Corrosion and Anodic Oxidation of Alloy 690 in Simulated Primary Coolant of a Small Modular Reactor Studied by In Situ Electrochemical Impedance Spectroscopy
Abstract
1. Introduction
2. Materials and Methods
2.1. Cells, Electrodes, and Electrolytes
2.2. Electrochemical Measurements
2.3. Ex Situ Characterization
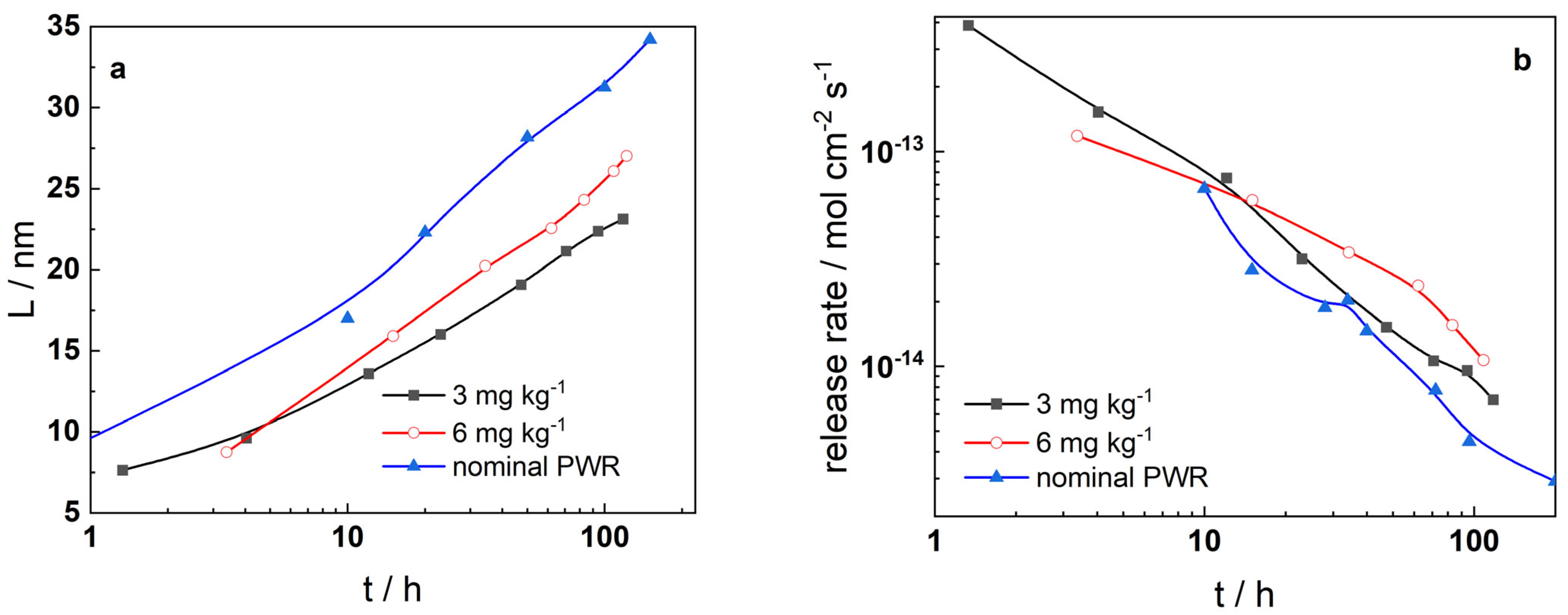
3. Results
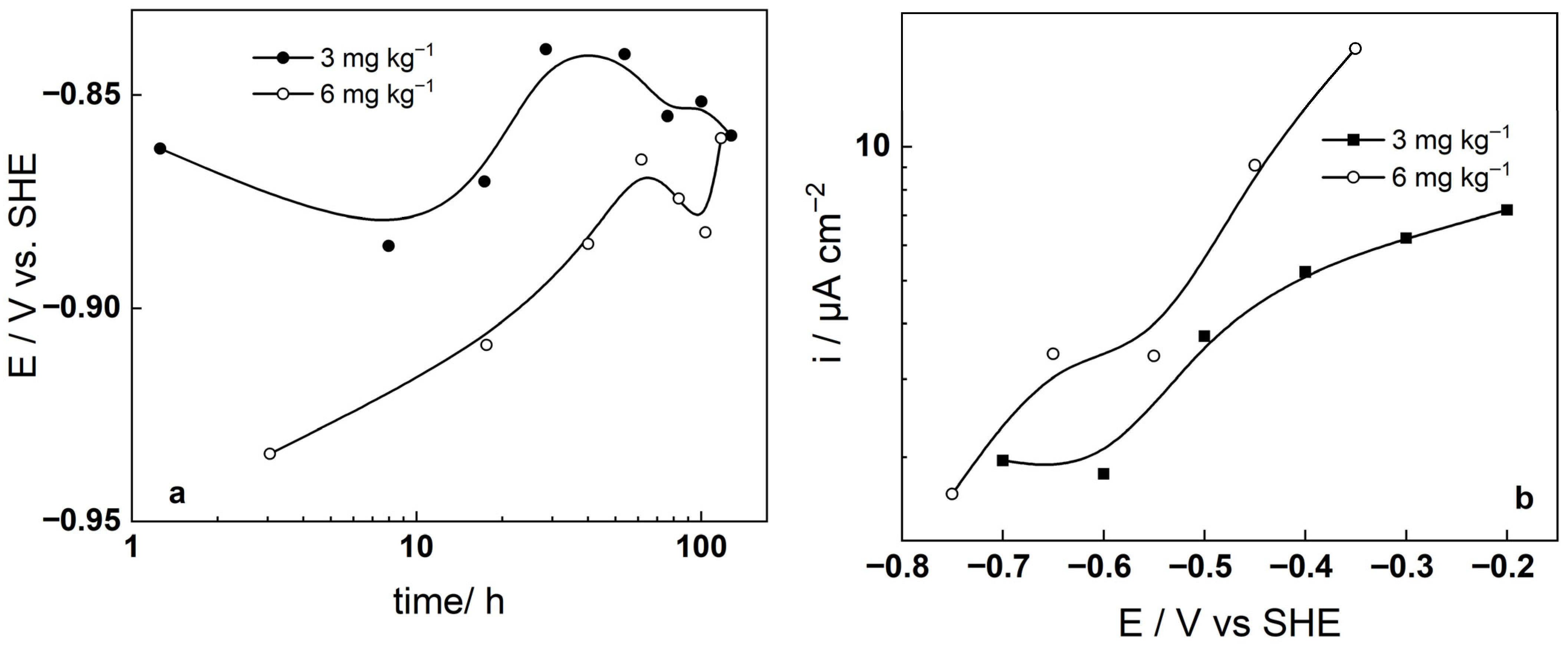
3.1. Corrosion Potential-Time and Current-Potential Curves
