Simultaneous Solvent Extraction of Co and Ni from Copper Raffinate Waste Solution
Abstract
1. Introduction
2. Experimental Procedures
2.1. Materials and Analysis
2.2. (DOE) Design of Experiments
2.3. Batch Experiments
3. Results and Discussion
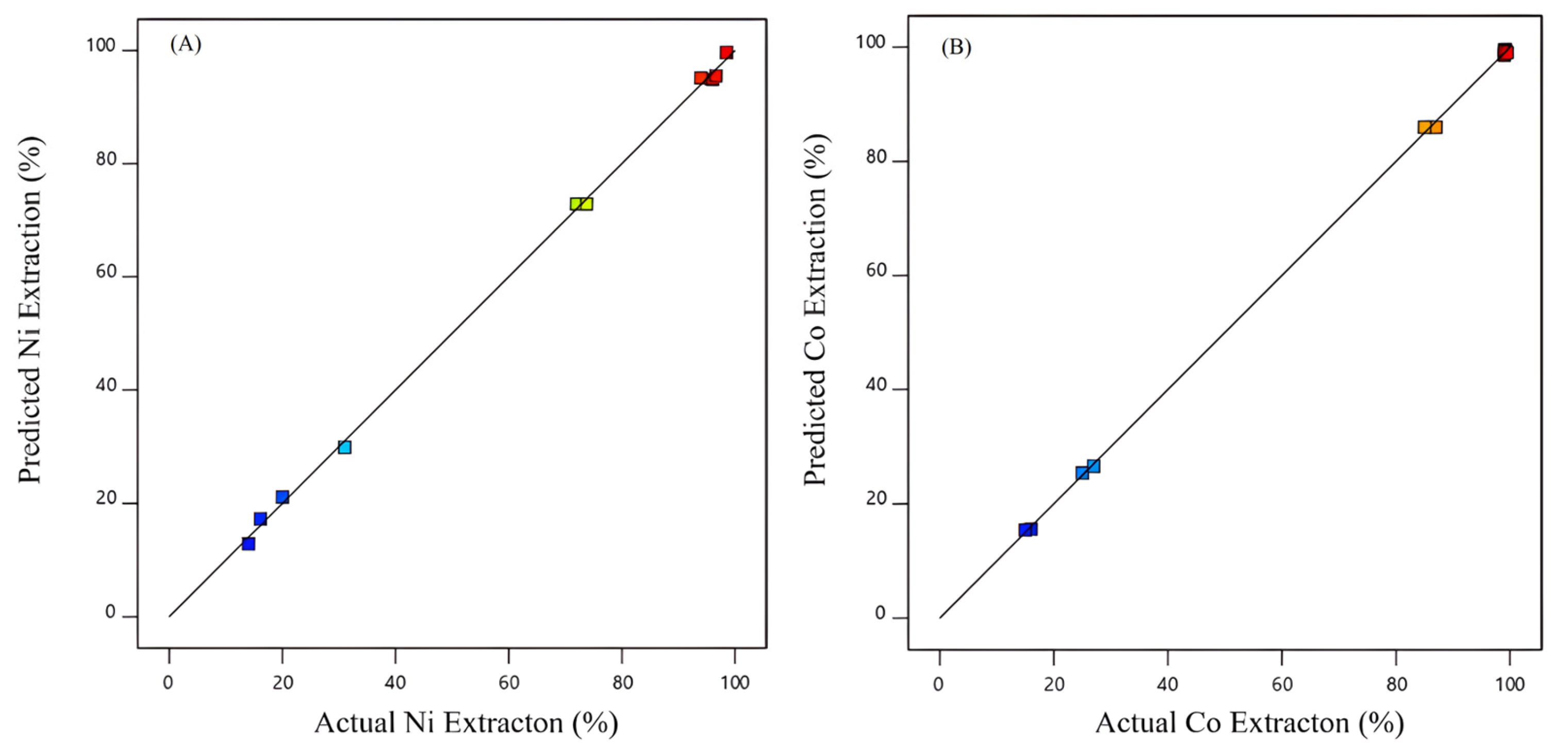
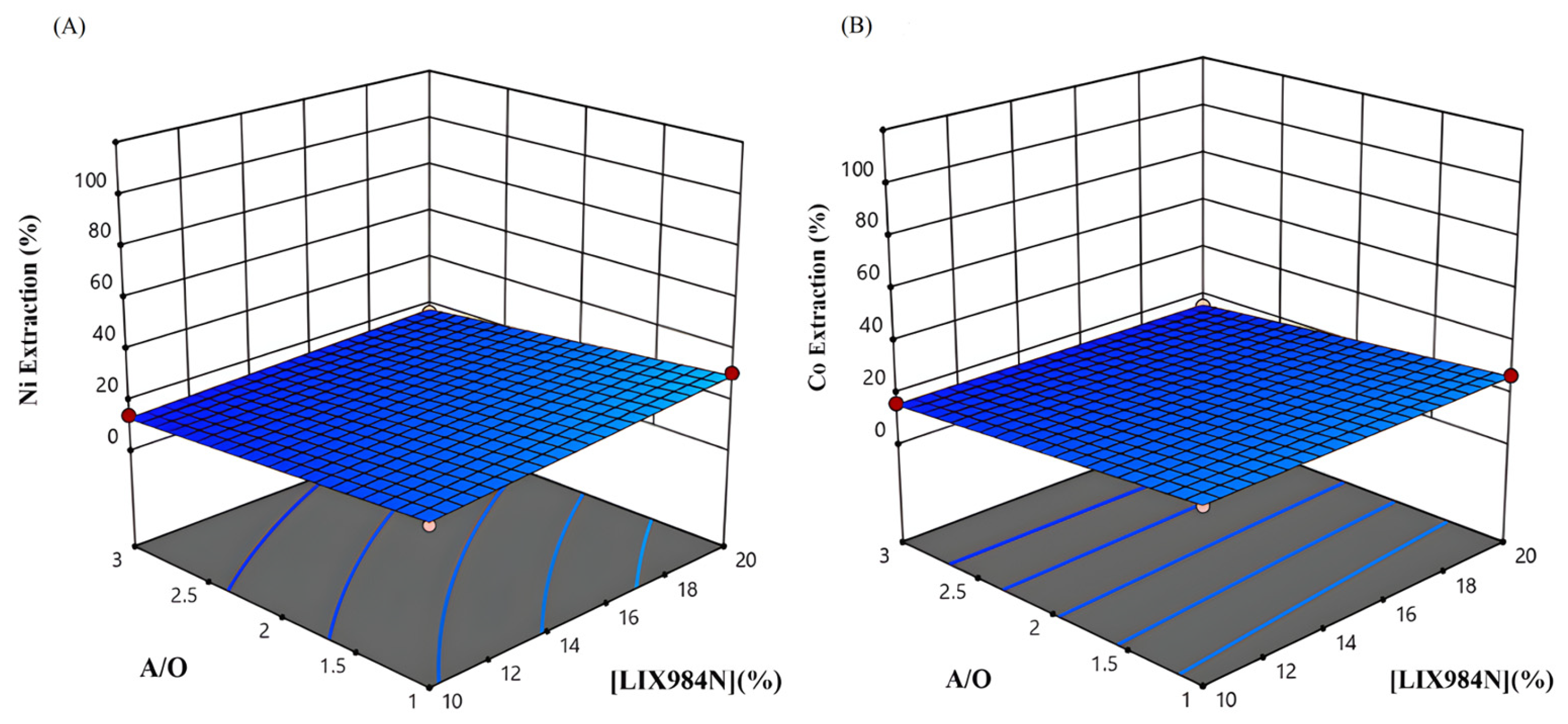
3.1. Data Analysis and Contour Plots
3.2. Diagrams of the Main Effects of Parameters on Nickel and Cobalt Extraction by LIX984N
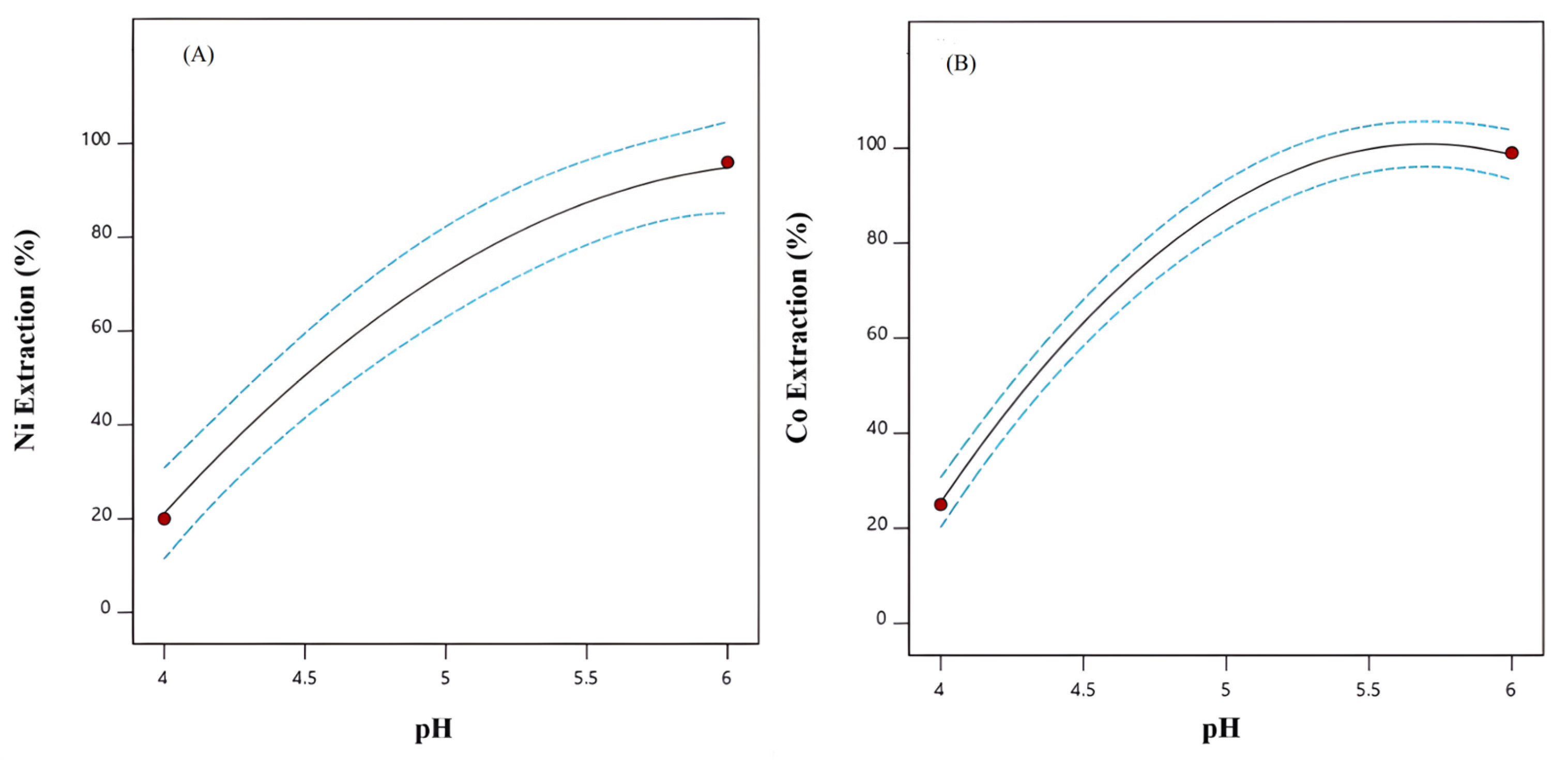
3.2.1. pH Effect
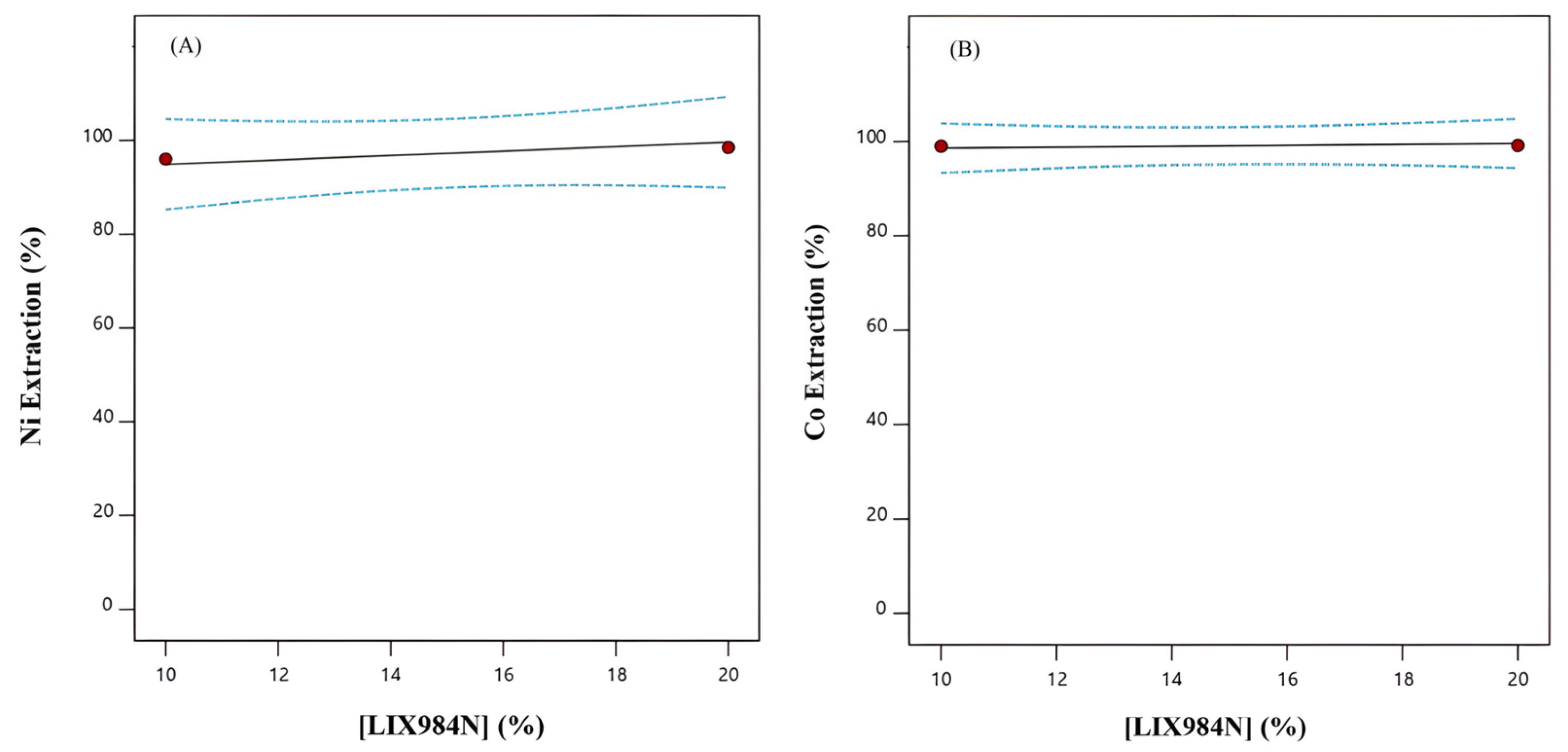
3.2.2. The Effect of LIX984N Extractant Concentration
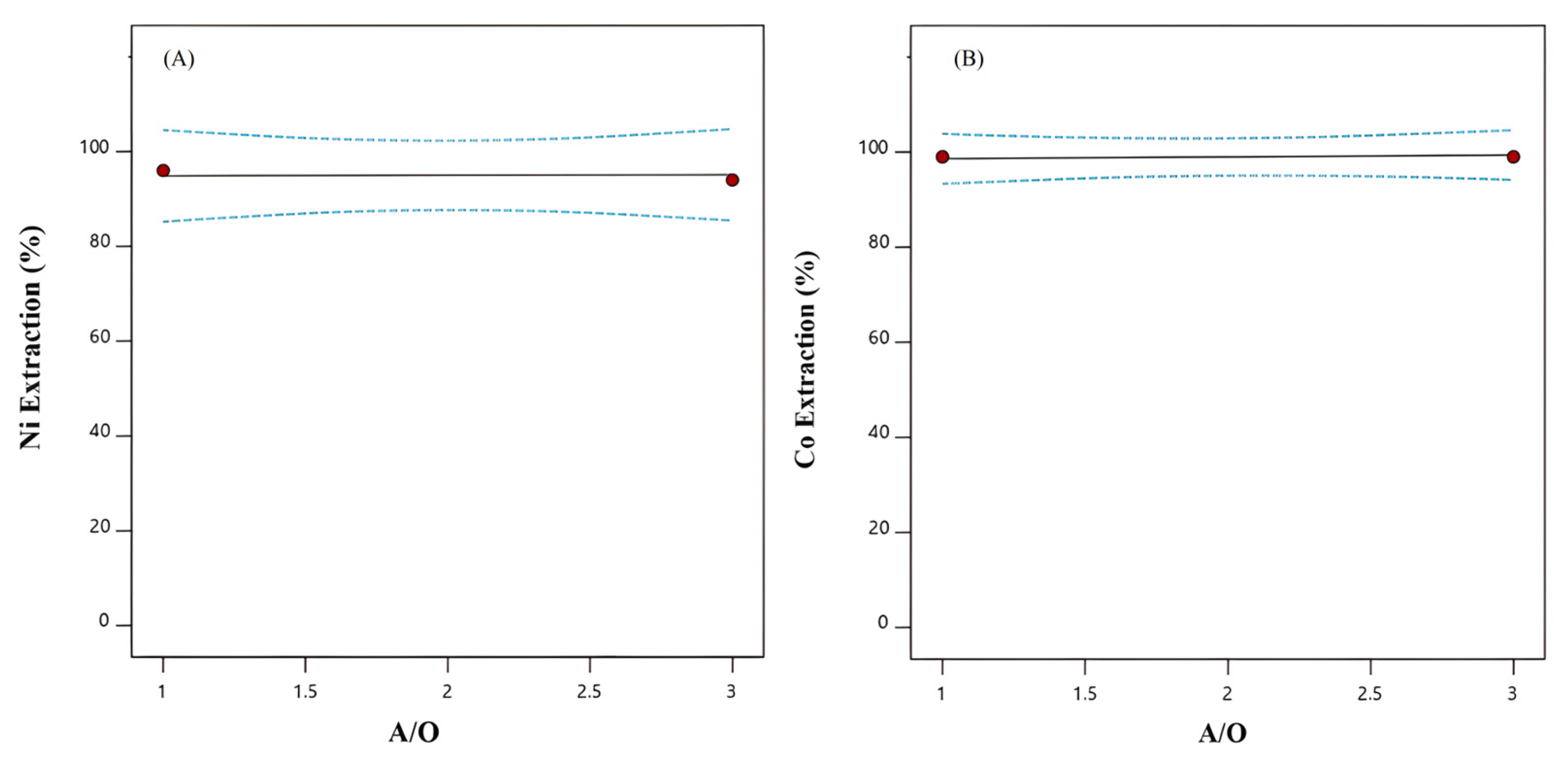
3.2.3. A/O Phase Ratio Effect
3.3. Synergistic Effect of the Studied Parameters on Nickel and Cobalt Extraction Using LIX984N
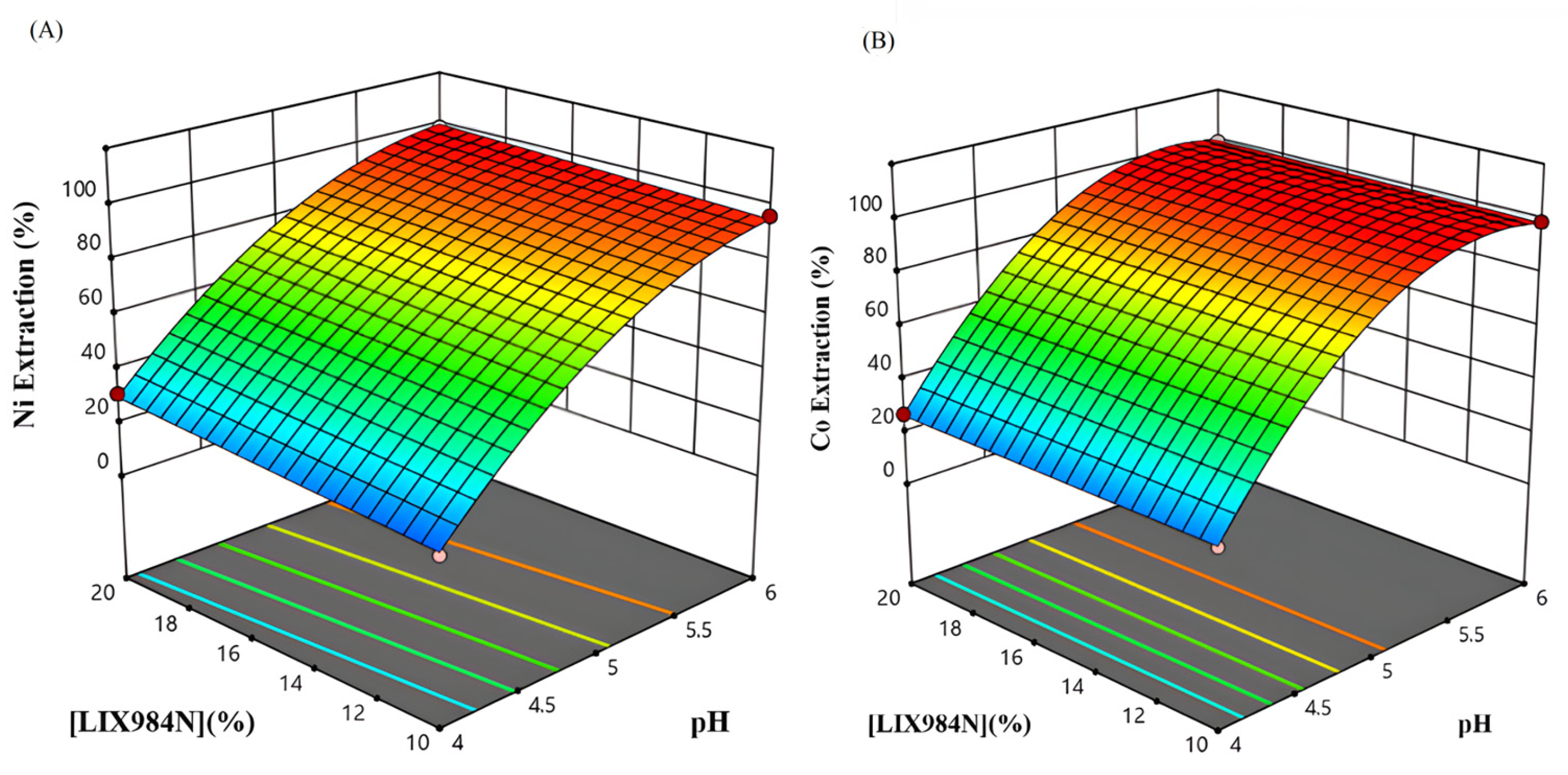
3.3.1. Effect of pH and Extractant Concentration
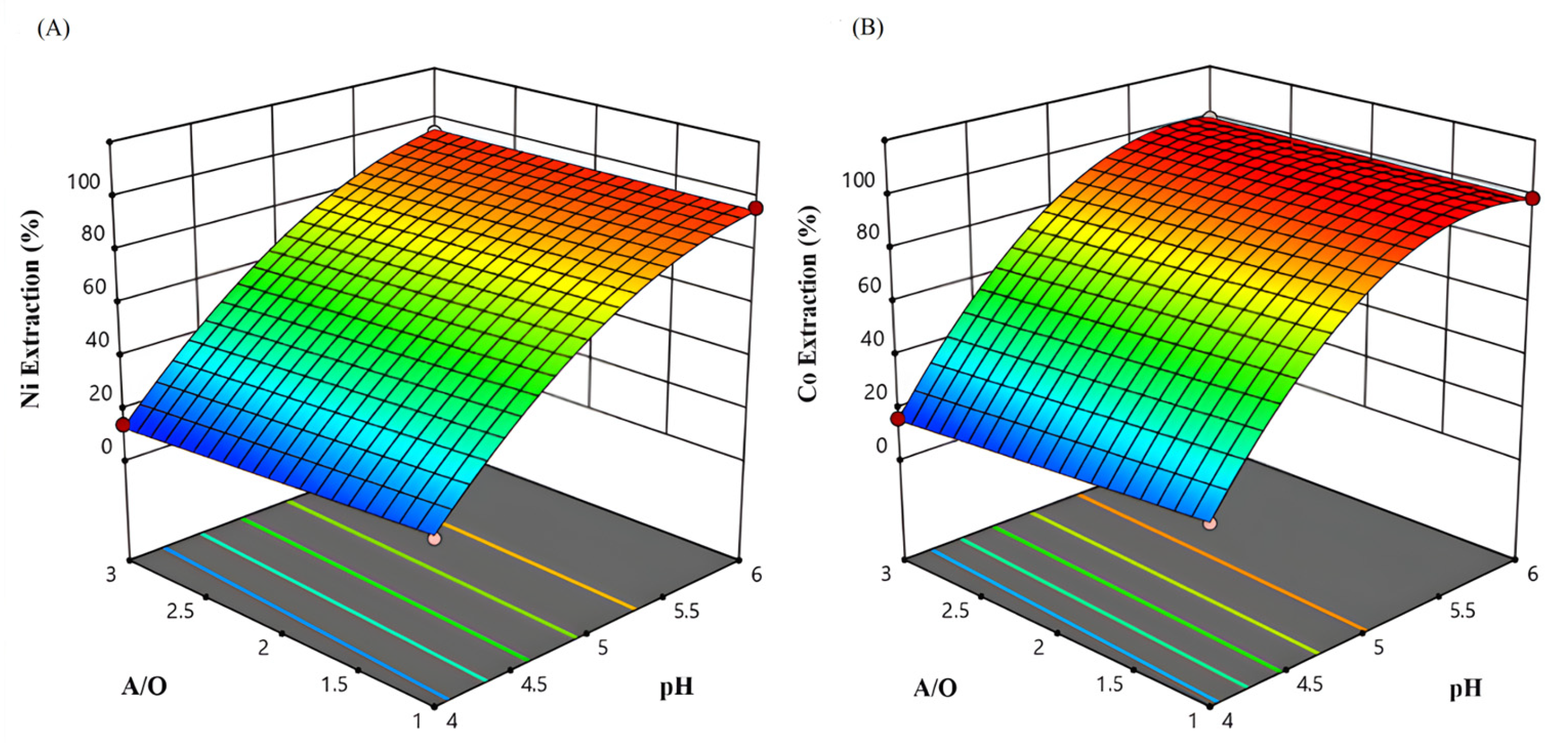
3.3.2. Effect of A/O Ratio, Extractant Concentration, and pH
3.4. Optimization of Process Parameters
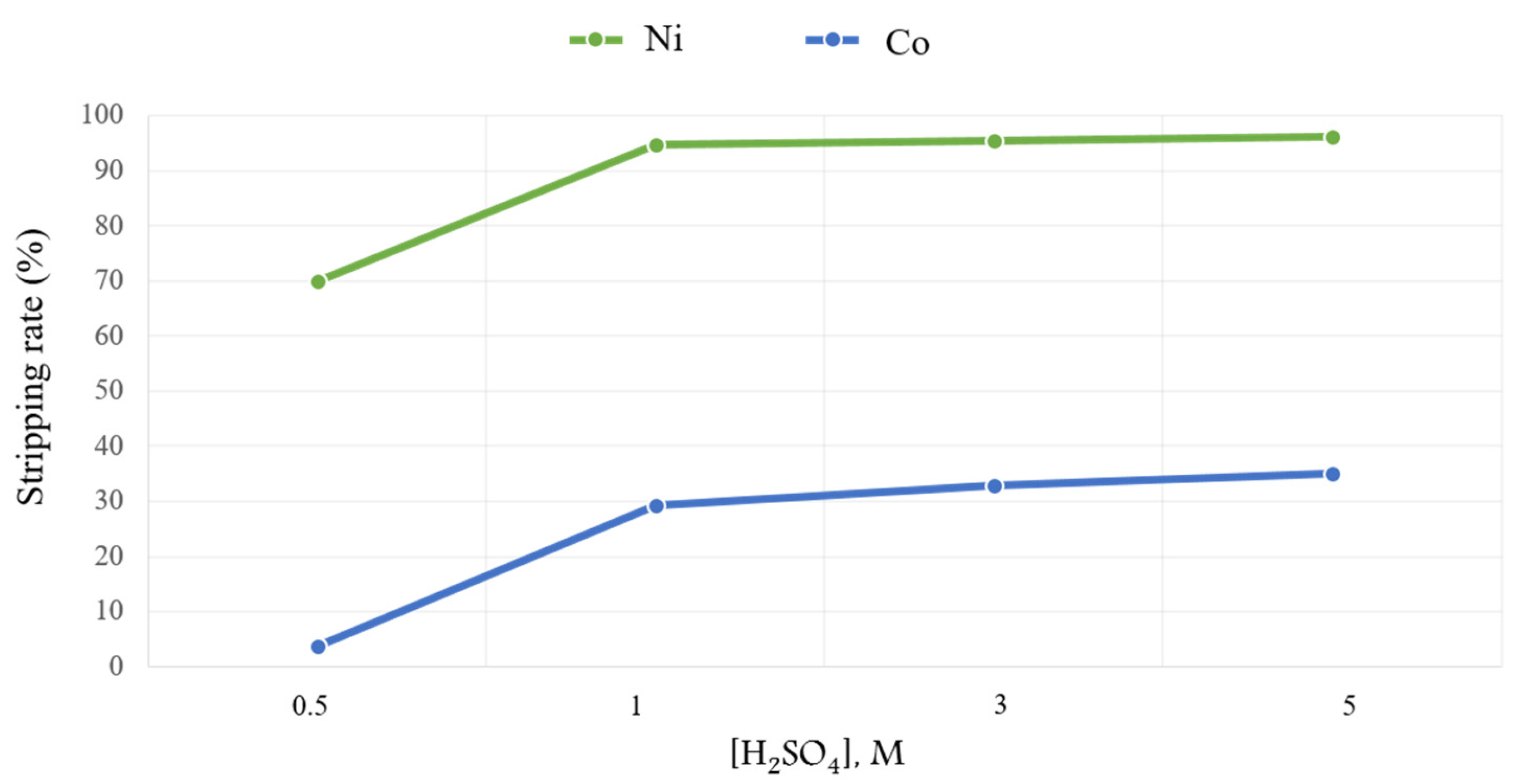
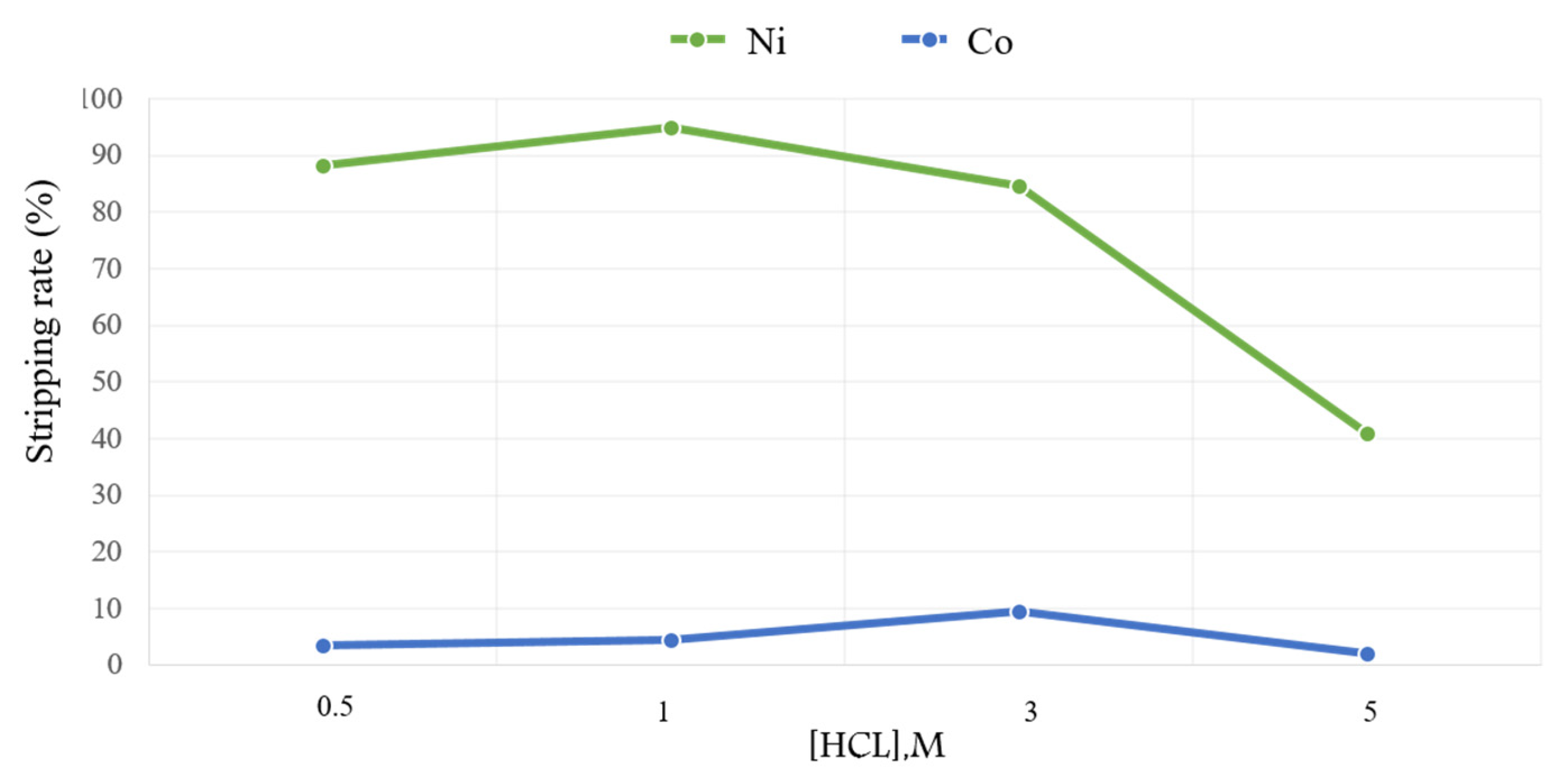
3.5. Stripping of the Loaded Organic Phase
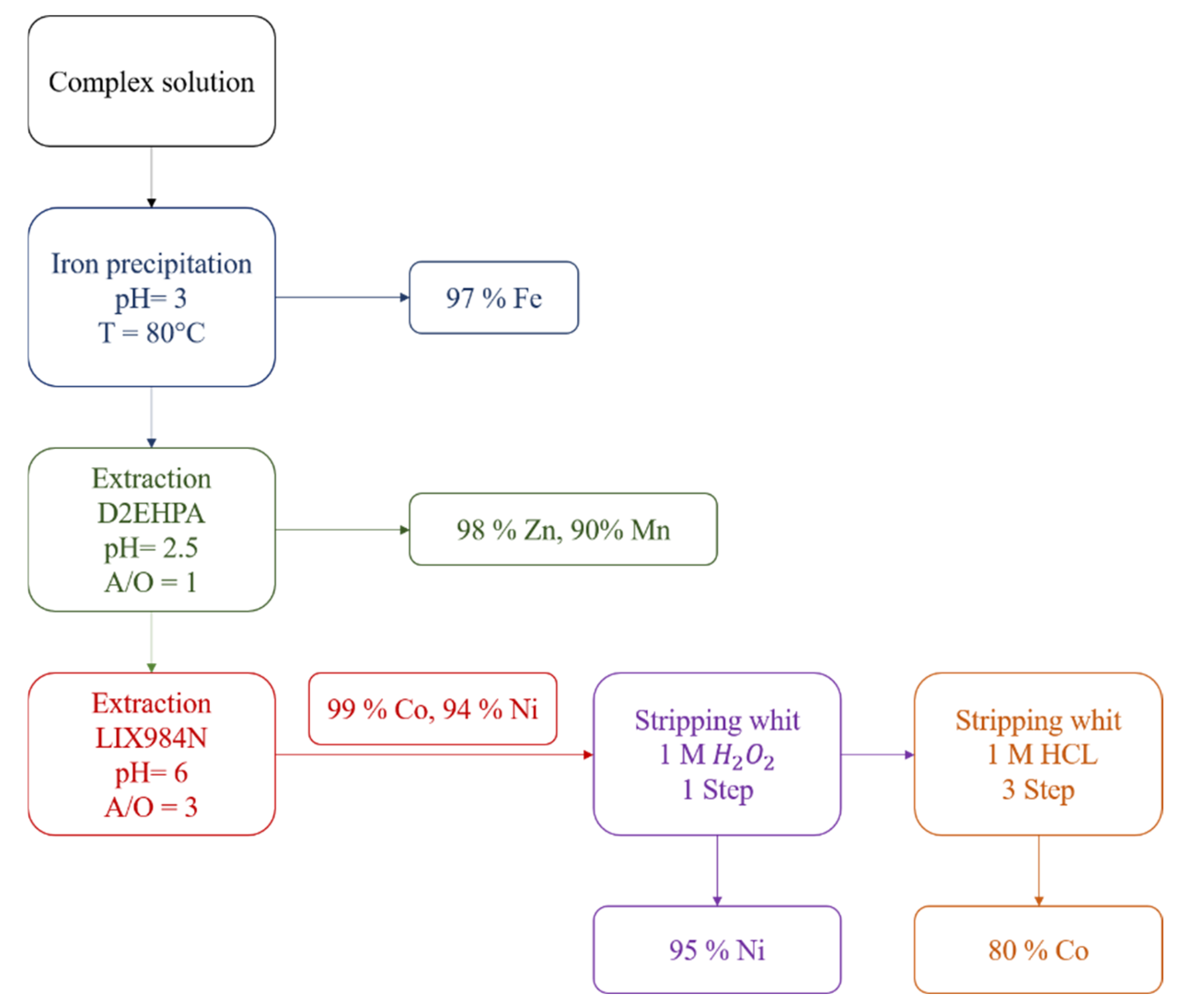
3.6. Flowsheet of Co and Ni Removal from Sarcheshmeh Copper Complex Raffinate
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, H.; Yu, M.; Wang, Y.; Shi, M. Influence of Process Parameters on Pulse Electroforming of Nickel-Rich Nickel-Cobalt Alloys from Sulfamate Electrolyte. In Advanced Materials Research; Trans Tech Publications Ltd.: Baech, Switzerland, 2013; pp. 440–443. [Google Scholar]
- Yu, M.; Li, H.; Wang, Y. Study on Present Situation and New Trends of the Electrodeposition of Nickel-Cobalt Alloy. In Advanced Materials Research; Trans Tech Publications Ltd.: Baech, Switzerland, 2012; pp. 973–976. [Google Scholar]
