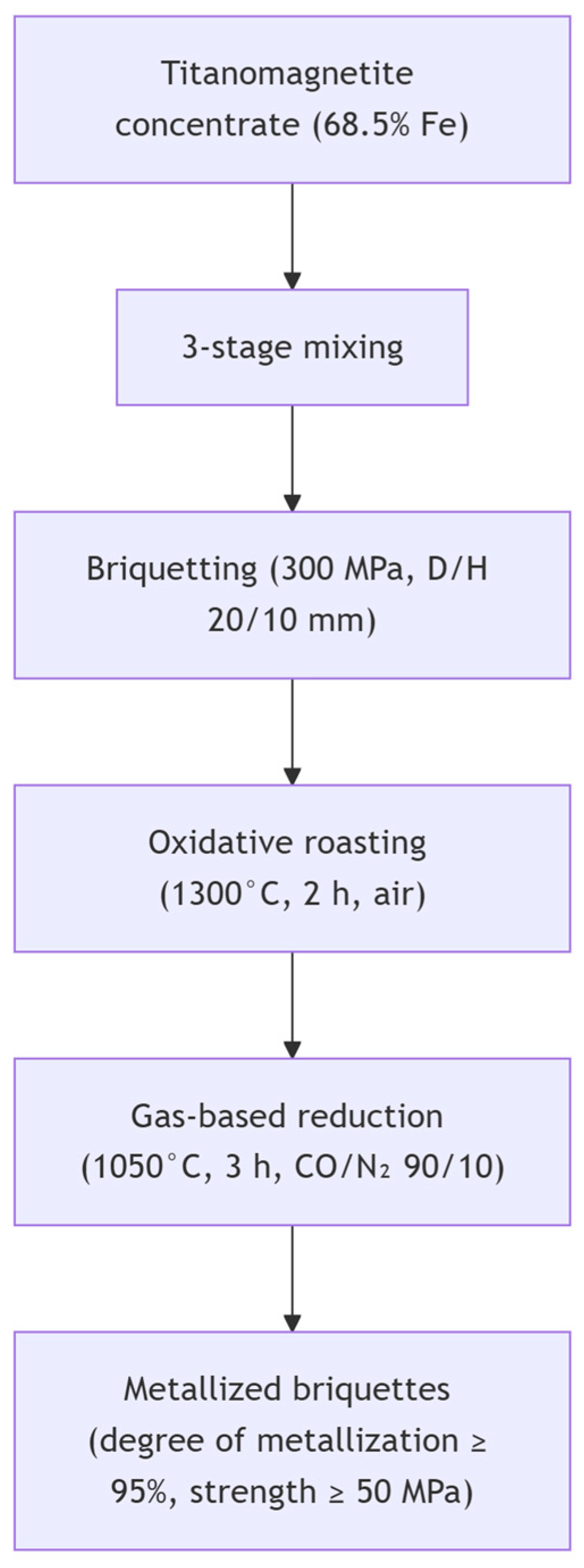
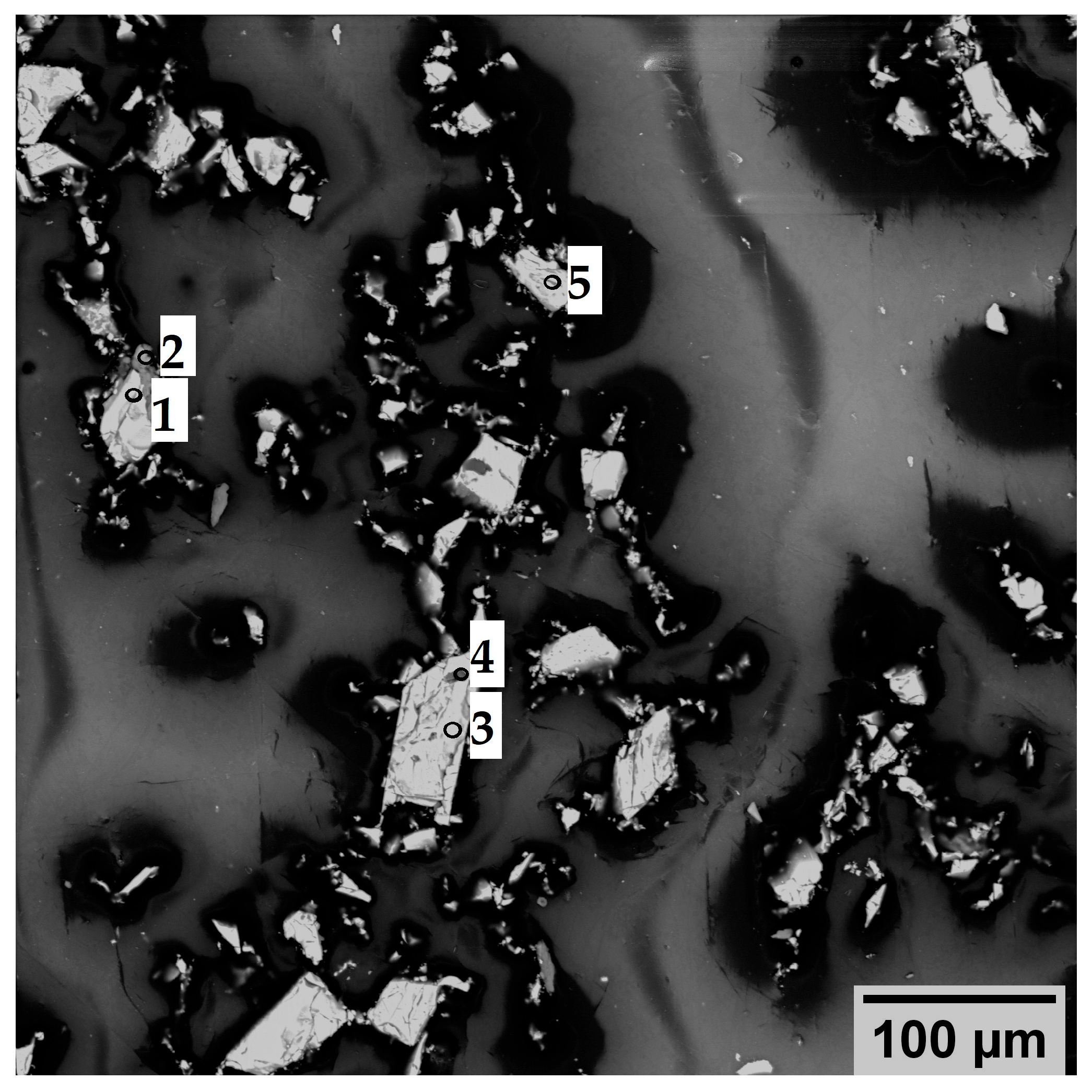
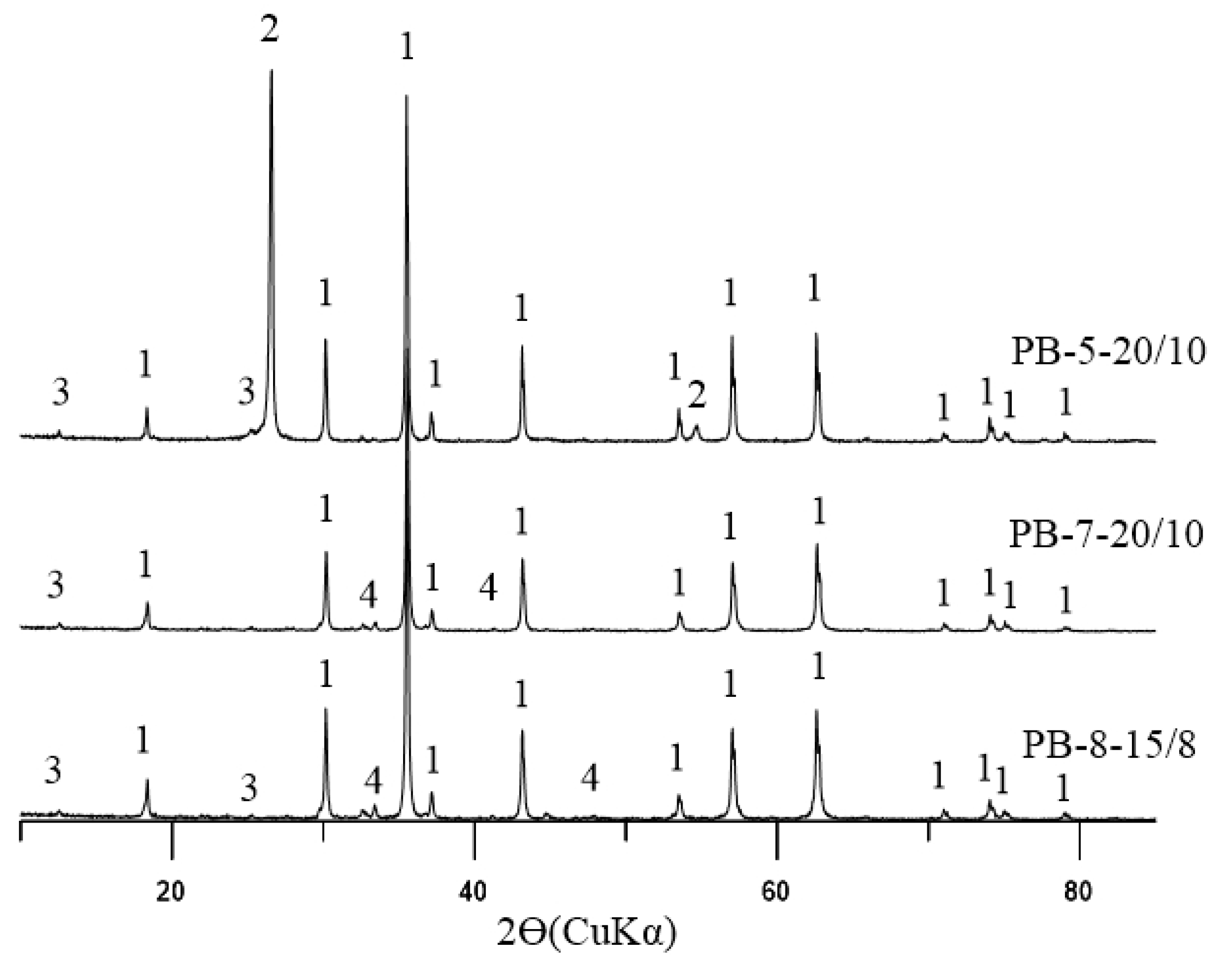
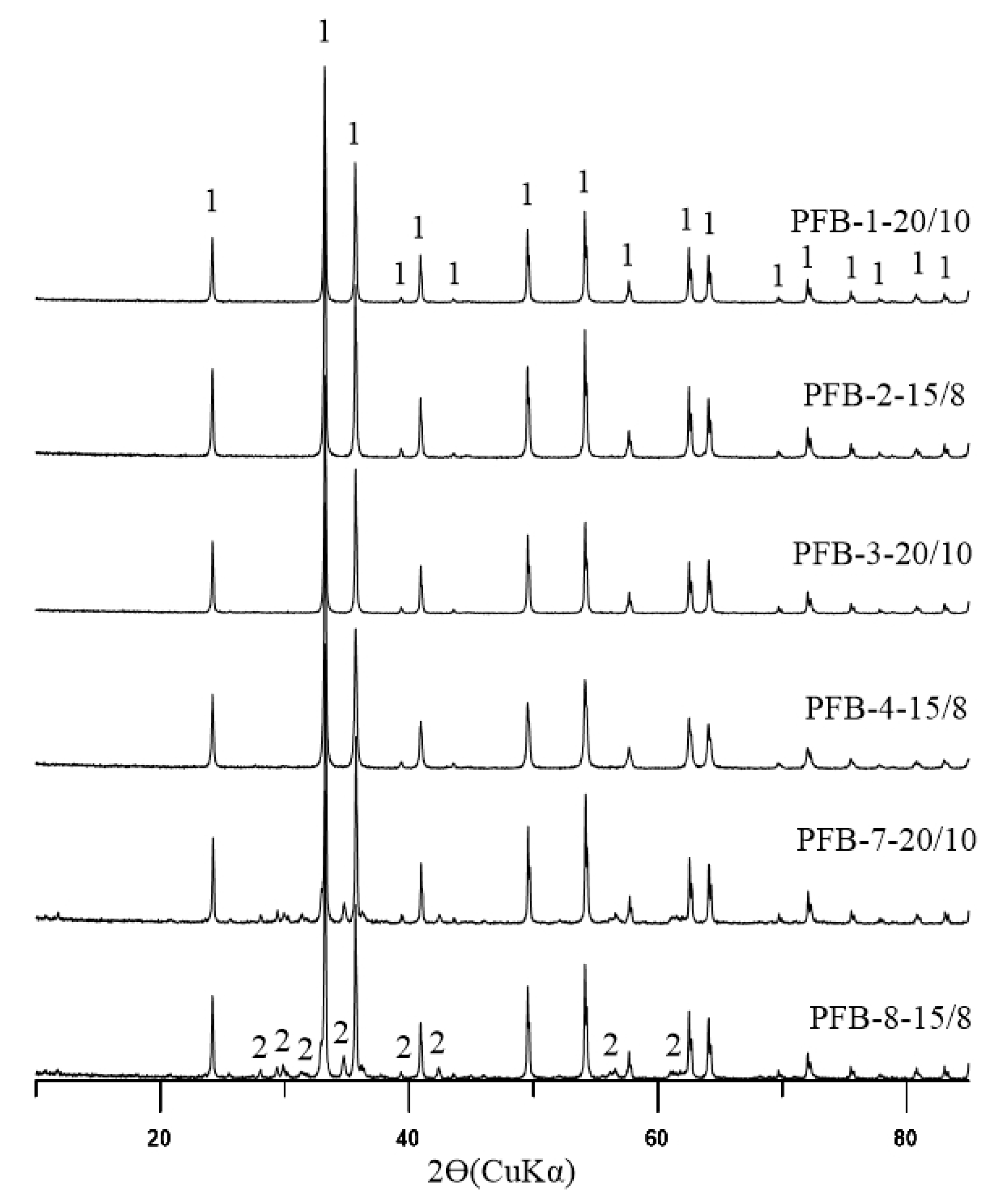
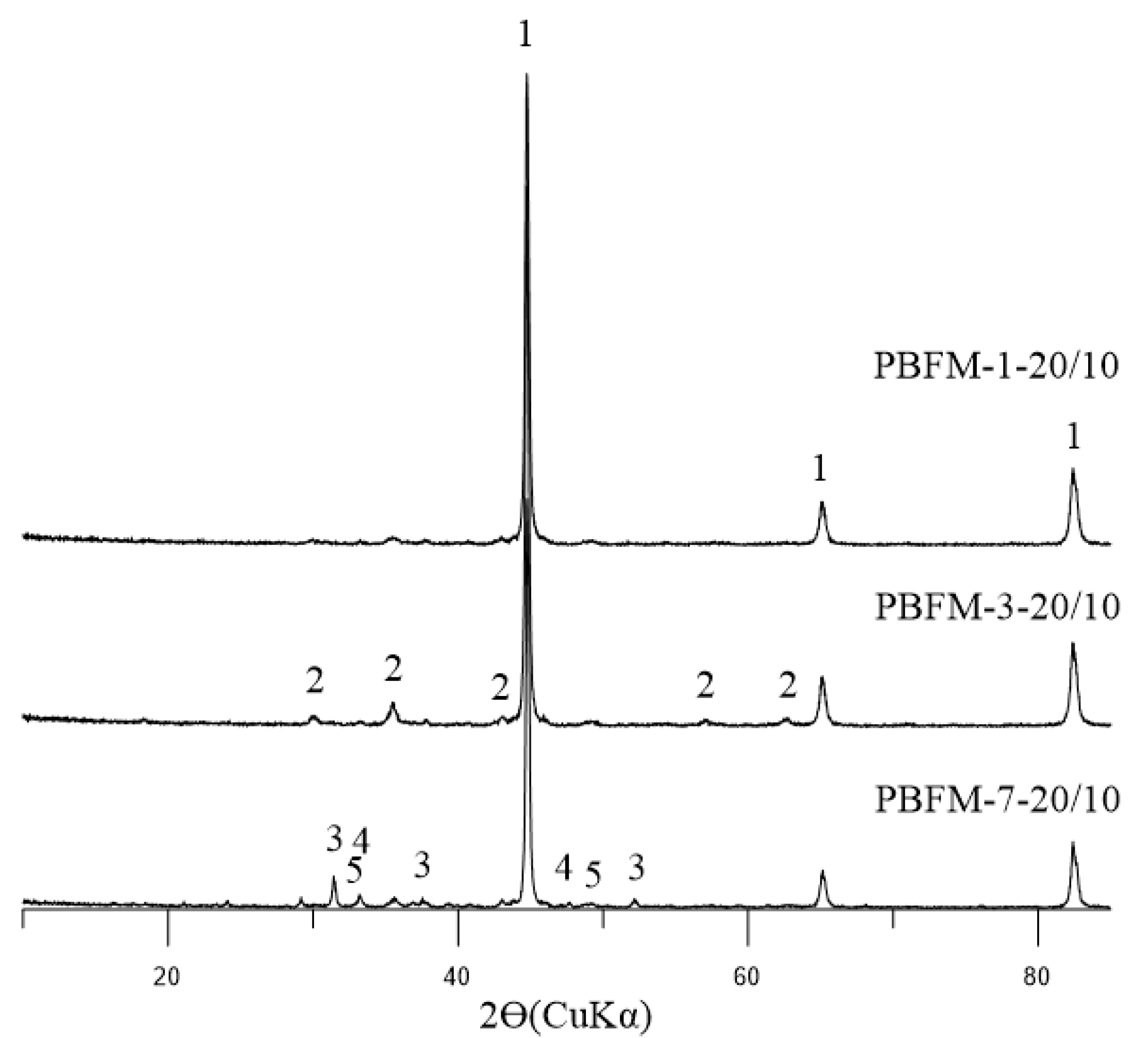
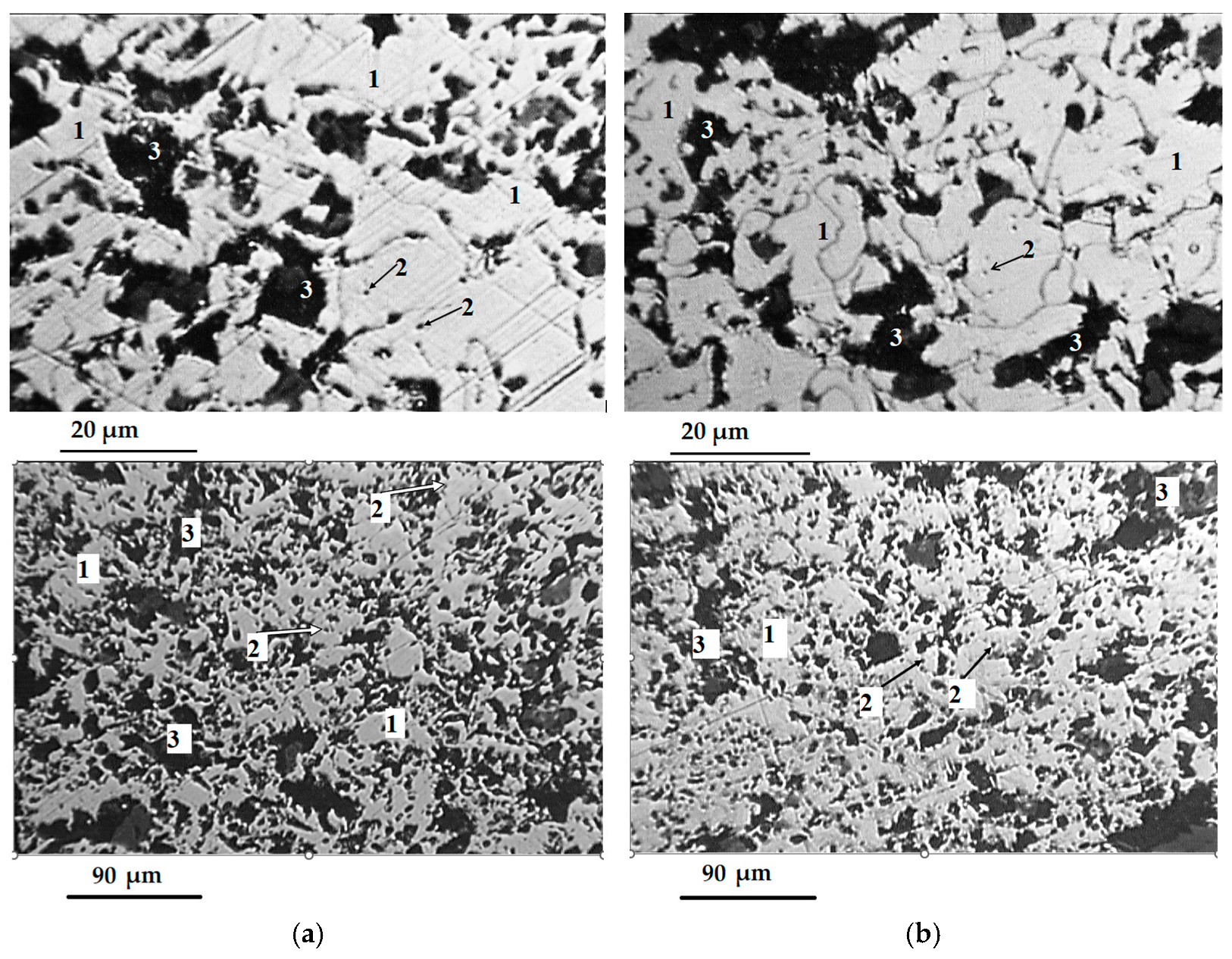