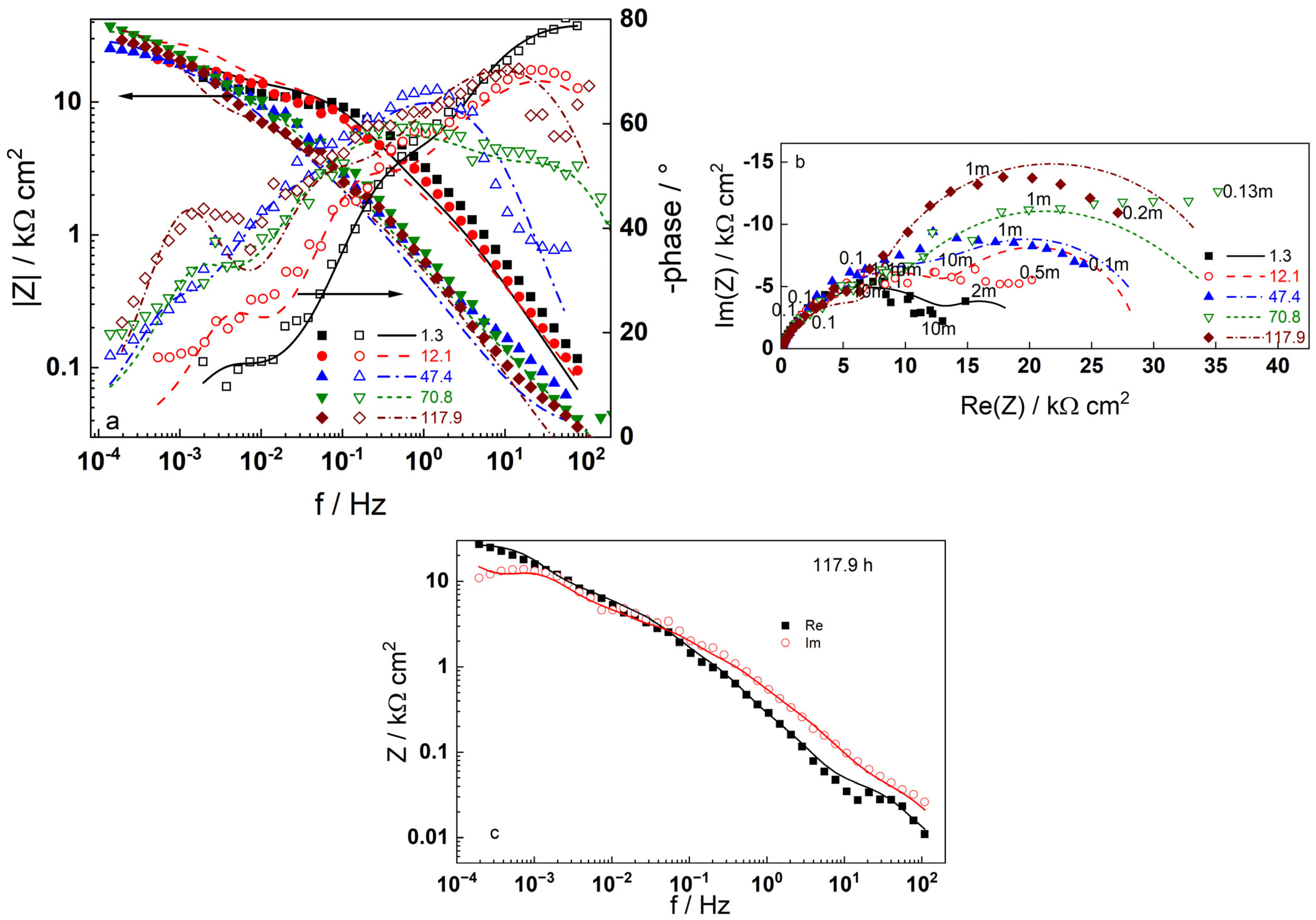
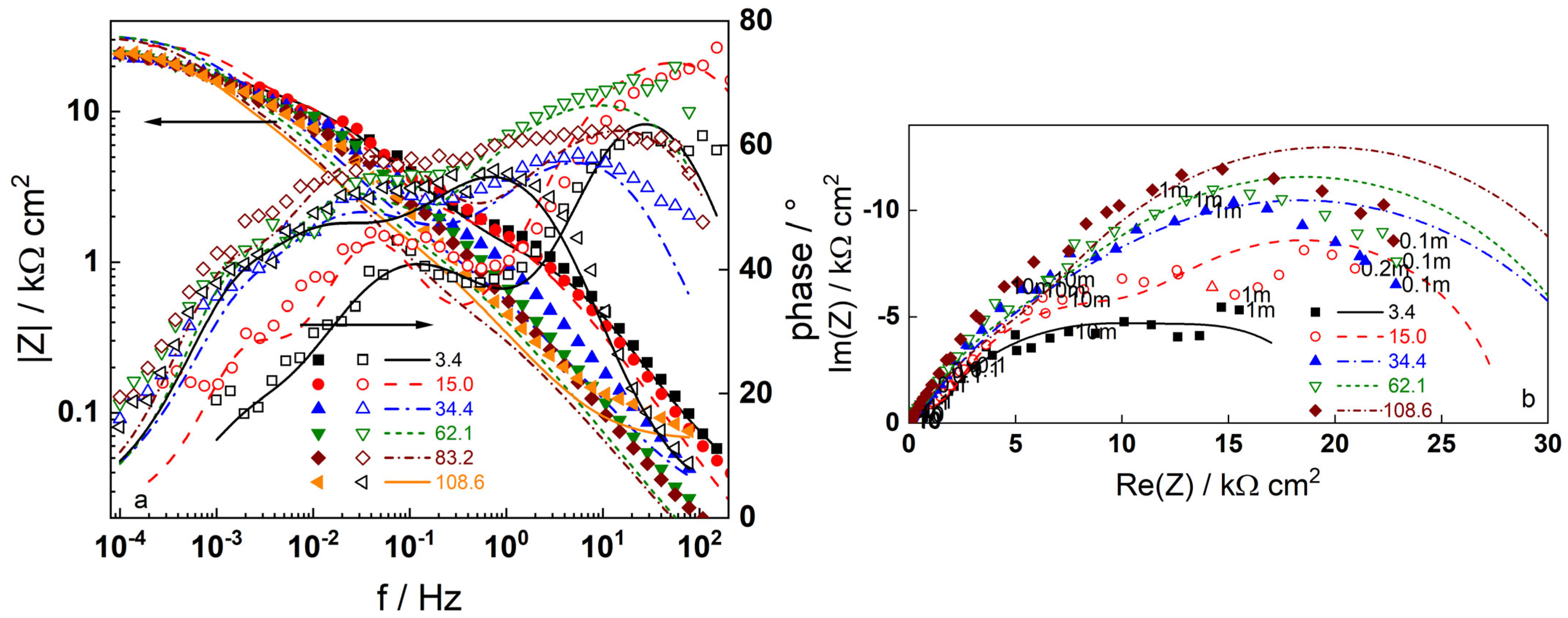
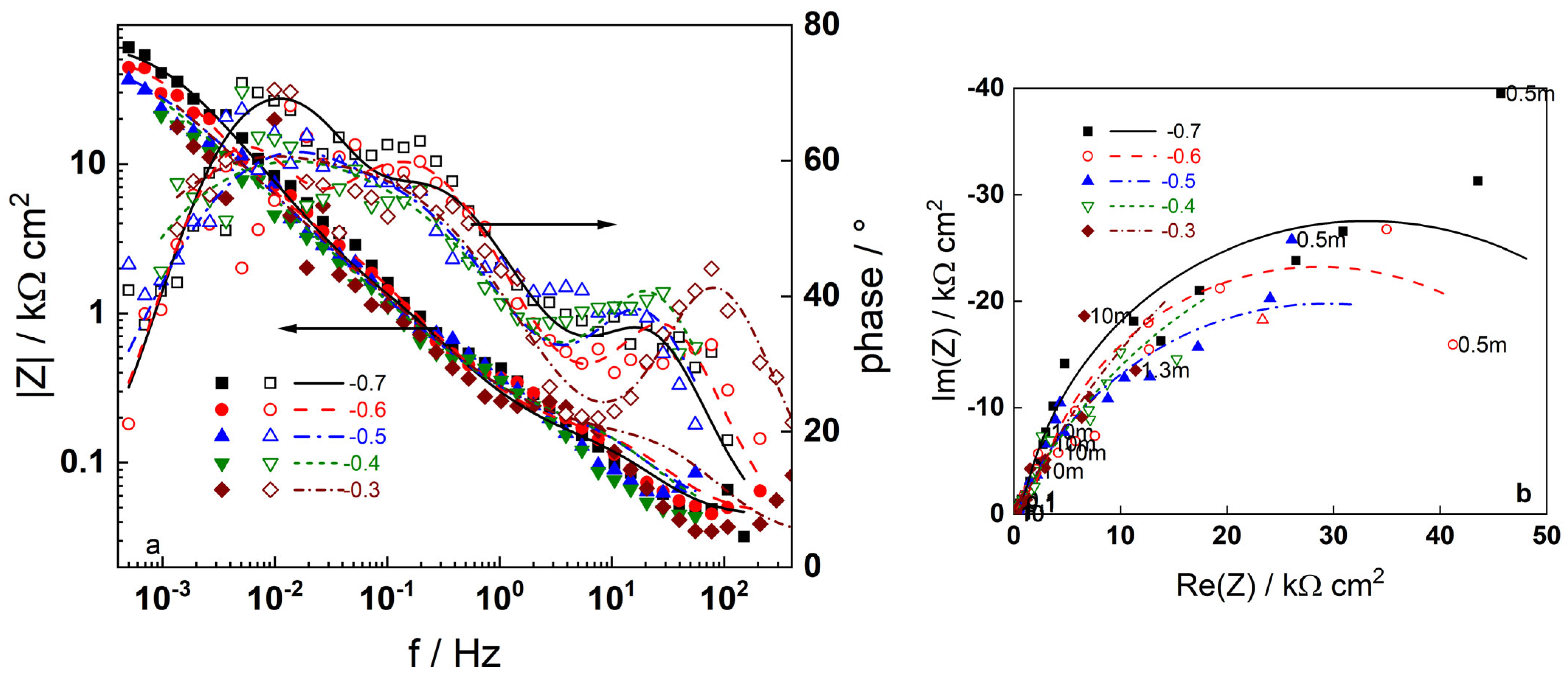
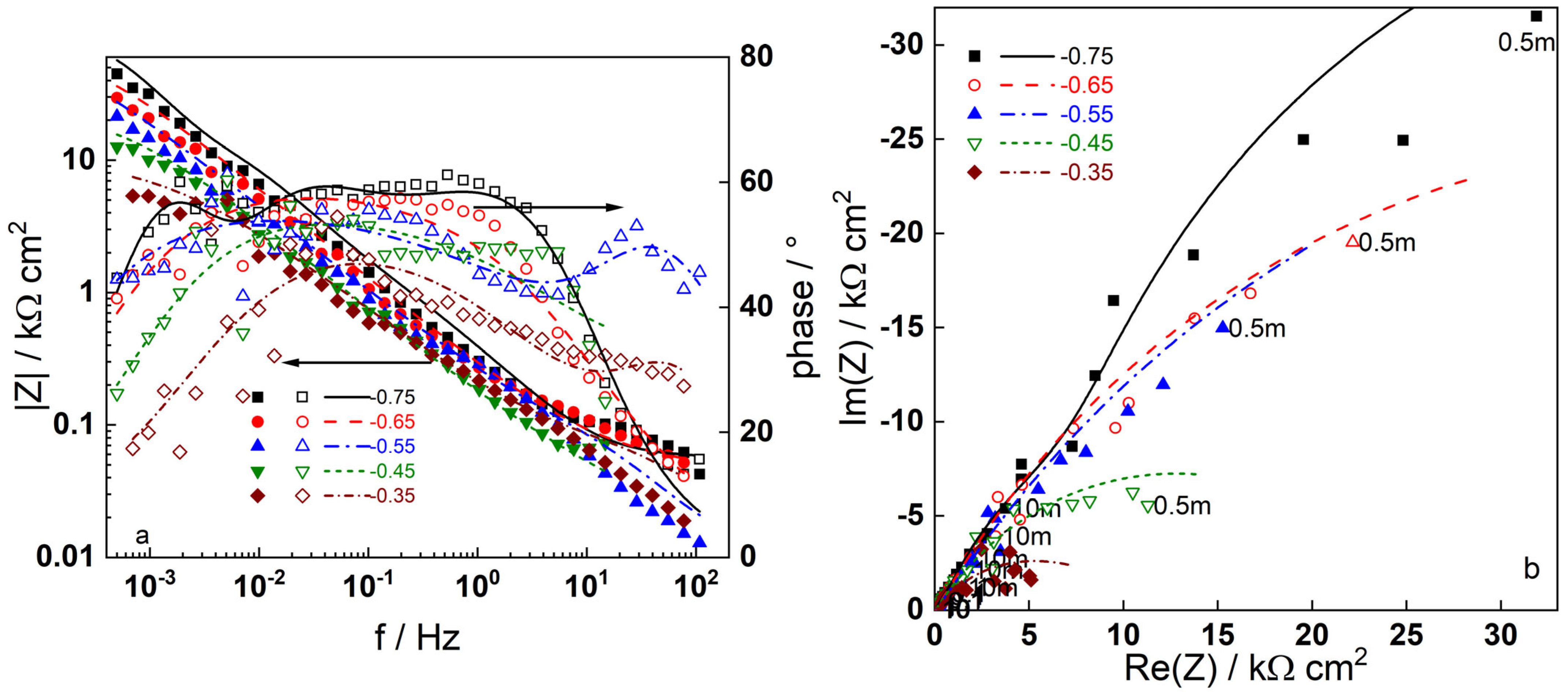
3.2. Electrochemical Impedance Spectroscopy
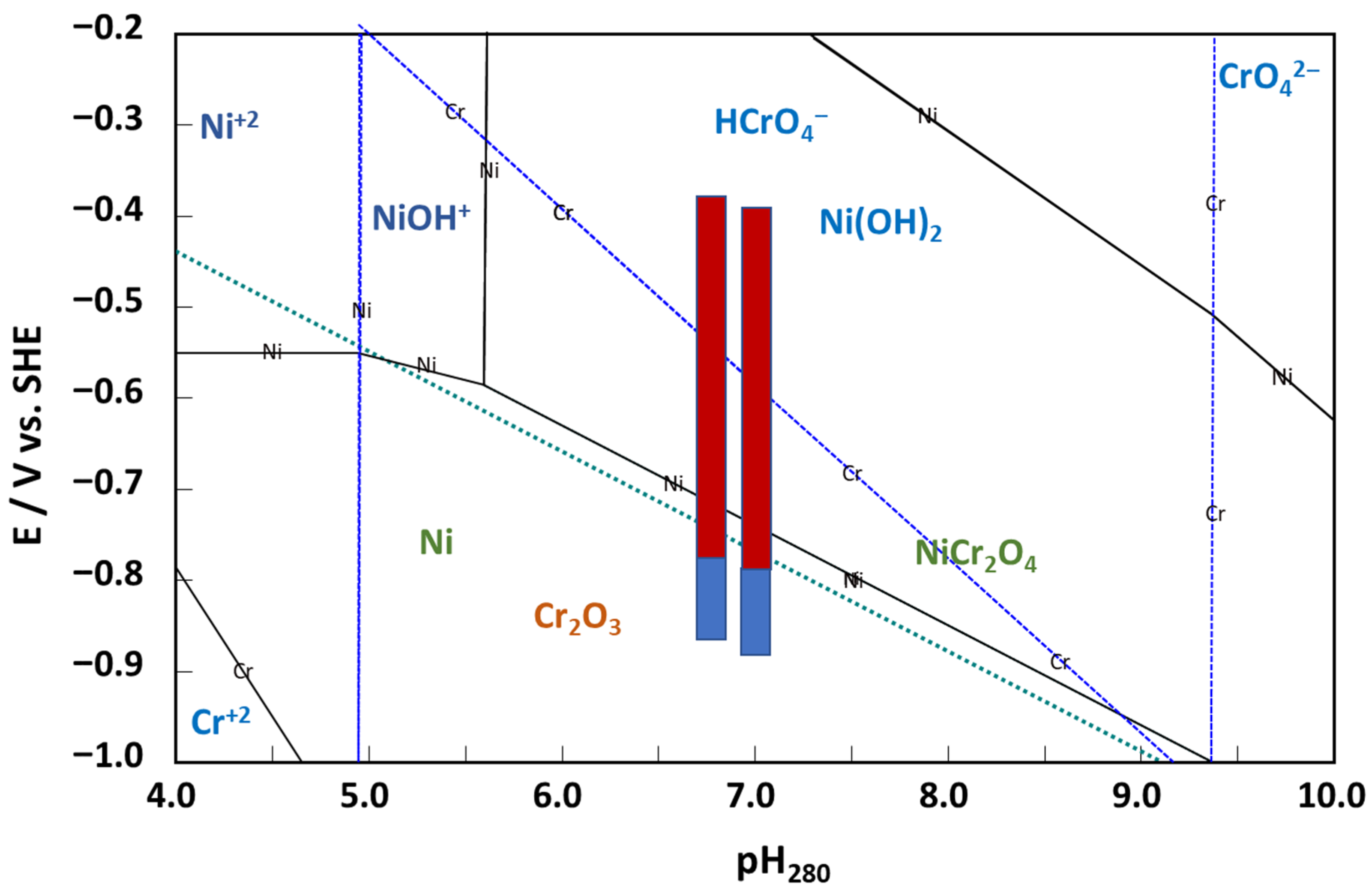
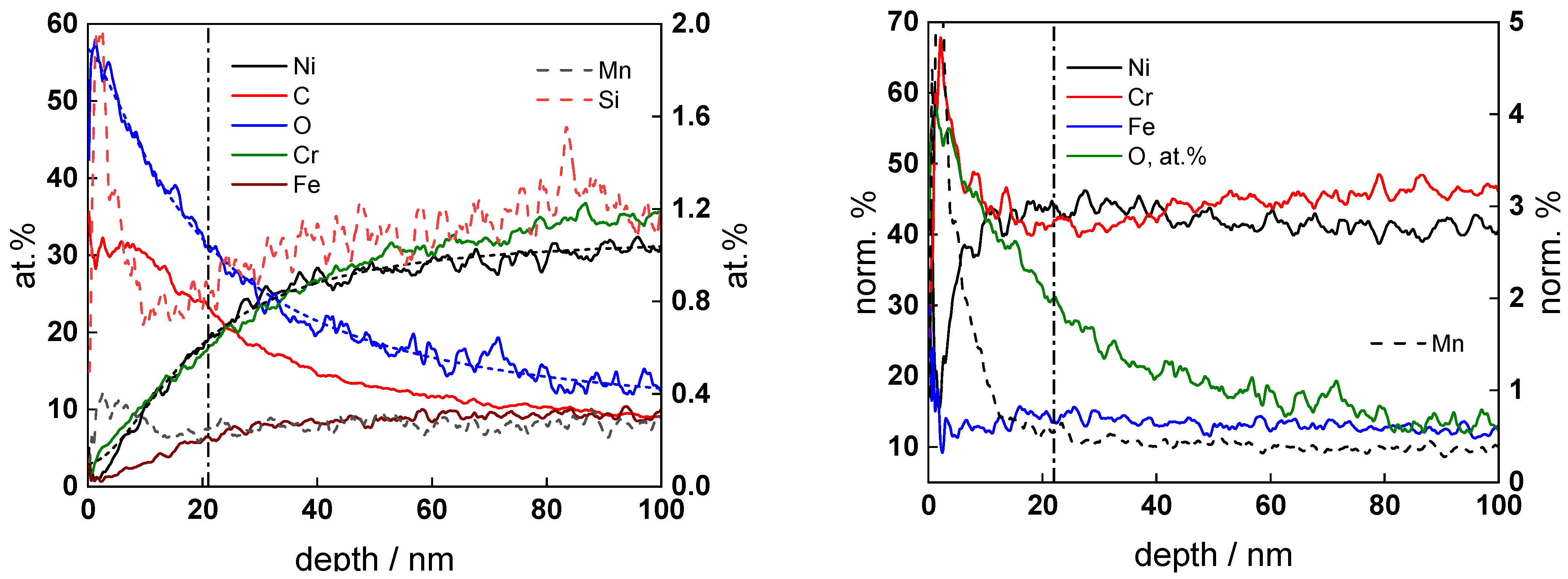
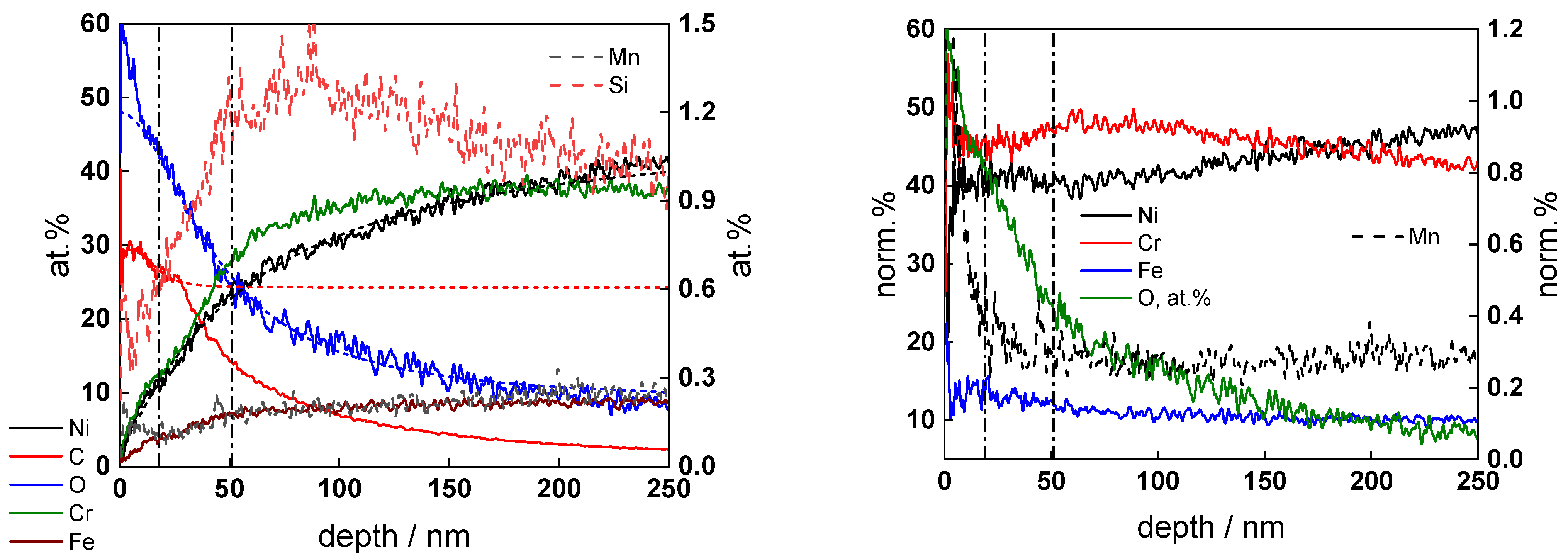
3.3. Ex Situ Analysis of the Corrosion Film
4. Discussion
Brief Description of the Kinetic Model
- Oxide formation
- Oxide dissolution