- Yu, M.M.; Li, H.Y.; Wang, Y. Effects of Process Parameters on the Morphologies and Composition of Pulse Electrodeposition of Nickel-Rich Co-Ni Alloys. In Advanced Materials Research; Trans Tech Publications Ltd.: Baech, Switzerland, 2013; pp. 565–569. [Google Scholar]
- Xiao, M.J.; Sun, H. Preparation of Nickel and Cobalt-Based Micro–Nano Structural Materials and Their Applications in Energy Storage and Conversion. Chem. Rec. 2025. Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Peng, H.; Yang, L.; Dong, H.; Xiao, P. Investigating the Effect of Sulfur and Selenium on the Electrochemical Properties of Nickel–Cobalt Oxides: Enhanced Charge Capacity and Composition–Property Relationships. J. Mater. Sci. 2016, 51, 7108–7118. [Google Scholar] [CrossRef]
- Chen, X.; Liu, Q.; Bai, T.; Wang, W.; He, F.; Ye, M. Nickel and Cobalt Sulfide-Based Nanostructured Materials for Electrochemical Energy Storage Devices. Chem. Eng. J. 2021, 409, 127237. [Google Scholar] [CrossRef]
- Ghannem, S.; Aouadi, B.; Yallese, M.A.; Ben Fathallah, B. The Machinability of Nickel and Cobalt Based Alloys: Brief Review. In Advances in Mechanical Engineering and Mechanics III; Lecture Notes in Mechanical Engineering; Springer: Cham, Switzerland, 2024; pp. 432–439. [Google Scholar]
- Skibińska, K.; Kula, A.; Kutyła, D.; Wojnicki, M.; Żabiński, P. Influence of Crystal Modifier Content on Ni-Cu Catalysts Dedicated to the Hydrogen Evolution Reaction. Materials 2025, 18, 2499. [Google Scholar] [CrossRef]