1.1. Problem Statement and Research Background
In the context of the escalating paucity of high-quality iron ore materials and the imposition of increasingly stringent environmental restrictions within the metallurgical industry, there is an imperative to identify alternative sources of iron and efficacious methods for their processing. Titanomagnetite ores represent a relatively prevalent titanium-vanadium iron ore raw material, with industrial reserves predominantly concentrated in Panzhihua (China), the Urals and Kola Peninsula (Russia), New Zealand, Malaysia, and Egypt [
1,
2,
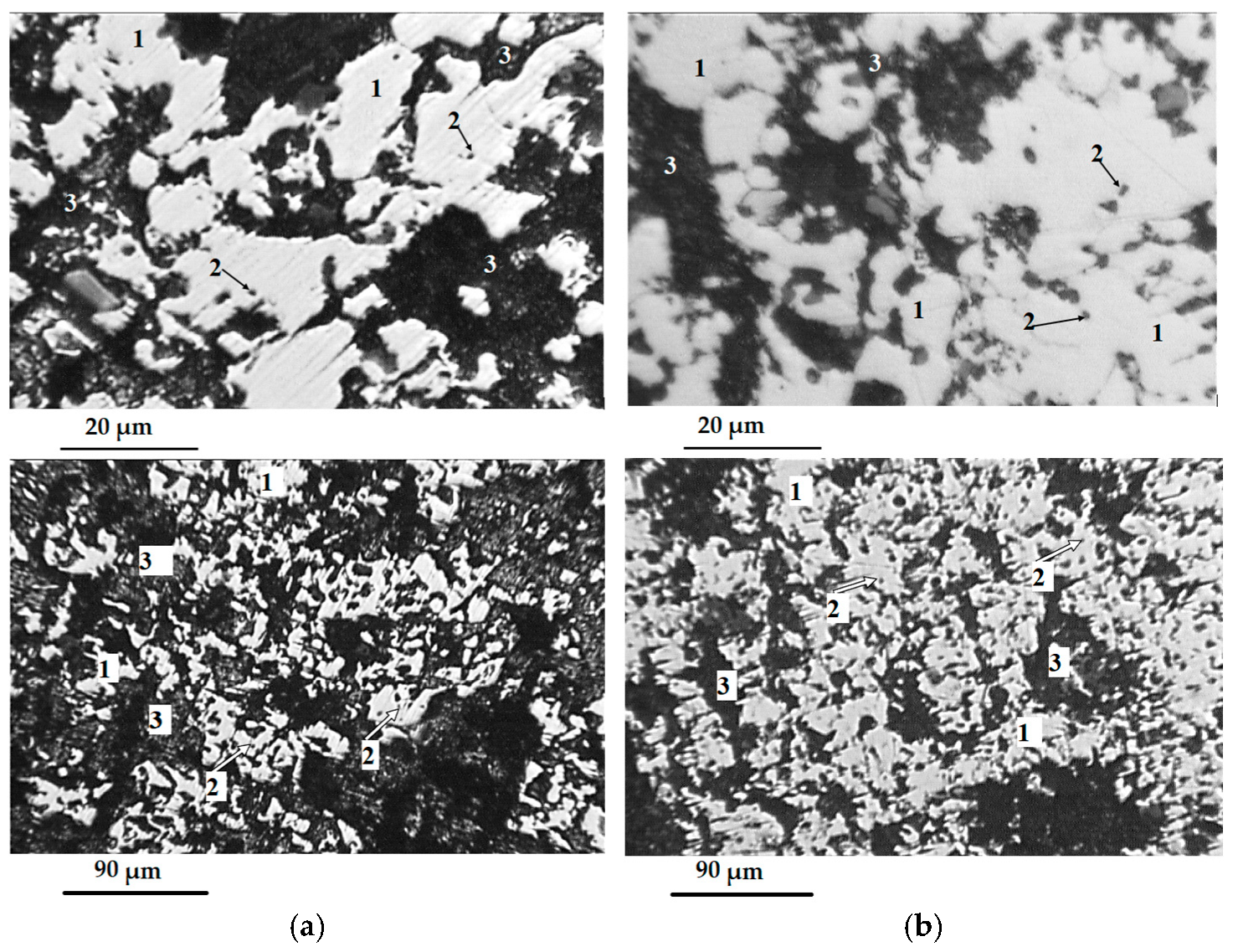
3]. The ores are constituted of fine-grained (20–150 µm) intergrown aggregates of magnetite and ulveshpinel Fe
2TiO
4 with ilmenite inclusions [
4]. The total iron content ranges from 52 to 62%, with titanium dioxide (TiO
2) ranging from 3 to 18% and vanadium pentoxide (V
2O
5) ranging from 0.4 to 1.8% [
1]. The composition of the ore in question renders titanomagnetite a valuable polymetallic resource; however, it simultaneously engenders technological difficulties in the context of conventional blast furnace processing.