- Corrosion release (Ni dissolution through the film)
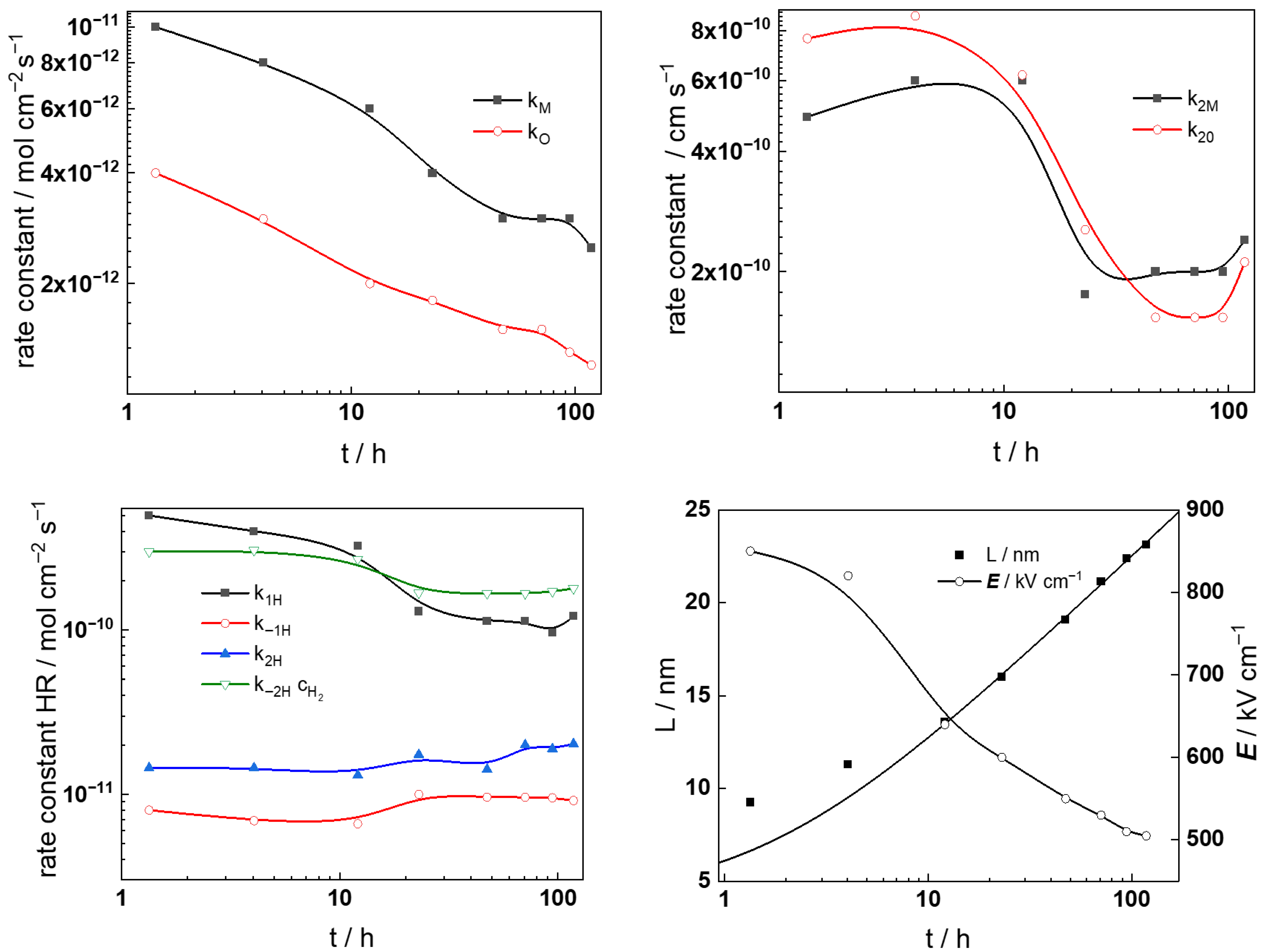
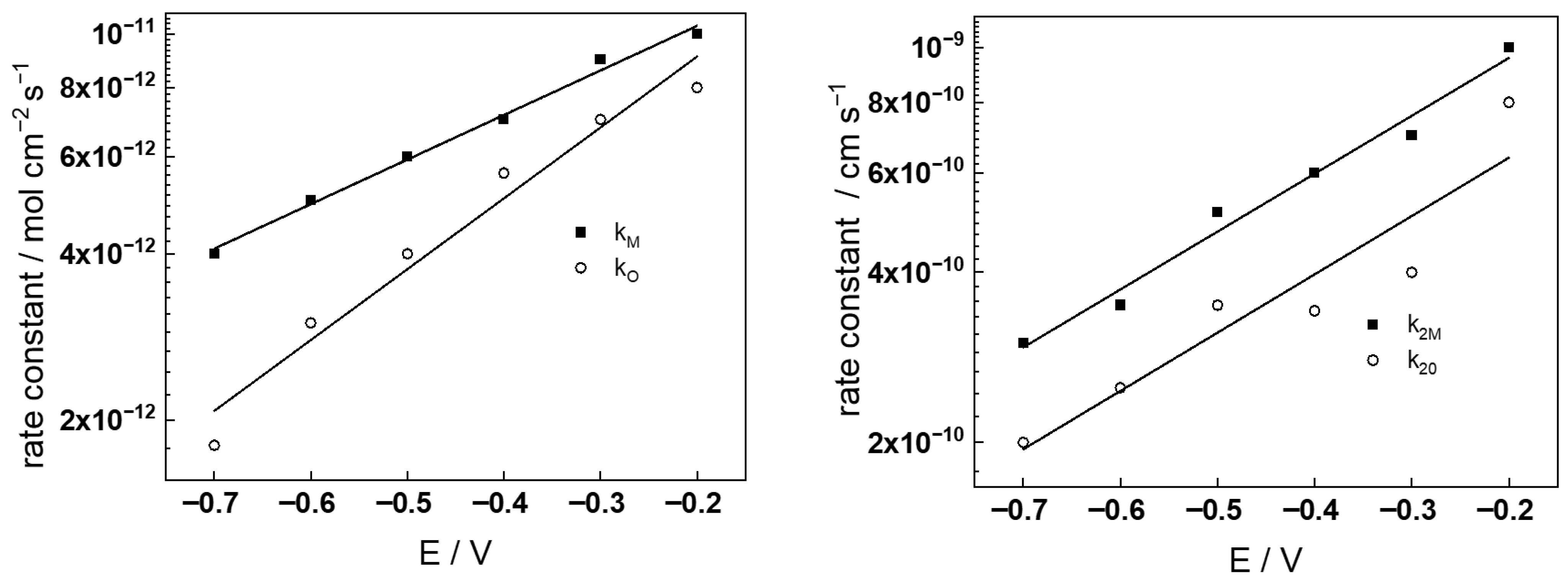
- The rate constants of the metal oxidation reactions (kM and kO) decrease with time according to a power law in both KOH concentrations. Taking into account that the film thickness increases logarithmically with time (third equation in (6)), an exponential decay of the rate constants with thickness is observed, as assumed in the model (first equation in (8)). Of course, since the field strength in the oxide is not constant with exposure, this dependence is only approximate. The rate constants in the two electrolytes are rather close to each other, the only point of note being the somewhat larger values of kO in the more concentrated KOH solution. Overall, soluble product release predominates over oxide formation since kM > kO for all exposure times in the two coolants studied.