- Kutyła, D.; Fukumoto, M.; Takahashi, H.; Wojnicki, M.; Żabiński, P. Catalytic Activity Evaluation of the Molten Salt-Modified Novel Ni Electrodes for Urea Electrooxidation in Alkaline Solutions. Metals 2024, 14, 904. [Google Scholar] [CrossRef]
- Fukumoto, M.; Takahashi, H.; Kutyła, D.; Wojnicki, M.; Żabiński, P. Morphological Investigation and Electrochemical Performance Evaluation of Novel Porous Ni–Pt Produced by Al-Deposition/Dissolution in Molten Salts for Hydrogen and Oxygen Evolution Reaction. Int. J. Hydrog. Energy 2024, 49, 754–765. [Google Scholar] [CrossRef]
- Alvial-Hein, G.; Mahandra, H.; Ghahreman, A. Separation and Recovery of Cobalt and Nickel from End of Life Products Via Solvent Extraction Technique: A Review. J. Clean. Prod. 2021, 297, 126592. [Google Scholar] [CrossRef]
- Dehaine, Q.; Tijsseling, L.T.; Glass, H.J.; Törmänen, T.; Butcher, A.R. Geometallurgy of Cobalt Ores: A Review. Miner. Eng. 2021, 160, 106656. [Google Scholar] [CrossRef]
- Arustamian, O.M.; Tkachyshin, V.S.; Arustamian, Y.A.; Aleksiichuk, O.Y.; Dumka, I.V. Features of Cobalt and Its Compounds Intoxication. Emerg. Med. 2022, 18, 6–11. [Google Scholar] [CrossRef]
- Kwon, Y.J.; Lee, B.H. Investigation of Color Mecchanism in Co-Doped Augite Purple for Color Glaze. Korean J. Mater. Res. 2013, 23, 271–275. [Google Scholar] [CrossRef]
- Rodrigo, M.; Gazulla, M.F.; Moreno, A.; Gilabert, J.; Gómez, P. Blue Inkjet Inks from E-Waste: Toward a Greener Ceramic Industry. J. Eur. Ceram. Soc. 2025, 45, 117696. [Google Scholar] [CrossRef]
- Ungureanu, A.; Sola, A.; Neri, P.; Rosa, R.; Ferrari, A.M. Systematic Life Cycle Assessment of Cobalt-Based Blue Ceramic Pigments: Evaluating CoAl2O4 and Lower-Cobalt Alternatives. Green Chem. 2025, 27, 12946–12958. [Google Scholar] [CrossRef]