The primary issue pertains to refractory slag, which is predominantly attributable to the presence of titanium carbides and oxynitrides. These compounds undergo vigorous formation during the interaction of slag with the coke gas burner. Consequently, the furnace becomes clogged with refractory titanium compounds, thereby reducing the filterability of coke gas burner and hindering the merging and flow of the liquid phases formed (i.e., drops of cast iron and slag) [
5]. Furthermore, the presence of vanadium in cast iron necessitates additional processing, thereby increasing the cost of steel [
2,
6]. Pellets and sinter produced from titanomagnetite are characterized by low cold strength (i.e., they have a strength of less than 1500 N/pellet when a minimum of 2200 N/pellet is required, i.e., a tumble index (TI) of less than 74%, with a required value of more than 78%, according to ISO 3271 [
7]). Furthermore, they are difficult to reduce (i.e., the degree of metallization from magnetite is up to 25%, since in the presence of TiO
2, the equilibrium pressure of CO
2/CO shifts, causing TiO
2 to form thin (1–3 μm) films on the FeO surface, thus blocking CO access) [
8]. Consequently, the quest for alternative methodologies for the preparation of raw materials for metallurgical processing continues to be a matter of significance.
One such method is briquetting, which involves the production of compressed materials (briquettes) of uniform mass and shape with specified physical and mechanical properties from a crushed mixture of ore (concentrate) and binder, without a sintering stage. The technology has the capacity to combine briquetting and reduction in a single process cycle (with a carbon-containing reducing agent) and allows for the precise composition of the final product [
9,
10,
11]. The application of pressure at a range of 50–200 MPa results in a briquette density of 1.8–3.4 g/cm
3, which is adequate for transportation and subsequent processing [
12,
13]. Self-hardening briquettes with cement or liquid glass binders have been observed to undergo a reduction in strength at temperatures ranging from 800 to 1000 °C [
10]. In contrast, hot ore-coal briquettes, which possess adequate strength, have been shown to achieve a metallization degree of 70–92% [
14,
15,
16].
The mechanism of carbothermal reduction of titanomagnetite at temperatures ranging from 900 to 1300 °C involves the sequential conversion of Fe
3O
4 to FeO and then Fe, whilst simultaneously undergoing the decomposition of Fe
2TiO
4 into metallic iron and titanium dioxide [
17,
18]. The activation energy of the transformation of FeO to Fe is reported to be within the range of 120–160 kJ/mol. However, the introduction of 2–3% CaF
2 has been shown to reduce this energy to 95 kJ/mol, a result attributable to the destruction of the refractory silicate film on the surface of the particles [
19]. At partial carbon monoxide pressures of less than 0.3, the formation of titanium carbides and carbonitrides may occur, thereby impeding the separation of iron and titanium [
20,
21].
The reduction of hydrogen at temperatures ranging from 700 to 1000 °C is selective in nature. This process leads to the complete reduction of iron oxides, while titanium dioxide (TiO
2) remains present in the slag. This phenomenon facilitates the subsequent extraction of vanadium and titanium into separate products [
21,
22,
23]. The degree of metallization achieved is 88–94%, yet this process necessitates protracted exposure times of 2–4 h, in addition to maintaining a low water vapor content of <5% [
22,
23]. In the context of industrial applications, the utilization of pure hydrogen is not currently a viable option. Consequently, the employment of hydrogen mixtures, comprising natural gas and hydrogen in a ratio of 1:1 (H
2/CH
4), is regarded as a potentially viable alternative. This approach is expected to yield a comparable degree of metallization while incurring a cost reduction of 20% [
21].
The nature of the reducing agent has been shown to have a significant effect on the reaction rate. Biochar and charcoal exhibit high reactivity (specific surface area of 250–400 m
2/g), yet contain up to 12% ash, which increases slag yield [
10,
24]. Coke and anthracite, on the other hand, are less active but provide a stable heat balance and low ash content [
3,
15]. It is considered that a mixed reducing agent is optimal, composed of 60% anthracite and 40% biochar, which results in 90% metallization with an ash content of <8% [
15,
24].