- The rate constants of ejection of interstitial cations (k2M) and the filling of oxygen vacancies (k2O) have comparatively large values at the beginning of exposure (up to 10–20 h), and decrease to ca. three times lower values for long oxidation times. This may indicate an alteration in the interface structure due to the evolution of the electropolished layer into a protective film. The values of k2M and k2O are larger in the more concentrated KOH solution regardless of exposure time. More detailed investigation of the evolution of the oxide interface with the coolant during exposure is needed in order to fully and quantitively explain the observed dependences.
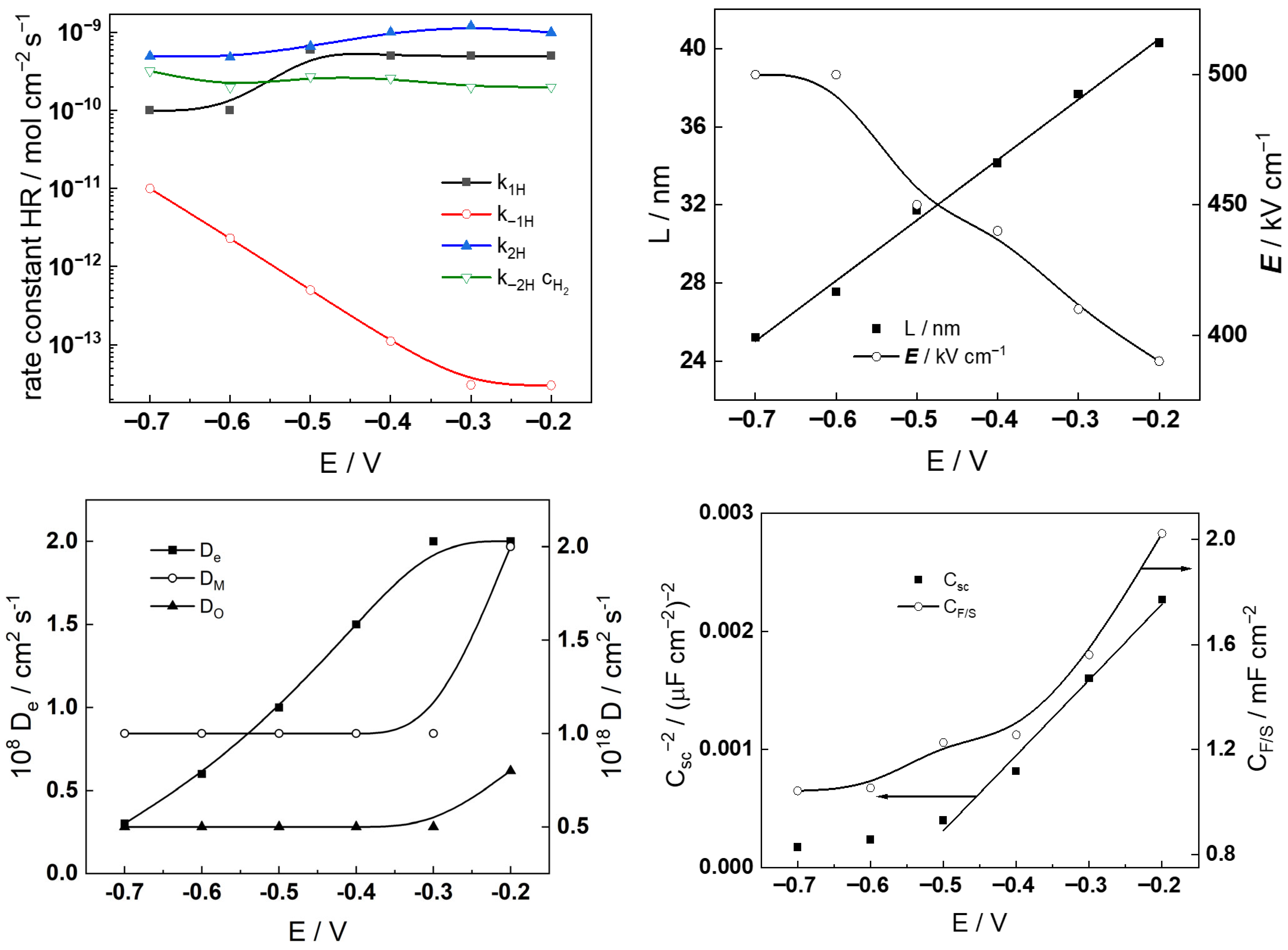
- The rate constants of the reactions associated with hydrogen evolution/oxidation do not show any appreciable dependence on the exposure time or KOH concentration, i.e., the kinetics of these reactions is not influenced by the evolution of the oxide or the coolant pH.
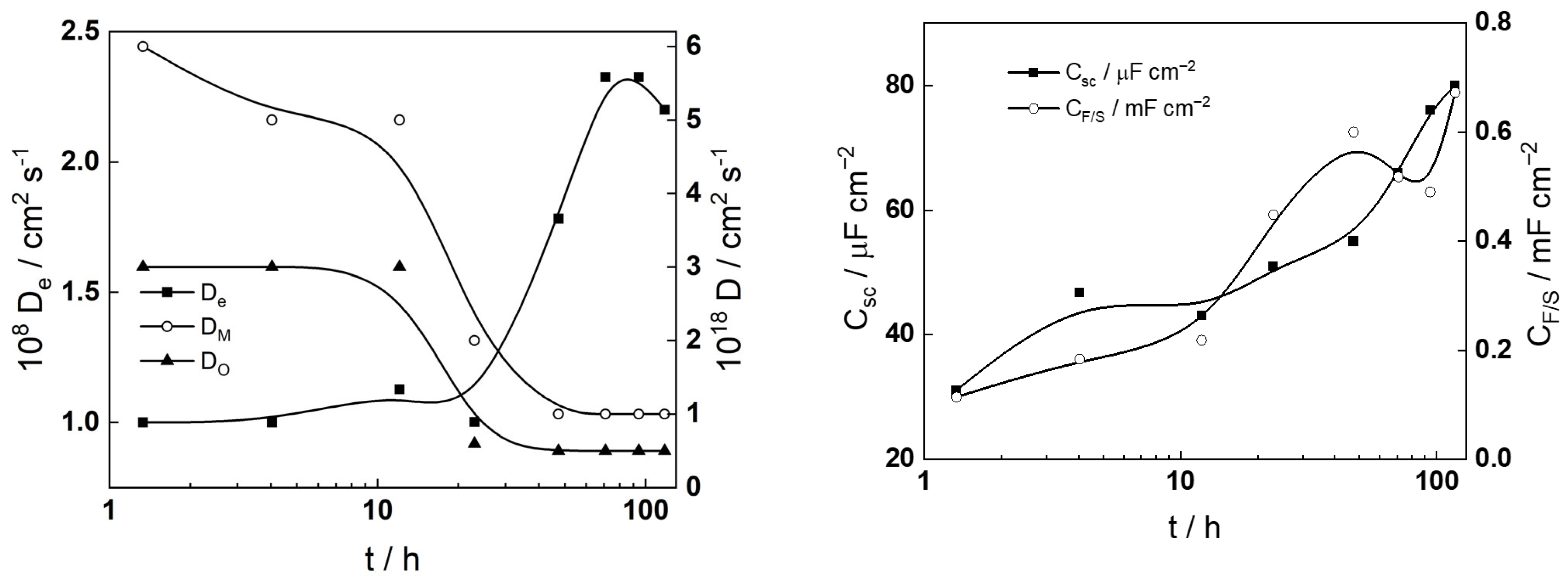
- The diffusivities of ionic defects are somewhat larger in the first 20 h of exposure and maintain constant values thereafter, indicating a quasi-steady state transport in the oxide for longer exposures. Conversely, the diffusivity of electronic carriers increases after 20 h of exposure in the more dilute KOH solution, whereas an opposite trend is detected in the other electrolyte. This feature is once again traced to the electropolished layer to corrosion film transformation and the associated alteration in semiconducting properties.
- The field strength in the oxide decreases with time and reaches quasi-constant values after 40–60 h. The values of this parameter are somewhat higher in the more concentrated KOH solution, in which the steady-state values are reached faster. This is in accordance with the faster oxide growth in that solution. As shown in a previous paper [19], such a dependence on exposure time (or, equivalently, on film thickness) can be explained by postulating a space charge formation in the oxide due to the large difference in electron and ion transport rates. If the total space charge is a sum of the concentration of mobile defects (oxygen vacancies and interstitial nickel cations) and the immobile charge of Ni(II) incorporated in Cr2O3, the following equation is obtained using the approach of Fromhold [31], x0 being the space charge screening parameter.
- Concerning the space charge and interfacial capacitances (Csc and CF/S), an increase in these two parameters is observed for times longer than 10–20 h in both studied electrolytes, which can also be traced to the alteration of the semiconducting properties of the oxide and the energetic homogeneity of the film/solution interface with time of exposure. The increase in the interfacial capacitance from values typical for the double layer (100–200 μF cm−2) could also indicate an accumulation of intermediate products of hydrogen reactions at that interface.