- Neklyudova, T.L.; Yugai, L.K.; Kryuchkov, Y.N. Effect of Underglaze Paints as Cobalt and Nickel Salt Solutions on Porcelain Structure. Glass Ceram. (Engl. Transl. Steklo I Keram.) 2020, 77, 226–230. [Google Scholar] [CrossRef]
- Shortland, A.J.; Hope, C.A.; Tite, M.S. Cobalt Blue Painted Pottery from 18th Dynasty Egypt. Geol. Soc. Spec. Publ. 2006, 257, 91–99. [Google Scholar] [CrossRef]
- Coates, G.; Cutler, P. Nickel-Containing Stainless Steels. Adv. Mater. Process. 2009, 167, 29–32. [Google Scholar]
- Miller, A. Breaking Free from the Norm. Mod. Met. 2006, 62, 50. [Google Scholar]
- Tanong, K.; Tran, L.-H.; Mercier, G.; Blais, J.-F. Recovery of Zn (II), Mn (II), Cd (II) and Ni (II) from the Unsorted Spent Batteries Using Solvent Extraction, Electrodeposition and Precipitation Methods. J. Clean. Prod. 2017, 148, 233–244. [Google Scholar] [CrossRef]
- Wang, L.Y.; Lee, M.S. Separation of Co (II) and Ni (II) from Chloride Leach Solution of Nickel Laterite Ore by Solvent Extraction with Cyanex 301. Int. J. Miner. Process. 2017, 166, 45–52. [Google Scholar] [CrossRef]
- Ncib, S.; Brahmi, K.; Othmen, K.; Lasaad, D.; Elaloui, E.; Bouguerra, W. Enhanced Ni(II) Recovery Via D2ehpa-Cellulose Triacetate-Based Polymer Inclusion Membranes: Optimized Extraction and Stability Analysis. Euro-Mediterr. J. Environ. Integr. 2025, 10, 2245–2263. [Google Scholar] [CrossRef]
- Pospiech, B. Separation of Co from Ni and Li from Chloride Media Using Polymer Inclusion Membrane System with Thiosalicylate Based Ionic Liquid. Physicochem. Probl. Miner. Process. 2022, 58, 152997. [Google Scholar] [CrossRef]
