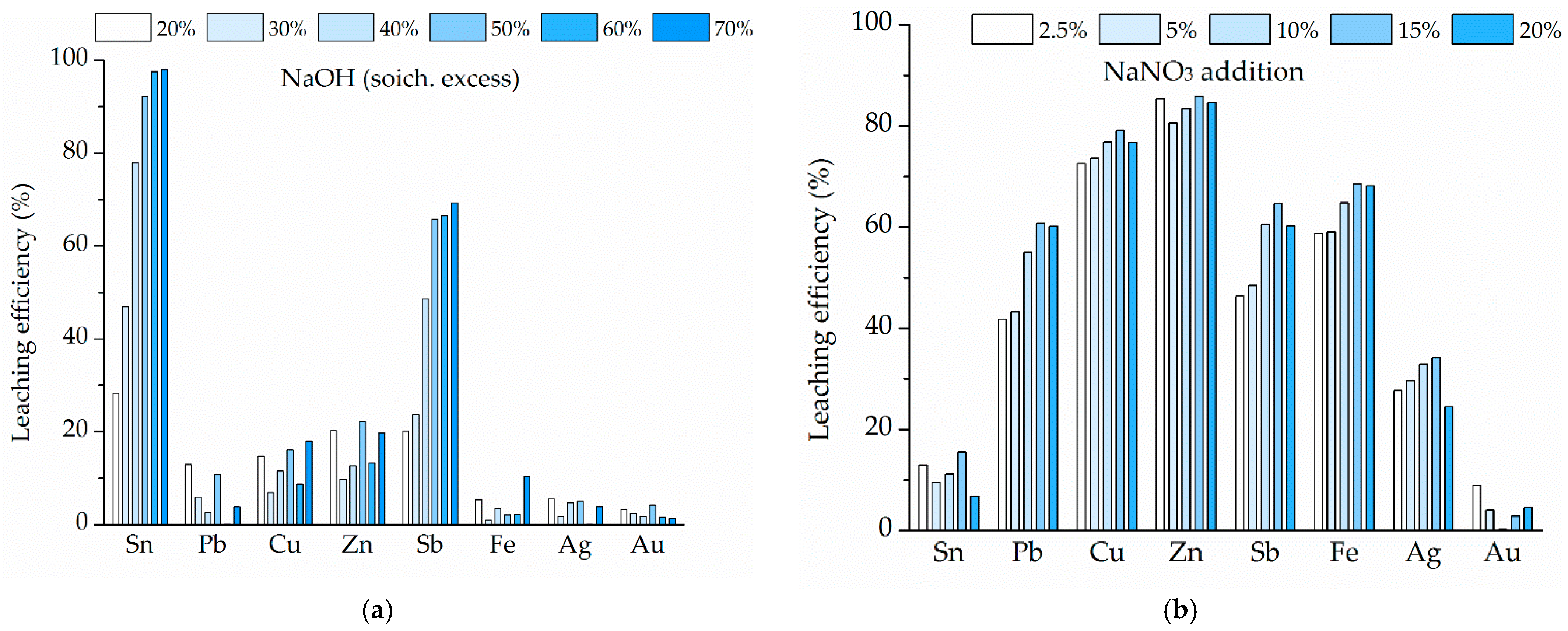
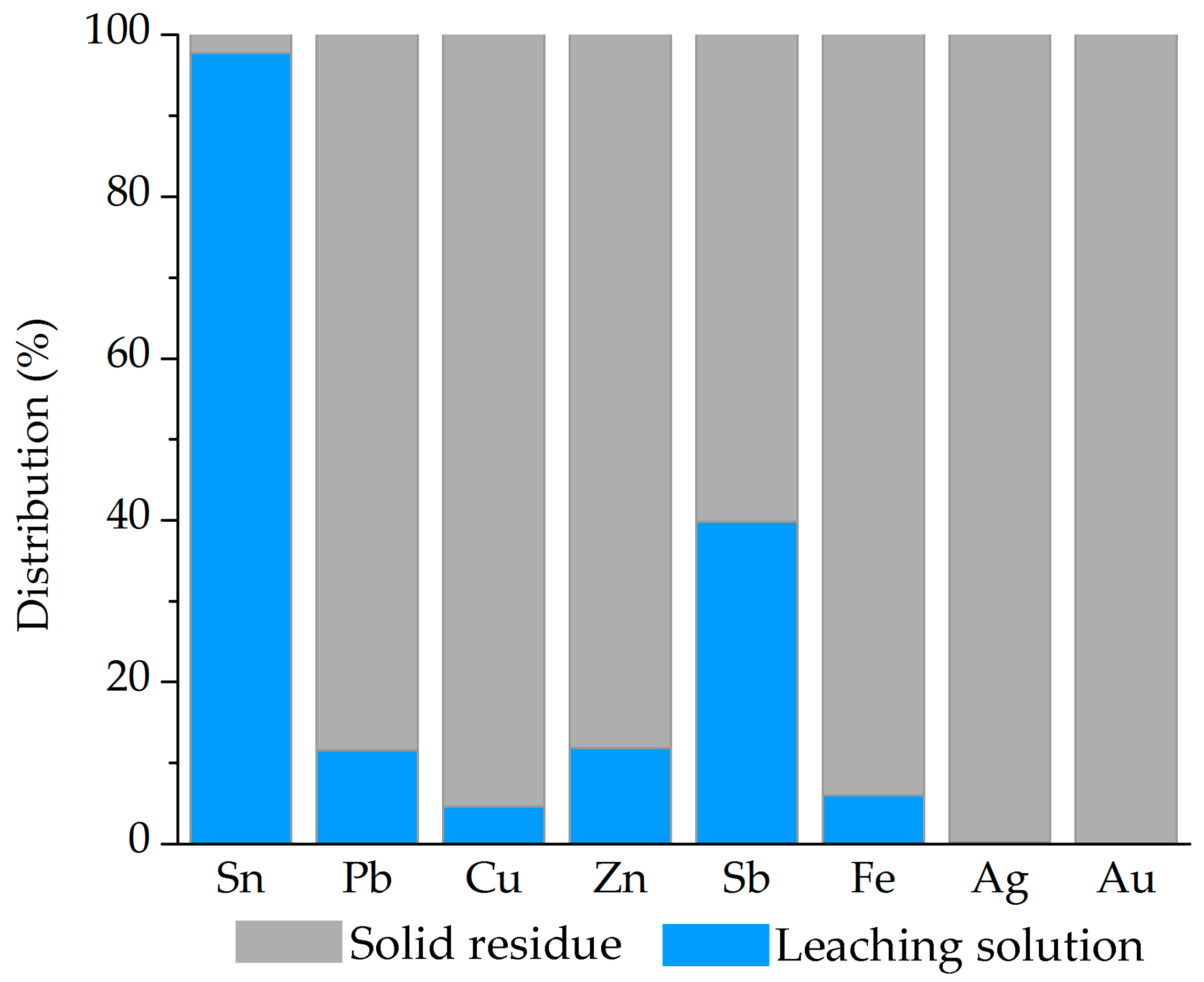
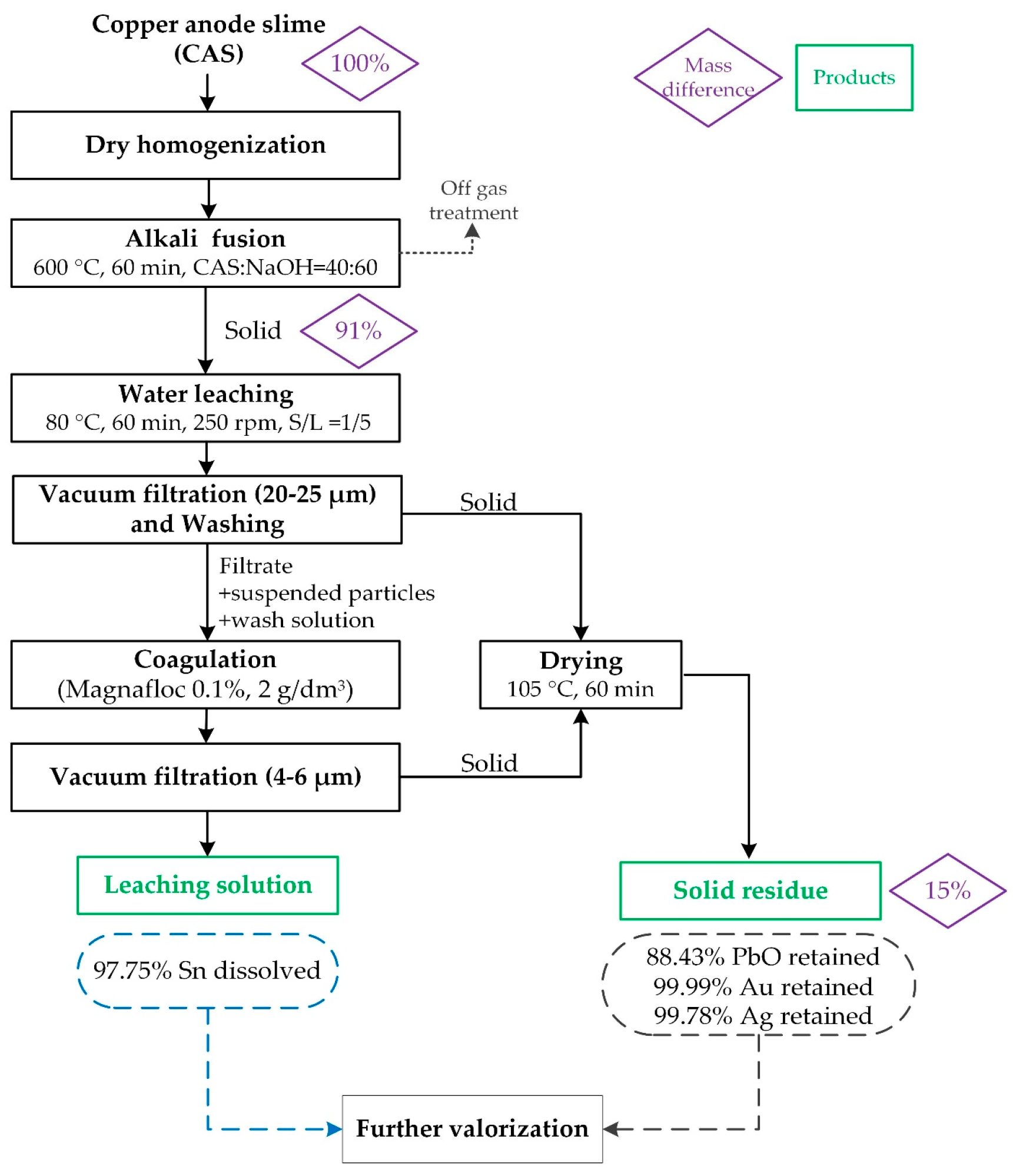
The optimized two-step process involved fusion at 600 °C for 60 min with a CAS:NaOH ratio of 40:60, followed by leaching at 80 °C for 60 min at 250 rpm, with a solid-to-liquid ratio of 200 g/dm3. High metal separation efficiency was achieved, with 97.58% of Sn leached, while Pb and precious metals remained in the solid residue. Additionally, accompanying base metals (Cu, Fe, Zn, and Sb) showed low solubility under optimal conditions, contributing to process selectivity.