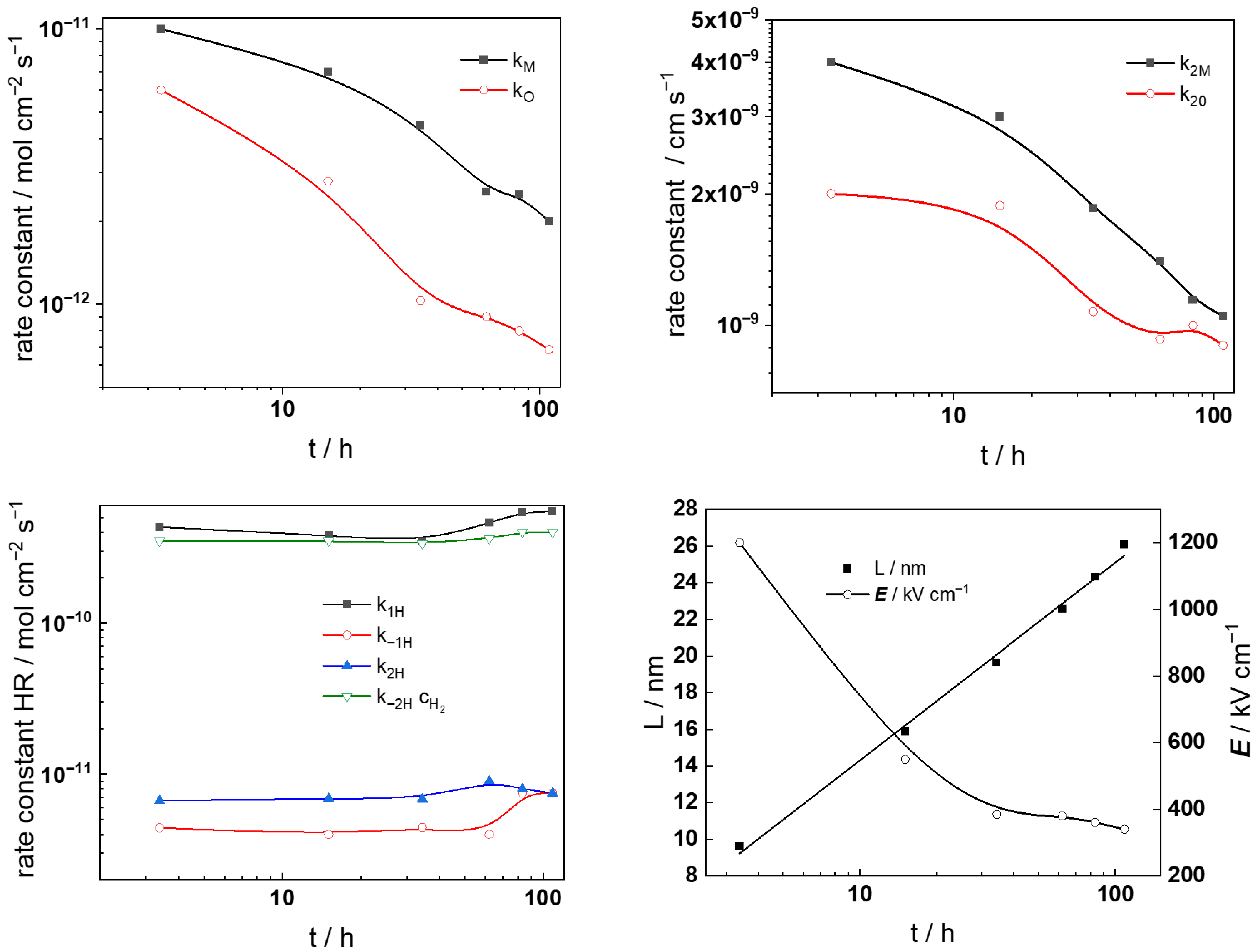
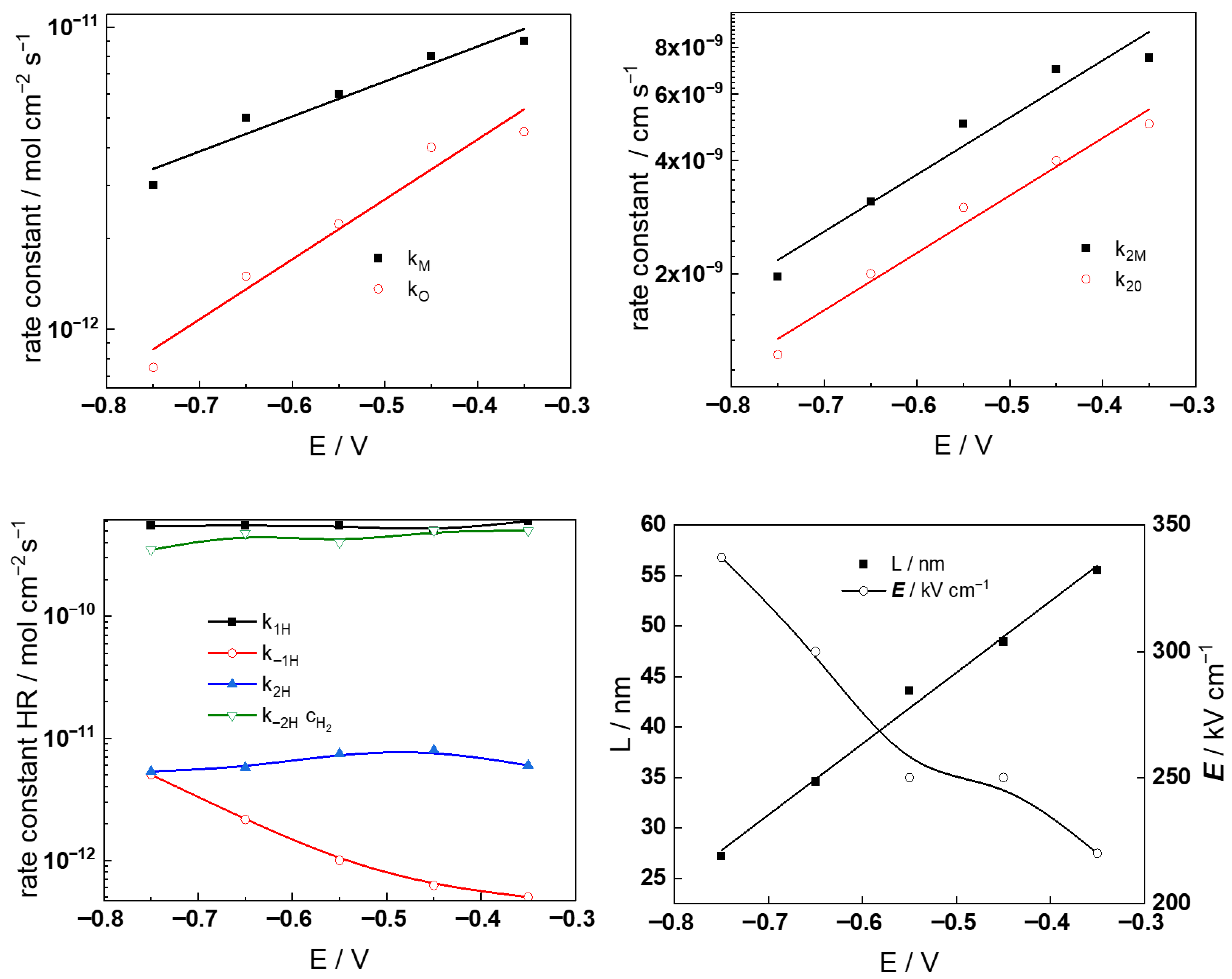
- The rate constants of hydrogen reactions at E = 0 do not vary with potential, with the exception of the rate constant of hydrogen oxidation (k−1H), which decreases significantly, indicating a change in mechanism for this reaction at higher potentials. This can be tentatively ascribed to the assumed change in the oxide from the corrosion film (Ni-doped Cr2O3) to NiCr2O4, eventually containing some Cr(VI) at potentials higher than −0.5 V vs. SHE.
- The thickness of the oxide increases quasi-linearly with increasing potential, which is in good correlation with the small (less than 20%) decrease in the field strength, also probably due to a change in the nature and concentration of mobile defects and immobile dopants in the oxide. It is worth noting that the reaction of transpassive oxidation is not taken into account within the frames of the present model; thus, the description of the processes at high anodic polarizations is semi-quantitative. However, it was judged premature to include new reactions in an already rather complex model at this stage.