- Alyani, I.; Ncib, S.; Kemla, O.; Mahmoud, H.; Bouguerra, W.; Elaloui, E. Synthesis and Application of Plasticized Polymer Membranes Containing Aliquat 336 and D2ehpa as Carriers for Efficient Cobalt Ion Separation: A Sustainable Approach for Metal Resource Recovery. Euro-Mediterr. J. Environ. Integr. 2024, 9, 1551–1569. [Google Scholar] [CrossRef]
- Soeezi, A. Extraction and Stripping of Cu and Ni from Synthetic and Industrial Solutions of Sarcheshmeh Copper Mine Containing Cu, Ni, Fe and Zn Ions. Trans. Nonferrous Met. Soc. China 2020, 30, 518–534. [Google Scholar] [CrossRef]
- Prasetyo, E.; Anderson, C. Isolation of Zinc, Copper, and Nickel from Glutamate Media by Solvent Extraction. J. Sustain. Metall. 2020, 6, 612–621. [Google Scholar] [CrossRef]
- Kursunoglu, S.; Ichlas, Z.T.; Kaya, M. Solvent Extraction Process for the Recovery of Nickel and Cobalt from Caldag Laterite Leach Solution: The First Bench Scale Study. Hydrometallurgy 2017, 169, 135–141. [Google Scholar] [CrossRef]
- Mishra, R.K.; Rout, P.C.; Sarangi, K.; Nathsarma, K.C. Solvent Extraction of Zinc, Manganese, Cobalt and Nickel from Nickel Laterite Bacterial Leach Liquor Using Sodium Salts of Tops-99 and Cyanex 272. Trans. Nonferrous Met. Soc. China 2016, 26, 301–309. [Google Scholar] [CrossRef]
- Zhu, Z.; Yoko, P.; Cheng, C.Y. Recovery of Cobalt and Manganese from Nickel Laterite Leach Solutions Containing Chloride by Solvent Extraction Using Cyphos Il 101. Hydrometallurgy 2017, 169, 213–218. [Google Scholar] [CrossRef]
- Fernandes, A.; Afonso, J.C.; Dutra, A.J.B. Separation of Nickel (II), Cobalt (II) and Lanthanides from Spent Ni-Mh Batteries by Hydrochloric Acid Leaching, Solvent Extraction and Precipitation. Hydrometallurgy 2013, 133, 37–43. [Google Scholar] [CrossRef]