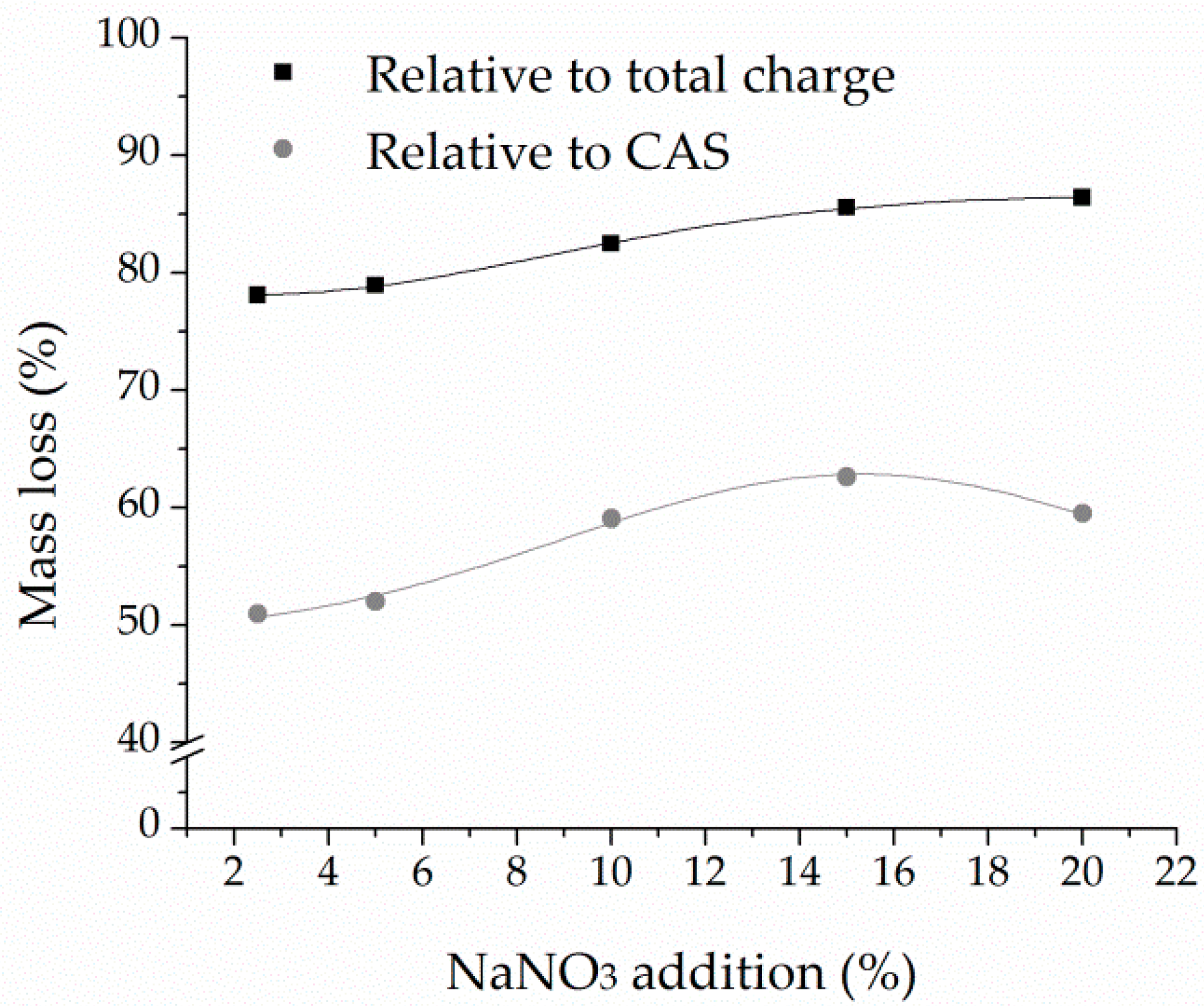
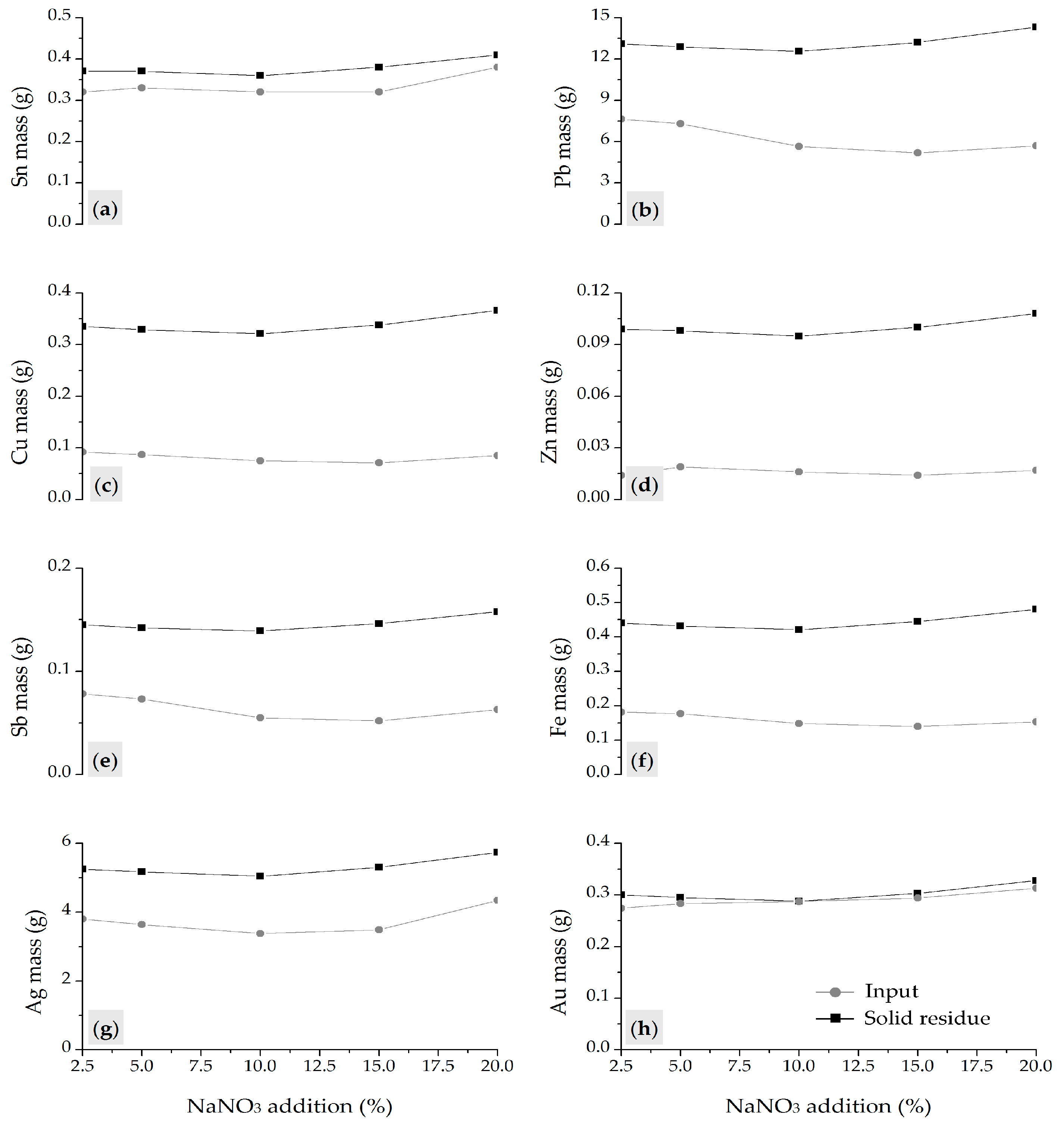
The addition of NaNO3 as an oxidant negatively affected the process by increasing the dissolution of base metals, promoting partial dissolution of Ag and Pb, and hindering the transformation of tin into soluble species. These results are in accordance with thermodynamic modeling, confirming reduced process efficiency and low selectivity in the presence of nitrate within the fusion system.
The proposed process was successfully validated through a scaled-up laboratory test, achieving 97.78% Sn dissolution and more than 99.99% of Au and 99.78% of Ag retention in the PbO-based solid residue, confirming the feasibility and reproducibility of the method. These findings define a selective, efficient, and scalable pyro-hydrometallurgical route for the recovery of tin and precious metals from non-standard CAS, addressing industrial needs related to increased e-waste co-processing and providing a framework which aligns with sustainable resource utilization.