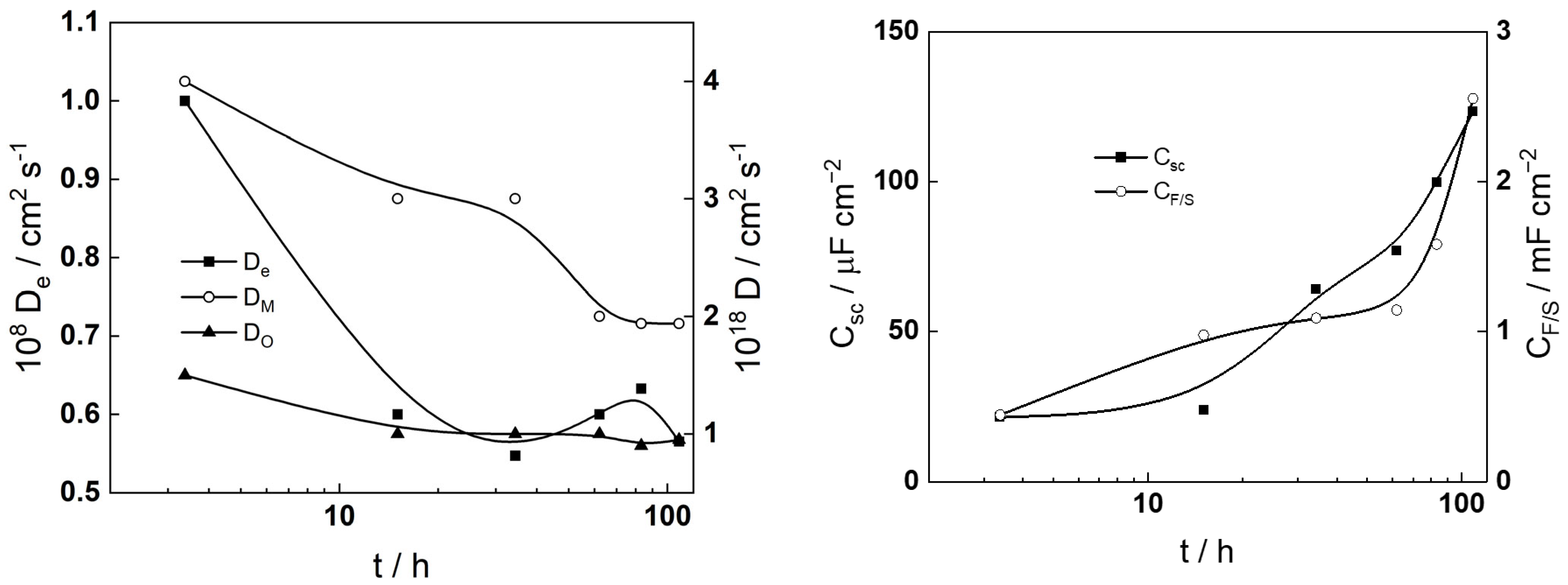
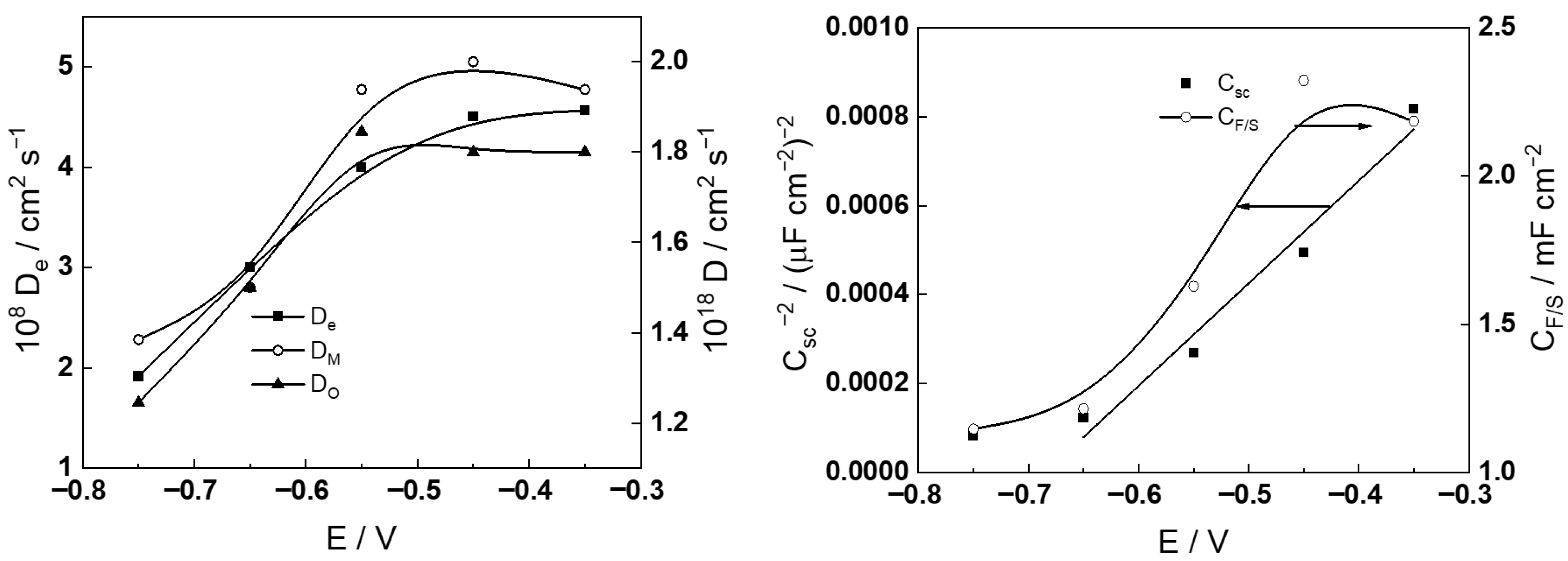
- The diffusivites of ionic and electronic defects increase with potential and reach constant values in the transpassive region, the values being somewhat lower in the more concentrated KOH solution. In general, the diffusivities are somewhat higher during anodic polarization in comparison to the values during free corrosion, which can also be taken as an indication of a different structure and composition of the film formed at anodic potentials. Further investigations are now in progress to elucidate that structure and composition.
- The capacitance of the depletion layer decreases with increasing potential, and, when plotted in Csc−2 vs. E coordinates, gives a quasi-linear evolution in the transpassive region. That indicates an n-type oxide, and rough estimates of the donor densities are (6–8) × 1020 cm−3, i.e., typical for a semiconductor at the edge of degeneration (the donor density is found to be higher in the more concentrated KOH solution). Further, the interfacial capacitance (CF/S) increases with potential and preserves values that are somewhat larger than a typical double layer capacitance, which can be due to the accumulation of intermediate products of both the hydrogen and transpassive oxidation reactions.
5. Conclusions
- There is a relatively small effect of KOH concentration in boron-free coolants on the conduction mechanism in the protective film, and soluble product release and electrochemical reactions are evidenced. Thus, no general corrosion problems are expected for Alloy 690 during the transition from B-Li and/or B-K-Li primary water chemistry to boron-free conditions.
- The conduction mechanism in the alloy/oxide/coolant system is described by a consistent set of parameters stemming from non-linear least squares fits to the impedance spectra, both during free corrosion and under anodic polarization. The evolution of kinetic constants and transport parameters with time and potential obey dependences that are imminently reasonable from general kinetic grounds and do not differ significantly in comparison to those estimated earlier in nominal PWR coolants.
- The oxides which formed in boron-free coolant are somewhat thinner than those in nominal PWR conditions, i.e., the rate of oxidation is to a certain extent lower in the absence of B. Soluble product release rates are rather similar in B-free and nominal PWR coolants, indicating that the overall general corrosion behavior does not differ significantly in the absence of boron. Thus, the present study demonstrates that there are no obstacles in also using Alloy 690 as steam generator tubing in SMRs.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ingersoll, D.T.; Carelli, M.D. (Eds.) Handbook of Small Modular Nuclear Reactors; Elsevier Woodhead Publishing: Cambridge, UK, 2021. [Google Scholar]
- Ricotti, M.E.; Fomin, R.V. Small modular reactors, Chapter 5. In Nuclear Reactor Technology Development and Utilization; Ud-Din Khan, S., Nakhabov, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 187–211. [Google Scholar]
- Zhang, Z.; Jiang, J. On load-following operations of small modular reactors (review). Prog. Nucl. Energy 2024, 173, 105274. [Google Scholar] [CrossRef]
- Liao, J.; Hu, Y.; Li, J.; Jin, D.; Meng, S.; Ruan, T.; Hu, Y.; Zhang, Z. Corrosion release behavior of alloy 690 and its application in high-temperature water with Zn injection. Nucl. Eng. Technol. 2022, 54, 984–990. [Google Scholar] [CrossRef]
- Flambard, J.; Carrette, F.; Monchy-Leroy, C.; Andrieu, E.; Laffont, L. Influence of the transient conditions on release of corrosion products and oxidation of alloy 690 tubes during pressurized water reactor restart after steam generators replacement. J. Nucl. Mater. 2021, 543, 152562. [Google Scholar] [CrossRef]
- Hur, D.H.; Lim, D.-S.; Jeon, S.-H. Corrosion behavior of Alloy 690 after cessation of zinc addition in simulated PWR primary water at 330 °C. J. Nucl. Mater. 2021, 555, 153147. [Google Scholar] [CrossRef]
- Jeon, S.-H.; Lim, D.-S.; Choi, J.; Song, K.-M.; Lee, J.-H.; Hur, D.-H. Effects of zinc addition on the corrosion behavior of pre-filmed alloy 690 in borated and lithiated water at 330 C. Materials 2021, 14, 4105. [Google Scholar] [CrossRef]
- Lim, D.-S.; Jeon, S.-H.; Bae, B.J.; Choi, J.; Song, K.-M.; Hur, D.-H. Effect of zinc addition scenarios on general corrosion of Alloy 690 in borated and lithiated water at 330 C. Corros. Sci. 2021, 189, 109627. [Google Scholar] [CrossRef]
- Wu, X.; Liu, X.; Zhang, Z.; Tan, J.; Han, E.-H.; Ke, W. Some fundamental understandings of Zn-injection water chemistry on material corrosion in pressurized water reactor primary circuit. Corros. Commun. 2022, 6, 52–61. [Google Scholar] [CrossRef]
- Ribière, N.; Engler, N.; Brimbal, D.; Skocic, M.; Andrieu, É.; Blanc, C.; Laffont, L. Multi-scale characterization of the inner surface of as-received steam generator tubes and correlation with the Ni release in primary water. Corros. Sci. 2023, 218, 111205. [Google Scholar] [CrossRef]
- Karimihaghighi, R.; Naghizadeh, M. Effect of alloying elements on aqueous corrosion of nickel-based alloys at high temperatures: A review. Mater. Corros. 2023, 74, 1246–1255. [Google Scholar] [CrossRef]
- Ribière, N.; Esvan, J.; Engler, N.; Brimbal, D.; Skocic, M.; Andrieu, É.; Blanc, C.; Laffont, L. An XPS and TEM study of the composition and structure of native oxides on the inner surface of as-received Ni base alloy steam generator tubes. Appl. Surf. Sci. 2024, 654, 159514. [Google Scholar] [CrossRef]
- Han, J.-Y.; Jeon, S.-H.; Ha, S.-J.; Shim, H.-S.; Sohn, I.; Kim, S.-W. Effect of plastic deformation on microstructure and general corrosion behavior of alloy 690 in SG crevice condition of PWR secondary system containing hydrogen. J. Mater. Res. Technol. 2024, 33, 9232–9248. [Google Scholar] [CrossRef]
- He, S.; Liu, F.; Li, Y.; Xia, R.; Wang, J.; Zhang, L. Effect of Zinc Addition on Corrosion and Metal Release Behavior of Nickel-based Alloy 690 in PWR Primary Water. At. Energy Sci. Technol. 2024, 58, 1514–1522. [Google Scholar]