- Joo, S.H.; ju Shin, D.; Oh, C.; Wang, J.P.; Senanayake, G.; Shin, S.M. Selective Extraction and Separation of Nickel from Cobalt, Manganese and Lithium in Pre-Treated Leach Liquors of Ternary Cathode Material of Spent Lithium-Ion Batteries Using Synergism Caused by Versatic 10 Acid and Lix 84-I. Hydrometallurgy 2016, 159, 65–74. [Google Scholar] [CrossRef]
- Cheng, C.; Boddy, G.; Zhang, W.; Godfrey, M.; Robinson, D.; Pranolo, Y.; Zhu, Z.; Wang, W. Recovery of Nickel and Cobalt from Laterite Leach Solutions Using Direct Solvent Extraction: Part 1—Selection of a Synergistic Sx System. Hydrometallurgy 2010, 104, 45–52. [Google Scholar] [CrossRef]
- Olivier, M.; Dorfling, C.; Eksteen, J. Evaluating a Solvent Extraction Process Route Incorporating Nickel Preloading of Cyanex 272 for the Removal of Cobalt and Iron from Nickel Sulphate Solutions. Miner. Eng. 2012, 27, 37–51. [Google Scholar] [CrossRef]
- Cheng, C.Y.; Urbani, M.D.; Davies, M.G.; Pranolo, Y.; Zhu, Z. Recovery of Nickel and Cobalt from Leach Solutions of Nickel Laterites Using a Synergistic System Consisting of Versatic 10 and Acorga Clx 50. Miner. Eng. 2015, 77, 17–24. [Google Scholar] [CrossRef]
- Guimarães, A.S.; Da Silva, P.S.; Mansur, M.B. Purification of Nickel from Multicomponent Aqueous Sulfuric Solutions by Synergistic Solvent Extraction Using Cyanex 272 and Versatic 10. Hydrometallurgy 2014, 150, 173–177. [Google Scholar] [CrossRef]
- Tsakiridis, P.; Agatzini, S. Process for the Recovery of Cobalt and Nickel in the Presence of Magnesium and Calcium from Sulphate Solutions by Versatic 10 and Cyanex 272. Miner. Eng. 2004, 17, 535–543. [Google Scholar] [CrossRef]