The addition of fluxes and catalysts to briquettes is a crucial step in the process, as it enables the precise control of slag mode and reduction rate. The addition of CaO, MgO, and SiO
2 to the slag results in a change in its basicity (CaO/SiO
2 = 0.8–1.4) and a consequent reduction in the softening temperature from 1280 to 1180 °C [
5,
25,
26]. The introduction of 0.3–0.5% Co
2O
3 or 0.2% NiO has been shown to accelerate reduction by forming defects in the Fe
3O
4 lattice [
15]. Vanadium itself exhibits catalytic activity due to the V
3+/V
4+ pair [
27]. The addition of organic binders, such as humic acids in concentrations ranging from 0.5 to 1%, has been shown to enhance the strength of briquettes in a dry state by 35–40%. This enhancement is attributed to the formation of a carbon framework that exhibits resistance to thermal shock [
28,
29].
Titanium magnetite briquettes with 12–15% carbon and a basicity of 1.1 have been observed to soften at 1160–1190 °C, subsequently melting at 80–100 °C (in contrast to the 150–180 °C melting point of pellets) and generating a gas flow resistance of 600–800 Pa, which is below the critical level of 1000 Pa for a blast furnace [
5,
12,
16]. The formation of the liquid metal phase is complete at 1300 °C, with the production of Fe-C melt containing 2.1–3.2% C and up to 0.6% V [
16,
26].
A summary of the results from over twenty laboratory and pilot experiments [
15,
16,
19,
20,
22,
30,
31] demonstrates that, at temperatures ranging from 1200 to 1250 °C and coal ash contents of less than 10%, the degree of metallization achieves a range of 85–92%. During the subsequent processing stage, metallic iron accounts for 55–60% of the briquette mass, while TiO
2 is concentrated in the slag (45–50%), which opens up the possibility of its further processing using the sulfate or chloride method [
2,
20]. The distribution of vanadium is as follows. It has been determined that 75–80% of the substance is absorbed by the metal, 15–20% remains in the slag, and less than 5% is released with the gas [
6,
32].
Metallized briquettes have been found to be a viable component in steelmaking units. In EAF, utilizing up to 30% of briquettes with a metallization degree >85% within the charge does not result in a reduction in productivity; there is merely an increase in specific energy consumption of 25 kWh/t, which is counterbalanced by the heat introduced by the carbon from the briquette [
24,
29]. In the context of oxygen converters (OC), the incorporation of 5–7% briquettes has been shown to result in a reduction of molten iron consumption by 15–20 kg/t, accompanied by a decrease in blowing time by 30–40 s [
13,
33]. The primary constraints pertain to the regulation of melt temperature (>1580 °C) and the viscosity of slag. In the context of induction and plasma furnaces, the utilization of briquettes (measuring 10–25 mm in diameter) has been demonstrated to be a successful methodology for the smelting of alloy steels. This approach has been shown to yield vanadium reducing rates of up to 90%, with titanium being introduced in the form of ferrotitanium [
6,
31].
From an environmental perspective, the substitution of 1 ton of iron ore pellets with metallized briquettes has been demonstrated to engender a reduction in CO
2 emissions of 180–220 kg, a consequence of the elimination of the calcination stage and the partial utilization of biochar [
10,
21,
24]. Furthermore, it has been demonstrated that dust emissions during transportation are reduced by 2.2–2.5 times as a consequence of the dense form of the briquette [
11,
29]. A review of the extant literature reveals that economic calculations for China and Kazakhstan demonstrate that the payback period for investments in briquetting is 3.5–4 years [
2,
13].
However, when transitioning to metallized briquettes, a number of issues must be addressed. There is an absence of long-term data concerning the behavior of briquettes in EAF with a capacity in excess of 150 MW·A and variable loads. There is no uniform classification of briquette quality for steelmaking (Fetotal, S, P, TiO2, ash content). The available information on the scalability of pressing (>50 t/h) and the thermal stability of molds during storage for periods exceeding six months is limited. To date, no comprehensive life cycle assessment (LCA) has been conducted for the transition to 100% briquettes in the charge. A systematic comparative analysis has not been undertaken from start to finish for the entire technological chain, which comprises the extraction and transport of raw materials, briquette production, smelting in EAF or OC, finished steel production and waste disposal. This analysis should include the introduction of metallized titanomagnetite briquettes into the charge in the range from 0 to 100%.