- Li, X.; Chen, H.; Xu, X.; Chen, J.; Cui, T.; Dong, H.; Wang, J.; Lu, Z. Corrosion Resistance of Alloy 690 in High-Temperature Water with Various Dissolved Hydrogen Concentrations. Corrosion 2025, 81, 205–215. [Google Scholar] [CrossRef]
- Wei, S.C.; Wang, X.; Li, X.; Xu, J.; Shoji, T. Atomically unveiling the initial stage of interfacial reactions of Alloy 690 in high temperature high pressure water environments by ab initio molecular dynamics. J. Nucl. Mater. 2025, 605, 155591. [Google Scholar] [CrossRef]
- Ribière, N.; Esvan, J.; Engler, N.; Brimbal, D.; Skocic, M.; Andrieu, É.; Blanc, C.; Laffont, L. XPS and TEM characterization of oxides formed in simulated primary water on steam generator tubes made of Ni based alloy. Corros. Sci. 2025, 249, 112823. [Google Scholar] [CrossRef]
- Yang, J.; Li, Y.; Xu, A.; Fekete, B.; Macdonald, D.D. The electrochemical properties of alloy 690 in simulated pressurized water reactor primary water: Effect of temperature. J. Nucl. Mater. 2019, 518, 305–315. [Google Scholar] [CrossRef]
- Bojinov, M.; Betova, I.; Karastoyanov, V. Corrosion Mechanism and Electrochemical Reactions on Alloy 690 in Simulated Primary Coolant of Water–Water Energy Reactors. Materials 2024, 17, 1846. [Google Scholar] [CrossRef]
- Bai, Z.; Li, Y.; Xing, L.; Gao, P.; Yu, Z.; Ding, S.; Macdonald, D.D.; Wang, S. In-Situ Monitoring Techniques and Analysis Theory for Electrochemical Corrosion in Subcritical and Supercritical Aqueous Systems. J. Electrochem. Soc. 2025, 172, 021504. [Google Scholar] [CrossRef]
- Jeon, S.-H.; Lim, D.-S.; Choi, J.; Song, K.M.; Hur, D.H. Influences of Zn Concentration on the General Corrosion Behavior of Alloy 690TT in Simulated Primary Water of PWRs. In Proceedings of the Transactions of the Korean Nuclear Society Virtual Spring Meeting, Virtual, 13–14 May 2021. paper 21S-227. [Google Scholar]
- Choi, W.; Baek, S.H.; Song, G.D.; Lim, D.S.; Son, Y.; Kim, Y.-J.; Bahn, C.B. Electrochemical Behavior of i-SMR Structural Materials in Simulated Soluble Boron-free KOH or LiOH Environments. In Proceedings of the Transactions of the Korean Nuclear Society Spring Meeting, Jeju, Republic of Korea, 18–19 May 2023. paper 23S-365. [Google Scholar]
- Shim, H.-S.; Jeon, S.-H.; Kim, J.S.; Hur, D.H. Corrosion Behaviors of Alloy 690 Steam Generator Tubes in Boron-Free Primary Coolant. In Proceedings of the Transactions of the Korean Nuclear Society Spring Meeting, Jeju, Republic of Korea, 18–19 May 2023. paper 23S-469. [Google Scholar]
- Mart, J.; Klein, A.; Soldatov, A. Feasibility Study of a Soluble Boron–Free Small Modular Integral Pressurized Water Reactor. Nucl. Technol. 2014, 188, 8–19. [Google Scholar] [CrossRef]
- Kang, H.O.; Lee, B.J.; Lim, S.G. Light water SMR development status in Korea. Nucl. Eng. Design 2024, 419, 112966. [Google Scholar] [CrossRef]
- Agarwal, P.; Orazem, M.E.; Garcia-Rubio, L.H. Application of measurement models to impedance spectroscopy III. Evaluation of consistency with the Kramers-Kronig relations. J. Electrochem. Soc. 1995, 142, 4159–4168. [Google Scholar] [CrossRef]
- You, C.; Zabara, M.; Orazem, M.; Ulgut, B. Application of the Kramers–Kronig Relations to Multi-Sine Electrochemical Impedance Measurements. J. Electrochem. Soc. 2020, 167, 020515. [Google Scholar] [CrossRef]
- Betova, I.; Bojinov, M.; Karastoyanov, V.; Kinnunen, P.; Saario, T. Effect of water chemistry on the oxide film on Alloy 690 during simulated hot functional testing of a pressurized water reactor. Corros. Sci. 2012, 58, 20–32. [Google Scholar] [CrossRef]
- Engler, N.; Marion, A.; Fournier, L.; Rahmouni, K.; El Euch, S.; Bojinov, M. Steam Generator Tube Release: Assessment by the Use of Electrochemical Impedance Spectroscopy. In Proceedings of the Nuclear Plant Chemistry Conference NPC 2016, Brighton, UK, 2–7 October 2016. paper 73. [Google Scholar]
- Wan, T.; Saccoccio, M.; Chen, C.; Ciucci, F. Influence of the Discretization Methods on the Distribution of Relaxation Times Deconvolution: Implementing Radial Basis Functions with DRTtools. Electrochim. Acta 2015, 184, 483–499. [Google Scholar] [CrossRef]
- Fromhold, A., Jr. Single Carrier Steady-State Theory for Formation of Anodic Films Under Conditions of High Space Charge in Very Large Electric Fields. J. Electrochem. Soc. 1977, 124, 538–549. [Google Scholar] [CrossRef]
- Sharifi-Asl, A.; Taylor, M.; Lu, Z.; Engelhardt, G.; Kursten, B.; Macdonald, D.D. Modeling of the electrochemical impedance spectroscopic behavior of passive iron using a genetic algorithm approach. Electrochim. Acta 2013, 102, 161–173. [Google Scholar] [CrossRef]
- Bai, Z.; Li, Y.; Zhu, W.; Wang, Q.; Gao, P.; Wang, S.; Ding, S. Temperature-dependent electrochemical corrosion mechanisms of alloy 690 in simulated primary coolant of PWRs: Insights from in-situ electrochemical characteristics and micro-nano scale dynamics. Electrochim. Acta 2025, 540, 147140. [Google Scholar] [CrossRef]
| Content, wt% | C | Fe | Cr | Cu | Mn | Ni | Al | Si | Mo |
|---|---|---|---|---|---|---|---|---|---|
| nominal | ≤0.03 | 9.0–10.0 | 29.0–31.0 | 0.05 | 0.10 | Bal. | ≤0.50 | 0.10 | 0.15 |
| analyzed | 0.025 | 9.1 | 29.5 | 0.03 | 0.07 | Bal. | 0.17 | 0.14 | 0.14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bojinov, M.; Betova, I.; Karastoyanov, V. Corrosion and Anodic Oxidation of Alloy 690 in Simulated Primary Coolant of a Small Modular Reactor Studied by In Situ Electrochemical Impedance Spectroscopy. Metals 2025, 15, 1242. https://doi.org/10.3390/met15111242
Bojinov M, Betova I, Karastoyanov V. Corrosion and Anodic Oxidation of Alloy 690 in Simulated Primary Coolant of a Small Modular Reactor Studied by In Situ Electrochemical Impedance Spectroscopy. Metals. 2025; 15(11):1242. https://doi.org/10.3390/met15111242
Chicago/Turabian StyleBojinov, Martin, Iva Betova, and Vasil Karastoyanov. 2025. "Corrosion and Anodic Oxidation of Alloy 690 in Simulated Primary Coolant of a Small Modular Reactor Studied by In Situ Electrochemical Impedance Spectroscopy" Metals 15, no. 11: 1242. https://doi.org/10.3390/met15111242
APA StyleBojinov, M., Betova, I., & Karastoyanov, V. (2025). Corrosion and Anodic Oxidation of Alloy 690 in Simulated Primary Coolant of a Small Modular Reactor Studied by In Situ Electrochemical Impedance Spectroscopy. Metals, 15(11), 1242. https://doi.org/10.3390/met15111242