- Balesini, A.A.; Zakeri, A.; Razavizadeh, H.; Khani, A. Nickel Solvent Extraction from Cold Purification Filter Cakes of Angouran Mine Concentrate Using Lix984n. Int. J. Miner. Metall. Mater. 2013, 20, 1029–1034. [Google Scholar] [CrossRef]
- Santanilla, A.J.; Aliprandini, P.; Benvenuti, J.; Tenorio, J.A.; Espinosa, D.C. Structure Investigation for Nickel and Cobalt Complexes Formed during Solvent Extraction with the Extractants Cyanex 272, Versatic 10 and Their Mixtures. Miner. Eng. 2021, 160, 106691. [Google Scholar] [CrossRef]
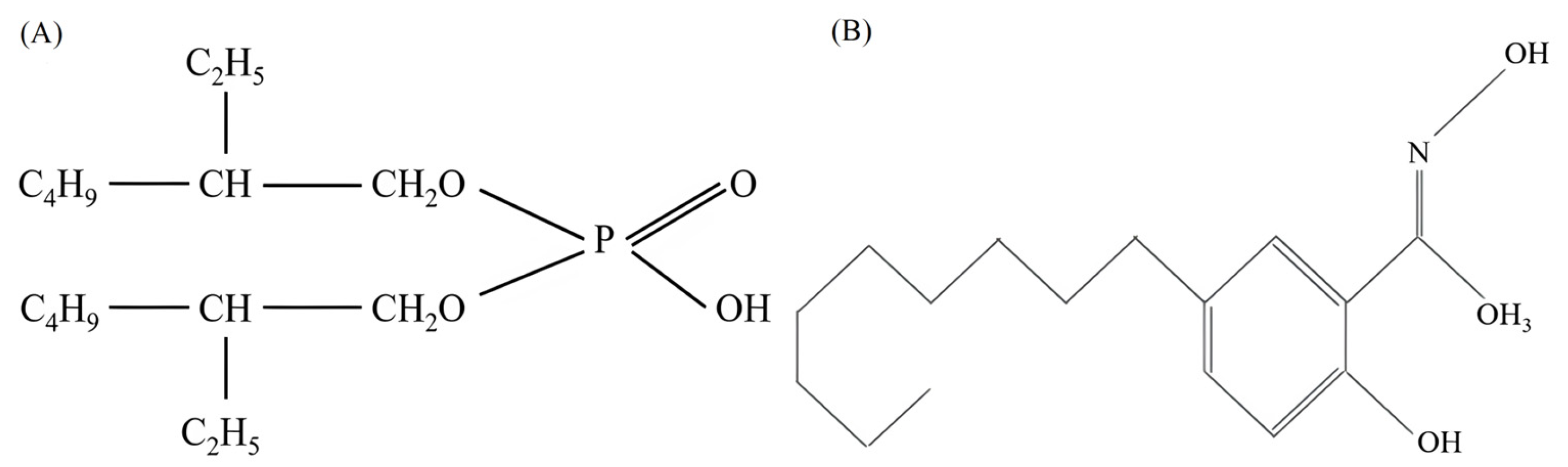
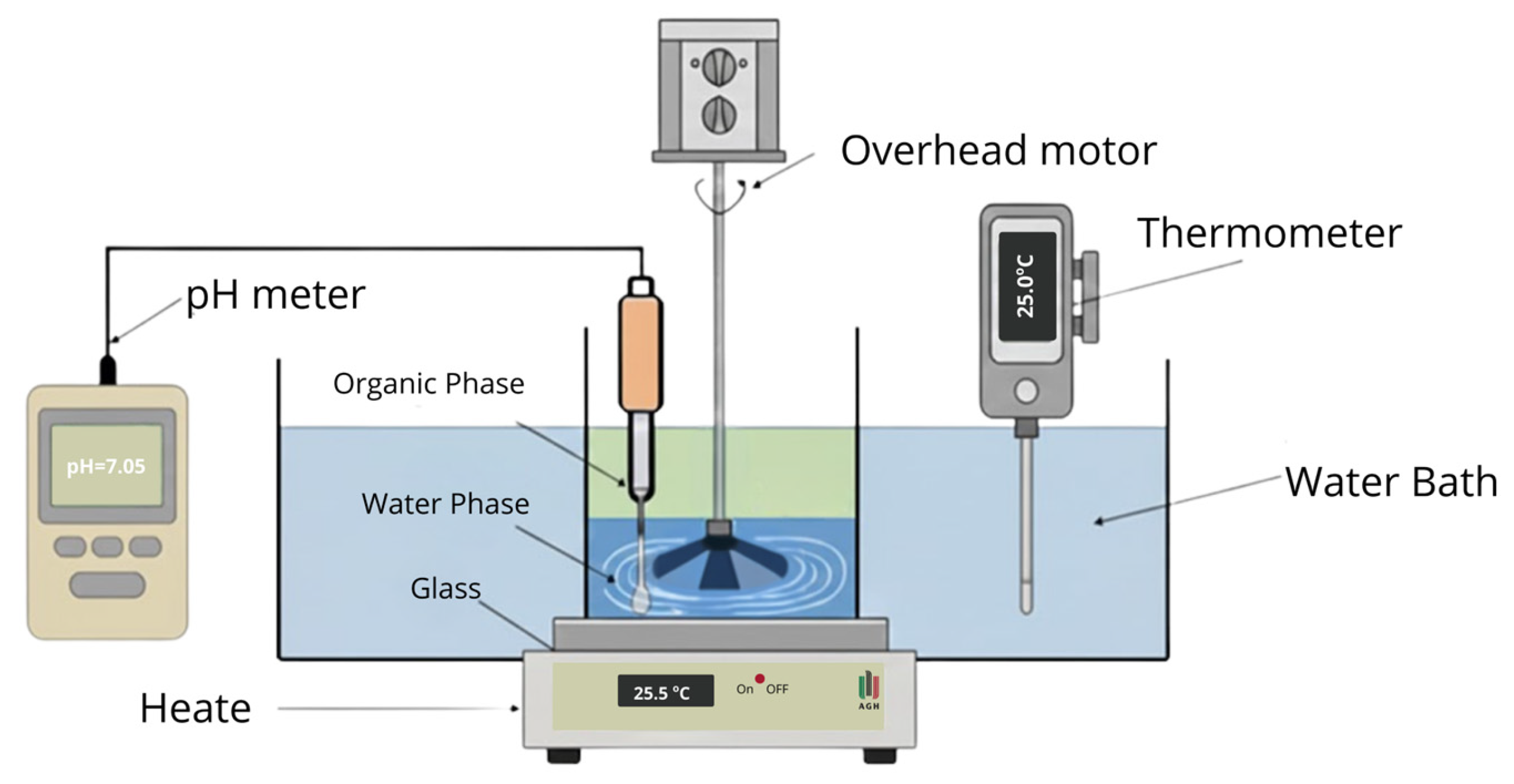
| Elements | Zn | Mg | Al | Co | Cu | Fe | Mn | Ni |
| Concentration, g/L | 2.4 | 8 | 11.37 | 0.06 | 0.14 | 10.72 | 4.83 | 0.15 |
| Row | Parameter 1: pH | Parameter 2: [LIX984N] (%) | Parameter 3: A/O | Co Ex (%) | Ni Ex (%) | Mn Ex (%) | Mg Ex (%) |
|---|---|---|---|---|---|---|---|
| 1 | 6 | 20 | 3 | 99.16 | 99.60 | 8.12 | 18.60 |
| 2 | 5 | 15 | 2 | 85.00 | 73.75 | 1.15 | 6.74 |
| 3 | 6 | 20 | 1 | 99.50 | 98.50 | 8.30 | 22.69 |
| 4 | 6 | 10 | 3 | 99.00 | 94.00 | 5.81 | 9.70 |
| 5 | 4 | 20 | 1 | 27.00 | 31.00 | 7.50 | 5.11 |
| 6 | 4 | 20 | 3 | 15.00 | 16.12 | 4.50 | 4.24 |
| 7 | 5 | 15 | 2 | 87.00 | 72.00 | 1.65 | 4.65 |
| 8 | 4 | 10 | 3 | 16.00 | 14.00 | 3.40 | 3.44 |
| 9 | 4 | 10 | 1 | 25.00 | 20.00 | 6.25 | 3.58 |
| 10 | 6 | 10 | 1 | 99.00 | 96.00 | 6.18 | 11.81 |
| Sum of Squares | Degrees of Freedom | Mean Square | F Value | p-Value Prob > F | |
|---|---|---|---|---|---|
| Model | 13,494.94 | 7 | 1927.85 | 1137.72 | 0.0009 |
| pH | 12,298.35 | 1 | 12,298.35 | 7258.04 | 0.0001 |
| [LIX984N] | 0.3472 | 1 | 0.3472 | 0.2049 | 0.6951 |
| A/O | 53.39 | 1 | 53.39 | 31.51 | 0.0303 |
| pH × [LIX984N] | 0.0139 | 1 | 0.0139 | 0.0082 | 0.9361 |
| pH × A/O | 56.89 | 1 | 56.89 | 33.57 | 0.0285 |
| A/O × [LIX984N] | 0.8889 | 1 | 0.8889 | 0.5246 | 0.5442 |
| pH × pH | 1085.07 | 1 | 1085.07 | 640.37 | 0.0016 |
| Sum of Squares | Degrees of Freedom | Mean Square | F Value | p-Value Prob > F | |
|---|---|---|---|---|---|
| Model | 12,063.39 | 7 | 1723.34 | 296.84 | 0.0034 |
| pH | 11,550.48 | 1 | 11,550.48 | 1989.52 | 0.0005 |
| [LIX984N] | 41.50 | 1 | 41.50 | 7.15 | 0.1161 |
| A/O | 76.76 | 1 | 76.76 | 13.22 | 0.0680 |
| pH × [LIX984N] | 8.04 | 1 | 8.04 | 1.38 | 0.3604 |
| pH × A/O | 36.04 | 1 | 36.04 | 6.21 | 0.1303 |
| A/O × [LIX984N] | 9.64 | 1 | 9.64 | 1.66 | 0.3266 |
| pH × pH | 340.94 | 1 | 340.94 | 58.73 | 0.0016 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rezaei, H.; Aboutalebi, M.R.; Seyedein, S.H.; Aghajani, H.; Wojnicki, M. Simultaneous Solvent Extraction of Co and Ni from Copper Raffinate Waste Solution. Metals 2025, 15, 1312. https://doi.org/10.3390/met15121312
Rezaei H, Aboutalebi MR, Seyedein SH, Aghajani H, Wojnicki M. Simultaneous Solvent Extraction of Co and Ni from Copper Raffinate Waste Solution. Metals. 2025; 15(12):1312. https://doi.org/10.3390/met15121312
Chicago/Turabian StyleRezaei, Hanieh, Mohammad Reza Aboutalebi, Seyed Hossein Seyedein, Hossein Aghajani, and Marek Wojnicki. 2025. "Simultaneous Solvent Extraction of Co and Ni from Copper Raffinate Waste Solution" Metals 15, no. 12: 1312. https://doi.org/10.3390/met15121312
APA StyleRezaei, H., Aboutalebi, M. R., Seyedein, S. H., Aghajani, H., & Wojnicki, M. (2025). Simultaneous Solvent Extraction of Co and Ni from Copper Raffinate Waste Solution. Metals, 15(12), 1312. https://doi.org/10.3390/met15121312







